Management of Pepper Weevil (Anthonomus eugenii Cano) (Coleoptera: Curculionidae) Using Biorational and Conventional Insecticides in South Florida
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
Study Area and Field Preparation
3. Experimental Design, Insecticide Application, and Evaluation of Treatments
4. Results
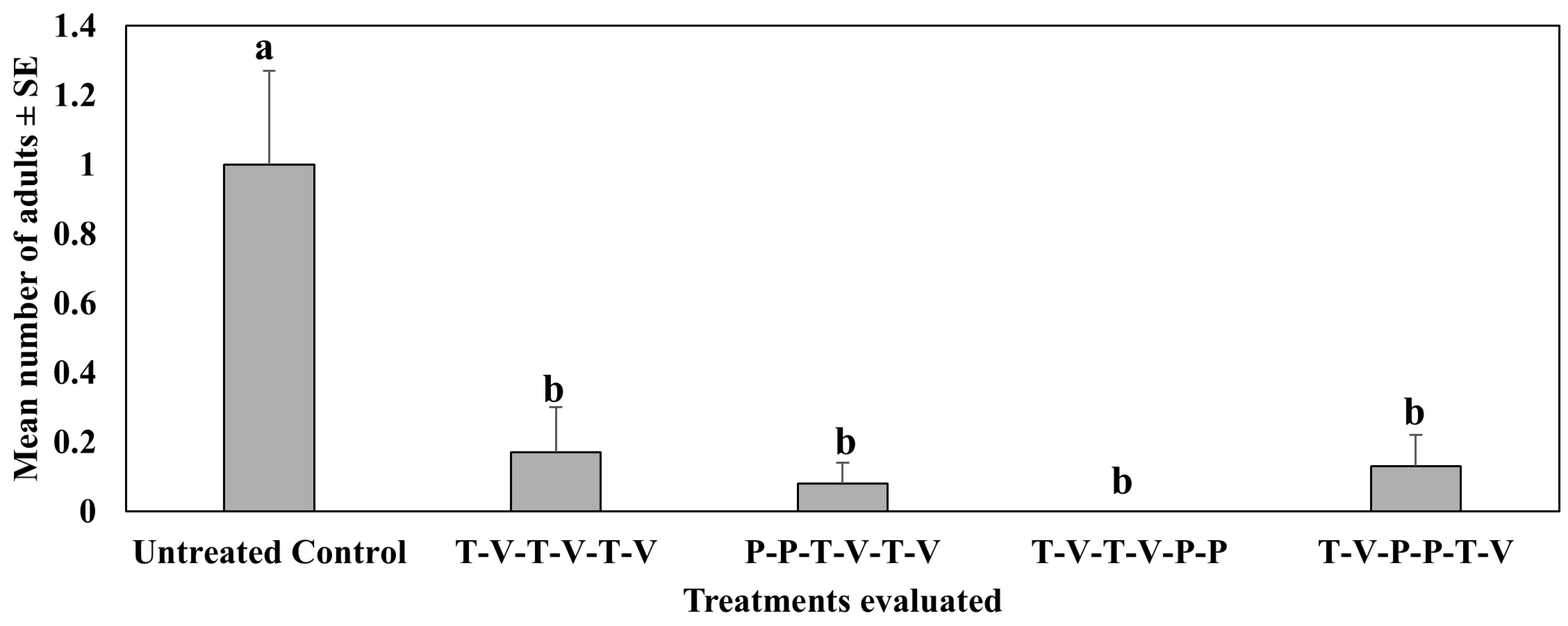
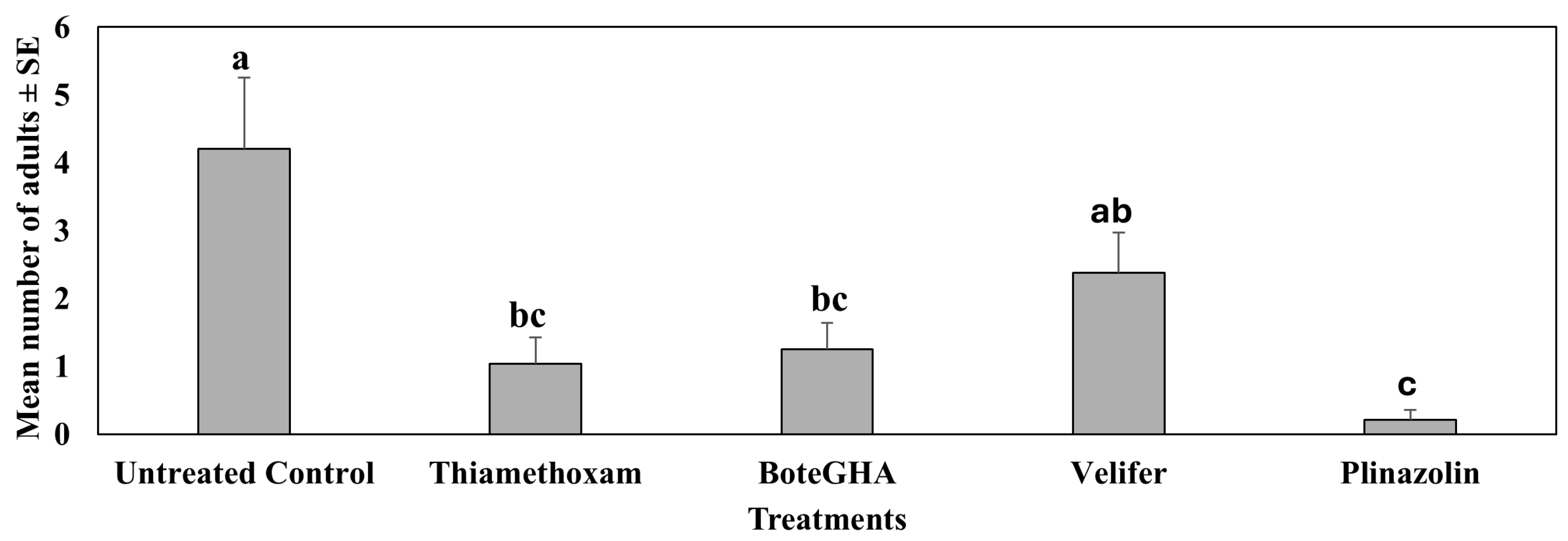
4.1. Treatment Effect on Pepper Weevil Adults on Foliage
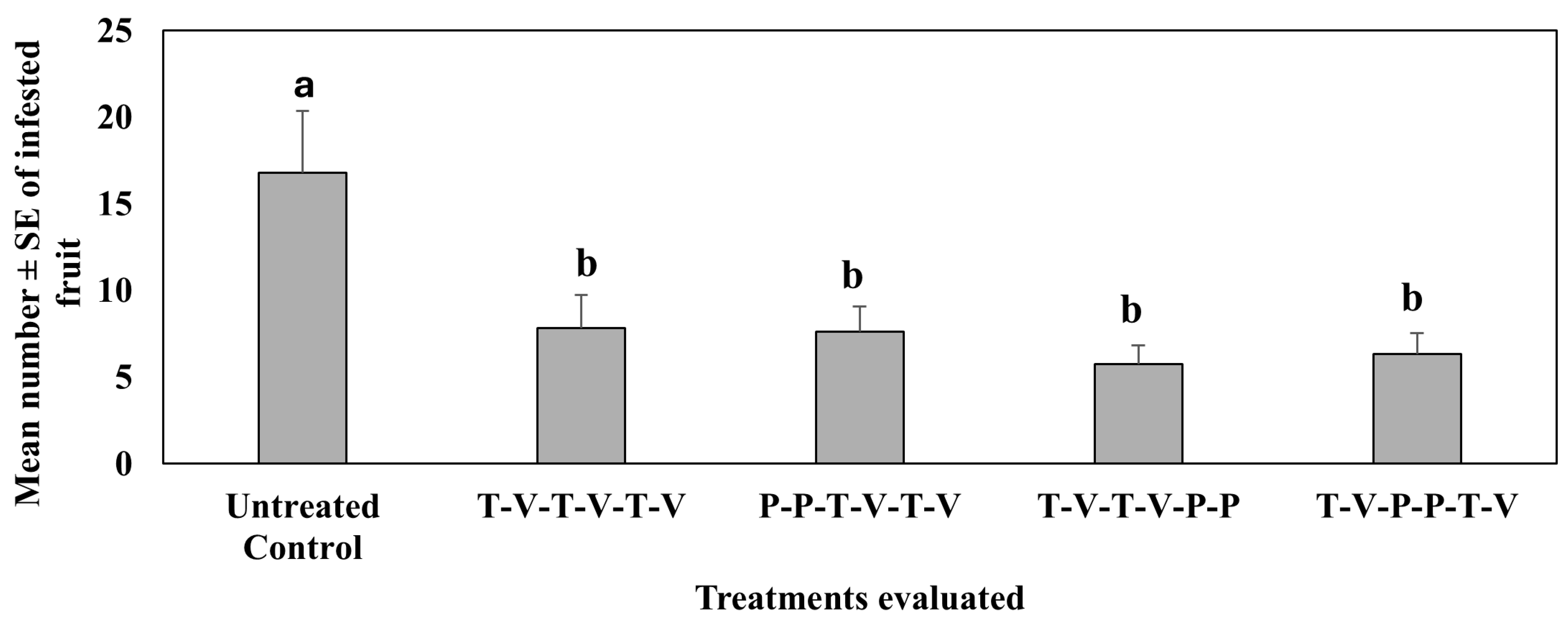
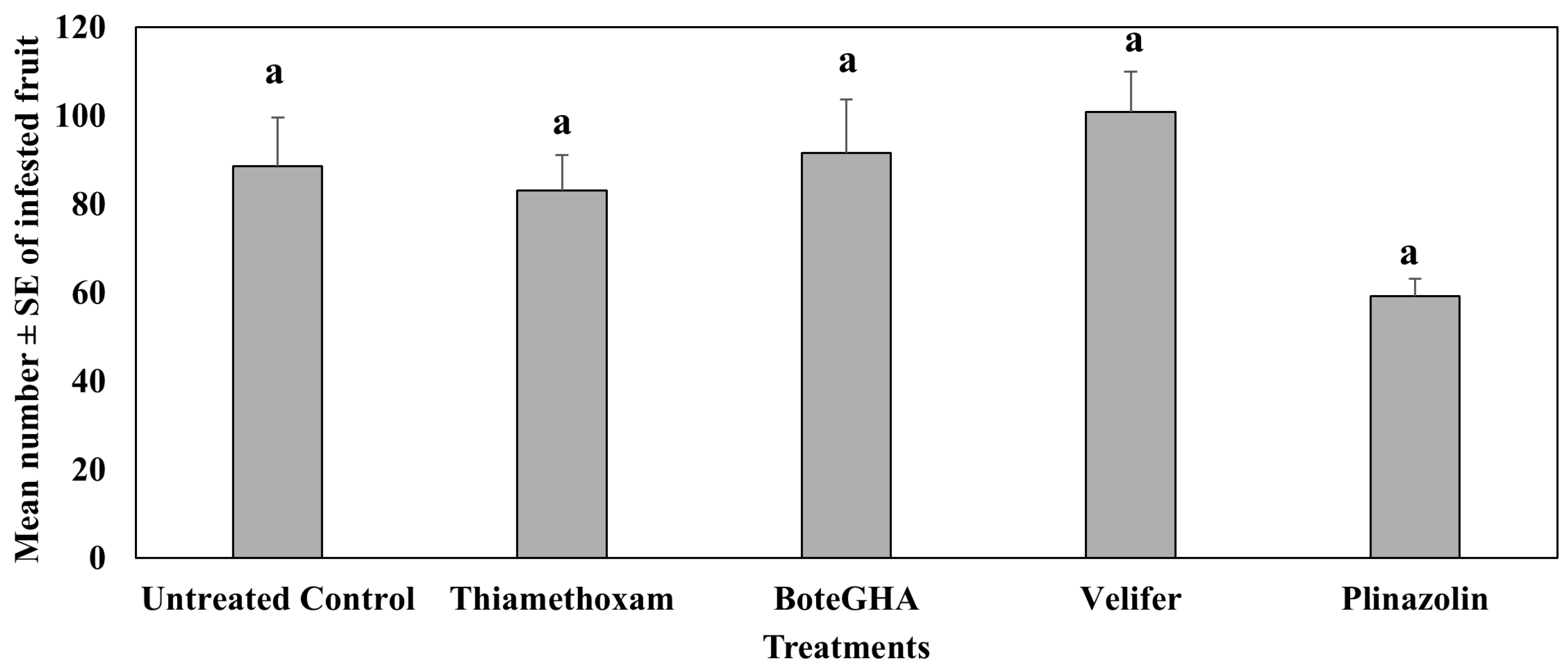
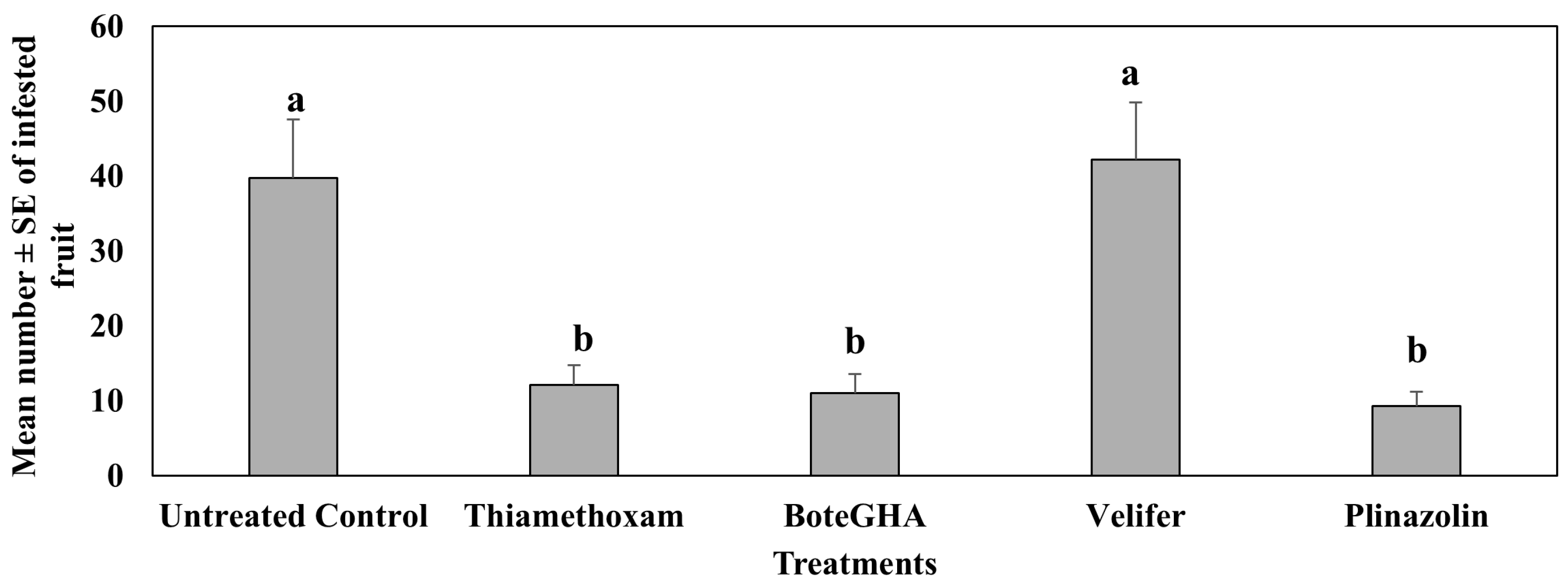
4.2. Treatment Effect on Pepper Weevil-Infested Fruit
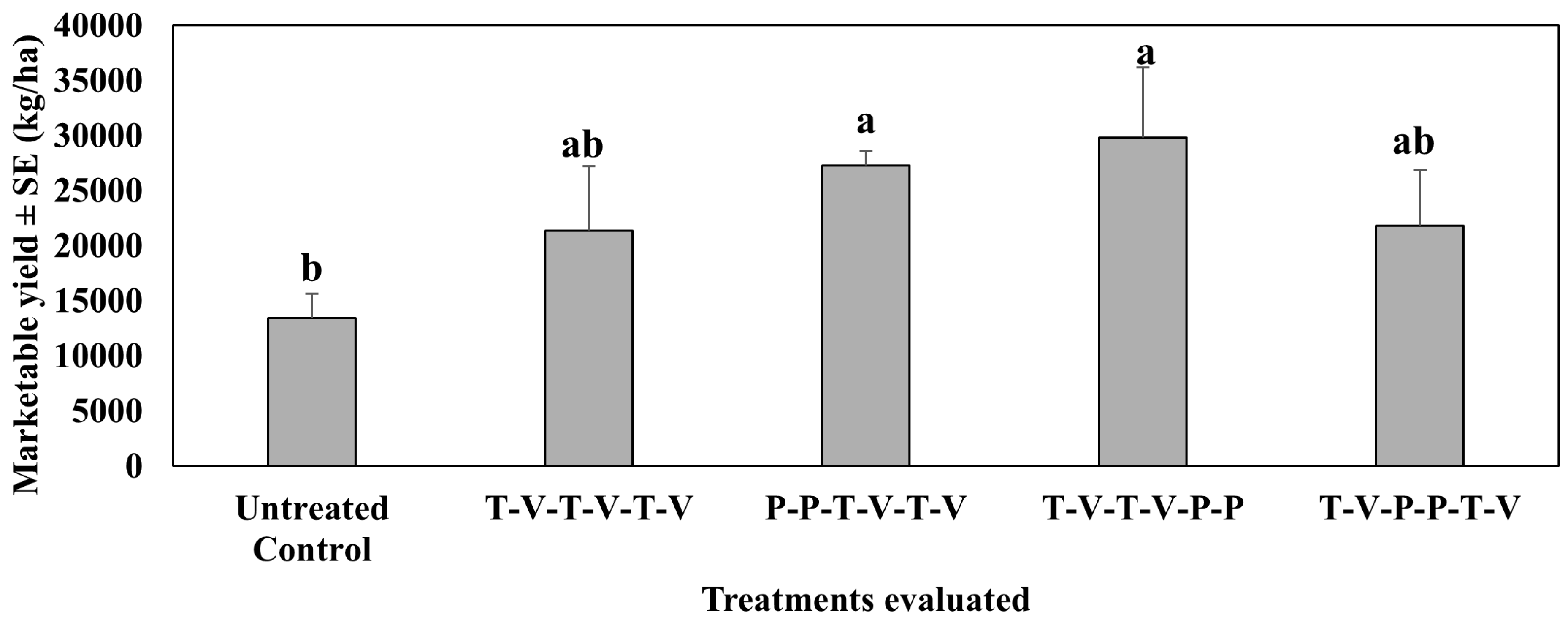
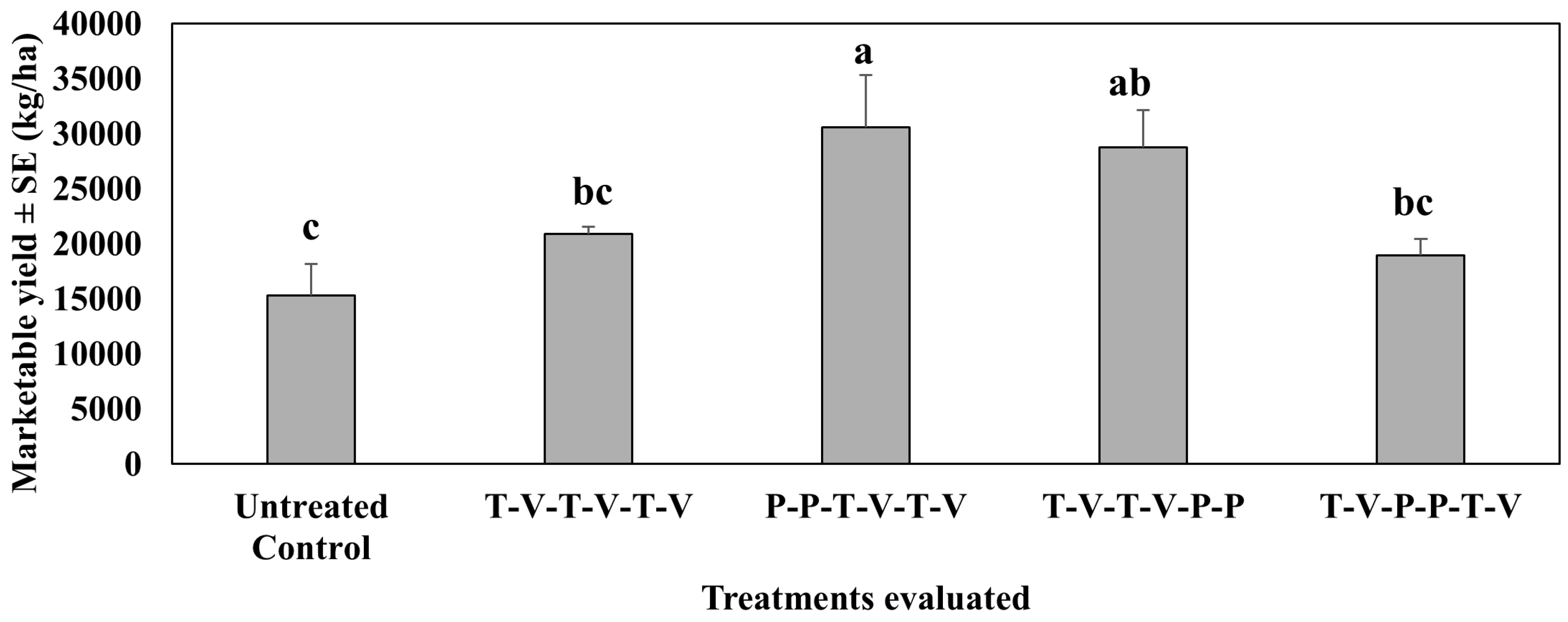
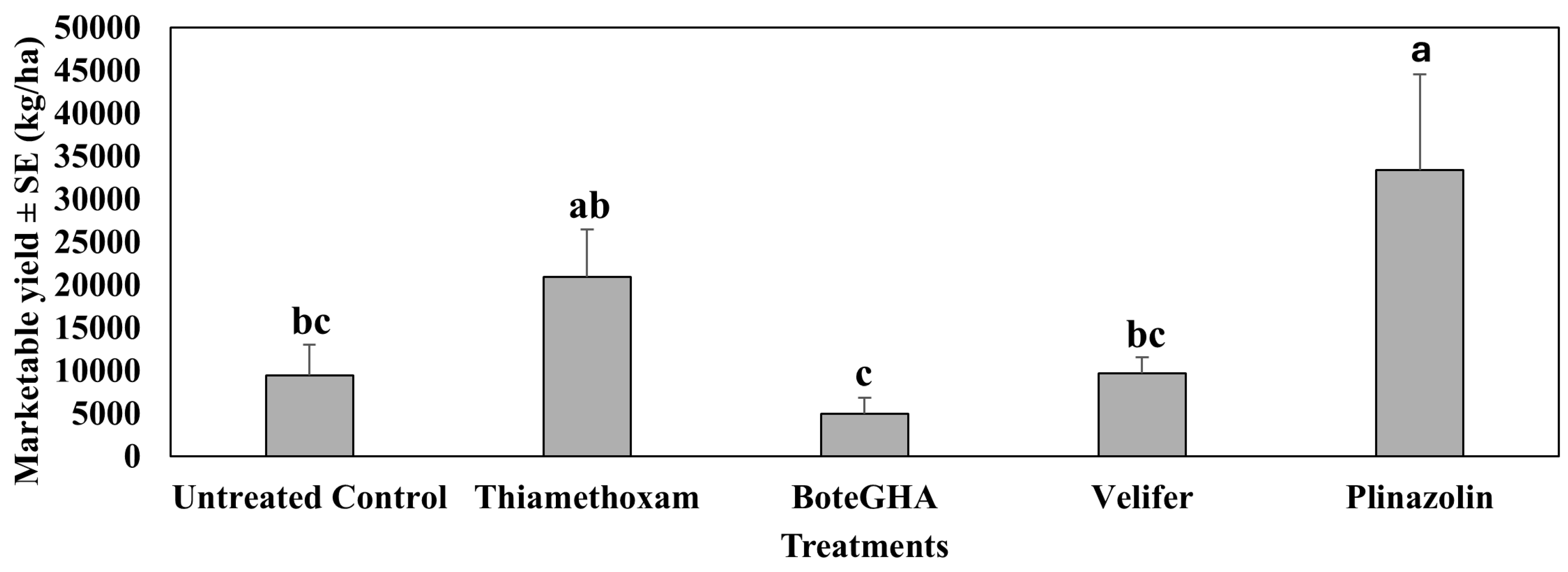
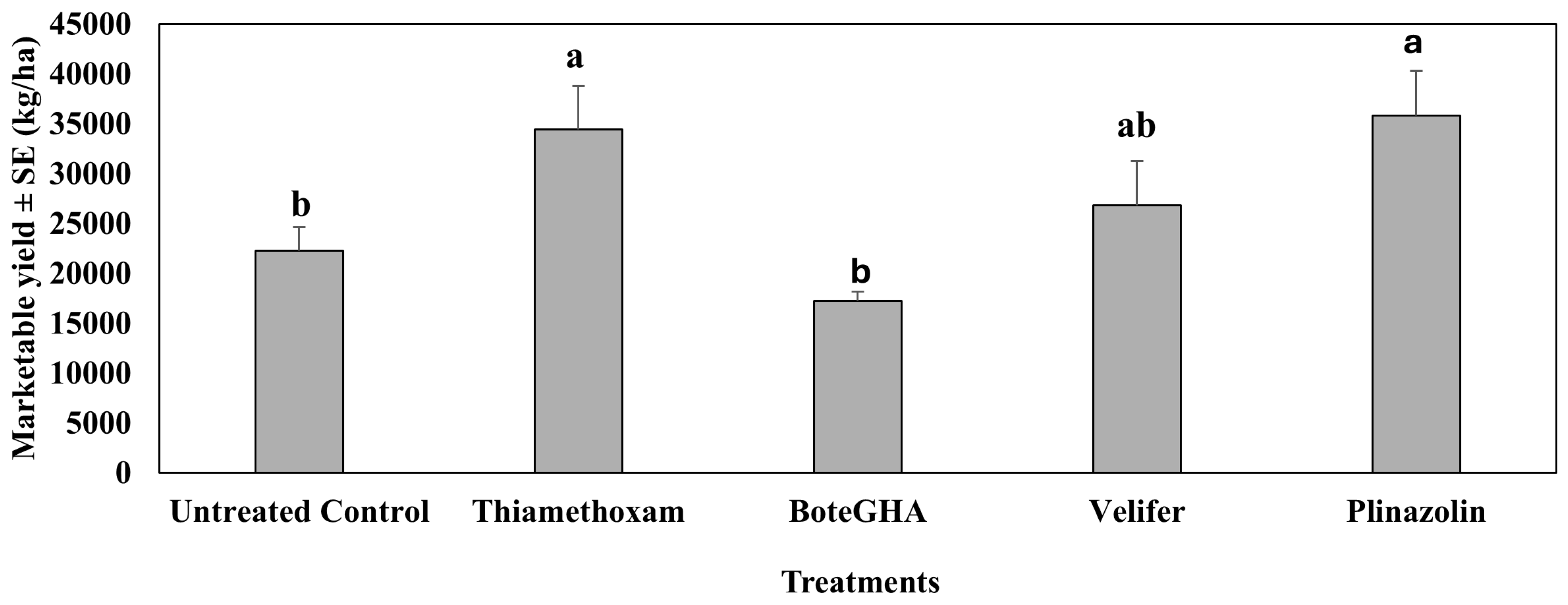
4.3. Marketable Yield
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andrews, K.L.; Rueda, A.; Gandini, G.; Evans, S.; Arango, A.; Avedillo, M. A Supervised control program for the pepper weevil, Anthonomus eugenii Cano, in Honduras, Central America. Int. J. Pest Manag. 1986, 32, 1–4. [Google Scholar]
- Patrock, R.J.; Schuster, D.J. Feeding, oviposition, and development of pepper weevil (Anthonomus eugenii Cano) on selected species of Solanaceae. Trop. Pest Manag. 1992, 38, 65–69. [Google Scholar] [CrossRef]
- Addesso, K.M.; McAuslane, H.J. Pepper weevil attraction to volatiles from host and nonhost plants. Environ. Entomol. 2009, 38, 216–224. [Google Scholar] [CrossRef]
- Seal, D.R.; Lamberts, M.L. Pepper Weevil, Anthonomus eugenii Cano (Coleoptera: Curculionidae), an Important Pest of Pepper; The Vegetarian Newsletter, No. 574; University of Florida: Gainesville, FL, USA, 2012. [Google Scholar]
- Seal, D.R.; Razzak, M.; Khan, R.A.; Sabines, C.M. Pepper weevil (PW), Anthonomus eugenii Cano (Col: Curculionidae), abundance in ‘jalapeño’ pepper under various management regimes. Proc. Fla. State Hort. Soc. 2016, 129, 167–171. [Google Scholar]
- Seal, D.R.; Martin, C.G. Laboratory rearing of pepper weevils (Coleoptera: Curculionidae) using artificial leaf balls and a boll weevil diet. J. Entomol. Sci. 2017, 52, 395–410. [Google Scholar] [CrossRef][Green Version]
- Elmore, J.C.; Davis, A.C.; Campbell, R.E. The Pepper Weevil; Technical Bulletin; USDA: Washington, DC, USA, 1934; Volume 447, p. 27. [Google Scholar][Green Version]
- Schultz, P.B.; Kuhar, T.P. First record of pepper weevil infestation in Virginia. Plant Health Progress 2008, 9, 49. [Google Scholar] [CrossRef]
- Van Der Gaag, D.J.; Loomans, R. Pest Risk Analysis for Anthonomus eugenii; Netherlands Food and Consumer Product Safety Authority: Utrecht, The Netherlands, 2013. [Google Scholar]
- Van De Vossenberg, B.T.L.H.; Warbroek, T.; Ingerson-Mahar, J.; Waalwijk, C.; Van Der Gouw, L.P.; Eichinger, B.; Loomans, A.J.M. Tracking outbreak populations of the pepper weevil Anthonomus eugenii (Coleoptera: Curculionidae) using complete mitochondrial genomes. PLoS ONE 2019, 14, e0221182. [Google Scholar] [CrossRef] [PubMed]
- Speranza, S.; Colonnelli, E.; Garonna, A.P.; Laudonia, S. First record of Anthonomus eugenii (Coleoptera: Curculionidae) in Italy. Fla. Entomol. 2014, 97, 844–845. [Google Scholar] [CrossRef]
- Costello, R.A.; Gillespie, D.R. The pepper weevil, Anthonomus eugenii Cano as a greenhouse pest in Canada. IOBC-WPRS Bull. 1993, 16, 3134. [Google Scholar]
- Fernandez, D.C.; Sinclair, B.; Vanlaerhoven, S.; Labbe, R. Biology and overwintering potential of the pepper weevil, Anthonomus eugenii (Coleoptera: Curculionidae). IOBC-WPRS Bull. 2017, 124, 224–229. [Google Scholar]
- Labbe, R.; Hilker, R.; Gagnier, D.; McCreary, C.; Gibson, G.A.P.; Fernandez-Triana, J.; Mason, P.G.; Gariepy, T.D. Natural enemies of Anthonomus eugenii (Coleoptera: Curculionidae) in Canada. Can. Entomol. 2018, 150, 404–411. [Google Scholar] [CrossRef]
- Seal, D.R.; Schuster, D.J. Control of the pepper weevil, Anthonomus eugenii, in west-central and south-Florida. Proc. Fla. State Hort. Soc. 1995, 108, 220–225. [Google Scholar]
- Garcia-Nevarez, G.; Quiñones-Pando, F.J.; Lujan-Favela, M.; Chavez-Sanchez, N. Manejo Integrado de Picudo del Chile; Folleto técnico numero 36; INIFAP-Delicias: Chihuahua, Mexico, 2010. [Google Scholar]
- Garcia-Nevarez, G.; Campos-Figueroa, M.; Chavez-Sanchez, N.; Quiñones-Pando, F.J. Efficacy of biorational and conventional insecticides against the pepper weevil, Anthonomus eugenii Cano (Coleoptera: Curculionidae) in the South-Central Chihuahua. Southwest. Entomol. 2012, 37, 391–401. [Google Scholar] [CrossRef]
- Servin-Villegas, R.; Garcia-Hernandez, J.; Tejas-Romero, A.; Martinez-Carrillo, J.L.; Toapanta, M.A. Susceptibility of pepper weevil (Anthonomus eugenii Cano) (Coleoptera: Curculionidae) to seven insecticides in rural areas of Baja California Sur, Mexico. Acta Zool. Mex. 2008, 24, 45–54. [Google Scholar] [CrossRef][Green Version]
- Eller, F.J.; Bartelt, R.J.; Shasha, B.S.; Schuster, D.J.; Riley, D.G.; Stansly, P.A.; Mueller, T.F.; Shuler, K.D.; Davis, J.H.; Sutherland, C.A. Aggregation pheromone for the pepper weevil, Anthonomus eugenii Cano (Coleoptera: Curculionidae): Identification and field activity. J. Chem. Ecol. 1994, 20, 1537–1555. [Google Scholar] [CrossRef] [PubMed]
- Labbé, R.M.; Gagnier, D.; Rizzato, R.; Tracey, A.; McCreary, C. Assessing new tools for management of the pepper weevil (Coleoptera: Curculionidae) in greenhouse and field pepper crops. J. Econ. Entomol. 2020, 113, 1903–1912. [Google Scholar] [CrossRef] [PubMed]
- Servin, R.; Aguilar, R.; Martinez, J.L.; Troyo, E.; Ortega, A. Monitoring of resistance to three insecticides on pepper weevil (Anthonomus eugenii) in populations from Baja California Sur, Mexico. Interciencia 2002, 27, 691–694. [Google Scholar]
- Ruiz-Sanchez, E.; Aguilar-Ochoa, O.; Alejo, J.C.; Tun Suarez, J.M.; Latournerie-Moreno, L.; Perz-Gutierrez, A. Comparación de la efectividad de un insecticida botánico y dos químicos convencionales en el control del picudo (Anthonomus eugenii Cano) (Coleoptera: Curculionidae) en chile habanero (Capsicum chinense Jacq.). Fitosanidad 2009, 13, 117–120. [Google Scholar]
- Caballero, R.; Schuster, D.J.; Smith, H.A.; Mangandi, J.; Portillo, H.E. A systemic bioassay to determine susceptibility of the pepper weevil, Anthonomus eugenii Cano (Coleoptera: Curculionidae) to cyantraniliprole and thiamethoxam. Crop Prot. 2015, 72, 16–21. [Google Scholar] [CrossRef]
- Avendaño-Meza, F.; Corrales-Madrid, J.L.; Parra-Terraza, S.; Medina-Lopez, R.; Gasper-Aguilar, S.S.; Avendaño-Jatomea, F.D. Baselines of susceptibility to three insecticides in pepper weevil Anthonomus eugenii Cano, 1824 (Coleoptera: Curculionidae) from Sinaloa State, Mexico. Entomol. Mex. 2016, 3, 775–780. [Google Scholar]
- Addesso, K.M.; Stansly, P.A.; Kostyk, B.C.; McAuslane, H.J. Organic treatments for control of pepper weevil (Coleoptera: Curculionidae). Fla. Entomol. 2014, 97, 1148–1156. [Google Scholar] [CrossRef]
- Nauen, R.; Bretschneider, T.; Elbert, A.; Fisher, R.; Reckmann, U.; van Waetermeulen, X. Biological and mechanistic considerations on the mode of action of Spirotetramat. In Proceedings of the 11th IUPAC International Congress of Pesticide Chemistry, Kobe, Japan, 6–11 August 2006; p. 109. [Google Scholar]
- Nauen, R.; Reckmann, U.; Thomzik, J.; Thielert, W. Biological profile of Spirotetramat (Movento®)—A new two-way systemic (ambimobile) insecticide against sucking pest species. Bayer Crop. J. 2008, 61, 245–278. [Google Scholar]
- Jeschke, P.; Haas, M.; Nauen, R.; Gutbrod, O.; Beck, M.E.; Matthiesen, S.; Velten, R. Sivanto®, a Novel Insecticide with a Sustainable Profile; Bayer CropScience AG, R&D: Monheim am Rhein, Germany, 2015. [Google Scholar]
- Nauen, R.; Denholm, I. Resistance of insect pests to neonicotinoid insecticides: Current status and prospects. Arch. Insect Biochem. Physiol. 2005, 58, 200–215. [Google Scholar] [CrossRef]
- Dayan, F.E.; Haesaert, G.; Van Leeuwen, T.; Holden-Dye, L.; Crossthwaite, A.; Nauen, R. Pesticides modes of action and resistance: A perspective from the 2019 IUPAC congress. Outlook Pest Manag. 2019, 30, 157–163. [Google Scholar] [CrossRef]
- Adeleye, V.A.; Seal, D.R.; Liburd, O.E.; McAuslane, H.; Alborn, H. Pepper weevil, Anthonomus eugenii (Coleoptera: Curculionidae) suppression on jalapeño pepper using non-host insect repellent plants. J. Crop Prot. 2022, 154, 105893. [Google Scholar] [CrossRef]
- Arthur, F.H.; Yue, B.; Wilde, G.E. Susceptibility of stored-product beetles on wheat and maize treated with thiamethoxam: Effects of concentration, exposure interval, and temperature. J. Stored Prod. Res. 2004, 40, 527–546. [Google Scholar] [CrossRef]
- Tsaganou, F.C.; Vassilakos, T.N.; Athanassiou, C.G. Knockdown and mortality of five stored product beetle species after short exposures of thiamethoxam. J. Econ. Entomol. 2014, 107, 2222–2228. [Google Scholar] [CrossRef]
- Maienfisch, P.; Huerlimann, H.; Rindlisbacher, V.; Gsell, L.; Dettwiler, H.; Haettenschwiler, J.; Sieger, E.; Walti, M. The discovery of thiamethoxam: A second-generation neonicotinoid. Pest Manag. Sci. 2001, 57, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, J.; Kostyk, B.C. Insecticidal Rotations for Control of Pepper Weevil, Fall 2019. Arthropod Manag. Tests 2021, 46, tsab050. [Google Scholar] [CrossRef]
- Sydnor, T.; McIntyre, K.; Bekelja, K.; Kuhar, T.P. Efficacy of selected insecticides against flea beetle and harlequin bug in cabbage in Virginia, 2022. Arthropod Manag. Tests 2023, 48, tsac142. [Google Scholar] [CrossRef]
- Natwick, E.T.; Lopez, M.I. Insecticide Efficacy Against Egyptian Alfalfa Weevil, 2014a. Arthropod Manag. Tests 2015, 40, F24. [Google Scholar] [CrossRef][Green Version]
- Owens, D.; Malone, M. Control of alfalfa weevil infesting alfalfa, 2022. Arthropod Manag. Tests 2024, 49. [Google Scholar] [CrossRef]
- Kuhar, T.P.; Doughty, H. Evaluation of soil and foliar insecticide treatments for the control of foliar insect pests in cabbage in Virginia, 2008. Arthropod Manag. Tests 2009, 34, E7. [Google Scholar] [CrossRef]
- Blythe, J.; Earley, F.G.; Piekarska-Hack, K.; Firth, L.; Bristow, J.; Hirst, E.A.; Goodchild, J.A.; Hillesheim, E.; Crossthwaite, A.J. The mode of action of isocycloseram: A novel isoxazoline insecticide. Pestic. Biochem. Physiol. 2022, 187, 105217. [Google Scholar] [CrossRef]
- Bekelja, K. A Study of Neonicotinoid Seed Treatments in Bt Maize: Insect Resistance Management, Efficacy, and Environmental Fate. Ph.D. Thesis, Virginia Tech, Blacksburg, VA, USA, 2022; 94p. [Google Scholar]
- Buzza, A.M.; Alyokhin, A. Control of Colorado potato beetle on potato by isocycloseram. Arthropod Manag. Tests 2023, 49, tsad139. [Google Scholar] [CrossRef]
- Bekelja, K.M.; Malone, S.; Mascarenhas, V.; Taylor, S. A novel insecticide, isocycloseram, shows promise as an alternative to chlorpyrifos against a direct pest of peanut, Diabrotica undecimpunctata howardi (Coleoptera: Chrysomelidae). J. Econ. Entomol. 2024, 117, 537–544. [Google Scholar] [CrossRef]
- Lira, R.; Nascimento, D.V.; Lopes, K.C.; Soares, M.R.; Torres, J.B. Assessment of boll weevil susceptibility to isocycloseram and ethiprole and differential toxicity to natural enemies. Neotrop. Entomol. 2024, 53, 682–693. [Google Scholar] [CrossRef]
- Tiwari, G.; Kaur, N.; Anderson, N.P.; Tanner, K.C.; Lightle, D.M.; Willette, A.R.; Donovan, B.C.; Dorman, S.J. Evaluating foliar insecticides and economic thresholds for Tychius picirostris (Coleoptera: Curculionidae) management in Oregon white clover seed production. J. Econ. Entomol. 2024, 117, toae163. [Google Scholar] [CrossRef]
- Qureshi, J.A.; Kostyk, B.C.; Stansly, P.A. Foliar sprays of Delegate WG and Close 240 SC with adjuvants for control of Asian citrus psyllid and citrus leafminer. Proc. Fla. State Hort. Sci. 2014, 127, 60–63. [Google Scholar]
- Stanley, H.M. Effects of Spray Adjuvants on Insecticide Application, Efficacy, Rainfastness, Penetration of the Plant Canopy, and Residual Activity. Master’s Thesis, Mississippi State University, Starkville, MS, USA, 2002. [Google Scholar]
- Song, Y.; Huang, Q.; Huang, G.; Liu, M.; Cao, L.; Li, F.; Zhao, P.; Cao, C. The effects of adjuvants on the wetting and deposition of insecticide solutions on hydrophobic wheat leaves. Agronomy. 2022, 9, 2148. [Google Scholar] [CrossRef]
- Nyoike, T.W.; Liburd, O.E.; Webb, S.E. Suppression of whiteflies, Bemisia tabaci (Hemiptera: Aleyrodidae) and incidence of Cucurbit leaf crumple virus, a whitefly-transmitted virus of zucchini squash new to Florida, using mulches and imidacloprid. Florida Entomol. 2008, 92, 460–465. [Google Scholar] [CrossRef]
- Kousik, C.S.; Adkins, S.T.; Turechek, W.; Roberts, P.D. Use of reflective plastic mulch and insecticide sprays to manage viral watermelon vine decline i Florida, 2007. Plant Dis. Manag. Rep. 2008, 2, V169. [Google Scholar]
- Adeleye, V.A.; Seal, D.R.; Liburd, O.E.; Martini, X.; Meru, G. Integrated approach using insecticides in combination with reflective plastic mulch for the management of pepper weevil, Anthonomus eugenii (Coleoptera: Curculionidae). J. Environ. Entomol. 2023, 52, 391–398. [Google Scholar] [CrossRef] [PubMed]


| Treatments | Insecticides | Rate [oz]/Acre (kg/ha) |
|---|---|---|
| 1 | Spirotetramat 240 SC + Dyne-amic | 5.0 (0.35) + 0.25% v/v |
| 2 | Flupyradifurone + Dyne-amic | 14.0 (0.98) + 0.25% v/v |
| 3 | Spirotetramat 240 SC + Dyne-amic rotated with Flupyradifurone HL + Dyne-amic | 5.0 (0.35) + 0.25% v/v 14.0 (0.98) + 0.25% v/v |
| 4 | Thiamethoxam | 4.0 (0.28) |
| 5 | Control |
| Insecticide | Product Name | Active Ingredient | Rate | Timing | Company | |
|---|---|---|---|---|---|---|
| 1 | Control | - | - | - | ||
| 2 | Actara | Thiamethoxam | 5 oz/acre | Every 7 days | 4A | Syngenta (Greensboro, NC, USA) |
| 3 | Vydate | Oxamyl | 2 pint/acre | Every 7 days | 1A | Dupont (Wilmington, DE, USA) |
| 4 | Plinazolin | Isocycloseram | 3.1fl oz/A | Every 7 days | 30 | Syngenta (Greensboro, NC, USA) |
| 5 | Velifer | Beauveria bassiana | 500 mL/L | Every 7 days | Omri listed | BASF (Florham Park, NJ, USA) |
| 6 | BoteGHA | Beauveria bassiana strain GHA | 1 qt/acre | Every 4 days | Omri listed | Certis USA (Columbia, MD, USA) |
| Dyne-amic | Methylated seed oils | 0.25 v/v | Helena Agri-Enterprises, LLC (Collierville, TN, USA) |
| Trt | Wk 1 | Wk 2 | Wk 3 | Wk 4 | Wk 5 | Wk 6 |
|---|---|---|---|---|---|---|
| 1 W | Control | Control | Control | Control | Control | Control |
| 2 R | Isocycloseram + Dyne-amic | Isocycloseram + Dyne-amic | Thiamethoxam | Oxamyl | Thiamethoxam | Oxamyl |
| 3 B | Thiamethoxam | Oxamyl | Isocycloseram + Dyne-amic | Isocycloseram + Dyne-amic | Thiamethoxam | Oxamyl |
| 4 G | Thiamethoxam | Oxamyl | Thiamethoxam | Oxamyl | Isocycloseram + Dyne-amic | Isocycloseram + Dyne-amic |
| 5 Y | Thiamethoxam | Oxamyl | Thiamethoxam | Oxamyl | Thiamethoxam | Oxamyl |
| Treatments | Rate [oz]/A | 21 Mar | 29 Mar | 4 Apr | 12 Apr | 19 Apr | 26 Apr |
|---|---|---|---|---|---|---|---|
| Spirotetramat 240 SC | 5.0 | 0 a | 0.00 b | 0.00 b | 0.10 ab | 0.00 a | 0.35 a |
| Flupyradifurone | 14.0 | 0 a | 0.00 b | 0.00 b | 0.10 ab | 0.10 a | 0.35 a |
| Spirotetramat 240 SC Rotated with Flupyradifurone HL | 5.0 14.0 | 0 a | 0.10 a | 0.05 ab | 0.35 a | 0.10 a | 0.25 a |
| Thiamethoxam | 4.0 | 0 a | 0.00 b | 0.00 b | 0.00 b | 0.10 a | 0.05 a |
| Control | 0 a | 0.00 b | 0.15 a | 0.25 ab | 0.25 a | 0.45 a |
| Treatments | Rate [oz]/A | 21 Mar | 29 Mar | 4 Apr | 12 Apr | 19 Apr | 26 Apr |
|---|---|---|---|---|---|---|---|
| Spirotetramat 240 SC | 5.0 | 0.00 b | 0.25 b | 0.00 b | 0.20 b | 0.15 c | 0.30 b |
| Flupyradifurone | 14.0 | 0.30 b | 0.30 b | 0.00 b | 0.25 b | 0.55 b | 0.30 b |
| Spirotetramat 240 SC rotated with Flupyradifurone HL | 5.0 14.0 | 0.00 b | 0.30 b | 0.10 b | 0.25 b | 0.10 c | 0.40 b |
| Thiamethoxam | 4.0 | 0.00 b | 0.30 b | 0.00 b | 0.00 b | 0.15 c | 0.15 b |
| Control | 0.95 a | 1.50 a | 1.45 a | 1.65 a | 2.15 a | 2.00 a |
| Treatments | Rate [oz]/A | Weight of Harvested Fruits/Plot (in lbs.) |
|---|---|---|
| Spirotetramat 240 SC | 5.0 | 4.50 ab |
| Flupyradifurone HL | 14.0 | 3.25 bc |
| Spirotetramat 240 SC rotated with Flupyradifurone HL | 5.0 14.0 | 2.75 c |
| Thiamethoxam | 4.0 | 5.18 a |
| Control | 4.12 abc |
| Study/Monthly Average (F) | Month 1 | Month 2 | Month 3 | Month 4 | Average for Growing Season (F) |
|---|---|---|---|---|---|
| 1 | 64.98 (Jan) | 71.40 (Feb) | 70.44 (Mar) | 75.02 (Apr) | 70.46 |
| 2 | 71.21 (Mar) | 73.83 (Apr) | 77.72 (May) | 79.32 (Jun) | 75.52 |
| 3 | 69.89 (Feb) | 73.13 (Mar) | 75.24 (Apr) | 77.96 (May) | 74.06 |
| 4 | 73.13 (Mar) | 75.24 (Apr) | 77.96 (May) | 79.33 (Jun) | 76.42 |
| 5 | 68.11 (Dec) | 66.89 (Jan) | 71.09 (Feb) | 72.42 (Mar) | 69.63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adeleye, V.; Seal, D.; Liburd, O. Management of Pepper Weevil (Anthonomus eugenii Cano) (Coleoptera: Curculionidae) Using Biorational and Conventional Insecticides in South Florida. Insects 2025, 16, 1101. https://doi.org/10.3390/insects16111101
Adeleye V, Seal D, Liburd O. Management of Pepper Weevil (Anthonomus eugenii Cano) (Coleoptera: Curculionidae) Using Biorational and Conventional Insecticides in South Florida. Insects. 2025; 16(11):1101. https://doi.org/10.3390/insects16111101
Chicago/Turabian StyleAdeleye, Victoria, Dakshina Seal, and Oscar Liburd. 2025. "Management of Pepper Weevil (Anthonomus eugenii Cano) (Coleoptera: Curculionidae) Using Biorational and Conventional Insecticides in South Florida" Insects 16, no. 11: 1101. https://doi.org/10.3390/insects16111101
APA StyleAdeleye, V., Seal, D., & Liburd, O. (2025). Management of Pepper Weevil (Anthonomus eugenii Cano) (Coleoptera: Curculionidae) Using Biorational and Conventional Insecticides in South Florida. Insects, 16(11), 1101. https://doi.org/10.3390/insects16111101







