Genome-Wide Analysis and Functional Correlation of Tomato JAZ Genes Under Tuta absoluta Infestation and Nanoparticle-Induced Defense
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Experimental Design
2.3. Assessment of Pest-Induced Leaf Damage, Larval Survival, and Leaf Mining
2.4. Genome-Wide Identification of JAZ Genes
2.5. Determination of Physicochemical Properties
2.6. Domain and Motif Analysis
2.7. Phylogenetic and Gene Structure Analysis
2.8. Promoter Cis-Regulatory Elements Analysis
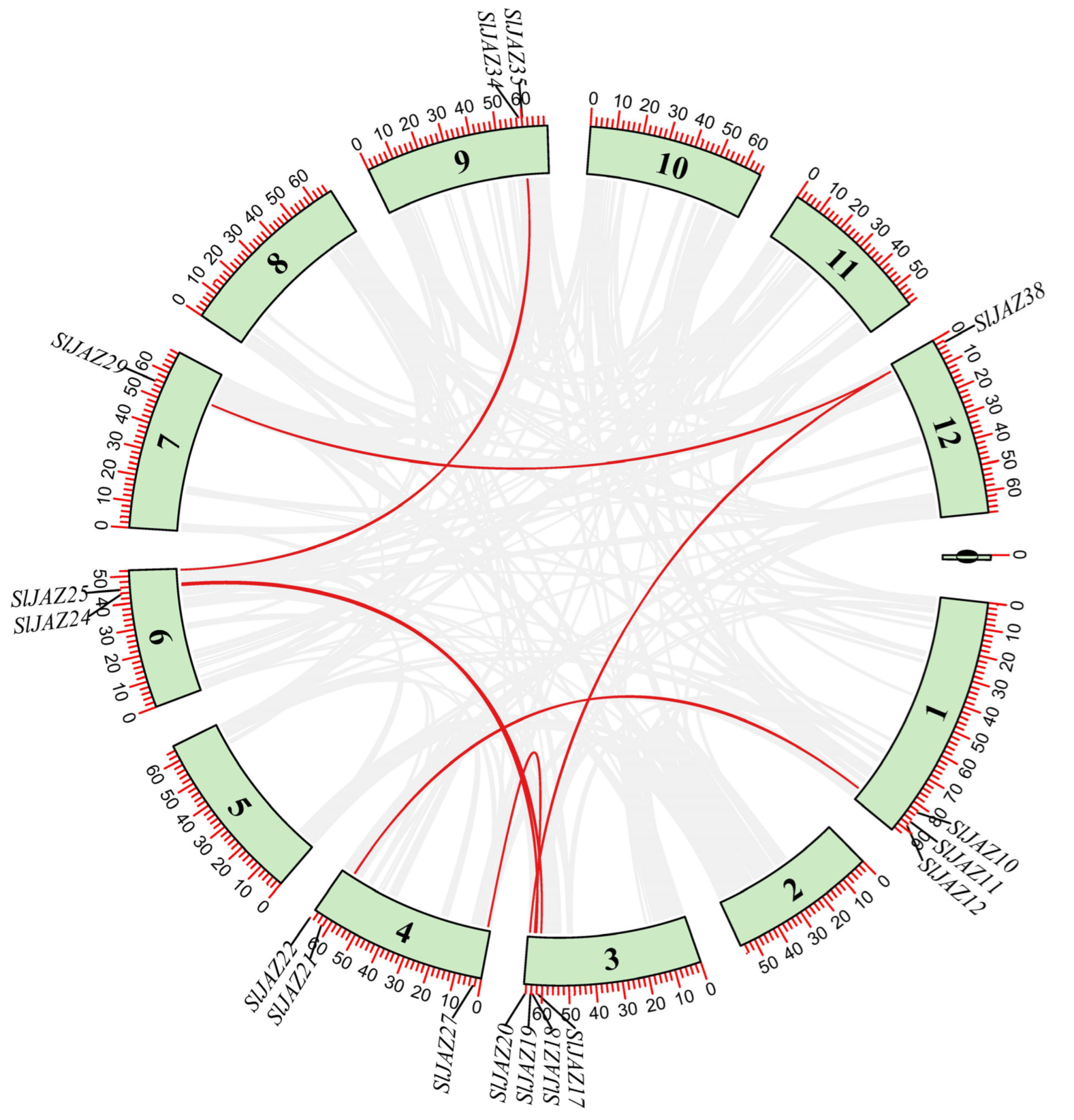
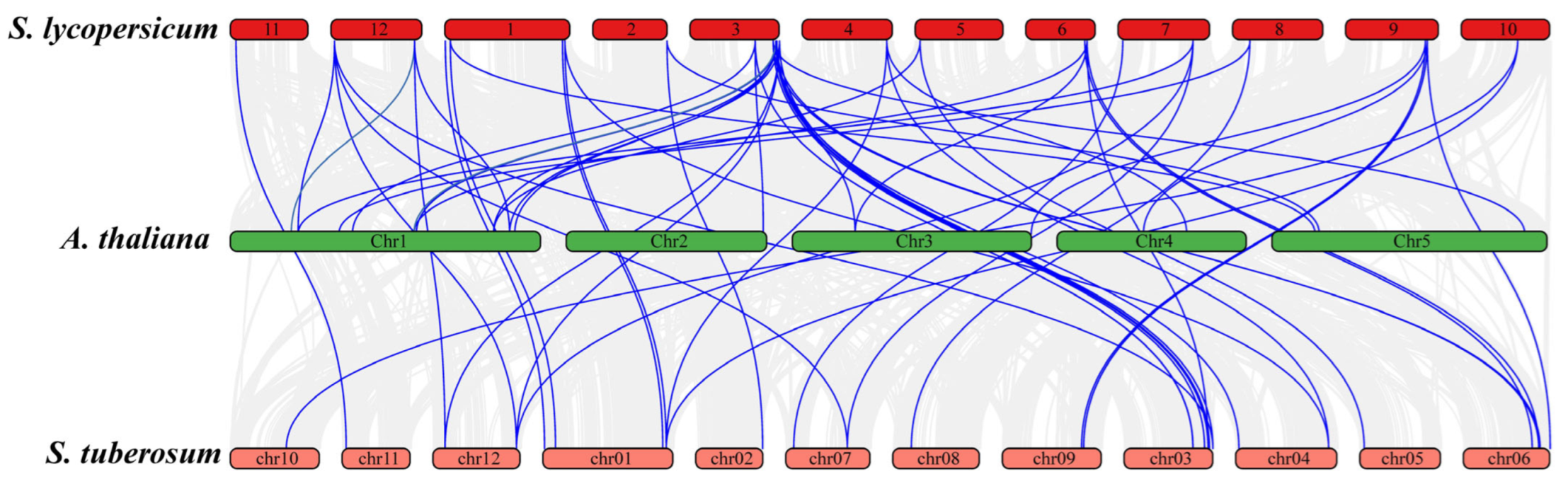
2.9. Gene Duplication and Synteny Analysis
2.10. Protein–Protein Interaction Analysis
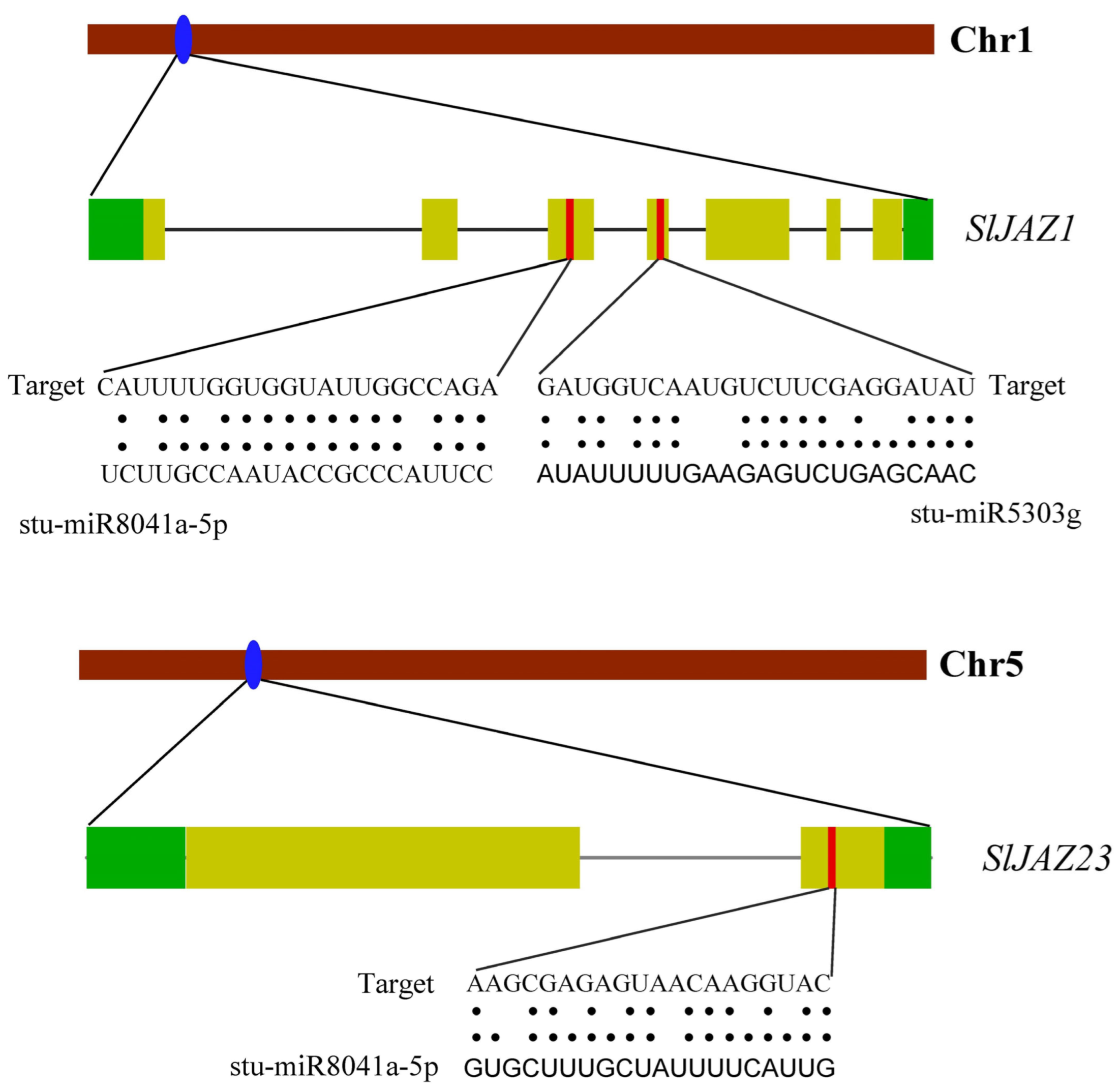
2.11. Prediction of microRNAs
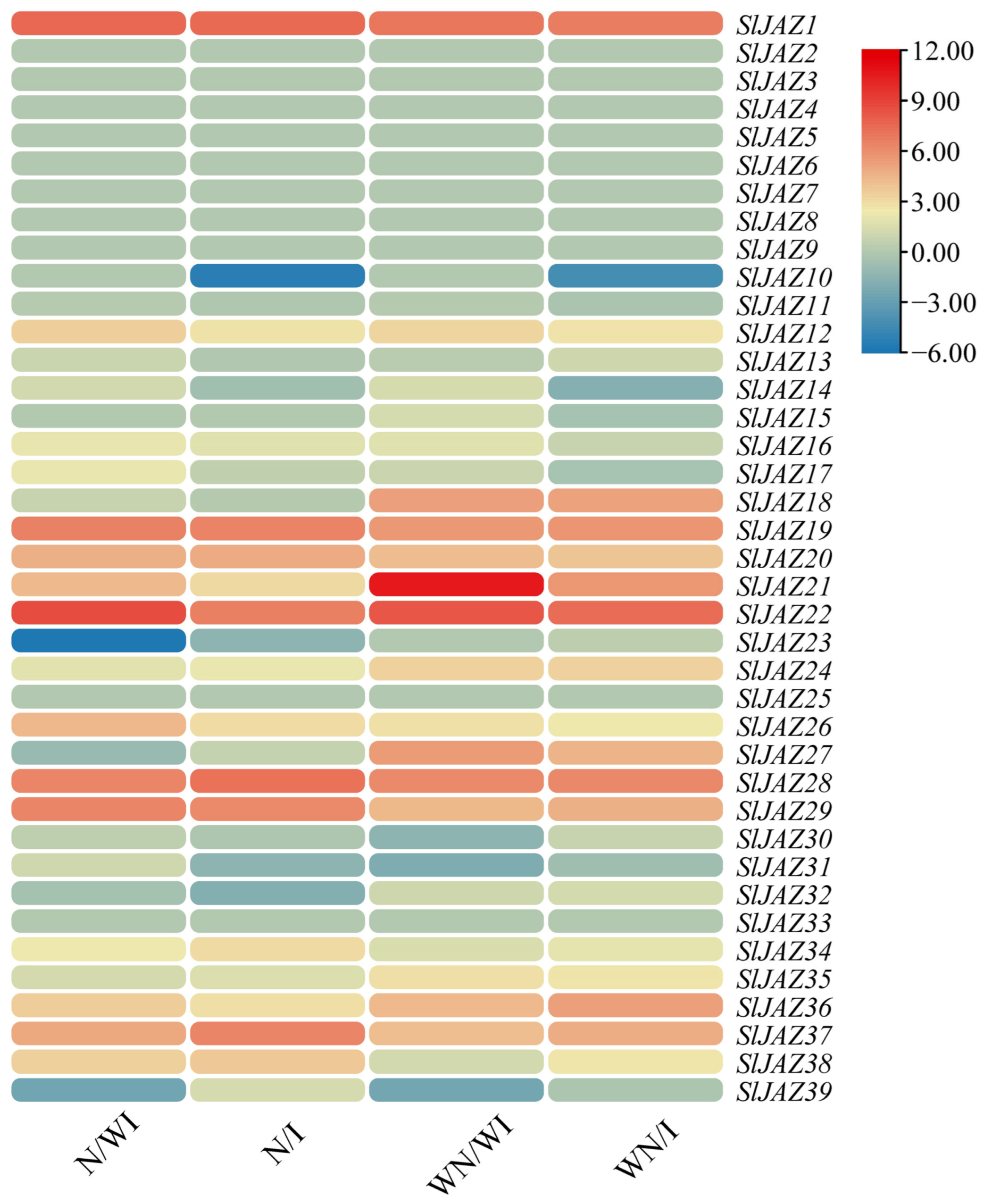
2.12. Gene Expression Analysis
2.13. qPCR-Based Expression Analysis
2.14. Statistical Analysis
3. Results
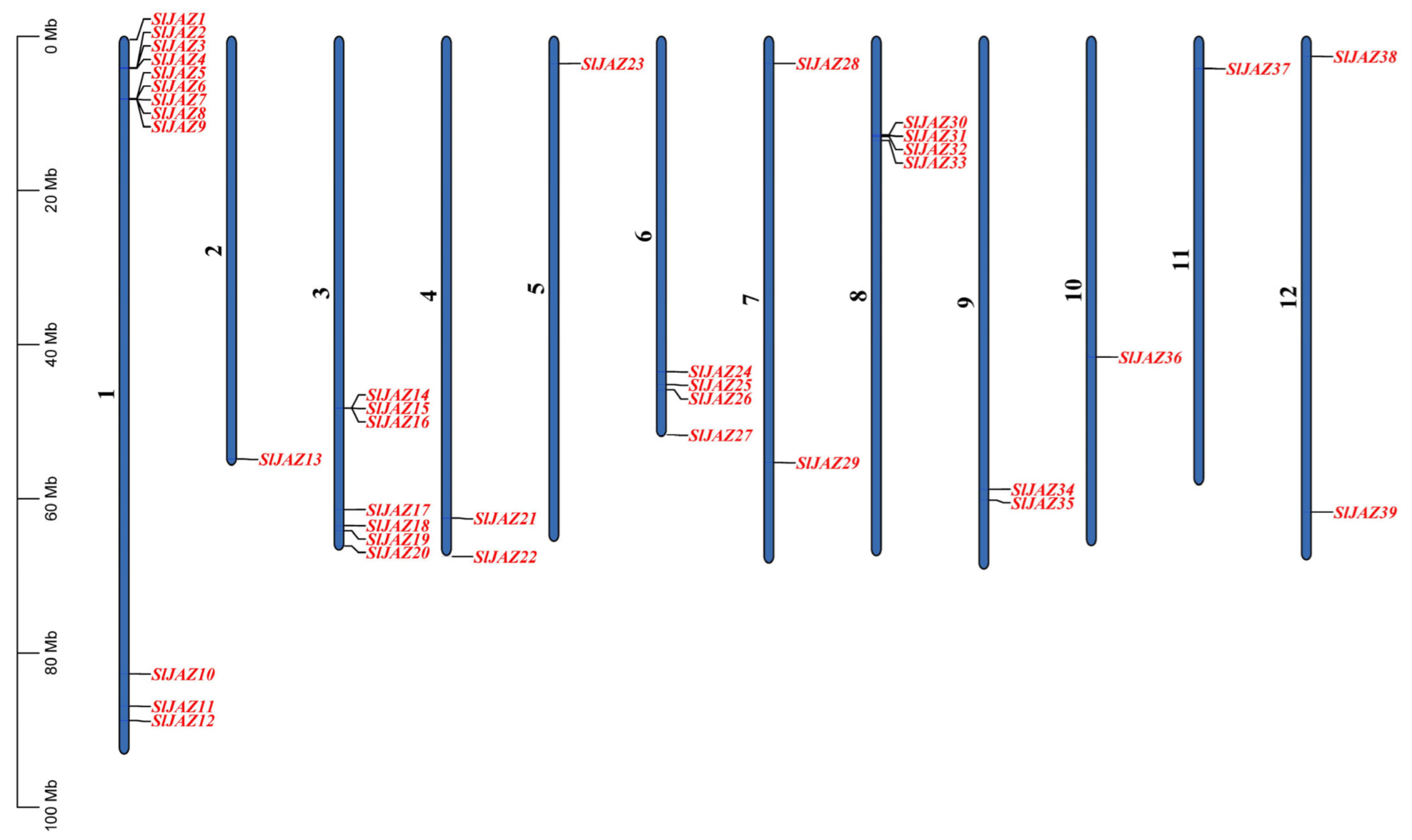
3.1. Identification and Distribution of JAZ Genes
3.2. Physicochemical Properties of SlJAZ Genes
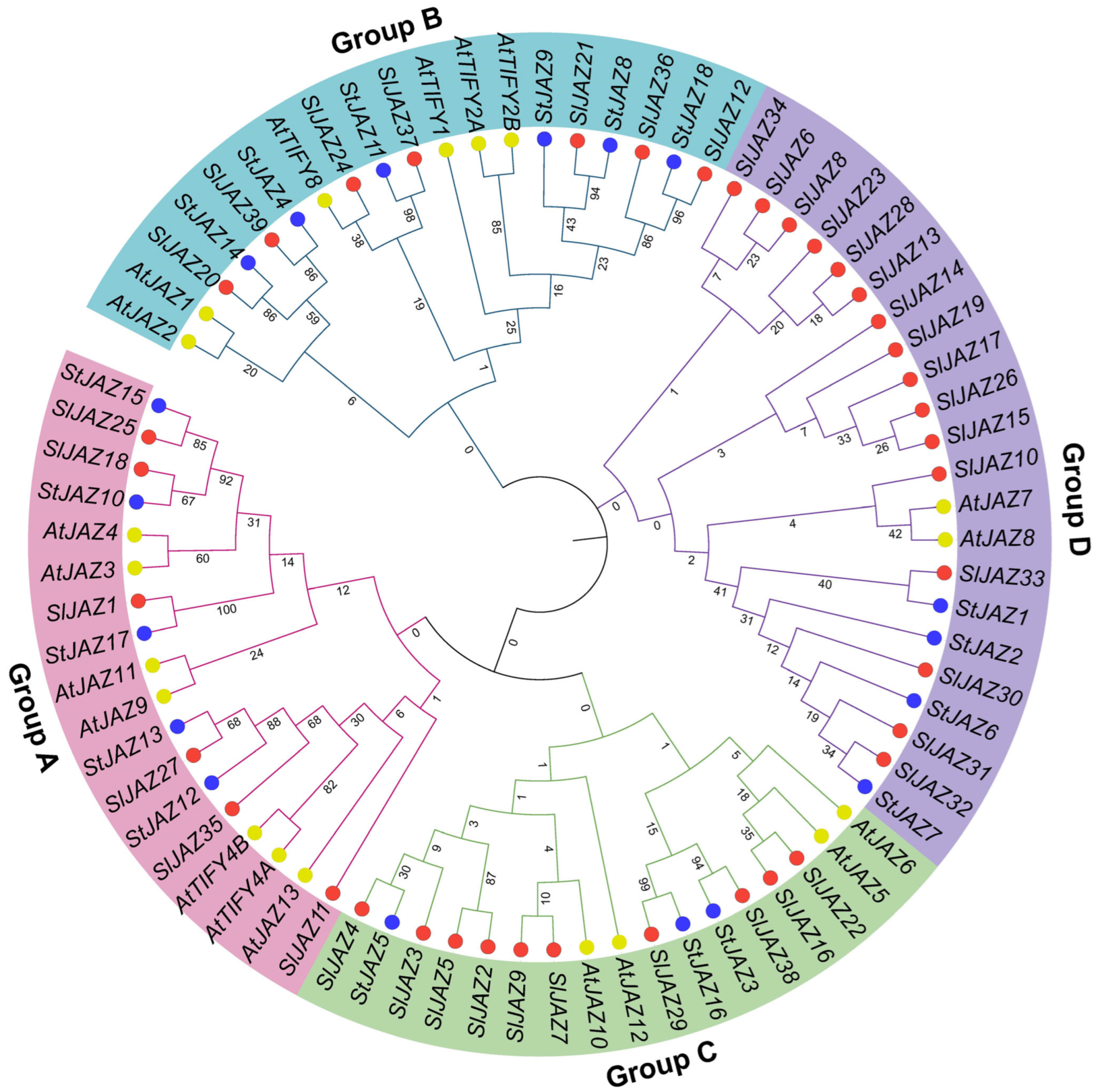
3.3. Phylogenetic Analysis
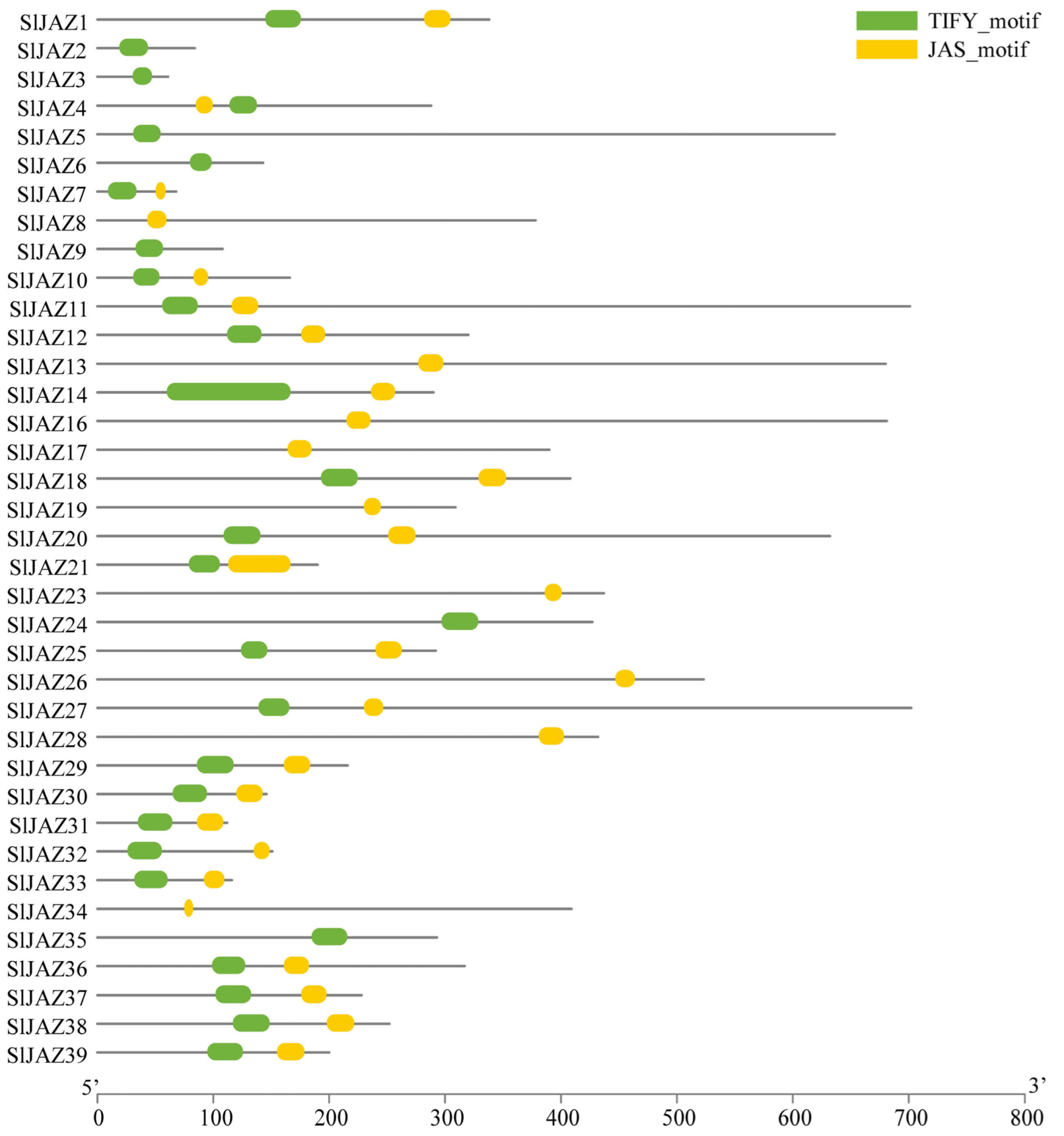
3.4. Motif, Gene Structure, and Domain Analysis
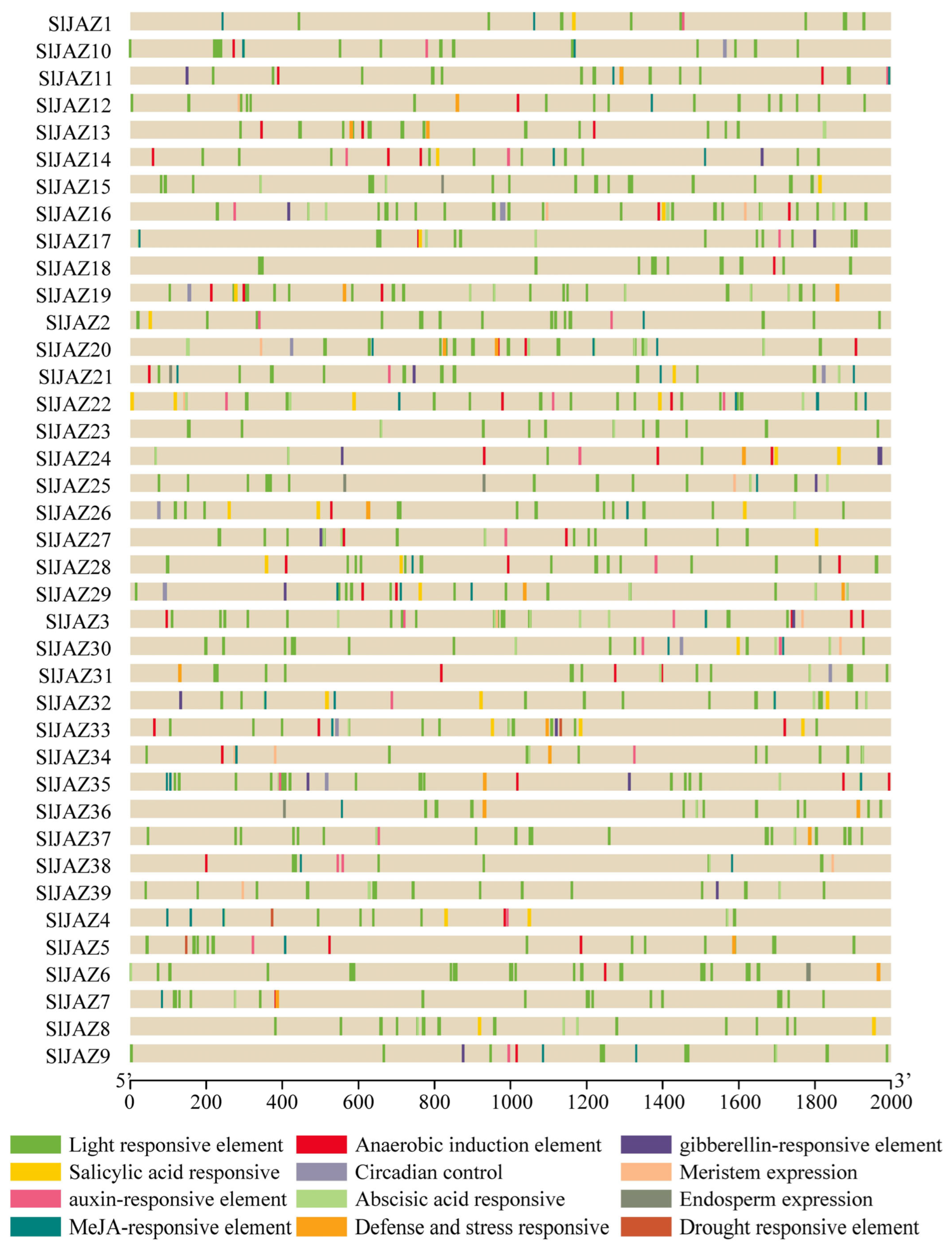
3.5. Cis-Regulatory Elements
3.6. Duplication and Synteny Analysis
3.7. miRNA Prediction
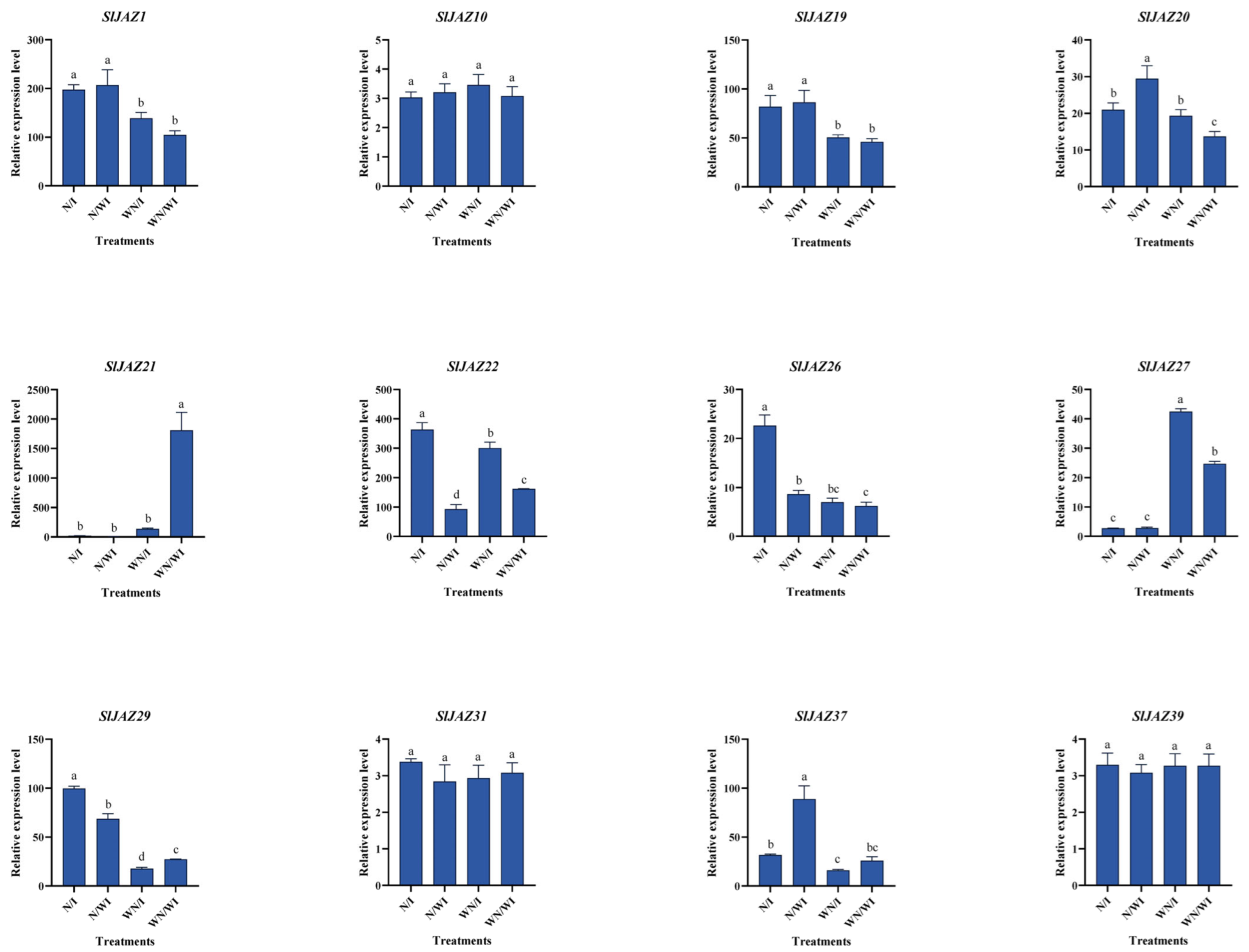
3.8. Expression Profiling of SlJAZ Genes Under Different Treatments
3.9. Phenotypic Effects of Tuta absoluta and MSNs on Tomato Plants
3.10. Regression Analysis of SlJAZ Gene Expression and Pest-Related Parameters
3.11. qPCR Validation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| JA | Jasmonic Acid |
| JAZ | Jasmonate ZIM-domain |
| MSNs | Mesoporous Silica Nanoparticles |
| qRT-PCR | Quantitative Real-Time Polymerase Chain Reaction |
| DEGs | Differentially Expressed Genes |
| SlJAZ | Solanum lycopersicum JAZ |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| miRNA | MicroRNA |
| LDI | Leaf Damage Index |
| LSR | Larval Survival Rate |
| LMN | Leaf Mine Number |
| R2 | Coefficient of Determination |
| FDR | False Discovery Rate |
| ANOVA | Analysis of Variance |
References
- Apostolova, E.L. Molecular Mechanisms of Plant Defense against Abiotic Stress. Int. J. Mol. Sci. 2023, 24, 10339. [Google Scholar] [CrossRef]
- Wang, Y.; Mostafa, S.; Zeng, W.; Jin, B. Function and mechanism of jasmonic acid in plant responses to abiotic and biotic stresses. Int. J. Mol. Sci. 2021, 22, 8568. [Google Scholar] [CrossRef]
- Pauwels, L.; Goossens, A. The JAZ proteins: A crucial interface in the jasmonate signaling cascade. Plant Cell 2011, 23, 3089–3100. [Google Scholar] [CrossRef] [PubMed]
- Browse, J. Jasmonate passes muster: A receptor and targets for the defense hormone. Annu. Rev. Plant Biol. 2009, 60, 183–205. [Google Scholar] [CrossRef]
- Wasternack, C.; Hause, B. Jasmonates: Biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann. Bot. 2013, 111, 1021–1058. [Google Scholar] [CrossRef]
- Kazan, K.; Manners, J.M. JAZ repressors and the orchestration of phytohormone crosstalk. Trends Plant Sci. 2012, 17, 22–31. [Google Scholar] [CrossRef]
- Song, S.; Qi, T.; Wasternack, C.; Xie, D. Jasmonate signaling and crosstalk with gibberellin and ethylene. Curr. Opin. Plant Biol. 2014, 21, 112–119. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, L.; Ma, Q.; Chen, M.; Cao, X.; Zhao, S.; Zhang, X. Jasmonate ZIM Domain Protein (JAZ) Gene SLJAZ15 Increases Resistance to Orobanche aegyptiaca in Tomato. Plants 2024, 13, 1493. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Liu, K.; Chen, D.; Zhang, Z.; Li, B.; El-Mogy, M.M.; Tian, S.; Chen, T. Solanum lycopersicum, a model plant for the studies in developmental biology, stress biology and food science. Foods 2022, 11, 2402. [Google Scholar] [CrossRef] [PubMed]
- Wasternack, C.; Song, S. Jasmonates: Biosynthesis, metabolism, and signaling by proteins activating and repressing transcription. J. Exp. Bot. 2016, 68, 1303–1321. [Google Scholar] [CrossRef]
- Panno, S.; Davino, S.; Caruso, A.G.; Bertacca, S.; Crnogorac, A.; Mandić, A.; Noris, E.; Matić, S. A review of the most common and economically important diseases that undermine the cultivation of tomato crop in the mediterranean basin. Agronomy 2021, 11, 2188. [Google Scholar] [CrossRef]
- Qi, T.; Huang, H.; Song, S.; Xie, D. Regulation of jasmonate-mediated stamen development and seed production by a bHLH-MYB complex in Arabidopsis. Plant Cell 2015, 27, 1620–1633. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, Y.; Dhanasekaran, S.; Wang, J.; Zhou, H.; Gu, X.; Li, B.; Zhao, L.; Zhang, H. Insights into the defense mechanisms involved in the induction of resistance against black spot of cherry tomatoes by Pichia caribbica. LWT 2022, 169, 113973. [Google Scholar] [CrossRef]
- Song, S.; Qi, T.; Fan, M.; Zhang, X.; Gao, H.; Huang, H.; Wu, D.; Guo, H.; Xie, D. The bHLH subgroup IIId factors negatively regulate jasmonate-mediated plant defense and development. PLoS Genet. 2013, 9, e1003653. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Li, M.; Achal, V. A comprehensive review on environmental and human health impacts of chemical pesticide usage. Emerg. Contam. 2024, 11, 100410. [Google Scholar] [CrossRef]
- Liu, B.; Seong, K.; Pang, S.; Song, J.; Gao, H.; Wang, C.; Zhai, J.; Zhang, Y.; Gao, S.; Li, X. Functional specificity, diversity, and redundancy of Arabidopsis JAZ family repressors in jasmonate and COI1—Regulated growth, development, and defense. New Phytol. 2021, 231, 1525–1545. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Liu, B.; Song, J.; Pang, S.; Song, T.; Gao, S.; Zhang, Y.; Huang, H.; Qi, T. A molecular framework for signaling crosstalk between jasmonate and ethylene in anthocyanin biosynthesis, trichome development, and defenses against insect herbivores in Arabidopsis. J. Integr. Plant Biol. 2022, 64, 1770–1788. [Google Scholar] [CrossRef]
- Song, J.; Pang, S.; Xue, B.; Rong, D.; Qi, T.; Huang, H.; Song, S. The AMS/DYT1–MYB module interacts with the MED25–MYC–MYB complexes to inhibit jasmonate—Regulated floral defense in Arabidopsis. J. Integr. Plant Biol. 2025, 67, 408–422. [Google Scholar] [CrossRef]
- Mariyam, S.; Upadhyay, S.K.; Chakraborty, K.; Verma, K.K.; Duhan, J.S.; Muneer, S.; Meena, M.; Sharma, R.K.; Ghodake, G.; Seth, C.S. Nanotechnology, a frontier in agricultural science, a novel approach in abiotic stress management and convergence with new age medicine-A review. Sci. Total Environ. 2023, 912, 169097. [Google Scholar] [CrossRef]
- Haq, I.U.; Cai, X.; Ali, H.; Akhtar, M.R.; Ghafar, M.A.; Hyder, M.; Hou, Y. Interactions Between Nanoparticles and Tomato Plants: Influencing Host Physiology and the Tomato Leafminer’s Molecular Response. Nanomaterials 2024, 14, 1788. [Google Scholar] [CrossRef]
- Okeke, E.S.; Nweze, E.J.; Ezike, T.C.; Nwuche, C.O.; Ezeorba, T.P.C.; Nwankwo, C.E.I. Silicon-based nanoparticles for mitigating the effect of potentially toxic elements and plant stress in agroecosystems: A sustainable pathway towards food security. Sci. Total Environ. 2023, 898, 165446. [Google Scholar] [CrossRef]
- Haq, I.U.; Liu, H.; Ghafar, M.A.; Zafar, S.; Subhan, M.; Abbasi, A.; Hyder, M.; Basit, A.; Rebouh, N.Y.; Hou, Y. Mesoporous Silica Nanoparticles Impair Physiology and Reproductive Fitness of Tuta absoluta Through Plant-Mediated Oxidative Stress and Enzymatic Disruption. Insects 2025, 16, 877. [Google Scholar] [CrossRef]
- Gao, Y.; Gao, D.; Shen, J.; Wang, Q. A review of mesoporous silica nanoparticle delivery systems in chemo-based combination cancer therapies. Front. Chem. 2020, 8, 598722. [Google Scholar] [CrossRef]
- Dilnawaz, F.; Misra, A.N.; Apostolova, E. Involvement of nanoparticles in mitigating plant’s abiotic stress. Plant Stress 2023, 10, 100280. [Google Scholar] [CrossRef]
- Torabian, S.; Zahedi, M.; Khoshgoftar, A.H. Effects of foliar spray of nano-particles of FeSO4 on the growth and ion content of sunflower under saline condition. J. Plant Nutr. 2017, 40, 615–623. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, C.; Shen, Y.; Gao, H.; Zhang, G.; Liu, W.; Jiang, H.; Zhang, Y. Life Table Parameters of the Tomato Leaf Miner Tuta absoluta (Lepidoptera: Gelechiidae) on Five Tomato Cultivars in China. Insects 2024, 15, 208. [Google Scholar] [CrossRef] [PubMed]
- Desneux, N.; Wajnberg, E.; Wyckhuys, K.A.; Burgio, G.; Arpaia, S.; Narváez-Vasquez, C.A.; González-Cabrera, J.; Catalán Ruescas, D.; Tabone, E.; Frandon, J. Biological invasion of European tomato crops by Tuta absoluta: Ecology, geographic expansion and prospects for biological control. J. Pest Sci. 2010, 83, 197–215. [Google Scholar] [CrossRef]
- Mahlangu, L.; Sibisi, P.; Nofemela, R.S.; Ngmenzuma, T.; Ntushelo, K. The differential effects of Tuta absoluta infestations on the physiological processes and growth of tomato, potato, and eggplant. Insects 2022, 13, 754. [Google Scholar] [CrossRef] [PubMed]
- Ong’onge, M.A.; Ajene, I.J.; Runo, S.; Sokame, B.M.; Khamis, F.M. Population dynamics and insecticide resistance in Tuta absoluta (Lepidoptera: Gelechiidae), an invasive pest on tomato in Kenya. Heliyon 2023, 9, e21465. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, M.; Mermer, S.; Kozaci, L.D.; Turgut, C. Insecticide resistance in two populations of Tuta absoluta (Meyrick, 1917) (Lepidoptera: Gelechiidae) from Turkey. Turk. J. Entomol. 2015, 39, 137–145. [Google Scholar]
- Tropea Garzia, G.; Siscaro, G.; Biondi, A.; Zappalà, L. Tuta absoluta, a South American pest of tomato now in the EPPO region: Biology, distribution and damage. EPPO Bull. 2012, 42, 205–210. [Google Scholar] [CrossRef]
- Guedes, R.N.C.; Walse, S.S.; Throne, J.E. Sublethal exposure, insecticide resistance, and community stress. Curr. Opin. Insect Sci. 2017, 21, 47–53. [Google Scholar] [CrossRef]
- Hajiahmadi, Z.; Shirzadian-Khorramabad, R.; Kazemzad, M.; Sohani, M.M. Enhancement of tomato resistance to Tuta absoluta using a new efficient mesoporous silica nanoparticle-mediated plant transient gene expression approach. Sci. Hortic. 2019, 243, 367–375. [Google Scholar] [CrossRef]
- Chen, S.; Guo, X.; Zhang, B.; Nie, D.; Rao, W.; Zhang, D.; Lü, J.; Guan, X.; Chen, Z.; Pan, X. Mesoporous silica nanoparticles induce intracellular peroxidation damage of Phytophthora infestans: A new type of green fungicide for late blight control. Environ. Sci. Technol. 2023, 57, 3980–3989. [Google Scholar] [CrossRef]
- Hussey, N.; Parr, W. The effect of glasshouse red spider mite (Tetranychus urticae Koch) on the yield of cucumbers. J. Hortic. Sci. 1963, 38, 255–263. [Google Scholar] [CrossRef]
- Škaloudová, B.; Křivan, V.; Zemek, R. Computer-assisted estimation of leaf damage caused by spider mites. Comput. Electron. Agric. 2006, 53, 81–91. [Google Scholar] [CrossRef]
- Rocandio-Rodríguez, M.; Torres-Castillo, J.A.; Juárez-Aragón, M.C.; Chacón-Hernández, J.C.; Moreno-Ramírez, Y.d.R.; Mora-Ravelo, S.G.; Delgado-Martínez, R.; Hernández-Juárez, A.; Heinz-Castro, R.T.Q.; Reyes-Zepeda, F. Evaluation of Resistance of Eleven Maize Races (Zea mays L.) to the Red Spider Mite (Tetranychus merganser, Boudreaux). Plants 2022, 11, 1414. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Park, J.-J.; Cho, K. Characterization of leaf mining damage of Liriomyza trifolii (Diptera: Agromyzidae) in Cherry-Tomato Greenhouse. J. Asia Pacif. Entomol. 2004, 7, 201–205. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Gasteiger, E.; Gattiker, A.; Hoogland, C.; Ivanyi, I.; Appel, R.D.; Bairoch, A. ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003, 31, 3784–3788. [Google Scholar]
- Yu, C.S.; Chen, Y.C.; Lu, C.H.; Hwang, J.K. Prediction of protein subcellular localization. Proteins Struct. Funct. Bioinform. 2006, 64, 643–651. [Google Scholar] [CrossRef]
- Bailey, T.L.; Johnson, J.; Grant, C.E.; Noble, W.S. The MEME suite. Nucleic Acids Res. 2015, 43, W39–W49. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Yuan, Y.; Bayer, P.E.; Scheben, A.; Chan, C.-K.K.; Edwards, D. BioNanoAnalyst: A visualisation tool to assess genome assembly quality using BioNano data. BMC Bioinform. 2017, 18, 323. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Zhuang, Z.; Zhao, P.X. psRNATarget: A plant small RNA target analysis server (2017 release). Nucleic Acids Res. 2018, 46, W49–W54. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Christensen, S.; Isakeit, T.; Engelberth, J.; Meeley, R.; Hayward, A.; Emery, R.N.; Kolomiets, M.V. Disruption of OPR7 and OPR8 reveals the versatile functions of jasmonic acid in maize development and defense. Plant Cell 2012, 24, 1420–1436. [Google Scholar] [CrossRef] [PubMed]
- Chini, A.; Fonseca, S.; Fernandez, G.; Adie, B.; Chico, J.M.; Lorenzo, O.; García-Casado, G.; López-Vidriero, I.; Lozano, F.; Ponce, M. The JAZ family of repressors is the missing link in jasmonate signalling. Nature 2007, 448, 666–671. [Google Scholar] [CrossRef]
- Campos, M.L.; De Almeida, M.; Rossi, M.L.; Martinelli, A.P.; Litholdo Junior, C.G.; Figueira, A.; Rampelotti-Ferreira, F.T.; Vendramim, J.D.; Benedito, V.A.; Pereira Peres, L.E. Brassinosteroids interact negatively with jasmonates in the formation of anti-herbivory traits in tomato. J. Exp. Bot. 2009, 60, 4347–4361. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, C.; Liu, Z.; Zhao, T.; Jiang, J.; Li, J.; Xu, X.; Yang, H. Genome-Wide Identification, Characterization and Expression Analysis of the JAZ Gene Family in Resistance to Gray Leaf Spots in Tomato. Int. J. Mol. Sci. 2021, 22, 9974. [Google Scholar] [CrossRef]
- Thines, B.; Katsir, L.; Melotto, M.; Niu, Y.; Mandaokar, A.; Liu, G.; Nomura, K.; He, S.Y.; Howe, G.A.; Browse, J. JAZ repressor proteins are targets of the SCFCOI1 complex during jasmonate signalling. Nature 2007, 448, 661–665. [Google Scholar] [CrossRef]
- Huang, Y.-L.; Zhang, L.-K.; Zhang, K.; Chen, S.-M.; Hu, J.-B.; Cheng, F. The impact of tandem duplication on gene evolution in Solanaceae species. J. Integr. Agric. 2022, 21, 1004–1014. [Google Scholar] [CrossRef]
- Koo, A.J.; Howe, G.A. The wound hormone jasmonate. Phytochemistry 2009, 70, 1571–1580. [Google Scholar] [CrossRef]
- Rossanese, A. Development of Multifunctional Mesoporous Silica Nanoparticles with Antioxidant Properties for Treating Inflammatory-Related Diseases. Ph.D. Thesis, Politecnico di Torino, Torino, Italy, 2023. [Google Scholar]
- Ranjan, A.; Sinha, R.; Bala, M.; Pareek, A.; Singla-Pareek, S.L.; Singh, A.K. Silicon-mediated abiotic and biotic stress mitigation in plants: Underlying mechanisms and potential for stress resilient agriculture. Plant Physiol. Biochem. 2021, 163, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.; Gupta, N.; Kumar, M.; Kumar, V.; Wang, S.; Abd-Elsalam, K.A. Nanomaterials act as plant defense mechanism. In Nanotechnology: Food and Environmental Paradigm; Springer: Singapore, 2017; pp. 253–269. [Google Scholar]
- Sun, D.; Hussain, H.I.; Yi, Z.; Rookes, J.E.; Kong, L.; Cahill, D.M. Mesoporous silica nanoparticles enhance seedling growth and photosynthesis in wheat and lupin. Chemosphere 2016, 152, 81–91. [Google Scholar] [CrossRef]
- Raliya, R.; Nair, R.; Chavalmane, S.; Wang, W.-N.; Biswas, P. Mechanistic evaluation of translocation and physiological impact of titanium dioxide and zinc oxide nanoparticles on the tomato (Solanum lycopersicum L.) plant. Metallomics 2015, 7, 1584–1594. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.H.; Al-Whaibi, M.H. Role of nano-SiO2 in germination of tomato (Lycopersicum esculentum seeds Mill.). Saudi J. Biol. Sci. 2014, 21, 13–17. [Google Scholar] [CrossRef]
- Guedes, R.; Smagghe, G.; Stark, J.; Desneux, N. Pesticide-induced stress in arthropod pests for optimized integrated pest management programs. Annu. Rev. Entomol. 2016, 61, 43–62. [Google Scholar] [CrossRef]
- Zappala, L.; Biondi, A.; Alma, A.; Al-Jboory, I.J.; Arno, J.; Bayram, A.; Chailleux, A.; El-Arnaouty, A.; Gerling, D.; Guenaoui, Y. Natural enemies of the South American moth, Tuta absoluta, in Europe, North Africa and Middle East, and their potential use in pest control strategies. J. Pest Sci. 2013, 86, 635–647. [Google Scholar] [CrossRef]
- Mostofa, M.G.; Rahman, M.M.; Ansary, M.M.U.; Keya, S.S.; Abdelrahman, M.; Miah, M.G.; Phan Tran, L.-S. Silicon in mitigation of abiotic stress-induced oxidative damage in plants. Crit. Rev. Biotechnol. 2021, 41, 918–934. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.S.; Koo, A.J.; Gao, X.; Jayanty, S.; Thines, B.; Jones, A.D.; Howe, G.A. Regulation and function of Arabidopsis JASMONATE ZIM-domain genes in response to wounding and herbivory. Plant Physiol. 2008, 146, 952–964. [Google Scholar] [CrossRef]
- Ye, H.; Du, H.; Tang, N.; Li, X.; Xiong, L. Identification and expression profiling analysis of TIFY family genes involved in stress and phytohormone responses in rice. Plant Mol. Biol. 2009, 71, 291–305. [Google Scholar] [CrossRef]
- Huang, H.; Zhao, W.; Qiao, H.; Li, C.; Sun, L.; Yang, R.; Ma, X.; Ma, J.; Song, S.; Wang, S. SlWRKY45 interacts with jasmonate-ZIM domain proteins to negatively regulate defense against the root-knot nematode Meloidogyne incognita in tomato. Hortic. Res. 2022, 9, uhac197. [Google Scholar] [CrossRef]
- Huang, H.; Ma, X.; Sun, L.; Wang, Y.; Ma, J.; Hong, Y.; Zhao, M.; Zhao, W.; Yang, R.; Song, S. SlVQ15 recruits SlWRKY30IIc to link with jasmonate pathway in regulating tomato defence against root—Knot nematodes. Plant Biotechnol. J. 2025, 23, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Conrath, U.; Beckers, G.J.; Langenbach, C.J.; Jaskiewicz, M.R. Priming for enhanced defense. Annu. Rev. Phytopathol. 2015, 53, 97–119. [Google Scholar] [CrossRef] [PubMed]
- Torabian, S.; Farhangi-Abriz, S.; Zahedi, M. Efficacy of FeSO 4 nano formulations on osmolytes and antioxidative enzymes of sunflower under salt stress. Indian J. Plant Physiol. 2018, 23, 305–315. [Google Scholar] [CrossRef]

| Gene-1 | Gene-2 | Ka | Ks | Ka_Ks | Selection Pressure | Duplication Type | T = Ks/2r (MYA) |
|---|---|---|---|---|---|---|---|
| SlJAZ12 | SlJAZ21 | 0.480957 | 1.872467 | 0.256858 | Purifying | Segmental | 144.0359 |
| SlJAZ38 | SlJAZ20 | 0.521719 | N/A | N/A | N/A | Segmental | N/A |
| SlJAZ38 | SlJAZ29 | 0.249556 | 1.284069 | 0.194348 | Purifying | Segmental | 98.77454 |
| SlJAZ19 | SlJAZ20 | 0.9835 | N/A | N/A | N/A | Tandem | N/A |
| SlJAZ18 | SlJAZ25 | 0.302624 | 0.860289 | 0.35177 | Purifying | Segmental | 66.17609 |
| SlJAZ17 | SlJAZ26 | 0.204479 | 0.567128 | 0.360552 | Purifying | Segmental | 43.62523 |
| SlJAZ27 | SlJAZ35 | 0.23627 | 0.725057 | 0.325864 | Purifying | Segmental | 55.77358 |
| Treatment Group | Leaf Damage Index (%) (Mean ± SE) | Larval Survival Rate (%) (Mean ± SE) | Number of Leaf Mines per Leaf (Mean ± SE) |
|---|---|---|---|
| WN/WI (Control: No Pest, No MSNs) | 0.0 ± 0.0 c | - | 0.0 ± 0.0 c |
| WN/I (Pest Only) | 45.2 ± 4.7 a | 84.6 ± 2.9 a | 12.4 ± 1.8 a |
| N/WI (MSNs Only) | 0.0 ± 0.0 c | - | 0.0 ± 0.0 c |
| N/I (MSNs + Pest) | 20.6 ± 3.8 b | 52.3 ± 4.4 b | 5.1 ± 1.2 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haq, I.U.; Basit, A.; Hyder, M.; Shahzad, M.N.; Abbasi, A.; Sharif, Y.; Ghafar, M.A.; Cai, X.; Rebouh, N.Y.; Hou, Y. Genome-Wide Analysis and Functional Correlation of Tomato JAZ Genes Under Tuta absoluta Infestation and Nanoparticle-Induced Defense. Insects 2025, 16, 1046. https://doi.org/10.3390/insects16101046
Haq IU, Basit A, Hyder M, Shahzad MN, Abbasi A, Sharif Y, Ghafar MA, Cai X, Rebouh NY, Hou Y. Genome-Wide Analysis and Functional Correlation of Tomato JAZ Genes Under Tuta absoluta Infestation and Nanoparticle-Induced Defense. Insects. 2025; 16(10):1046. https://doi.org/10.3390/insects16101046
Chicago/Turabian StyleHaq, Inzamam Ul, Abdul Basit, Moazam Hyder, Mirza Naveed Shahzad, Asim Abbasi, Yasir Sharif, Muhammad Adeel Ghafar, Xiangyun Cai, Nazih Y. Rebouh, and Youming Hou. 2025. "Genome-Wide Analysis and Functional Correlation of Tomato JAZ Genes Under Tuta absoluta Infestation and Nanoparticle-Induced Defense" Insects 16, no. 10: 1046. https://doi.org/10.3390/insects16101046
APA StyleHaq, I. U., Basit, A., Hyder, M., Shahzad, M. N., Abbasi, A., Sharif, Y., Ghafar, M. A., Cai, X., Rebouh, N. Y., & Hou, Y. (2025). Genome-Wide Analysis and Functional Correlation of Tomato JAZ Genes Under Tuta absoluta Infestation and Nanoparticle-Induced Defense. Insects, 16(10), 1046. https://doi.org/10.3390/insects16101046










