The Mitochondrial Hsp90 Homolog PmTRAP1 Mediates Thermal Tolerance in the Papaya Mealybug, Paracoccus marginatus
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing
2.2. Total RNA Extraction and cDNA Synthesis
2.3. Identification and Molecular Cloning of PmHsp90 cDNA
2.3.1. Candidate Gene Screening
2.3.2. Molecular Cloning of PmHsp90 Isoforms
2.3.3. Bioinformatic Characterization of PmHsp90 Isoforms
2.4. Thermal Stress Response of PmHsp90 Expression
2.5. Functional Validation of PmTRAP1 via RNA Interference
2.5.1. Double-Stranded RNA Synthesis
2.5.2. Microinjection and Sampling
2.5.3. Silencing Efficiency Validation
2.6. Thermal Tolerance Assay Following PmTRAP1 Silencing
3. Results
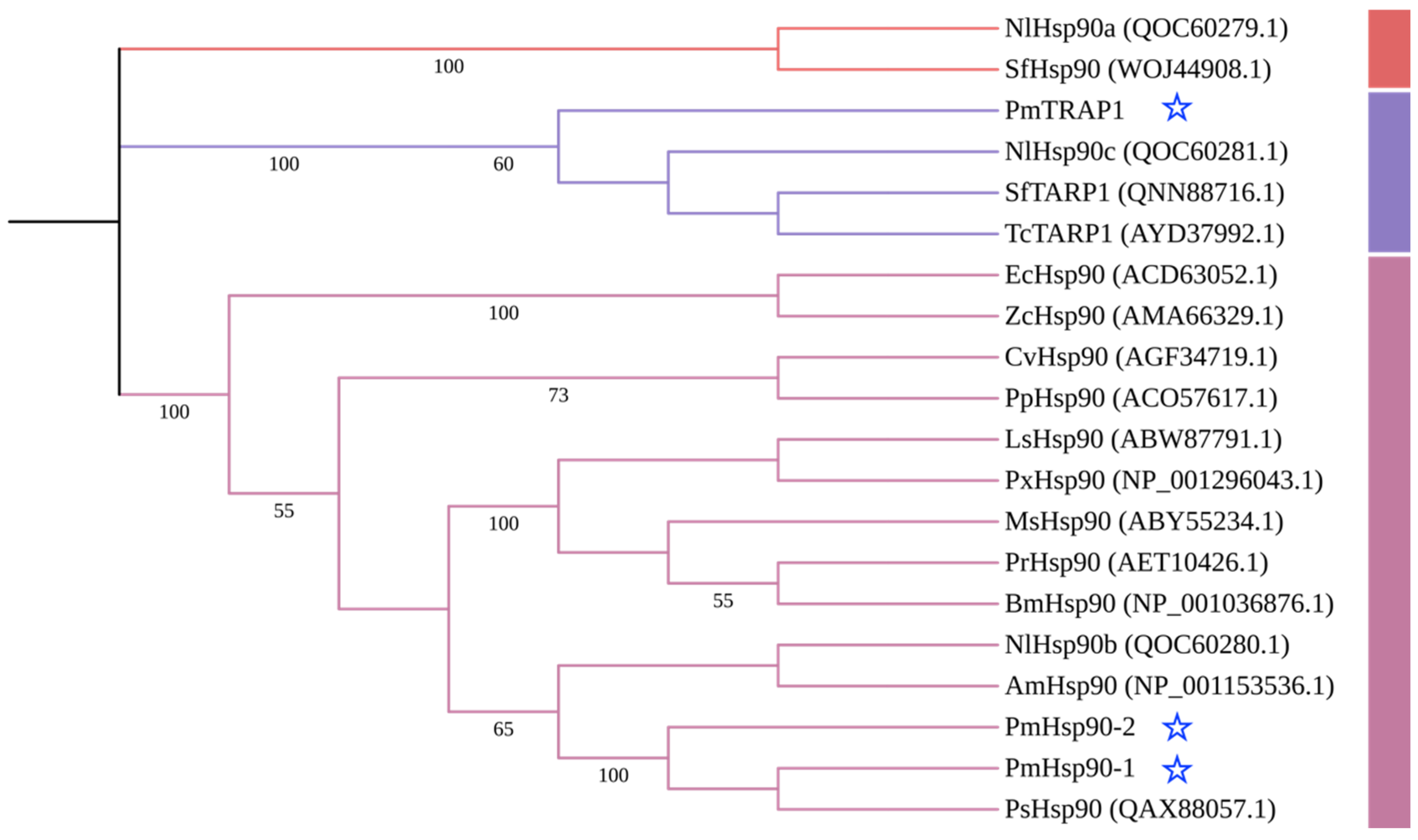
3.1. Characterization of PmHsp90 Isoforms in P. marginatus
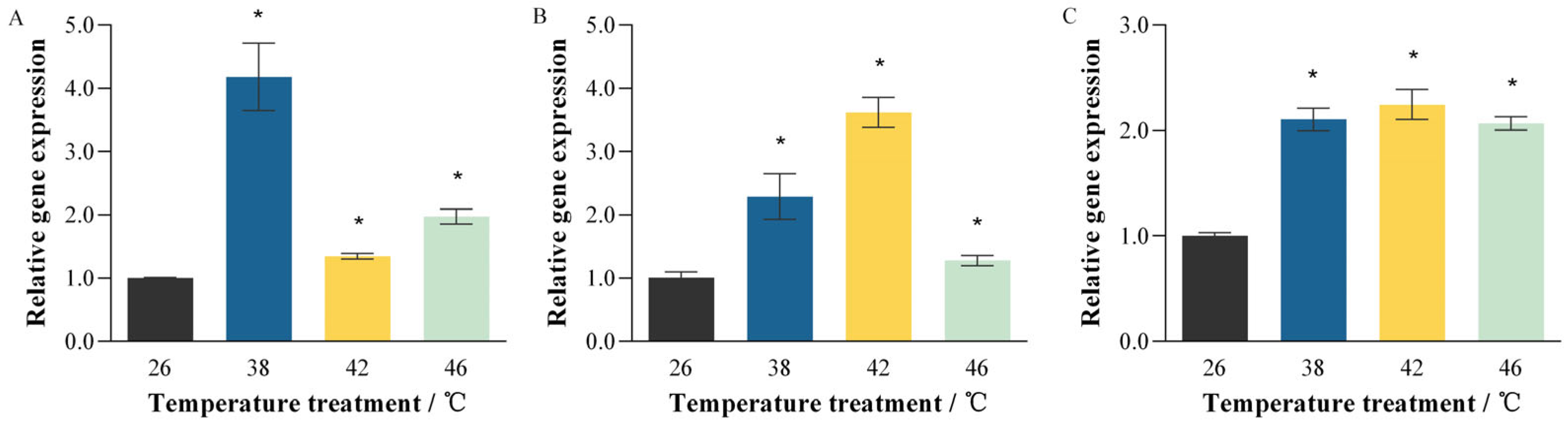
3.2. Thermal Induction of PmHsp90 Isoform Expression
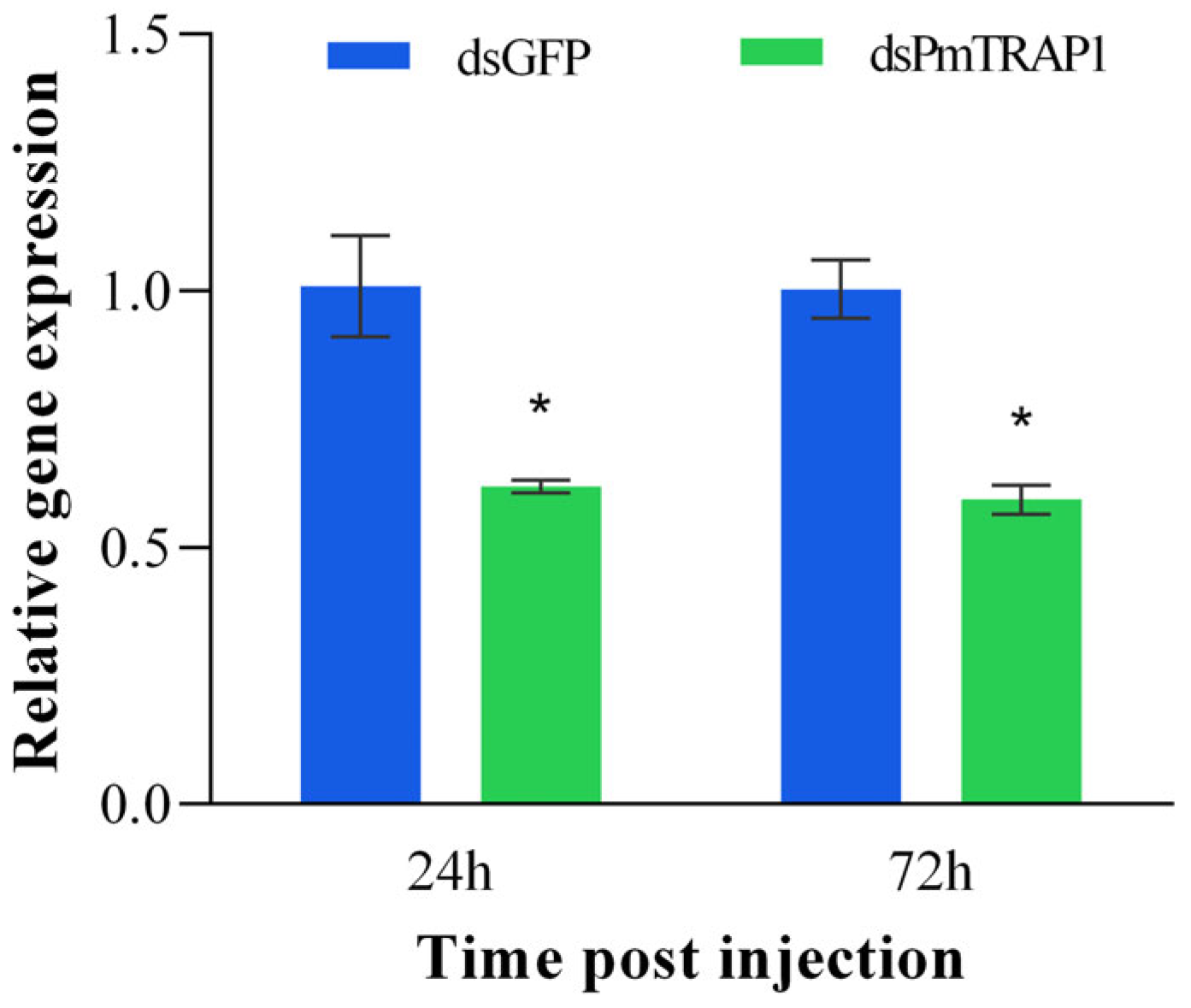
3.3. RNAi Silencing Efficiency of PmTRAP1 in P. marginatus
3.4. Impact of PmTRAP1 Silencing on Thermal Tolerance
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Miller, D.R.; Miller, G.L. Redescription of Paracoccus marginatus Williams and Granara de Willink (Hemiptera: Coccoidea: Pseudococcidae), including descriptions of the immature stages and adult male. Proc. Entomol. Soc. Wash. 2002, 104, 1–23. [Google Scholar]
- Myrick, S.; Norton, G.W.; Selvaraj, K.N.; Natarajan, K.; Muniappan, R. Economic impact of classical biological control of papaya mealybug in India. Crop Prot. 2014, 56, 82–86. [Google Scholar] [CrossRef]
- Seni, A.; Chongtham, S. Papaya mealybug Paracoccus marginatus williams & granara de willink (Hemiptera: Pseudococcidae), a current threat to agriculture—A review. Agric. Rev. 2013, 34, 216–222. [Google Scholar]
- CABI. Crop Protection Compendium. Available online: http://www.cabi.org/cpc (accessed on 10 July 2025).
- Tanwar, R.K. Papaya mealybug and its management strategies. Technol. Bull (Natl. Cent. Integr. Pest Manag.) 2010, 22, 1–22. [Google Scholar]
- Chen, Y.; Shi, M.; Fu, J.; Zhao, Z.; Liu, W.; Li, J. Prediction of potential suitable habitats for Paracoccus marginatus in China under climate change conditions. J. Plant Prot. 2023, 50, 1491–1498. [Google Scholar]
- Amarasekare, K.G.; Chong, J.H.; Epsky, N.D.; Mannion, C.M. Effect of temperature on the life history of the mealybug Paracoccus marginatus (Hemiptera: Pseudococcidae). J. Econ. Entomol. 2008, 101, 1798–1804. [Google Scholar] [CrossRef]
- Amutha, M.; Dharajothi, B. Life table and population parameters of Paracoccus marginatus at varying temperatures on cotton. Indian J. Plant Prot. 2016, 44, 24–29. [Google Scholar]
- Chen, Q.; Liang, X.; Wu, C.-l.; Wang, Y.-r.; Zhao, H.-p.; Chen, Q. Influence of different temperatures on protective enzyme activities of Paracoccus marginatus (Hemiptera: Pseudococcidae). Genom. Appl. Biol. 2020, 39, 241–245. [Google Scholar]
- Chen, Y.; Zhao, J.; Shi, M.; Ruan, F.; Fu, J.; Liu, W.; Li, J. Characterization and expression patterns of heat shock protein 70 genes from Paracoccus marginatus in response to temperature and insecticide stress. Agriculture 2024, 14, 2164. [Google Scholar] [CrossRef]
- Banfi, D.; Bianchi, T.; Mastore, M.; Brivio, M.F. The role of heat shock proteins in insect stress response, immunity, and climate adaptation. Insects 2025, 16, 741. [Google Scholar] [CrossRef]
- Morimoto, R. Cells in stress: Transcriptional activation of heat shock genes. Science 1993, 259, 1409–1410. [Google Scholar] [CrossRef]
- Abdullah, H.; Marwan, E.S.; Hassan, N. The Hsp90 family: Structure, regulation, function, and implications in health and disease. Int. J. Mol. Sci. 2018, 19, 2560. [Google Scholar] [CrossRef]
- Zhao, R.; Houry, W.A. Molecular interaction network of the Hsp90 chaperone system. Adv. Exp. Med. Biol. 2007, 594, 27–36. [Google Scholar]
- Johnson, J.L. Evolution and function of diverse Hsp90 homologs and cochaperone proteins. Biochim. Biophys. Acta. 2012, 1823, 607–613. [Google Scholar] [CrossRef]
- Ding, J.H.; Zheng, L.X.; Chu, J.; Sheng, S. Characterization, and functional analysis of Hsp70 and Hsp90 gene families in Glyphodes pyloalis (Walker) (Lepidoptera: Pyralidae). Front. Physiol. 2021, 12, 753914. [Google Scholar] [CrossRef]
- Garcia-Gomez, B.; Sánchez, T.A.; Cano, S.N.; Nascimento, N.A.D.; Bravo, A.; Soberón, M. Insect chaperones Hsp70 and Hsp90 cooperatively enhance toxicity of Bacillus thuringiensis Cry1A toxins and counteract insect resistance. Front. Immunol. 2023, 14, 1151943. [Google Scholar] [CrossRef]
- Will, T.; Schmidtberg, H.; Škaljac, M.; Vilcinskas, A. Heat shock protein 83 plays pleiotropic roles in embryogenesis, longevity, and fecundity of the Pea Aphid Acyrthosiphon pisum. Dev. Genes Evol. 2017, 227, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Zhai, M.; Yu, X.; Wei, L.; Mao, J.; Liu, J.; Xie, J.; Li, B. Comparative RNA-sequencing analysis of ER-based HSP90 functions and signal pathways in Tribolium castaneum. Cell Stress Chaperones 2017, 23, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.J.; Feng, J.N. Comparisons of expression levels of heat shock proteins (Hsp70 and Hsp90) from Anaphothrips obscurus (Thysanoptera: Thripidae) in polymorphic adults exposed to different heat shock treatments. J. Insect Sci. 2018, 18, 15. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Li, D.; Wang, Y.; Liu, Y.; Zhu-Salzman, K. Cloning of heat shock protein genes (hsp70, hsc70 and hsp90) and their expression in response to larval diapause and thermal stress in the wheat blossom midge, Sitodiplosis mosellana. J. Insect Physiol. 2016, 95, 66–77. [Google Scholar] [CrossRef]
- Chen, X.; Li, Z.D.; Dai, Y.T.; Jiang, M.X.; Zhang, C.X. Identification and characterization of three heat shock protein 90 (Hsp90) homologs in the Brown planthopper. Genes 2020, 11, 1074. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Fei, S.; Awais, M.M.; Sun, J. Heat Shock Protein 75 (TRAP1) facilitate the proliferation of the Bombyx mori nucleopolyhedrovirus. Int. J. Biol. Macromol. 2021, 175, 372–378. [Google Scholar] [CrossRef]
- Partridge, J.R.; Lavery, L.A.; Elnatan, D.; Naber, N.; Cooke, R.; Agard, D.A. A novel N-terminal extension in mitochondrial TRAP1 serves as a thermal regulator of chaperone activity. eLife 2014, 3, e03487. [Google Scholar] [CrossRef]
- Wang, X.; Li, S.; Yao, L.; Liu, Y.; Chen, Y.; Yang, H.; Fan, D. The stress-induced expression of the Aghsp75 gene in Aphis glycines in response to imidacloprid and temperature stress. Chin. J. Biol. Control 2020, 36, 913–919. [Google Scholar]
- Liang, X.; Chen, Q.; Wu, C.; Liu, Y.; Han, Z.; Wu, M. Reference gene selection for analyzing the transcription patterns of two fatty acyl-CoA reductase genes from Paracoccus marginatus (Hemiptera: Pseudococcidae). J. Insect Sci. 2021, 21, 11. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- King, A.M.; Macrae, T.H. Insect heat shock proteins during stress and diapause. Annu. Rev. Entomol. 2015, 60, 59–75. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Shang, Q.; Huang, H.; Zhang, S.; Zhong, J.; Hou, Q.; Guo, X. Quantitative proteomics analysis provides insight into the biological role of Hsp90 in BmNPV infection in Bombyx mori. J. Proteom. 2019, 203, 103379. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Li, D.; Ai, H.; Xie, X.; Jiang, X.; Afrasiyab; Zhang, H.; Xu, J.; Huang, S. Glucose-regulated protein 94 facilitates the proliferation of the Bombyx mori nucleopolyhedrovirus via inhibiting apoptosis. Int. J. Biol. Macromol. 2023, 253, 127158. [Google Scholar] [CrossRef] [PubMed]
- Ruan, H.Y.; Zhou, L.; Yang, L.; Meng, J.Y.; Zhang, C.Y. Functional analysis of two SfHsp90 genes in response to high temperature and insecticide stress in Spodoptera frugiperda (Lepidoptera: Noctuidae). Eur. J. Entomol. 2024, 121, 54–63. [Google Scholar] [CrossRef]
- Li, J.; Buchner, J. Structure, function and regulation of the Hsp90 machinery. Biomed J. 2013, 36, 106–117. [Google Scholar]
- Schopf, F.H.; Biebl, M.M.; Buchner, J. The HSP90 chaperone machinery. Nat. Rev. Mol. Cell Biol. 2017, 18, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.; Ito, T.; Picard, D.; Neckers, L. The mitochondrial HSP90 paralog TRAP1: Structural dynamics, interactome, role in metabolic regulation, and inhibitors. Biomolecules 2022, 12, 880. [Google Scholar] [CrossRef]
- Sgrò, C.M.; Terblanche, J.S.; Hoffmann, A.A. What can plasticity contribute to insect responses to climate change? Annu. Rev. Entomol. 2016, 61, 433–451. [Google Scholar] [CrossRef] [PubMed]
- González-Tokman, D.; Córdoba-Aguilar, A.; Dáttilo, W.; Lira-Noriega, A.; Sánchez-Guillén, R.A.; Villalobos, F. Insect responses to heat: Physiological mechanisms, evolution and ecological implications in a warming world. Biol. Rev. 2020, 95, 802–821. [Google Scholar] [CrossRef]
- Zhao, L.; Jones, W.A. Expression of heat shock protein genes in insect stress responses. Invertebrate. Surviv. J. 2012, 9, 93–101. [Google Scholar]
- Nguyen, M.C.; Tu, G.H.; Koprivnikar, K.E.; Gonzalez-Edick, M.; Jooss, K.U.; Harding, T.C. Antibody responses to galectin-8, TARP and TRAP1 in prostate cancer patients treated with a GM-CSF-secreting cellular immunotherapy. Cancer Immunol. Immunother. 2010, 59, 1313–1323. [Google Scholar] [CrossRef]
- Xie, S.; Wang, X.; Gan, S.; Tang, X.; Zhu, S. The mitochondrial chaperone TRAP1 as a candidate target of oncotherapy. Front. Oncol. 2021, 10, 585047. [Google Scholar] [CrossRef]
- Hua, G.; Zhang, Q.; Fan, Z. Heat shock protein 75 (TRAP1) antagonizes reactive oxygen species generation and protects cells from granzyme M-mediated apoptosis. J. Biol. Chem. 2007, 282, 20553–20560. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.C.; Loh, S.H.Y.; Martins, L.M. Drosophila Trap1 protects against mitochondrial dysfunction in a PINK1/parkin model of Parkinson’s disease. Cell Death Dis. 2013, 4, e467. [Google Scholar] [CrossRef]
- Baqri, R.M.; Pietron, A.V.; Gokhale, R.H.; Turner, B.A.; Kaguni, L.S.; Shingleton, A.W.; Kunes, S.; Miller, K.E. Mitochondrial chaperone TRAP1 activates the mitochondrial UPR and extends healthspan in Drosophila. Mech. Ageing Dev. 2014, 141–142, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Chrétien, D.; Bénit, P.; Hyung-Ho, H.; Susanne, K.; Riyad, E.K.; Young-Tae, C.; Martin, J.; Jacobs, H.T.; Pierre, R.; Malgorzata, R. Mitochondria are physiologically maintained at close to 50 °C. PLoS Biol. 2018, 16, e2003992. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Yang, Z.; Sui, M.; Cui, C.; Hu, Y.; Hou, X.; Xing, Q.; Huang, X.; Bao, Z. Identification and characterization of hsp90 gene family reveals involvement of hsp90, grp94 and not TRAP1 in heat stress response in Chlamys farreri. Genes 2021, 12, 1592. [Google Scholar] [CrossRef] [PubMed]


| Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) | Usage |
|---|---|---|---|
| PmHsp90-1 | ATGCCGGAAGACGTAGCTATG | TCAATCGACTTCTTCCATTCT | cDNA sequence cloning |
| PmHsp90-2 | ATGCCTGAAGACGTAGCAATG | TTAATCGACCTCTTCCATTCT | |
| PmTRAP1 | ATGATTAGTTTAATCAGTCGT | TTAATGTTTTTCCAAAGCTAA | |
| PmHsp90-1 | AGACAGAAAGCCGAAGCAGAT | CTGCTTCGGCTTTCTGTCTCA | Fluorescence quantitative PCR |
| PmHsp90-2 | GAAGCCAACTCCGAATTGACCA | GGTATGACGAAAGCCGACCTTG | |
| PmTRAP1 | GGTATGACGAAAGCCGACCTTG | GCTGTAGAAACCGACACCGAAT | |
| Pmβ-actin | CATCCTGCGTTTGGATTTAG | TCCAAAGCAACATAGCACAAT | |
| PmTRAP1 | TAATACGACTCACTATAGG AAGGAAACGTATCTGAAGAGC | TAATACGACTCACTATAGG CCAATAATACCCCTTTGCTTC | dsRNA synthesis |
| GFP | AATACGACTCACTATAGGGCACAAGTTCAGCGTGTCC | TAATACGACTCACTATAGGGGGTGCTCAGGTAGTGGTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Zhao, X.; Lv, C.; Zhao, J.; Shi, M.; Fu, J.; Li, J. The Mitochondrial Hsp90 Homolog PmTRAP1 Mediates Thermal Tolerance in the Papaya Mealybug, Paracoccus marginatus. Insects 2025, 16, 1064. https://doi.org/10.3390/insects16101064
Chen Y, Zhao X, Lv C, Zhao J, Shi M, Fu J, Li J. The Mitochondrial Hsp90 Homolog PmTRAP1 Mediates Thermal Tolerance in the Papaya Mealybug, Paracoccus marginatus. Insects. 2025; 16(10):1064. https://doi.org/10.3390/insects16101064
Chicago/Turabian StyleChen, Yanting, Xiaomin Zhao, Chenyu Lv, Jianwei Zhao, Mengzhu Shi, Jianwei Fu, and Jianyu Li. 2025. "The Mitochondrial Hsp90 Homolog PmTRAP1 Mediates Thermal Tolerance in the Papaya Mealybug, Paracoccus marginatus" Insects 16, no. 10: 1064. https://doi.org/10.3390/insects16101064
APA StyleChen, Y., Zhao, X., Lv, C., Zhao, J., Shi, M., Fu, J., & Li, J. (2025). The Mitochondrial Hsp90 Homolog PmTRAP1 Mediates Thermal Tolerance in the Papaya Mealybug, Paracoccus marginatus. Insects, 16(10), 1064. https://doi.org/10.3390/insects16101064







