Integrated Transcriptomic and Metabolomic Insights into “I See You” (ISY) Defensive Behavior in Apis cerana Against Vespa velutina
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Honeybees
2.2. RNA Extraction, Library Construction, and Sequencing
2.3. Fluorescent Real-Time Quantitative PCR (qRT-PCR)
2.4. Data Analysis
2.5. Integrated Metabolomic and Transcriptomic Analysis
2.6. Statistical Analysis
3. Results
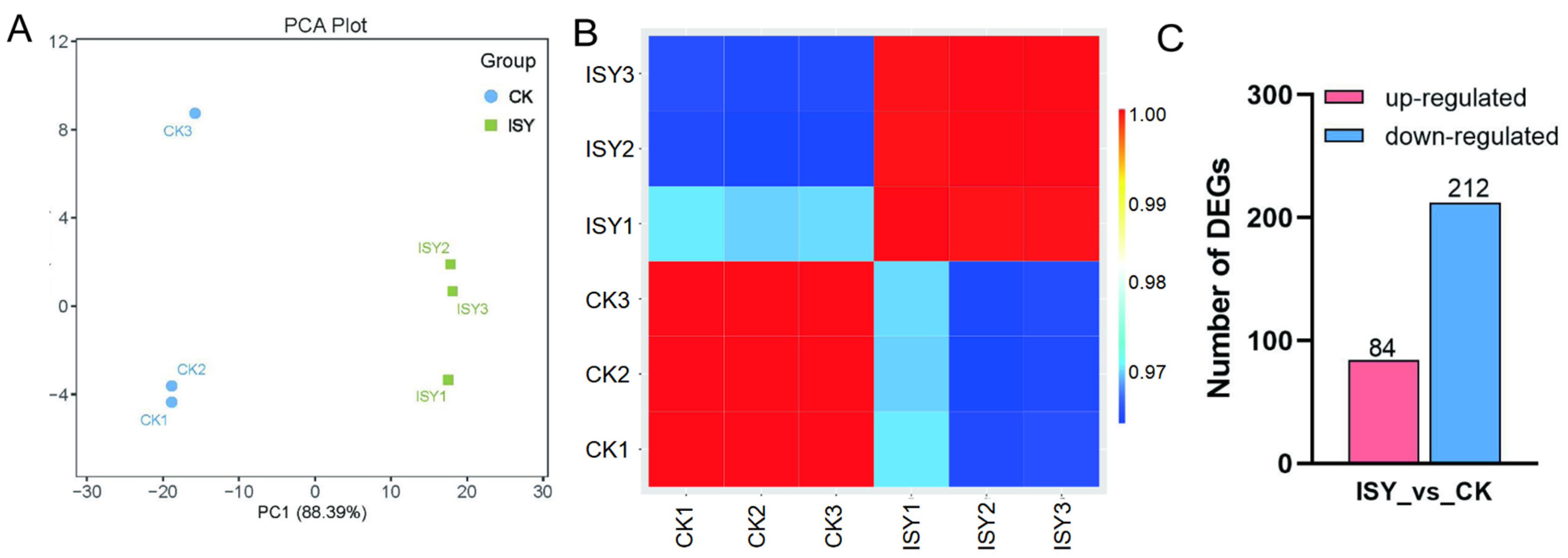
3.1. Transcriptome Sequencing Results and Quality Analysis
3.2. Functional Enrichment Analysis of DEGs
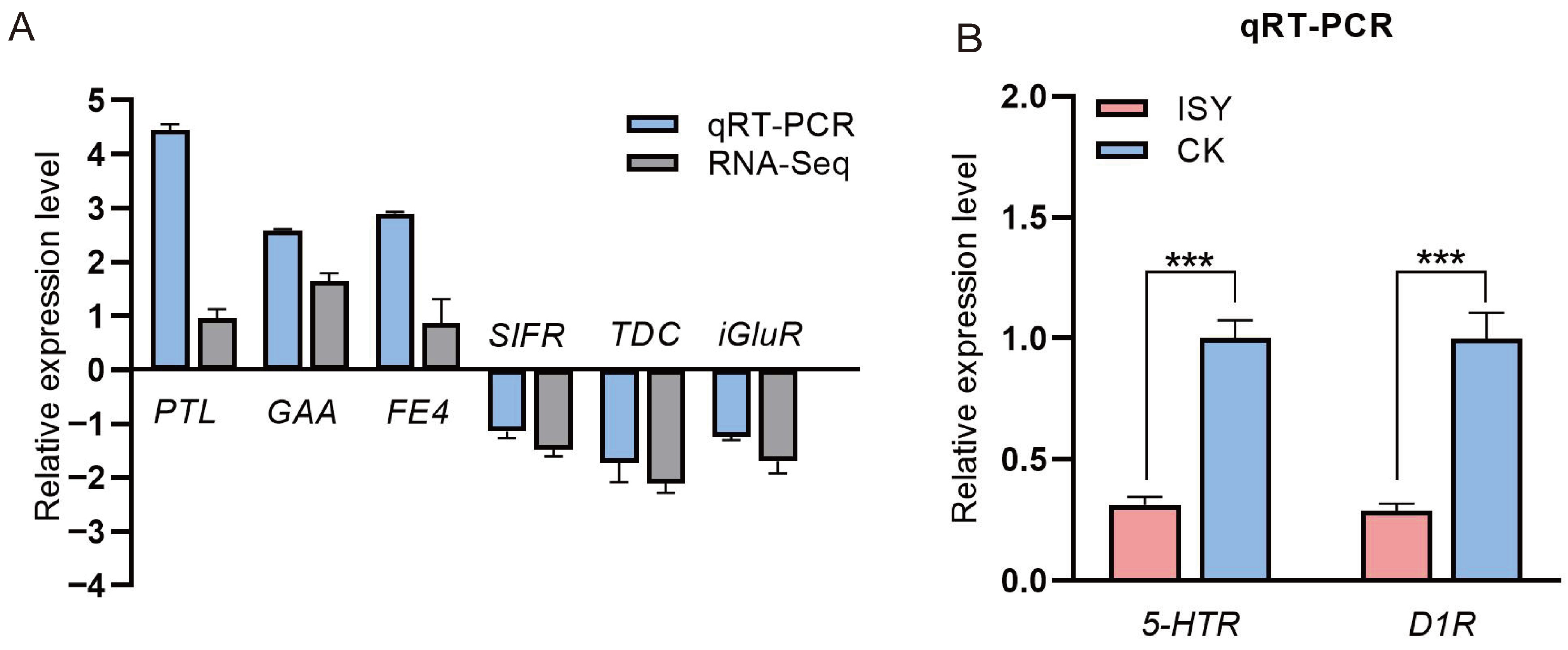
3.3. Validation of Transcriptomic Data by Quantitative Real-Time PCR (qRT-PCR)
3.4. Metabolomic Analysis
3.5. Integrated Analysis of Metabolomic and Transcriptomic Data
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nie, P.; Cao, R.; Yang, R.; Feng, J. Future range dynamics of Asian yellow-legged hornets (Vespa velutina) and their range overlap with Western honey bees (Apis mellifera) reveal major challenges for bee conservation in Europe. Pest Manag. Sci. 2024, 80, 2785–2795. [Google Scholar] [PubMed]
- Cappa, F.; Cini, A.; Bortolotti, L.; Poidatz, J.; Cervo, R. Hornets and honey bees: A coevolutionary arms race between ancient adaptations and new invasive threats. Insects 2021, 12, 1037. [Google Scholar] [CrossRef]
- Zhang, X.; Nie, P.; Hu, X.; Feng, J. Future range expansions of invasive wasps suggest their increasing impacts on global apiculture. Insects 2024, 15, 546. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Moreno, D.; Galizia, C.G.; Nouvian, M. Division of labour during honeybee colony defence: Poetic and scientific views. Philos. Trans. B 2025, 380, 20230272. [Google Scholar] [CrossRef]
- Baracchi, D.; Cusseau, G.; Pradella, D.; Turillazzi, S. Defence reactions of Apis mellifera ligustica against attacks from the European hornet Vespa crabro. Ethol. Ecol. Evol. 2010, 22, 281–294. [Google Scholar]
- Ken, T.; Hepburn, H.R.; Radloff, S.E.; Yusheng, Y.; Yiqiu, L.; Danyin, Z.; Neumann, P. Heat-balling wasps by honeybees. Naturwissenschaften 2005, 92, 492–495. [Google Scholar]
- Gu, G.; Meng, Y.; Tan, K.; Dong, S.; Nieh, J.C. Lethality of honey bee stings to heavily armored hornets. Biology 2021, 10, 484. [Google Scholar] [CrossRef]
- Tan, K.; Radloff, S.E.; Li, J.J.; Hepburn, H.R.; Yang, M.X.; Zhang, L.J.; Neumann, P. Bee-hawking by the wasp, Vespa velutina, on the honeybees A. cerana and A. mellifera. Naturwissenschaften 2007, 94, 469–472. [Google Scholar]
- Kastberger, G.; Weihmann, F.; Hoetzl, T.; Weiss, S.E.; Maurer, M.; Kranner, I. How to join a wave: Decision-making processes in shimmering behavior of giant honeybees (Apis dorsata). PLoS ONE 2012, 7, e36736. [Google Scholar]
- Kastberger, G.; Hoetzl, T.; Maurer, M.; Kranner, I.; Weiss, S.; Weihmann, F. Speeding up social waves. Propagation mechanisms of shimmering in giant honeybees. PLoS ONE 2014, 9, e86315. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.; Dong, S.; Li, X.; Liu, X.; Wang, C.; Li, J.; Nieh, J.C. Honey bee inhibitory signaling is tuned to threat severity and can act as a colony alarm signal. PLoS Biol. 2016, 14, e1002423. [Google Scholar] [CrossRef]
- Tan, K.; Wang, Z.; Chen, W.; Hu, Z.; Oldroyd, B.P. The ‘I see you’prey–predator signal of Apis cerana is innate. Naturwissenschaften 2013, 100, 245–248. [Google Scholar] [CrossRef]
- Dong, S.; Tan, K.; Nieh, J.C. Visual contagion in prey defence signals can enhance honest defence. J. Anim. Ecol. 2021, 90, 594–601. [Google Scholar] [CrossRef]
- Kastberger, G.; Schmelzer, E.; Kranner, I. Social waves in giant honeybees repel hornets. PLoS ONE 2008, 3, e3141. [Google Scholar] [CrossRef]
- Schmelzer, E.; Kastberger, G. ‘Special agents’ trigger social waves in giant honeybees (Apis dorsata). Naturwissenschaften 2009, 96, 1431–1441. [Google Scholar] [CrossRef] [PubMed]
- Kamioka, T.; Suzuki, H.C.; Ugajin, A.; Yamaguchi, Y.; Nishimura, M.; Sasaki, T.; Kawata, M. Genes associated with hot defensive bee ball in the Japanese honeybee. Apis cerana japonica. BMC Ecol. Evol. 2022, 22, 31. [Google Scholar]
- Yamaguchi, Y.; Ugajin, A.; Utagawa, S.; Nishimura, M.; Hattori, M.; Ono, M. Double-edged heat: Honeybee participation in a hot defensive bee ball reduces life expectancy with an increased likelihood of engaging in future defense. Behav. Ecol. Sociobiol. 2018, 72, 123. [Google Scholar] [CrossRef]
- Vijayan, S.; Warrant, E.J.; Somanathan, H. Defensive shimmering responses in Apis dorsata are triggered by dark stimuli moving against a bright background. J. Exp. Biol. 2022, 225, jeb244716. [Google Scholar] [CrossRef]
- Shorter, J.R.; Arechavaleta-Velasco, M.; Robles-Rios, C.; Hunt, G.J. A genetic analysis of the stinging and guarding behaviors of the honey bee. Behav. Genet. 2012, 42, 663–674. [Google Scholar] [CrossRef]
- Nouvian, M.; Mandal, S.; Jamme, C.; Claudianos, C.; D’Ettorre, P.; Reinhard, J.; Giurfa, M. Cooperative defence operates by social modulation of biogenic amine levels in the honey bee brain. Proc. R. Soc. B Biol. Sci. 2018, 285, 20172653. [Google Scholar] [CrossRef]
- Hunt, G.J. Flight and fight: A comparative view of the neurophysiology and genetics of honey bee defensive behavior. J. Insect Physiol. 2007, 53, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Momohara, Y.; Kanai, A.; Nagayama, T. Aminergic control of social status in crayfish agonistic encounters. PLoS ONE 2013, 8, e74489. [Google Scholar] [CrossRef]
- Momohara, Y.; Yoshida, M.; Nagayama, T. Serotonergic modulation of social status-dependent behavioural plasticity of the crayfish avoidance reaction. J. Comp. Physiol. A 2015, 201, 1063–1074. [Google Scholar] [CrossRef] [PubMed]
- Dierick, H.A.; Greenspan, R.J. Serotonin and neuropeptide F have opposite modulatory effects on fly aggression. Nat. Genet. 2007, 39, 678–682. [Google Scholar] [CrossRef]
- Hu, S.W.; Yang, Y.T.; Sun, Y.; Zhan, Y.P.; Zhu, Y. Serotonin signals overcome loser mentality in Drosophila. iScience 2020, 23, 101651. [Google Scholar] [CrossRef] [PubMed]
- Nouvian, M.; Deisig, N.; Reinhard, J.; Giurfa, M. Seasonality, alarm pheromone and serotonin: Insights on the neurobiology of honeybee defence from winter bees. Biol. Lett. 2018, 14, 20180337. [Google Scholar] [CrossRef]
- De Boer, S.F.; Olivier, B.; Veening, J.; Koolhaas, J.M. The neurobiology of offensive aggression: Revealing a modular view. Physiol. Behav. 2015, 146, 111–127. [Google Scholar] [CrossRef]
- Bubak, A.N.; Watt, M.J.; Yaeger, J.D.; Renner, K.J.; Swallow, J.G. The stalk-eyed fly as a model for aggression–is there a conserved role for 5-HT between vertebrates and invertebrates? J. Exp. Biol. 2020, 223, jeb132159. [Google Scholar] [CrossRef]
- Nouvian, M.; Reinhard, J.; Giurfa, M. The defensive response of the honeybee Apis mellifera. J. Exp. Biol. 2016, 219, 3505–3517. [Google Scholar] [CrossRef]
- Tedjakumala, S.R.; Aimable, M.; Giurfa, M. Pharmacological modulation of aversive responsiveness in honey bees. Front. Behav. Neurosci. 2014, 7, 221. [Google Scholar] [CrossRef]
- Chen, Y.L.; Hung, Y.S.; Yang, E.C. Biogenic amine levels change in the brains of stressed honeybees. Arch. Insect Biochem. Physiol. 2008, 68, 241–250. [Google Scholar] [CrossRef]
- Stubbendorff, C.; Hale, E.; Cassaday, H.J.; Bast, T.; Stevenson, C.W. Dopamine D1-like receptors in the dorsomedial prefrontal cortex regulate contextual fear conditioning. Psychopharmacology 2019, 236, 1771–1782. [Google Scholar] [CrossRef]
- Huang, C.; Luo, J.; Woo, S.J.; Roitman, L.A.; Li, J.; Pieribone, V.A.; Kannan, M.; Vasan, G.; Schnitzer, M.J. Dopamine-mediated interactions between short- and long-term memory dynamics. Nature 2024, 634, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
- Pribadi, A.; Rieger, M.A.; Rosales, K.; Reddy, K.C.; Chalasani, S.H. Dopamine signaling regulates predator-driven changes in Caenorhabditis elegans’ egg laying behavior. eLife 2023, 12, e83957. [Google Scholar] [CrossRef]
- Gu, G.; Wang, Z.; Lin, T.; Wang, S.; Li, J.; Dong, S.; Tan, K. Bee fear responses are mediated by dopamine and influence cognition. J. Anim. Ecol. 2025, 94, 112–124. [Google Scholar] [CrossRef]
- Wang, Z.; Qu, Y.; Dong, S.; Wen, P.; Li, J.; Tan, K.; Menzel, R. Honey bees modulate their olfactory learning in the presence of hornet predators and alarm component. PLoS ONE 2016, 11, e0150399. [Google Scholar] [CrossRef] [PubMed]
- Rittschof, C.C.; Vekaria, H.J.; Palmer, J.H.; Sullivan, P.G. Biogenic amines and activity levels alter the neural energetic response to aggressive social cues in the honey bee Apis mellifera. J. Neurosci. Res. 2019, 97, 991–1003. [Google Scholar] [CrossRef]
- Aonuma, H. Serotonergic control in initiating defensive responses to unexpected tactile stimuli in the trap-jaw ant Odontomachus kuroiwae. J. Exp. Biol. 2020, 223, jeb228874. [Google Scholar]
- Gibson, W.T.; Gonzalez, C.R.; Fernandez, C.; Ramasamy, L.; Tabachnik, T.; Du, R.R.; Felsen, P.D.; Maire, M.R.; Perona, P.; Anderson, D.J. Behavioral responses to a repetitive visual threat stimulus express a persistent state of defensive arousal in Drosophila. Curr. Biol. 2015, 25, 1401–1415. [Google Scholar] [CrossRef]
- Chouhan, N.S.; Mohan, K.; Ghose, A. cAMP signaling mediates behavioral flexibility and consolidation of social status in Drosophila aggression. J. Exp. Biol. 2017, 220, 4502–4514. [Google Scholar] [PubMed]
- Wachten, S.; Schlenstedt, J.; Gauss, R.; Baumann, A. Molecular identification and functional characterization of an adenylyl cyclase from the honeybee. J. Neurochem. 2006, 96, 1580–1590. [Google Scholar] [CrossRef] [PubMed]
- Beggs, K.T.; Tyndall, J.D.; Mercer, A.R. Honey bee dopamine and octopamine receptors linked to intracellular calcium signaling have a close phylogenetic and pharmacological relationship. PLoS ONE 2011, 6, e26809. [Google Scholar] [CrossRef]
- Mattila, H.R.; Kernen, H.G.; Otis, G.W.; Nguyen, L.T.; Pham, H.D.; Knight, O.M.; Phan, N.T. Giant hornet (Vespa soror) attacks trigger frenetic antipredator signalling in honeybee (Apis cerana) colonies. R. Soc. Open Sci. 2021, 8, 211215. [Google Scholar] [CrossRef]
- Rittschof, C.C.; Vekaria, H.J.; Palmer, J.H.; Sullivan, P.G. Brain mitochondrial bioenergetics change with rapid and prolonged shifts in aggression in the honey bee, Apis mellifera. J. Exp. Biol. 2018, 221, jeb176917. [Google Scholar] [CrossRef]
- Tait, C.; Chicco, A.J.; Naug, D. Brain energy metabolism as an underlying basis of slow and fast cognitive phenotypes in honeybees. J. Exp. Biol. 2024, 227, jeb247835. [Google Scholar] [CrossRef]
- Placais, P.Y.; de Tredern, É.; Scheunemann, L.; Trannoy, S.; Goguel, V.; Han, K.A.; Preat, T. Upregulated energy metabolism in the Drosophila mushroom body is the trigger for long-term memory. Nat. Commun. 2017, 8, 15510. [Google Scholar] [CrossRef] [PubMed]
- Ugajin, A.; Kiya, T.; Kunieda, T.; Ono, M.; Yoshida, T.; Kubo, T. Detection of neural activity in the brains of Japanese honeybee workers during the formation of a “hot defensive bee ball”. PLoS ONE 2012, 7, e32902. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, S.; Rittschof, C.C.; Djukovic, D.; Gu, H.; Raftery, D.; Price, N.D.; Robinson, G.E. Aggression is associated with aerobic glycolysis in the honey bee brain1. Genes Brain Behav. 2015, 14, 158–166. [Google Scholar] [CrossRef]
- Li-Byarlay, H.; Rittschof, C.C.; Massey, J.H.; Pittendrigh, B.R.; Robinson, G.E. Socially responsive effects of brain oxidative metabolism on aggression. Proc. Natl. Acad. Sci. USA 2014, 111, 12533–12537. [Google Scholar] [CrossRef]
- Bavaresco, C.S.; Ben, J.; Chiarani, F.; Netto, C.A.; de Souza Wyse, A.T. Intrastriatal injection of hypoxanthine impairs memory formation of step-down inhibitory avoidance task in rats. Pharmacol. Biochem. Behav. 2008, 90, 594–597. [Google Scholar] [CrossRef]
- Si, A.; Zhang, S.W.; Maleszka, R. Effects of caffeine on olfactory and visual learning in the honey bee (Apis mellifera). Pharmacol. Biochem. Behav. 2005, 82, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sun, L.; Zhao, D.; Hou, C.; Xia, X.; Cai, Y.; Chen, Y. Adenosine and L-proline can possibly hinder Chinese Sacbrood virus infection in honey bees via immune modulation. Virology 2022, 573, 29–38. [Google Scholar] [CrossRef]
- Lin, C.Y.; Lin, Y.C.; Lu, Y.H.; Chen, S.J.; Lin, Y.H.; Tseng, Y.K.; Huang, R.N. Synergistic impacts of propargite exposure and deformed wing virus infection on the health of western honey bees. Ecotoxicol. Environ. Saf. 2025, 289, 117430. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Tang, C.K.; Lin, Y.H.; Tsai, C.H.; Lu, Y.H.; Wu, Y.L. Snellenius manilae bracovirus suppresses the host immune system by regulating extracellular adenosine levels in Spodoptera litura. Sci. Rep. 2020, 10, 2096. [Google Scholar] [CrossRef]
- Lin, Y.H.; Tai, C.C.; Brož, V.; Tang, C.K.; Chen, P.; Wu, C.P.; Wu, Y.L. Adenosine receptor modulates permissiveness of baculovirus (budded virus) infection via regulation of energy metabolism in Bombyx mori. Front. Immunol. 2020, 11, 763. [Google Scholar] [CrossRef]
- Lin, Y.C.; Lu, Y.H.; Tang, C.K.; Yang, E.C.; Wu, Y.L. Honey bee foraging ability suppressed by imidacloprid can be ameliorated by adding adenosine. Environ. Pollut. 2023, 332, 121920. [Google Scholar] [CrossRef]
- Bajgar, A.; Dolezal, T. Extracellular adenosine modulates host-pathogen interactions through regulation of systemic metabolism during immune response in Drosophila. PLoS Pathog. 2018, 14, e1007022. [Google Scholar] [CrossRef]
- Chen, P.; Lu, Y.H.; Lin, Y.H.; Wu, C.P.; Tang, C.K.; Wei, S.C.; Wu, Y.L. Deformed wing virus infection affects the neurological function of Apis mellifera by altering extracellular adenosine signaling. Insect Biochem. Mol. Biol. 2021, 139, 103674. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Xu, X.; Li, Y.; Ji, N.; Ruan, Y.; Yang, M.; Huang, H.; Yang, L.; Cao, X.; Li, J. Integrated Transcriptomic and Metabolomic Insights into “I See You” (ISY) Defensive Behavior in Apis cerana Against Vespa velutina. Insects 2025, 16, 1047. https://doi.org/10.3390/insects16101047
Chen Y, Xu X, Li Y, Ji N, Ruan Y, Yang M, Huang H, Yang L, Cao X, Li J. Integrated Transcriptomic and Metabolomic Insights into “I See You” (ISY) Defensive Behavior in Apis cerana Against Vespa velutina. Insects. 2025; 16(10):1047. https://doi.org/10.3390/insects16101047
Chicago/Turabian StyleChen, Yijie, Xueling Xu, Yingjiao Li, Ning Ji, Yiwei Ruan, Mei Yang, Hongji Huang, Liulin Yang, Xiaoyu Cao, and Jianghong Li. 2025. "Integrated Transcriptomic and Metabolomic Insights into “I See You” (ISY) Defensive Behavior in Apis cerana Against Vespa velutina" Insects 16, no. 10: 1047. https://doi.org/10.3390/insects16101047
APA StyleChen, Y., Xu, X., Li, Y., Ji, N., Ruan, Y., Yang, M., Huang, H., Yang, L., Cao, X., & Li, J. (2025). Integrated Transcriptomic and Metabolomic Insights into “I See You” (ISY) Defensive Behavior in Apis cerana Against Vespa velutina. Insects, 16(10), 1047. https://doi.org/10.3390/insects16101047




