Drylands Under Pressure: Responses of Insect Density to Land-Use Change in a Tropical Desert
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
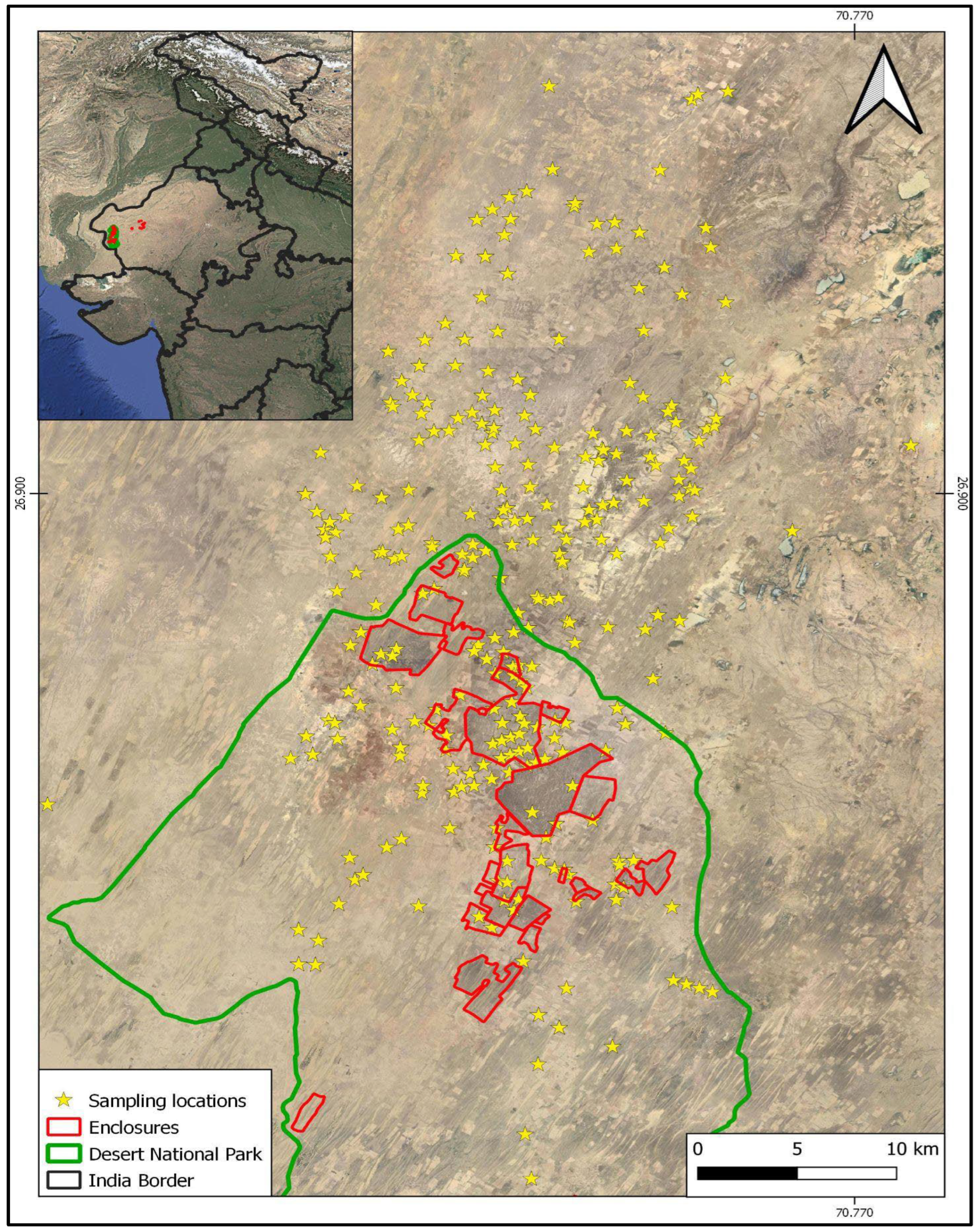
2.1. Study Area
2.2. Field Sampling
2.3. Statistical Analyses
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ceballos, G.; Ehrlich, P.R.; Barnosky, A.D.; García, A.; Pringle, R.M.; Palmer, T.M. Biological annihilation via the ongoing sixth mass extinction signalled by vertebrate population losses and declines. Proc. Natl. Acad. Sci. USA 2017, 114, E6089–E6096. [Google Scholar] [CrossRef]
- Maxwell, S.L.; Fuller, R.A.; Brooks, T.M.; Watson, J.E.M. The ravages of guns, nets and bulldozers. Nature 2016, 536, 143–145. [Google Scholar] [CrossRef] [PubMed]
- Diamond, J.M. Overview: Laboratory experiments, field experiments, and natural experiments. In Community Ecology; Diamond, J.M., Case, T.J., Eds.; Harper & Row: New York, NY, USA, 1989; pp. 3–22. [Google Scholar]
- Li, L.; Wang, L.; Qi, Z. The spatiotemporal variation of farmland use transition and its critical influential factors in coordinated urban-rural regions: A case of Chongqing in western China. Sustain. Cities Soc. 2021, 70, 102921. [Google Scholar] [CrossRef]
- Li, S.; Qin, Z.; Zhao, S.; Gao, M.; Li, S.; Liao, Q.; Du, W. Spatiotemporal Variation of Land Surface Temperature in Henan Province of China from 2003 to 2021. Land 2022, 11, 1104. [Google Scholar] [CrossRef]
- Ye, Y.; Yu, X.; Liu, S. Land use and land cover changes in Northeast China (1780–1908). Landsc. Ecol. 2011, 26, 1097–1109. [Google Scholar]
- Green, R.E.; Cornell, S.J.; Scharlemann, J.P.W.; Balmford, A. Farming and the fate of wild nature. Science 2005, 307, 550–555. [Google Scholar] [CrossRef]
- Tilman, D.; Cassman, K.G.; Matson, P.A.; Naylor, R.; Polasky, S. Agricultural sustainability and intensive production practices. Nature 2001, 418, 671–677. [Google Scholar] [CrossRef]
- Pielke, R.A., Sr.; Pitman, A.J. The influence of land-use change and landscape dynamics on the climate system: Relevance to climate-change policy beyond the radiative effect of greenhouse gases. Philos. Trans. R. Soc. A 2002, 360, 1705–1719. [Google Scholar] [CrossRef] [PubMed]
- Bounoua, L.; DeFries, R.S.; Collatz, G.J.; Sellers, P.; Khan, H. Effects of land cover conversion on surface climate. Clim. Change 2002, 52, 29–64. [Google Scholar] [CrossRef]
- White, R.; Murray, S.; Rohweder, M. Pilot Analysis of Global Ecosystems (PAGE): Grassland Ecosystems; World Resources Institute (WRI): Washington, DC, USA, 2000. [Google Scholar]
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef]
- Grixti, J.C.; Wong, L.T.; Cameron, S.A.; Favret, C. Decline of bumble bees (Bombus) in the North American Midwest. Biol. Conserv. 2009, 142, 75–84. [Google Scholar] [CrossRef]
- Roy, P.S.; Roy, A.; Joshi, P.K.; Kale, M.P.; Srivastava, V.K.; Srivastava, S.K.; Dwevidi, R.S.; Joshi, C.; Behera, M.D.; Meiyappan, P.; et al. Development of Decadal (1985–1995–2005) Land Use and Land Cover Database for India. Remote Sens. 2015, 7, 2401–2430. [Google Scholar] [CrossRef]
- Tian, H.; Banger, K.; Bo, T.; Dadhwal, V.K. History of Land Use in India During 1880–2010: Large-Scale Land Transformations Reconstructed from Satellite Data and Historical Archives. Glob. Planet. Change 2014, 121, 78–88. [Google Scholar] [CrossRef]
- Bakhshi, J.; Javadi, S.A.; Tavili, A.; Arzani, H. Study on the effects of different levels of grazing and exclosure on vegetation and soil properties in semi-arid rangelands of Iran. Acta Ecol. Sin. 2020, 40, 425–431. [Google Scholar] [CrossRef]
- Arroyo, A.I.; Pueyo, Y.; Barrantes, O.; Alados, C.L. Interplay between Livestock Grazing and Aridity on the Ecological and Nutritional Value of Forage in Semi-arid Mediterranean Rangelands (NE Spain). Environ. Manag. 2024, 73, 1005–1015. [Google Scholar] [CrossRef]
- Raven, P.H.; Wagner, D.L. Agricultural intensification and climate change are rapidly decreasing insect biodiversity. Proc. Natl. Acad. Sci. USA 2021, 118, e2002548117. [Google Scholar] [CrossRef] [PubMed]
- Dlamini, P.; Chaplot, V. The Impact of Land Degradation on the Quality of Soils in a South African Communal Rangeland. In Land Degradation and Desertification—A Global Crisis; IntechOpen: London, UK, 2016. [Google Scholar] [CrossRef]
- Bai, Z.G.; Dent, D.L.; Olsson, L.; Schaepman, M.E. Assessment of Land Degradation and Improvement. 1. Identification by Remote Sensing; Report 2008/01; ISRIC–World Soil Information: Wageningen, The Netherlands, 2008. [Google Scholar]
- Rahmani, A.R. Need to Start Project Bustards; Bombay Natural History Society: Mumbai, India, 2006. [Google Scholar]
- Dutta, S.; Rahmani, A.R.; Gautam, P.; Kasambe, R.; Narwade, S.; Narayan, G.; Jhala, Y.V. Guidelines for State Action Plan for Resident Bustards’ Recovery Programme; Ministry of Environment & Forests, Government of India: New Delhi, India, 2013. [Google Scholar]
- Tewari, V.P.; Arya, R. Degradation of Arid Rangelands in Thar Desert, India: A Review. Arid Land Res. Manag. 2004, 19, 1–12. [Google Scholar] [CrossRef]
- Rawat, G.S.; Adhikari, B.S. Ecology and Management of Grassland Habitats in India. In ENVIS Bulletin: Wildlife & Protected Areas; Wildlife Institute of India: Dehradun, India, 2015; Volume 17. [Google Scholar]
- Dutta, S.; Rahmani, A.R.; Jhala, Y.V. Running out of time ? The great Indian bustard Ardeotis nigriceps—Status, viability, and conservation strategies. Eur. J. Wildl. Research. 2011, 57, 615–625. [Google Scholar] [CrossRef]
- Fleishman, E.; Murphy, D.D. A Realistic Assessment of the Indicator Potential of Butterflies and Other Charismatic Taxonomic Groups. Conserv. Biol. 2009, 23, 1109–1116. [Google Scholar] [CrossRef]
- Matenaar, D.; Bazelet, C.S.; Hochkirch, A. Simple Tool for the Evaluation of Protected Areas for the Conservation of Grasshoppers. Biol. Conserv. 2015, 192, 192–199. [Google Scholar] [CrossRef]
- Burner, R.C.; Drag, L.; Stephan, J.; Birkemoe, T.; Wetherbee, R.; Muller, J.; Siitonen, J.; Snäll, T.; Skarpaas, O.; Potterf, M.; et al. Functional Structure of European Forest Beetle Communities Is Enhanced by Rare Species. Biol. Conserv. 2022, 267, 109491. [Google Scholar] [CrossRef]
- Powney, G.D.; Carvell, C.; Edwards, M.; Morris, R.K.A.; Roy, H.E.; Woodcock, B.A.; Isaac, N.J.B. Widespread Losses of Pollinating Insects in Britain. Nat. Commun. 2019, 10, 1018. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Bayo, F.; Wyckhuys, K.A.G. Worldwide Decline of the Entomofauna: A Review of Its Drivers. Biol. Conserv. 2019, 232, 8–27. [Google Scholar] [CrossRef]
- Uhler, J.; Redlich, S.; Zhang, J.; Hothorn, T.; Tobisch, C.; Ewald, J.; Thorn, S.; Seibold, S.; Mitesser, O.; Morinière, J.; et al. Relationship of Insect Biomass and Richness with Land Use Along a Climate Gradient. Nat. Commun. 2021, 12, 5946. [Google Scholar] [CrossRef]
- Raghavendra, K.V.; Bhoopathi, T.; Gowthami, R.; Keerthi, M.C.; Suroshe, S.S.; Ramesh, K.B.; Thammayya, S.K.; Shivaramu, S.; Chander, S. Insects: Biodiversity, Threat Status and Conservation Approaches. Curr. Sci. 2022, 122, 1374–1384. [Google Scholar] [CrossRef]
- Van der Sluijs, J.P. Insect Decline, an Emerging Global Environmental Risk. Curr. Opin. Environ. Sustain. 2020, 46, 39–42. [Google Scholar] [CrossRef]
- Pati, A.; Kundu, S.; Sharma, A.; Dubey, V.K.; Ghosh, M.; Dasgupta, S.; Banerjee, S. Diversity of Macroinvertebrates in Aquatic Bodies of West Bengal: Implications of Vector Control. Acta Ecol. Sin. 2023, 43, 560–575. [Google Scholar] [CrossRef]
- Chowdhury, S.; Dubey, V.K.; Choudhury, S.; Das, A.; Jeengar, D.; Sujatha, B.; Kumar, A.; Kumar, N.; Semwal, A.; Kumar, V. Insects as Bioindicator: A Hidden Gem for Environmental Monitoring. Front. Environ. Sci. 2023, 11, 1146052. [Google Scholar] [CrossRef]
- Poonia, S.; Rao, A.S. Climate and Climate Change Scenarios in the Indian Thar Region. In Handbook of Climate Change Resilience; Leal Filho, W., Ed.; Springer: Cham, Switzerland, 2018; pp. 1–14. [Google Scholar] [CrossRef]
- Rao, A.S.; Singh, R.S. Climatic Features and Crop Production. In Fifty Years of Arid Zone Research in India; Faroda, A.S., Singh, M., Eds.; CAZRI: Jodhpur, India, 1998; pp. 17–38. [Google Scholar]
- Bhati, T.K.; Kumar, S.; Haileslassie, A.; Whitbread, A.M. Assessment of Agricultural Technologies for Dryland Systems in South Asia; ICRISAT Monograph; ICRISAT: Patancheru, Hyderabad, India, 2017. [Google Scholar]
- Dutta, S.; Bipin, C.M.; Bhardwaj, G.S.; Anoop, K.R.; Jhala, Y.V. Status of Great Indian Bustard and Associated Wildlife in Thar; Wildlife Institute of India & Rajasthan Forest Department: Dehradun & Jaipur, India, 2016. [Google Scholar]
- Song, Y.; Cang, X.; He, W.; Zhang, H.; Wu, K. Migration Activity of Spodoptera litura Between China and South-Southeast Asia. Insects 2024, 15, 335. [Google Scholar] [CrossRef] [PubMed]
- Roffey, J.; Popov, G. Environmental and Behavioural Processes in a Desert Locust Outbreak. Nature 1968, 219, 446–450. [Google Scholar] [CrossRef]
- Sharma, K.K.; Mehra, S.P. The Thar of Rajasthan: Ecology and Conservation of a Desert Ecosystem. In Faunal Ecology and Conservation of the Great Indian Desert; Sivaperuman, C., Baqri, Q.H., Ramaswamy, G., Naseema, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 1–11. [Google Scholar] [CrossRef]
- Islam, M.Z.; Rahmani, A.R. Important Bird Areas in India: Priority Sites for Conservation; Indian Bird Conservation Network; Bombay Natural History Society: Mumbai, India; Birdlife International: Cambridge, UK, 2004. [Google Scholar]
- Nyundo, B.A.; Yarro, J.G. An Assessment of Methods for Sampling Carabid Beetles (Coleoptera: Carabidae) in a Montane Rain Forest. Tanzan. J. Sci. 2009, 33, 41–49. [Google Scholar] [CrossRef]
- Southwood, T.R.E.; Henderson, P.A. Ecological Methods, 3rd ed.; Blackwell Science Ltd.: Oxford, UK, 2000. [Google Scholar]
- Bolker, B.M.; Brooks, M.E.; Clark, C.J.; Geange, S.W.; Poulsen, J.R.; Stevens, M.H.; White, J.S. Generalized Linear Mixed Models: A Practical Guide for Ecology and Evolution. Trends Ecol. Evol. 2009, 24, 127–135. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Bullock, J.M.; Lavorel, S.; Manning, P.; Schaffner, U.; Ostle, N.; Chomel, M.; Durigan, G.; Fry, E.L.; Johnson, D.; et al. Combatting Global Grassland Degradation. Nat. Rev. Earth Environ. 2021, 2, 10. [Google Scholar] [CrossRef]
- Millennium Ecosystem Assessment. Ecosystems and Human Well-Being: Biodiversity Synthesis; World Resources Institute: Washington, DC, USA, 2005. [Google Scholar]
- Zabel, F.; Delzeit, R.; Schneider, J.M.; Seppelt, R.; Mauser, W.; Václavík, T. Global Impacts of Future Cropland Expansion and Intensification on Agricultural Markets and Biodiversity. Nat. Commun. 2019, 10, 2844. [Google Scholar] [CrossRef] [PubMed]
- Fartmann, T.; Brüggeshemke, J.; Poniatowski, D.; Löffler, F. Summer Drought Affects Abundance of Grassland Grasshoppers Differently Along an Elevation Gradient. Ecol. Entomol. 2022, 47, 778–790. [Google Scholar] [CrossRef]
- Poniatowski, D.; Beckmann, C.; Helbing, F.; Löffler, F.; Münsch, T.; Samways, M.J.; Fartmann, T. Relative Impacts of Land-Use and Climate Change on Grasshopper Range Shifts Have Changed Over Time. Glob. Ecol. Biogeogr. 2020, 29, 2190–2202. [Google Scholar] [CrossRef]
- Lahiri, S.; Roy, A.; Fleischman, F.D. Grassland Conservation and Restoration in India: A Governance Crisis. Restor. Ecol. 2023, 31, e13858. [Google Scholar] [CrossRef]
- Madhusudan, M.D.; Vanak, A.T. Mapping the Distribution and Extent of India’s Semi-Arid Open Natural Ecosystems. J. Biogeogr. 2022, 50, 1377–1387. [Google Scholar] [CrossRef]
- Zoltán, K.; Cservenka, J. Effects of Climate Change and Various Grassland Management Practices on Grasshopper (Orthoptera) Assemblages. Adv. Ecol. 2014, 10, 601813. [Google Scholar] [CrossRef]
- O’Neill, K.M.; Olson, B.E.; Rolston, M.G.; Wallander, R.; Larson, D.P.; Seibert, C.E. Effects of Livestock Grazing on Rangeland Grasshopper (Orthoptera: Acrididae) Abundance. Agric. Ecosyst. Environ. 2003, 97, 51–64. [Google Scholar] [CrossRef]
- Hallmann, C.A.; Sorg, M.; Jongejans, E.; Siepel, H.; Hofland, N.; Schwan, H.; Stenmans, W.; Müller, A.; Sumser, H.; Hörren, T.; et al. More than 75 Percent Decline Over 27 Years in Total Flying Insect Biomass in Protected Areas. PLoS ONE 2017, 12, e0185809. [Google Scholar] [CrossRef]
- Pati, A.; Paul, I.; Duta, S. Survey of Orthoptera in the Desert National Park, Rajasthan, India. J. Threat. Taxa 2025, 17, 26421–26425. [Google Scholar] [CrossRef]
- Haridas, C.V.; Meinke, L.J.; Hibbard, B.E.; Siegfried, B.D.; Tenhumberg, B. Effects of Temporal Variation in Temperature and Density Dependence on Insect Population Dynamics. Ecosphere 2016, 7, e01287. [Google Scholar] [CrossRef]
- Hou, Y.; Weng, Z. Temperature-Dependent Development and Life Table Parameters of Octodonta nipae (Coleoptera: Chrysomelidae). Environ. Entomol. 2010, 39, 1676–1684. [Google Scholar] [CrossRef] [PubMed]
- Ragland, G.J.; Kingsolver, J.G. Evolution of Thermotolerance in Seasonal Environments: The Effects of Annual Temperature Variation and Life-History Timing in Wyeomyia smithii. Evolution 2008, 62, 1345–1357. [Google Scholar] [CrossRef]
- Chown, S.L.; Nicolson, S.W. Insect Physiological Ecology: Mechanisms and Patterns; Oxford University Press: Oxford, UK, 2004. [Google Scholar]
- Uvarov, B.P. Grasshoppers and Locusts: A Handbook of General Acridology; Cambridge University Press: Cambridge, UK, 1966; Volume I. [Google Scholar]
- Tauber, C.A.; Tauber, M.J. Insect Seasonal Cycles: Genetics and Evolution. Annu. Rev. Ecol. Syst. 1981, 12, 281–308. [Google Scholar] [CrossRef]
- Aranzamendi, N.H.; Hall, M.; Kingma, S.A.; Van de Pol, M.; Peters, A. Rapid Plastic Breeding Response to Rain Matches Peak Prey Abundance in a Tropical Savanna Bird. J. Anim. Ecol. 2019, 88, 1799–1811. [Google Scholar] [CrossRef]
- Viswanathan, A.; Thrikkadeeri, K.; Koulgi, P.; Praveen, J.; Deomurari, A.; Jha, A.; Warudkar, A.; Suryawanshi, K.; Madhusudan, M.D.; Kaushik, M.; et al. State of India’s Birds 2023: A Framework to Leverage Semi-Structured Citizen Science for Bird Conservation. Ecosphere 2025, 16, e70290. [Google Scholar] [CrossRef]
- SoIB. State of India’s Birds, 2023: Range, Trends, and Conservation Status; The SoIB Partnership: Bengaluru, India, 2023. [Google Scholar] [CrossRef]
- Dutta, S. Ecology of the Great Indian Bustard (Ardeotis nigriceps) in the Thar Landscape. Ph.D. Thesis, Wildlife Institute of India, Dehradun, India, 2012. [Google Scholar]

| Model | K | logL | AICc | delta | Ak. wt |
|---|---|---|---|---|---|
| (Orthoptera) | |||||
| Y ~ hab + grz + (1|year/season) | 8 | −709.20 | 1434.7 | 0.00 | 0.74 |
| Y ~ hab + (1|year/season) | 7 | −711.26 | 1436.7 | 2.08 | 0.26 |
| Y ~ 1 + (1|year/season) | 4 | −765.89 | 1539.9 | 105.2 | 0.00 |
| Y ~ grz + (1|year/season) | 5 | −765.03 | 1540.2 | 105.5 | 0.00 |
| (Coleoptera) | |||||
| Y ~ 1 + (1|year/season) | 4 | −354.05 | 716.2 | 0.00 | 0.40 |
| Y ~ grz + (1|year/season) | 5 | −353.19 | 716.5 | 0.32 | 0.34 |
| Y ~ hab + (1|year/season) | 7 | −351.95 | 718.1 | 1.95 | 0.15 |
| Y ~ hab + grz + (1|year/season) | 8 | −351.25 | 718.8 | 2.60 | 0.11 |
| (Other insects) | |||||
| Y ~ hab + (1|year/season) | 7 | −595.71 | 1205.6 | 0.00 | 0.87 |
| Y ~ hab + grz + (1|year/season) | 8 | −596.98 | 1210.2 | 4.59 | 0.09 |
| Y ~ 1 + (1|year/season) | 4 | −602.00 | 1212.1 | 6.44 | 0.04 |
| Y ~ grz + (1|year/season) | 5 | −603.16 | 1216.4 | 10.80 | 0.00 |
| Random Effect | Fixed Effect | |||||||
|---|---|---|---|---|---|---|---|---|
| SD of Groups | Estimate (SE) | |||||||
| Insect Order | Season: Year | Year | Residual | Intercept | Barren | Grassland | Scrubland | Livestock Grazing |
| Orthoptera | 0.71 | 0.20 | 0.88 | 0.98 (0.40) | −0.27 (0.14) | 0.99 (0.11) | 0.25 (0.11) | −0.29 (0.11) |
| Coleoptera | 0.18 | 0.09 | 0.46 | 0.21 (0.12) | - | - | - | - |
| Others | 0.30 | 0.37 | 0.72 | 0.71 (0.32) | −0.34 (0.12) | −0.27 (0.09) | 0.03 (0.09) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pati, A.; Paul, I.; Dutta, S. Drylands Under Pressure: Responses of Insect Density to Land-Use Change in a Tropical Desert. Insects 2025, 16, 1043. https://doi.org/10.3390/insects16101043
Pati A, Paul I, Dutta S. Drylands Under Pressure: Responses of Insect Density to Land-Use Change in a Tropical Desert. Insects. 2025; 16(10):1043. https://doi.org/10.3390/insects16101043
Chicago/Turabian StylePati, Anshuman, Indranil Paul, and Sutirtha Dutta. 2025. "Drylands Under Pressure: Responses of Insect Density to Land-Use Change in a Tropical Desert" Insects 16, no. 10: 1043. https://doi.org/10.3390/insects16101043
APA StylePati, A., Paul, I., & Dutta, S. (2025). Drylands Under Pressure: Responses of Insect Density to Land-Use Change in a Tropical Desert. Insects, 16(10), 1043. https://doi.org/10.3390/insects16101043






