Comparison of Protein and Carbohydrate Consumption and Processing in Emerging Workers, Gynes and Males of the Wasp Polistes metricus
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Wasp Collections
2.2. Wasp Rearing and Feeding Measurements
2.3. Nutrient Measurements
2.4. Data Analysis
2.4.1. Analysis of Food Consumption
2.4.2. Analysis of Nutrient Intake
2.4.3. Analysis of Nutrient Levels
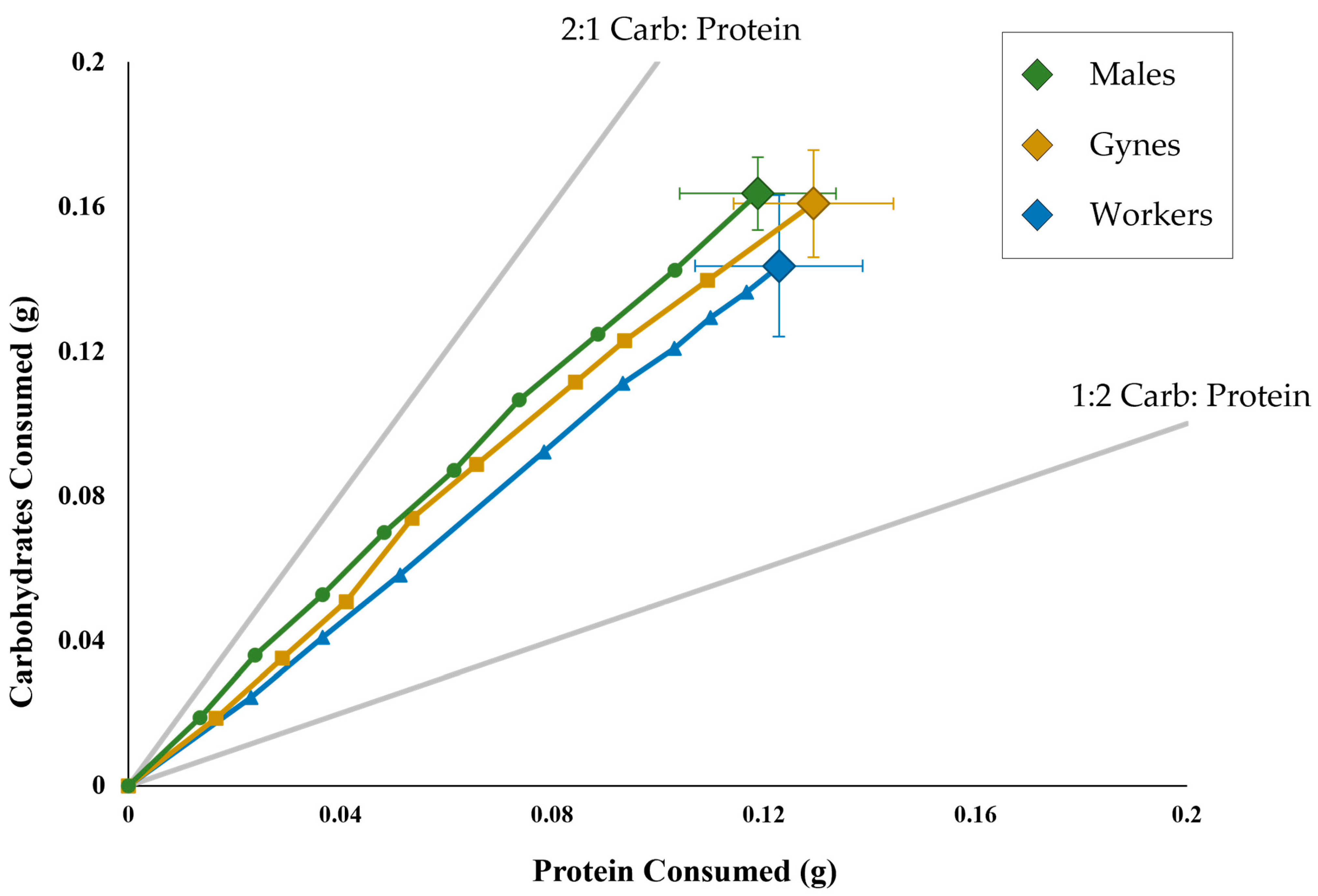
3. Results
3.1. Food Consumption
3.2. Comparison of Nutrient Intake
3.3. Comparison of Nutrient Levels
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Libbrecht, R.; Corona, M.; Wende, F.; Azevedo, D.O.; Serrão, J.E.; Keller, L. Interplay between insulin signaling, juvenile hormone, and vitellogenin regulates maternal effects on polyphenism in ants. Proc. Natl. Acad. Sci. USA 2013, 110, 11050–11055. [Google Scholar] [CrossRef]
- Hunt, J.H.; O’donnell, S.; Chernoff, N.; Brownie, C. Observations on Two Neotropical Swarm-Founding Wasps, Agelaia yepocapa and A. panamaensis (Hymenoptera: Vespidae). Ann. Entomol. Soc. Am. 2001, 94, 555–562. [Google Scholar] [CrossRef]
- Mutti, N.S.; Dolezal, A.G.; Wolschin, F.; Mutti, J.S.; Gill, K.S.; Amdam, G.V. IRS and TOR nutrient-signaling pathways act via juvenile hormone to influence honey bee caste fate. J. Exp. Biol. 2011, 214, 3977–3984. [Google Scholar] [CrossRef]
- Patel, A.; Fondrk, M.K.; Kaftanoglu, O.; Emore, C.; Hunt, G.; Frederick, K.; Amdam, G.V. The making of a queen: TOR pathway is a key player in diphenic caste development. PLoS ONE 2007, 2, e509. [Google Scholar] [CrossRef]
- Smith, C.R.; Anderson, K.E.; Tillberg, C.V.; Gadau, J.; Suarez, A.V. Caste determination in a polymorphic social insect: Nutritional, social, and genetic factors. Am. Nat. 2008, 172, 497–507. [Google Scholar] [CrossRef]
- Wheeler, D.E. Developmental, and physiological determinants of caste in social Hymenoptera: Evolutionary implications. Am. Nat. 1986, 128, 13–34. [Google Scholar] [CrossRef]
- Wheeler, D.E.; Buck, N.; Evans, J.D. Expression of insulin pathway genes during the period of caste determination in the honey bee, Apis mellifera. Insect Mol. Biol. 2006, 15, 597–602. [Google Scholar] [CrossRef]
- Hunt, J.H. A conceptual model for the origin of worker behaviour and adaptation of eusociality. J. Evol. Biol. 2012, 25, 1–19. [Google Scholar] [CrossRef]
- Hunt, J.H. The Evolution of Social Wasps; Oxford University Press: New York, NY, USA, 2007; p. 259. [Google Scholar]
- Strambi, A. Physiological aspects of caste differentiation in social wasps. In Caste Differentiation in Social Insects; Watson, J.A.L., Okot-Kotber, B.M., Noirot, C., Eds.; Pergamon Press: Oxford, UK, 1985; pp. 371–384. [Google Scholar]
- Toth, A.L.; Bilof, K.B.J.; Henshaw, M.T.; Hunt, J.H.; Robinson, G.E. Lipid stores, ovary development, and brain gene expression in Polistes metricus females. Insectes Sociaux 2009, 56, 77–84. [Google Scholar] [CrossRef]
- Strassmann, J.E. Evolutionary implications of early male and satellite nest production in Polistes exclamans colony cycles. Behav. Ecol. Sociobiol. 1981, 8, 55–64. [Google Scholar] [CrossRef]
- Reeve, H.K.; Peters, J.M.; Nonacs, P.; Starks, P.T. Dispersal of first “workers” in social wasps: Causes and implications of an alternative reproductive strategy. Proc. Natl. Acad. Sci. USA 1998, 95, 13737–13742. [Google Scholar] [CrossRef]
- Judd, T.M. Effect of the presence of brood on the behavior and nutrient levels of emerging individuals in field colonies of Polistes metricus. Insectes Sociaux 2018, 65, 171–182. [Google Scholar] [CrossRef]
- Reeve, H.K. Polistes. In The Social Biology of Wasps; Ross, K., Mathews, R., Eds.; Comstock Publishing Associates: Ithaca, NY, USA, 1991; pp. 99–148. [Google Scholar]
- Judd, T.M.; Teal, P.E.A.; Hernandez, E.J.; Choudhury, T.; Hunt, J.H. Quantitative differences in nourishment affect caste-related physiology and development in the paper wasp Polistes metricus. PLoS ONE 2015, 10, e0116199. [Google Scholar] [CrossRef] [PubMed]
- Suryanarayanan, S.; Jeanne, R.L. Antennal drumming, trophallaxis, and colony development in the social wasp Polistes fuscatus (Hymenoptera: Vespidae). Ethology 2008, 114, 1201–1209. [Google Scholar] [CrossRef]
- Yoshimura, H.; Yamada, Y.Y.; Sasaki, K. Identification of biogenic amines involved in photoperiod-dependent caste-fate determination during the adult stage in a temperate paper wasp. J. Insect Physiol. 2021, 131, 104223. [Google Scholar] [CrossRef] [PubMed]
- Solis, C.R.; Strassmann, J.E. Presence of brood affects caste differentiation in the social wasp, Polistes exclamans Viereck (Hymenoptera: Vespidae). Funct. Ecol. 1990, 4, 531–541. [Google Scholar] [CrossRef]
- Yoshimura, H.; Yamada, Y.Y. Caste-fate determination primarily occurs after adult emergence in a primitively eusocial paper wasp: Significance of the photoperiod during the adult stage. Sci. Nat. 2018, 105, 15. [Google Scholar] [CrossRef]
- Hunt, J.H. Trophallaxis and the evolution of eusocial Hymenoptera. In The Biology of Social Insects; Breed, M.D., Michener, C.D., Evans, H.E., Eds.; Westview Press: Boulder, CO, USA, 1982; pp. 201–205. [Google Scholar]
- Hunt, J.H.; Baker, I.; Baker, H.G. Similarity of amino acids in nectar and larval saliva: The nutritional basis for trophallaxis in social wasps. Evolution 1982, 36, 1318–1322. [Google Scholar] [CrossRef]
- Jeanne, R.L. Evolution of social behavior in the Vespidae. Annu. Rev. Entomol. 1980, 25, 371–396. [Google Scholar] [CrossRef]
- O’Donnell, S.; Fiocca, K.; Campbell, M.; Bulova, S.; Zelanko, P.; Velinsky, D. Adult nutrition and reproductive physiology: A stable isotope analysis in a eusocial paper wasp (Mischocyttarus mastigophorus, Hymenoptera: Vespidae). Behav. Ecol. Sociobiol. 2018, 72, 86. [Google Scholar] [CrossRef]
- Hunt, J.H. Adult nourishment during larval provisioning in a primitively eusocial wasp, Polistes metricus Say. Insectes Sociaux 1984, 31, 452–460. [Google Scholar] [CrossRef]
- Gamboa, G.J.; Foster, R.L.; Scope, J.A.; Bitterman, A.M. Effects of stage of colony cycle, contex, and intercolony distance on conspecific tolerance by papers wasps (Polistes fuscatus). Behav. Ecol. Sociobiol. 1991, 29, 87–92. [Google Scholar] [CrossRef]
- Hunt, J.H.; Buck, N.A.; Wheeler, D.E. Storage proteins in vespid wasps: Characterization, developmental pattern, and occurrence in adults. J. Insect Physiol. 2003, 49, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Judd, T.M.; Magnus, R.M.; Fasnacht, M.P. A nutritional profile of the social wasp Polistes metricus: Differences in nutrient levels between castes and changes within castes during the annual life cycle. J. Insect Physiol. 2010, 56, 42–56. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, H.; Yamada, Y.Y. The first brood emerges smaller, lighter, and with lower lipid stores in the paper wasp Polistes jokahamae (Hymenoptera: Vespidae). Insectes Sociaux 2018, 65, 473–481. [Google Scholar] [CrossRef]
- Simpson, S.J.; Raubenheimer, D. A framework for the study of macronutrient intake in fish. Aquac. Res. 2001, 32, 421–432. [Google Scholar] [CrossRef]
- de Souza, A.R.; Lino-Neto, J.; Tibbetts, E.A.; Turillazzi, S.; Beani, L. The leks of Polistes dominula paper wasps: Tiny abdominal spots play a critical role in male attacks toward potential rivals. Ethol. Ecol. Evol. 2017, 29, 410–419. [Google Scholar] [CrossRef]
- Izzo, A.; Tibbetts, E.A. Heightened condition dependence of a sexually selected signal in male Polistes dominulus paper wasps. Ethology 2015, 121, 586–592. [Google Scholar] [CrossRef]
- Lee, J.X.Q.; Starr, C.K. Violent combat among Polistes gigas males (Hymenoptera: Vespidae). Sociobiology 2007, 50, 337–342. [Google Scholar]
- Polak, M. Large-size advantage and assessment of resource holding potential in male Polistes fuscatus (F.) (Hymenoptera: Vespidae). Anim. Behav. 1994, 48, 1231–1234. [Google Scholar] [CrossRef]
- Hunt, J.H.; Noonan, K.M. Larval feeding by male Polistes fuscatus and Polistes metricus (Hymenoptera: Vespidae). Insectes Sociaux 1979, 26, 247–251. [Google Scholar] [CrossRef]
- Makino, S.i. Sexual differences in larval feeding behavior in a paper wasp, Polistes jadwigae (Hymenoptera, Vespidae). J. Ethol. 1993, 11, 73–75. [Google Scholar] [CrossRef]
- Bohm, M.K. Effects of environment and juvenile hormone on ovaries of the wasp, Polistes metricus. J. Insect Physiol. 1972, 18, 1875–1883. [Google Scholar] [CrossRef]
- Strassmann, J.E.; Lee, R.E., Jr.; Rojas, R.R.; Baust, J.G. Caste and sex differences in cold-hardiness in the social wasps, Polistes annularis and P. exclamans (Hymenoptera: Vespidae). Insectes Sociaux 1984, 31, 291–301. [Google Scholar] [CrossRef]
- Jandt, J.M.; Thomson, J.L.; Geffre, A.C.; Toth, A.L. Lab rearing environment perturbs social traits: A case study with Polistes wasps. Behav. Ecol. 2015, 26, 1274–1284. [Google Scholar] [CrossRef]
- Hunt, J.H. Nourishment and evolution in wasps sensu lato. In Nourishment and Evolution in Insect Societies; Hunt, J.H., Nalepa, C.A., Eds.; Westview Press: Boulder, CO, USA, 1994; pp. 211–245. [Google Scholar]
- Hunt, J.H. Origin of an evolutionary novelty: The worker phenotype of eusocial wasps. Insectes Sociaux 2021, 68, 303–318. [Google Scholar] [CrossRef]
- Hunt, J.H.; Dove, M.A. Nourishment affects colony demographics in the paper wasp Polistes metricus. Ecol. Entomol. 2002, 27, 467–474. [Google Scholar] [CrossRef]
- Raubenheimer, D.; Simpson, S.J. Nutritional ecology and foraging theory. Curr. Opin. Insect Sci. 2018, 27, 38–45. [Google Scholar] [CrossRef]
- Hayward, A.; Gillooly, J.F. The Cost of sex: Quantifying energetic investment in gamete production by males and females. PLoS ONE 2011, 6, e16557. [Google Scholar] [CrossRef]
- Correa-Fernandez, F.; Cruz-Landim, C. Differential flight muscle development in workers, queens and males of the eusocial bees, Apis mellifera and Scaptotrigona postica. J. Insect Sci. 2010, 10, 85. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gay, D.R.; Judd, T.M. Comparison of Protein and Carbohydrate Consumption and Processing in Emerging Workers, Gynes and Males of the Wasp Polistes metricus. Insects 2023, 14, 617. https://doi.org/10.3390/insects14070617
Gay DR, Judd TM. Comparison of Protein and Carbohydrate Consumption and Processing in Emerging Workers, Gynes and Males of the Wasp Polistes metricus. Insects. 2023; 14(7):617. https://doi.org/10.3390/insects14070617
Chicago/Turabian StyleGay, Daniel R., and Timothy M. Judd. 2023. "Comparison of Protein and Carbohydrate Consumption and Processing in Emerging Workers, Gynes and Males of the Wasp Polistes metricus" Insects 14, no. 7: 617. https://doi.org/10.3390/insects14070617
APA StyleGay, D. R., & Judd, T. M. (2023). Comparison of Protein and Carbohydrate Consumption and Processing in Emerging Workers, Gynes and Males of the Wasp Polistes metricus. Insects, 14(7), 617. https://doi.org/10.3390/insects14070617





