Qualitative Analyses of Textile Damage (Cuts and Tears) Applied to Fabrics Exposed to the Decomposition of Carcasses and Associated Insect Activity in an Austral Summer
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Piglet and Fabric Preparation
2.2. Field Setup
2.3. Collection and Analyses of Piglets and Fabrics
3. Results
3.1. Environmental Conditions
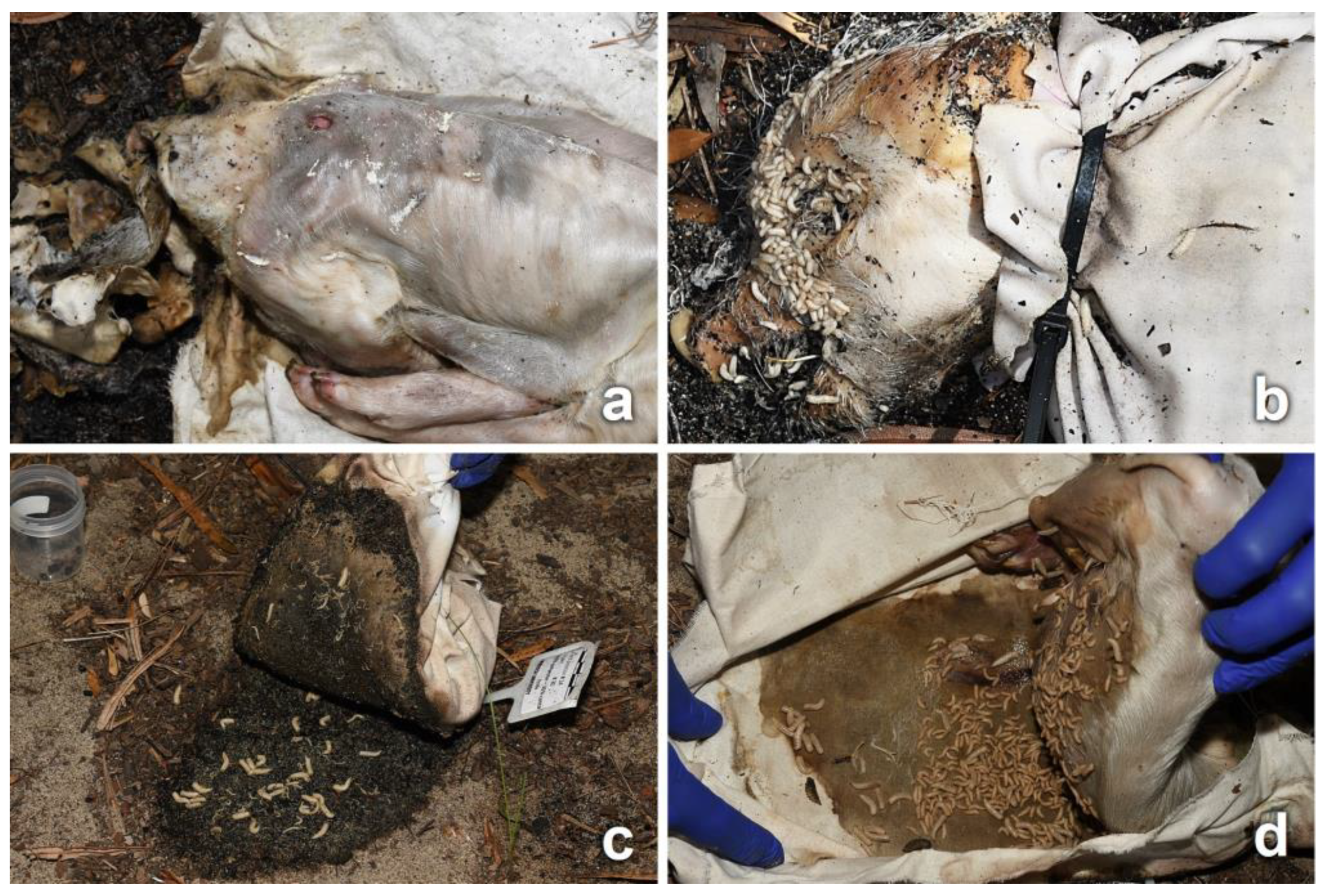
3.2. Decomposition Process
3.3. Entomological Data
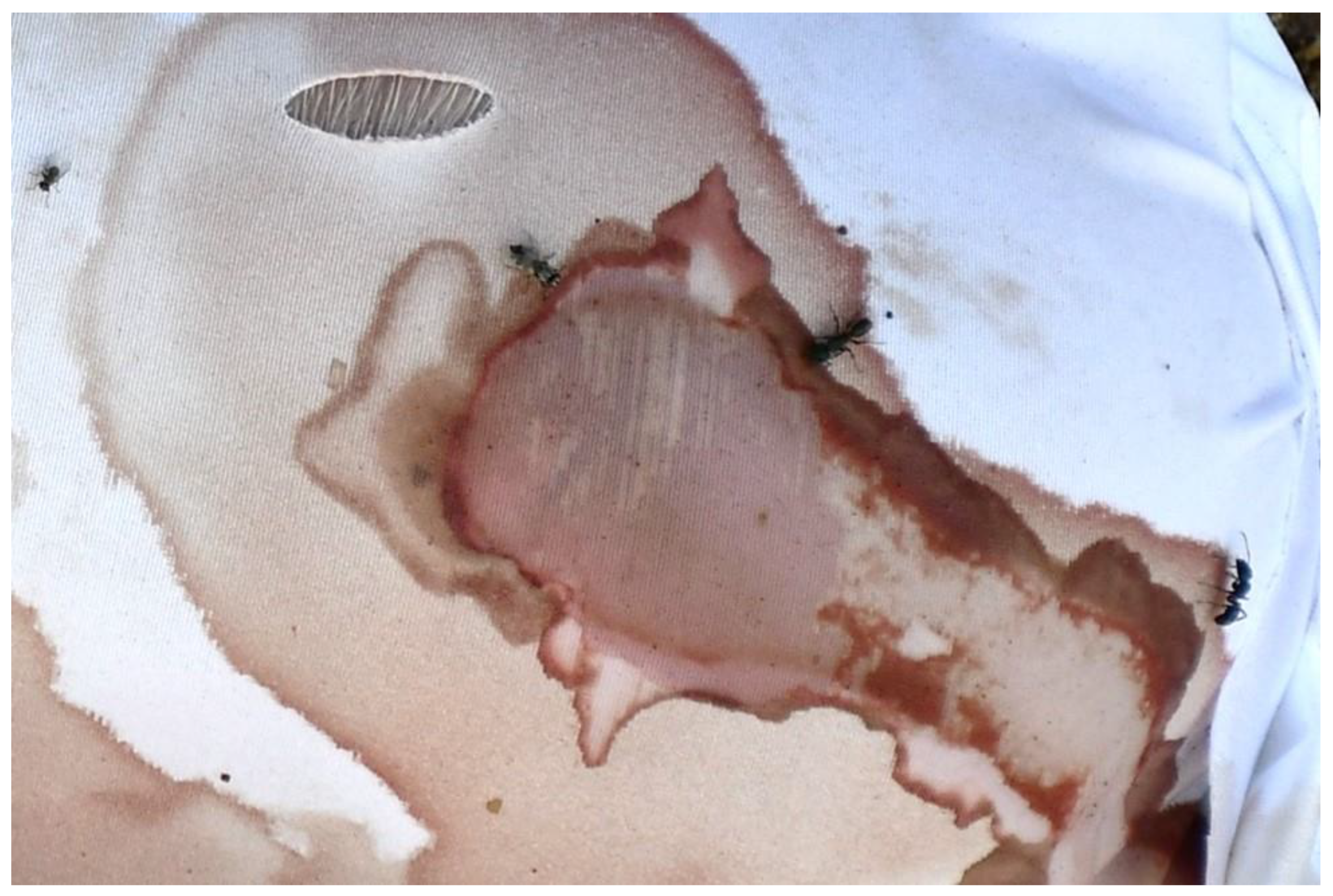
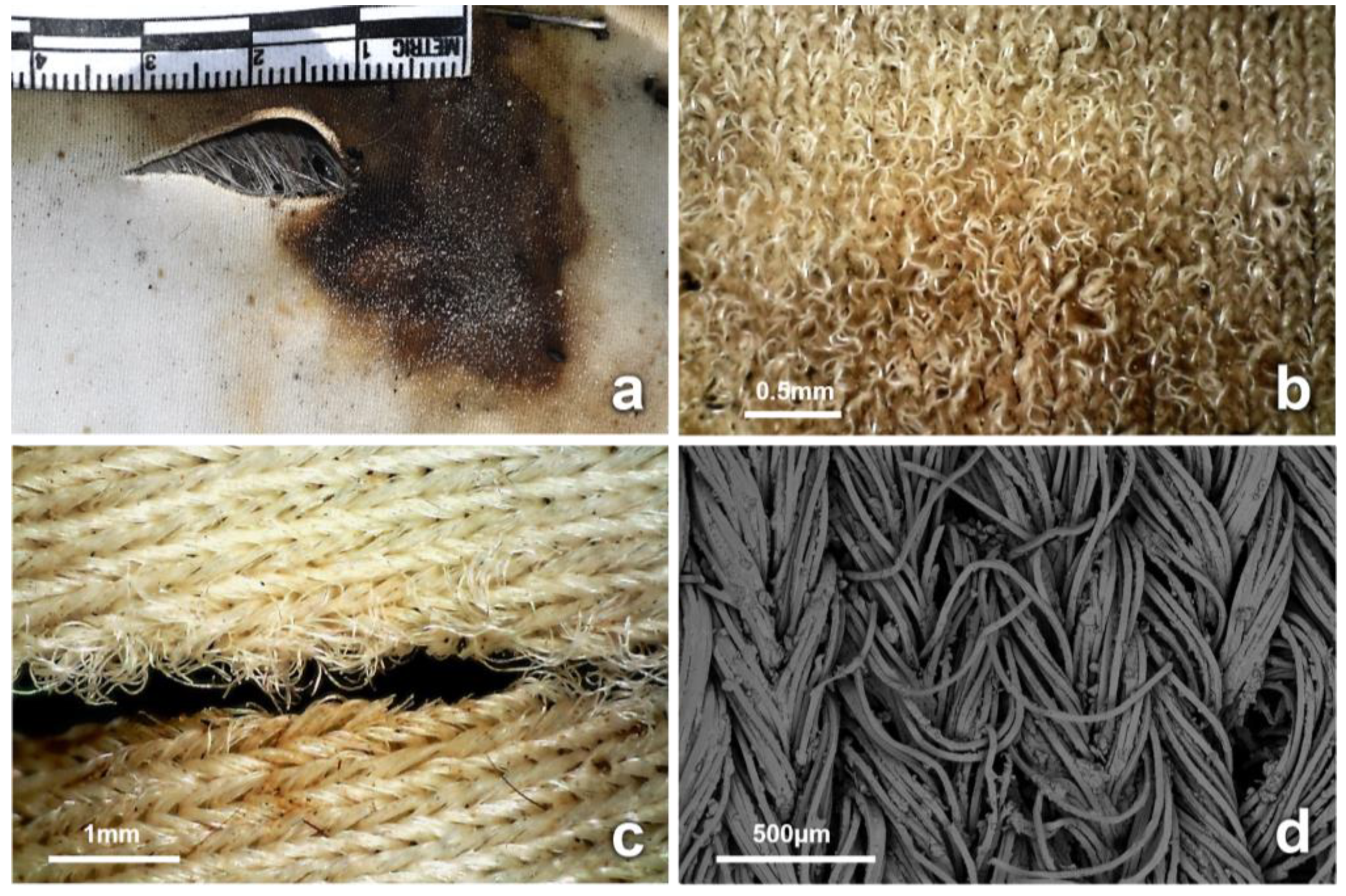
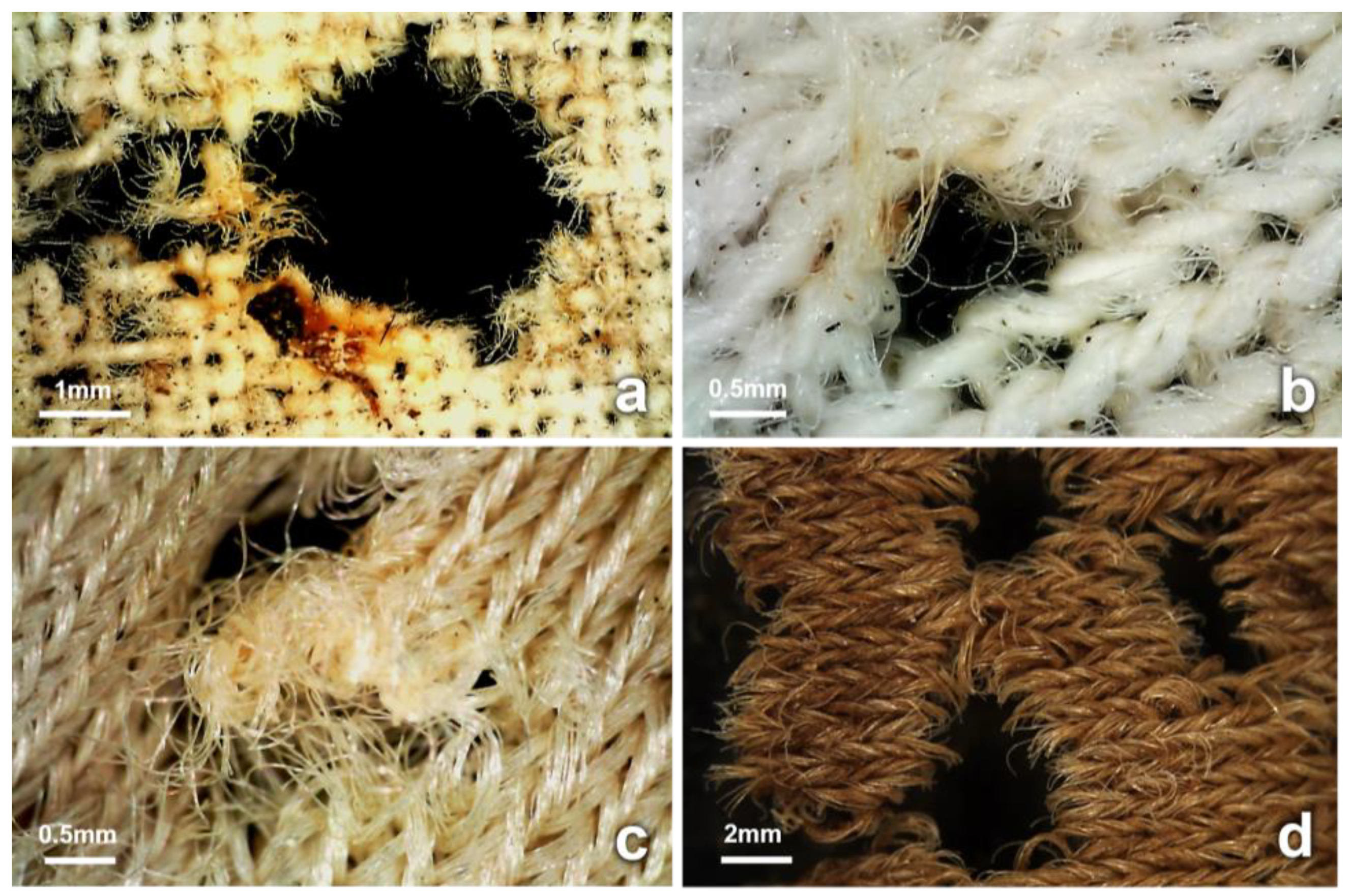
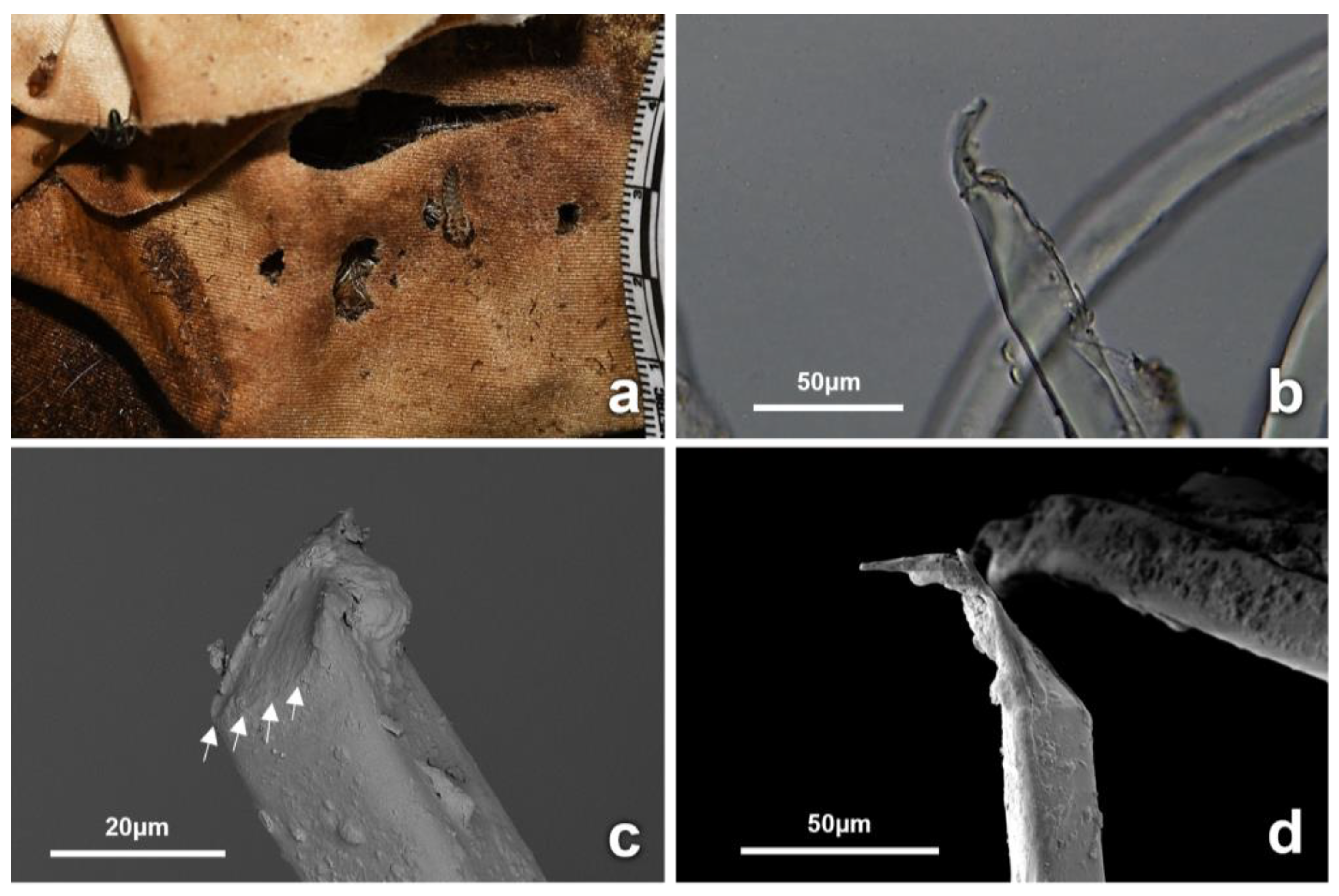
3.4. Observations on Fabrics
4. Discussion
4.1. Inflicted Damage (Cuts and Tears)
4.2. Insect-Induced Modifications
4.3. Biodegradation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taupin, J.M.; Cwiklik, C. Scientific Protocols for Forensic Examination of Clothing; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Bier, C.; Dusenbury, M.M. Textiles. In Encyclopedia of Archaeology; Elsevier: Amsterdam, The Netherlands, 2008; pp. 2119–2125. [Google Scholar]
- Carr, D.J. Chapter One—Fibres, Yarns and Fabrics. In Forensic Textile Science; Carr, D., Ed.; Woodhead Publishing: Sawston, UK, 2017; pp. 3–14. [Google Scholar] [CrossRef]
- Harrison, K.; Ries, S. Chapter Three—Fabrics as Forensic Evidence. In Forensic Textile Science; Carr, D., Ed.; Woodhead Publishing: Sawston, UK, 2017; pp. 27–37. [Google Scholar] [CrossRef]
- Bartels, L. Knife crime: Recent Data on Carriage and Use. In Trends Issues Crime Crim. Justice no. 417; Australian Institute of Criminology: Canberra, Australia, 2011; pp. 1–6. [Google Scholar]
- Kemp, S.E.; Carr, D.J.; Kieser, J.; Niven, B.E.; Taylor, M.C. Forensic evidence in apparel fabrics due to stab events. Forensic Sci. Int. 2009, 191, 86–96. [Google Scholar] [CrossRef]
- Dann, T.; Carr, D.J.; Laing, R.; Niven, B.E.; Kieser, J. Tearing of knicker fabrics. Forensic Sci. Int. 2011, 217, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Sloan, K.; Fergusson, M.; Robertson, J. Australian forensic textile damage examinations—Finding a way forward since PCAST. Sci. Justice 2019, 59, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Goff, M.L. Estimation of Postmortem Interval Using Arthropod Development and Successional Patterns. Forensic Sci. Rev. 1993, 5, 81–94. [Google Scholar] [PubMed]
- Payne, J.A. A Summer Carrion Study of the Baby Pig Sus Scrofa Linnaeus. Ecology 1965, 46, 592–602. [Google Scholar] [CrossRef]
- Byrd, J.H.; Tomberlin, J.K. Forensic Entomology: The Utility of Arthropods in Legal Investigations, 3rd ed.; CRC Press; Taylor & Francis Group: Boca Raton, FL, USA, 2020. [Google Scholar]
- Mann, R.W.; Bass, W.M.; Meadows, L. Time since death and decomposition of the human body: Variables and observations in case and experimental field studies. J. Forensic Sci. 1990, 35, 103–111. [Google Scholar] [CrossRef]
- Campobasso, C.P.; Di Vella, G.; Introna, F. Factors affecting decomposition and Diptera colonization. Forensic Sci. Int. 2001, 120, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Goff, M. Problems in Estimation of Postmortem Interval Resulting from Wrapping of the Corpse: A Case Study from Hawaii’. J. Agri. Entomol. 1992, 9, 237–243. [Google Scholar]
- Magni, P.A.; Dhaliwal, S.S.; Dadour, I.R. Effect of Continuous and Cyclic Exposure to a Cold Environment on the Development of Larvae of Lucilia sericata (Diptera: Calliphoridae) in Different Sized Larval Masses. J. Med. Entomol. 2016, 53, 782–789. [Google Scholar] [CrossRef]
- Adolf, F.P.; Hearle, J. Textile damage in forensic investigations. In Atlas of Fibre Fracture and Damage to Textiles, 2nd ed.; Hearle, J.W.S., Lomas, B., Cooke, W.D., Eds.; Woodhead Publishing: Sawston, UK, 1998; pp. 397–405. [Google Scholar] [CrossRef]
- Kemp, S.E. Forensic Analysis of Sharp Weapon Damage to Textile Products. In Forensic Textile Science; Carr, D., Ed.; Woodhead Publishing: Sawston, UK, 2017; pp. 71–97. [Google Scholar] [CrossRef]
- Magni, P.A.; Voss, S.C.; Testi, R.; Borrini, M.; Dadour, I.R. A Biological and Procedural Review of Forensically Significant Dermestes Species (Coleoptera: Dermestidae). J. Med. Entomol. 2015, 52, 755–769. [Google Scholar] [CrossRef]
- Mills, J.; Klausmeier, R.E. The biodeterioration of synthetic polymers and plasticizers. C R C Crit. Rev. Environ. Control. 1974, 4, 341–351. [Google Scholar] [CrossRef]
- Mallis, A.; Miller, A.C.; Hill, R.C. The Attraction of Stains to Three Species of Fabric Pests. J. Econ. Entomol. 1959, 52, 382–384. [Google Scholar] [CrossRef]
- Finley, E.L.; McDermott, F.G.; Gross, H.R. Degradation of Fabric by American Cockroach, House Cricket, and Striped Earwig. J. Econ. Entomol. 1968, 61, 1552–1557. [Google Scholar] [CrossRef]
- Mallis, A.; Miller, A.C.; Hill, R.C. Feeding of Four Species of Fabric Pests on Natural and Synthetic Textiles. J. Econ. Entomol. 1958, 51, 248–249. [Google Scholar] [CrossRef]
- Haglund, W.D. Forensic Taphonomy: The Postmortem Fate of Human Remains; CRC Press: Boca Raton, FL, USA, 1996. [Google Scholar]
- Komar, D.; Beattie, O. Postmortem insect activity may mimic perimortem sexual assault clothing patterns. J. Forensic Sci. 1998, 43, 792. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.S. Comparison of decomposition rates and faunal colonization of carrion in indoor and outdoor environments. J. Forensic Sci. 2011, 56, 136–142. [Google Scholar] [CrossRef] [PubMed]
- De Souza, A.A.F.; De Rosa, C.T.A.; Arantes, L.C.; Pujol-Luz, J.R. Artifacts Caused by Leaf-Cutting Ants of the Genus Atta (Hymenoptera: Formicidae): Postmortem Bite Injuries and the Tearing of Clothes. J. Forensic Sci. 2020, 65, 1012–1015. [Google Scholar] [CrossRef]
- Bostock, E.; Parkes, G.M.B.; Williams, G. The effect of insect activity on clothing damage evidence following a period of decomposition. Crime Secur. Soc. 2018, 1, 37–41. [Google Scholar] [CrossRef]
- Bostock, E.; Parkes, G.M.B.; Williams, G. Effect of decomposition on clothing damage evidence: A preliminary study. Crime Secur. Soc. 2018, 1, 1–4. [Google Scholar] [CrossRef]
- Taupin, J.M. Testing conflicting scenarios—A role for simulation experiments in damage analysis of clothing. J. Forensic Sci. 1998, 43, 891. [Google Scholar] [CrossRef]
- Williams, G.A. Forensic textile damage analysis: Recent advances. Res. Rep. Forensic Med. Sci. 2018, 8, 1–8. [Google Scholar] [CrossRef]
- Smith, M.J.; Thompson, K. Forensic Analysis of Textile Degradation and Natural Damage. In Forensic Textile Science; Carr, D., Ed.; Woodhead Publishing: Sawston, UK, 2017; pp. 41–69. [Google Scholar] [CrossRef]
- Mitchell, J.L.; Carr, D.J.; Niven, B.E.; Harrison, K.; Girvan, E. Physical and mechanical degradation of shirting fabrics in burial conditions. Forensic Sci. Int. 2012, 222, 94–101. [Google Scholar] [CrossRef]
- Schotsmans, E.M.; Márquez-Grant, N.; Forbes, S.L. Taphonomy of Human Remains: Forensic Analysis of the Dead and the Depositional Environment; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Janaway, R.C. Degradation of clothing and other dress materials associated with buried bodies of archaeological and forensic interest. In Advances in Forensic Taphonomy; CRC Press: Boca Raton, FL, USA, 2001; pp. 379–402. [Google Scholar]
- Ueland, M.; Nizio, K.D.; Forbes, S.L.; Stuart, B.H. The interactive effect of the degradation of cotton clothing and decomposition fluid production associated with decaying remains. Forensic Sci. Int. 2015, 255, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J.; Roux, C.; Wiggins, K.G. Forensic Examination of Fibres, 3rd ed.; Taylor and Francis: Boca Raton, FL, USA, 2017. [Google Scholar]
- Hill, M.A.; Pokines, J.T. Comparative analysis of fetal pig decomposition processes in burials of variable depths and wrapping. J. Forensic Sci. 2022, 67, 2192–2202. [Google Scholar] [CrossRef] [PubMed]
- Korn, E.D. An Introduction to the Chemistry and Biochemistry of Fatty Acids and Their Glycerides. F. D. Gunstone. Chapman and Hall, London, 1967 Second edition of Introduction to the Chemistry of Fats and Fatty Acids. Science 1968, 160, 1214. [Google Scholar] [CrossRef]
- Schoenly, K.; Griest, K.; Rhine, S. An experimental field protocol for investigating the postmortem interval using multidisciplinary indicators. J. Forensic Sci. 1991, 36, 1395. [Google Scholar] [CrossRef] [PubMed]
- Schoenly, K.G.; Hall, R.D. Testing Reliability of Animal Models in Research and Training Programs in Forensic Entomology, Part II; National Criminal Justice Reference Service (NCJRS): Washington, DC, USA, 2002; p. 32. [Google Scholar]
- Matuszewski, S.; Hall, M.J.R.; Moreau, G.; Schoenly, K.G.; Tarone, A.M.; Villet, M.H. Pigs vs people: The use of pigs as analogues for humans in forensic entomology and taphonomy research. Int. J. Legal Med. 2020, 134, 793–810. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, P.T.; Jones, M.D.; James, D.S.; Leadbeatter, S.; Holt, C.A.; Nokes, L.D. Dynamics of stab wounds: Force required for penetration of various cadaveric human tissues. Forensic Sci. Int. 1999, 104, 173–178. [Google Scholar] [CrossRef]
- Megyesi, M.S.; Nawrocki, S.P.; Haskell, N.H. Using accumulated degree-days to estimate the postmortem interval from decomposed human remains. J. Forensic Sci. 2005, 50, 618–626. [Google Scholar] [CrossRef]
- Millar, R.B.; Anderson, M.J. Remedies for pseudoreplication. Fish. Res. 2004, 70, 397–407. [Google Scholar] [CrossRef]
- Amendt, J.; Campobasso, C.P.; Gaudry, E.; Reiter, C.; LeBlanc, H.N.; Hall, M.J. Best practice in forensic entomology–standards and guidelines. Int. J. Legal Med. 2007, 121, 90–104. [Google Scholar] [CrossRef]
- Wallman, J.F. A key to the adults of species of blowflies in southern Australia known or suspected to breed in carrion. Med. Vet. Entomol. 2001, 15, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.F.; Ślipiński, S.A. Australian Beetles. Volume 1: Morphology, Classification and Keys; CSIRO Publishing: Collingwood, Australia, 2013. [Google Scholar]
- Entomology, C.D.O. The Insects of Australia: A Textbook for Students and Research Workers, 2nd ed.; Melbourne University Press: Carlton South, Australia, 1991. [Google Scholar]
- Voss, S.C.; Spafford, H.; Dadour, I.R. Temperature-dependant development of Nasonia vitripennis on five forensically important carrion fly species. Entomol. Exp. Et Appl. 2010, 135, 37–47. [Google Scholar] [CrossRef]
- Frederickx, C.; Dekeirsschieter, J.; Verheggen, F.J.; Haubruge, E. Depth and type of substrate influence the ability of Nasonia vitripennis to locate a host. J. Insect. Sci. 2014, 14, 58. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mair, M.M.; Ruther, J. Chemical Ecology of the Parasitoid Wasp Genus Nasonia (Hymenoptera, Pteromalidae). Front. Ecol. Evol. 2019, 7, 184. [Google Scholar] [CrossRef]
- Eubanks, M.; Lin, C.; Tarone, A. The role of ants in vertebrate carrion decomposition. Food Webs 2018, 18, e00109. [Google Scholar] [CrossRef]
- Voss, S.C.; Cook, D.F.; Dadour, I.R. Decomposition and insect succession of clothed and unclothed carcasses in Western Australia. Forensic Sci. Int. 2011, 211, 67–75. [Google Scholar] [CrossRef]
- Schotman, T.; Soedhoe, R.; Van der Weerd, J. Toward a systematic classification of textile damages. Forensic Sci. Rev. 2018, 30, 51–75. [Google Scholar]
- Monahan, D.L.; Harding, H.W.J. Damage to Clothing—Cuts and Tears. J. Forensic Sci. 1990, 35, 12903J. [Google Scholar] [CrossRef]
- Stoffolano, J.G. Fly foregut and transmission of microbes. Adv. In. Insect. Phys. 2019, 57, 27–95. [Google Scholar] [CrossRef]
- Mullen, G.R.; Durden, L.A. Medical and Veterinary Entomology, 3rd ed.; Academic Press: Cambridge, MA, USA; Elsevier: London, UK, 2019. [Google Scholar]
- Sukontason, K.; Sukontason, K.L.; Vogtsberger, R.C.; Boonchu, N.; Chaiwong, T.; Piangjai, S. Prestomal teeth of some flies of medical importance. Micron 2003, 34, 449–452. [Google Scholar] [CrossRef]
- Lehnert, M.S.; Tarver, L.A.; Feng, J. Material Properties and Morphology of Prestomal Teeth in Relation to the Feeding Habits of Diptera (Brachycera). Insects 2022, 13, 207. [Google Scholar] [CrossRef]
- Kovacs, F.; Medveczky, I.; Papp, L.; Gondar, E. Role of prestomal teeth in feeding of the house fly, Musca domestica (Diptera; Muscidae). Med. Vet. Entomol. 1990, 4, 331. [Google Scholar]
- Ukponmwan, J.O.; Mukhopadhyay, A.; Chatterjee, K.N. Pilling. Text. Prog. 1998, 28, 1–57. [Google Scholar] [CrossRef]
- Gintis, D.; Mead, E.J. The mechanism of pilling. Text. Res. J. 1959, 29, 578–585. [Google Scholar] [CrossRef]
- Szpila, K.; Wallman, J.F. Morphology and identification of first instar larvae of Australian blowflies of the genus Chrysomya of forensic importance. Acta Trop. 2016, 162, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Sukontason, K.L.; Sukontason, K.; Lertthamnongtham, S.; Kuntalue, B.; Thijuk, N.; Vogtsberger, R.C.; Olson, J.K. Surface ultrastructure of Chrysomya rufifacies (Macquart) larvae (Diptera: Calliphoridae). J. Med. Entomol. 2003, 40, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Rivers, D.B.; Thompson, C.; Brogan, R. Physiological trade-offs of forming maggot masses by necrophagous flies on vertebrate carrion. Bull. Entomol. Res. 2011, 101, 599–611. [Google Scholar] [CrossRef] [PubMed]
- Rivers, D.; Dahlem, G. The Science of Forensic Entomology, 1st ed.; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Walker, G.; Yulf, A.B.; Ratcliffe, J. The adhesive organ of the blowfly, Calliphora vomitoria: A functional approach (Diptera: Calliphoridae). J. Zool. 1985, 205, 297–307. [Google Scholar] [CrossRef]
- Sukontason, K.L.; Bunchu, N.; Methanitikorn, R.; Chaiwong, T.; Kuntalue, B.; Sukontason, K. Ultrastructure of adhesive device in fly in families calliphoridae, muscidae and sarcophagidae, and their implication as mechanical carriers of pathogens. Parasitol. Res. 2006, 98, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.A.; Hoskinson, R.M. Scanning Electron Microscopy of Insect-damaged Wool Fibres. J. Text. Inst. 1970, 61, 355–358. [Google Scholar] [CrossRef]
- Hammers, I.; Arns, W.; Zahn, H. Scanning Electron Microscopic Investigations of the Breakdown of Wool by Insect Larvae. Text. Res. J. 1987, 57, 401–406. [Google Scholar] [CrossRef]
- Hearle, J.W.S.; Lomas, B.; Cooke, W.D. Wool and human hair. In Atlas of Fibre Fracture and Damage to Textiles, 2nd ed.; Hearle, J.W.S., Lomas, B., Cooke, W.D., Eds.; Woodhead Publishing: Sawston, UK, 1998; pp. 138–151. [Google Scholar] [CrossRef]
- Mazzarelli, D.; Vanin, S.; Gibelli, D.; Maistrello, L.; Porta, D.; Rizzi, A.; Cattaneo, C. Splitting hairs: Differentiating between entomological activity, taphonomy, and sharp force trauma on hair. Forensic Sci. Med. Pathol. 2015, 11, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Lowe, A.C.; Beresford, D.V.; Carter, D.O.; Gaspari, F.; O’Brien, R.C.; Stuart, B.H.; Forbes, S.L. The effect of soil texture on the degradation of textiles associated with buried bodies. Forensic Sci. Int. 2013, 231, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Stuart, B.H.; Ueland, M. Degradation of Clothing in Depositional Environments. In Taphonomy of Human Remains: Forensic Analysis of the Dead and the Depositional Environment; John Wiley & Sons, Ltd.: Chichester, UK, 2017; pp. 120–133. [Google Scholar] [CrossRef]
- Szostak-Kotowa, J. Biodeterioration of textiles. Int. Biodeterior. Biodegrad. 2004, 53, 165–170. [Google Scholar] [CrossRef]
- Hearle, J.W.S.; Lomas, B.; Cooke, W.D. Degraded fibres. In Atlas of Fibre Fracture and Damage to Textiles, 2nd ed.; Hearle, J.W.S., Lomas, B., Cooke, W.D., Eds.; Woodhead Publishing: Sawston, UK, 1998; pp. 116–126. [Google Scholar] [CrossRef]
- Pelton, W.R. Distinguishing the Cause of Textile Fiber Damage Using the Scanning Electron Microscope (SEM). J. Forensic Sci. 1995, 40, 874–882. [Google Scholar] [CrossRef]
- Pelton, W.; Ukpabi, P. Using the Scanning Electron Microscope to Identify the Cause of Fibre Damage Part II: An Exploratory Study. Can. Soc. Forensic Sci. J. 1995, 28, 189–200. [Google Scholar] [CrossRef]
- Boland, C.A.; McDermott, S.D.; Ryan, J. Clothing damage analysis in alleged sexual assaults--The need for a systematic approach. Forensic Sci. Int. 2007, 167, 110–115. [Google Scholar] [CrossRef]
- Sloan, K.; Fergusson, M.; Robertson, J. Textile damage examinations on the cutting edge—An Australian perspective. Aust. J. Forensic Sci. 2018, 50, 1–7. [Google Scholar] [CrossRef]
- Michaud, J.-P.; Schoenly, K.G.; Moreau, G. Sampling flies or sampling flaws? Experimental design and inference strength in forensic entomology. J. Med. Entomol. 2012, 49, 1–10. [Google Scholar] [CrossRef]
- Boudreau, D.R.; Moreau, G. Is Forensic Entomology Lost in Space? Insects 2022, 13, 11. [Google Scholar] [CrossRef] [PubMed]
- Shahid, S.A.; Schoenly, K.; Haskell, N.H.; Hall, R.D.; Zhang, W. Carcass Enrichment Does Not Alter Decay Rates or Arthropod Community Structure: A Test of the Arthropod Saturation Hypothesis at the Anthropology Research Facility in Knoxville, Tennessee. J. Med. Entomol. 2003, 40, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Schoenly, K.G.; Shahid, S.A.; Haskell, N.H.; Hall, R.D. Does carcass enrichment alter community structure of predaceous and parasitic arthropods? A second test of the arthropod saturation hypothesis at the Anthropology Research Facility in Knoxville, Tennessee. J. Forensic Sci. 2005, 50, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Pechal, J.L.; Benbow, M.E.; Crippen, T.L.; Tarone, A.M.; Tomberlin, J.K. Delayed insect access alters carrion decomposition and necrophagous insect community assembly. Ecosphere 2014, 5, art45. [Google Scholar] [CrossRef]
- Perez, A.E.; Haskell, N.H.; Wells, J.D. Commonly Used Intercarcass Distances Appear to Be Sufficient to Ensure Independence of Carrion Insect Succession Pattern. Ann. Entomol. Soc. Am. 2015, 109, 72–80. [Google Scholar] [CrossRef]
- Tomberlin, J.; Barton, B.; Lashley, M.; Jordan, H. Mass mortality events and the role of necrophagous invertebrates. Curr. Opin. Insect Sci. 2017, 23, 7–12. [Google Scholar] [CrossRef]




| Fabric Type and Characteristics | Damage Type | Number of Piglets | Number of Swatches |
|---|---|---|---|
| 100% cotton; Natural; Plain weave (1:1); No stretch | Cut | 15 | 4 |
| Tear | 15 | 4 | |
| Nil | 3 | 4 | |
| 65% polyester–35% cotton; Blend (synthetic/natural); Jersey knit; Uni-directional stretch | Cut | 15 | 4 |
| Tear | 15 | 4 | |
| Nil | 3 | 4 | |
| 80% nylon–20% spandex; Synthetic (all-synthetic blend); Jersey knit; Multi-directional stretch | Cut | 15 | 4 |
| Tear | 15 | 4 | |
| Nil | 3 | 4 | |
| Unclothed; (No fabric) | Cut | 3 | n/a |
| Nil | 3 | n/a | |
| Total | - | 105 | 36 |
| Order | Family | Species | Day 6 | Day 11 | Day 17 | Day 25 | Day 46 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UCC | AFT | UCC | AFT | UCC | AFT | UCC | AFT | UCC | AFT | |||
| Diptera | Calliphoridae | Chrysomya rufifacies | L1, L2, L3 | L3 | L3, P | L3, P, PF | n/a | P, Pu | n/a | P, Pu, PP | ||
| Chrysomya varipes | L1, L2, L3 | L3, P | A, L3, P | P, Pu | P, Pu, PP | |||||||
| Calliphora dubia | L2, L3 | L2, L3 | L3 | P | L, PP | P | ||||||
| Lucilia sericata | L2, L3 | L3 | ||||||||||
| Sarcophagidae | spp. | L3 | L3 | L3 | ||||||||
| Muscidae | Musca vetustissima | L3 | L3 | L3 | L3 | L3, PF, P | L3, PF, P | L3, PF, P | ||||
| Australophyra rostrata | L3 | L3 | L3, A | L3 | L3, PF, P | L3, PF, P | L3, PF, P | |||||
| Musca domestica | L3 | L3 | L3, A | L3 | L3, PF, P | L3, PF, P | L3, PF, P | |||||
| Phoridae | spp. | A | P | P | P | |||||||
| Fanniidae | Fannia canicularis | A, L, P | L | A, L, P | A, L3, P | L | ||||||
| Coleoptera | Dermestidae | Dermestes spp. | A | L | A, L, P | A, L | A, L, P | |||||
| Histeridae | Saprinus spp. | A | A | A | A | A | A | A | ||||
| Staphylinidae | Creophilus erythrocephalus | A | A | A | A | |||||||
| Cleridae | Necrobia rufipes | A | A | A | A | |||||||
| Trogidae | Omorgus tatei | A | ||||||||||
| Others | E | E, ants, earwigs | Millipedes, mites, ants | Mites, ants | Mites, ants, dung beetles | Millipedes, mites, ants, earwigs | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziogos, S.; Dadour, I.R.; Pitts, K.; Magni, P.A. Qualitative Analyses of Textile Damage (Cuts and Tears) Applied to Fabrics Exposed to the Decomposition of Carcasses and Associated Insect Activity in an Austral Summer. Insects 2023, 14, 618. https://doi.org/10.3390/insects14070618
Ziogos S, Dadour IR, Pitts K, Magni PA. Qualitative Analyses of Textile Damage (Cuts and Tears) Applied to Fabrics Exposed to the Decomposition of Carcasses and Associated Insect Activity in an Austral Summer. Insects. 2023; 14(7):618. https://doi.org/10.3390/insects14070618
Chicago/Turabian StyleZiogos, Sotirios, Ian R. Dadour, Kari Pitts, and Paola A. Magni. 2023. "Qualitative Analyses of Textile Damage (Cuts and Tears) Applied to Fabrics Exposed to the Decomposition of Carcasses and Associated Insect Activity in an Austral Summer" Insects 14, no. 7: 618. https://doi.org/10.3390/insects14070618
APA StyleZiogos, S., Dadour, I. R., Pitts, K., & Magni, P. A. (2023). Qualitative Analyses of Textile Damage (Cuts and Tears) Applied to Fabrics Exposed to the Decomposition of Carcasses and Associated Insect Activity in an Austral Summer. Insects, 14(7), 618. https://doi.org/10.3390/insects14070618








