Wide Range of Brachyceran Fly Taxa Attracted to Synthetic and Semi-Synthetic Generic Noctuid Lures and the Description of New Attractants for Sciomyzidae and Heleomyzidae Families
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Baits and Sampling
2.3. Statistical Analyses
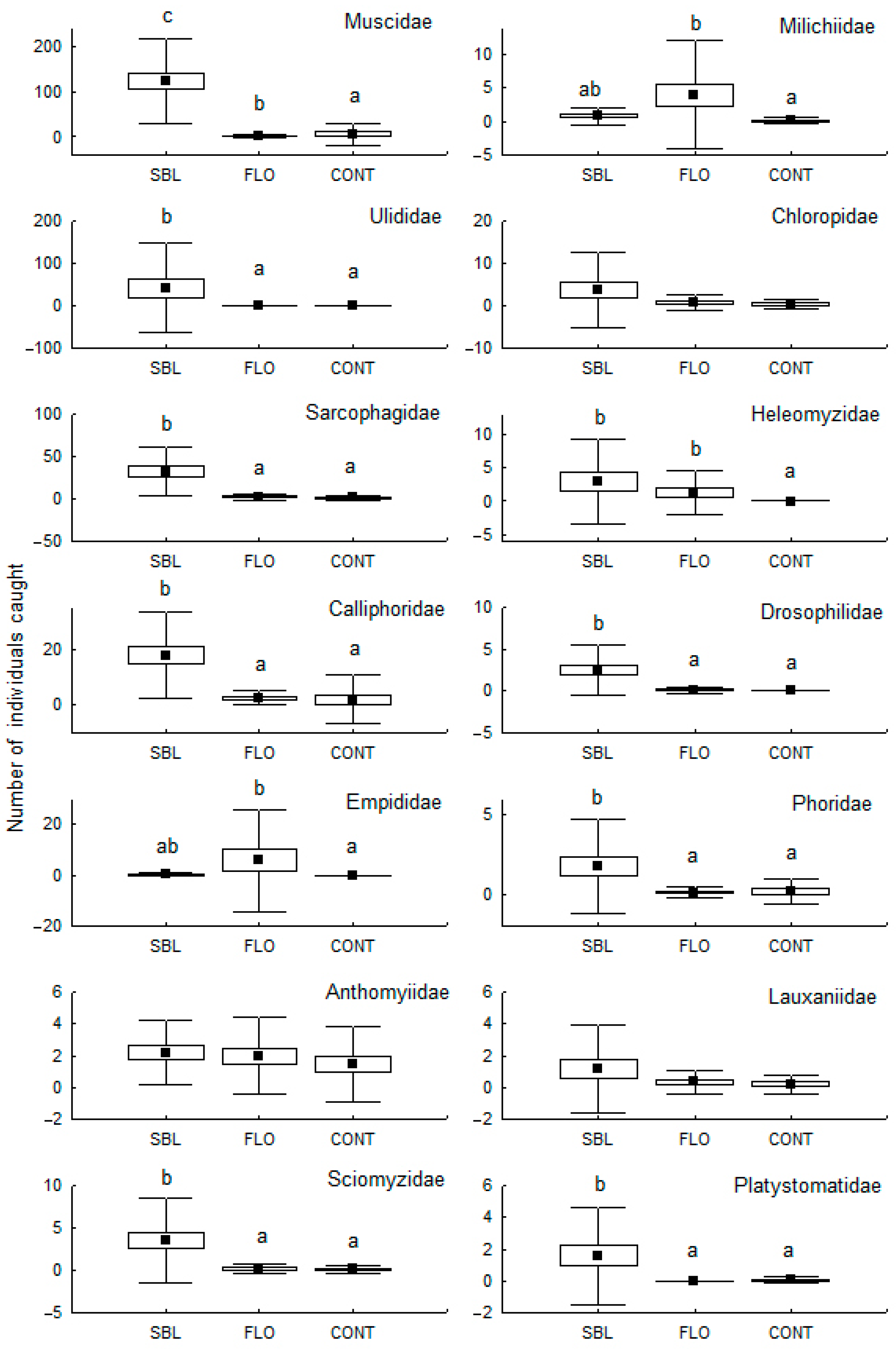
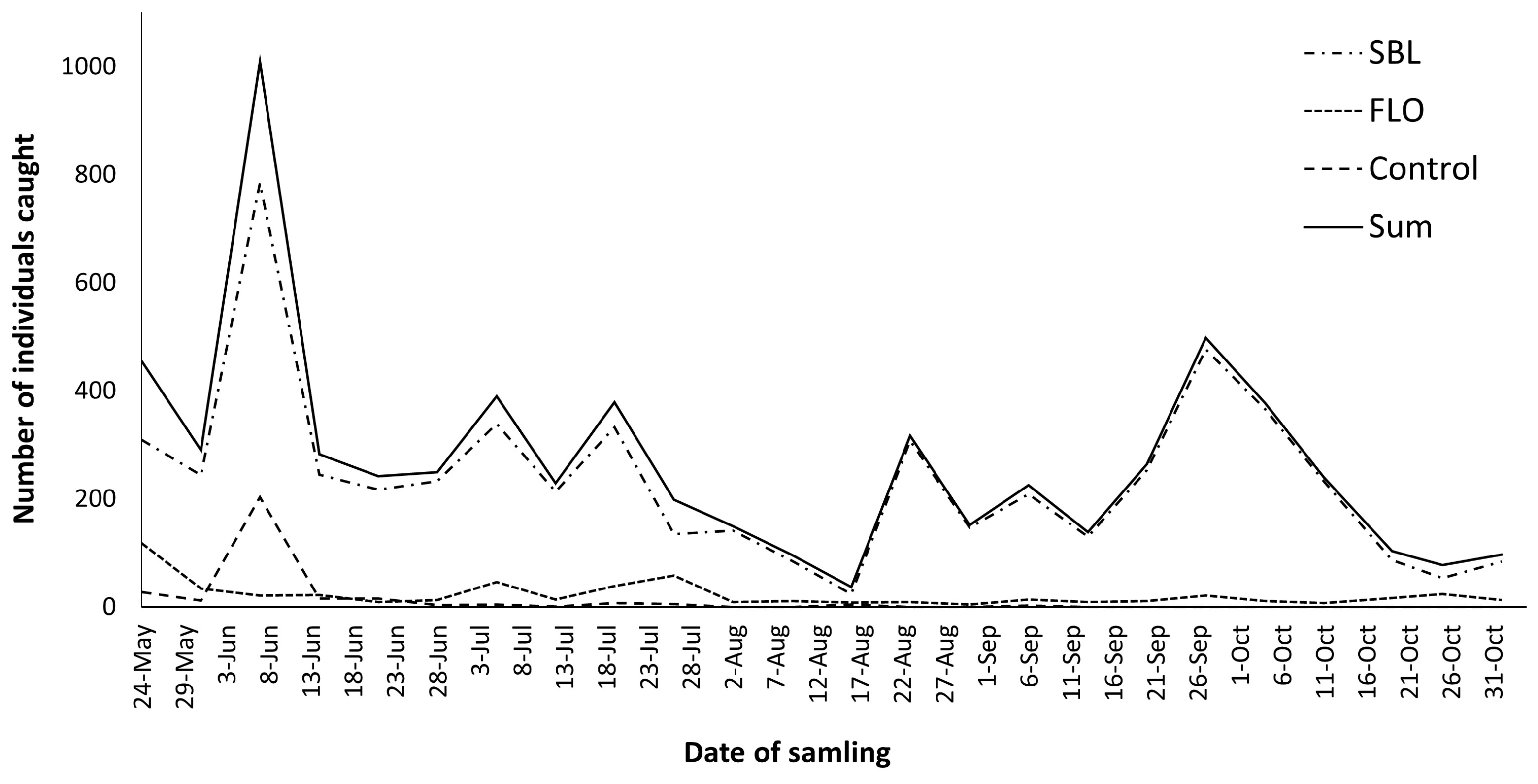
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Papp, L.; Darvas, B. Contributions to a Manual of Palaearctic Diptera, Vol. 1. General and Applied Dipterology; Science Herald: Budapest, Hungary, 2000; pp. 1–978. [Google Scholar]
- Marshall, S.A. Flies: The Natural History and Diversity of Diptera; Firefly Books Ltd.: Buffalo, NY, USA, 2012; pp. 1–616. [Google Scholar]
- Shelly, T.E. Trapping Male Oriental Fruit Flies (Diptera: Tephritidae): Does Feeding on a Natural Source of Methyl Eugenol Reduce Capture Probability? Fla. Entomol. 2000, 83, 109–111. [Google Scholar] [CrossRef]
- El-Sayed, A.M.; Sucking, D.M.; Wearing, C.H.; Byers, J.A. Potential of Mass Trapping for Long-Term Pest Management and Eradication of Invasive Species. J. Econ. Entomol. 2006, 99, 1550–1564. [Google Scholar] [CrossRef] [PubMed]
- Manko, P.; Demková, L.; Kohútová, M.; Oboňa, J. Efficiency of traps in collecting selected Diptera families according to the used bait: Comparison of baits and mixtures in a field experiment. Eur. J. Ecol. 2018, 4, 92–99. [Google Scholar] [CrossRef]
- Colacci, M.; Trematerra, P.; Sciarretta, A. Evaluation of Trap Devices for Mass Trapping of Ceratitis capitata (Diptera: Tephritidae) Populations. Insects 2022, 13, 941. [Google Scholar] [CrossRef] [PubMed]
- Facanha, B.L.B.; Esposito, M.C.; Juen, L. Trap and bait efficiency for catching Calliphoridae and Mesembrinellidae (Insecta, Diptera) at different heights. An. Acad. Bras. Ciências 2022, 94, e20210763. [Google Scholar] [CrossRef]
- Antonatos, S.; Anastasaki, E.; Balayiannis, G.; Michaelakis, A.; Magiatis, P.; Milonas, P.; Papadopoulos, N.T.; Papachristos, D.P. Identification of volatile compounds from fruits aroma and citrus essential oils and their effect on oviposition of Ceratitis capitata (Diptera: Tephritidae). Environ. Entomol. 2023, 52, 327–340. [Google Scholar] [CrossRef]
- Walsh, D.B.; Bolda, M.P.; Goodhue, R.E.; Dreves, A.J.; Lee, J.; Bruck, D.J.; Walton, V.M.; O’Neal, S.D.; Zalom, F.G. Drosophila suzukii (Diptera: Drosophilidae): Invasive Pest of Ripening Soft Fruit Expanding its Geographic Range and Damage Potential. J. Integr. Pest Manag. 2011, 2, G1–G7. [Google Scholar] [CrossRef]
- Tait, G.; Mermer, S.; Stockton, D.; Lee, J.; Avosani, S.; Abrieux, A.; Anfora, G.; Beers, E.; Biondi, A.; Burrack, H.; et al. Drosophila suzukii (Diptera: Drosophilidae): A Decade of Research Towards a Sustainable Integrated Pest Management Program. J. Econ. Entomol. 2021, 114, 1950–1974. [Google Scholar] [CrossRef]
- The Pherobase: Database of Pheromones and Semiochemicals. Available online: http://www.pherobase.com (accessed on 6 April 2023).
- Dreistadt, S.H.; Newman, J.P.; Robb, K.L. Sticky Trap Monitoring of Insect Pest, Publication 21572; University of California, Division of Agriculture and Natural Resources: Berkeley, CA, USA, 1998; pp. 1–8. [Google Scholar]
- Finch, F.; Skinner, G. Trapping cabbage root flies in traps baited with plant extracts and with natural and synthetic isothiocyanates. Entomol. Exp. Appl. 1982, 31, 133–139. [Google Scholar] [CrossRef]
- Tóth, M.; Voigt, E.; Baric, B.; Pajac, I.; Subic, M.; Baufeld, P.; Lerche, S. Importance of application of synthetic food lures in trapping of Rhagoletis spp. and Strauzia longipennis Wiedemann. Acta Phytopath. Entomol. Hung. 2014, 49, 25–35. [Google Scholar] [CrossRef]
- Sundar, S.T.; Latha, B.R.; Vijayashanthi, R.; Pandian, S.S. (Z)-9-Tricosene based Musca domestica lure study on a garbage dump yard using plywood sticky trap baited with fish meal. J. Parasit. Dis. 2016, 40, 32–35. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ishikawa, Y.; Ikeshoji, T.; Matsumoto, Y.; Tsutsumi, M.; Mitsui, Y. 2-Phenylethanol: An attractant for the onion and seed-corn flies, Hylemya antiqua and H. platura (Diptera: Anthomyiidae). Appl. Entomol. Zool. 1983, 18, 270–277. [Google Scholar]
- Görnitz, K. Cantharidin als Gift und Anlockungsmittel für Insekten. Arb. Phys. Angew. Ent. Berlin Dahlem 1937, 4, 116–157. (In German) [Google Scholar]
- Sellenschlo, U. Beifänge in borkenkäfer-pheromonfallen in Norddeutschland. Anz. Für Schadl. Pflanzenschutz Umweltschutz 1986, 59, 152–156. (In German) [Google Scholar]
- Hemp, C.; Hemp, A.; Dettner, K. Canthariphilous insects in East Africa. J. East Afr. Nat. Hist. 1999, 88, 1–15. [Google Scholar]
- Hemp, C.; Dettner, K. Compilation of canthariphilus insects. Contrib. Entomol. 2001, 51, 231–245. [Google Scholar] [CrossRef]
- Nagy, A.; Szarukán, I.; Gém, F.; Nyitrai, R.; Füsti-Molnár, B.; Németh, A.; Kozák, L.; Molnár, A.; Katona, K.; Szanyi, S.; et al. Preliminary data on the effect of semi-synthetic baits for Noctuidea (Lepidoptera) on the non-target Lepidoptera species. Acta Agrar. Debr. 2015, 66, 71–80. [Google Scholar] [CrossRef]
- Tóth, M.; Szarukán, I.; Nagy, A.; Ábri, T.; Katona, V.; Körösi, S.; Nagy, T.; Szarvas, Á.; Koczor, S. An improved female-targeted semiochemical lure for the European corn borer Ostrinia nubilalis Hbn. Acta Phytopathol. Entomol. Hung. 2016, 51, 247–254. [Google Scholar] [CrossRef]
- Tóth, M.; Szarukán, I.; Nagy, A.; Furlan, L.; Benvegnù, I.; Rak, C.; Ábri, T.; Kéki, T.; Körösi, S.; Pogonyi, A.; et al. European corn borer (Ostrinia nubilalis Hbn., Lepidoptera: Crambidae): Comparing the performance of a new bisexual lure with that of synthetic sex pheromone in five countries. Pest Manag. Sci. 2017, 73, 2504–2508. [Google Scholar] [CrossRef]
- Tóth, M.; Landolt, P.; Szarukán, I.; Nagy, A.; Jósvai, J. Improving bisexual lures for the Silver Y Moth Autographa gamma L. and related Plusiinae (Lepidoptera: Noctuidae). Acta Phytopathol. Entomol. Hung. 2019, 54, 137–146. [Google Scholar] [CrossRef][Green Version]
- Tóth, M.; Nagy, A.; Szarukán, I.; Ary, K.; Cserenyecz, A.; Fenyődi, B.; Gombás, D.; Lajkó, T.; Merva, L.; Szabó, J.; et al. One Decade’s Research Efforts in Hungary to Develop a Bisexual Lure for the Cotton Bollworm Helicoverpa armigera Hübner. Acta Phytopathol. Entomol. Hung. 2020, 55, 79–88. [Google Scholar] [CrossRef]
- Szanyi, S.; Nagy, A.; Molnár, A.; Katona, K.; Tóth, M.; Varga, Z. Night-active Macroheterocera species in traps with synthetic attractants in the Velyka Dobron’ Game Reserve (Ukraine, Transcarpathia). Acta Zool. Acad. Sci. Hung. 2017, 63, 97–114. [Google Scholar] [CrossRef]
- Szanyi, S.; Szarukán, I.; Nagy, A.; Jósvai, J.; Imrei, Z.; Varga, Z.; Tóth, M. Comparing Performance of Synthetic Sex Attractants and a Semisynthetic Bisexual Lure in Orthosia and Conistra Species (Lepidoptera: Noctuidae). Acta Phytopathol. Entomol. Hung. 2020, 55, 115–122. [Google Scholar] [CrossRef]
- Szanyi, S.; Varga, Z.; Nagy, A.; Jósvai, J.K.; Imrei, Z.; Tóth, M. Bisexual lures and their comparison with synthetic sex attractants for trapping Orthosia species (Lepidoptera: Noctuidae). J. Appl. Entomol. 2022, 146, 1109–1115. [Google Scholar] [CrossRef]
- Nagy-Szalárdi, T.; Szanyi, S.; Szarukán, I.; Tóth, M.; Nagy, A. Semiochemical baited traps of lepidopteran pests of economic importance can deliver reliable data also on wide range of non-target species: Case study in the Hajdúság Region of East Pannonian Lowland (East Hungary). Biodivers. Data J. 2021, 9, e72305. [Google Scholar] [CrossRef]
- Szanyi, S.; Nagy, A.; Molnár, A.; Tóth, M.; Varga, Z. Pest species of Macrolepidoptera in the Game Reserve of Velyka Dobron’ (Transcarpathia, Ukraine). Acta Agrar. Debr. 2015, 66, 58–64. [Google Scholar] [CrossRef]
- Tóth, M.; Imrei, Z.; Szőcs, G. Non-sticky, non-saturable, high capacity new pheromone traps for Diabrotica virgifera virgifera (Coleoptera: Chrysomelidae) and Helicoverpa (Heliothis) armigera (Lepidoptera: Noctuidae). Integr. Term. Kert. Szántóf. Kult. 2000, 21, 44–49. (In Hungarian) [Google Scholar]
- Tóth, M.; Répási, V.; Szőcs, G. Chemical attractants for females of pest pyralids and phycitids (Lepidoptera: Pyralidae, Phycitidae). Acta Phytopathol. Entomol. Hung. 2002, 37, 375–384. [Google Scholar] [CrossRef]
- Tóth, M.; Szarukán, I.; Nagy, A.; Gém, F.; Nyitrai, R.; Kecskés, Z.; Krakkó, L.; Jósvai, J.; Bélai, I. Félszintetikus "biszex" csalétkek kártevő rovarok nőstényeinek és hímjeinek fogására. Növényvédelem 2015, 51, 197–205. (In Hungarian) [Google Scholar]
- Oosterbroek, P. The European Families of the Diptera. Identification, Diagnosis, Biology; KNNV Publishing: Utrecht, The Netherlands, 2006; pp. 1–208. [Google Scholar]
- Pape, T.; Beuk, P.; Pont, A.; Shatalkin, A.; Ozerov, A.; Woźnica, A.; Merz, B.; Bystrowski, C.; Raper, C.; Bergström, C.; et al. Fauna Europaea: Diptera—Brachycera. Biodivers. Data J. 2015, 3, e4187. [Google Scholar] [CrossRef]
- Ketskeméty, L.; Izsó, L.; Könyves Tóth, E. Bevezetés az IBM SPSS Statistics Programrendszerbe; Artéria Stúdió Kft: Budapest, Hungary, 2011. (In Hungarian) [Google Scholar]
- Podani, J. SYNTAX 5.1.: A new version of PC and Macinthos computers. Coenoses 1997, 12, 149–152. [Google Scholar]
- Szanyi, S.; Molnár, A.; Kozák, L.; Nagy-Szalárdi, T.; Varga, Z.; Tóth, M.; Nagy, A. Nyírségi Macroheterocera együttesek vizsgálata illatanyagcsapdák alkalmazásával. Erdtud. Közl. 2019, 9, 51–68. [Google Scholar] [CrossRef]
- Kido, M.H.; Asquith, A.; Vargas, R.I. Nontarget insect attraction to methyl eugenol traps used in male annihilation of the oriental fruit fly (Diptera: Tephritidae) in Riparian Hawaiian stream habitat. Environ. Entomol. 1996, 25, 1279–1289. [Google Scholar] [CrossRef]
- Uchida, G.K.; McInnis, D.O.; Vargas, R.I.; Kumashiro, B.R.; Jang, E. Nontarget arthropods captured in cue-lure baited bucket traps at area-wide pest management implementation sites in Kamuela and Kula, Hawaiian islands. Proc. Hawaii. Entomol. Soc. 2003, 36, 135–143. [Google Scholar]
- Nuessly, G.S.; Capinera, J.L. Cornsilk Fly. In Featured Creatures. University of Florida, Entomology and Nematology Department; 2001; EENY-224. Available online: https://entnemdept.ufl.edu/creatures/field/cornsilk_fly.htm (accessed on 15 August 2001).
- Bjerke, J.M.; Anderson, A.W.; Freeman, T.P. Morphology of the larval stages of Tetanops myopaeformis (Röder) (Diptera: Otitidae). J. Kans. Entomol. 1992, 65, 59–65. [Google Scholar]
- Nagy, A.; Ősz, A.; Tóth, M.; Rácz, I.A.; Kovács, S.; Szanyi, S. Nontarget catches of traps with chemical lures may refer to the flower-visitation, probable pollination, and feeding of bush crickets (Ensifera: Tettigoniidae). Ecol. Evol. 2023, 13, e10249. [Google Scholar] [CrossRef]
- Heiduk, A.; Brake, I.; von Tschirnhaus, M.; Haenni, J.P.; Miller, R.; Hash, J.; Prieto-Benítez, S.; Jürgens, A.; Johnson, S.D.; Schulz, S.; et al. Floral scent and pollinators of Ceropegia trap flower. Flora 2017, 232, 169–182. [Google Scholar] [CrossRef]
- Davis, T.S.; Landolt, P.J. A survey of insect assemblages responding to volatiles from a ubiquitous fungus in an agricultural landscape. J. Chem. Ecol. 2013, 39, 860–868. [Google Scholar] [CrossRef]
- Epsky, N.D.; Heath, R.R.; Dueben, B.D.; Lauzon, C.R.; Proveaux, A.T.; MacCollom, G.B. Attraction of 3-methyl-1-butanol and ammonia identified from Enterobacter agglomerans to Anastrepha suspensa. J. Chem. Ecol. 1998, 24, 1867–1880. [Google Scholar] [CrossRef]
- Cha, D.H.; Olsson, S.B.; Yee, W.L.; Goughnour, R.B.; Hood, G.R.; Mattsson, M.; Schwarz, D.; Feder, J.L.; Linn, C.E., Jr. Identification of host fruit volatiles from snowberry (Symphoricarpos albus), attractive to Rhagoletis zephyria flies from the Western United States. J. Chem. Ecol. 2017, 43, 188–197. [Google Scholar] [CrossRef]
- Warthen, J.D., Jr.; Cunningham, R.T.; DeMilo, A.B.; Spencer, S. Trans-ceralure isomers: Differences in attraction for Mediterranean fruit fly, Ceratitis capitata (Wied.) (Diptera: Tephritidae). J. Chem. Ecol. 1994, 20, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Nojima, S.; Linn, C., Jr.; Roelofs, W.J. Identification of host fruit volatiles from flowering dogwood (Cornus florida) attractive to dogwood-origin Rhagoletis pomonella flies. J. Chem. Ecol. 2003, 29, 2347–2357. [Google Scholar] [CrossRef]
- Nojima, S.; Linn, C., Jr.; Morris, B.; Zhang, A.; Roelofs, W.J. Identification of host fruit volatiles from hawthorn (Crataegus spp.) attractive to hawthorn-origin Rhagoletis pomonella flies. J. Chem. Ecol. 2003, 29, 321–336. [Google Scholar] [CrossRef]
- Perkins, M.V.; Kitching, W.; Drew, R.A.I.; Moore, C.J.; König, W.A. Chemistry of fruit flies: Composition of the male rectal gland secretions of some species of South-East Asian Dacinae. Re-examination of Dacus cucurbitae (melon fly). J. Chem. Soc. Perkin Trans. 1990, 1, 1111–1117. [Google Scholar] [CrossRef]
- Hwang, Y.S.; Mulla, M.S.; Axelrod, H. Attractants for synanthropic flies. Identification of attractants and coattractants for Hippelates eye gnats (Diptera: Chloropidae). J. Agric. Food Chem. 1976, 24, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.W.; Park, K.C.; Baker, T.C.J. Identification of odors from overripe mango that attract vinegar flies, Drosophila melanogaster. J. Chem. Ecol. 2003, 29, 899–909. [Google Scholar] [CrossRef] [PubMed]
- Becher, P.G.; Bengtsson, M.; Hansson, B.S.; Witzgall, P. Flying the fly: Long-range flight behaviour of Drosophila melanogaster to attractive odors. J. Chem. Ecol. 2010, 36, 599–607. [Google Scholar] [CrossRef]
- Landolt, P.J.; Adams, T.; Rogg, H. Trapping spotted wing drosophila, Drosophila suzukii (Matsumura) (Diptera: Drosophilidae), with combinations of vinegar and wine, and acetic acid and ethanol. J. Appl. Entomol. 2012, 136, 148–154. [Google Scholar] [CrossRef]
- Cha, D.H.; Adams, T.; Werle, C.T.; Sampson, B.J.; Adamczyk, J.J., Jr.; Rogg, H.; Landolt, P.J. A four-component synthetic attractant for Drosophila suzukii (Diptera: Drosophilidae) isolated from fermented bait headspace. Pest Manag. Sci. 2014, 70, 324–331. [Google Scholar] [CrossRef]
- Casana-Giner, V.; Gandia-Balaguer, A.; Primo-Yufera, E. Field trial of an attractant mixture for dipterous, including the pest Ceratitis capitata (Wiedemann) (Diptera: Tephritidae), in Valencia, Spain. J. Appl. Entomol. 1999, 123, 47–48. [Google Scholar] [CrossRef]
- Robacker, D.C.; Massa, M.J.; Sacchetti, P.; Bartelt, R.J. A novel attractant for Anastrepha ludens (Diptera: Tephritidae) from a concord grape product. J. Econ. Entomol. 2011, 104, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
- Landolt, P.J. Chemical attractants for trapping yellowjackets Vespula germanica and Vespula pensylvanica (Hymenoptera: Vespidae). Environ. Entomol. 1998, 27, 1229–1234. [Google Scholar] [CrossRef]
- Landolt, P.J.; Reed, H.C.; Aldrich, J.R.; Antonelli, A.L.; Dickey, C. Social wasps (Hymenoptera: Vespidae) trapped with acetic acid and isobutanol. Fla. Entomol. 1999, 82, 609–614. [Google Scholar] [CrossRef]
- Landolt, P.J.; Smithhisler, C.S.; Reed, H.C.; McDonough, L.M. Trapping social wasps (Hymenoptera: Vespidae) with acetic acid and saturated short chain alcohols. J. Econ. Entomol. 2000, 93, 1613–1618. [Google Scholar] [CrossRef] [PubMed]
- Landolt, P.J.; Tóth, M.; Jósvai, J. First European report of social wasps trapped in response to acetic acid, isobutanol, 2-methyl-2-propanol and heptyl butyrate in tests conducted in Hungary. Bull. Insectol. 2007, 60, 7–11. [Google Scholar]
- Day, S.E.; Jeanne, R.L. Food volatiles as attractants for yellowjackets (Hymenoptera: Vespidae). Environ. Entomol. 2001, 30, 157–165. [Google Scholar] [CrossRef]
- Reed, H.C.; Landolt, P.J. Trap response of Michigan social wasps (Hymenoptera: Vespidae) to the feeding attractants acetic acid, isobutanol, and heptyl butyrate. Great Lakes Entomol. 2002, 35, 71–77. [Google Scholar]
- Landolt, P.J.; Zhang, Q.H. Discovery and development of chemical attractants used to trap pestiferous social wasps (Hymenoptera: Vespidae). J. Chem. Ecol. 2016, 42, 655–665. [Google Scholar] [CrossRef]
- Landolt, P.J. New chemical attractants for trapping Lacanobia subjuncta, Mamestra configurata, and Xestia c-nigrum (Lepidoptera: Noctuidae). J. Econ. Entomol. 2000, 93, 101–106. [Google Scholar] [CrossRef]
- Landolt, P.J.; Alfaro, J.F. Trapping Lacanobia subjuncta, Xestia c-nigrum and Mamestra configurata (Lepidoptera: Noctuidae) with acetic acid and 3-methyl-1-butanol in controlled relesase dispensers. Environ. Entomol. 2001, 30, 656–662. [Google Scholar] [CrossRef]
- Landolt, P.J.; Highbee, B.S. Both sexes of the true armyworm (Lepidoptera: Noctuide) trapped with the feeding attractant composed of acetic acid and 3-methyl-1-butanol. Fla. Entomol. 2002, 85, 182–185. [Google Scholar] [CrossRef]
- Landolt, P.J.; Pantoja, A.; Hagerty, A.; Crabo, L.; Green, D. Moths trapped in Alaska with feeding attractant lures and the seasonal flight patterns of potential agricultural pests. Can. Entomol. 2007, 139, 278–291. [Google Scholar] [CrossRef]
- Tóth, M.; Szarukán, I.; Dorogi, B.; Gulyás, A.; Nagy, P.; Rozgonyi, Z. Male and female noctuid moths attracted to synthetic lures in Europe. J. Chem. Ecol. 2010, 36, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Shamshev, I.V.; Selitskaya, O.G. Methyl salicylate as an attractant for the dance fly Rhamphomyia gibba (Fallén) (Diptera, Empididae). Entomol. Rev. 2016, 96, 1003–1007. [Google Scholar] [CrossRef]
- Heiduk, A.; Brake, I.; von Tschirnhaus, M.; Göhl, M.; Jürgens, A.; Johnson, S.D.; Meve, U.; Dötterl, S. Ceropegia sandersonii Mimics Attacked Honeybees to Attract Kleptoparasitic Flies for Pollination. Curr. Biol. 2016, 26, 2787–2793. [Google Scholar] [CrossRef]
- Creighton, C.S.; Mcfadden, T.L.; Cuthbert, E.R. Supplementary data on phenylacetaldehyde: An attractant for Lepidoptera. J. Econ. Entomol. 1973, 66, 114–115. [Google Scholar] [CrossRef]
- Cantelo, W.W.; Jacobson, M. Phenylacetaldehyde attracts moths to bladder flower and blacklight traps. Environ. Entomol. 1979, 8, 444–447. [Google Scholar] [CrossRef]
- Meagher, R.L. Collection of soybean looper and other noctuids in phenylacetaldehyde-baited field traps. Fla. Entomol. 2001, 84, 154–155. [Google Scholar] [CrossRef]
- Eby, C.; Gardiner, M.G.; Gries, R.; Judd, G.J.; Khaskin, G.; Gries, G. Phenylacetaldehyde attracts male and female apple clearwing moths, Synanthedon myopaeformis, to inflorescences of showy milkweed, Asclepias speciosa. Entomol. Exp. Appl. 2013, 147, 82–92. [Google Scholar] [CrossRef]
- Landolt, P.; Cha, D.; Davis, T.S. Attraction of the orange mint moth and false celery leaftier moth (Lepidoptera: Crambidae) to floral chemical lures. J. Econ. Entomol. 2014, 107, 654–660. [Google Scholar] [CrossRef]
- Molnár, B.; Kárpáti, Z.; Nagy, A.; Szarukán, I.; Csabai, J.; Koczor, S.; Tóth, M. Development of a Female-Targeted Lure for the Box Tree Moth Cydalima perspectalis (Lepidoptera: Crambidae): A Preliminary Report. J. Chem. Ecol. 2019, 45, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Tóth, M.; Bozsik, A.; Szentkirályi, F.; Letardi, A.; Tabilio, M.R.; Verdinelli, M.; Zandigiacomo, P.; Jekisa, J.; Szarukán, I. Phenylacetaldehyde: A chemical attractant for common green lacewings (Chrysoperla carnea s.l., Neuroptera: Chrysopidae). Eur. J. Entomol. 2006, 103, 267–271. [Google Scholar] [CrossRef]
- Arthurs, S.P.; Tofangsazi, N.; Meagher, R.L.; Cherry, R.H. Attraction of Plecia nearctica (Diptera: Bibionidae) to floral lures containing phenylacetaldehyde. Fla. Entomol. 2012, 95, 199–201. [Google Scholar] [CrossRef]
- Otienoburu, P.E.; Ebrahimi, B.; Phelan, P.L.; Foster, W.A. Analysis and optimization of a synthetic milkweed floral attractant for mosquitoes. J. Chem. Ecol. 2012, 38, 873–881. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Heiduk, A.; Haenni, J.P.; Meve, U.; Schulz, S.; Dötterl, S. Flower scent of Ceropegia stenantha: Electrophysiological activity and synthesis of novel components. J. Comp. Physiol. A 2019, 205, 301–310. [Google Scholar] [CrossRef]
- Vartak, P.H.; Tungikar, V.B.; Sharma, R.N. Comparative repellent properties of certain chemicals against mosquitoes, house flies and cockroaches using modified techniques. J. Commun. Dis. 1994, 26, 156–160. [Google Scholar]
- Alagarmalai, J.; Nestel, D.; Dragushich, D.; Nemny-Lavy, E.; Anshelevich, L.; Zada, A.; Soroker, V. Identification of host attractants for the Ethiopian fruit fly, Dacus ciliatus Loew. J. Chem. Ecol. 2009, 35, 542–551. [Google Scholar] [CrossRef]
| Family | Total | CONTROL | SBL | FLO |
|---|---|---|---|---|
| Total number of individuals | 6501 | 306 | 5656 | 543 |
| Muscidae | 3137 | 147 | 2946 | 44 |
| Ulidiidae | 1001 | 9 | 990 | 2 |
| Sarcophagidae | 873 | 35 | 780 | 58 |
| Calliphoridae | 536 | 48 | 425 | 63 |
| Empididae | 145 | 0 | 4 | 141 |
| Anthomyiidae | 136 | 35 | 53 | 48 |
| Sciomyzidae | 118 | 3 | 85 | 5 |
| Milichiidae | 115 | 3 | 19 | 96 |
| Chloropidae | 100 | 10 | 89 | 16 |
| Heleomyzidae | 93 | 0 | 70 | 30 |
| Drosophilidae | 63 | 0 | 60 | 3 |
| Phoridae | 49 | 4 | 42 | 3 |
| Lauxaniidae | 41 | 5 | 28 | 8 |
| Platystomatidae | 39 | 1 | 38 | 0 |
| Syrphidae | 18 | 0 | 4 | 14 |
| Tachinidae | 13 | 0 | 4 | 9 |
| Microphoridae | 7 | 2 | 4 | 1 |
| Odinidae | 4 | 0 | 4 | 0 |
| Piophilidae | 3 | 3 | 0 | 0 |
| Dolichopodidae | 2 | 1 | 0 | 1 |
| Periscelididae | 2 | 0 | 2 | 0 |
| Pallopteridae | 2 | 0 | 2 | 0 |
| Statiomyidae | 1 | 0 | 0 | 1 |
| Tabanidae | 1 | 0 | 1 | 0 |
| Hybotidae | 1 | 0 | 1 | 0 |
| Scathophagidae | 1 | 0 | 1 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagy, A.; Katona, P.; Molnár, A.; Rádai, Z.; Tóth, M.; Szanyi, K.; Szanyi, S. Wide Range of Brachyceran Fly Taxa Attracted to Synthetic and Semi-Synthetic Generic Noctuid Lures and the Description of New Attractants for Sciomyzidae and Heleomyzidae Families. Insects 2023, 14, 705. https://doi.org/10.3390/insects14080705
Nagy A, Katona P, Molnár A, Rádai Z, Tóth M, Szanyi K, Szanyi S. Wide Range of Brachyceran Fly Taxa Attracted to Synthetic and Semi-Synthetic Generic Noctuid Lures and the Description of New Attractants for Sciomyzidae and Heleomyzidae Families. Insects. 2023; 14(8):705. https://doi.org/10.3390/insects14080705
Chicago/Turabian StyleNagy, Antal, Patrik Katona, Attila Molnár, Zoltán Rádai, Miklós Tóth, Kálmán Szanyi, and Szabolcs Szanyi. 2023. "Wide Range of Brachyceran Fly Taxa Attracted to Synthetic and Semi-Synthetic Generic Noctuid Lures and the Description of New Attractants for Sciomyzidae and Heleomyzidae Families" Insects 14, no. 8: 705. https://doi.org/10.3390/insects14080705
APA StyleNagy, A., Katona, P., Molnár, A., Rádai, Z., Tóth, M., Szanyi, K., & Szanyi, S. (2023). Wide Range of Brachyceran Fly Taxa Attracted to Synthetic and Semi-Synthetic Generic Noctuid Lures and the Description of New Attractants for Sciomyzidae and Heleomyzidae Families. Insects, 14(8), 705. https://doi.org/10.3390/insects14080705









