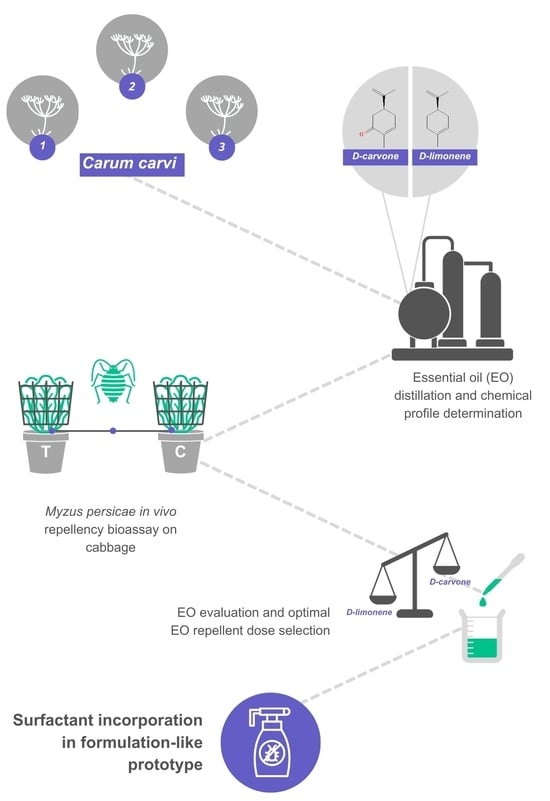
In Vivo Bioassay of the Repellent Activity of Caraway Essential Oil against Green Peach Aphid
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
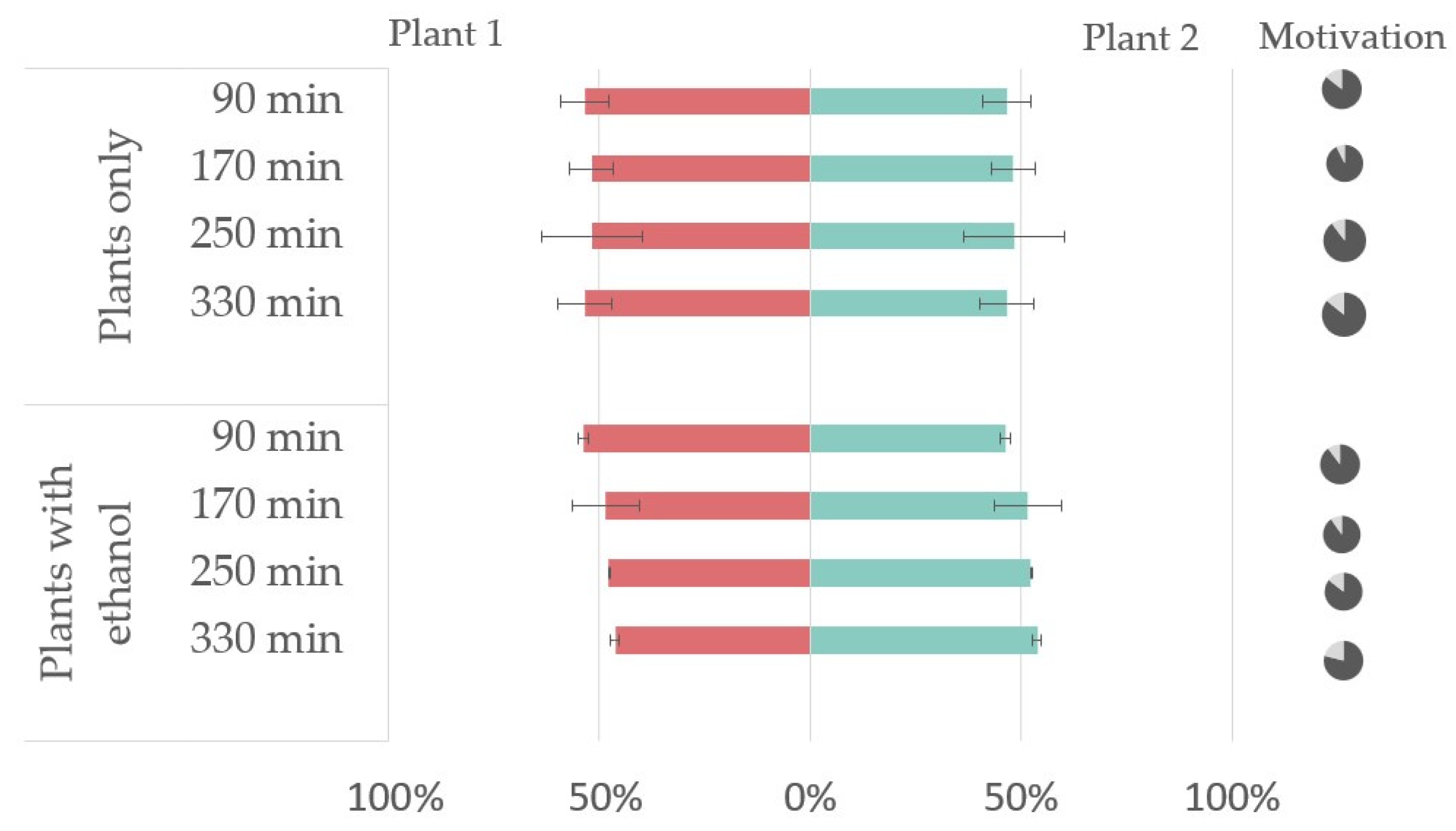
2.1. Plant Material
2.2. Chemicals
2.3. Essential Oil Distillation
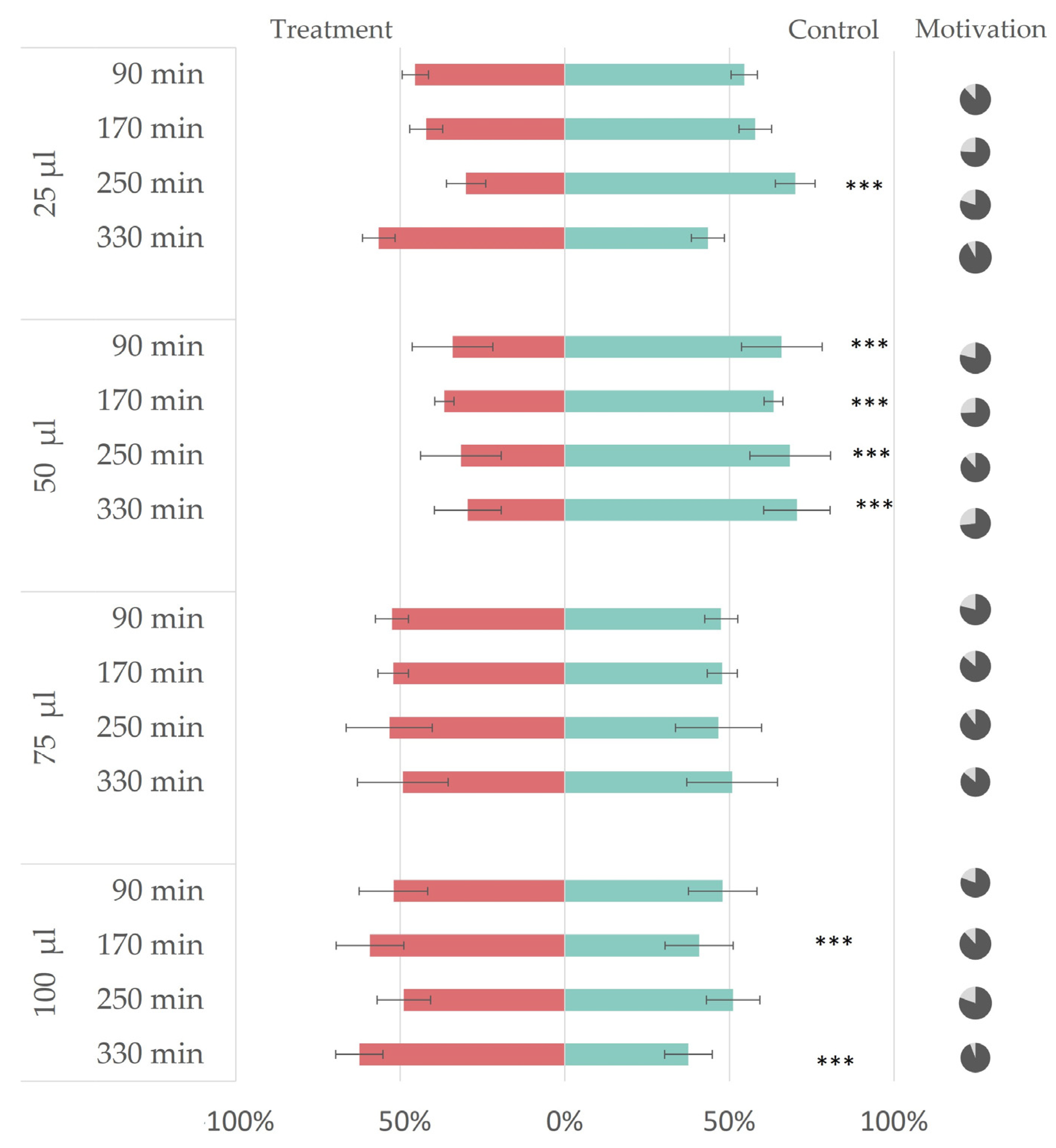
2.4. Essential Oil and Surfactant Mixture
2.5. GC/MS Analysis of Essential Oils
2.6. Insect Rearing
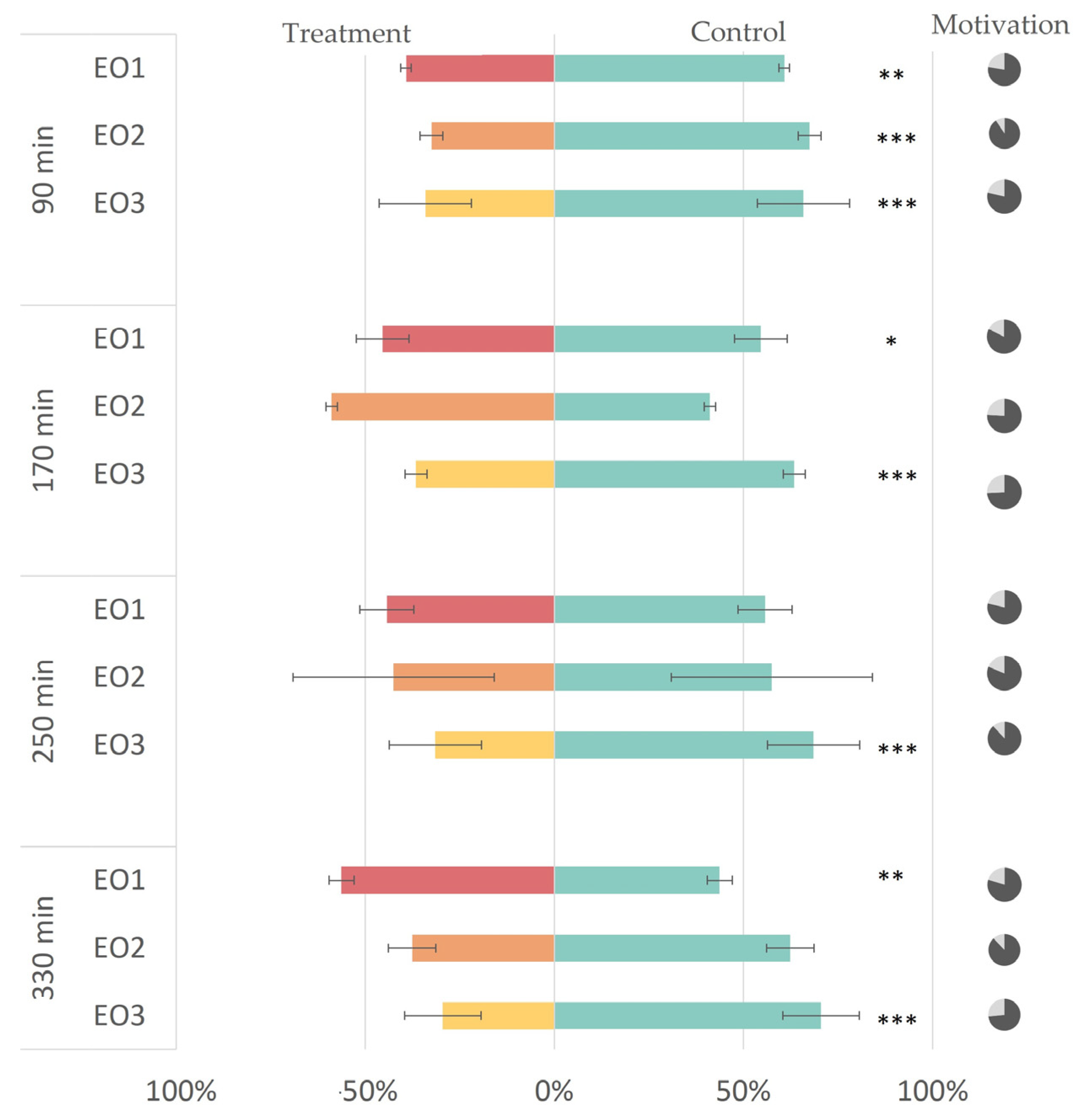
2.7. In Vivo Repellency Bioassay
2.8. Statistical Analysis
3. Results
3.1. Composition of Caraway Seed Essential Oils
3.2. In Vivo Repellency Bioassay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Myzus persicae (Green Peach Aphid)|CABI Compendium. Available online: https://www.cabidigitallibrary.org/doi/10.1079/cabicompendium.35642 (accessed on 4 October 2023).
- Ali, J.; Bayram, A.; Mukarram, M.; Zhou, F.; Karim, M.F.; Hafez, M.M.A.; Mahamood, M.; Yusuf, A.A.; King, P.J.H.; Adil, M.F.; et al. Peach–Potato Aphid Myzus persicae: Current Management Strategies, Challenges, and Proposed Solutions. Sustainability 2023, 15, 11150. [Google Scholar] [CrossRef]
- van Emden, H.F.; Eastop, V.F.; Hughes, R.D.; Way, M.J. The Ecology of Myzus persicae. Annu. Rev. Entomol. 1969, 14, 197–270. [Google Scholar] [CrossRef]
- Powell, G.; Hardie, J. The Chemical Ecology of Aphid Host Alternation: How Do Return Migrants Find the Primary Host Plant? Appl. Entomol. Zool. 2001, 36, 259–267. [Google Scholar] [CrossRef]
- Webster, B.; Bruce, T.; Pickett, J.; Hardie, J. Volatiles Functioning as Host Cues in a Blend Become Nonhost Cues When Presented Alone to the Black Bean Aphid. Anim. Behav. 2010, 79, 451–457. [Google Scholar] [CrossRef]
- Döring, T.F. How Aphids Find Their Host Plants, and How They Don’t. Ann. Appl. Biol. 2014, 165, 3–26. [Google Scholar] [CrossRef]
- van Emden, H.F.; Harrington, R. Aphids as Crop Pests; Cabi: Wallingford, UK, 2017; ISBN 1780647107. [Google Scholar]
- Bass, C.; Puinean, A.M.; Zimmer, C.T.; Denholm, I.; Field, L.M.; Foster, S.P.; Gutbrod, O.; Nauen, R.; Slater, R.; Williamson, M.S. The Evolution of Insecticide Resistance in the Peach Potato Aphid, Myzus persicae. Insect Biochem. Mol. Biol. 2014, 51, 41–51. [Google Scholar] [CrossRef] [PubMed]
- van der Werf, H.M.G. Assessing the Impact of Pesticides on the Environment. Agric. Ecosyst. Environ. 1996, 60, 81–96. [Google Scholar] [CrossRef]
- Alavanja, M.C.R.; Hoppin, J.A.; Kamel, F. Health Effects of Chronic Pesticide Exposure: Cancer and Neurotoxicity. Annu. Rev. Public Health 2004, 25, 155–197. [Google Scholar] [CrossRef]
- Khater Prospects of Botanical Biopesticides in Insect Pest Management. J. Appl. Pharm. Sci. 2012, 3, 641–656. [CrossRef]
- Regnault-Roger, C.; Vincent, C.; Arnason, J.T. Essential Oils in Insect Control: Low-Risk Products in a High-Stakes World. Annu. Rev. Entomol. 2011, 57, 405–424. [Google Scholar] [CrossRef]
- Devrnja, N.; Milutinović, M.; Savić, J. When Scent Becomes a Weapon—Plant Essential Oils as Potent Bioinsecticides. Sustainability 2022, 14, 6847. [Google Scholar] [CrossRef]
- Hikal, W.M.; Baeshen, R.S.; Said-Al Ahl, H.A.H. Botanical Insecticide as Simple Extractives for Pest Control. Cogent Biol. 2017, 3, 1404274. [Google Scholar] [CrossRef]
- Pascual-Villalobos, M.J.; Cantó-Tejero, M.; Vallejo, R.; Guirao, P.; Rodríguez-Rojo, S.; Cocero, M.J. Use of Nanoemulsions of Plant Essential Oils as Aphid Repellents. Ind. Crops Prod. 2017, 110, 45–57. [Google Scholar] [CrossRef]
- Toledo, P.F.S.; Ferreira, T.P.; Bastos, I.M.A.S.; Rezende, S.M.; Viteri Jumbo, L.O.; Didonet, J.; Andrade, B.S.; Melo, T.S.; Smagghe, G.; Oliveira, E.E.; et al. Essential Oil from Negramina (Siparuna guianensis) Plants Controls Aphids without Impairing Survival and Predatory Abilities of Non-Target Ladybeetles. Environ. Pollut. 2019, 255, 113153. [Google Scholar] [CrossRef] [PubMed]
- Sayed, S.; Soliman, M.M.; Al-Otaibi, S.; Hassan, M.M.; Elarrnaouty, S.-A.A.; Abozeid, S.M.; El-Shehawi, A.M. Toxicity, Deterrent and Repellent Activities of Four Essential Oils on Aphis punicae (Hemiptera: Aphididae). Plants 2022, 11, 463. [Google Scholar] [CrossRef]
- Lacotte, V.; Rey, M.; Peignier, S.; Mercier, P.-E.; Rahioui, I.; Sivignon, C.; Razy, L.; Benhamou, S.; Livi, S.; da Silva, P. Bioactivity and Chemical Composition of Forty Plant Essential Oils against the Pea Aphid Acyrthosiphon Pisum Revealed Peppermint Oil as a Promising Biorepellent. Ind. Crops Prod. 2023, 197, 116610. [Google Scholar] [CrossRef]
- Deletre, E.; Schatz, B.; Bourguet, D.; Chandre, F.; Williams, L.; Ratnadass, A.; Martin, T. Prospects for Repellent in Pest Control: Current Developments and Future Challenges. Chemoecology 2016, 26, 127–142. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological Effects of Essential Oils—A Review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Blackwell, A.; Stuart, A.E.; Estambale, B.A. The Repellant and Antifeedant Activity of Oil of Myrica Gale against Aedes Aegypti Mosquitoes and Its Enhancement by the Addition of Salicyluric Acid. Proc. R. Coll. Phys. Edinburgh 2003, 33, 209–214. [Google Scholar]
- Pavela, R. Essential Oils from Foeniculum Vulgare Miller as a Safe Environmental Insecticide against the Aphid Myzus persicae Sulzer. Environ. Sci. Pollut. Res. 2018, 25, 10904–10910. [Google Scholar] [CrossRef]
- Ali, S.A.M.; Saleh, A.A.A.; Saleh, F.M. Bioefficacy of Plant Extracts and Entomopathogenic Fungi (Trichoderma Album) in Controling Myzus persicae and Bemisia tabaci. Plant Arch. 2020, 20, 1450–1459. [Google Scholar]
- Costa, A.V.; Pinheiro, P.F.; de Queiroz, V.T.; Rondelli, V.M.; Marins, A.K.; Valbon, W.R.; Pratissoli, D. Chemical Composition of Essential Oil from Eucalyptus Citriodora Leaves and Insecticidal Activity against Myzus persicae and Frankliniella schultzei. J. Essent. Oil-Bearing Plants 2015, 18, 374–381. [Google Scholar] [CrossRef]
- Petrakis, E.A.; Kimbaris, A.C.; Perdikis, D.C.; Lykouressis, D.P.; Tarantilis, P.A.; Polissiou, M.G. Responses of Myzus persicae (Sulzer) to Three Lamiaceae Essential Oils Obtained by Microwave-Assisted and Conventional Hydrodistillation. Ind. Crops Prod. 2014, 62, 272–279. [Google Scholar] [CrossRef]
- Pascual-Villalobos, M.; Canto-Tejero, M.; Guirao, P.; Lopez, M.D. Fumigant Toxicity in Myzus persicae Sulzer (Hemiptera:Aphididae): Cotrolled Release of (E)-Anethole from Microspheres. Plants 2020, 9, 124. [Google Scholar] [CrossRef] [PubMed]
- Digilio, M.C.; Mancini, E.; Voto, E.; De Feo, V. Insecticide Activity of Mediterranean Essential Oils. J. Plant Interact. 2008, 3, 17–23. [Google Scholar] [CrossRef]
- Hori, M. Antifeeding, Settling Inhibitory and Toxic Activities of Labiate Essential Oils against the Green Peach Aphid, Myzus persicae (Sulzer) (Homoptera: Aphididae). Appl. Entomol. Zool. 1999, 34, 113–118. [Google Scholar] [CrossRef][Green Version]
- Dehliz, A.; Lakhdari, W.; Mlik, R.; Chahbar, N.; Acheuk, F.; Mekhadmi, N.E.H.; Benyahia, I.; Fethallah, R.; Hammi, H.; Mohammed, B.; et al. Chemical Composition and Bioactivity of Essential Oil against the Green Peach Aphid (Myzus persicae). Org. Agric. 2022, 12, 411–418. [Google Scholar] [CrossRef]
- Dancewicz, K.; Kordan, B.; Szumny, A.; Gabrys, B. Aphid Behaviour-Modifying Activity of Essential Oils from Lamiaceae and Apiaceae. Aphids Other Hemipterous Insects 2012, 18, 93–100. [Google Scholar]
- Bailer, J.; Aichinger, T.; Hackl, G.; de Hueber, K.; Dachler, M. Essential Oil Content and Composition in Commercially Available Dill Cultivars in Comparison to Caraway. Ind. Crops Prod. 2001, 14, 229–239. [Google Scholar] [CrossRef]
- Franzios, G.; Mirotsou, M.; Hatziapostolou, E.; Kral, J.; Scouras, Z.G.; Mavragani-Tsipidou, P. Insecticidal and Genotoxic Activities of Oregano Essential Oils. J. Agric. Food Chem. 1997, 45, 2690–2694. [Google Scholar] [CrossRef]
- De Carvalho, C.C.C.R.; Da Fonseca, M.M.R. Carvone: Why and How Should One Bother to Produce This Terpene. Food Chem. 2006, 95, 413–422. [Google Scholar] [CrossRef]
- López, M.D.; Jordán, M.J.; Pascual-Villalobos, M.J. Toxic Compounds in Essential Oils of Coriander, Caraway and Basil Active against Stored Rice Pests. J. Stored Prod. Res. 2008, 44, 273–278. [Google Scholar] [CrossRef]
- Morcia, C.; Tumino, G.; Ghizzoni, R.; Terzi, V. Carvone (Mentha Spicata L.) Oils; Elsevier Inc.: Amsterdam, The Netherlands, 2016; ISBN 9780124166417. [Google Scholar]
- Lee, S.; Tsao, R.; Peterson, C.; Coats, J.R. Insecticidal Activity of Monoterpenoids to Western Corn. J. Econ. Entomol. 1997, 90, 883–892. [Google Scholar] [CrossRef]
- Tripathi, A.K.; Prajapati, V.; Kumar, S. Bioactivities of L-Carvone, d-Carvone, and Dihydrocarvone Toward Three Stored Product Beetles. J. Econ. Entomol. 2003, 96, 1594–1601. [Google Scholar] [CrossRef] [PubMed]
- Gámiz, B.; Celis, R. S-Carvone Formulation Based on Granules of Organoclay to Modulate Its Losses and Phytotoxicity in Soil. Agronomy 2021, 11, 1593. [Google Scholar] [CrossRef]
- Nerio, L.S.; Olivero-Verbel, J.; Stashenko, E. Repellent Activity of Essential Oils: A Review. Bioresour. Technol. 2010, 101, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.K.; Prajapati, V.; Khanuja, S.P.S.; Kumar, S. Effect of D-Limonene on Three Stored-Product Beetles. J. Econ. Entomol. 2003, 96, 990–995. [Google Scholar] [CrossRef]
- Fang, R.; Jiang, C.H.; Wang, X.Y.; Zhang, H.M.; Liu, Z.L.; Zhou, L.; Du, S.S.; Deng, Z.W. Insecticidal Activity of Essential Oil of Carum carvi Fruits from China and Its Main Components against Two Grain Storage Insects. Molecules 2010, 15, 9391–9402. [Google Scholar] [CrossRef] [PubMed]
- Yoon, C.; Kang, S.H.; Jang, S.A.; Kim, Y.J.; Kim, G.H. Repellent Efficacy of Caraway and Grapefruit Oils for Sitophilus oryzae (Coleoptera: Curculionidae). J. Asia. Pac. Entomol. 2007, 10, 263–267. [Google Scholar] [CrossRef]
- Wróblewska-Kurdyk, A.; Nowak, L.; Dancewicz, K.; Szumny, A.; Gabryś, B. In Search of Biopesticides: The Effect of Caraway Carum carvi Essential Oil and Its Major Constituents on Peach Potato Aphid Myzus persicae Probing Behavior. Acta Biol. 2015, 22, 51–62. [Google Scholar] [CrossRef][Green Version]
- Pavoni, L.; Perinelli, D.R.; Bonacucina, G.; Cespi, M.; Palmieri, G.F. An Overview of Micro-and Nanoemulsions as Vehicles for Essential Oils: Formulation, Preparation and Stability. Nanomaterials 2020, 10, 135. [Google Scholar] [CrossRef]
- Sousa, V.I.; Parente, J.F.; Marques, J.F.; Forte, M.A.; Tavares, C.J. Microencapsulation of Essential Oils: A Review. Polymers 2022, 14, 1730. [Google Scholar] [CrossRef] [PubMed]
- Mahran, H.A. Using Nanoemulsions of the Essential Oils of a Selection of Medicinal Plants from Jazan, Saudi Arabia, as a Green Larvicidal against Culex pipiens. PLoS ONE 2022, 17, e0267150. [Google Scholar] [CrossRef]
- Perinelli, D.R.; Pavela, R.; Bonacucina, G.; Baldassarri, C.; Spinozzi, E.; Torresi, J.; Petrelli, R.; Morshedloo, M.R.; Maggi, F.; Benelli, G.; et al. Development, Characterization, Insecticidal and Sublethal Effects of Bunium persicum and Ziziphora Clinopodioides-Based Essential Oil Nanoemulsions on Culex Quinquefasciatus. Ind. Crops Prod. 2022, 186, 115249. [Google Scholar] [CrossRef]
- Abdelaal, K.; Essawy, M.; Quraytam, A.; Abdallah, F.; Mostafa, H.; Shoueir, K.; Fouad, H.; Hassan, F.A.S.; Hafez, Y. Toxicity of Essential Oils Nanoemulsion against Aphis Craccivora and Their Inhibitory Activity on Insect Enzymes. Processes 2021, 9, 624. [Google Scholar] [CrossRef]
- Draz, K.A.; Tabikha, R.M.; Eldosouky, M.I.; Darwish, A.A.; Abdelnasser, M. Biotoxicity of Essential Oils and Their Nano-Emulsions against the Coleopteran Stored Product Insect Pests Sitophilus oryzae L. and Tribolium castaneum Herbst. Int. J. Pest Manag. 2022, 1–15. [Google Scholar] [CrossRef]
- López, A.; Castro, S.; Andina, M.J.; Ures, X.; Munguía, B.; Llabot, J.M.; Elder, H.; Dellacassa, E.; Palma, S.; Domínguez, L. Insecticidal Activity of Microencapsulated Schinus molle Essential Oil. Ind. Crops Prod. 2014, 53, 209–216. [Google Scholar] [CrossRef]
- Ahsaei, S.M.; Rodríguez-Rojo, S.; Salgado, M.; Cocero, M.J.; Talebi-Jahromi, K.; Amoabediny, G. Insecticidal Activity of Spray Dried Microencapsulated Essential Oils of Rosmarinus Officinalis and Zataria multiflora against Tribolium confusum. Crop Prot. 2020, 128, 104996. [Google Scholar] [CrossRef]
- Tapondjou, A.L.; Adler, C.; Fontem, D.A.; Bouda, H.; Reichmuth, C. Bioactivities of Cymol and Essential Oils of Cupressus sempervirens and Eucalyptus saligna against Sitophilus zeamais Motschulsky and Tribolium confusum Du Val. J. Stored Prod. Res. 2005, 41, 91–102. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, F.; Zhou, X.M.; Niu, C.Y.; Lei, C.L. Repellent and Fumigant Activity of Essential Oil from Artemisia Vulgaris to Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). J. Stored Prod. Res. 2006, 42, 339–347. [Google Scholar] [CrossRef]
- Cosimi, S.; Rossi, E.; Cioni, P.L.; Canale, A. Bioactivity and Qualitative Analysis of Some Essential Oils from Mediterranean Plants against Stored-Product Pests: Evaluation of Repellency against Sitophilus zeamais Motschulsky, Cryptolestes ferrugineus (Stephens) and Tenebrio molitor (L.). J. Stored Prod. Res. 2009, 45, 125–132. [Google Scholar] [CrossRef]
- Conti, B.; Canale, A.; Cioni, P.L.; Flamini, G. Repellence of Essential Oils from Tropical and Mediterranean Lamiaceae against Sitophilus zeamais. Bull. Insectology 2010, 63, 197–202. [Google Scholar]
- Barbosa-Cornelio, R.; Cantor, F.; Coy-Barrera, E.; Rodríguez, D. Tools in the Investigation of Volatile Semiochemicals on Insects: From Sampling to Statistical Analysis. Insects 2019, 10, 241. [Google Scholar] [CrossRef] [PubMed]
- Dardouri, T.; Gautier, H.; Costagliola, G.; Gomez, L. How French Marigold (Tagetes patula L.) Volatiles Can Affect the Performance of Green Peach Aphid. Integr. Prot. Fruit Crop. IOBC-WPRS Bull. 2017, 123, 71–78. [Google Scholar]
- Powell, G.; Tosh, C.R.; Hardie, J. Host Plant Selection by Aphids: Behavioral, Evolutionary, and Applied Perspectives. Annu. Rev. Entomol. 2006, 51, 309–330. [Google Scholar] [CrossRef] [PubMed]
- Khaled-Gasmi, W.; Ben Hamouda, A.; Chaieb, I.; Souissi, R.; Ascrizzi, R.; Flamini, G.; Boukhris-Bouhachem, S. Natural Repellents Based on Three Botanical Species Essential Oils as an Eco-Friendly Approach against Aphids. South Afr. J. Bot. 2021, 141, 133–141. [Google Scholar] [CrossRef]
- McDonald, L.L.; Guy, R.H.; Speirs, R.D. Preliminary Evalution of New Candidate Materials as Toxicants, Repellents, and Attractants Against Store-Products Insects. Agric. Res. Serv. 1970, 882, 398–494. [Google Scholar]
- Czarnobai De Jorge, B.; Hummel, H.E.; Gross, J. Repellent Activity of Clove Essential Oil Volatiles and Development of Nanofiber-Based Dispensers against Pear Psyllids (Hemiptera: Psyllidae). Insects 2022, 13, 743. [Google Scholar] [CrossRef]
- Boncan, D.A.T.; Tsang, S.S.K.; Li, C.; Lee, I.H.T.; Lam, H.-M.; Chan, T.-F.; Hui, J.H.L. Terpenes and Terpenoids in Plants: Interactions with Environment and Insects. Int. J. Mol. Sci. 2020, 21, 7382. [Google Scholar] [CrossRef]
- Ben-Issa, R.; Gomez, L.; Gautier, H. Companion Plants for Aphid Pest Management. Insects 2017, 8, 112. [Google Scholar] [CrossRef]
- Ahmed, Q.; Agarwal, M.; Alobaidi, R.; Zhang, H.; Ren, Y. Response of Aphid Parasitoids to Volatile Organic Compounds from Undamaged and Infested Brassica Oleracea with Myzus persicae. Molecules 2022, 27, 1522. [Google Scholar] [CrossRef]
- Seo, S.M.; Kim, J.; Lee, S.G.; Shin, C.H.; Shin, S.C.; Park, I.K. Fumigant Antitermitic Activity of Plant Essential Oils and Components from Ajowan (Trachyspermum ammi), Allspice (Pimenta dioica), Caraway (Carum carvi), Dill (Anethum graveoiens), Geranium (Pelargonium graveoiens), and Litsea (Litsea cubeba) Oils Against. J. Agric. Food Chem. 2009, 57, 6596–6602. [Google Scholar] [CrossRef]
- Giatropoulos, A.; Papachristos, D.P.; Kimbaris, A.; Koliopoulos, G.; Polissiou, M.G.; Emmanouel, N.; Michaelakis, A. Evaluation of Bioefficacy of Three Citrus Essential Oils against the Dengue Vector Aedes Albopictus (Diptera: Culicidae) in Correlation to Their Components Enantiomeric Distribution. Parasitol. Res. 2012, 111, 2253–2263. [Google Scholar] [CrossRef]
- Webster, B. The Role of Olfaction in Aphid Host Location. Physiol. Entomol. 2012, 37, 10–18. [Google Scholar] [CrossRef]
- Ngumbi, E.; Eigenbrode, S.D.; Bosque-Pérez, N.A.; Ding, H.; Rodriguez, A. Myzus persicae Is Arrested More by Blends than by Individual Compounds Elevated in Headspace of Plrv-Infected Potato. J. Chem. Ecol. 2007, 33, 1733–1747. [Google Scholar] [CrossRef]
- Deletre, E.; Chandre, F.; Barkman, B.; Menut, C.; Martin, T. Naturally Occurring Bioactive Compounds from Four Repellent Essential Oils against Bemisia tabaci Whiteflies. Pest Manag. Sci. 2015, 72, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Cantó-Tejero, M.; Casas, J.L.; Marcos-García, M.Á.; Pascual-Villalobos, M.J.; Florencio-Ortiz, V.; Guirao, P. Essential Oils-Based Repellents for the Management of Myzus persicae and Macrosiphum euphorbiae. J. Pest Sci. 2022, 95, 365–379. [Google Scholar] [CrossRef]
- Xu, Y.L.; He, P.; Zhang, L.; Fang, S.Q.; Dong, S.L.; Zhang, Y.J.; Li, F. Large-Scale Identification of Odorant-Binding Proteins and Chemosensory Proteins from Expressed Sequence Tags in Insects. BMC Genom. 2009, 10, 632. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.P.; Zhao, L.J.; Sun, L.; Zhang, S.G.; Ban, L.P. Immunolocalization of Odorant-Binding Proteins on Antennal Chemosensilla of the Peach Aphid Myzus persicae (Sulzer). Chem. Senses 2013, 38, 129–136. [Google Scholar] [CrossRef][Green Version]
- Zhou, S.; Jander, G. Molecular Ecology of Plant Volatiles in Interactions with Insect Herbivores. J. Exp. Bot. 2022, 73, 449–462. [Google Scholar] [CrossRef]
- Hao, H.; Sun, J.; Dai, J. Dose-Dependent Behavioral Response of the Mosquito Aedes Albopictus to Floral Odorous Compounds. J. Insect Sci. 2013, 13, 127. [Google Scholar] [CrossRef]
- Rizzo, R.; Pistillo, M.; Germinara, G.S.; Lo Verde, G.; Sinacori, M.; Maggi, F.; Petrelli, R.; Spinozzi, E.; Cappellacci, L.; Zeni, V.; et al. Bioactivity of Carlina acaulis Essential Oil and Its Main Component towards the Olive Fruit Fly, Bactrocera oleae: Ingestion Toxicity, Electrophysiological and Behavioral Insights. Insects 2021, 12, 880. [Google Scholar] [CrossRef]
- Badji, C.A.; Dorland, J.; Kheloul, L.; Bréard, D.; Richomme, P.; Kellouche, A.; De Souza, C.R.A.; Bezerra, A.L.; Anton, S. Behavioral and Antennal Responses of Tribolium confusum to Varronia globosa Essential Oil and Its Main Constituents: Perspective for Their Use as Repellent. Molecules 2021, 26, 439. [Google Scholar] [CrossRef] [PubMed]
- Licciardello, F.; Muratore, G.; Suma, P.; Russo, A.; Nerín, C. Effectiveness of a Novel Insect-Repellent Food Packaging Incorporating Essential Oils against the Red Flour Beetle (Tribolium castaneum). Innov. Food Sci. Emerg. Technol. 2013, 19, 173–180. [Google Scholar] [CrossRef]
- Naik, D.G.; Vaidya-Kannur, H.; Deshpande, P.V.; Dandge, C.N.; Reddy, G.V.P. Potential Use of an Essential Oil from the Flower of Swertia densifolia as a Repellent for Apis florea (Hymenoptera: Apidae). Ann. Entomol. Soc. Am. 2015, 108, 18–25. [Google Scholar] [CrossRef]
- Rudolfi, T.A.; Schedrina, M.M.; Mindlin, L.O. Determination of the Evaporation Rate of Essential Oils and Perfumery Compositions Using Gas Chromatography. Chromatographia 1988, 25, 520–522. [Google Scholar] [CrossRef]
- Inouye, S.; Takizawa, T.; Yamaguchi, H. Antibacterial Activity of Essential Oils and Their Major Constituents against Respiratory Tract Pathogens by Gaseous Contact. J. Antimicrob. Chemother. 2001, 47, 565–573. [Google Scholar] [CrossRef]
- Brosset, A.; Blande, J.D. Volatile-Mediated Plant-Plant Interactions: Volatile Organic Compounds as Modulators of Receiver Plant Defence, Growth, and Reproduction. J. Exp. Bot. 2022, 73, 511–528. [Google Scholar] [CrossRef]
- Maffei, M.; Bossi, S.; Spiteller, D.; Mithöfer, A.; Boland, W. Effects of Feeding Spodoptera littoralis on Lima Bean Leaves. I. Membrane Potentials, Intracellular Calcium Variations, Oral Secretions, and Regurgitate Components. Plant Physiol. 2004, 134, 1752–1762. [Google Scholar] [CrossRef]
- Werrie, P.Y.; Durenne, B.; Delaplace, P.; Fauconnier, M.L. Phytotoxicity of Essential Oils: Opportunities and Constraints for the Development of Biopesticides. A Review. Foods 2020, 9, 1291. [Google Scholar] [CrossRef]
- Dunan, L.; Malanga, T.; Bearez, P.; Benhamou, S.; Monticelli, L.S.; Desneux, N.; Michel, T.; Lavoir, A.V. Biopesticide Evaluation from Lab to Greenhouse Scale of Essential Oils Used against Macrosiphum euphorbiae. Agriculture 2021, 11, 867. [Google Scholar] [CrossRef]
- Isman, M.B. Botanical Insecticides in the Twenty-First Century-Fulfilling Their Promise? Annu. Rev. Entomol. 2020, 65, 233–249. [Google Scholar] [CrossRef]
- Denoirjean, T.; Rivière, M.; Doury, G.; Le Goff, G.J.; Ameline, A. Behavioral Disruption of Two Orchard Hemipteran Pests by Garlic Essential Oil. Entomol. Exp. Appl. 2022, 170, 782–791. [Google Scholar] [CrossRef]
- Mežaka, I.; Kronberga, A.; Berga, M.; Kaļāne, L.; Pastare, L.; Skudriņš, G.; Nakurte, I. Biochemical and Physiological Responses of Cucumis sativus L. to Application of Potential Bioinsecticides—Aqueous Carum carvi L. Seed Distillation By-Product Based Extracts. Agriculture 2023, 13, 17. [Google Scholar] [CrossRef]
- Baudry, X.; Doury, G.; Couty, A.; Fourdrain, Y.; van Havermaet, R.; Lateur, M.; Ameline, A. Antagonist Effects of the Leek Allium Porrum as a Companion Plant on Aphid Host Plant Colonization. Sci. Rep. 2021, 11, 4032. [Google Scholar] [CrossRef] [PubMed]
- Gupta, I.; Singh, R.; Muthusamy, S.; Sharma, M.; Grewal, K.; Singh, H.P.; Batish, D.R. Plant Essential Oils as Biopesticides: Applications, Mechanisms, Innovations, and Constraints. Plants 2023, 12, 2916. [Google Scholar] [CrossRef]
- Isman, M.B. Bridging the Gap: Moving Botanical Insecticides from the Laboratory to the Farm. Ind. Crops Prod. 2017, 110, 10–14. [Google Scholar] [CrossRef]
- Javed, K.; Javed, H.; Qiu, D. Extracted from Brevibacillus Laterosporus Strain A60 and Its Capacity in the Induction of Defense Process against Cucumber Aphid (Myzus persicae). Biology 2020, 9, 179. [Google Scholar] [CrossRef]
- Dardouri, T.; Gautier, H.; Ben Issa, R.; Costagliola, G.; Gomez, L. Repellence of Myzus persicae (Sulzer): Evidence of Two Modes of Action of Volatiles from Selected Living Aromatic Plants. Pest Manag. Sci. 2019, 75, 1571–1584. [Google Scholar] [CrossRef] [PubMed]
- Alqarni, M.H.; Foudah, A.I.; Aodah, A.H.; Alkholifi, F.K.; Salkini, M.A.; Alam, A. Caraway Nanoemulsion Gel: A Potential Antibacterial Treatment against Escherichia Coli and Staphylococcus Aureus. Gels 2023, 9, 193. [Google Scholar] [CrossRef] [PubMed]
- Partanen, R.; Ahro, M.; Hakala, M.; Kallio, H.; Forssell, P. Microencapsulation of Caraway Extract in β-Cyclodextrin and Modified Starches. Eur. Food Res. Technol. 2002, 214, 242–247. [Google Scholar] [CrossRef]
- Baranauskiene, R.; Rutkaite, R.; Pečiulyte, L.; Kazernavičiūte, R.; Venskutonis, P.R. Preparation and Characterization of Single and Dual Propylene Oxide and Octenyl Succinic Anhydride Modified Starch Carriers for the Microencapsulation of Essential Oils. Food Funct. 2016, 7, 3555–3565. [Google Scholar] [CrossRef]
- Chmiel, M.; Drzymała, G.; Bocianowski, J.; Komnenić, A.; Baran, A.; Synowiec, A. Maltodextrin-Coated Peppermint and Caraway Essential Oils Effects on Soil Microbiota. Plants 2022, 11, 3343. [Google Scholar] [CrossRef] [PubMed]
- Bylaitë, E.; Rimantas Venskutonis, P.; Maþdþierienë, R. Properties of Caraway (Carum carvi L.) Essential Oil Encapsulated into Milk Protein-Based Matrices. Eur. Food Res. Technol. 2001, 212, 661–670. [Google Scholar] [CrossRef]
- Ziaee, M.; Moharramipour, S.; Mohsenifar, A. MA-Chitosan Nanogel Loaded with Cuminum Cyminum Essential Oil for Efficient Management of Two Stored Product Beetle Pests. J. Pest Sci. 2014, 87, 691–699. [Google Scholar] [CrossRef]



| Commercial Name | Common Name | Manufacturer | HLB Value | Share in the Mixture |
|---|---|---|---|---|
| - | Ethanol, 96% | Kalsnavas elevators | - | 50% |
| Polysorbate 20 | Polyoxyethylene (20) sorbitan monolaurate | BBFactory | 16.7 | 50% |
| Coco glucoside | C8–C14 fatty alcohol glucoside | BBFactory | 13.5 | 50% |
| PolySol ® PGA | Ester of Polyglyceryl-6 with Caprylic Acid and Proline | Socri | 15 | 66% |
| Contact | Ethoxylated alcohols C9–11 > 90% | AgroDan | 12.4 | 50% |
| No | Retention Indexes * | Compound | EO1 | EO2 | EO3 |
|---|---|---|---|---|---|
| 1 | 1118 | β-Thujene | <LOD | <LOD | 0.10 ± 0.00 |
| 2 | 1160 | β-Myrcene | 0.17 ± 0.01 | 0.19 ± 0.02 | 0.41 ± 0.05 |
| 3 | 1197 | D-Limonene | 25.12 ± 0.72 | 28.94 ± 0.91 | 51.89 ± 1.02 |
| 4 | 1593 | Caryophyllene | <LOD | <LOD | 0.13 ± 0.01 |
| 5 | 1617 | Dihydrocarvone | <LOD | 0.12 ± 0.02 | <LOD |
| 6 | 1624 | trans-Dihydrocarvone | <LOD | 0.12 ± 0.01 | <LOD |
| 7 | 1699 | α-Terpineol | 0.10 ± 0.00 | <LOD | <LOD |
| 8 | 1740 | D-Carvone | 74.38 ± 1.17 | 70.38 ± 1.00 | 47.33 ± 0.89 |
| 9 | 1792 | Perylla aldehyde | 0.24 ± 0.03 | 0.25 ± 0.02 | 0.13 ± 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Girardi, J.; Berķe-Ļubinska, K.; Mežaka, I.; Nakurte, I.; Skudriņš, G.; Pastare, L. In Vivo Bioassay of the Repellent Activity of Caraway Essential Oil against Green Peach Aphid. Insects 2023, 14, 876. https://doi.org/10.3390/insects14110876
Girardi J, Berķe-Ļubinska K, Mežaka I, Nakurte I, Skudriņš G, Pastare L. In Vivo Bioassay of the Repellent Activity of Caraway Essential Oil against Green Peach Aphid. Insects. 2023; 14(11):876. https://doi.org/10.3390/insects14110876
Chicago/Turabian StyleGirardi, Jessica, Kristīne Berķe-Ļubinska, Ieva Mežaka, Ilva Nakurte, Gundars Skudriņš, and Laura Pastare. 2023. "In Vivo Bioassay of the Repellent Activity of Caraway Essential Oil against Green Peach Aphid" Insects 14, no. 11: 876. https://doi.org/10.3390/insects14110876
APA StyleGirardi, J., Berķe-Ļubinska, K., Mežaka, I., Nakurte, I., Skudriņš, G., & Pastare, L. (2023). In Vivo Bioassay of the Repellent Activity of Caraway Essential Oil against Green Peach Aphid. Insects, 14(11), 876. https://doi.org/10.3390/insects14110876