Perennial Flowering Plants Sustain Natural Enemy Populations in Gobi Desert Oases of Southern Xinjiang, China
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Plant and Predator Survey
2.3. Data Analyses
3. Results
3.1. Floral Composition
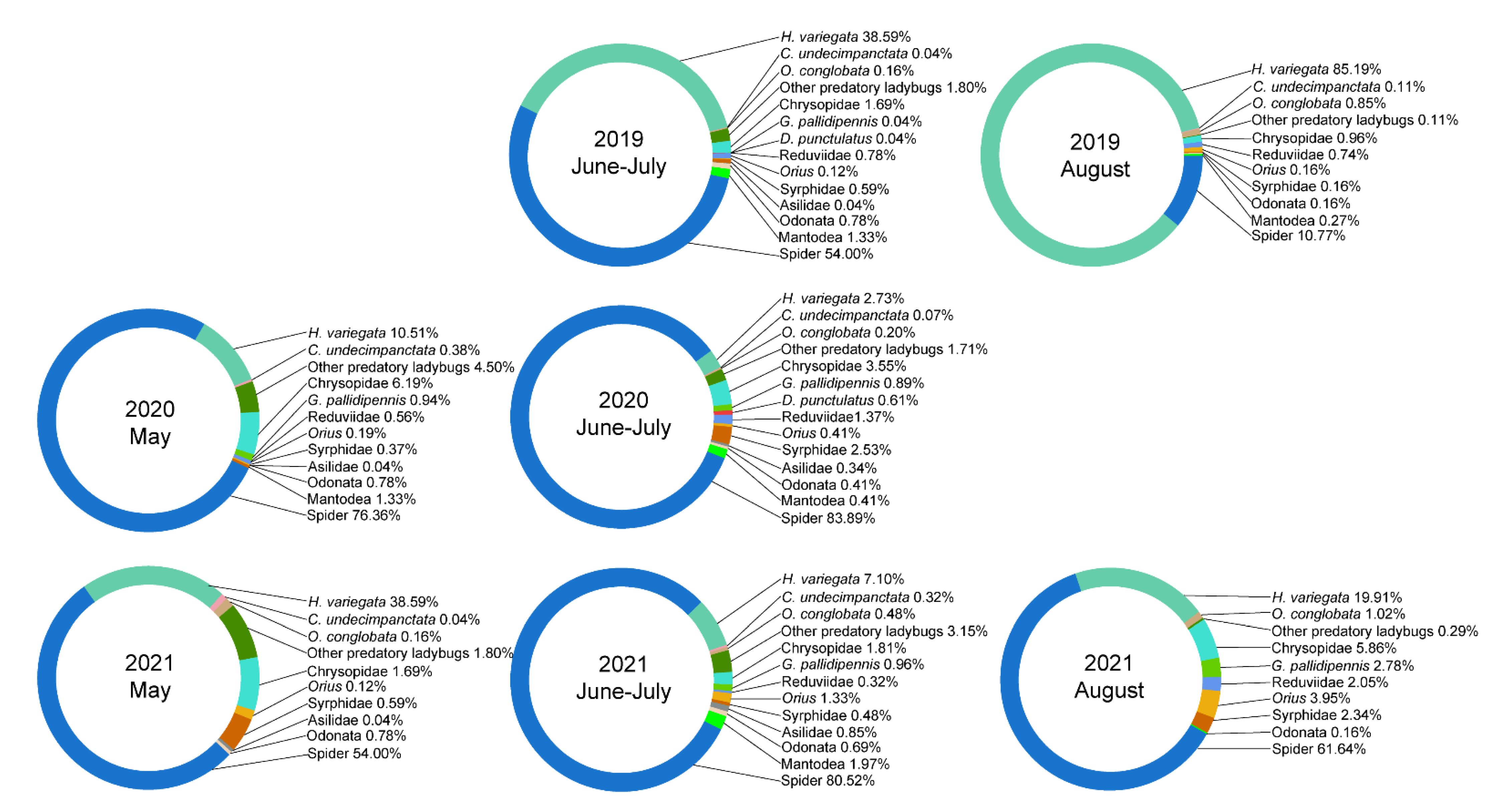
3.2. Predator Composition
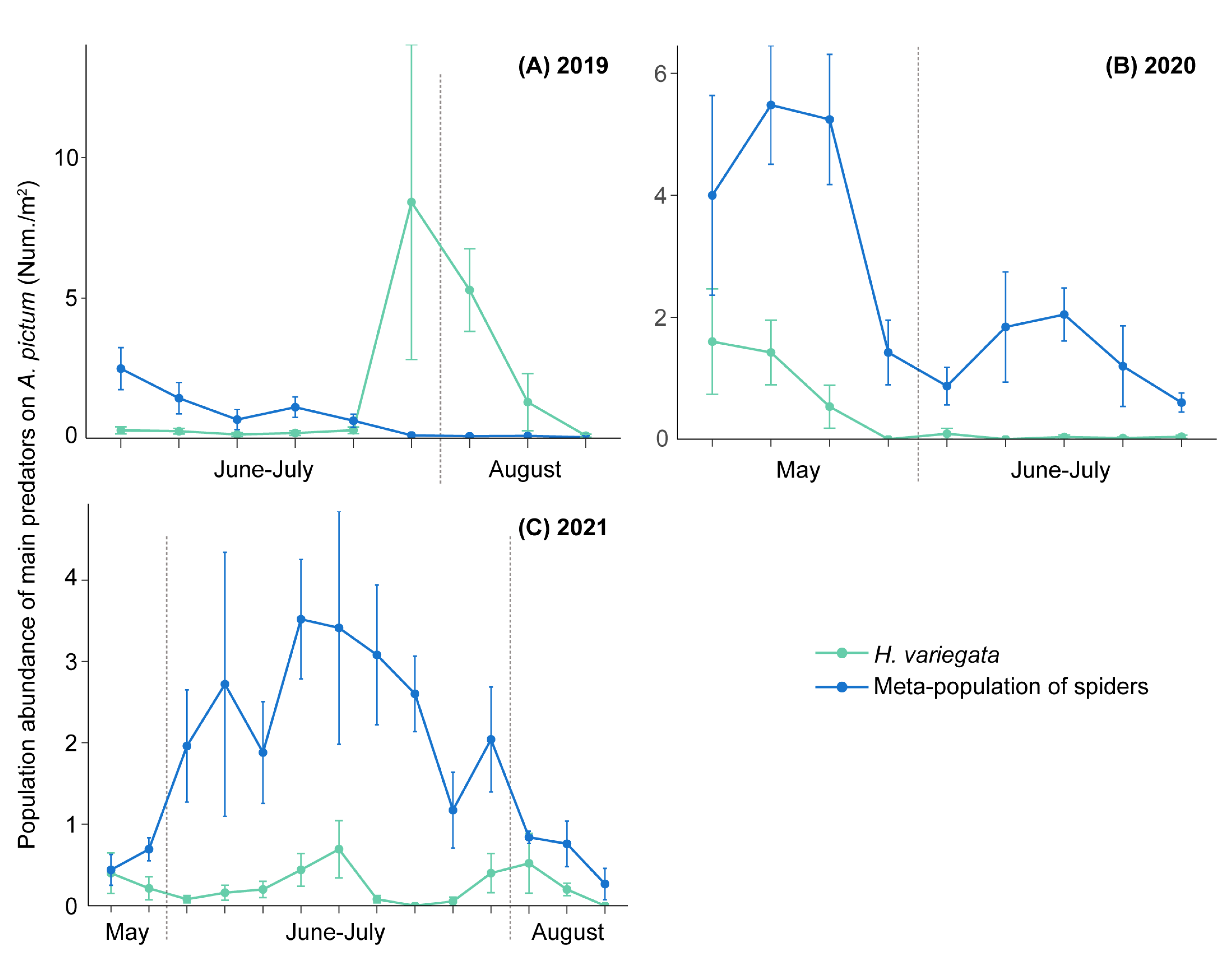
3.3. Temporal Predator Patterns
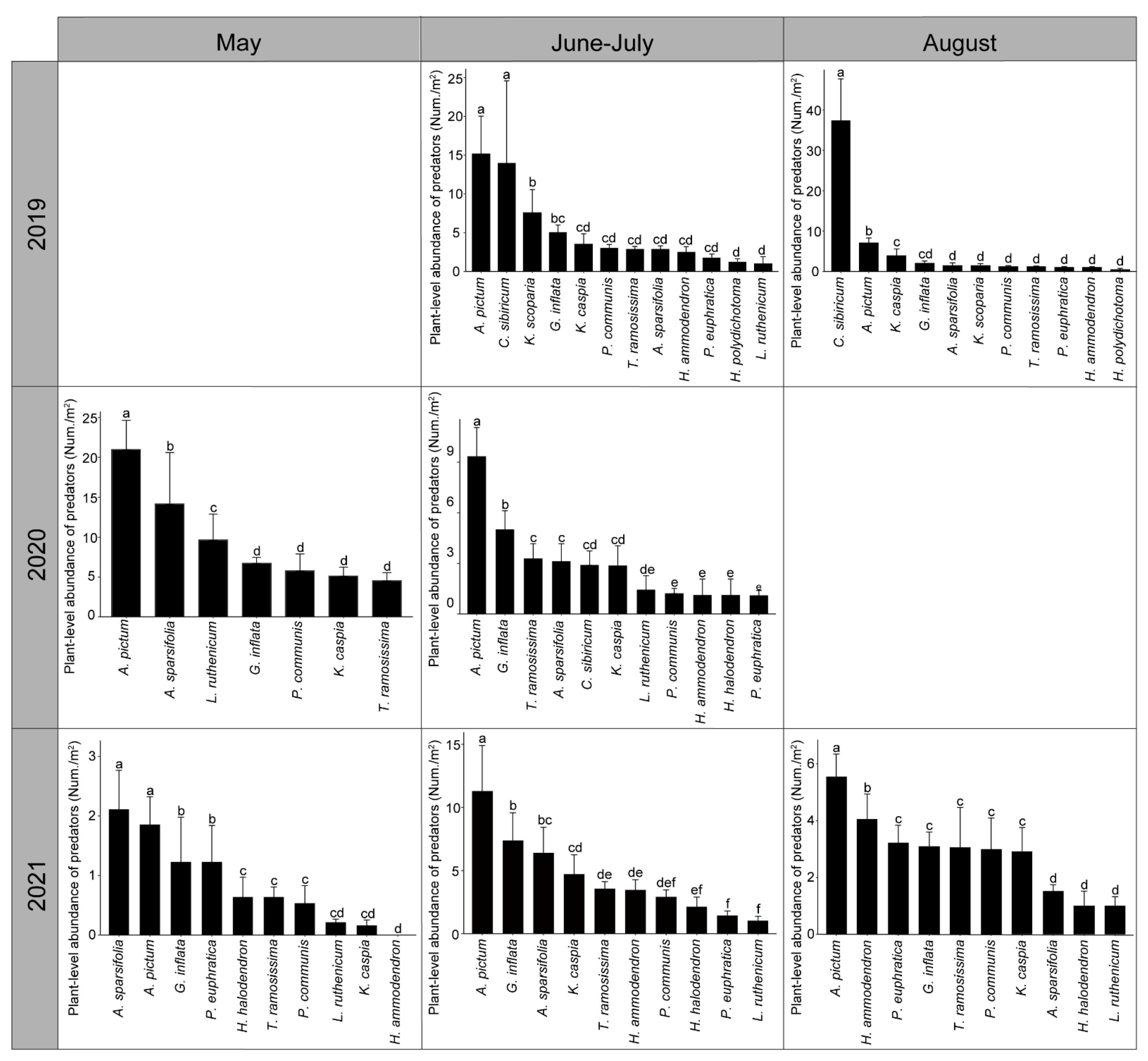
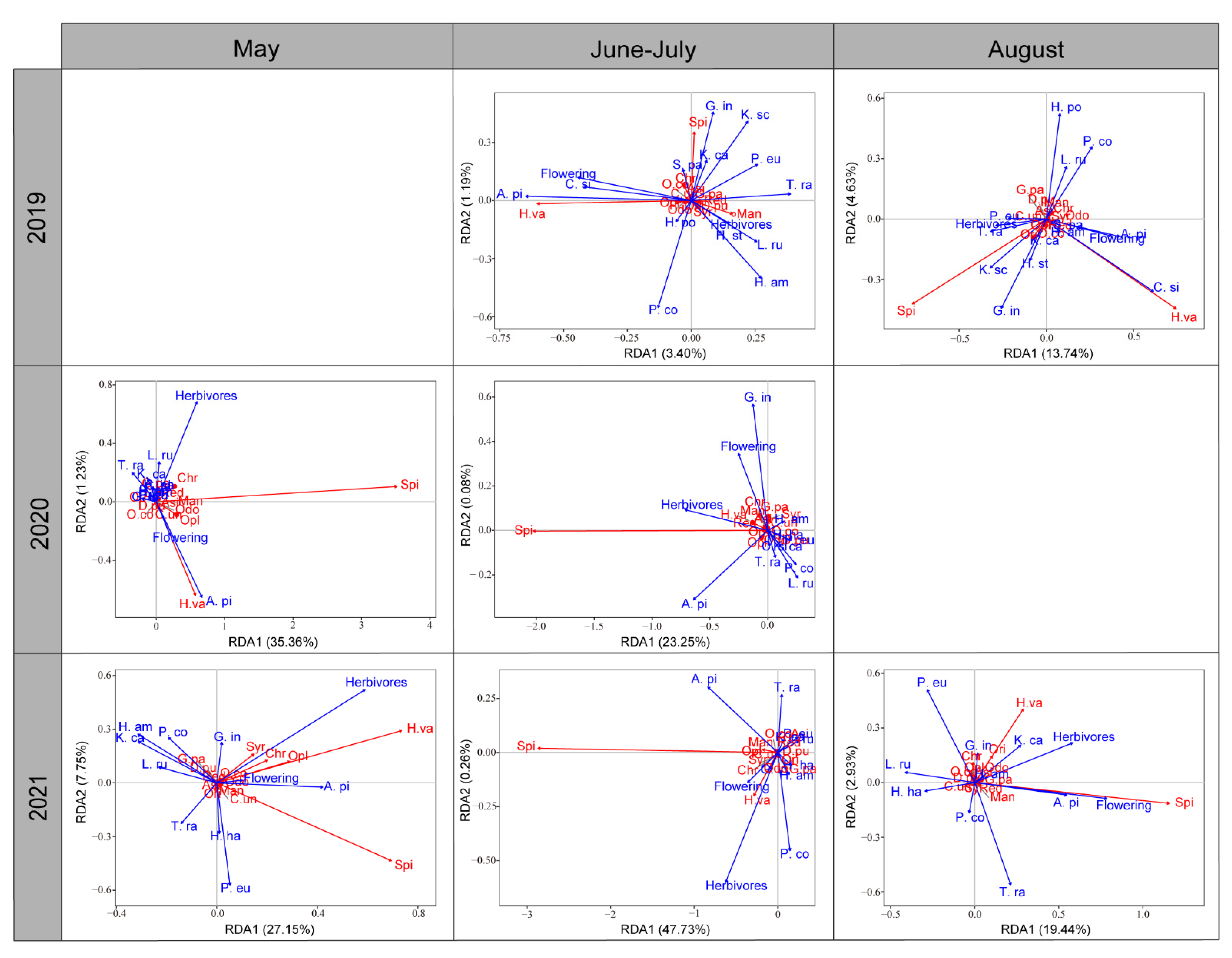
3.4. Plant Effects on Resident Predator Community
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tscharntke, T.; Karp, D.S.; Chaplin-Kramer, R.; Batary, P.; DeClerck, F.; Gratton, C.; Hunt, L.; Ives, A.; Jonsson, M.; Larsen, A.; et al. When natural habitat fails to enhance biological pest control—Five hypotheses. Biol. Conserv. 2016, 204, 449–458. [Google Scholar] [CrossRef] [Green Version]
- Gurr, G.M.; Wratten, S.D.; Landis, D.A.; You, M. Habitat management to suppress pest populations: Progress and prospects. Annu. Rev. Entomol. 2017, 62, 91–109. [Google Scholar] [CrossRef] [PubMed]
- Heimoana, V.; Pilkington, L.J.; Raman, A.; Mitchell, A.; Nicol, H.I.; Johnson, A.C.; Gurr, G.M. Integrating spatially explicit molecular and ecological methods to explore the significance of non-crop vegetation to predators of brassica pests. Agric. Ecosyst. Environ. 2017, 239, 12–19. [Google Scholar] [CrossRef]
- Holland, J.M.; Bianchi, F.J.J.A.; Entling, M.H.; Moonen, A.C.; Smith, B.M.; Jeanneret, P. Structure, function and management of semi-natural habitats for conservation biological control: A review of European studies. Pest Manag. Sci. 2016, 72, 1638–1651. [Google Scholar] [CrossRef]
- Yang, L.; Zeng, Y.D.; Xu, L.; Liu, B.; Zhang, Q.; Lu, Y.H. Change in ladybeetle abundance and biological control of wheat aphids over time in agricultural landscape. Agric. Ecosyst. Environ. 2018, 255, 102–110. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Q.; Liu, B.; Zeng, Y.; Pan, Y.; Li, M.; Lu, Y. Mixed effects of landscape complexity and insecticide use on ladybeetle abundance in wheat fields. Pest Manag. Sci. 2019, 75, 1638–1645. [Google Scholar] [CrossRef]
- Matteson, P.C. Insect pest management in tropical Asian irrigated rice. Annu. Rev. Entomol. 2000, 45, 549–574. [Google Scholar] [CrossRef]
- Landis, D.A.; Wratten, S.D.; Gurr, G.M. Habitat management to conserve natural enemies of arthropod pests in agriculture. Annu. Rev. Entomol. 2000, 45, 175–201. [Google Scholar] [CrossRef]
- Han, P.; Lavoir, A.V.; Rodriguez-Saona, C.; Desneux, N. Bottom-up forces in agroecosystems and their potential impact on arthropod pest management. Annu. Rev. Entomol. 2021, 67, 239–259. [Google Scholar] [CrossRef]
- Martin, E.A.; Seo, B.; Park, C.R.; Reineking, B.; Steffan-Dewenter, I. Scale-dependent effects of landscape composition and configuration on natural enemy diversity, crop herbivory, and yields. Ecol. Appl. 2016, 26, 448–462. [Google Scholar] [CrossRef]
- Gonzalez, E.; Landis, D.A.; Knapp, M.; Valladares, G. Forest cover and proximity decrease herbivory and increase crop yield via enhanced natural enemies in soybean fields. J. Appl. Ecol. 2020, 57, 2296–2306. [Google Scholar] [CrossRef]
- Middleton, E.G.; MacRae, I.V. Wildflower plantings in commercial agroecosystems promote generalist predators of Colorado potato beetle. Biol. Control 2021, 152, 104463. [Google Scholar] [CrossRef]
- Karp, D.S.; Chaplin-Kramer, R.; Meehan, T.D.; Martin, E.A.; DeClerck, F.; Grab, H.; Gratton, C.; Hunt, L.; Larsen, A.E.; Martinez-Salinas, A.; et al. Crop pests and predators exhibit inconsistent responses to surrounding landscape composition. Proc. Nat. Acad. Sci. USA 2018, 115, 7863–7870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuldiner-Harpaz, T.; Coll, M. Estimating the effect of plant-provided food supplements on pest consumption by omnivorous predators: Lessons from two coccinellid beetles. Pest Manag. Sci. 2017, 73, 976–983. [Google Scholar] [CrossRef]
- Xiu, C.L.; Xu, B.; Pan, H.S.; Zhang, W.; Yang, Y.Z.; Lu, Y.H. Volatiles from Sophora japonica flowers attract Harmonia axyridis adults (Coleoptera: Coccinellidae). J. Integr. Agric. 2019, 18, 873–883. [Google Scholar] [CrossRef] [Green Version]
- Van Wyk, J.I.; Krimmel, B.A.; Crova, L.; Pearse, I.S. Plants trap pollen to feed predatory arthropods as an indirect resistance against herbivory. Ecology 2019, 100, e02867. [Google Scholar] [CrossRef] [Green Version]
- Turlings, T.C.J.; Erb, M. Tritrophic interactions mediated by herbivore-induced plant volatiles: Mechanisms, ecological relevance, and application potential. Annu. Rev. Entomol. 2018, 63, 433–452. [Google Scholar] [CrossRef]
- Xiu, C.L.; Zhang, W.; Xu, B.; Wyckhuys, K.A.G.; Cai, X.M.; Su, H.H.; Lu, Y.H. Volatiles from aphid-infested plants attract adults of the multicolored Asian lady beetle Harmonia axyridis. Biol. Control 2019, 129, 1–11. [Google Scholar] [CrossRef]
- Landis, D.A. Designing agricultural landscapes for biodiversity-based ecosystem services. Basic Appl. Ecol. 2017, 18, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Perović, D.J.; Gámez-Virués, S.; Landis, D.A.; Wäckers, F.; Gurr, G.M.; Wratten, S.D.; You, M.-S.; Desneux, N. Managing biological control services through multi-trophic trait interactions: Review and guidelines for implementation at local and landscape scales. Biol. Rev. 2018, 93, 306–321. [Google Scholar] [CrossRef] [Green Version]
- Blaix, C.; Moonen, A.C.; Dostatny, D.F.; Izquierdo, J.; Le Corff, J.; Morrison, J.; Von Redwitz, C.; Schumacher, M.; Westerman, P.R. Quantification of regulating ecosystem services provided by weeds in annual cropping systems using a systematic map approach. Weed Res. 2018, 58, 151–164. [Google Scholar] [CrossRef]
- Li, S.; Jaworski, C.C.; Hatt, S.; Zhang, F.; Desneux, N.; Wang, S. Flower strips adjacent to greenhouses help reduce pest populations and insecticide applications inside organic commercial greenhouses. J. Pest Sci. 2021, 94, 679–689. [Google Scholar] [CrossRef]
- Hoffmann, U.S.; Jauker, F.; Lanzen, J.; Warzecha, D.; Wolters, V.; Diekotter, T. Prey-dependent benefits of sown wildflower strips on solitary wasps in agroecosystems. Insect Conserv. Diver. 2018, 11, 42–49. [Google Scholar] [CrossRef]
- Sutter, L.; Albrecht, M.; Jeanneret, P. Landscape greening and local creation of wildflower strips and hedgerows promote multiple ecosystem services. J. Appl. Ecol. 2018, 55, 612–620. [Google Scholar] [CrossRef]
- Mateos-Fierro, Z.; Fountain, M.T.; Garratt, M.P.D.; Ashbrook, K.; Westbury, D.B. Active management of wildflower strips in commercial sweet cherry orchards enhances natural enemies and pest regulation services. Agric. Ecosyst. Environ. 2021, 317, 107485. [Google Scholar] [CrossRef]
- Huang, R.; Hu, H.; Wu, W.; Fan, Z.; Suo, F. Formation and evolution of desert insects in Xinjiang and its adjacent regions. Arid Land Geogr. 2005, 28, 38–44. [Google Scholar]
- Li, F.R.; Liu, J.L.; Sun, T.S.; Jin, B.W.; Chen, L.J. Converting natural vegetation to farmland alters functional structure of ground-dwelling beetles and spiders in a desert oasis. J. Insect Conserv. 2014, 18, 57–67. [Google Scholar] [CrossRef]
- Wei, H.J.; Xu, Z.H.; Liu, H.M.; Ren, J.H.; Fan, W.G.; Lu, N.C.; Dong, X.B. Evaluation on dynamic change and interrelations of ecosystem services in a typical mountain-oasis-desert region. Ecol. Indic. 2018, 93, 917–929. [Google Scholar] [CrossRef]
- Wang, H.F.; Lv, G.H.; Cai, Y.; Zhang, X.N.; Jiang, L.M.; Yang, X.D. Determining the effects of biotic and abiotic factors on the ecosystem multifunctionality in a desert-oasis ecotone. Ecol. Indic. 2021, 128, 107830. [Google Scholar] [CrossRef]
- Liu, J.L.; Li, F.R.; Liu, C.A.; Liu, Q.J. Influences of shrub vegetation on distribution and diversity of a ground beetle community in a Gobi desert ecosystem. Biodivers. Conserv. 2012, 21, 2601–2619. [Google Scholar] [CrossRef]
- Liu, G.; Ma, Q.; Yao, J.; Li, X.; Wang, D.; Song, Z. Study on dynamic of dominant spider species and Aphis gossypii in transitional zone between cotton field and its near desert in Xinjiang. Xinjiang Agric. Sci. 2007, 44, 298–302. [Google Scholar]
- Li, X.; Ma, Q.; Yao, J.; Liu, G. The characterizes of predatory natural enemy communities in farmland and the transitional zone between farmland and desert. China Cotton 2007, 34, 8–10. [Google Scholar]
- Su, J.; Li, J.; Li, T.; Han, G.; Xu, Z.; Li, X.; Zhang, J. Occurrence regularity of ladybugs in ecotone and farmland in southern marginal zone of Gurbantunggut desert. J. Shihezi Univ. (Nat. Sci.) 2018, 36, 603–608. [Google Scholar]
- Vuong, N.; Greenville, A.C.; Dickman, C.R.; Wardle, G.M. On the validity of visual cover estimates for time series analyses: A case study of hummock grasslands. Plant Ecol. 2015, 216, 975–988. [Google Scholar]
- Berger, W.H.; Parker, F.L. Diversity of Planktonic Foraminifera in Deep-Sea Sediments. Science 1970, 168, 1345–1347. [Google Scholar] [CrossRef]
- Legendre, P.; Gallagher, E.D. Ecologically meaningful transformations for ordination of species data. Oecologia 2001, 129, 271–280. [Google Scholar] [CrossRef]
- Bale, J.S.; van Lenteren, J.C.; Bigler, F. Biological control and sustainable food production. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 761–776. [Google Scholar] [CrossRef] [Green Version]
- Wyckhuys, K.A.G.; Lu, Y.; Morales, H.; Vazquez, L.L.; Legaspi, J.C.; Eliopoulos, P.A.; Hernandez, L.M. Current status and potential of conservation biological control for agriculture in the developing world. Biol. Control 2013, 65, 152–167. [Google Scholar] [CrossRef]
- Wyckhuys, K.A.G.; Lu, Y.; Zhou, W.; Cock, M.J.W.; Naranjo, S.E.; Fereti, A.; Williams, F.E.; Furlong, M.J. Ecological pest control fortifies agricultural growth in Asia–Pacific economies. Nat. Ecol. Evol. 2020, 4, 1522–1530. [Google Scholar] [CrossRef]
- Mason, P. Biological Control: Global Impacts, Challenges and Future Directions of Pest Management; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]
- Pekar, S.; Michalko, R.; Loverre, P.; Liznarova, E.; Cernecka, L. Biological control in winter: Novel evidence for the importance of generalist predators. J. Appl. Ecol. 2015, 52, 270–279. [Google Scholar] [CrossRef]
- Jonsson, M.; Kaartinen, R.; Straub, C.S. Relationships between natural enemy diversity and biological control. Curr. Opin. Insect Sci. 2017, 20, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Yang, L.; Zeng, Y.; Yang, F.; Yang, Y.; Lu, Y. Secondary crops and non-crop habitats within landscapes enhance the abundance and diversity of generalist predators. Agric. Ecosyst. Environ. 2018, 258, 30–39. [Google Scholar] [CrossRef]
- Lu, Y.H.; Wu, K.M.; Jiang, Y.Y.; Guo, Y.Y.; Desneux, N. Widespread adoption of Bt cotton and insecticide decrease promotes biocontrol services. Nature 2012, 487, 362–365. [Google Scholar] [CrossRef]
- Bouvet, J.P.R.; Urbaneja, A.; Monzo, C. Aphid predators in citrus crops: The least voracious predators are the most effective. J. Pest Sci. 2021, 94, 321–333. [Google Scholar] [CrossRef]
- Ostandie, N.; Muneret, L.; Giffard, B.; Thiery, D.; Rusch, A. The shape of the predator biomass distribution affects biological pest control services in agricultural landscapes. Funct. Ecol. 2021, 35, 193–204. [Google Scholar] [CrossRef]
- Wan, N.F.; Cai, Y.M.; Shen, Y.J.; Ji, X.Y.; Wu, X.W.; Zheng, X.R.; Cheng, W.; Li, J.; Jiang, Y.P.; Chen, X.; et al. Increasing plant diversity with border crops reduces insecticide use and increases crop yield in urban agriculture. Elife 2018, 7, e35103. [Google Scholar] [CrossRef]
- Zhang, W.; Lu, Y.H.; van der Werf, W.; Huang, J.K.; Wu, F.; Zhou, K.; Deng, X.Z.; Jiang, Y.Y.; Wu, K.M.; Rosegrant, M.W. Multidecadal, county-level analysis of the effects of land use, Bt cotton, and weather on cotton pests in China. Proc. Nat. Acad. Sci. USA 2018, 115, E7700–E7709. [Google Scholar] [CrossRef] [Green Version]
- Rusch, A.; Chaplin-Kramer, R.; Gardiner, M.M.; Hawro, V.; Holland, J.; Landis, D.; Thies, C.; Tscharntke, T.; Weisser, W.W.; Winqvist, C. Agricultural landscape simplification reduces natural pest control: A quantitative synthesis. Agric. Ecosyst. Environ. 2016, 221, 198–204. [Google Scholar] [CrossRef] [Green Version]
- Dainese, M.; Montecchiari, S.; Sitzia, T.; Sigura, M.; Marini, L. High cover of hedgerows in the landscape supports multiple ecosystem services in Mediterranean cereal fields. J. Appl. Ecol. 2017, 54, 380–388. [Google Scholar] [CrossRef] [Green Version]
- Tang, F.H.M.; Lenzen, M.; McBratney, A.; Maggi, F. Risk of pesticide pollution at the global scale. Nat. Geosci. 2021, 14, 206–210. [Google Scholar] [CrossRef]
- Bommarco, R.; Kleijn, D.; Potts, S.G. Ecological intensification: Harnessing ecosystem services for food security. Trends Ecol. Evol. 2013, 28, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.H.; Yoldas, Z. Intraguild predation between two aphidophagous coccinellids, Hippodamia variegata (G.) and Coccinella septempunctata L. (Coleoptera: Coccinellidae): The role of prey abundance. Biol. Control 2018, 126, 7–14. [Google Scholar] [CrossRef]
- Skouras, P.J.; Brokaki, M.; Stathas, G.J.; Demopoulos, V.; Louloudakis, G.; Margaritopoulos, J.T. Lethal and sub-lethal effects of imidacloprid on the aphidophagous coccinellid Hippodamia variegata. Chemosphere 2019, 229, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.N.; Sun, Y.X.; Liu, C.Z. Functional morphology of the mouthparts of lady beetle Hippodamia variegata (Coleoptera: Coccinellidae), with reference to their feeding mechanism. Zoomorphology 2020, 139, 199–212. [Google Scholar] [CrossRef]
- Ranjbar, F.; Michaud, J.P.; Jalali, M.A.; Ziaaddini, M. Intraguild predation between two lady beetle predators is more sensitive to density than species of extraguild prey. Biol. Control 2020, 65, 713–725. [Google Scholar] [CrossRef]
- Wyckhuys, K.A.G.; O’Neil, R.J. Population dynamics of Spodoptera frugiperda Smith (Lepidoptera: Noctuidae) and associated arthropod natural enemies in Honduran subsistence maize. Crop Prot. 2006, 25, 1180–1190. [Google Scholar] [CrossRef]
- Marko, V.; Keresztes, B. Flowers for better pest control? Ground cover plants enhance apple orchard spiders (Araneae), but not necessarily their impact on pests. Biocontrol Sci. Technol. 2014, 24, 574–596. [Google Scholar] [CrossRef]
- Michalko, R.; Pekar, S.; Entling, M.H. An updated perspective on spiders as generalist predators in biological control. Oecologia 2019, 189, 21–36. [Google Scholar] [CrossRef]
- Liang, Y.; Chen, X.; Dai, H.; Wang, J.; Guo, X.; Wang, S.; Jaworski, C.C. Flower provision reduces intraguild predation between predators and increases aphid biocontrol in tomato. J. Pest Sci. 2022, 95, 461–472. [Google Scholar] [CrossRef]
- Root, R.B. Organization of a plant-arthropod association in simple and diverse habitats: The fauna of Collards (Brassica Oleracea). Ecol. Monogr. 1973, 43, 95–124. [Google Scholar] [CrossRef]
- Wiedenmann, R.N.; Smith, J.W. Attributes of natural enemies in ephemeral crop habitats. Biol. Control 1997, 10, 16–22. [Google Scholar] [CrossRef]
- Buchanan, A.; Grieshop, M.; Szendrei, Z. Assessing annual and perennial flowering plants for biological control in asparagus. Biol. Control 2018, 127, 1–8. [Google Scholar] [CrossRef]
- He, X.Q.; Kiaer, L.P.; Jensen, P.M.; Sigsgaard, L. The effect of floral resources on predator longevity and fecundity: A systematic review and meta-analysis. Biol. Control 2021, 153, 104476. [Google Scholar] [CrossRef]
- Patt, J.M.; Moreno, A.M.T.; Niedz, R.P. Response surface methodology reveals proportionality effects of plant species in conservation plantings on occurrence of generalist predatory arthropods. PLoS ONE 2020, 15, e0231471. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.M.; Pfannenstiel, R.S. Nectar feeding by wandering spiders on cotton plants. Environ. Entomol. 2008, 37, 996–1002. [Google Scholar] [CrossRef]
- Narváez, A.; Cancino, J.; Daza, N.C.; Wyckhuys, K.A.G. Effect of different dietary resources on longevity, carbohydrate metabolism, and ovarian dynamics in two fruit fly parasitoids. Arthropod-Plant Interact. 2012, 6, 361–374. [Google Scholar] [CrossRef]
- Cahenzli, F.; Sigsgaard, L.; Daniel, C.; Herz, A.; Jamar, L.; Kelderer, M.; Jacobsen, S.K.; Kruczynska, D.; Matray, S.; Porcel, M.; et al. Perennial flower strips for pest control in organic apple orchards—A pan-European study. Agric. Ecosyst. Environ. 2019, 278, 43–53. [Google Scholar] [CrossRef]
- Pan, H.S.; Xiu, C.L.; Liu, B.; Wyckhuys, K.A.G.; Lu, Y.H. Whorl-stage maize provides a microclimate refuge for predatory ladybeetles. Biol. Control 2020, 142, 104162. [Google Scholar] [CrossRef]
- Woltz, J.M.; Landis, D.A. Coccinellid immigration to infested host patches influences suppression of Aphis glycines in soybean. Biol. Control 2013, 64, 330–337. [Google Scholar] [CrossRef]
- Bianchi, F.J.J.A.; Walters, B.J.; Cunningham, S.A.; Hemerik, L.; Schellhorn, N.A. Landscape-scale mass-action of spiders explains early-season immigration rates in crops. Landscape Ecol. 2017, 32, 1257–1267. [Google Scholar] [CrossRef] [Green Version]
- Campbell, A.J.; Wilby, A.; Sutton, P.; Wackers, F. Getting more power from your flowers: Multi-functional flower strips enhance pollinators and pest vontrol agents in apple orchards. Insects 2017, 8, 101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatt, S.; Uytenbroeck, R.; Lopes, T.; Mouchon, P.; Osawa, N.; Piqueray, J.; Monty, A.; Francis, F. Identification of flower functional traits affecting abundance of generalist predators in perennial multiple species wildflower strips. Arthropod-Plant Interact. 2019, 13, 127–137. [Google Scholar] [CrossRef]
- Liang, T.; Yue, W.; Li, Q. Comparison of the phenolic content and antioxidant activities of Apocynum venetum L. (Luo-Bu-Ma) and two of its alternative species. Int. J. Mol. Sci. 2010, 11, 4452–4464. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Li, G.; Zhang, R.; Zheng, J.; Suo, Y.; You, J.; Liu, Y.-J. A validated HPLC-DAD-MS method for identitifying and determining the bioactive components of two kinds of Luobuma. J. Liq. Chromatogr. Relat. Technol. 2011, 34, 537–547. [Google Scholar] [CrossRef]
- Kebede, Y.; Bianchi, F.; Baudron, F.; Abraham, K.; de Valenca, A.; Tittonell, P. Implications of changes in land cover and landscape structure for the biocontrol potential of stemborers in Ethiopia. Biol. Control 2018, 122, 1–10. [Google Scholar] [CrossRef]
- Toivonen, M.; Huusela-Veistola, E.; Herzon, I. Perennial fallow strips support biological pest control in spring cereal in Northern Europe. Biol. Control 2018, 121, 109–118. [Google Scholar] [CrossRef] [Green Version]

| Family | Species | Flowering Phase | Life | Flower | Coverage Proportion | Dominance | ||
|---|---|---|---|---|---|---|---|---|
| Cycle | Structure | 2019 | 2020 | 2021 | Level | |||
| Apocynaceae | Apocynum pictum | Early-May to unknown time | P | O | 11.33% | 9.91% | 6.06% | Ds |
| Asclepiadaceae | Cynanchum sibiricum | Mid-May to unknown time | P | O | 3.01% | 6.56% | 0.23% | |
| Asteraceae | Hexinia polydichotoma | Mid-May to early-August | P | O | 0.73% | <0.01% | <0.01% | |
| Inula salsoloides | Late-June to early-August | P | O | 0.15% | <0.01% | — | ||
| Karelinia caspia | Late-June to unknown time | P | O | 9.98% | 10.36% | 19.01% | Ds | |
| Scorzonera divaricata | Late-June to late-July | P | O | <0.01% | <0.01% | — | ||
| Chenopodiaceae | Haloxylon strobilaceum | Early-August to unknown time | P | O | 0.08% | <0.01% | 3.30% | |
| Halocnemum ammodendron | Early-August to unknown time | P | O | 3.70% | 0.21% | 6.50% | ||
| Kochia prostrata | Late-July to unknown time | P | O | 1.66% | — | — | ||
| Suaeda paradoxa | Late-August to unknown time | P | O | 0.15% | — | — | ||
| Elaeagnaceae | Elaeagnus angustifolia | Mid-May to early-June | P | O | — | 0.29% | — | |
| Gramineae | Phragmites communis | Late-August to unknown time | A | O | 28.27% | 46.23% | 11.76% | Ds |
| Leguminosae | Alhagi sparsifolia | Mid-May to mid-July | P | C | 8.19% | 3.84% | 4.12% | |
| Glycyrrhiza inflata | Mid-June to late-July | P | C | 5.47% | 4.54% | 9.02% | ||
| Halimodendron halodendron | Mid-May to early-June | P | C | <0.01% | 0.25% | 1.17% | ||
| Tamaricaceae | Tamarix ramosissima | Mid-May to unknown time | P | O | 19.10% | 11.81% | 20.38% | Ds |
| Salicaceae | Populus euphratica | Unrecorded | P | O | 6.51% | 3.72% | 9.70% | |
| Solanaceae | Lycium ruthenicum | Mid-June to mid-July | P | O | 1.65% | 2.27% | 8.74% | |
| Year | Period | Factors | df1 | df2 | F | P |
|---|---|---|---|---|---|---|
| 2019 | June–July | Plant species | 11 | 78 | 2.76 | 0.005 |
| August | 10 | 66 | 20.35 | ˂0.001 | ||
| 2020 | May | Plant species | 6 | 42 | 6.86 | ˂0.001 |
| June–July | 10 | 53 | 4.41 | ˂0.001 | ||
| 2021 | May | Plant species | 9 | 26 | 2.66 | 0.025 |
| June–July | 9 | 56 | 3.37 | 0.002 | ||
| August | 9 | 30 | 3.42 | 0.005 |
| Year | Period | Factors | Df1 | Df2 | F | P | Radj |
|---|---|---|---|---|---|---|---|
| 2019 | June–July | Plant species | 13 | 408 | 2.10 | ˂0.001 | 0.03 |
| Herbivore abundance | 1 | 421 | 0.85 | 0.469 | |||
| Flowering status | 1 | 421 | 0.36 | 0.865 | |||
| August | Plant species | 13 | 193 | 3.33 | ˂0.001 | 0.11 | |
| Herbivore abundance | 1 | 206 | 0.84 | 0.511 | |||
| Flowering status | 1 | 206 | 0.15 | 0.978 | |||
| 2020 | May | Plant species | 10 | 128 | 4.40 | ˂0.001 | 0.29 |
| Herbivore abundance | 1 | 137 | 29.11 | ˂0.001 | |||
| Flowering status | 1 | 137 | 0.28 | 0.756 | |||
| June–July | Plant species | 10 | 300 | 5.38 | ˂0.001 | 0.20 | |
| Herbivore abundance | 1 | 205 | 63.10 | ˂0.001 | |||
| Flowering status | 1 | 205 | 1.53 | 0.192 | |||
| 2021 | May | Plant species | 9 | 49 | 2.83 | ˂0.001 | 0.31 |
| Herbivore abundance | 1 | 57 | 7.76 | 0.005 | |||
| Flowering status | 1 | 57 | 3.39 | 0.023 | |||
| June–July | Plant species | 10 | 307 | 23.88 | ˂0.001 | 0.44 | |
| Herbivore abundance | 1 | 310 | 38.70 | ˂0.001 | |||
| Flowering status | 1 | 310 | 2.75 | 0.074 | |||
| August | Plant species | 9 | 125 | 3.52 | ˂0.001 | 0.19 | |
| Herbivore abundance | 1 | 133 | 7.43 | ˂0.001 | |||
| Flowering status | 1 | 133 | 4.43 | 0.015 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Liu, B.; Li, Q.; Sun, M.; Li, M.; Wyckhuys, K.A.G.; Wang, P.; Lu, Y. Perennial Flowering Plants Sustain Natural Enemy Populations in Gobi Desert Oases of Southern Xinjiang, China. Insects 2022, 13, 399. https://doi.org/10.3390/insects13050399
Liu Y, Liu B, Li Q, Sun M, Li M, Wyckhuys KAG, Wang P, Lu Y. Perennial Flowering Plants Sustain Natural Enemy Populations in Gobi Desert Oases of Southern Xinjiang, China. Insects. 2022; 13(5):399. https://doi.org/10.3390/insects13050399
Chicago/Turabian StyleLiu, Yangtian, Bing Liu, Qian Li, Mengxiao Sun, Minlong Li, Kris A. G. Wyckhuys, Peiling Wang, and Yanhui Lu. 2022. "Perennial Flowering Plants Sustain Natural Enemy Populations in Gobi Desert Oases of Southern Xinjiang, China" Insects 13, no. 5: 399. https://doi.org/10.3390/insects13050399
APA StyleLiu, Y., Liu, B., Li, Q., Sun, M., Li, M., Wyckhuys, K. A. G., Wang, P., & Lu, Y. (2022). Perennial Flowering Plants Sustain Natural Enemy Populations in Gobi Desert Oases of Southern Xinjiang, China. Insects, 13(5), 399. https://doi.org/10.3390/insects13050399







