Characterization of Two Aldehyde Oxidases from the Greater Wax Moth, Galleria mellonella Linnaeus. (Lepidoptera: Pyralidae) with Potential Role as Odorant-Degrading Enzymes
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing
2.2. Collection of Honeycomb Volatiles
2.3. Honeycomb Volatile Identification by GC/MS
2.4. Identification of AOX Transcripts by Comparing Two Transcriptome Data of G. mellonella
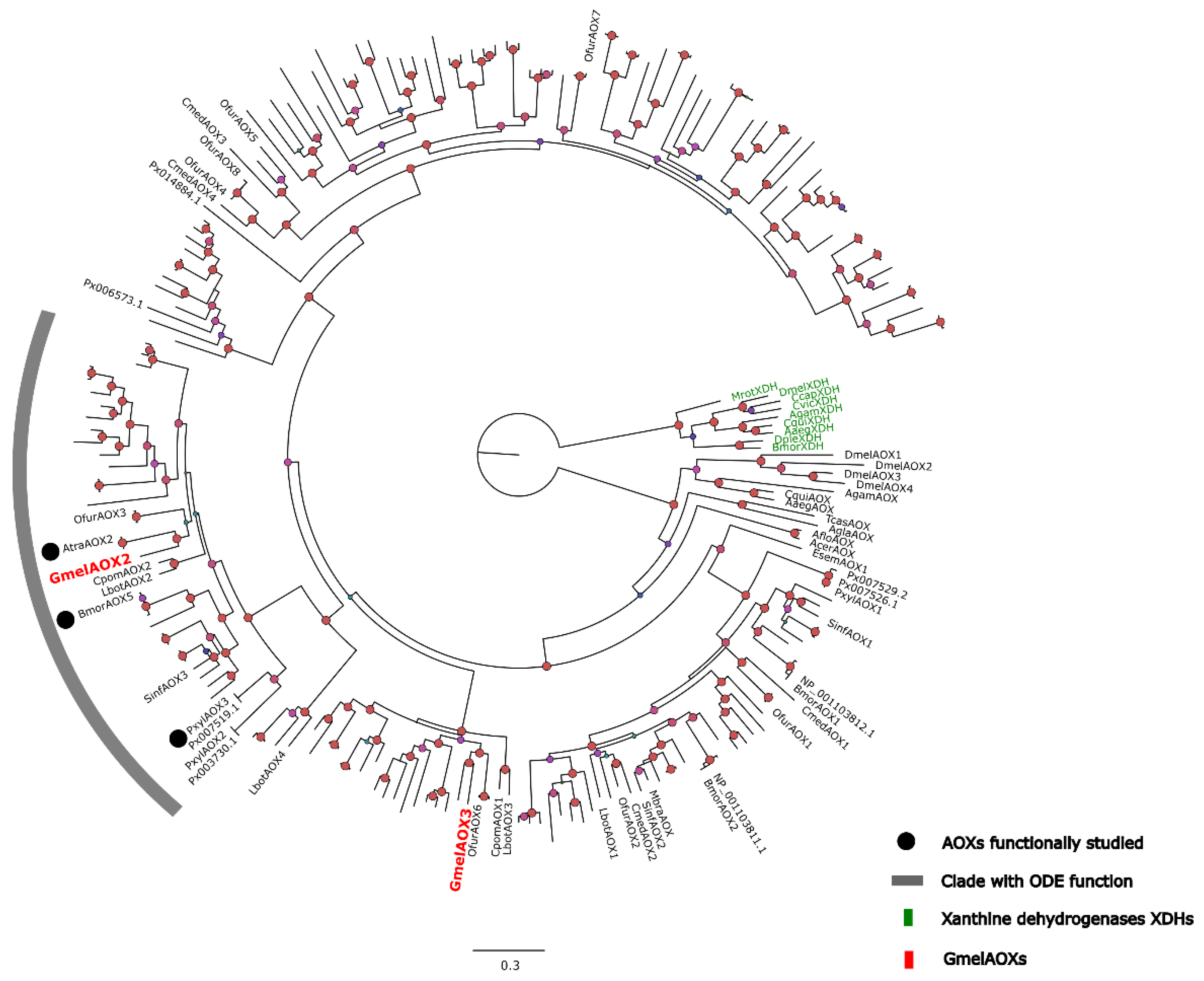
2.5. Phylogenetic Analysis of G. mellonella AOXs
2.6. Total RNA Extraction, cDNA Synthesis, and Primer Design
2.7. Analysis of Tissue Distribution by Semi-Quantitative RT-PCR
2.8. Analysis of GmelAOXs Relative Expression by qRT-PCR
2.9. AOX Activity from Antennal Extract
3. Results
3.1. Volatile Organic Compounds in Honeycombs
3.2. AOX-Related Transcripts Obtained by Comparing Two Transcriptomes
3.3. Tissue Distribution and Relative Expression Levels of GmelAOX2 and GmelAOX3
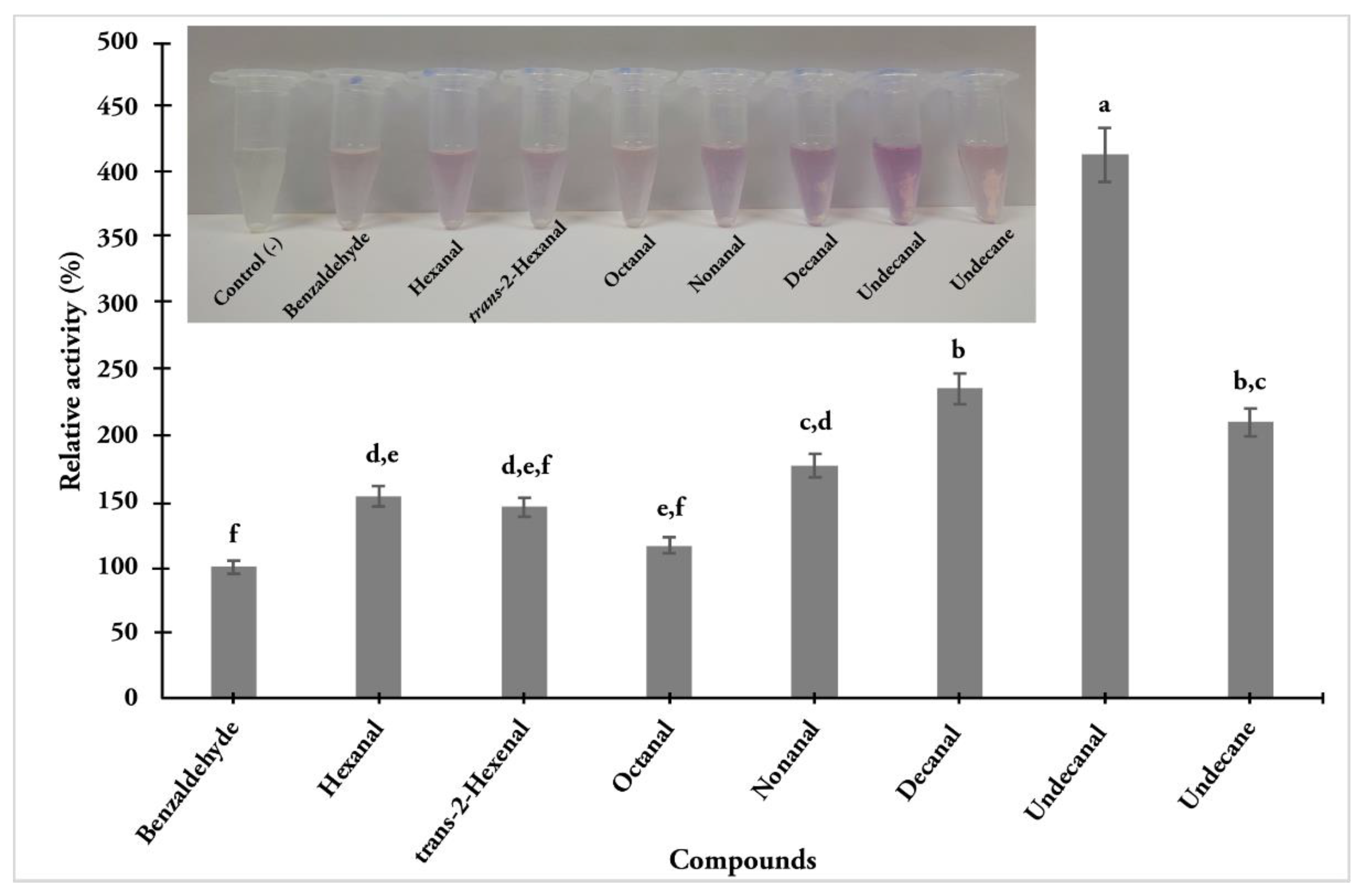
3.4. AOXs Activity from Antennal Extract
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sato, K.; Touhara, K. Insect Olfaction: Receptors, Signal Transduction, and Behavior. In Results and Problems in Cell Differentiation; Korsching, S., Meyerhof, W., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 203–220. [Google Scholar] [CrossRef]
- Zhou, J.J. Odorant-Binding Proteins in Insects. In Vitamins and Hormones; Litwack, G., Ed.; Elsevier: London, UK, 2010; pp. 241–272. [Google Scholar] [CrossRef]
- Kaissling, K.E. Kinetics of olfactory responses might largely depend on the odorant-receptor interaction and the odorant deactivation postulated for flux detectors. J. Comp. Physiol. 2013, 199, 879–896. [Google Scholar] [CrossRef] [PubMed]
- Leal, W. Odorant Reception in Insects: Roles of receptors, binding proteins, and degrading enzymes. Annu. Rev. Entomol. 2013, 58, 373–391. [Google Scholar] [CrossRef] [PubMed]
- Pelosi, P.; Iovinella, I.; Zhu, J.; Wang, G.; Dani, F. Beyond chemoreception: Diverse tasks of soluble olfactory proteins in insects. Biol. Rev. 2017, 93, 184–200. [Google Scholar] [CrossRef]
- Chertemps, T.; Maïbèche, M. Odor Degrading Enzymes and Signal Termination. In Insect Pheromone Biochemistry and Molecular Biology; Blomquist, G.J., Vogt, R.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 619–644. [Google Scholar]
- Ishida, Y.; Leal, W.S. Rapid inactivation of a moth pheromone. Proc. Natl. Acad. Sci. USA 2005, 102, 14075–14079. [Google Scholar] [CrossRef] [PubMed]
- Chertemps, T.; Younus, F.; Steiner, C.; Durand, N.; Coppin, C.W.; Pandey, G.; Oakeshott, J.G.; Maïbèche, M. An antennal carboxylesterase from Drosophila melanogaster, esterase 6, is a candidate odorant-degrading enzyme toward food odorant. Front. Physiol. 2015, 6, 315. [Google Scholar] [CrossRef]
- Vogt, R.; Riddiford, L.M. Pheromone binding and inactivation by moth antennae. Nature 1981, 293, 161–163. [Google Scholar] [CrossRef]
- Vogt, R.G.; Riddiford, L.M.; Prestwich, G.D. Kinetic properties of a sex pheromone-degrading enzyme: The sensillar esterase of Antheraea polyphemus. Proc. Natl. Acad. Sci. USA 1985, 82, 8827–8831. [Google Scholar] [CrossRef]
- Ishida, Y.; Leal, W.S. Chiral discrimination of the Japanese beetle sex pheromone and a behavioral antagonist by a pheromone-degrading enzyme. Proc. Natl. Acad. Sci. USA 2008, 105, 9076–9080. [Google Scholar] [CrossRef]
- Rybczynski, R.; Reagan, J.; Lerner, M. A pheromone-degrading aldehyde oxidase in the antennae of the moth Manduca sexta. J. Neurosci. 1989, 9, 1341–1353. [Google Scholar] [CrossRef] [PubMed]
- Merlin, C.; François, M.C.; Bozzolan, F.; Pelletier, J.; Jacquin-Joly, E.; Maïbèche-Coisne, C. A new aldehyde oxidase selectively expressed in chemosensory organs of insects. Biochem. Biophys. Res. Commun. 2005, 332, 4–10. [Google Scholar] [CrossRef]
- Guerrero, A.; Rosell, G. Biorational approaches for insect control by enzymatic inhibition. Curr. Med. Chem. 2005, 12, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Ando, T.; Taguchi, K.; Uchiyama, M.; Ujiye, T.; Kuroko, H. (7Z,11Z)-7,11-Hexadecadienal: Sex Attractant of the Citrus Leafminer Moth, Phyllocnistis citrella Stainton (Lepidoptera, Phyllocnistidae). Agric. Biol. Chem. 1985, 49, 3633–3635. [Google Scholar] [CrossRef][Green Version]
- Gries, G.; Li, J.; Gries, R.; Bowers, W.; West, R.; Wimalaratne, P.; Khaskin, G.; Skip-King, G.G.; Slessor, K.N. (E)-11,13-Tetradecadienal: Major sex pheromone component of the eastern blackheaded budworm, Acleris variana (Fem.) (Lepidoptera: Tortricidae). J. Chem. Ecol. 1994, 20, 1–8. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gago, R.; Allison, J.D.; McElfresh, J.S.; Haynes, K.F.; McKenney, J.; Guerrero, A.; Millar, J.G. A tetraene aldehyde as the major sex pheromone component of the promethea moth (Callosamia promethea (Drury)). J. Chem. Ecol. 2013, 39, 1263–1272. [Google Scholar] [CrossRef] [PubMed]
- Kochansky, J.; Tette, J.; Taschenberg, E.F.; Cardé, R.T.; Kaissling, K.E.; Röelofs, W.L. Sex pheromone of the Moth Antheraea polyphemus. J. Insect Physiol. 1975, 21, 1977–1983. [Google Scholar] [CrossRef]
- Ebbinghaus, D.; Lösel, P.M.; Lindemann, M.; Scherkenbeck, J.; Zebitz, C.P.W. Detection of major and minor sex pheromone components by the male Codling Moth Cydia pomonella (Lepidoptera: Tortricidae). J. Insect Physiol. 1997, 44, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Downham, M.C.A.; Hall, D.R.; Chamberlain, D.J.; Cork, A.; Farman, D.I.; Tamò, M.; Dahounto, D.; Datinon, B.; Adetonah, S. Minor components in the sex pheromone of the legume podborer: Maruca vitrata. Development of an attractive blend. J. Chem. Ecol. 2003, 29, 989–1011. [Google Scholar] [CrossRef]
- Kaissling, K.E.; Kasang, G. A new pheromone of the silkworm moth Bombyx mori. Sensory pathway and behavioral effect. Naturwissenschaften 1978, 65, 382–384. [Google Scholar] [CrossRef]
- Rybczynski, R.; Vogt, R.G.; Lerner, M.R. Antennal-specific pheromone-degrading aldehyde oxidases from the Moths Antheraea polyphermus and Bombyx mori. J. Biol. Chem. 1990, 265, 19712–19715. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Wang, W.L.; Li, M.Y.; Li, S.G.; Liu, L.S. Identification of putative carboxylesterase and aldehyde oxidase genes from the antennae of the rice leaffolder, Cnaphalocrosis medinalis (Lepidoptera: Pyralidae). J. Asia-Pac. Entomol. 2017, 20, 907–913. [Google Scholar] [CrossRef]
- Xu, W.; Liao, Y. Identification and characterization of aldehyde oxidases (AOXs) in the cotton bollworm. Sci. Nat. 2017, 104, 94. [Google Scholar] [CrossRef] [PubMed]
- Kwadha, C.; Ong´amo, G.; Ndegwa, P.; Raina, S.; Fombong, A. The biology and control of the greater wax Moth, Galleria mellonella. Insects 2017, 8, 61. [Google Scholar] [CrossRef]
- Williams, J.L. Insects: Lepidoptera (Moths). In Honey Bee Pests, Predators, and Diseases; Morse, R., Flottum, K., Eds.; AI Root Company: Medina, OH, USA, 1997; pp. 121–141. [Google Scholar]
- Leyrer, R.; Monroe, R. Isolation and identification of the scent of the moth, Galleria mellonella, and a revaluation of its sex pheromone. J. Insect Physiol. 1973, 19, 2267–2271. [Google Scholar] [CrossRef]
- Butenandt, A.; Beckman, R.; Stamm, D.; Hecker, E. Über den sexuallockstoff des seidenspinners Bombyx mori. Reindarstellung und konstitution. Z. Naturforsch. B 1959, 14, 283–284. [Google Scholar] [CrossRef]
- Choo, Y.M.; Pelletier, J.; Atungulu, E.; Leal, W.S. Identification and characterization of an antennae-specific aldehyde oxidase from the Navel Orangeworm. PLoS ONE 2013, 8, e67794. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Xia, Y.H.; Zhu, J.Y.; Li, S.Y.; Dong, S.L. Putative Pathway of Sex Pheromone Biosynthesis and Degradation by Expression Patterns of genes Identified from Female Pheromone Gland and Adult Antenna of Sesamia inferens (Walker). J. Chem. Ecol. 2014, 40, 439–451. [Google Scholar] [CrossRef]
- Foster, S.; Anderson, K. Production and distribution of aldehyde and alcohol sex pheromone components in the pheromone gland of females of the Moth Chloridea virescens. J. Chem. Ecol. 2018, 47, 9–17. [Google Scholar] [CrossRef]
- Hao, C.Y.; Fan, R.; Qin, X.W.; Hu, L.S.; Tan, L.H.; Xu, F.; Wu, B.W. Characterization of volatile compounds in ten Piper species cultivated in Hainan Island, South China. Int. J. Food Prop. 2018, 21, 633–644. [Google Scholar] [CrossRef]
- González-Mas, M.C.; Rambla, J.L.; López-Gresa, M.P.; Blázquez, M.A.; Granell, A. Volatile compounds in Citrus essential oils: A comprehensive review. Front. Plant Sci. 2019, 10, 12. [Google Scholar] [CrossRef]
- Giuffrè, A.M.; Capocasale, M.; Macrì, R.; Caracciolo, M.; Zappia, C.; Poiana, M. Volatile profiles of extra virgin olive oil, olive pomace oil, soybean oil and palm oil in different heating conditions. LWT 2020, 117, 108631. [Google Scholar] [CrossRef]
- Castro-Vázquez, L.; Díaz-Maroto, M.C.; Pérez-Coello, M.S. Aroma composition and new chemical markers of Spanish citrus honeys. Food Chem. 2007, 103, 601–606. [Google Scholar] [CrossRef]
- Lima-Neto, J.; Lopes, J.A.; Moita-Neto, J.M.; Lima, S.G.; Luz, C.F.; Citó, A.M. Volatile compounds and palynological analysis from pollen pots of stingless bees from the mid-north region of Brazil. Braz. J. Pharm. Sci. 2017, 53, 1–9. [Google Scholar] [CrossRef]
- Pattamayutanon, P.; Angeli, S.; Thakeow, P.; Abraham, J.; Disayathanoowat, T.; Chantawannakul, P. Volatile organic compounds of Thai honeys produced from several floral sources by different honey bee species. PLoS ONE 2017, 12, e0172099. [Google Scholar] [CrossRef]
- Abd El-Wahed, A.A.; Khalifa, S.A.M.; Sheikh, B.Y.; Farag, M.A.; Saeed, A.; Larik, F.A.; Koca-Caliskan, U.; AlAjmi, M.F.; Hassan, M.; Wahabi, H.A.; et al. Bee Venom Composition: From Chemistry to Biological Activity. In Studies in Natural Products Chemistry; Rahman, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 459–484. [Google Scholar]
- Ritter, W.; Akratanakul, P. Honey Bee Diseases and Pests: A Practical Guide; FAO, Ed.; FAO: Rome, Italy, 2006; pp. 1–42. Available online: https://www.fao.org/3/a0849e/A0849E.pdf (accessed on 16 July 2022).
- Zhao, H.X.; Xiao, W.Y.; Ji, C.H.; Ren, Q.; Xia, X.S.; Zhang, X.F.; Huang, W.Z. Candidate chemosensory genes identified from the grater wax moth, Galleria mellonella, through a transcriptomic analysis. Sci. Rep. 2019, 9, 10032. [Google Scholar] [CrossRef] [PubMed]
- Lizana, P.; Machuca, J.; Larama, G.; Quiroz, A.; Mutis, A.; Venthur, H. Mating-based regulation and ligand binding of an odorant-binding protein support the inverse sexual communication of the greater wax moth, Galleria mellonella (Lepidoptera: Pyralidae). Insect Mol. Biol. 2020, 29, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Zamorano, D. Utilización de un extracto alcohólico de Neem (Azadirachta indica A. Juss.) para el control de Galleria mellonella L. (Lepidoptera: Pyralidae). Ph.D. Thesis, Universidad Austral de Chile, Facultad de Ciencias Agrarias, Escuela de Agronomía, Valdivia, Chile, 2011. Available online: http://cybertesis.uach.cl/tesis/uach/2011/fac764u/doc/fac764u.pdf (accessed on 20 July 2022).
- Vernon, R.S.; Borden, J.H.; Pierce, H.D.; Oehlschlager, A.C. Host Selection by Hylemya antiqua. Laboratory bioassay and methods of obtaining host volatiles. J. Chem. Ecol. 1977, 3, 359–368. [Google Scholar] [CrossRef]
- Agelopoulos, N.G.; Hooper, A.M.; Maniar, S.P.; Pickett, J.A.; Wadhams, L.J. A novel approach for isolation of volatile chemicals released by individual leaves of a plant in situ. J. Chem. Ecol. 1999, 25, 1411–1425. [Google Scholar] [CrossRef]
- Parra, L.; Mutis, A.; Ceballos, R.; Lizama, M.; Pardo, F.; Perich, F.; Quiroz, A. Volatiles released from Vaccinium corymbosum were attractive to Aegorhinus superciliosus (Coleoptera: Curculionidae) in an olfatometric bioassay. Environ. Entomol. 2009, 38, 781–789. [Google Scholar] [CrossRef]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.K. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef]
- Anderson, I.; Brass, A. Searching DNA databases for similarities to DNA sequences: When is a match significant? Bioinformatics 1998, 14, 349–356. [Google Scholar] [CrossRef]
- Godoy, R.; Mutis, A.; Carabajal-Paladino, L.; Venthur, H. Genome-Wide identification of aldehyde oxidase genes in moths and butterflies suggests new insights into their function as odorant-degrading enzymes. Front. Ecol. Evol. 2022, 10, 823119. [Google Scholar] [CrossRef]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef]
- Sela, I.; Ashkenazy, H.; Katoh, K.; Pupko, T. GUIDANCE2: Accurate detection of unreliable alignment regions accounting for the uncertainty of multiple parameters. Nucleic Acids Res. 2015, 43, W7–W14. [Google Scholar] [CrossRef]
- Price, M.; Dehal, P.; Arkin, A. FastTree 2—Approximately maximum- likelihood trees for large alignments. PLoS ONE 2010, 5, e9490. [Google Scholar] [CrossRef]
- Gu, S.H.; Zhou, J.J.; Gao, S.; Wang, D.H.; Li, X.C.; Guo, Y.Y.; Zhang, Y.J. Identification and comparative expression analysis of odorant binding protein genes in the tobacco cutworm Spodoptera litura. Sci. Rep. 2015, 5, 13800. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, 16–21. [Google Scholar] [CrossRef]
- Wang, M.M.; He, M.; Wang, H.; Ma, Y.F.; Dewer, Y.; Zhang, F.; He, P. A candidate aldehyde oxidase in the antennae of the diamondback moth, Plutella xylostella (L.), is potentially involved in the degradation of pheromones, plant-derived volatiles and the detoxification of xenobiotics. Pestic. Biochem. Phys. 2021, 171, 104726. [Google Scholar] [CrossRef]
- Ferber, C.E.; Nursten, H.E. The aroma of beeswax. J. Sci. Food Agric. 1977, 28, 511–518. [Google Scholar] [CrossRef]
- Radovic, B.S.; Careri, M.; Mangia, A.; Musci, M.; Gerboles, M.; Anklam, E. Contribution of dynamic headspace GC-MS analysis of aroma compounds to authenticity testing of honey. Food Chem. 2001, 72, 511–520. [Google Scholar] [CrossRef]
- Torto, B.; Suazo, A.; Alborn, H.; Tumlinson, J.; Teal, P. Response of the small beetle (Aethina tumida) to a blend of chemicals identified from honeybee (Apis mellifera) volatiles. Apidologie 2005, 36, 523–532. [Google Scholar] [CrossRef]
- Zehentbauer, G.; Reineccius, G.A. Determination of key aroma components of Cheddar cheese using dynamic headspace dilution assay. Flavour Fragr. J. 2002, 17, 300–305. [Google Scholar] [CrossRef]
- Schmitt, T.; Herzner, G.; Weckerle, B.; Schreier, P.; Strohm, E. Volatiles of foraging honeybees Apis mellifera (Hymenoptera: Apidae) and their potential role as semiochemicals. Apidologie 2007, 38, 164–170. [Google Scholar] [CrossRef]
- Mohtar, L.; Rodríguez, S.; Nazareno, M. Comparative analysis of volatile compounds profiles of propolis from different provenances. J. Sci. Food Agric. 2017, 98, 3409–3415. [Google Scholar] [CrossRef] [PubMed]
- Marques, F.; McElfresh, J.S.; Millar, J. Kováts Retention Indexes of Monounsaturated C12, C14, and C16 Alcohols, Acetates and Aldehydes Commonly Found in Lepidopteran Pheromone Blends. J. Braz. Chem. Soc. 2000, 11, 592–599. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured Publishing: Carol Stream, IL, USA, 2007; ISBN 1932633219. [Google Scholar]
- Flint, H.; Merkle, J. Mating behavior, sex pheromone responses, and radiation sterilization of the greater wax moth (Lepidoptera: Pyralidae). J. Econ. Entomol. 1983, 76, 467–472. [Google Scholar] [CrossRef]
- Chisholm, M.; Jell, J.; Cass, D. Characterization of the major odorants found in the peel oil of Citrus reticulata Blanco cv. Clementine using gas chromatography-olfactometry. Flavour Fragr. J. 2003, 18, 275–281. [Google Scholar] [CrossRef]
- Boo, K.S. Variation in sex pheromone composition of a few selected lepidopteran species. J. Asia-Pac. Entomol. 1998, 1, 17–23. [Google Scholar] [CrossRef]
- El-Sayed, A.M.; Gibb, A.R.; Mitchell, V.J.; Manning, L.A.; Revell, J.; Thistleton, B.; Suckling, D.M. Identification of the sex pheromone of Conogethes pluto: A pest of Alpinia. Chemoecology 2013, 23, 93–101. [Google Scholar] [CrossRef]
- Nielsen, R.A.; Brister, D. The greater wax moth: Adult behavior. Ann. Entomol. Soc. Am. 1977, 70, 101–103. [Google Scholar] [CrossRef]
- Durand, N.; Carot-Sans, G.; Bozzolan, F.; Rosell, G.; Siaussat, D.; Debernand, S.; Chertemps, T.; Maïbèche-Coisne, M. Degradation of pheromone and plant volatile components by a same Odorant-degrading enzyme in the Cotton Leafworm, Spodoptera littoralis. PLoS ONE 2011, 6, e29147. [Google Scholar] [CrossRef]
- He, P.; Zhang, Y.N.; Yang, K.; Li, Z.Q.; Dong, S.L. An antenna-biased carboxylesterase is specifically active to plant volatiles in Spodoptera exigua. Pestic. Biochem. Phys. 2015, 123, 93–100. [Google Scholar] [CrossRef]
- Huang, X.; Liu, L.; Su, X.; Feng, J. Identification of biotransformation enzymes in the antennae of codling moth Cydia pomonella. Gene 2016, 580, 73–79. [Google Scholar] [CrossRef]
- Kurosaki, M.; Bolis, M.; Fratelli, M.; Barzago, M.M.; Pattini, L.; Perretta, G.; Terao, M.; Garattini, E. Structure and evolution of vertebrate aldehyde oxidases: From gene duplication to gene suppression. Cell. Mol. Life Sci. 2013, 70, 1807–1830. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Y.; Shen, G.; Mao, X.; Jiao, M.; Lin, Y. Identification and characterization of aldehyde oxidase 5 in the pheromone gland of the Silkworm (Lepidoptera: Bombycidae). J. Insect Sci. 2020, 20, 31. [Google Scholar] [CrossRef]
- Coleman, M.; Vontas, J.G.; Hemingway, J. Molecular characterization of the amplified aldehyde oxidase from insecticide resistance Culex quinquefasciatus. Eur. J. Biochem. 2002, 269, 768–779. [Google Scholar] [CrossRef]
- Ando, T.; Inomata, S.I.; Yamamoto, M. Lepidopteran Sex Pheromones. In The Chemistry of Pheromones and Other Semiochemicals I; Schulz, S., Ed.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 51–96. [Google Scholar]
- Newman, J.; Ghaemmaghami, S.; Ihmels, J.; Breslow, D.; Noble, M.; DeRisi, J.; Weissman, J.S. Single-cell proteomic analysis of S. cerevisiae reveals the architecture of biological noise. Nature 2006, 441, 840–846. [Google Scholar] [CrossRef]
- Legal, L.; Moulin, B.; Jallon, J.M. The Relation between Structures and Toxicity of Oxygenated Aliphatic Compounds Homologous to the Insecticide Octanoic Acid and the Chemotaxis of Two Species of Drosophila. Pestic. Biochem. Physiol. 1999, 65, 90–101. [Google Scholar] [CrossRef]
- Tian, Y.; Fan, H.; Wang, Y.; Zheng, Y.; Hu, D.; Du, S. Insecticidal and Repellent Activities of Volatile Constituents from Litsea dilleniifolia P. Y. Pai et P. H. Huang Against Stored-Product Insects. Rec. Nat. Prod. 2022, 16, 398–403. [Google Scholar] [CrossRef]
- Flores-Macías, A.; Flores-Sánchez, M.A.; León-Herrera, L.R.; Mondragón-Olguín, V.M.; Zavala-Gómez, C.E.; Tapia-Pérez, A.D.; Campos-Guillén, J.; Amaro-Reyes, A.; Sandoval-Cárdenas, D.; Romero-Gómez, S.; et al. Activity of chloroformic extract from Salvia connivens (Lamiales: Lamiaceae) and its principal compounds against Spodoptera frugiperda (Lepidoptera: Noctuidae). Appl. Sci. 2021, 11, 11813. [Google Scholar] [CrossRef]
- Omolo, M.O.; Ndiege, I.O.; Hassanali, A. Semiochemical signatures associated with differential attraction of Anopheles gambiae to human feet. PLoS ONE 2021, 16, e0260149. [Google Scholar] [CrossRef]
- Cui, K.; He, L.; Cui, G.; Zhang, T.; Chen, Y.; Zhang, T.; Mu, W.; Liu, F. Biological activity of trans-2-Hexanal against the storage insect pest Tribolium castaneum (Coleoptera: Tenebrionidae) and mycotoxigenic storange fungi. J. Econ. Entomol. 2021, 114, 979–987. [Google Scholar] [CrossRef]
- Wojtasek, H.; Leal, W.S. Degradation of an alkaloid pheromone from the pale-brown chafer, Phyllopertha diversa (Coleoptera: Scarabaeidae), by an insect olfactory cytochrome P450. FEBS Lett. 1999, 458, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.G.; Wen, Z. Cytochromes P450 of insects: The tip of the iceberg. Pest Manag. Sci. 2001, 57, 958–967. [Google Scholar] [CrossRef] [PubMed]
- Pino, J.; Marbot, R.; Delgado, A.; Zumárraga, C.; Sauri, E. Volatile constituents of propolis from honey bees and stingless bees from Yucatán. J. Essent. Oil Res. 2006, 18, 53–56. [Google Scholar] [CrossRef]
- Choi, H.S. Character impact odorants of Citrus hallabong [(C. unshiu Marcov x C. sinensis Osbeck) x C. reticulata Blanco] Cold-Pressed Peel Oil. J. Agric. Food. Chem. 2003, 51, 2687–2692. [Google Scholar] [CrossRef] [PubMed]
- Panseri, S.; Manzo, A.; Chiesa, L.; Giorgi, A. Melissopalynological and volatile compounds analysis of buckwheat honey from different geographical origins and their role in botanical determination. J. Chem. 2013, 904202, 1–11. [Google Scholar] [CrossRef]
- Adams, R.P. The leaf essential oils and chemotaxonomy of Juniperus sect. Juniperus. Biochem. System. Ecol. 1998, 26, 637–645. [Google Scholar] [CrossRef]
- Karioti, A.; Skaltsa, H.; Demetzos, C.; Perdetzoglou, D.; Economakis, C.D.; Salem, A.B. Effect of nitrogen concentration of the nutrient solution on the volatile constituents of leaves of Salvia fruticosa Mill. in solution culture. J. Agric. Food Chem. 2003, 51, 6505–6508. [Google Scholar] [CrossRef]
- Kaskoniene, V.; Venskutonis, P. Floral markers in honey of various botanical and geographic origins: A review. Compr. Rev. Food Sci. Food Saf. 2010, 9, 620–634. [Google Scholar] [CrossRef]
- Hamm, S.; Bleton, J.; Connan, J.; Tchapla, A. A chemical investigation by headspace SPME and GC-MS of volatile and semi-volatile terpenes in various olibanum samples. Phytochemistry 2005, 66, 1499–1514. [Google Scholar] [CrossRef]
- Skaltsa, H.D.; Demetzos, C.; Lazari, D.; Sokovic, M. Essential oil analysis and antimicrobial activity of eight Stachys species from Greece. Phytochemistry 2003, 64, 743–752. [Google Scholar] [CrossRef] [PubMed]
- De Kraker, J.W.; Schurink, M.; Franssen, M.C.; König, W.A.; De Groot, A.; Bouwmeester, H.J. Hydroxylation of sesquiterpenes by enzymes from chicory (Cichorium intybus L.) roots. Tetrahedron 2003, 59, 409–418. [Google Scholar] [CrossRef]
- Tellez, M.R.; Canel, C.; Rimando, A.M.; Duke, S.O. Differential accumulation of isoprenoids in glanded and glandless Artemisia annua L. Phytochemistry 1999, 52, 1035–1040. [Google Scholar] [CrossRef]
- Apel, M.A.; Sobral, M.; Schapoval, E.E.; Henriques, A.T.; Menut, C.; Bessiere, J.M. Essential oil composition of Eugenia florida and Eugenia mansoi. J. Essent. Oil Res. 2004, 16, 321–322. [Google Scholar] [CrossRef]
- Zahn, D.K.; Moreira, J.A.; Millar, J.G. Identification, synthesis, and bioassay of a male-specific aggregation pheromone from the harlequin bug, Murgantia histrionica. J. Chem. Ecol. 2008, 34, 238–251. [Google Scholar] [CrossRef]
- Jordán, M.J.; Margaria, C.A.; Shaw, P.E.; Goodner, K.L. Volatile components and aroma active compounds in aqueous essence and fresh pink Guava fruit puree (Psidium guajava L.) by GC-MS and multidimensional GC/GC-O. J. Agric. Food Chem. 2003, 51, 1421–1426. [Google Scholar] [CrossRef]
- Sotomayor, J.A.; Martinez, R.M.; Garcia, A.J.; Jordan, M.J. Thymus zygis subsp. Gracilis: Watering level effect on phytomass production and essential oil quality. J. Agric. Food Chem. 2004, 52, 5418–5424. [Google Scholar] [CrossRef]
- Tepe, B.; Donmez, E.; Unlu, M.; Candan, F.; Daferera, D.; Vardar-Unlu, G.; Polissiou, M.; Sokmen, A. Antimicrobial and antioxidative activities of the essential oils and methanol extracts of Salvia cryptantha (Montbret et Aucher ex Benth.) and Salvia multicaulis (Vahl). Food Chem. 2004, 84, 519–525. [Google Scholar] [CrossRef]
- Adams, R.P.; Dev, V. Synthesis and GC-MS analysis of angelates and tiglates as an aid to identification of these components in essential oils. Flavour Fragr. J. 2010, 25, 71–74. [Google Scholar] [CrossRef]
- Ali, N.A.; Wurster, M.; Arnold, N.; Teicher, A.; Schmidt, J.; Lindequist, U.; Wessjohann, L. Chemical composition and biological activity of essential oils from the Oleogum resins of three endemic soqotraen Boswellia species. Rec. Nat. Prod. 2008, 2, 6–12. [Google Scholar]
- Demyttenaere, J.C.; Sánchez-Martínez, J.I.; Verhé, R.; Sandra, P.; De Kimpe, N. Analysis of volatiles of malt whisky by solid-phase microextraction and stir bar sorptive extraction. J. Chromatogr. A. 2003, 985, 221–232. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, A.M.; Heppelthwaite, V.J.; Manning, L.M.; Gibb, A.R.; Suckling, D.M. Volatile constituents of fermented sugar baits and their attraction to lepidopteran species. J. Agric. Food Chem. 2005, 53, 953–958. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, Y.; Zhao, X.; Zhu, B.; Nan, P.; Zhao, J.; Wang, L.; Chen, F.; Liu, Z.; Zhong, Y. Chemical variation in the essential oil of Ephedra sinica from Northeastern China. Food Chem. 2006, 98, 52–58. [Google Scholar] [CrossRef]
- McDaniel, C.A.; Schmidt, J.O.; Howard, R.W. Mandibular gland secretions of the male beewolves Philanthus crabroniformis, P. barbatus, and P. pulcher (Hymenoptera: Sphecidae). J. Chem. Ecol. 1992, 18, 27–37. [Google Scholar] [CrossRef]
- De Simon, B.F.; Estruelas, E.; Munoz, A.M.; Cadahia, E.; Sanz, M. Volatile compounds in acacia, chestnut, cherry, ash, and oak woods, with a view to their use in cooperage. J. Agri. Food Chem. 2009, 57, 3217–3227. [Google Scholar] [CrossRef]
- Witte, L.; Rubiolo, P.; Bicchi, C.; Hartmann, T. Comparative analysis of pyrrolizidine alkaloids from natural sources by gas chromatography-mass spectrometry. Phytochemistry 1993, 32, 187–196. [Google Scholar] [CrossRef]
- DeGrandi-Hoffman, G.; Chambers, M.; Hooper, J.; Schneider, S. Description of an intermorph between a worker and queen in african honey bees Apis mellifera scutellata (Hymenoptera: Apidae). Ann. Entomol. Soc. Am. 2004, 97, 1299–1305. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, J.; Xu, Y.; Duan, H.; Fan, W.; Zhao, G. Extraction, preparation and identification of volatile compounds in Changyu XO Brandy. Chin. J. Chromatogr. 2008, 26, 212–222. [Google Scholar] [CrossRef]
- Morteza-Semnani, K.; Akbarzadeh, M.; Moshiri, K. The essential oil composition of Eupatorium cannabinum L. from Iran. Flavour Fragr. J. 2006, 21, 521–523. [Google Scholar] [CrossRef]
- Gómez, E.; Ledbetter, C.A.; Hartsell, P.L. Volatile compounds in apricot, plum, and their interspecific hybrids. J. Agric. Food Chem. 1993, 41, 1669–1676. [Google Scholar] [CrossRef]
- Suwannapong, G.; Benbow, M.; Chinokul, C.; Seanbualuang, P.; Sivaram, V. Bioassay of the mandibular gland pheromones of Apis florea on the foraging activity of dwarf honey bees. J. Apic. Res. 2011, 50, 212–217. [Google Scholar] [CrossRef]
- Leffingwell, J.C.; Alford, E.D. Volatile constituents of perique tobacco. Elec. J. Env. Agricult. Food Chem. 2005, 4, 899–915. Available online: http://www.leffingwell.com/download/Volatile%20Constituents%20of%20Perique%20Tobacco4.pdf (accessed on 1 September 2022).
- Rostad, C.E.; Pereira, W.E. Kovats and Lee Retention Indices determined by Gas Chromatography/Mass Spectrometry for organic compounds of environmental interest. J. High. Resolut Chromatogr. Chromatogr. Commun. 1986, 9, 328–334. [Google Scholar] [CrossRef]
- Jaramillo, J.; Torto, B.; Mwenda, D.; Troeger, A.; Borgemeister, C.; Poehling, H.M.; Francke, W. Coffee berry borer joins bark beetles in coffee klatch. PLoS ONE 2013, 8, e74277. [Google Scholar] [CrossRef] [PubMed]
- Harrison, B.M.; Priest, F.G. Composition of peaks used in the preparation of malt for Scotch Whisky production - influence of geographical source and extraction depth. J. Agric. Food Chem. 2009, 57, 2385–2391. [Google Scholar] [CrossRef]
- Graham, J. The Attraction of Bumble Bee (Hymenoptera: Apidae, Bombus impatiens Cresson) Colonies to Small Hive Beetles (Coleoptera: Nitidulidae, Aethina tumida Murray). 2009; A thesis presented to the graduate school of the University of Florida. Available online: http://ufdc.ufl.edu/UFE0024736/00001 (accessed on 1 September 2022).
- Lai, W.C.; Song, C. Temperature-programmed retention indices for GC. and GC-MS analysis of coal- and petroleum-derived liquid fuels. Fuel. 1995, 74, 1436–1451. [Google Scholar] [CrossRef]
- Zaikin, V.G.; Borisov, R.S. Chromatographic-mass spectrometric analysis of Fischer-Tropsch synthesis products. J. Anal. Chem. 2002, 57, 544–551. [Google Scholar] [CrossRef]
- Jordán, M.J.; Margaría, C.A.; Shaw, P.E.; Goodner, K.L. Aroma active components in aqueous kiwi fruit essence and kiwi fruit puree by GC-MS and multidimensional GC/GC-O. J. Agric. Food Chem. 2002, 50, 5386–5390. [Google Scholar] [CrossRef]
- Karabadagias, I.; Karabadagias, V.; Badeka, A. The honey volatile code: A collective study and extended version. Foods. 2019, 8, 508. [Google Scholar] [CrossRef]
- Pino, J.A.; Mesa, J.; Munoz, Y.; Marti, M.P.; Marbot, R. Volatile components from mango (Mangifera indica L.) cultivars. J. Agric. Food Chem. 2005, 53, 2213–2223. [Google Scholar] [CrossRef]
- Kim, K.R.; Kim, H. Gas chromatographic profiling and screening for phenols as isobutoxycarbonyl derivatives in aqueous samples. J. Chromatogr. A 2000, 866, 87–96. [Google Scholar] [CrossRef]
 ) female; (
) female; (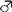 ) male.
) male.
 ) female; (
) female; ( ) male.
) male.

| Compound | Source | Source Reference | ng/Kg | RT (min) | Kexp | Kref | Identification Reference |
|---|---|---|---|---|---|---|---|
| Octanal | Honey, honeybees, wax | [55,56,57] | 1.45 | 12.69 | 999 | 999 | Standard *; [58] |
| Nonanal | Honeybees, G. mellonella pheromone, wax | [25,55,59] | 22 | 15.27 | 1085 | 1084 | Standard * |
| (E)-2-Nonenal | Propolis | [60] | - | 17.26 | 1153 | 1155 | [58] |
| Undecanal | Honeybees, G. mellonella pheromone | [25,59] | 35.3 | 21.00 | 1284 | 1284 | Standard * |
| (Z)-2-Dodecenal | - | - | - | 25.70 | 1467 | 1467 | [61] |
| α-Sinensal | Honey | [35] | - | 32.16 | 1752 | 1752 | [62] |
| (Z)-10-Hexadecenal | - | - | - | 33.27 | 1804 | 1804 | [61] |
| (E,E,Z,Z)-4,6,11,13-Hexadecatetraenal | - | - | - | 35.78 | 1926 | 1926 | [17] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Godoy, R.; Arias, I.; Venthur, H.; Quiroz, A.; Mutis, A. Characterization of Two Aldehyde Oxidases from the Greater Wax Moth, Galleria mellonella Linnaeus. (Lepidoptera: Pyralidae) with Potential Role as Odorant-Degrading Enzymes. Insects 2022, 13, 1143. https://doi.org/10.3390/insects13121143
Godoy R, Arias I, Venthur H, Quiroz A, Mutis A. Characterization of Two Aldehyde Oxidases from the Greater Wax Moth, Galleria mellonella Linnaeus. (Lepidoptera: Pyralidae) with Potential Role as Odorant-Degrading Enzymes. Insects. 2022; 13(12):1143. https://doi.org/10.3390/insects13121143
Chicago/Turabian StyleGodoy, Ricardo, Ignacio Arias, Herbert Venthur, Andrés Quiroz, and Ana Mutis. 2022. "Characterization of Two Aldehyde Oxidases from the Greater Wax Moth, Galleria mellonella Linnaeus. (Lepidoptera: Pyralidae) with Potential Role as Odorant-Degrading Enzymes" Insects 13, no. 12: 1143. https://doi.org/10.3390/insects13121143
APA StyleGodoy, R., Arias, I., Venthur, H., Quiroz, A., & Mutis, A. (2022). Characterization of Two Aldehyde Oxidases from the Greater Wax Moth, Galleria mellonella Linnaeus. (Lepidoptera: Pyralidae) with Potential Role as Odorant-Degrading Enzymes. Insects, 13(12), 1143. https://doi.org/10.3390/insects13121143






