Double-Stranded RNA-Degrading Enzymes Reduce the Efficiency of RNA Interference in Plutella xylostella
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing
2.2. Isolation and Sequencing of PxdsRNase cDNAs
2.3. Amino Acid Sequence Analysis of PxdsRNases
2.4. Determination of Gene Expression of PxdsRNase Genes in Different Tissues and Developmental Stages
2.5. In Vitro Incubation of dsRNA with Hemolymph/Gut Fluid
2.6. RNAi Response after dsRNA Injection or Oral Delivery
2.7. Heterologous Expression of PxdsRNases
2.8. Determination of PxdsRNase Activity
3. Results
3.1. Identification of DsRNases in P. xylostella
3.2. Stage-Specific and Tissue-Specific Expression of PxdsRNase Genes
3.3. DsRNA Degradation by the Proteins Extracted from the Gut and Hemolymph of Larvae
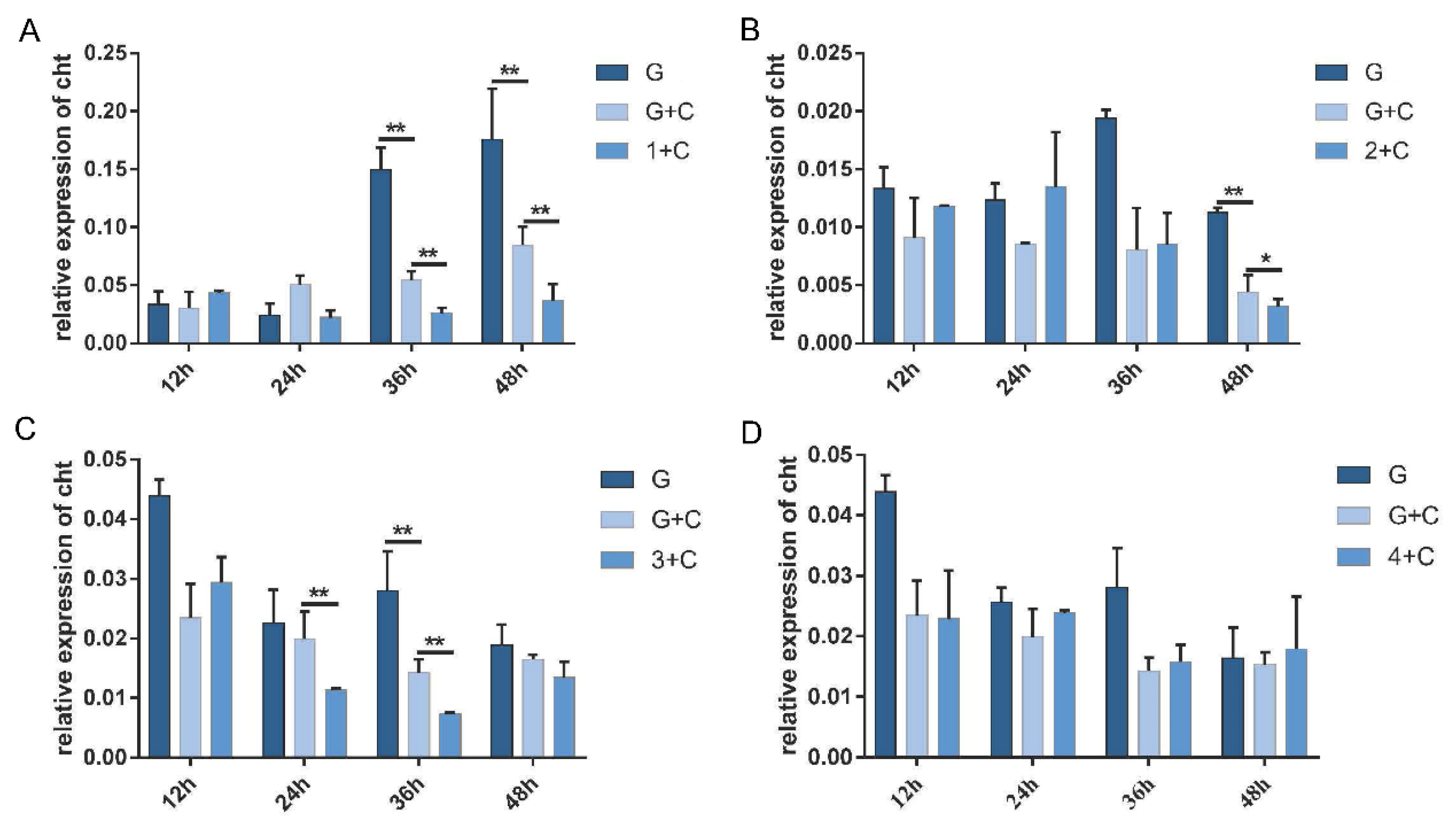
3.4. Effects of PxdsRNase Suppression on RNAi Efficiency
3.5. Enzymatic Activities of PxdsRNases
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Terenius, O.; Papanicolaou, A.; Garbutt, J.S.; Eleftherianos, I.; Huvenne, H.; Kanginakudru, S.; Albrechtsen, M.; An, C.; Aymeric, J.L.; Barthel, A.; et al. RNA interference in Lepidoptera: An overview of successful and unsuccessful studies and implications for experimental design. J. Insect Physiol. 2011, 57, 231–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, Y.; Wang, X.; Wang, X.; Yu, D.; Chen, B.; Kang, L. Differential responses of migratory locusts to systemic RNA interference via double-stranded RNA injection and feeding. Insect Mol. Biol. 2013, 22, 574–583. [Google Scholar] [CrossRef]
- Shukla, J.N.; Kalsi, M.; Sethi, A.; Narva, K.E.; Fishilevich, E.; Singh, S.; Mogilicherla, K.; Palli, S.R. Reduced stability and intracellular transport of dsRNA contribute to poor RNAi response in lepidopteran insects. RNA Biol. 2016, 13, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Vatanparast, M.; Kim, Y. Optimization of recombinant bacteria expressing dsRNA to enhance insecticidal activity against a lepidopteran insect, Spodoptera exigua. PLoS ONE 2017, 12, e0183054. [Google Scholar] [CrossRef] [Green Version]
- Cappelle, K.; de Oliveira, C.F.R.; Van Eynde, B.; Christiaens, O.; Smagghe, G. The involvement of clathrin-mediated endocytosis and two Sid-1-like transmembrane proteins in doublestranded RNA uptake in the Colorado potato beetle midgut. Insect Mol. Biol. 2016, 25, 315–323. [Google Scholar] [CrossRef]
- Bolognesi, R.; Ramaseshadri, P.; Anderson, J.; Bachman, P.; Clinton, W.; Flannagan, R.; Ilagan, O.; Lawrence, C.; Levine, S.; Moar, W.; et al. Characterizing the mechanism of action of double-stranded RNA activity against western corn rootworm (Diabrotica virgifera virgifera LeConte). PLoS ONE 2012, 7, e47534. [Google Scholar] [CrossRef]
- Wang, K.; Peng, Y.; Fu, W.; Shen, Z.; Han, Z. Key factors determining variations in RNA interference efficacy mediated by different double-stranded RNA lengths in Tribolium castaneum. Insect Mol. Biol. 2019, 28, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Arimatsu, Y.; Kotani, E.; Sugimura, Y.; Furusawa, T. Molecular characterization of a cDNA encoding extracellular dsRNase and its expression in the silkworm, Bombyx mori. Insect Biochem. Mol. Biol. 2007, 37, 176–183. [Google Scholar] [CrossRef]
- Liu, J.H.; Swevers, L.; Iatrou, K.; Huvenne, H.; Smagghe, G. Bombyx mori DNA/RNA non-specific nuclease: Expression of isoforms in insect culture cells, subcellular localization and functional assays. J. Insect Physiol. 2012, 58, 1166–1176. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.L.; Walker, W.B. Saliva of Lygus lineolaris digests double stranded ribonucleic acids. J. Insect Physiol. 2012, 58, 391–396. [Google Scholar] [CrossRef]
- Garbutt, J.S.; Belles, X.; Richards, E.H.; Reynolds, S.E. Persistence of double-stranded RNA in insect hemolymph as a potential determiner of RNA interference success: Evidence from Manduca sexta and Blattella germanica. J. Insect Physiol. 2013, 59, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Christiaens, O.; Swevers, L.; Smagghe, G. DsRNA degradation in the pea aphid (Acyrthosiphon pisum) associated with lack of response in RNAi feeding and injection assay. Peptides 2014, 53, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Wynant, N.; Santos, D.; Verdonck, R.; Spit, J.; Van Wielendaele, P.; Vanden Broeck, J. Identification, functional characterization and phylogenetic analysis of double stranded RNA degrading enzymes present in the gut of the desert locust, Schistocerca gregaria. Insect Biochem. Mol. Biol. 2014, 46, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.S.; Gurusamy, D.; Palli, S.R. Accumulation of dsRNA in endosomes contributes to inefficient RNA interference in the fall armyworm, Spodoptera frugiperda. Insect Biochem. Mol. Biol. 2017, 90, 53–60. [Google Scholar] [CrossRef]
- Song, H.; Zhang, J.; Li, D.; Cooper, A.M.W.; Silver, K.; Li, T.; Liu, X.; Ma, E.; Zhu, K.Y.; Zhang, J. A double-stranded RNA degrading enzyme reduces the efficiency of oral RNA interference in migratory locust. Insect Biochem. Mol. Biol. 2017, 86, 68–80. [Google Scholar] [CrossRef]
- Garcia, R.A.; Macedo, L.L.P.; Do Nascimento, D.C.; Gillet, F.X.; Moreira-Pinto, C.E.; Faheem, M.; Basso, A.M.M.; Silva, M.C.M.; Grossi-de-Sa, M.F. Nucleases as a barrier to gene silencing in the cotton boll weevil, Anthonomus grandis. PLoS ONE 2017, 12, e0189600. [Google Scholar]
- Peng, Y.C.; Wang, K.X.; Fu, W.X.; Sheng, C.W.; Han, Z.J. Biochemical comparison of dsRNA degrading nucleases in four different insects. Front. Physiol. 2018, 9, 624. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.C.; Wang, K.X.; Zhu, G.H.; Han, Q.X.; Chen, J.S.; Elzaki, M.E.A.; Sheng, C.W.; Zhao, C.Q.; Palli, S.R.; Han, Z.J. Identification and characterization of multiple dsRNases from a lepidopteran insect, the tobacco cutworm, Spodoptera litura (Lepidoptera: Noctuidae). Pest. Biochem. Physiol. 2020, 162, 86–95. [Google Scholar] [CrossRef]
- Guan, R.B.; Li, H.C.; Fan, Y.J.; Hu, S.R.; Christiaens, O.; Smagghe, G.; Miao, X.X. A nuclease specific to lepidopteran insects suppresses RNAi. J. Biol. Chem. 2018, 293, 6011–6021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.P.; Wang, Y.J.; Zeng, B.S.; Liu, Z.X.; Xu, X.J.; Meng, Q.; Huang, Y.P.; Yang, G.; Vasseur, L.; Gurr, G.M.; et al. Functional characterization of Pol III U6 promoters for gene knockdown and knockout in Plutella xylostella. Insect Biochem. Mol. Biol. 2017, 89, 71–78. [Google Scholar] [CrossRef]
- He, Z.X.; Chen, J.; Ran, X.Y.; Chen, X.; Bai, Y.; Xie, X.M.; Hou, T.W. Prokaryotic expression and immunogenicity analysis of phosphoglycerate kinase 1 of Candida albicans. Chin. J. Cell. Mol. Immunol. 2013, 29, 1079–1081. [Google Scholar] [CrossRef]
- Grabski, A.C. Advances in preparation of biological extracts for protein purification. In Guide to Protein Purification, 2nd ed.; Burgess, R.R., Deutscher, M.P., Eds.; Elsevier Academic Press Inc.: San Diego, CA, USA, 2009; Volume 463, pp. 285–303. [Google Scholar]
- Peng, Y.C.; Wang, K.X.; Chen, J.S.; Wang, J.D.; Zhang, H.N.; Ze, L.J.; Zhu, G.H.; Zhao, C.Q.; Xiao, H.J.; Han, Z.J. Identification of a double-stranded RNA-degrading nuclease influencing both ingestion and injection RNA interference efficiency in the red flour beetle Tribolium castaneum. Insect Biochem. Mol. Biol. 2020, 125, 103440. [Google Scholar] [CrossRef] [PubMed]
- Lomate, P.R.; Bonning, B.C. Proteases and nucleases involved in the biphasic digestion process of the brown marmorated stink bug, Halyomorpha halys (Hemiptera: Pentatomidae). Arch. Insect Biochem. Physiol. 2018, 98, e21459. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Peng, Y.; Pu, J.; Fu, W.; Wang, J.; Han, Z. Variation in RNAi efficacy among insect species is attributable to dsRNA degradation in vivo. Insect Biochem. Mol. Biol. 2016, 77, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Joga, M.R.; Zotti, M.J.; Smagghe, G.; Christiaens, O. RNAi efficiency, systemic properties, and novel delivery methods for pest insect control: What we know so far. Front. Physiol. 2016, 7, 553. [Google Scholar] [CrossRef] [Green Version]
- Sapountzis, P.; Duport, G.; Balmand, S.; Gaget, K.; Jaubert-Possamai, S.; Febvay, G.; Charles, H.; Rahbe, Y.; Colella, S.; Calevro, F. New insight into the RNA interference response against cathepsin-L gene in the pea aphid, Acyrthosiphon pisum: Molting or gut phenotypes specifically induced by injection or feeding treatments. Insect Biochem. Mol. Biol. 2014, 51, 20–32. [Google Scholar] [CrossRef]
- Spit, J.; Philips, A.; Wynant, N.; Santos, D.; Plaetinck, G.; Broeck, J.V. Knockdown of nuclease activity in the gut enhances RNAi efficiency in the Colorado potato beetle, Leptinotarsa decemlineata, but not in the desert locust, Schistocerca gregaria. Insect Biochem. Mol. Biol. 2017, 81, 103–116. [Google Scholar] [CrossRef]
- Prentice, K.; Smagghe, G.; Gheysen, G.; Christians, O. Nuclease activity decreases the RNAi response in the sweetpotato weevil Cylas puncticollis. Insect Biochem. Mol. Biol. 2019, 110, 80–89. [Google Scholar] [CrossRef]
- Yu, N.; Christiaens, O.; Liu, J.; Niu, J.; Cappelle, K.; Caccia, S.; Huvenne, H.; Smagghe, G. Delivery of dsRNA for RNAi in insects: An overview and future directions. Insect Sci. 2013, 20, 4–14. [Google Scholar] [CrossRef]
- Machado, V.; Rodriguez-Garcia, M.J.; Sanchez-Garcia, F.J.; Galian, J. RNA Interference: A new strategy in the evolutionary arms race between human control strategies and insect pests. Folia Biol. Krakow 2014, 62, 335–343. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, T.B.; Figueira, A. Management of insect pest by RNAi—A new tool for crop protection. In RNA Interference; Abdurakhmonov, I.Y., Ed.; InTech: London, UK, 2016; pp. 371–390. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhang, J.; Zhu, K.Y. Chitosan/double-stranded RNA nanoparticle-mediated RNA interference to silence chitin synthase genes through larval feeding in the African malaria mosquito (Anopheles gambiae). Insect Mol. Biol. 2010, 19, 683–693. [Google Scholar] [CrossRef] [PubMed]
- He, B.C.; Chu, Y.; Yin, M.Z.; Mullen, K.; An, C.J.; Shen, J. Fluorescent nanoparticle delivered dsRNA toward genetic control of insect pests. Adv. Mater. 2013, 25, 4580–4584. [Google Scholar] [CrossRef] [PubMed]
- Sanitt, P.; Apiratikul, N.; Niyomtham, N.; Yingyongnarongkul, B.E.; Assavalapsakul, W.; Panyim, S.; Udomkit, A. Cholesterol-based cationic liposome increases dsRNA protection of yellow head virus infection in Penaeus vannamei. J. Biotechnol. 2016, 228, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Han, Z.J. Efficiency of different methods for dsRNA delivery in cotton bollworm (Helicoverpa armigera). J. Integr. Agric. 2014, 13, 115–123. [Google Scholar] [CrossRef] [Green Version]








| Use of Primers | Gene | Primer Sequence (5′-3′) |
|---|---|---|
| cDNA cloning | PxdsRNase1 | F: ATGCTTCACAAATGGGTTCTAATG |
| R: TCACAGTAGTAATCCAGTGACTTTG | ||
| PxdsRNase2 | F: ATGATGTTGCGTGGTTGTGTGC | |
| R: TTAAACTAAAAGCCCATTCACGGTAAAAG | ||
| PxdsRNase3 | F: ATGCTGCGTCCTTGTCTGC | |
| R: TTAAGCCAACAGCCCATTAACTTTG | ||
| PxdsRNase4 | F: ATGATTCACAAAAAACTTTTACAAG | |
| R: TTACACTTTCTTTCCGTTGATGCTAG | ||
| qPCR primer | PxdsRNase1 | F: GCGCGAATTGTACCCTCTGT |
| R: GGACTCAGCAGCCAATCAAC | ||
| PxdsRNase2 | F: GGGCGTATGGAGTCTTCCTG | |
| R: CGAACTCCTCTTCTCCCAGC | ||
| PxdsRNase3 | F: CGGACACTCCTTCCACGAC | |
| R: TGTAGCCGACCCTGATGACC | ||
| PxdsRNase4 | F: AAGTAGCCCAACTCAGTGCC | |
| R: AGCAGACACGGTCCCGAATA | ||
| EF1 | F: GCCTCCCTACAGCGAATC | |
| R: CCTTGAACCAGGGCATCT | ||
| PxCht | F: AGACTTGATGGTGTTCGCGT | |
| R: GTCCACCTTCTGCCCTATCG | ||
| dsRNA primer | dsPxdsRNase1 | F: TAATACGACTCACTATAGGGTAGATGCTCCTCACCTCCA |
| R: TAATACGACTCACTATAGGGTCTCTCCGAACGCAAACAC | ||
| dsPxdsRNase2 | F: TAATACGACTCACTATAGGGGTGGAGGATGGTAAAGCGAC | |
| R: TAATACGACTCACTATAGGGGTGGCGAACACAAAGTCCGT | ||
| dsPxdsRNase3 | F: TAATACGACTCACTATAGGGTCCAAGACTGTGGCTACTGC | |
| R: TAATACGACTCACTATAGGGGTCAGATGCCCTCGGTTCAA | ||
| dsPxdsRNase4 | F: TAATACGACTCACTATAGGGCGGGTTCCTAACTGGGTGTT | |
| R: TAATACGACTCACTATAGGGCCACCGAGTTGCCTCCTATC | ||
| dsPxCht | F: TAATACGACTCACTATAGGGAACGAAAAGGTCTGGATCTG | |
| R: TAATACGACTCACTATAGGGGGGTGGTCTCAGCCTAACAT | ||
| dsGFP | F: TAATACGACTCACTATAGGGGCTTCTCGTTGGGGTCTTTG | |
| R: TAATACGACTCACTATAGGGACCACATGAAGCAGCACGAC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.-Z.; Jiang, Y.-X.; Li, M.-W.; Li, J.-W.; Zha, B.-H.; Yang, G. Double-Stranded RNA-Degrading Enzymes Reduce the Efficiency of RNA Interference in Plutella xylostella. Insects 2021, 12, 712. https://doi.org/10.3390/insects12080712
Chen J-Z, Jiang Y-X, Li M-W, Li J-W, Zha B-H, Yang G. Double-Stranded RNA-Degrading Enzymes Reduce the Efficiency of RNA Interference in Plutella xylostella. Insects. 2021; 12(8):712. https://doi.org/10.3390/insects12080712
Chicago/Turabian StyleChen, Jin-Zhi, Ying-Xia Jiang, Miao-Wen Li, Jian-Wen Li, Ben-Hu Zha, and Guang Yang. 2021. "Double-Stranded RNA-Degrading Enzymes Reduce the Efficiency of RNA Interference in Plutella xylostella" Insects 12, no. 8: 712. https://doi.org/10.3390/insects12080712
APA StyleChen, J.-Z., Jiang, Y.-X., Li, M.-W., Li, J.-W., Zha, B.-H., & Yang, G. (2021). Double-Stranded RNA-Degrading Enzymes Reduce the Efficiency of RNA Interference in Plutella xylostella. Insects, 12(8), 712. https://doi.org/10.3390/insects12080712






