Field Cage Assessment of Feeding Damage by Riptortus pedestris on Soybeans in China
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Rearing of R. pedestris
2.2. Field Management of Soybean Plants
2.3. Releasing Different Densities of R. pedestris onto Three Development Stages of Soybeans
2.4. Syndromes of Soybean Plants and Seeds Influenced by R. pedestris Feeding Damage
2.5. Soybean Quality Influenced by R. pedestris Injury
2.6. Data Analysis
3. Results
3.1. Number of Staygreen Leaves
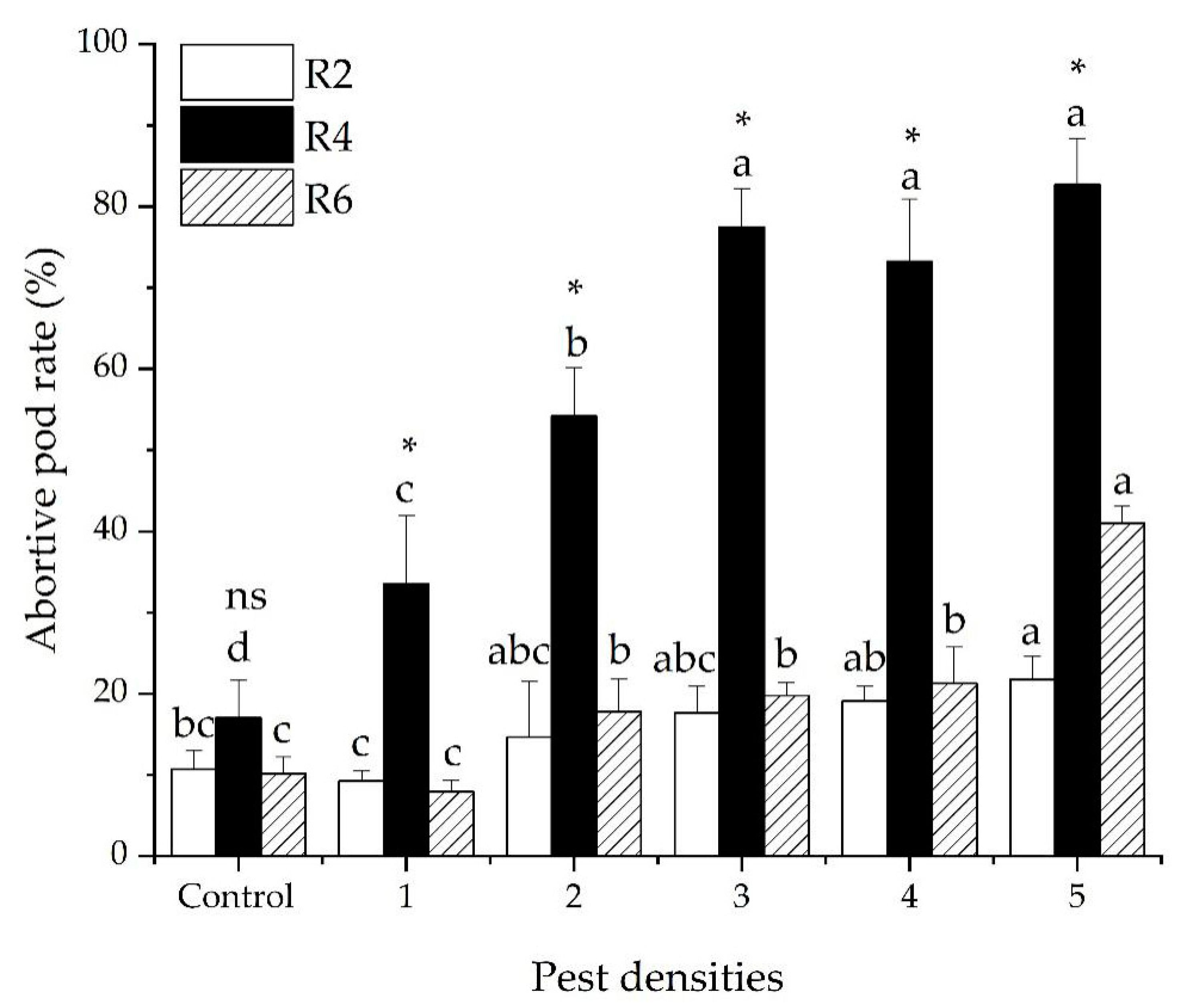
3.2. The Abortive Pod Rate
3.3. The Abortive Seed Rate
3.4. The Dry Weight
3.5. The Nutrient Transfer Rate
3.6. Evaluation of the Nutrient Content of Soybean Plants and Seeds Caused by Five R. pedestris on the R4 Stage of Soybeans
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, H.S.; Chung, B.K.; Kim, T.S.; Kwon, J.H.; Song, W.D.; Rho, C.W. Damage of sweet persimmon fruit by the inoculation date and number of stink bugs, Riptortus clavatus, Halyomorpha halys and Plautia stali. Korean J. Appl. Entomol. 2009, 48, 485–491. [Google Scholar] [CrossRef] [Green Version]
- Kim, G.H.; Ahn, Y.J.; Cho, K.Y. Effect of diflubenzuronon longevity and reproduction of adult bean bug (Hemiptera: Alydidae). J. Econ. Entomol. 1992, 85, 664–668. [Google Scholar] [CrossRef]
- Lim, U.T. Occurrence and control method of Riptortus pedestris (Hemiptera: Alydidae): Korean perspectives. Korean J. Appl. Entomol. 2013, 52, 437–448. [Google Scholar] [CrossRef]
- Parvathy, V. Ecological Perspectives and Host Plant Resistance Studies of Pod Feeders in Field Bean, Lablab purpureus (L.) Sweet. Bachelor’s Thesis, Acharya N.G. Ranga Agricultural University, Pradesh, India, 2011. [Google Scholar]
- Qi, Y.Y.; Zhao, C.X.; Shao, W.X.; Cui, S.Y.; Zhang, G.Z.; Hua, J.X. Occurrence and control techniques of soybean stink bug Riptortus pedestris in Langfang area. Mod. Rural Sci. Technol. 2017, 9, 34. [Google Scholar]
- Gao, Y.; Chen, J.H.; Shi, S.S. Research progress on soybean stink bug (Riptortus pedestris). Chin. J. Oil Crop Sci. 2019, 41, 804–815. [Google Scholar]
- Xie, H.; Chen, L.J.; Han, J.; Wang, C. Damage characteristics and control Methods of Riptortus pedestris in Soybea. Soybean Sci. Technol. 2016, 6, 11–13. [Google Scholar]
- Chen, J.H.; Bi, R.; Huang, J.M.; Cui, J.; Shi, S.S. Analysis on the different effects of different stinkbugs infestations on growth and yield of soybean. Soybean Sci. 2018, 37, 585–589. [Google Scholar]
- Miles, P.W. The saliva of Hemiptera. Adv. Insect Physiol. 1972, 9, 183–255. [Google Scholar]
- Gao, Y.; Shi, S.S. The relationship between staygreen syndrome in soybean and stink bugs and preventive strategy. Soybean Sci. 2019, 38, 650–655. [Google Scholar]
- Zhang, X.X.; Wang, M.; Wu, T.; Wu, C.X.; Jiang, B.J.; Guo, C.H.; Han, T.F. Physiological and Molecular Studies of Staygreen Caused by Pod Removal and Seed Injury in Soybean. Master’s Thesis, Harbin Normal University, Harbin, China, 2016. [Google Scholar]
- Kimura, S.; Tokumaru, S.; Kikuchi, A. Carrying and transmission of Eremothecium coryli (Peglion) Kurtzman as a causal pathogen of yeast-spot disease in soybeans by Riptortus clavatus (Thunberg), Nezara antennata Scott, Piezodorus hybneri (Gmelin) and Dolycoris baccarum (Linnaeus). Jpn. J. Appl. Entomol. Zool. 2008, 52, 13–18. [Google Scholar] [CrossRef]
- Li, K.; Zhang, X.X.; Guo, J.Q.; Hannah, P.; Wu, T.T.; Li, L.; Jiang, H.; Chang, L.D.; Wu, C.X.; Han, T.F. Feeding of Riptortus pedestris on soybean plants, the primary cause of soybean staygreen syndrome in the Huang-Huai-Hai river basin. Crop J. 2019, 7, 360–367. [Google Scholar] [CrossRef]
- Boethel, D.J.; Russin, J.S.; Wier, A.T.; Layton, M.B.; Mink, J.S.; Boyd, M.L. Delayed maturity associated with southern green stink bug (Heteroptera: Pentatomidae) injury at various soybean phenological stages. J. Econ. Entomol. 2000, 93, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Fehr, W.R.; Caviness, C.E. Stages of Soybean Development; Iowa State University: Ames, IA, USA, 1977; pp. 1–12. [Google Scholar]
- Kim, S.; Lim, U.T. Seasonal occurrence pattern and within-plant egg distribution of bean bug, Riptortus pedestris (Fabricius) (Hemiptera: Alydidae), and its egg parasitoids in soybean fields. Appl. Entomol. Zool. 2010, 45, 457–464. [Google Scholar] [CrossRef] [Green Version]
- Mainali, B.P.; Lim, U.T. Annual pattern of occurrence of Riptortus pedestris (Hemiptera: Alydidae) and its egg parasitoids Ooencyrtus nezarae Ishii and Gryon japonicum (Ashmead) in Andong, Korea. Crop Prot. 2012, 36, 37–42. [Google Scholar] [CrossRef]
- Li, F.; Zhang, L.J.; Fu, J.Y.; Huang, W.H.; Wang, Y.J.; Zhang, G.H.; Xu, R.; Li, K.F. Survey and analysis of damages of soybean Riptortus pedestris in Qingyan. Shaanxi J. Agric. Sci. 2020, 66, 81–82. [Google Scholar]
- Wittenbach, V.A. Purification and characterization of a soybean leaf storage glycoprotein. Plant Physiol. 1983, 73, 125–129. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.; Lim, U.T. Fruits of apple and sweet persimmon are not essential food sources for Riptortus pedestris (Hemiptera: Alydidae) which causes fruit-spotting. J. Asia-Pac. Entomol. 2012, 15, 203–206. [Google Scholar] [CrossRef]
- Mainali, B.P.; Kim, H.J.; Yoon, Y.N.; Oh, I.S.; Bae, S.D. Evaluation of different leguminous seeds as food sources for the bean bug Riptortus pedestris. J. Asia-Pac. Entomol. 2014, 17, 115–117. [Google Scholar] [CrossRef]
- Corrêa-Ferreira, B.S.; Azevedo, J.D. Soybean seed damage by different species of stink bugs. Agric. For. Entomol. 2002, 4, 145–150. [Google Scholar] [CrossRef]
- Bae, S.D.; Kim, H.J.; Mainali, B.P. Infestation of Riptortus pedestris (Fabricius) decreases the nutritional quality and germination potential of soybean seeds. J. Asia-Pac. Entomol. 2014, 17, 477–481. [Google Scholar] [CrossRef]
- Soxhlet Extraction Method- Estimation of Fat in Food. Available online: https://discoverfoodtech.com/soxhlet-extraction-method/ (accessed on 19 August 2020).
- Walker, J.M. The bicinchoninic acid (BCA) assay for protein quantitation. In The Protein Protocols Handbook, Walker, J.M., Ed.; Humana Press: New York, NY, USA, 1994; pp. 11–14. [Google Scholar]
- Zhang, J.; Li, C.Y.; Li, J.P.; Pan, J.; Xiang, D.X. Determination of polysaccharide in rhizoma of Panax japonicus by anthrone sulfuric acid method and phenol sulfuric method. Cent. South Pharm. 2012, 10, 421–424. [Google Scholar]
- Owens, D.R.; Herbert, D.A.; Dively, G.P.; Reisig, D.D.; Kuhar, T.P. Does feeding by Halyomorpha halys (Hemiptera: Pentatomidae) reduce soybean seed quality and yield. J. Econ. Entomol. 2013, 106, 1317–1323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, H.M.; Zheng, H.B. Research advance on physiological changes respond to alteration of source sink relationship in soybean. Soybean Sci. 2009, 28, 736–739. [Google Scholar]
- Daugherty, D.H.; Nuestadt, M.H.; Gehrke, C.W.; Cavanah, L.E.; Williams, L.F.; Green, D.E. An evaluation of damage to soybean by brown and green stink bugs. J. Econ. Entomol. 1964, 57, 719–722. [Google Scholar] [CrossRef]
- Todd, J.W.; Turnipseed, S.G. Effect of southern green stink bug damage on yield and quality of soybeans. J. Econ. Entomol. 1974, 67, 421–426. [Google Scholar] [CrossRef]
- Kim, S.; Lim, U.T. New soybean variety, Agakong, as a host of Riptortus pedestris (Fabricius): Study on field occurrence and biological attributes in the laboratory. J. Asia-Pac. Entomol. 2010, 13, 261–265. [Google Scholar] [CrossRef]
- Endo, N.; Wada, T.; Sasaki, R. Seasonal synchrony between pheromone trap catches of the bean bug, Riptortus pedestris (Heteroptera: Alydidae) and the timing of invasion of soybean fields. Appl. Entomol. Zool. 2011, 46, 477–482. [Google Scholar] [CrossRef]
- Tabuchi, K.; Moriya, S.; Mizutani, N. Seasonal catches of the bean bug, Riptortus clavatus (Thunberg) (Heteroptera: Alydidae), in water-pan traps with synthetic attractants. Jpn. J. Appl. Entomol. Zool. 2005, 49, 99–104. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.M.; Kim, E.; Kim, D.; Bhuyain, M.M.; Lim, U.T. Use of aggregation pheromone traps increases infestation of adult Riptortus pedestris (Hemiptera: Alydidae) in soybean fields. Pest Manag. Sci. 2018, 74, 2578–2588. [Google Scholar] [CrossRef]
- Wang, Z.J.; Tian, X.Y.; Li, W.B.; Gao, Y.; Shi, S.S. Indoor biological activity and field effect of 5 kinds of insecticides to Riptortus pedestris. Agrochemicals 2020, 59, 537–540. [Google Scholar]



| Dry Weight | Development Stage of Soybean | Pest Densities | |||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | ||
| Soybean plants (g) | R2 | 3.76 ± 0.28 a A | 3.24 ± 0.43 a A | 3.97 ± 0.29 a B | 3.60 ± 0.289 a B | 4.26 ± 0.44 a C | 3.56 ± 0.25 a C |
| R4 | 4.66 ± 0.38 b A | 6.41 ± 2.19 b A | 7.72 ± 1.19 b A | 14.02 ± 3.01 ab A | 24.95 ± 7.44 a A | 27.44 ± 3.82 a A | |
| R6 | 4.52 ± 0.64 b A | 4.63 ± 0.75 b A | 6.89 ± 1.23 b AB | 7.01 ± 0.39 b B | 8.34 ± 1.82 b B | 13.33 ± 0.83 a B | |
| Soybean seeds (g) | R2 | 13.84 ± 1.81 a A | 11.89 ± 1.66 a A | 13.26 ± 1.89 a A | 10.63 ± 0.64 a A | 10.83 ± 0.84 a A | 9.48 ± 0.50 a A |
| R4 | 13.29 ± 0.95 a A | 6.31 ± 1.30 b A | 2.60 ± 0.70 c B | 1.31 ± 1.72 c B | 1.12 ± 0.84 c B | 0.96 ± 0.45 c C | |
| R6 | 12.66 ± 2.40 a A | 12.08 ± 1.93 a A | 8.74 ± 2.52 a AB | 7.67 ± 1.24 a A | 8.23 ± 1.45 a A | 7.04 ± 0.85 a B | |
| Soybean | Treatment | Protein (g/mL) | Lipid (μg/mL) | Carbohydrate (μg/mL) |
|---|---|---|---|---|
| Seed | treatment | 0.16 ± 0.02 a | 0.11 ± 0.01 b | 68.91 ± 21.47 a |
| control | 0.24 ± 0.05 a | 0.18 ± 0.00 a | 94.87 ± 25.68 a | |
| Plant | treatment | 0.23 ± 0.04 a | 0.04 ± 0.01 a | 73.29 ± 7.77 a |
| control | 0.05 ± 0.00 b | 0.01 ± 0.00 b | 81.73 ± 4.07 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Gao, Y.; Hu, Y.; Chen, J.; Zhang, J.; Shi, S. Field Cage Assessment of Feeding Damage by Riptortus pedestris on Soybeans in China. Insects 2021, 12, 255. https://doi.org/10.3390/insects12030255
Li W, Gao Y, Hu Y, Chen J, Zhang J, Shi S. Field Cage Assessment of Feeding Damage by Riptortus pedestris on Soybeans in China. Insects. 2021; 12(3):255. https://doi.org/10.3390/insects12030255
Chicago/Turabian StyleLi, Wenjing, Yu Gao, Yinglu Hu, Juhong Chen, Jinping Zhang, and Shusen Shi. 2021. "Field Cage Assessment of Feeding Damage by Riptortus pedestris on Soybeans in China" Insects 12, no. 3: 255. https://doi.org/10.3390/insects12030255
APA StyleLi, W., Gao, Y., Hu, Y., Chen, J., Zhang, J., & Shi, S. (2021). Field Cage Assessment of Feeding Damage by Riptortus pedestris on Soybeans in China. Insects, 12(3), 255. https://doi.org/10.3390/insects12030255








