Direct and Indirect Effect via Endophytism of Entomopathogenic Fungi on the Fitness of Myzus persicae and Its Ability to Spread PLRV on Tobacco
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plants, Insects and Virus
2.2. Entomopathogenic Fungi
2.3. Aphid Fitness Bioassays
2.4. Virus Spread Bioassay
2.5. Confirmation of Endophytic Colonization of Tobacco by EPF
2.6. Statistical Analysis
3. Results
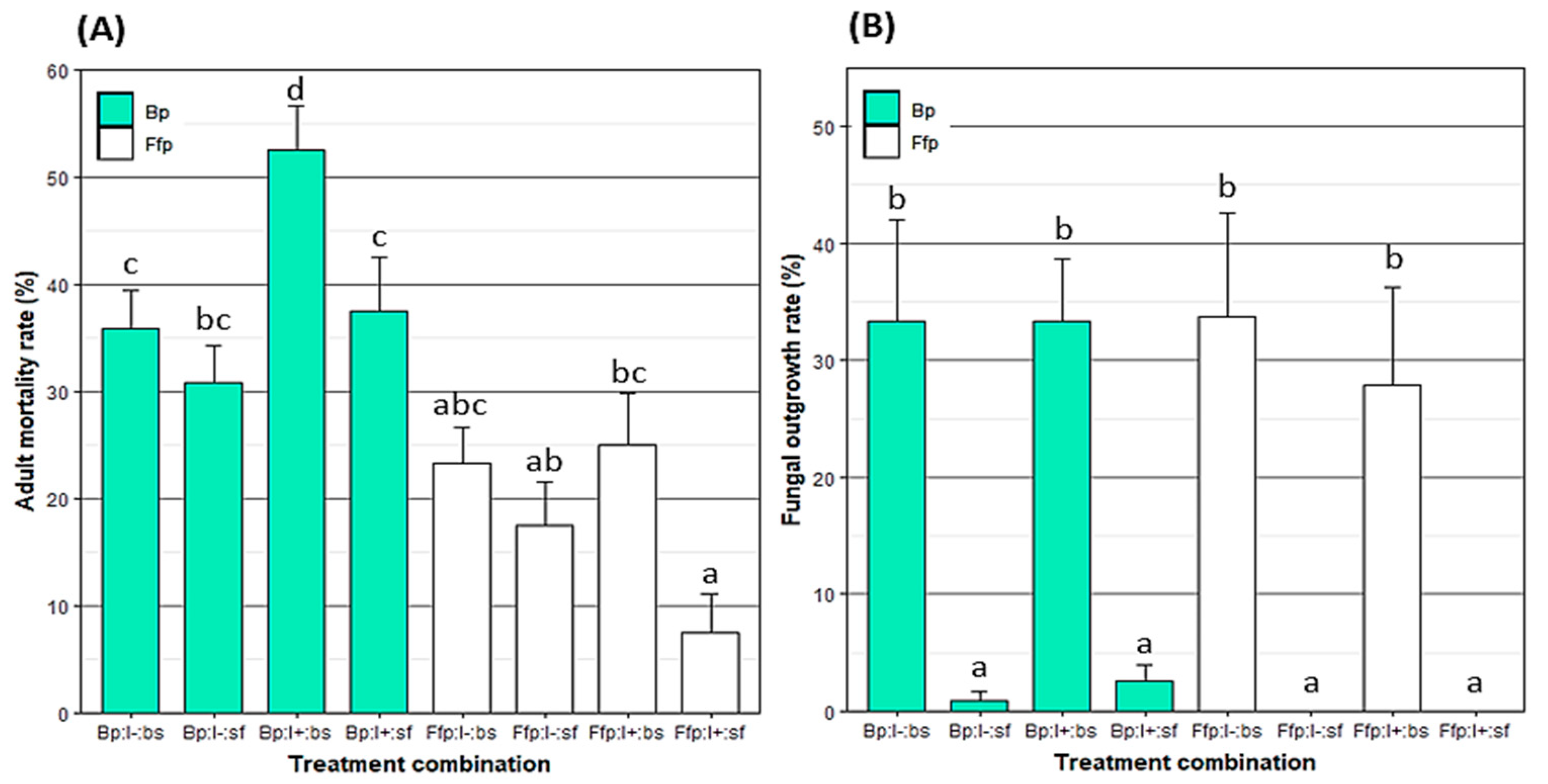
3.1. Endophytic Colonization of Tobacco Plants
3.2. Direct and Indirect Effect of EPF on Aphid Mortality
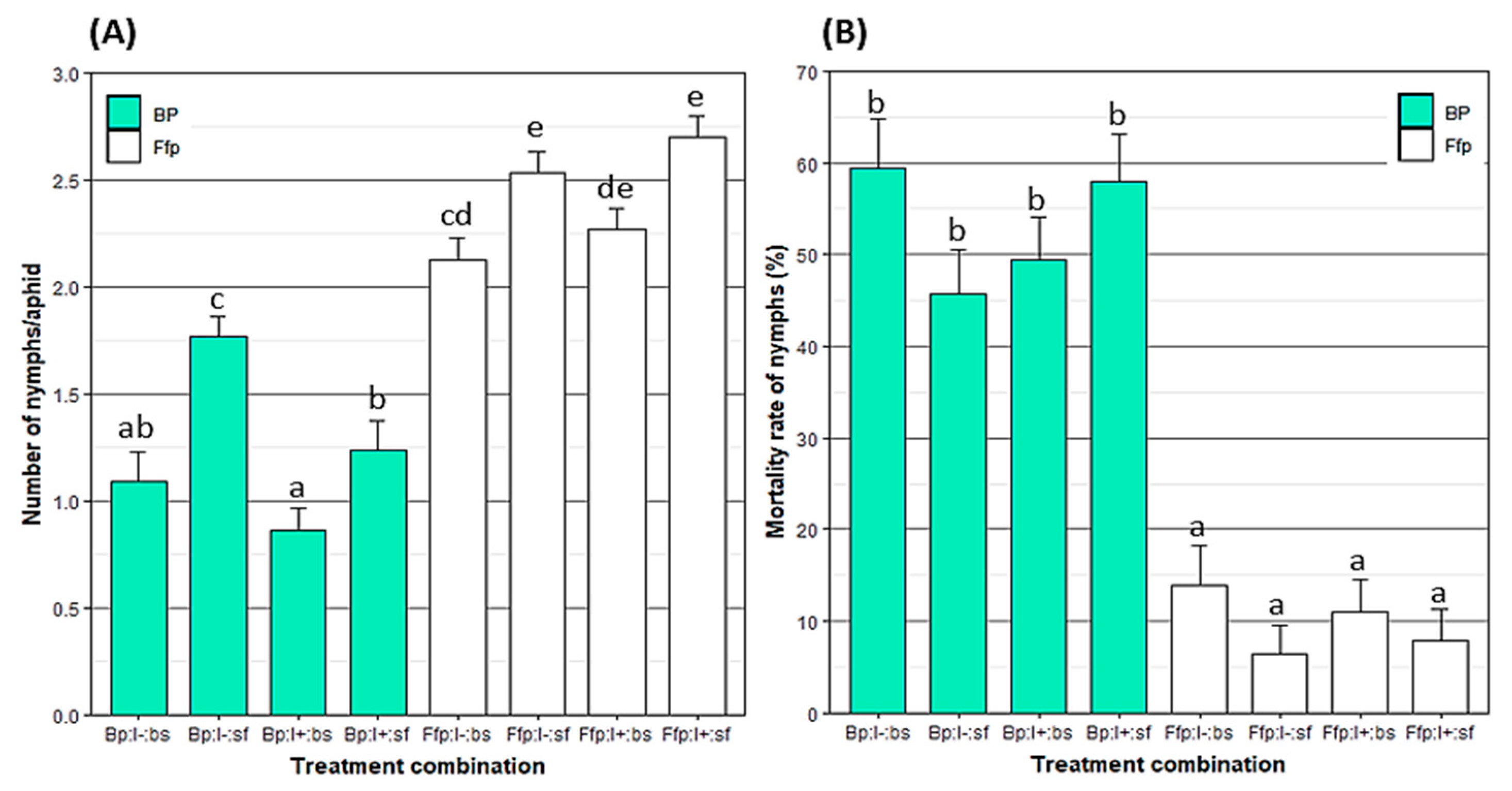
3.3. Direct and Indirect Effect of EPF on Aphid Fecundity and Nymph Mortality
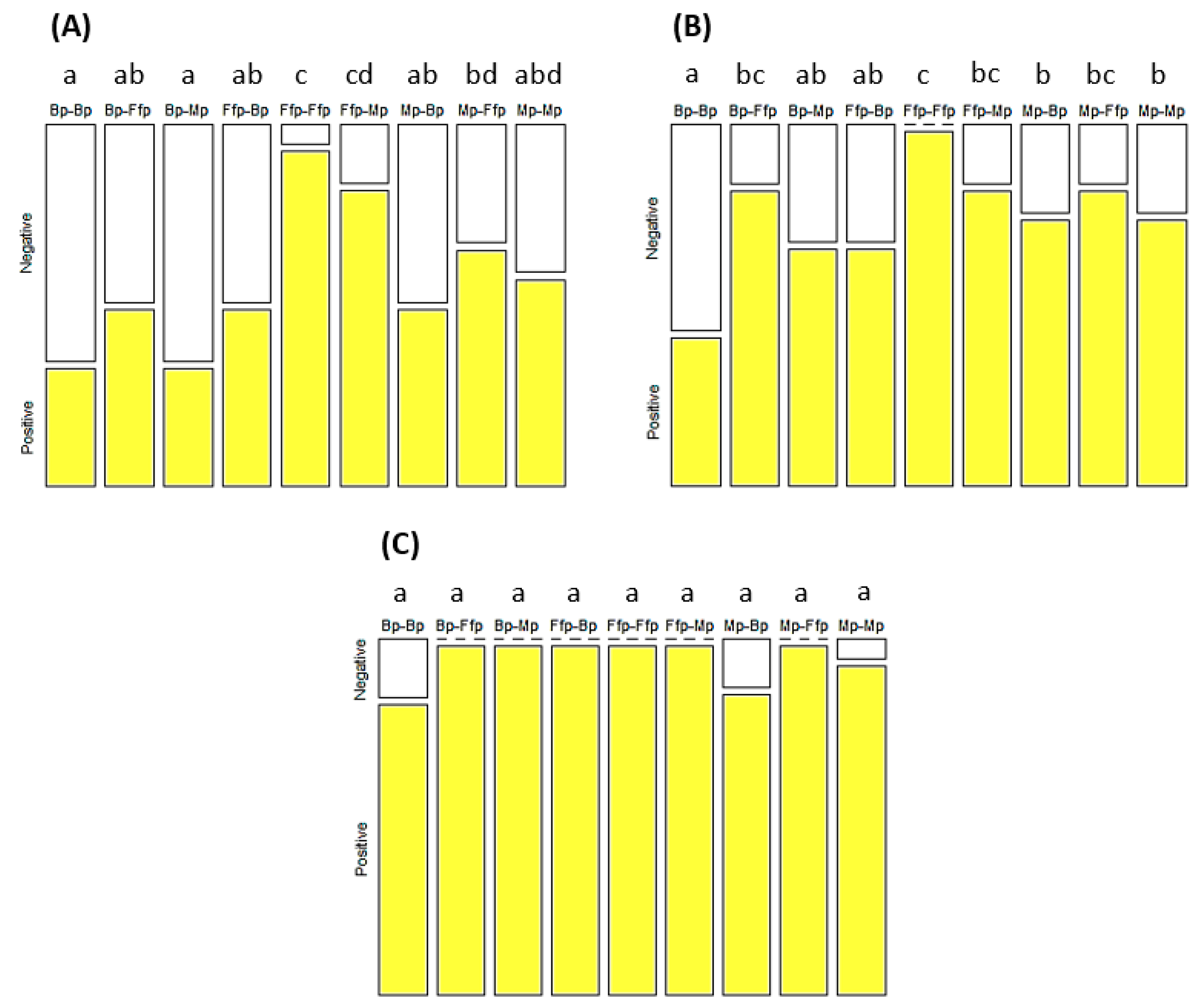
3.4. Effect of EEPF Colonization on Virus Spread by Aphids
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, F.J.; Bosquée, E.; Liu, Y.J.; Chen, J.L.; Yong, L.; Francis, F. Impact of aphid alarm pheromone release on virus transmission efficiency: When pest control strategy could induce higher virus dispersion. J. Virol. Methods 2016, 235, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Whitworth, J.L.; Nolte, P.; McIntosh, C.; Davidson, R. Effect of Potato virus Y on yield of three potato cultivars grown under different nitrogen levels. Plant Dis. 2006, 90, 73–76. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Capinera, J.L. Green Peach Aphid, Myzus persicae (Sulzer) (Hemiptera: Aphididae). In Encyclopedia of Entomology; Capinera, J.L., Ed.; Springer: Dordrecht, The Netherlands, 2008; pp. 1727–1730. [Google Scholar] [CrossRef]
- Moreno, A.; Tjallingii, W.F.; Fernandez-Mata, G.; Fereres, A. Differences in the mechanism of inoculation between a semi-persistent and a non-persistent aphid-transmitted plant virus. J. Gen. Virol. 2012, 93, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Radcliffe, E.B.; Ragsdale, D.W. Aphid-transmitted potato viruses: The importance of understanding vector biology. Am. J. Potato Res. 2002, 79, 353–386. [Google Scholar] [CrossRef]
- Eskandari, F.; Sylvester, E.S.; Richardson, J. Evidence for lack of propagation of Potato leaf roll virus in its aphid vector, Myzus persicae. Phytopathology 1979, 69, 45. [Google Scholar] [CrossRef]
- Garret, A.; Kerlan, C.; Thomas, D. Ultrastructural study of acquisition and retention of potato leafroll luteovirus in the alimentary canal of its aphid vector, Myzus persicae Sulz. Arch. Virol. 1996, 141, 1279–1292. [Google Scholar] [CrossRef]
- Kotzampigikis, A.T.; Hristova, D.; Tasheva-Terzieva, E. Virus-vector relationship between Potato leafroll virus PLRV and Myzus persicae Sulzer. Bulg. J. Agric. Sci. 2010, 16, 412–421. [Google Scholar]
- Loebenstein, G. Potato leafroll virus (PLRV; Genus Polerovirus; Family Luteoviridae). In Virus and Virus-like Diseases of Potatoes and Production of Seed-Potatoes; Loebenstein, G., Brunt, A.A., Eds.; Kluwer Academic Publishers: Amsterdam, The Netherlands; Springer Netherlands: Dordrecht, The Netherlands, 2001; pp. 69–75. [Google Scholar] [CrossRef]
- Tanaka, S.; Shiota, H. Latent period of Potato leaf roll virus in the green peach aphid (Myzus persicae Sulzer). Jpn. J. Phytopathol. 1970, 36, 106–111. [Google Scholar] [CrossRef]
- Mowry, T.M. Insecticidal reduction of Potato leafroll virus transmission by Myzus persicae. Ann. Appl. Biol. 2005, 146, 81–88. [Google Scholar] [CrossRef]
- Alvarez, J.M.; Srinivasan, R. Evaluation of Hairy Nightshade as an Inoculum Source for Aphid-Mediated Transmission of Potato Leafroll Virus. J. Econ. Entomol. 2005, 98, 1101–1108. [Google Scholar] [CrossRef]
- DiFonzo, C.D.; Ragsdale, D.W.; Radcliffe, E.B. Potato leafroll virus spread in differentially resistant potato cultivars under varying aphid densities. Am. Potato J. 1995, 72, 119–132. [Google Scholar] [CrossRef]
- Agrios, G.N. Plant Pathology, 5th ed.; Elsevier: Burlington, NJ, USA, 2005; Volume 1. [Google Scholar] [CrossRef]
- Shah, P.A.; Pell, J.K. Entomopathogenic fungi as biological control agents. Appl. Microbiol. Biotechnol. 2003, 61, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Peshin, R.; Vasanthakumar, J.; Kalra, R. Diffusion of innovation theory and integrated pest management. In Integrated Pest Managment: Dissemination and Impact; Peshin, R., Dhawan, A.K., Eds.; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2009; Volume 2, pp. 1–30. [Google Scholar]
- Vernon, R.S.; van Herk, W.G. Wireworms as Pests of Potato. In Insect Pests of Potato; Elsevier: Amsterdam, The Netherlands, 2013; pp. 103–164. [Google Scholar] [CrossRef]
- Khan, S. Bioassay and enzymatic comparison of six entomopathogenic fungal isolates for virulence or toxicity against green peach aphids Myzus persicae. Afr. J. Biotechnol. 2012, 11, 14193–14203. [Google Scholar] [CrossRef]
- Roy, H.E.; Baverstock, J.; Chamberlain, K.; Pell, J.K. Do aphids infected with entomopathogenic fungi continue to produce and respond to alarm pheromone? Biocontrol. Sci. Technol. 2005, 15, 859–866. [Google Scholar] [CrossRef]
- Seye, F.; Bawin, T.; Boukraa, S.; Zimmer, J.Y.; Ndiaye, M.; Delvigne, F.; Francis, F. Effect of entomopathogenic Aspergillus strains against the pea aphid, Acyrthosiphon pisum (Hemiptera: Aphididae). Appl. Entomol. Zool. 2014, 49, 453–458. [Google Scholar] [CrossRef]
- Clifton, E.H.; Jaronski, S.T.; Coates, B.S.; Hodgson, E.W.; Gassmann, A.J. Effects of endophytic entomopathogenic fungi on soybean aphid and identification of Metarhizium isolates from agricultural fields. PLoS ONE 2018, 13, e0194815. [Google Scholar] [CrossRef] [PubMed]
- Hatano, E.; Baverstock, J.; Kunert, G.; Pell, J.K.; Weisser, W.W. Entomopathogenic fungi stimulate transgenerational wing induction in pea aphids, Acyrthosiphon pisum (Hemiptera: Aphididae). Ecol. Entomol. 2012, 37, 75–82. [Google Scholar] [CrossRef]
- Vu, V.H.; Hong, S., II; Kim, K. Selection of entomopathogenic fungi for aphid control. J. Biosci. Bioeng. 2007, 104, 498–505. [Google Scholar] [CrossRef]
- Koch, K.A. The Role of Entomopathogenic Fungi in the Management of Soybean Aphid. Ph.D. Thesis, University of Minnesota, Minneapolis, MN, USA, 2011. [Google Scholar]
- Saranya, S.; Ushakumari, R.; Jacob, S.; Philip, B.M. Efficacy of different entomopathogenic fungi against cowpea aphid, Aphis craccivora (Koch). J. Biopestic. 2010, 3, 138–142. [Google Scholar]
- Manoussopoulos, Y.; Mantzoukas, S.; Lagogiannis, I.; Goudoudaki, S.; Kambouris, M. Effects of three strawberry entomopathogenic fungi on the prefeeding behavior of the aphid Myzus persicae. J. Insect Behav. 2019, 32, 99–108. [Google Scholar] [CrossRef]
- González-Mas, N.; Sánchez-Ortiz, A.; Valverde-García, P.; Quesada-Moraga, E. Effects of endophytic entomopathogenic ascomycetes on the life-history traits of Aphis gossypii Glover and its interactions with melon plants. Insects 2019, 10, 165. [Google Scholar] [CrossRef] [PubMed]
- Jaber, L.R.; Araj, S.-E. Interactions among endophytic fungal entomopathogens (Ascomycota: Hypocreales), the green peach aphid Myzus persicae Sulzer (Homoptera: Aphididae), and the aphid endoparasitoid Aphidius colemani Viereck (Hymenoptera: Braconidae). Biol. Control 2018, 116, 53–61. [Google Scholar] [CrossRef]
- González-Mas, N.; Medina, M.C.; Sánchez, F.G.; Moraga, E.Q. Bottom—Up effects of endophytic Beauveria bassiana on multitrophic interactions between the cotton aphid, Aphis gossypii, and its natural enemies in melon. J. Pest Sci. 2019, 92, 1271–1281. [Google Scholar] [CrossRef]
- Lopez, D.C.; Zhu-Salzman, K.; Ek-Ramos, M.J.; Sword, G.A. The entomopathogenic fungal endophytes Purpureocillium lilacinum (formerly Paecilomyces lilacinus) and Beauveria bassiana negatively affect cotton aphid reproduction under both greenhouse and field conditions. PLoS ONE 2014, 9, e103891. [Google Scholar] [CrossRef]
- Vidal, S.; Jaber, L.R. Entomopathogenic fungi as endophytes: Plant–endophyte–herbivore interactions and prospects for use in biological control. Curr. Sci. 2015, 109, 46–54. [Google Scholar] [CrossRef]
- Gurulingappa, P.; McGee, P.A.; Sword, G. Endophytic Lecanicillium lecanii and Beauveria bassiana reduce the survival and fecundity of Aphis gossypii following contact with conidia and secondary metabolites. Crop Prot. 2011, 30, 349–353. [Google Scholar] [CrossRef]
- Skinner, M.; Parker, B.L.; Kim, J.S. Chapter 10—Role of Entomopathogenic Fungi in Integrated Pest Management Margaret; Elsevier Inc.: Amsterdam, The Netherlands, 2014. [Google Scholar] [CrossRef]
- Jaronski, S.T. Chapter 11—Mass Production of Entomopathogenic Fungi: State of the Art. In Mass Production of Beneficial Organisms; Morales-Ramos, J.A., Rojas, M.G., Shapiro-Ilan, D.I., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 357–413. [Google Scholar] [CrossRef]
- Chandler, D. Chapter 5—Basic and Applied Research on Entomopathogenic Fungi; Elsevier Inc.: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Klieber, J.; Reineke, A. The entomopathogen Beauveria bassiana has epiphytic and endophytic activity against the tomato leaf miner Tuta absoluta. J. Appl. Entomol. 2016, 140, 580–589. [Google Scholar] [CrossRef]
- Lacey, L.A. Chapter 1—Entomopathogens Used as Microbial Control Agents; Elsevier Inc.: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Pedrini, N.; Crespo, R.; Juárez, M.P. Biochemistry of insect epicuticle degradation by entomopathogenic fungi. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2007, 146, 124–137. [Google Scholar] [CrossRef]
- Bawin, T.; Seye, F.; Boukraa, S.; Zimmer, J.-Y.; Delvigne, F.; Francis, F. La lutte contre les moustiques (Diptera: Culicidae): Diversité des approches et application du contrôle biologique. Can. Entomol. 2015, 147, 476–500. [Google Scholar] [CrossRef]
- Jaronski, S.T.; Mascarin, G.M. Mass Production of Fungal Entomopathogens. In Microbial Control of Insect and Mite Pests; Elsevier: Amsterdam, The Netherlands, 2017; pp. 141–155. [Google Scholar] [CrossRef]
- Jaronski, S.T.; Jackson, M.A. Mass Production of Entomopathogenic Hypocreales, 2nd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2012. [Google Scholar] [CrossRef]
- Guesmi-Jouini, J.; Garrido-Jurado, I.; López-Díaz, C.; Ben Halima-Kamel, M.; Quesada-Moraga, E. Establishment of fungal entomopathogens Beauveria bassiana and Bionectria ochroleuca (Ascomycota: Hypocreales) as endophytes on artichoke Cynara scolymus. J. Invertebr. Pathol. 2014, 119, 1–4. [Google Scholar] [CrossRef]
- Rondot, Y.; Reineke, A. Endophytic Beauveria bassiana in grapevine Vitis vinifera (L.) reduces infestation with piercing-sucking insects. Biol. Control 2018, 116, 82–89. [Google Scholar] [CrossRef]
- Elliot, S.L.; Sabelis, M.W.; Janssen, A.; Van der Geest, L.P.S.; Beerling, E.A.M.; Fransen, J. Can plants use entomopathogens as bodyguards? Ecol. Lett. 2000, 3, 228–235. [Google Scholar] [CrossRef]
- Vega, F.E.; Posada, F.; Catherine Aime, M.; Pava-Ripoll, M.; Infante, F.; Rehner, S.A. Entomopathogenic fungal endophytes. Biol. Control 2008, 46, 72–82. [Google Scholar] [CrossRef]
- Biswas, C.; Dey, P.; Satpathy, S.; Satya, P. Establishment of the fungal entomopathogen Beauveria bassiana as a season long endophyte in jute (Corchorus olitorius) and its rapid detection using SCAR marker. BioControl 2012, 57, 565–571. [Google Scholar] [CrossRef]
- Russo, M.L.; Pelizza, S.A.; Cabello, M.N.; Stenglein, S.A.; Scorsetti, A.C. Endophytic colonisation of tobacco, corn, wheat and soybeans by the fungal entomopathogen Beauveria bassiana (Ascomycota, Hypocreales). Biocontrol Sci. Technol. 2015, 25, 475–480. [Google Scholar] [CrossRef]
- Rúa, M.A.; McCulley, R.L.; Mitchell, C.E. Fungal endophyte infection and host genetic background jointly modulate host response to an aphid-transmitted viral pathogen. J. Ecol. 2013, 101, 1007–1018. [Google Scholar] [CrossRef]
- Lehtonen, P.T.; Helander, M.; Siddiqui, S.A.; Lehto, K.; Saikkonen, K. Endophytic fungus decreases plant virus infections in meadow ryegrass (Lolium pratense). Biol. Lett. 2006, 2, 620–623. [Google Scholar] [CrossRef] [PubMed][Green Version]
- González-Mas, N.; Quesada-Moraga, E.; Plaza, M.; Fereres, A.; Moreno, A. Changes in feeding behaviour are not related to the reduction in the transmission rate of plant viruses by Aphis gossypii (Homoptera: Aphididae) to melon plants colonized by Beauveria bassiana (Ascomycota: Hypocreales). Biol. Control 2019, 130, 95–103. [Google Scholar] [CrossRef]
- Guy, P.L. Barley yellow dwarf viruses in Japanese pasture grasses and lack of correlation with the presence of fungal endophytes. Plant Pathol. 1993, 42, 1–5. [Google Scholar] [CrossRef]
- Shan, L.T.; Feng, M.G. Evaluation of the biocontrol potential of various Metarhizium isolates against green peach aphid Myzus persicae (Homoptera: Aphididae). Pest Manag. Sci. 2010, 66, 669–675. [Google Scholar] [CrossRef]
- Sookar, P.; Bhagwant, S.; Awuor Ouna, E. Isolation of entomopathogenic fungi from the soil and their pathogenicity to two fruit fly species (Diptera: Tephritidae). J. Appl. Entomol. 2008, 132, 778–788. [Google Scholar] [CrossRef]
- Baverstock, J.; Clark, S.J.; Alderson, P.G.; Pell, J.K. Intraguild interactions between the entomopathogenic fungus Pandora neoaphidis and an aphid predator and parasitoid at the population scale. J. Invertebr. Pathol. 2009, 102, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Quintela, E.D.; Mascarin, G.M.; da Silva, R.A.; Barrigossi, J.A.F.; da Martins, J.F.S. Enhanced susceptibility of Tibraca limbativentris (Heteroptera: Pentatomidae) to Metarhizium anisopliae with sublethal doses of chemical insecticides. Biol. Control 2013, 66, 56–64. [Google Scholar] [CrossRef]
- Mbata, G.N.; Shapiro-Ilan, D. The potential for controlling Pangaeus bilineatus (Heteroptera: Cydnidae) using a combination of entomopathogens and an insecticide. J. Econ. Entomol. 2013, 106, 2072–2076. [Google Scholar] [CrossRef] [PubMed]
- Klinger, E. Susceptibility of Adult Colorado Potato Beetle (Leptinotarsa decemlineata) to the Fungal Entomopathogen Beauveria bassiana. Master’s Thesis, The University of Maine, Orono, ME, USA, 2003. [Google Scholar]
- Inglis, G.D.; Johnson, D.L.; Goettel, M.S. Effects of temperature and thermoregulation on mycosis by Beauveria bassiana in grasshoppers. Biol. Control 1996, 7, 131–139. [Google Scholar] [CrossRef]
- Fargues, J. Étude des conditions d’infection des larves de doryphore, Leptinotarsa decemlineata say, par Beauveria bassiana (Bals.) Vuill. [Fungi imperfecti]. Entomophaga 1972, 17, 319–337. [Google Scholar] [CrossRef]
- Liu, B.; Tzeng, Y. Development and applications of destruxins: A review. Biotechnol. Adv. 2012, 30, 1242–1254. [Google Scholar] [CrossRef]
- Jaber, L.R.; Ownley, B.H. Can we use entomopathogenic fungi as endophytes for dual biological control of insect pests and plant pathogens? Biol. Control 2017, 107, 50–59. [Google Scholar] [CrossRef]
- Van Wees, S.C.; Van der Ent, S.; Pieterse, C.M. Plant immune responses triggered by beneficial microbes. Curr. Opin. Plant Biol. 2008, 11, 443–448. [Google Scholar] [CrossRef]
- Aragón, S.M. How Entomopathogenic Endophytic Fungi Modulate Plant-Insect Interactions. Ph.D. Thesis, Georg-August-University Goettingen, Göttingen, Germany, 2016. [Google Scholar]
- Rajabaskar, D.; Bosque-Pérez, N.A.; Eigenbrode, S.D. Preference by a virus vector for infected plants is reversed after virus acquisition. Virus Res. 2014, 186, 32–37. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Donkersley, P.; Dong, Y.; Chen, X.; Zang, Y.; Xu, P.; Ren, G. Preference of the aphid Myzus persicae (Hemiptera: Aphididae) for tobacco plants at specific stages of potato virus Y infection. Arch. Virol. 2019, 164, 1567–1573. [Google Scholar] [CrossRef] [PubMed]
- Fingu-Mabola, J.C.; Martin, C.; Bawin, T.; Verheggen, F.J.; Francis, F. Does the infectious status of aphids influence their preference towards healthy, virus-infected and endophytically colonized plants? Insects 2020, 11, 435. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, R.L.; Moscardini, V.F.; Gontijo, P.C.; Sâmia, R.R.; Marucci, R.C.; Budia, F.; Carvalho, G.A. Life history parameters and feeding preference of the green lacewing Ceraeochrysa cubana fed with virus-free and potato leafroll virus-infected Myzus persicae. BioControl 2016, 61, 671–679. [Google Scholar] [CrossRef]
- Haas, J.; Lozano, E.R.; Poppy, G.M. A simple, light clip-cage for experiments with aphids. Agric. For. Entomol. 2018, 20, 589–592. [Google Scholar] [CrossRef]
- van den Heuvel, J.F.J.M.; Boerma, T.M.; Peters, D. Transmission of Potato leafroll virus from plants and artificial diets by Myzus persicae. Phytopathology 1991, 81, 150–154. [Google Scholar] [CrossRef]
- Humber, R.A. Fungi. In Manual of Techniques in Insect Pathology; Elsevier: Amsterdam, The Netherlands, 1997; pp. 153–185. [Google Scholar] [CrossRef]
- Driver, F.; Milner, R.J.; Trueman, J.W.H. A taxonomic revision of Metarhizium based on a phylogenetic analysis of rDNA sequence data. Mycol. Res. 2000, 104, 134–150. [Google Scholar] [CrossRef]
- Lenth, R. V Least-Squares Means: The R Package lsmeans. J. Stat. Softw. 2016, 69. [Google Scholar] [CrossRef]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef]
- Kaplan, E.L.; Meier, P. Nonparametric estimation from incomplete observations. J. Am. Stat. Assoc. 1958, 53, 457. [Google Scholar] [CrossRef]
- Therneau, T.M.; Grambsch, P.M. Modeling Survival Data: Extending the Cox Model; Statistics for Biology and Health; Springer New York: New York, NY, USA, 2000. [Google Scholar] [CrossRef]
- Cox, D.R. Regression models and life-tables. J. R. Stat. Soc. Ser. B 1972, 34, 187–220. [Google Scholar] [CrossRef]
- Best, D.J.; Roberts, D.E. Algorithm AS 89: The Upper Tail Probabilities of Spearman’s Rho. J. R. Stat. Soc. 1975, 24, 377–379. [Google Scholar] [CrossRef]
- Hollander, M.; Wolfe, D.A.; Chicken, E. Nonparametric Statistical Methods, Wiley Series in Probability and Statistics; Wiley: Hoboken, NJ, USA, 2015. [Google Scholar] [CrossRef]
- Mangiafico, S.S. Summary and Analysis of Extension Program Evaluation in R; Version 1.18.1.; Rutgers Co.: New Brunswick, NJ, USA, 2016. [Google Scholar]
- Al-alawi, M.S.; Obeidat, M. Selection of Beauveria bassiana (Balsamo) Vuillemin isolates for management of Myzus persicae (sultzar) (hom.: Aphidae) based on virulence and growth related characteristics. Am. J. Agric. Biol. Sci. 2014, 9, 94–100. [Google Scholar] [CrossRef]
- Allegrucci, N.; Velazquez, M.S.; Russo, M.L.; Vianna, M.F.; Abarca, C.; Scorsetti, A.C. Establishment of the entomopathogenic fungus Beauveria bassiana as an endophyte in Capsicum annuum and its effects on the aphid pest Myzus persicae (Homoptera: Aphididae). Rev. Biol. Trop. 2020, 68, 1084–1094. [Google Scholar] [CrossRef]
- Akbari, S.; Ali Safavi, S.; Ghosta, Y. Efficacy of Beauveria bassiana (Blas.) Vuill. against cabbage aphid Brevicoryne brassicae L. (Hem.: Aphididae) in laboratory condition. Arch. Phytopathol. Plant Prot. 2014, 47, 1454–1458. [Google Scholar] [CrossRef]
- Abd El-Salam, A.M.E.; El-Hawary, F.M.A. Lethal and pathogenic effects of Beauveria bassiana and Lecanicillium lecanii on the adult and nymph of Aphis craccivora Koch. Arch. Phytopathol. Plant Prot. 2011, 44, 57–66. [Google Scholar] [CrossRef]
- Knudsen, G.R.; Schotzko, D.J. Spatial simulation of epizootics caused by Beauveria bassiana in russian wheat aphid populations. Biol. Control 1999, 16, 318–326. [Google Scholar] [CrossRef]
- Dara, S.K.; Dara, S.S. Endophytic colonization and pest management potential of Beauveria bassiana in strawberries. J. Berry Res. 2013, 3, 203–211. [Google Scholar] [CrossRef]
- Baverstock, J.; Roy, H.E.; Clark, S.J.; Alderson, P.G.; Pell, J.K. Effect of fungal infection on the reproductive potential of aphids and their progeny. J. Invertebr. Pathol. 2006, 91, 136–139. [Google Scholar] [CrossRef]
- Shrestha, G.; Enkegaard, A.; Steenberg, T. Laboratory and semi-field evaluation of Beauveria bassiana (Ascomycota: Hypocreales) against the lettuce aphid, Nasonovia ribisnigri (Hemiptera: Aphididae). Biol. Control 2015, 85, 37–45. [Google Scholar] [CrossRef]
- Rashki, M.; Kharazi-pakdel, A.; Allahyari, H.; van Alphen, J.J.M. Interactions among the entomopathogenic fungus, Beauveria bassiana (Ascomycota: Hypocreales), the parasitoid, Aphidius matricariae (Hymenoptera: Braconidae), and its host, Myzus persicae (Homoptera: Aphididae). Biol. Control 2009, 50, 324–328. [Google Scholar] [CrossRef]
- Bayındır Erol, A.; Abdelaziz, O.; Birgücü, A.K.; Senoussi, M.M.; Oufroukh, A.; Karaca, İ. Effects of some entomopathogenic fungi on the aphid species, Aphis gossypii Glover (Hemiptera: Aphididae). Egypt. J. Biol. Pest Control 2020, 30, 3–6. [Google Scholar] [CrossRef]
- Sahayaraj, K.; Namachivayam, S. Field evaluation of three entomopathogenic fungi on groundnut pests. Tropicultura 2011, 29, 143–147. [Google Scholar]
- Kaushal, K.S.; Ajoy, K.C.; Priyanka, K. Entomopathogenic Fungi. In Ecofriendly Pest Management for Food Security; Omkar, Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 475–505. [Google Scholar] [CrossRef]
- Toledo, A.V.; de Remes Lenicov, A.M.M.; López Lastra, C.C. Pathogenicity of fungal isolates (Ascomycota: Hypocreales) against Peregrinus maidis, Delphacodes kuscheli (Hemiptera: Delphacidae), and Dalbulus maidis (Hemiptera: Cicadellidae), vectors of corn diseases. Mycopathologia 2007, 163, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Bawin, T.; Seye, F.; Boukraa, S.; Zimmer, J.Y.; Raharimalala, F.N.; Ndiaye, M.; Compere, P.; Delvigne, F.; Francis, F. Histopathological effects of Aspergillus clavatus (Ascomycota: Trichocomaceae) on larvae of the southern house mosquito, Culex quinquefasciatus (Diptera: Culicidae). Fungal Biol. 2016, 120, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Gurulingappa, P.; Sword, G.A.; Murdoch, G.; McGee, P.A. Colonization of crop plants by fungal entomopathogens and their effects on two insect pests when in planta. Biol. Control 2010, 55, 34–41. [Google Scholar] [CrossRef]
- Jallow, M.F.A.; Dugassa-Gobena, D.; Vidal, S. Influence of an endophytic fungus on host plant selection by a polyphagous moth via volatile spectrum changes. Arthropod. Plant. Interact. 2008, 2, 53–62. [Google Scholar] [CrossRef]
- Reddy, N.P.; Ali Khan, A.P.; Devi, U.K.; Sharma, H.C.; Reineke, A. Treatment of millet crop plant (Sorghum bicolor) with the entomopathogenic fungus (Beauveria bassiana) to combat infestation by the stem borer, Chilo partellus Swinhoe (Lepidoptera: Pyralidae). J. Asia Pac. Entomol. 2009, 12, 221–226. [Google Scholar] [CrossRef][Green Version]
- Quesada-Moraga, E.; Munoz-Ledesma, F.J.; Santiago-Alvarez, C. Systemic protection of Papaver somniferum L. against Iraella luteipes (Hymenoptera: Cynipidae) by an endophytic strain of Beauveria bassiana (Ascomycota: Hypocreales). Environ. Entomol. 2009, 38, 723–730. [Google Scholar] [CrossRef]
- Akutse, K.S.; Maniania, N.K.; Fiaboe, K.K.M.; Berg, J.V.A.N.D.E.N.; Ekesi, S. Endophytic colonization of Vicia faba and Phaseolus vulgaris (Fabaceae) by fungal pathogens and their effects on the life- history parameters of Liriomyza huidobrensis (Diptera: Agromyzidae). Fungal Ecol. 2013, 6, 293–301. [Google Scholar] [CrossRef]
- Powell, W.A.; Klingeman, W.E.; Ownley, B.H.; Gwinn, K.D. Evidence of endophytic Beauveria bassiana in seed-treated tomato plants acting as a systemic entomopathogen to larval Helicoverpa zea (Lepidoptera: Noctuidae). J. Entomol. Sci. 2009, 44, 391–396. [Google Scholar] [CrossRef]
- Wagner, B.L.; Lewis, L.C. Colonization of corn, Zea mays, by the entomopathogenic fungus Beauveria bassiana. Appl. Environ. Microbiol. 2000, 66, 3468–3473. [Google Scholar] [CrossRef]
- Meister, B.; Krauss, J.; Härri, S.A.; Victoria Schneider, M.; Müller, C.B. Fungal endosymbionts affect aphid population size by reduction of adult life span and fecundity. Basic Appl. Ecol. 2006, 7, 244–252. [Google Scholar] [CrossRef]
- Collinson, N.P.; Mann, R.C.; Giri, K.; Malipatil, M.; Kaur, J.; Spangenberg, G.; Valenzuela, I. Novel bioassay to assess antibiotic effects of fungal endophytes on aphids. PLoS ONE 2020, 15, e0228813. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, F.; Vidal, S.; Rautenbach, F.; Lewu, F.; Nchu, F. Effects of Beauveria bassiana (Hypocreales) on plant growth and secondary metabolites of extracts of hydroponically cultivated chive (Allium schoenoprasum L. [Amaryllidaceae]). Heliyon 2019, 5, e03038. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, G. Review on safety of the entomopathogenic fungi Beauveria bassiana and Beauveria brongniartii. Biocontrol Sci. Technol. 2007, 17, 553–596. [Google Scholar] [CrossRef]
- Liu, H.; Xie, L.; Wang, J.; Guo, Q.; Yang, S.; Liang, P.; Wang, C.; Lin, M.; Xu, Y.; Zhang, L. The stress-responsive and host-oriented role of nonribosomal peptide synthetases in an entomopathogenic fungus, Beauveria bassiana. J. Microbiol. Biotechnol. 2017, 27, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Prince, G.; Chandler, D. Susceptibility of Myzus persicae, Brevicoryne brassicae and Nasonovia ribisnigri to fungal biopesticides in laboratory and field experiments. Insects 2020, 11, 55. [Google Scholar] [CrossRef]
- Jandricic, S.E.; Filotas, M.; Sanderson, J.P.; Wraight, S.P. Pathogenicity of conidia-based preparations of entomopathogenic fungi against the greenhouse pest aphids Myzus persicae, Aphis gossypii, and Aulacorthum solani (Hemiptera: Aphididae). J. Invertebr. Pathol. 2014, 118, 34–46. [Google Scholar] [CrossRef]
- de Oliveira, C.F.; Long, E.Y.; Finke, D.L. A negative effect of a pathogen on its vector? A plant pathogen increases the vulnerability of its vector to attack by natural enemies. Oecologia 2014, 174, 1169–1177. [Google Scholar] [CrossRef][Green Version]
- Mauck, K.E.; De Moraes, C.M.; Mescher, M.C. Biochemical and physiological mechanisms underlying effects of Cucumber mosaic virus on host-plant traits that mediate transmission by aphid vectors. Plant Cell Environ. 2014, 37, 1427–1439. [Google Scholar] [CrossRef]
- Souza, P.F.N.; Garcia-Ruiz, H.; Carvalho, F.E.L. What proteomics can reveal about plant–virus interactions? Photosynthesis-related proteins on the spotligh. Physiol. Behav. 2017, 176, 139–148. [Google Scholar] [CrossRef]
- Mauck, K.E.; De Moraes, C.M.; Mescher, M.C. Effects of pathogens on sensory-mediated interactions between plants and insect vectors. Curr. Opin. Plant Biol. 2016, 32, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Jaber, L.R.; Salem, N.M. Endophytic colonisation of squash by the fungal entomopathogen Beauveria bassiana (Ascomycota: Hypocreales) for managing Zucchini yellow mosaic virus in cucurbits. Biocontrol Sci. Technol. 2014, 24, 1096–1109. [Google Scholar] [CrossRef]
- Muvea, A.M.; Subramanian, S.; Maniania, N.K.; Poehling, H.M.; Ekesi, S.; Meyhöfer, R. Endophytic colonization of onions induces resistance against viruliferous thrips and virus replication. Front. Plant Sci. 2018, 871, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ownley, B.H.; Gwinn, K.D.; Vega, F.E. Endophytic fungal entomopathogens with activity against plant pathogens: Ecology and evolution. BioControl 2010, 55, 113–128. [Google Scholar] [CrossRef]
- Vega, F.E. The use of fungal entomopathogens as endophytes in biological control: A review. Mycologia 2018, 110, 4–30. [Google Scholar] [CrossRef] [PubMed]
- Golo, P.S.; Gardner, D.R.; Grilley, M.M.; Takemoto, J.Y.; Krasnoff, S.B.; Pires, M.S.; Fernandes, E.K.K.; Bittencourt, V.R.E.P.; Roberts, D.W. Production of destruxins from Metarhizium spp. fungi in artificial medium and in endophytically colonized cowpea plants. PLoS ONE 2014, 9, e104946. [Google Scholar] [CrossRef]
- Loerenstein, G. Localization and induced resistance in virus-infected plants. Annu. Rev. Phytopathol. 1972, 10, 177–206. [Google Scholar] [CrossRef]
- Behie, S.W.; Jones, S.J.; Bidochka, M.J. Plant tissue localization of the endophytic insect pathogenic fungi Metarhizium and Beauveria. Fungal Ecol. 2015, 13, 112–119. [Google Scholar] [CrossRef]
- Quesada-Moraga, E.; Landa, B.B.; Muñoz-Ledesma, J.; Jiménez-Diáz, R.M.; Santiago-Álvarez, C. Endophytic colonisation of opium poppy, Papaver somniferum, by an entomopathogenic Beauveria bassiana strain. Mycopathologia 2006, 161, 323–329. [Google Scholar] [CrossRef]

| Plant Treatment | Insect | Treatment | Description | |
|---|---|---|---|---|
| Infectious Status | EPF Treatment | |||
| Fungal-free (Ffp) | Non-Viruliferous (I−) | Spore-free (sf) | 1. Ffp:I−:sf | Non-viruliferous insects, sprayed with spore-free solution, released on a fungal-free plant |
| Beauveria bassiana spores (bs) | 2. Ffp:I−:bs | Non-viruliferous insects, sprayed with B. bassiana spore suspension, released on a fungal-free plant | ||
| Metarhizium acridum spores (ms) | 3. Ffp:I−:ms | Non-viruliferous insects, sprayed with M. acridum spore suspension, released on a fungal-free plant | ||
| Viruliferous (I+) | Spore-free (sf) | 4. Ffp:I+:sf | Viruliferous insects, sprayed with spore-free solution, released on a fungal-free plant | |
| B. bassiana spores (bs) | 5. Ffp:I+:bs | Viruliferous insects, sprayed with B. bassiana spore suspension, released on a fungal-free plant | ||
| M. acridum spores (ms) | 6. Ffp:I+:ms | Viruliferous insects, sprayed with M. acridum spore suspension, released on a fungal-free plant | ||
| B. bassiana-inoculated (Bp) | Non-Viruliferous (I−) | Spore-free (sf) | 7. Bp:I−:sf | Non-viruliferous insects, sprayed with spore-free solution, released on a B. bassiana-inoculated plant |
| B. bassiana spores (bs) | 8. Bp:I−:bs | Non-viruliferous insects, sprayed with B. bassiana spore suspension, released on a B. basiana-inoculated plant | ||
| Viruliferous (I+) | Spore-free (sf) | 9. Bp:I+:sf | Viruliferous insects, sprayed with spore-free solution, released on a B. bassiana-inoculated plant | |
| B. bassiana spores (bs) | 10. Bp:I+:bs | Viruliferous insects, sprayed with B. bassiana spore suspension, released on a B. bassiana-inoculated plant | ||
| M. acridum-inoculated (Mp) | Non-Viruliferous (I−) | Spore-free (sf) | 11. Mp:I−:sf | Non-viruliferous insects, sprayed with spore-free solution, released on a M. acridum-inoculated plant |
| M. acridum spores (ms+) | 12. Mp:I−:ms | Non-viruliferous insects, sprayed with M. acridum spore suspension, released on a M. acridum-inoculated plant | ||
| Viruliferous (I+) | Spore-free (sf) | 13. Mp:I+:sf | Viruliferous insects, sprayed with spore-free solution, released on a M. acridum-inoculated plant | |
| M. acridum spores (ms) | 14. Mp:I+:ms | Viruliferous insects, sprayed with M. acridum spore suspension, released on a M. acridum-inoculated plant | ||
| Source Plant | Recipient Plant | Treatment Combination | Description |
|---|---|---|---|
| Fungal-free (Ffp) | 1. Fungal-free (Ffp) | 1. Ffp–Ffp | Vectors from fungal-free plant released on fungal-free plants (control) |
| 2. B. bassiana plant (Bp) | 2. Ffp–Bp | Vectors from fungal-free plant released on B. bassiana-inoculated plants | |
| 3. M. acridum plant (Mp) | 3. Ffp–Mp | Vectors from fungal-free plant released on M. acridum-inoculated plants | |
| B. bassiana plant (Bp) | 4. Fungal-free (Ffp) | 4. Bp–Ffp | Vectors from B. bassiana-inoculated plant released on fungal-free plants |
| 5. B. bassiana plant (Bp) | 5. Bp–Bp | Vectors from B. bassiana-inoculated plant released on B. bassiana-inoculated plants | |
| 6. M. acridum plant (Mp) | 6. Bp–Mp | Vectors from B. bassiana-inoculated plant released on M. acridum-inoculated plants | |
| M. acridum plant (Mp) | 7. Fungal-free (Ffp) | 7. Mp–Ffp | Vectors from M. acridum-inoculated plant released on fungal-free plants |
| 8. B. bassiana plant (Bp) | 8. Mp–Bp | Vectors from M. acridum-inoculated plant released on B. bassiana-inoculated plants | |
| 9. M. acridum plant (Mp) | 9. Mp–Mp | Vectors from M. acridum-inoculated plant released on M. acridum-inoculated plants |
| Bp:I−:sf | |||||||
| Bp:I−:bs | - | Bp:I−:bs | |||||
| Bp:I+:sf | - | - | Bp:I+:sf | ||||
| Bp:I+:bs | *** | * | ** | Bp:I+:bs | |||
| Ffp:I−:sf | * | ** | ** | **** | Ffp:I−:sf | ||
| Ffp:I−:bs | - | + | + | **** | - | Ffp:I−:bs | |
| Ffp:I+:sf | **** | **** | **** | **** | * | ** | Ffp:I+:sf |
| Ffp:I+:bs | - | + | - | **** | - | - | *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fingu-Mabola, J.C.; Bawin, T.; Francis, F. Direct and Indirect Effect via Endophytism of Entomopathogenic Fungi on the Fitness of Myzus persicae and Its Ability to Spread PLRV on Tobacco. Insects 2021, 12, 89. https://doi.org/10.3390/insects12020089
Fingu-Mabola JC, Bawin T, Francis F. Direct and Indirect Effect via Endophytism of Entomopathogenic Fungi on the Fitness of Myzus persicae and Its Ability to Spread PLRV on Tobacco. Insects. 2021; 12(2):89. https://doi.org/10.3390/insects12020089
Chicago/Turabian StyleFingu-Mabola, Junior Corneille, Thomas Bawin, and Frédéric Francis. 2021. "Direct and Indirect Effect via Endophytism of Entomopathogenic Fungi on the Fitness of Myzus persicae and Its Ability to Spread PLRV on Tobacco" Insects 12, no. 2: 89. https://doi.org/10.3390/insects12020089
APA StyleFingu-Mabola, J. C., Bawin, T., & Francis, F. (2021). Direct and Indirect Effect via Endophytism of Entomopathogenic Fungi on the Fitness of Myzus persicae and Its Ability to Spread PLRV on Tobacco. Insects, 12(2), 89. https://doi.org/10.3390/insects12020089







