Stink Bug Communication and Signal Detection in a Plant Environment
Abstract
:Simple Summary
Abstract
1. Introduction
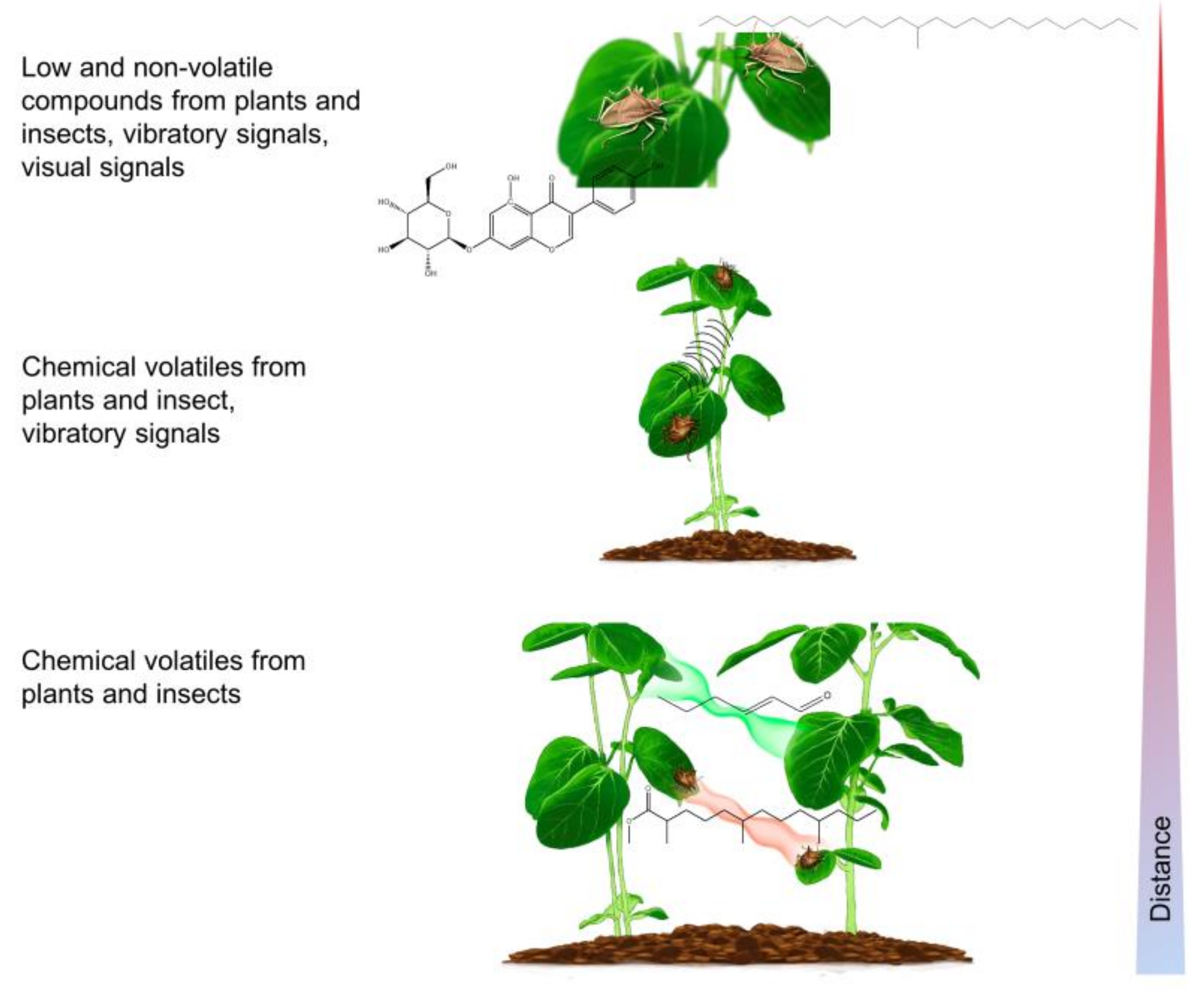
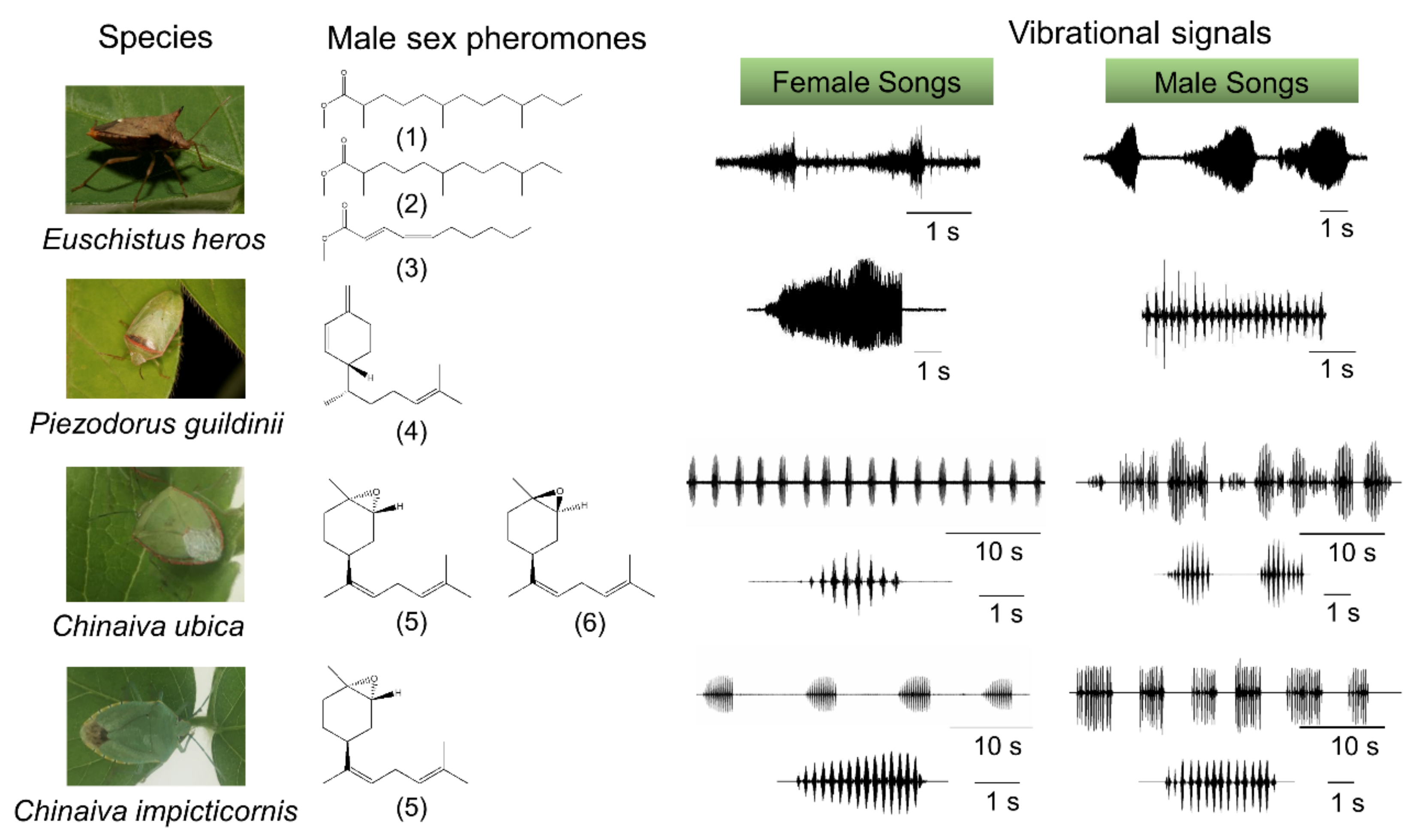
2. Long-Range Sex Pheromone Communication in Stink Bugs in a Plant Environment
Neuronal Basis of the Perception of Semiochemicals in Stink Bugs
3. Multimodal Communication on a Plant
3.1. Communication during Calling, Courtship and Rivalry
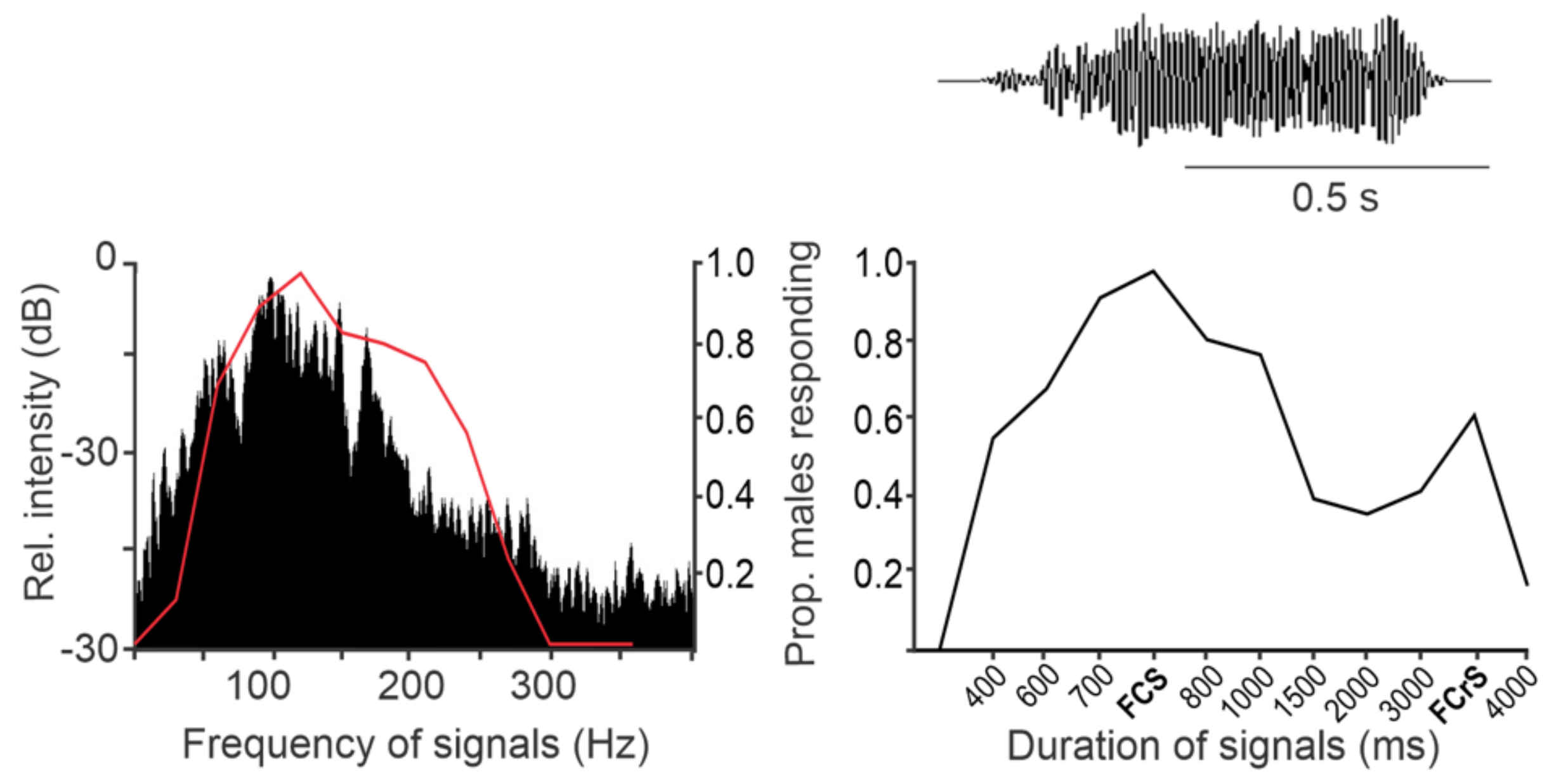
3.2. Plants as the Vibratory Signal Transmission Medium
3.3. The Effect of Noise on Signal Transmission on Plants
4. Detection of Vibratory Signals on a Plant
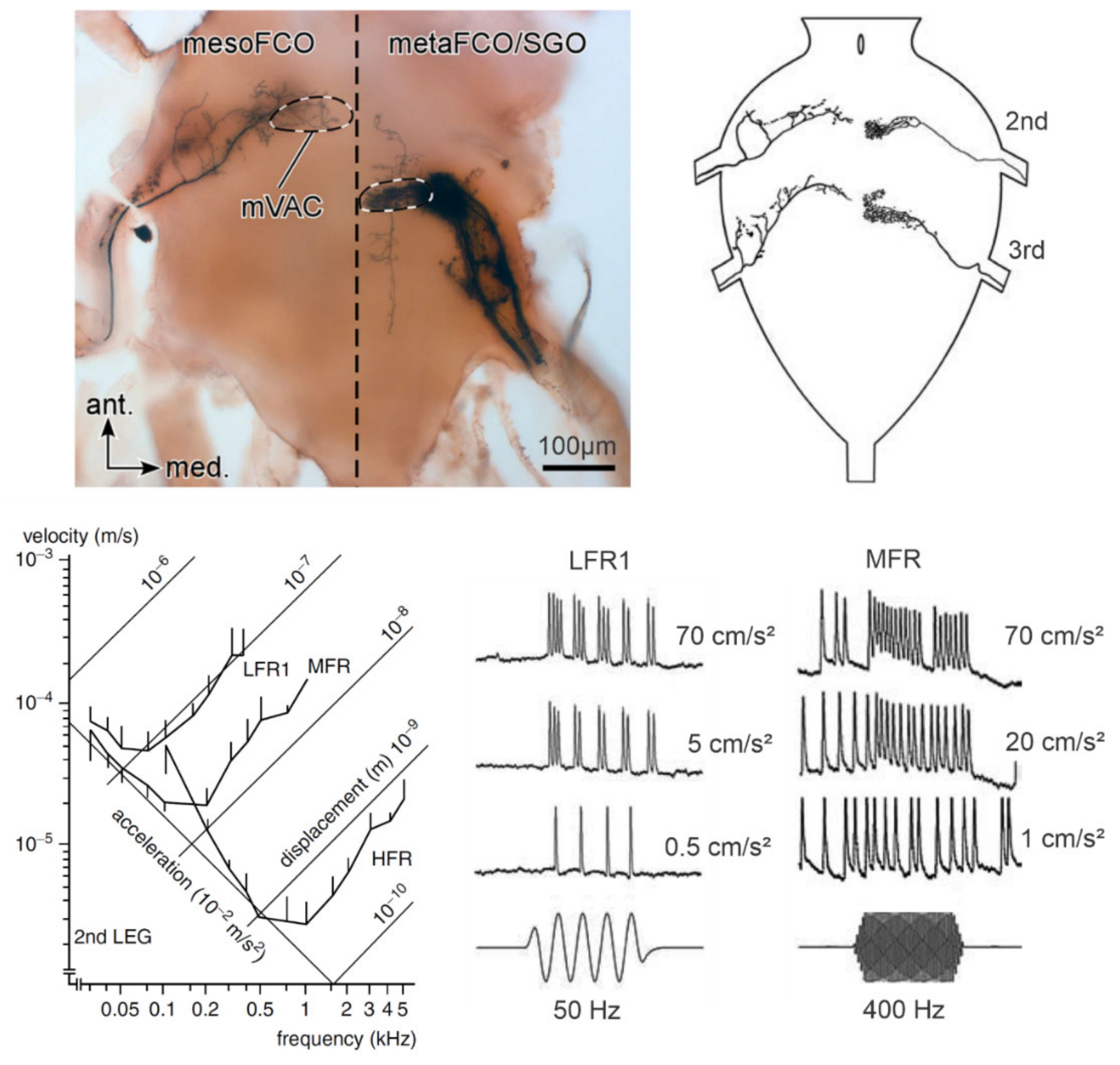
4.1. Vibration Receptor Organs
4.2. Central Projections of the Vibration Receptor Neurons
5. Processing of Vibratory Input in the Ventral Nerve Cord
5.1. Frequency and Time Pattern Coding in Ventral Nerve Cord Neurons
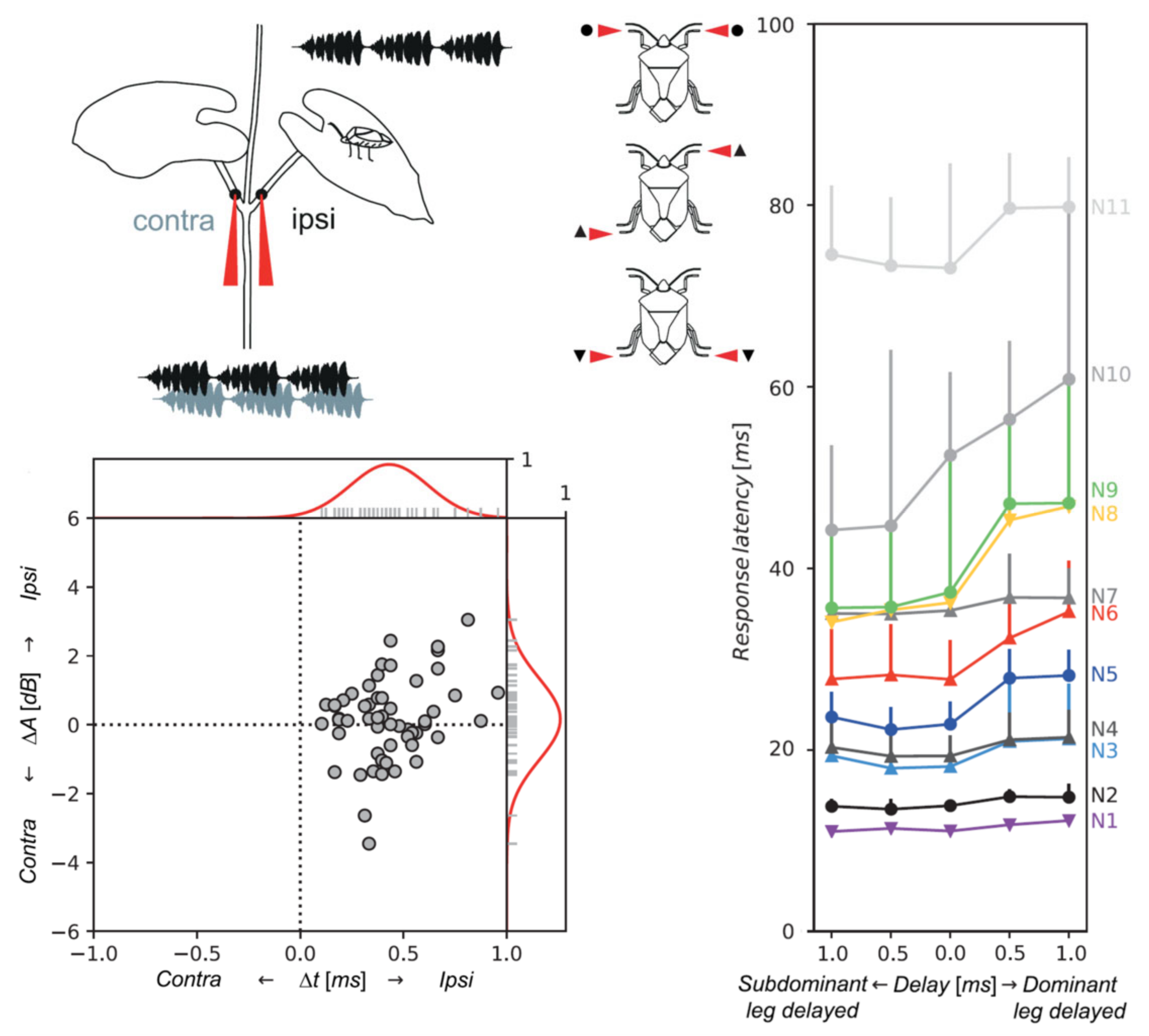
5.2. Neuronal Basis of Vibrational Directionality and Mate Recognition on a Plant
6. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Grazia, J.; Schwertner, L.A. Stink Bug Classification, Phylogeny, Biology and Reproductive Behavior. In Biorational Control Based on Communication Processes, 1st ed.; Čokl, A., Borges, M., Eds.; CRC Press: Boca Raton, FL, USA, 2017; Volume 1, pp. 1–30. [Google Scholar]
- Grazia, J.; Panizzi, A.R.; Schwertner, L.A.; Campos, L.A.; Garbelotto, T.A. True Bugs (Heteroptera) of the Neotropics. In Short Views on Insect Genomics and Proteomics; Springer: Singapore, 2015; pp. 681–756. [Google Scholar]
- Panizzi, A.R.; Lucini, T. Host plant-stink bug relationships. In Biorational Control Based on Communication Processes; CRC Press: Boca Raton, FL, USA, 2017; Volume 5, pp. 31–85. [Google Scholar]
- Laumann, R.A.; Bottura Maccagnan, D.H.; Čokl, A. Use of Vibratory Signals for Stink Bug Monitoring and Control. In Biorational Control Based on Communication Processes; CRC Press: Boca Raton, FL, USA, 2017; Volume 1, pp. 226–245. [Google Scholar]
- Panizzi, A.R.; McPherson, J.E.; James, D.G.; Javahery, M.; McPherson, R.M. Stink bugs (Pentatomidae). In Heteroptera of Economic Importance, 1st ed.; Schaefer, C.W., Panizzi, A.R., Eds.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2000; pp. 421–474. ISBN 9780849306952. [Google Scholar]
- McPherson, J.E.; McPherson, R. Stink Bugs of Economic Importance in America North of Mexico; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Smaniotto, L.F.; Panizzi, A.R. Interactions of Selected Species of Stink Bugs (Hemiptera: Heteroptera: Pentatomidae) from Leguminous Crops with Plants in the Neotropics. Fla. Èntomol. 2015, 98, 7–17. [Google Scholar] [CrossRef]
- Esquivel, J.F.; Musolin, D.L.; Jones, W.A.; Rabitsch, W.; Greene, J.K.; Toews, M.D.; Schwertner, C.F.; Grazia, J.; McPherson, R.M. Nezara viridula (L.). In Invasive Stink Bugs and Related Species (Pentatomoidea); CRC Press: Boca Raton, FL, USA, 2018; pp. 351–424. [Google Scholar]
- Esquivel, J.F.; Medrano, E.G. Ingestion of a Marked Bacterial Pathogen of Cotton Conclusively Demonstrates Feeding by First Instar Southern Green Stink Bug (Hemiptera: Pentatomidae): Table 1. Environ. Èntomol. 2014, 43, 110–115. [Google Scholar] [CrossRef] [Green Version]
- Kester, K.M.; Smith, C.M. Effects of diet on growth, fecundity and duration of tethered flight of Nezara viridula. Èntomol. Exp. Appl. 1984, 35, 75–81. [Google Scholar] [CrossRef]
- Panizzi, A.R.; Slansky, S.I. Performance of nymphal and adult southern green stink bug on an overwintering host and impact of nymph to adult food-switch. Entomol. Exp. Appl. 1991, 68, 109–115. [Google Scholar] [CrossRef]
- Da Silva, C.C.A.; Blassioli-Moraes, M.C.; Borges, M.; Laumann, R.A. Food diversification with associated plants increases the performance of the Neotropical stink bug, Chinavia impicticornis (Hemiptera: Pentatomidae). Arthropod Plant Interact. 2018, 13, 423–429. [Google Scholar] [CrossRef]
- Čokl, A.; Laumann, R.A.; Stritih, N. Substrate-borne vibratory communication. In Biorational Control Based on Communication Processes; CRC Press: Boca Raton, FL, USA, 2017; Volume 5, pp. 125–164. [Google Scholar]
- Čokl, A.; Blassioli-Moraes, M.C.; Laumann, R.A.; Žunič, A.; Borges, M. Stinkbugs—multisensory communication with chemical and vibratory signals transmitted through different media. In Biotremology—Studying Vibrational Behavior, 1st ed.; Hill, P.S.M., Lakes-Harlan, R., Mazzoni, V., Narins, P.M., Virant-Doberlet, M., Wessel, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 91–122. [Google Scholar] [CrossRef]
- Čokl, A.; Žunič-Kosi, A.; Laumann, R.A. Stink Bug Communication with Multimodal Signals Transmitted through Air and Substrate. Emerg. Sci. J. 2019, 3, 407–424. [Google Scholar] [CrossRef] [Green Version]
- Borges, M.; Blassioli-Moraes, M.C. The semiochemistry of Pentatomidae in stinkbugs. In Biorational Control Based on Communication Processes; CRC Press: Boca Raton, FL, USA, 2017; Volume 5, pp. 95–124. [Google Scholar]
- Borges, M.; Jepson, P.C.; Howse, P.E. Long-range mate location and close-range courtship behaviour of the Green Stink Bug, Nezara viridula and its mediation by sex pheromones. Èntomol. Exp. Appl. 1987, 44, 205–212. [Google Scholar] [CrossRef]
- Millar, J.G. Pheromones of True Bugs. Top. Curr. Chem. 2004, 240, 37–84. [Google Scholar] [CrossRef]
- McBrien, H.; Millar, J.G. Phytophagous bugs. In Pheromone of Non-Lepidopteran Insects Associated with Agriculture Plants, 1st ed.; Hardie, J., Minks, A., Eds.; Cabi Publishing: New York, NY, USA, 1999; pp. 237–304. ISBN 0-85199-3451. [Google Scholar]
- Aldrich, J. Chemical Ecology of the Heteroptera. Annu. Rev. Èntomol. 1988, 33, 211–238. [Google Scholar] [CrossRef]
- Borges, M.; Schmidt, F.G.V.; Sujii, E.R.; Medeiros, M.A.; Mori, K.; Zarbin, P.H.G.; Ferreira, J.T.B. Field responses of stink bugs to the natural and synthetic pheromone of the Neotropical brown stink bug, Euschistus heros (Heteroptera: Pentatomidae). Physiol. Èntomol. 1998, 23, 202–207. [Google Scholar] [CrossRef]
- Borges, M.; Moraes, M.C.B.; Peixoto, M.F.; Pires, C.S.S.; Sujii, E.R.; Laumann, R.A. Monitoring the Neotropical brown stink bug Euschistus heros (F.) (Hemiptera: Pentatomidae) with pheromone-baited traps in soybean fields. J. Appl. Èntomol. 2011, 135, 68–80. [Google Scholar] [CrossRef] [Green Version]
- Leal, W.S.; Kuwahara, S.; Shi, X.; Higuchi, H.; Marino, C.; Ono, M.; Meinwald, J. Male-Released Sex Pheromone of the Stink Bug Piezodorus hybneri. J. Chem. Ecol. 1998, 24, 1817–1829. [Google Scholar] [CrossRef]
- Zahn, D.K.; Moreira, J.A.; Millar, J.G. Erratum to: Identification, Synthesis, and Bioassay of a Male-Specific Aggregation Pheromone from the Harlequin Bug, Murgantia histrionica. J. Chem. Ecol. 2012, 38, 126. [Google Scholar] [CrossRef]
- Khrimian, A.; Zhang, A.; Weber, D.C.; Ho, H.-Y.; Aldrich, J.R.; Vermillion, K.; Siegler, M.A.; Shirali, S.; Guzman, F.; Leskey, T.C. Discovery of the Aggregation Pheromone of the Brown Marmorated Stink Bug (Halyomorpha halys) through the Creation of Stereoisomeric Libraries of 1-Bisabolen-3-ols. J. Nat. Prod. 2014, 77, 1708–1717. [Google Scholar] [CrossRef]
- Moliterno, A.A.C.; De Melo, D.J.; Zarbin, P.H.G. Identification of Zingiberenol and Murgantiol as Components of the Aggregation-Sex Pheromone of the Rice Stink Bug, Mormidea v-luteum (Heteroptera: Pentatomidae). J. Chem. Ecol. 2021, 47, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Arif, M.A.; Guarino, S.; Colazza, S.; Peri, E. The Role of (E)-2-octenyl Acetate as a Pheromone of Bagrada hilaris (Burmeister): Laboratory and Field Evaluation. Insects 2020, 11, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.Y.; Seo, M.H.; Lee, S.C. Male-Produced Aggregation Pheromone of the Sloe Bug, Dolycoris baccarum L. (Hemiptera: Heteroptera: Pentatomidae). J. Chem. Ecol. 2019, 45, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Blassioli-Moraes, M.C.; Khrimian, A.; Michereff, M.F.F.; Magalhães, D.M.; Hickel, E.; De Freitas, T.F.S.; Barrigossi, J.A.F.; Laumann, R.A.; Silva, A.T.; Guggilapu, S.D.; et al. Male-Produced Sex Pheromone of Tibraca limbativentris Revisited: Absolute Configurations of Zingiberenol Stereoisomers and their Influence on Chemotaxis Behavior of Conspecific Females. J. Chem. Ecol. 2019, 46, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Khrimian, A.; Guggilapu, S.D.; Guzman, F.; Blassioli-Moraes, M.C.; Borge, M. Absolute Configurtions of Stink Bug- and Plant-Produced Sesquipiperitols Synthesis of All Stereoisomers. J. Nat. Prod. 2020, 83, 2281–2286. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, J.; Lehner, B.; Khrimian, A.; Muchlinski, A.; Luck, K.; Köllner, T.; Weber, D.C.; Gundersen-Rindal, D.E.; Tholl, D. An IDS-Type Sesquiterpene Synthase Produces the Pheromone Precursor (Z)-α-Bisabolene in Nezara viridula. J. Chem. Ecol. 2018, 45, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, J.; Khrimian, A.; Young, S.; Lehner, B.; Luck, K.; Wallingford, A.; Ghosh, S.K.B.; Zerbe, P.; Muchlinski, A.; Marek, P.E.; et al. De novo formation of an aggregation pheromone precursor by an isoprenyl diphosphate synthase-related terpene synthase in the harlequin bug. Proc. Natl. Acad. Sci. USA 2018, 115, E8634–E8641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ulhoa, L.A.; Barrigossi, J.A.F.; Borges, M.; Laumann, R.A.; Blassioli-Moraes, M.C. Differential induction of volatiles in rice plants by two stink bug species influence behaviour of conspecifics and their natural enemy Telenomus podisi. Èntomol. Exp. Appl. 2020, 168, 76–90. [Google Scholar] [CrossRef]
- Machado, R.M.; Sant’Ana, J.; Blassioli-Moraes, M.; Laumann, R.; Borges, M. Herbivory-induced plant volatiles from Oryza sativa and their influence on chemotaxis behaviour of Tibraca limbativentris Stal. (Hemiptera: Pentatomidae) and egg parasitoids. Bull. Èntomol. Res. 2014, 104, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Njihia, T.N.; Torto, B.; Murungi, L.K.; Irungu, J.; Mwenda, D.M.; Babin, R. Identification of kairomones of second instar nymphs of the variegated coffee bug Antestiopsis thunbergii (Heteroptera: Pentatomidae). Chemoecology 2017, 27, 239–248. [Google Scholar] [CrossRef]
- Arriola, K.; Guarino, S.; Schlawis, C.; Arif, M.A.; Colazza, S.; Peri, E.; Schulz, S.; Millar, J.G. Identification of Brassicadiene, a Diterpene Hydrocarbon Attractive to the Invasive Stink Bug Bagrada hilaris, from Volatiles of Cauliflower Seedlings, Brassica oleracea var. botrytis. Org. Lett. 2020, 22, 2972–2975. [Google Scholar] [CrossRef] [PubMed]
- Guarino, S.; Arif, M.A.; Millar, J.G.; Colazza, S.; Peri, E. Volatile unsaturated hydrocarbons emitted by seedlings of Brassica species provide host location cues to Bagrada hilaris. PLoS ONE 2018, 13, e0209870. [Google Scholar] [CrossRef]
- Bouwmeester, H.; Schuurink, R.C.; Bleeker, P.M.; Schiestl, F. The role of volatiles in plant communication. Plant J. 2019, 100, 892–907. [Google Scholar] [CrossRef] [Green Version]
- Guarino, S.; Peri, E.; Colazza, S. Plant and Stink Bug Interactions at Different Trophic Levels. In Biorational Control Based on Communication Processes; CRC Press: Boca Raton, FL, USA, 2017; Volume 1, pp. 180–199. [Google Scholar]
- Reddy, V.P.; Guerrero, A. Interactions of insect pheromones and plant semiochemicals. Trends Plant Sci. 2004, 9, 253–261. [Google Scholar] [CrossRef]
- Michereff, M.F.F.; Laumann, R.A.; Borges, M.; Michereff-Filho, M.; Diniz, I.R.; Neto, A.L.F.; Moraes, M.C.B. Volatiles Mediating a Plant-Herbivore-Natural Enemy Interaction in Resistant and Susceptible Soybean Cultivars. J. Chem. Ecol. 2011, 37, 273–285. [Google Scholar] [CrossRef] [Green Version]
- Williams, L.; Rodriguez-Saona, C.; Paré, P.W.; Crafts-Brandner, S.J. The piercing-sucking herbivores Lygus hesperus and Nezara viridula induce volatile emissions in plants. Arch. Insect Biochem. Physiol. 2005, 58, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Michereff, M.F.F.; Borges, M.; Laumann, R.; Diniz, I.; Blassioli-Moraes, M.C. Influence of volatile compounds from herbivore-damaged soybean plants on searching behavior of the egg parasitoid Telenomus podisi. Èntomol. Exp. Appl. 2013, 147, 9–17. [Google Scholar] [CrossRef]
- Helms, A.M.; De Moraes, C.M.; Tooker, J.; Mescher, M.C. Exposure of Solidago altissima plants to volatile emissions of an insect antagonist (Eurosta solidaginis) deters subsequent herbivory. Proc. Natl. Acad. Sci. USA 2013, 110, 199–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magalhães, D.M.; Da Silva, I.T.F.A.; Borges, M.; Laumann, R.A.; Blassioli-Moraes, M.C. Anthonomus grandis aggregation pheromone induces cotton indirect defence and attracts the parasitic wasp Bracon vulgaris. J. Exp. Bot. 2019, 70, 1891–1901. [Google Scholar] [CrossRef] [PubMed]
- De Bruyne, M.; Baker, T.C. Odor Detection in Insects: Volatile Codes. J. Chem. Ecol. 2008, 34, 882–897. [Google Scholar] [CrossRef] [PubMed]
- Su, C.-Y.; Menuz, K.; Carlson, J.R. Olfactory Perception: Receptors, Cells, and Circuits. Cell 2009, 139, 45–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brézot, P.; Tauban, D.; Renou, M. Dense organs on the antennae flagellum of the green stink bug, Nezara viridula (L.) (Heteroptera: Pentatomidae): Sensilla types and numerical growth during the post-embryonic development. Int. Insect Morphol. Embryol. 1997, 25, 427–441. [Google Scholar] [CrossRef]
- Rani, P.U.; Madhavendra, S.S. External morphology of antennal and rostral sensillae in four hemipteran insects and their possible role in host plant selection. Int. J. Trop. Insect Sci. 2005, 25, 198–207. [Google Scholar] [CrossRef]
- Silva, C.C.; de Capdeville, G.; Moraes, M.C.B.; Falcão, R.; Solino, L.F.; Laumann, R.A.; Silva, J.P.; Borges, M. Morphology, distribution and abundance of antennal sensilla in three stink bug species (Hemiptera: Pentatomidae). Micron 2010, 41, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Renou, M.; Anton, S. Insect olfactory communication in a complex and changing world. Curr. Opin. Insect Sci. 2020, 42, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Farias, L.R.; Schmmelpfeng, P.H.C.; Togawa, R.C.; Costa, M.M.C.; Grynberg, P.; Martins, N.F.; Blassioli-Moraes, M.C.; Laumann, R.A.; Báo, S.N.; Paula, P.D. Transcriptomo-base identification of highly similar odorant-binding proteins among neotropical stink bugs and their egg parasitoid. PLoS ONE 2015. [Google Scholar] [CrossRef]
- Wu, Z.-Z.; Qu, M.-Q.; Pu, X.-H.; Cui, Y.; Xiao, W.-Y.; Zhao, H.-X.; Bin, S.-Y.; Lin, J.-T. Transcriptome sequencing of Tessaratoma papillosa antennae to identify and analyze expression patterns of putative olfaction genes. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Sun, D.; Huang, Y.; Qin, Z.; Zhan, H.; Zhang, J.; Liu, Y.; Yang, S. Identification of Candidate Olfactory Genes in the Antennal Transcriptome of the Stink Bug Halyomorpha halys. Front. Physiol. 2020, 11, 876. [Google Scholar] [CrossRef]
- Kristoffersen, L.; Hansson, B.S.; Anderbrant, O.; Larsson, M.C. Aglomerular Hemipteran Antennal Lobes—Basic Neuroanatomy of a Small Nose. Chem. Senses 2008, 33, 771–778. [Google Scholar] [CrossRef] [Green Version]
- Xie, G.-Y.; Ma, B.-W.; Liu, X.-L.; Chang, Y.-J.; Chen, W.-B.; Li, G.-P.; Feng, H.-Q.; Zhang, Y.-J.; Berg, B.G.; Zhao, X.-C. Brain Organization of Apolygus lucorum: A Hemipteran Species With Prominent Atennal Lobes. Front. Neuroanat. 2019, 13, 70. [Google Scholar] [CrossRef] [Green Version]
- Xie, G.-Y.; Zhao, X.-C.; Ma, B.-W.; Guo, P.; Li, G.-P.; Feng, H.-Q.; Wu, G.-L. Central Projection of Antennal Sensory Neurons in the Central Nervous System of the Mirid Bug Apolygus lucorum (Meyer-Dür). PLoS ONE 2016, 11, e0160161. [Google Scholar] [CrossRef] [PubMed]
- Zgonik, V.; Čokl, A. The role of signals of different modalities in initiating vibratory communication in Nezara viridula. Open Life Sci. 2014, 9, 200–211. [Google Scholar] [CrossRef] [Green Version]
- Kavčič, A.; Čokl, A.; Laumann, R.A.; Blassioli-Moraes, M.C.; Borges, M. Tremulatory and Abdomen Vibration Signals Enable Communication through Air in the Stink Bug Euschistus heros. PLoS ONE 2013, 8, e56503. [Google Scholar] [CrossRef] [Green Version]
- Markl, H. Die Verständigung durch Stridulationssignale bei Blattschneiderameisen. II. Erzeugung und Eigenschaften der Signale (Communication by stridulatory signals in leaf- cutting ants. II. Production and characteristics of the signals). Z. Vergl. Physiol. 1968, 60, 103–150. [Google Scholar] [CrossRef]
- Brownell, P.; Farley, R.D. Orientation to vibrations in sand by the nocturnal scorpion Paruroctonus mesaensis: Mechanism of target localization. J. Comp. Physiol. A 1979, 131, 31–38. [Google Scholar] [CrossRef]
- Aicher, B. Vibrational communication in the fiddler crab, Uca pugilator. J. Comp. Physiol. A 1990, 166, 345–353. [Google Scholar] [CrossRef]
- Čokl, A.; Nardi, C.; Bento, J.M.S.; Hirose, E.; Panizzi, A.R. Transmission of stridulatory signals of the burrower bugs, Scaptocoris castanea and Scaptocoris carvalhoi (Heteroptera: Cydnidae) through the soil and soybean. Physiol. Èntomol. 2006, 31, 371–381. [Google Scholar] [CrossRef]
- Žunič, A.; Virant-Doberlet, M.; Čokl, A. Species Recognition During Substrate-Borne Communication in Nezara viridula (L.) (Pentatomidae: Heteroptera). J. Insect Behav. 2011, 24, 468–487. [Google Scholar] [CrossRef]
- Kuštor, V. Activity of Muscles of the Vibration Producing Organ of the Bug Nezara Viridula. Master’s Thesis, University of Ljubljana Slovenia, Ljubljana, Slovenija, 1988. [Google Scholar]
- Amon, T. Electrical brain stimulation elicits singing in the bug Nezara viridula. Naturwissenschaften 1990, 77, 291–292. [Google Scholar] [CrossRef]
- Čokl, A.; Virant-Doberlet, M. Communication with Substrate-Borne Signals in Small Plant-Dwelling Insects. Annu. Rev. Èntomol. 2003, 48, 29–50. [Google Scholar] [CrossRef] [PubMed]
- Ota, D.; Čokl, A. Mate location in the southern green stink bug, Nezara viridula (Heteroptera: Pentatomidae), mediated through substrate-borne signals on ivy. J. Insect Behav. 1991, 4, 441–447. [Google Scholar] [CrossRef]
- Čokl, A.; Doberlet, M.V.; McDowell, A. Vibrational directionality in the southern green stink bug, Nezara viridula (L.), is mediated by female song. Anim. Behav. 1999, 58, 1277–1283. [Google Scholar] [CrossRef] [Green Version]
- Prešern, J.; Polajnar, J.; de Groot, M.; Zorović, M.; Virant-Doberlet, M. On the spot: Utilization of directional cues in vibrational communication of a stink bug. Sci. Rep. 2018, 8, 5418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laumann, R.A.; Čokl, A.; Blassioli-Moraes, M.C.; Borges, M. Vibratory Communication and its Relevance to Reproductive Isolation in two Sympatric Stink Bug Species (Hemiptera: Pentatomidae: Pentatominae). J. Insect Behav. 2016, 29, 643–665. [Google Scholar] [CrossRef] [Green Version]
- Da Silveira, S.; Dias, A.M.; Lagoa, A.C.G.; Blassioli-Moraes, M.C.; Borges, M.; Čokl, A.; Laumann, R.A. Specificity of Male Responses to Female Vibratory Signals in two Chinavia Species (Hemiptera: Pentatomidae) is Based on Signal Structure and Narrow Temporal Parameters. Anim. Behav. Cogn. 2019, 6, 1–12. [Google Scholar] [CrossRef]
- Čokl, A.; Kosi, A.; Žunič, A.; Laumann, R.A.; Doberlet, M.V. Female competition for availability of males in insects: The Nezara viridula (Linnaeus, 1758) model. Insect Sci. 2020, 27, 801–814. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.; Borges, M.; Moraes, M.B.; Coelho, M.L.F.; Čokl, A.; Laumann, R. Inhibitory Copulation Effect of Vibrational Rival Female Signals of Three Stink Bug Species as a Tool for Mating Disruption. Insects 2021, 12, 177. [Google Scholar] [CrossRef] [PubMed]
- Kon, M.; Oe, A.; Numata, H.; Hidaka, T. Comparison of the mating behaviour between two sympatric species, Nezara antennata and N. viridula (Heteroptera: Pentatomidae), with special reference to sound emission. J. Ethol. 1988, 6, 91–98. [Google Scholar] [CrossRef]
- Blassioli-Moraes, M.C.; Laumann, R.A.; Čokl, A. Vibratory signals of four Neotropical stink bug species. Physiol. Entomol. 2005, 30, 175–188. [Google Scholar] [CrossRef] [Green Version]
- Čokl, A.; Zorović, M.; Millar, J.G. Vibrational communication along plants by the stink bugs Nezara viridula and Murgantia histrionica. Behav. Process. 2007, 75, 40–54. [Google Scholar] [CrossRef]
- Cˇokl, A.; Virant-Doberlet, M.; Stritih, N. The structure and function of songs emitted by southern green stink bugs from Brazil, Florida, Italy and Slovenia. Physiol. Èntomol. 2000, 25, 196–205. [Google Scholar] [CrossRef]
- Kavar, T.; Pavlovčič, P.; Sušnik, S.; Meglič, V.; Virant-Doberlet, M. Genetic differentiation of geographically separated populations of the southern green stink bug Nezara viridula (Hemiptera: Pentatomidae). Bull. Èntomol. Res. 2006, 96, 117–128. [Google Scholar] [CrossRef] [Green Version]
- Hrabar, N.; Virant-Doberlet, M.; Čokl, A. Species specificity of male southern green stink bug Nezara viridula (L.) reactions to the female calling song. Acta Zool. Sinica. 2004, 50, 566–575. [Google Scholar]
- Čokl, A.; Kosi Žunič, A.; Moraes, M.C.B.; Borges, M.; Laumann, R.A. Stink Bug Inter-Plant Communication with Signals Produced by Vibration of Lifted Wings. J. Insect Behav. 2021, 34, 194–210. [Google Scholar] [CrossRef]
- McBrien, H.L.; Millar, J.G. Substrate-borne vibrational signals of the Consperse stink bug (Hemiptera: Pentatomidae). Can. Èntomol. 2003, 135, 555–567. [Google Scholar] [CrossRef]
- Colazza, S.; Aquila, G.; De Pasquale, C.; Peri, E.; Millar, J.G. The egg parasitoids Trissolcus basalis uses n-nonadecane, a cuticular hydrocarbon from its stink bug host Nezara viridula, to discriminate between female and male hosts. J. Chem. Ecol. 2007, 33, 1405–1420. [Google Scholar] [CrossRef] [Green Version]
- Silveira, S. Isolamento Reprodutivo em Duas Espécies Simpátricas de Chinavia (Orian (Hemiptera: Pentatomidae): Importância da Comunicação Vibracional e Composição Química da Cutícula. Master’s Thesis, Universidade de Brasília, Brasilia, Brazil, 2015. [Google Scholar]
- Guarino, S.; De Pasquale, C.; Peri, E.; Alonzo, G.; Colazza, S. Role of volatile and contact pheromones in the mating behaviour of Bagrada hilaris (Heteroptera: Pentatomidae). Eur. J. Èntomol. 2008, 105, 613–617. [Google Scholar] [CrossRef] [Green Version]
- Colazza, S.; Bue, M.L.; Giudice, D.L.; Peri, E. The response of Trissolcus basalis to footprint contact kairomones from Nezara viridula females is mediated by leaf epicuticular waxes. Naturwissenschaften 2009, 96, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Cocroft, R.B.; Rodriguez, R.L. The behavioral ecology of insect vibrational communication. Bioscience 2005, 55, 323–334. [Google Scholar] [CrossRef]
- Barth, F.G. A Spider’s World; Springer: Singapore, 2002; pp. 35–329. [Google Scholar]
- Čokl, A.; Borges, M. Biorational Control Based on Communication Processes; CRC Press: Boca Raton, FL, USA, 2017; pp. 1–255. [Google Scholar]
- Cocroft, R.G.; Gogala, M.; Hill, P.S.M.; Wessel, A. Studying Vibrational Communication, 1st ed.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA; London, UK, 2014; pp. 3–395. ISBN 978-3-662-43607-3. [Google Scholar]
- Cremer, L.; Heckl, M. Structure-Borne Sound; Springer: Berlin/Heidelberg, Germany, 1973; p. 528. [Google Scholar]
- Michelsen, A.; Fink, F.; Gogala, M.; Traue, D. Plants as transmission channels for insect vibrational songs. Behav. Ecol. Sociobiol. 1982, 11, 269–281. [Google Scholar] [CrossRef]
- Polajnar, J.; Svenšek, D.; Čokl, A. Resonance in herbaceous plant stems as a factor in vibrational communication of pentatomide bugs (Heteroptera: Pentatomidae). J. Roy. Soc. Interf. 2012, 9, 1898–1907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cokl, A.; Presern, J.; Virant-Doberlet, M.; Bagwell, G.J.; Millar, J.G. Vibratory signals of the harlequin bug and their transmission through plants. Physiol. Èntomol. 2004, 29, 372–380. [Google Scholar] [CrossRef]
- Cokl, A.; Žunič, A.; Millar, J. Transmission of Podisus maculiventris tremulatory signals through plants. Open Life Sci. 2009, 4, 585–594. [Google Scholar] [CrossRef]
- Žunič, A.; Čokl, A. Predatory Stink Bugs (Asopinae) and the Role of Substrate-borne Vibrational Signals in Intra- and Interspecific Interactions. In Biorational Control Based on Communication Processes; CRC Press: Boca Raton, FL, USA, 2017; Volume 1, pp. 59–77. [Google Scholar]
- Gogala, M.; Razpotnik, R. A method of oscillographic sonagraphy for bio-acoustic. Res. Biol. Vestnik. 1974, 22, 209–216. [Google Scholar]
- Čokl, A.; Laumann, R.; Kosi Žunič, A.; Blassioli-Moraes, M.C.; Doberlet, M.V.; Borges, M. Interference of Overlapping Insect Vibratory Communication Signals: An Eushistus heros Model. PLoS ONE 2015, 10, e0130775. [Google Scholar] [CrossRef] [Green Version]
- Polajnar, J.; Eriksson, A.; Doberlet, M.V.; Mazzoni, V. Mating disruption of a grapevine pest using mechanical vibrations: From laboratory to the field. J. Pest Sci. 2016, 89, 909–921. [Google Scholar] [CrossRef]
- Čokl, A. Vibratory signal transmission in plants as measured by laser vibrometry. Period. Biol. 1988, 90, 193–196. [Google Scholar]
- Casas, J.; Bacher, S.; Tautz, J.; Meyhöfer, R.; Pierre, D. Leaf Vibrations and Air Movements in a Leafminer–Parasitoid System. Biol. Control. 1998, 11, 147–153. [Google Scholar] [CrossRef] [Green Version]
- Koczor, S.; Čokl, A. Percussion signals of Lygus rugulipennis Poppius (Heteroptera: Miridae). Open Life Sci. 2014, 9, 543–549. [Google Scholar] [CrossRef] [Green Version]
- Miklas, N.; Stritih, N.; Čokl, A.; Virant-Doberlet, M.; Renou, M. The Influence of Substrate on Male Responsiveness to the Female Calling Song in Nezara viridula. J. Insect Behav. 2001, 14, 313–332. [Google Scholar] [CrossRef]
- de Groot, M.; Čokl, A.; Doberlet, M.V. Effects of heterospecific and conspecific vibrational signal overlap and signal-to-noise ratio on male responsiveness in Nezara viridula (L.). J. Exp. Biol. 2010, 213, 3213–3222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Endler, J. Signals, Signal Conditions, and the Direction of Evolution. Am. Nat. 1992, 139, S125–S153. [Google Scholar] [CrossRef] [Green Version]
- Endler, J. Some general comments on the evolution and design of animal communication systems. Philos. Trans. R. Soc. B Biol. Sci. 1993, 340, 215–225. [Google Scholar] [CrossRef]
- Barth, F.G. The vibrational sense in spiders. In Handbook of Auditory Research: Insects, 1st ed.; Hoy, R.R., Popper, R.R., Fay, A.N., Eds.; Springer: New York, NY, USA, 1988; pp. 228–278. ISBN 978-1-4612-0585-2. [Google Scholar]
- McVean, A.; Field, L.H. Communication by substratum vibration in the New Zealand tree weta, Hemideina femorata (Stenopelmatidae: Orthoptera). J. Zoöl. 1996, 239, 101–122. [Google Scholar] [CrossRef]
- Tishechkin, D. Vibratory communication in Psylloidea (Hemiptera). In Insect Sounds and Vibration: Physiology, Behaviour, Ecology and Evolution, 1st ed.; Drosopoulos, S., Claridge, M.F., Eds.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2007; pp. 357–363. ISBN 0-84963-2060-7. [Google Scholar]
- McNett, G.D.; Cocroft, R.B. Host shifts favor vibrational signal divergence in Enchenopa binotata treehoppers. Behav. Ecol. 2008, 19, 650–656. [Google Scholar] [CrossRef] [Green Version]
- Šturm, R.; Polajnar, J.; Doberlet, M.V. Practical Issues in Studying Natural Vibroscape and Biotic Noise. In Coding Strategies in Vertebrate Acoustic Communication; Springer: Singapore, 2019; pp. 125–148. [Google Scholar]
- Velilla, E.; Muñoz, M.; Quiroga, N.; Symes, L.; Ter Hofstede, H.M.; Page, R.A.; Simon, R.; Ellers, J.; Halfwerk, W. Gone with the wind: Is signal timing in a neotropical katydid an adaptive response to variation in wind-induced vibratory noise? Behav. Ecol. Sociobiol. 2020, 74, 1–11. [Google Scholar] [CrossRef]
- Polajnar, J.; Čokl, A. The effect of vibratory disturbance on sexual behaviour of the southern green stink bug Nezara viridula (Heteroptera, Pentatomidae). Open Life Sci. 2008, 3, 189–197. [Google Scholar] [CrossRef]
- Laumann, R.A.; Maccagnan, D.H.B.; Čokl, A.; Blassioli-Moraes, M.C.; Borges, M. Substrate-borne vibrations disrupt the mating behaviors of the neotropical brown stink bug, Euschistus heros: Implications for pest management. J. Pest Sci. 2018, 91, 995–1004. [Google Scholar] [CrossRef] [Green Version]
- Spezia, S.; Curcio, L.; Fiasconaro, A.; Pizzolato, N.; Valenti, D.; Spagnolo, B.; Bue, P.L.; Peri, E.; Colazza, S. Evidence of stochastic resonance in the mating behavior of Nezara viridula (L.). Eur. Phys. J. B 2008, 65, 453–458. [Google Scholar] [CrossRef] [Green Version]
- Nishino, H.; Mukai, H.; Takanashi, T. Chordotonal organs in hemipteran insects: Unique peripheral structures but conserved central organization revealed by comparative neuroanatomy. Cell Tissue Res. 2016, 366, 549–572. [Google Scholar] [CrossRef] [PubMed]
- Čokl, A. Functional poperties of viboreceptors in the legs of Nezara viridula (L.) (Heteroptera, Pentatomidae). J. Comp. Physiol. A 1983, 150, 261–269. [Google Scholar] [CrossRef]
- Zorović, M. Morphological and Physiological Properties of Vibrational Neurons in Thoracic Ganglia of the Stink Bug Nezara Viridula (L.) (Heteroptera: Pentatomidae). PhD Thesis, University of Ljubljana, Ljubljana, Slovenia, 2005. [Google Scholar]
- Michel, K.; Amon, T.; Čokl, A. The morphology of the leg scolopidial organs in Nezara viridula (L.) (Heteroptera, Pentatomidae). Rev. Can. Biol. Exp. 1983, 42, 130–150. [Google Scholar]
- Clarac, F.; Cattaert, D. Functional multimodality of axonal tree in invertebrate neurons. J. Physiol. 1999, 93, 319–327. [Google Scholar] [CrossRef]
- Poulet, J.; Hedwig, B. A corollary discharge maintains auditory sensitivity during sound production. Nat. Cell Biol. 2002, 418, 872–876. [Google Scholar] [CrossRef]
- Poulet, J.; Hedwig, B. A Corollary Discharge Mechanism Modulates Central Auditory Processing in Singing Crickets. J. Neurophysiol. 2003, 89, 1528–1540. [Google Scholar] [CrossRef] [Green Version]
- Sauer, A.E.; Büschges, A.; Stein, W. Role of presynaptic inputs to proprioreceptive afferents in tuning sensorimotor pathways of an insect joint control network. J. Neurobiol. 1997, 32, 359–376. [Google Scholar] [CrossRef]
- Čokl, A.; Virant-Doberlet, M.; Zorović, M. Sense Organs Involved in the Vibratory Communication of Bugs. In Insect Sounds and Communication: Physiology, Behaviour, Ecology and Evolution; Drosopoulos, S., Claridge, M.F., Eds.; CRC Taylor & Francis: Boca Raton, FL, USA, 2006; pp. 71–80. [Google Scholar]
- Lakes-Harlan, R.; Strauß, J. Functional Morphology and Evolutionary Diversity of Vibration Receptors in Insects. In Coding Strategies in Vertebrate Acoustic Communication; Springer: Singapore, 2014; Volume 1, pp. 277–302. [Google Scholar]
- Lipovšek, S.; Pabst, M.; Devetak, D. Femoral chordotonal organ in the legs of an insect, Chrysoperla carnea (Neuroptera). Tissue Cell 1999, 31, 154–162. [Google Scholar] [CrossRef]
- Sharma, K.R.; Enzmann, B.L.; Schmidt, Y.; Moore, D.; Jones, G.R.; Parker, J.; Berger, S.; Reinberg, D.; Zwiebel, L.J.; Breit, B.; et al. Cuticular Hydrocarbon Pheromones for Social Behavior and Their Coding in the Ant Antenna. Cell Rep. 2015, 12, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Jeram, S.; Pabst, M. Johnston’s organ and central organ in Nezara viridula (L.) (Heteroptera, Pentatomidae). Tissue Cell 1996, 28, 227–235. [Google Scholar] [CrossRef]
- Jeram, S. Structure and Function of Johnston’s Organ in the Bug Species Nezara Viridula (L.). Bachelor’s Thesis, University of Ljubljana, Ljubljana, Slovenia, 1996. [Google Scholar]
- Simmons, P.; Young, D. The tymbal mechanism and song patterns of the Bladder Cicada, Cystosoma saundersii. J. Exp. Biol. 1978, 76, 27–45. [Google Scholar] [CrossRef]
- Halex, H.; Kaiser, W.; Kalmring, K. Projection areas and branching patterns of the tympanal receptor cells in migratory locusts, Locusta migratoria and Schistocerca gregaria. Z. Für Zellforsch. Und Mikrosk. Anat. 1988, 253, 517–528. [Google Scholar] [CrossRef]
- Barth, F.G. A Spiders World; Springer: Berlin/Heidelberg, Germany, 2002; pp. 85–111. [Google Scholar]
- Škorjanc, A.; Zupančič, G.; Drašlar, K. Multiple mechanisms generate the resting activity of filiform sensilla in the firebug (Pyrrhocoris apterus L.: Heteroptera). J. Comp. Physiol. A Sens. Neural Behav. Physiol. 2009, 195, 651–661. [Google Scholar] [CrossRef]
- Drašlar, K.; Škorjanc, A. Functional Properties of Trichobotria in the Bug Pyrrhocoris Apterus, The neurosciences from basic research to therapy; Elsner, N., Zimmermann, H., Eds.; Georg Thieme Verlag: Stuttgart, Germany, 2003; p. 359. ISBN 3-13-137351-2. [Google Scholar]
- Zorović, M.; Prešern, J.; Čokl, A. Morphology and physiology of vibratory interneurons in the thoracic ganglia of the southern green stink bug Nezara viridula (L.). J. Comp. Neurol. 2008, 508, 365–381. [Google Scholar] [CrossRef]
- Ibrahim, A.; Giovannini, I.; Anfora, G.; Rossi Stacconis, M.V.; Malek, R.; Maistrello, L.; Guidetti, R.; Romani, R. A closer look at the antennae of the invasive Halyomorpha halys: Fine structure of the sensilla. Bull. Insectology 2019, 72, 187–199. [Google Scholar]
- Stumpner, A.; Ronacher, B. Auditory interneurons in the metathoracic ganglion of the grasshopper Chorthippus biguttulus. I. Morphological and physiological characterization. J. Exp. Biol. 1991, 158, 391–410. Available online: https://www.researchgate.net/publication/255665563 (accessed on 23 September 2021). [CrossRef]
- Stritih, N. Anatomy and physiology of a set of low-frequency vibratory interneurons in a nonhearing ensiferan (Troglophilus neglectus, Rhaphidophoridae). J. Comp. Neurol. 2009, 516, 519–532. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, P.C.; Seifert, M.; Stumpner, A. Auditory DUM neurons in a bush-cricket: A filter bank for carrier frequency. J. Comp. Neurol. 2018, 526, 1166–1182. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, E.; Seki, H.; Asai, T.; Morimoto, T.; Miyakawa, H.; Ito, K.; Kamikouchi, A. Organization of projection neurons and localneurons of the primary auditory center in the fruit fly Drosophila melanogaster. J. Comp. Neurol. 2016, 524, 1099–1164. [Google Scholar] [CrossRef] [PubMed]
- Schröter, U.; Malun, D.; Menzel, R. Innervation pattern of suboesophageal ventral unpaired median neurones in the honeybee brain. Cell Tissue Res. 2006, 327, 647–667. [Google Scholar] [CrossRef]
- Stumpner, A.; Gubert, S.; Knorr, D.Y.; Göpfert, M.C. Auditory DUM neurons in a bush-cricket: Inhibited inhibitors. J. Comp. Physiol. A 2020, 206, 793–807. [Google Scholar] [CrossRef]
- Strauβ, J.; Stritih-Peljhan, N.; Nieri, R.; Virant-Doberlet, M.; Mazzoni, V. Communication by substrate-borne mechanical waves in insects: From basic to applied biotremology. Adv. Insect Phys. 2021, 61, 189–307. [Google Scholar] [CrossRef]
- Hergenröder, R.; Barth, F.G. Vibratory signals and spider behavior: How does sensory input from the eight legs interact in orientation? J. Comp. Physiol. A. 1983, 152, 361–371. [Google Scholar] [CrossRef]
- Speck-Hergenröder, J.; Barth, F.G. Vibration sensitive hairs on the spider leg. Cell. Mol. Life Sci. 1988, 44, 13–14. [Google Scholar] [CrossRef]
- Speck-Hergenröder, J.; Barth, F.G. Tuning of vibration sensitive neurons in the central nervous system of a wandering spider, Cupiennius salei Keys. J. Comp. Physiol. 1987, 160, 467–475. [Google Scholar] [CrossRef]
- Čokl, A.; Otto, C.; Kalmring, K. The processing of directional vibratory signals in the ventral nerve cord of Locusta migratoria. J. Comp. Physiol. A 1985, 156, 45–52. [Google Scholar] [CrossRef]
- Wirth, E. Die Bedeutung von Zeit- und Amplituden-Unterschieden für die Orientierung Nach Vibratorischen Signalen bei Spinnen. Master’s Thesis, Goethe University, Germany, 1984. [Google Scholar]
- Barth, F.G. Neuroethology of the Spider Vibration Sense. In Neurobiology of Arachnids; Springer: Singapore, 1985; pp. 203–229. [Google Scholar]
- Ronacher, B.; Stumpner, A. Filtering of behaviourally relevant temporal parameters of a grasshopper’s song by an auditory interneuron. J. Comp. Physiol. A 1988, 163, 517–523. [Google Scholar] [CrossRef]
- Stumpner, A.; Ronacher, R.; von Helversen, O. Auditory interneurons in the metathoracic ganglion of the grasshopper Chorthippus bigutulus. II. Processing of temporal patterns of the song of the male. J. Exp. Biol. 1991, 158, 411–430. [Google Scholar] [CrossRef]
- Hedwig, B.; Pollack, G.S. Invertebrate auditory pathways. In The Senses: A Comprehensive Reference, 1st ed.; Basbaum, A.I., Kaneko, A., Shepherd, G.M., Westheimer, G., Eds.; Academic Press: San Diego, CA, USA, 2008; pp. 525–564. ISBN 9780126394825. [Google Scholar]
- Stumpner, A.; Nowotny, M. Neural Processing in the Bush-Cricket Auditory Pathway. In Coding Strategies in Vertebrate Acoustic Communication; Springer: Singapore, 2014; pp. 143–166. [Google Scholar]
- Ronacher, B. Processing of species-specific signals in the auditory pathway of grasshoppers. In Insect Hearing and Acoustic Communication, 1st ed.; Hedwig, B., Ed.; Springer: Berlin, Germany, 2014; pp. 185–204. ISBN 978-3-642-40462-7. [Google Scholar]
- Hedwig, B.; Stumpner, A. Central neural processing of sound signals in insects. In Insect Hearing, Springer Handbook of Auditory Research 55, 1st ed.; Pollack, G.S., Mason, A.C., Popper, A.N., Fay., R.R., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; pp. 177–214. [Google Scholar]
- Pollack, G.S.; Hedwig, B. The Cricket Auditory Pathway: Neural Processing of Acoustic Signals. In The Cricket as a Model Organism; Springer: Singapore, 2017; Volume 11, pp. 155–167. [Google Scholar]
- Stumpner, A.; Lefebvre, P.C.; Seifert, M.; Ostrowski, T.D. Temporal processing properties of auditory DUM neurons in a bush-cricket. J. Comp. Physiol. A 2019, 205, 717–733. [Google Scholar] [CrossRef]
- Čokl, A.; Kalmring, K.; Wittig, H. The responses of auditory ventral-cord neurons of Locusta migratoria to vibration stimuli. J. Comp. Physiol. A 1977, 120, 161–172. [Google Scholar] [CrossRef]
- Kalmring, K.; Jatho, M.; Rössler, W. The Auditory ‚Äì Vibratory Sensory System in Bushcrickets. Insect Symbiosis 2005, 1, 35–69. [Google Scholar] [CrossRef]
- Zorović, M. Temporal Processing of Vibratory Communication Signals at the Level of Ascending Interneurons in Nezara viridula (Hemiptera: Pentatomidae). PLoS ONE 2011, 6, e26843. [Google Scholar] [CrossRef] [Green Version]
- Hedwig, B.; Poulet, J.F.A. Complex auditory behaviour emerges from simple reactive steering. Nat. Cell Biol. 2004, 430, 781–785. [Google Scholar] [CrossRef]
- Bush, S.L.; Schul, J. Pulse-rate recognition in an insect: Evidence of a role for oscillatory neurons. J. Comp. Physiol. A 2006, 192, 113–121. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Čokl, A.; Žunič-Kosi, A.; Stritih-Peljhan, N.; Blassioli-Moraes, M.C.; Laumann, R.A.; Borges, M. Stink Bug Communication and Signal Detection in a Plant Environment. Insects 2021, 12, 1058. https://doi.org/10.3390/insects12121058
Čokl A, Žunič-Kosi A, Stritih-Peljhan N, Blassioli-Moraes MC, Laumann RA, Borges M. Stink Bug Communication and Signal Detection in a Plant Environment. Insects. 2021; 12(12):1058. https://doi.org/10.3390/insects12121058
Chicago/Turabian StyleČokl, Andrej, Alenka Žunič-Kosi, Nataša Stritih-Peljhan, Maria Carolina Blassioli-Moraes, Raúl Alberto Laumann, and Miguel Borges. 2021. "Stink Bug Communication and Signal Detection in a Plant Environment" Insects 12, no. 12: 1058. https://doi.org/10.3390/insects12121058





