Variation in Plant–Pollinator Network Structure along the Elevational Gradient of the San Francisco Peaks, Arizona
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Location and Sampling Methods
2.2. Species Identification
2.3. Comparisons of Plant and Pollinator Richness and Abundance across Life Zones
2.4. Network Indices Calculations
2.5. Core Generalist Species Calculations
2.6. Generalist Contribution to Network Stability at a High Elevation Plant–Pollinator Community
3. Results
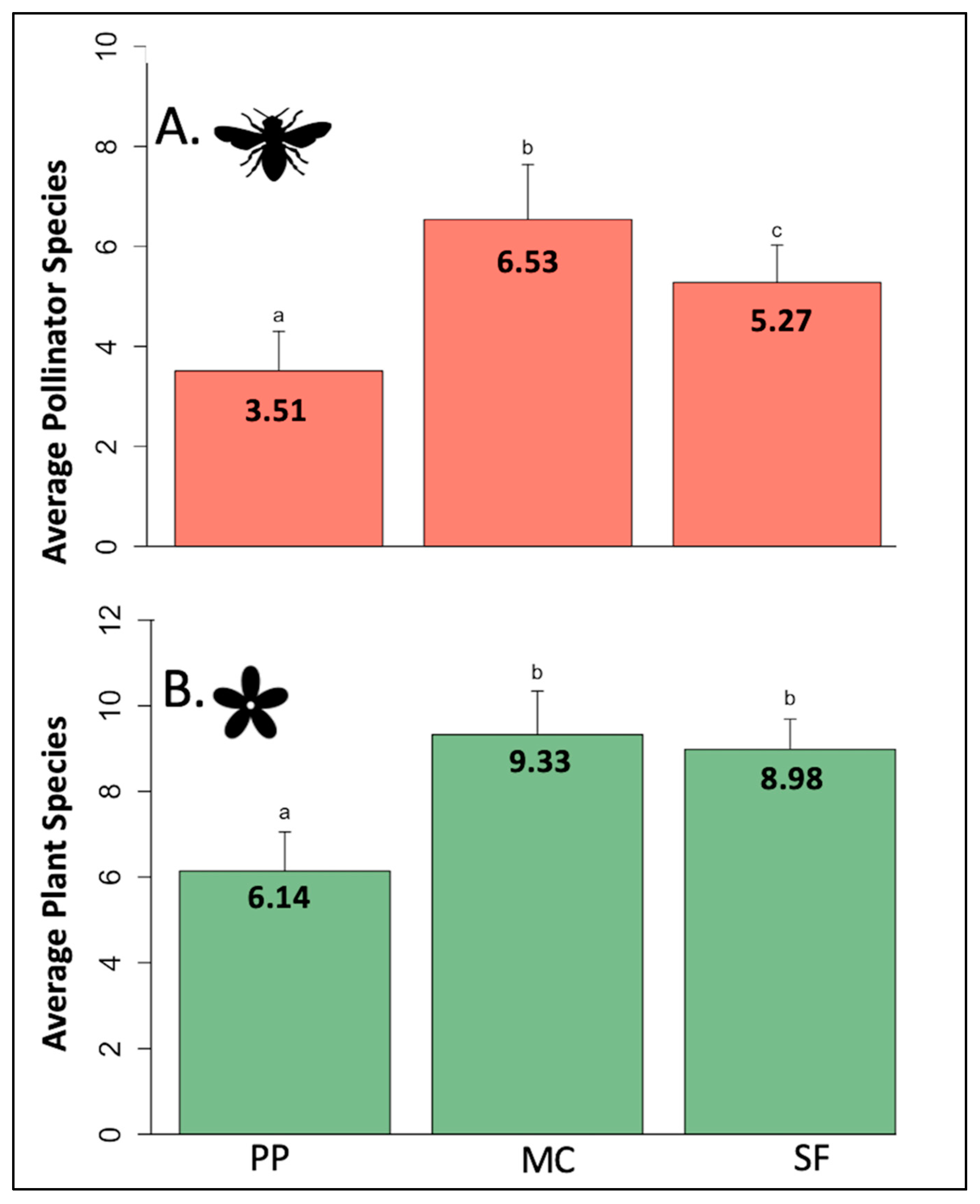
3.1. Pollinator and Plant Species Richness by Life Zone
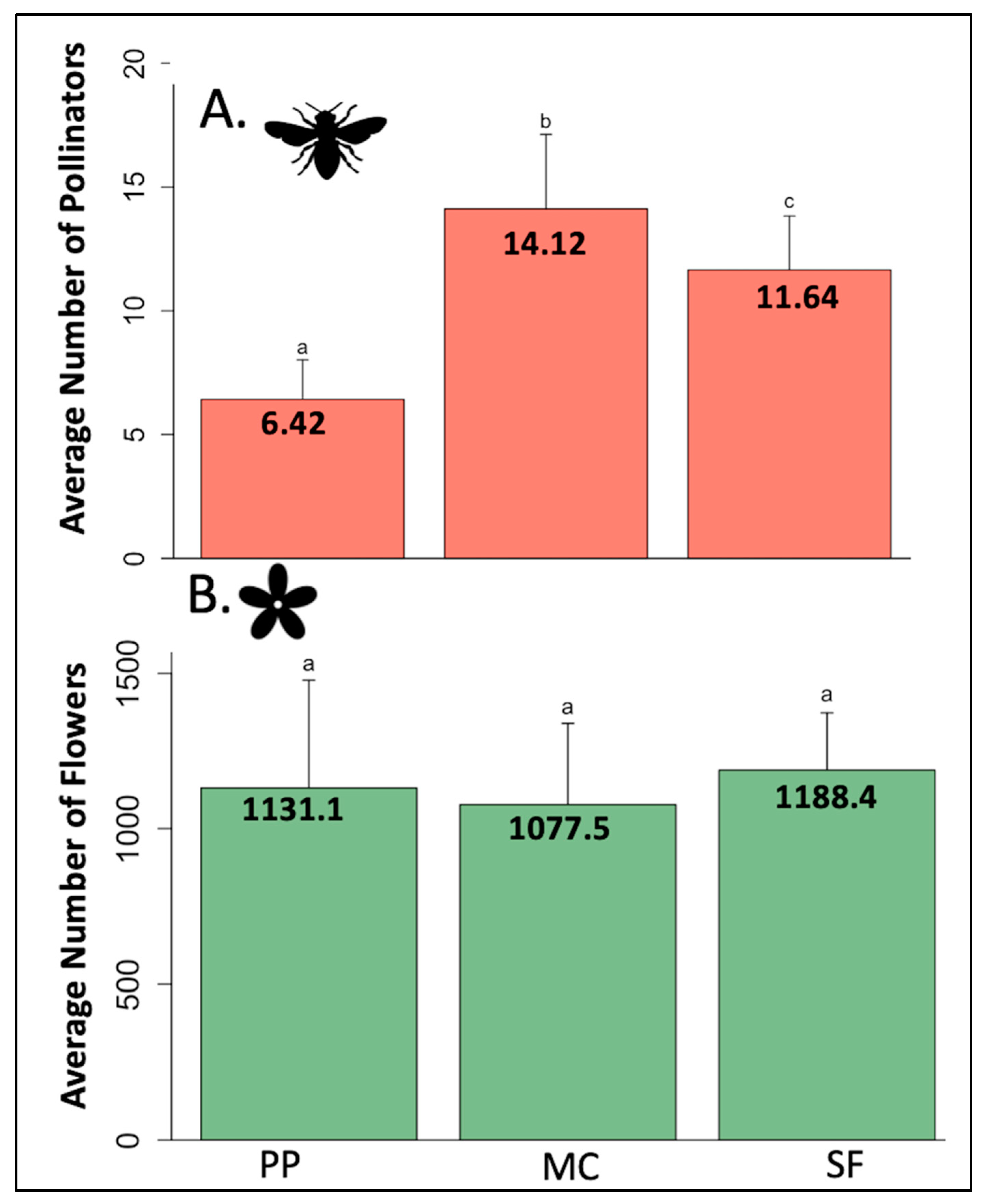
3.2. Pollinator and Flower Abundance by Life Zone
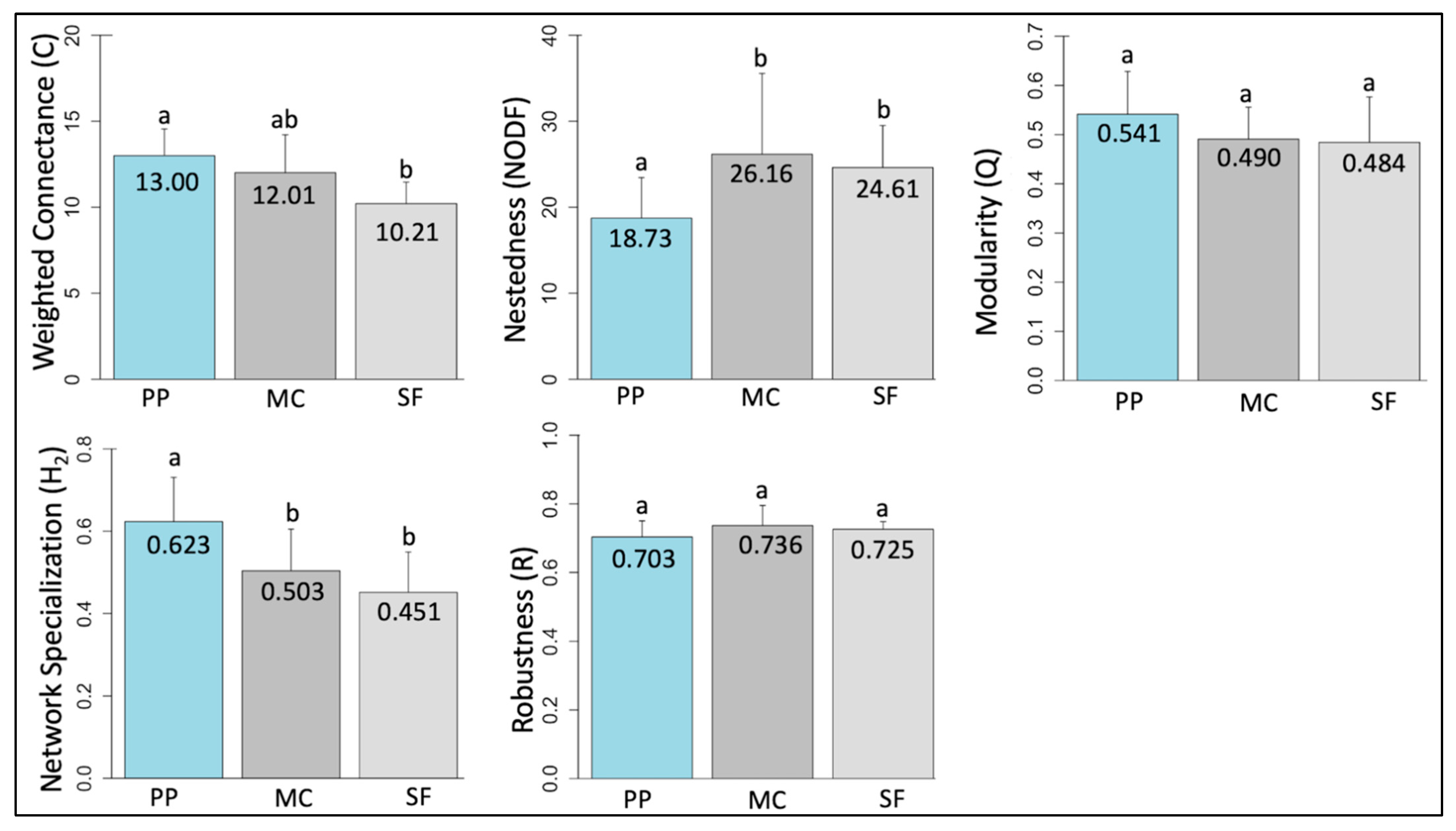
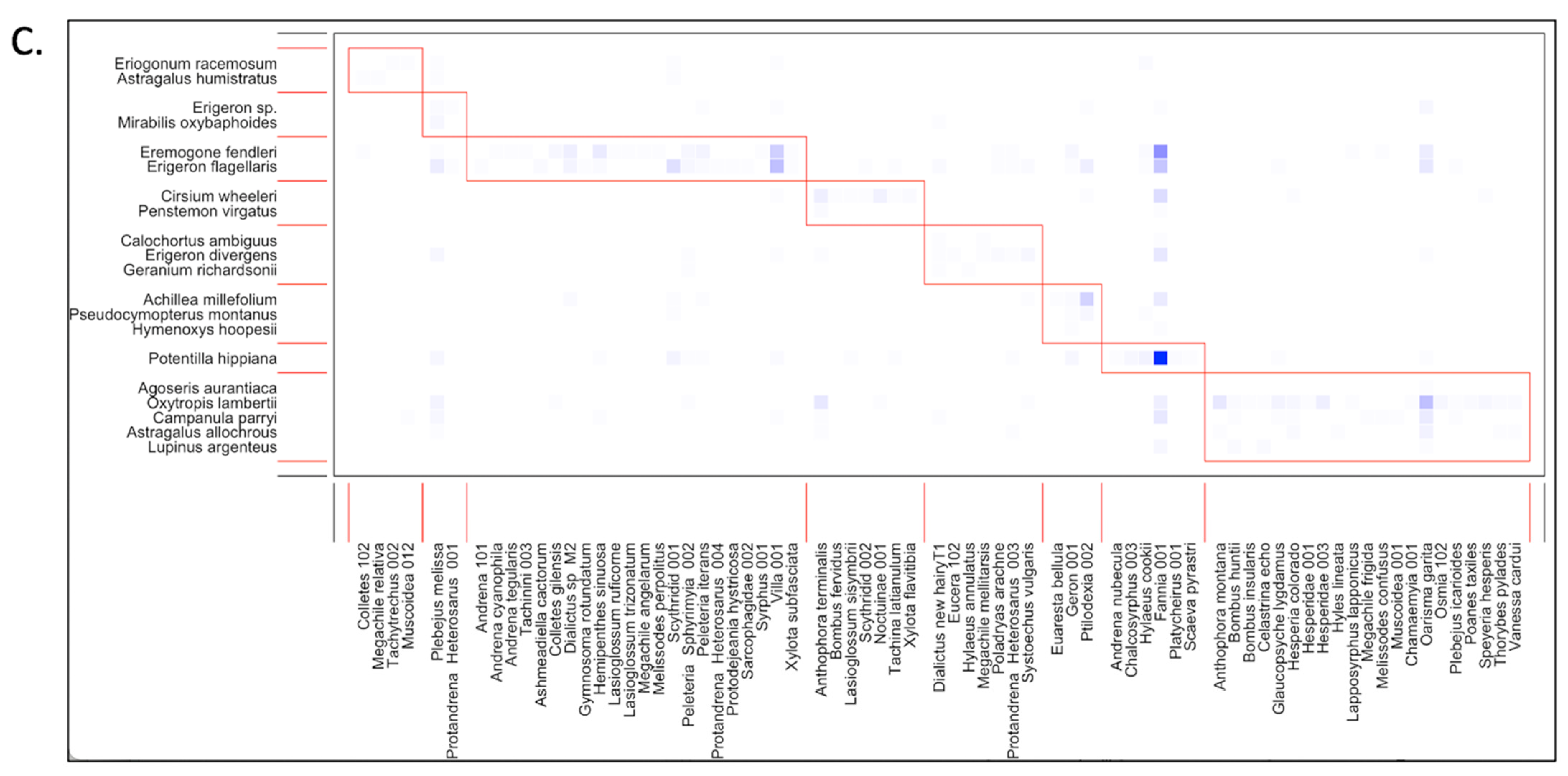
3.3. Network Structure by Life Zone
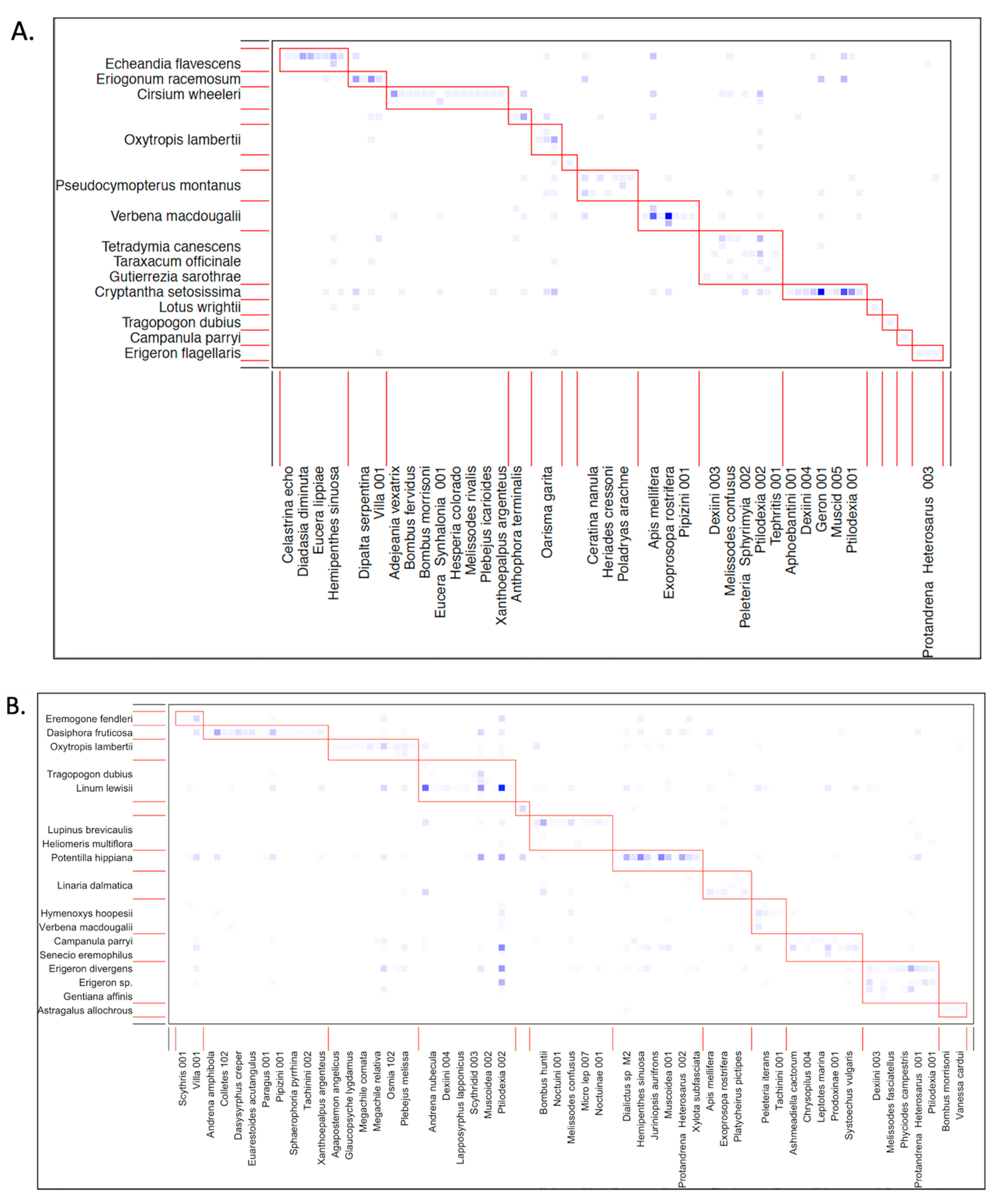
3.4. Core Generalist Pollinators by Life Zone
3.5. Generalist Importance at the High Elevation Plant–Pollinator Community
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Osorio-Canadas, S.; Flores-Hernández, N.; Sánchez-Ortiz, T.; Valiente-Banuet, A. Changes in the structure and composition of the ‘Mexical’scrubland bee community along an elevational gradient. PLoS ONE 2021, 16, e0254072. [Google Scholar] [CrossRef]
- Klein, A.M.; Vaissiere, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B Biol. Sci. 2007, 274, 303–313. [Google Scholar] [CrossRef] [PubMed]
- McCabe, L.M.; Colella, E.; Chesshire, P.; Smith, D.; Cobb, N.S. The transition from bee-to-fly dominated communities with increasing elevation and greater forest canopy cover. PloS ONE 2019, 14, e0217198. [Google Scholar] [CrossRef]
- Minachilis, K.; Kantsa, A.; Devalez, J.; Trigas, P.; Tscheulin, T.; Petanidou, T. Bumblebee diversity and pollination networks along the elevation gradient of Mount Olympus, Greece. Divers. Distrib. 2020, 26, 1566–1581. [Google Scholar] [CrossRef]
- Kearns, C.A. Anthophilous fly distribution across an elevation gradient. Am. Midl. Nat. 1992, 127, 172–182. [Google Scholar] [CrossRef]
- Klecka, J.; Hadrava, J.; Biella, P.; Akter, A. Flower visitation by hoverflies (Diptera: Syrphidae) in a temperate plant-pollinator network. PeerJ 2018, 6, e6025. [Google Scholar] [CrossRef]
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef]
- Jamieson, M.A.; Carper, A.L.; Wilson, C.J.; Scott, V.L.; Gibbs, J. Geographic biases in bee research limits understanding of species distribution and response to anthropogenic disturbance. Front. Ecol. Evol. 2019, 7, 194. [Google Scholar] [CrossRef]
- Kovács-Hostyánszki, A.; Földesi, R.; Báldi, A.; Endrédi, A.; Jordán, F. The vulnerability of plant-pollinator communities to honeybee decline: A comparative network analysis in different habitat types. Ecol. Indic. 2019, 97, 35–50. [Google Scholar] [CrossRef]
- Colla, S.R.; Ratti, C.M. Evidence for the decline of the western bumble bee (Bombus occidentalis Greene) in British Columbia. Pan-Pac. Entomol. 2010, 86, 32–34. [Google Scholar] [CrossRef]
- Colla, S.R.; Packer, L. Evidence for decline in eastern North American bumblebees (Hymenoptera: Apidae), with special focus on Bombus affinis Cresson. Biodivers. Conserv. 2008, 17, 1379–1391. [Google Scholar] [CrossRef]
- Allen, M.R.; Babiker, M.; Chen, Y.; de Coninck, H.; Connors, S.; van Diemen, R.; Dube, O.P.; Ebi, K.L.; Engelbrecht, F.; Ferrat, M. Summary for policymakers. In Global Warming of 1.5: An IPCC Special Report on the Impacts of Global Warming of 1.5\C above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; IPCC: Geneva, Switzerland, 2018. [Google Scholar]
- Hegland, S.J.; Nielsen, A.; Lázaro, A.; Bjerknes, A.L.; Totland, Ø. How does climate warming affect plant-pollinator interactions? Ecol. Lett. 2009, 12, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Memmott, J.; Craze, P.G.; Waser, N.M.; Price, M.V. Global warming and the disruption of plant—Pollinator interactions. Ecol. Lett. 2007, 10, 710–717. [Google Scholar] [CrossRef]
- Schenk, M.; Krauss, J.; Holzschuh, A. Desynchronizations in bee—Plant interactions cause severe fitness losses in solitary bees. J. Anim. Ecol. 2018, 87, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Adedoja, O.A.; Kehinde, T.; Samways, M.J. Insect-flower interaction networks vary among endemic pollinator taxa over an elevation gradient. PLoS ONE 2018, 13, e0207453. [Google Scholar] [CrossRef]
- Hewitt, G. The genetic legacy of the Quaternary ice ages. Nature 2000, 405, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Wiens, J.J.; Camacho, A.; Goldberg, A.; Jezkova, T.; Kaplan, M.E.; Lambert, S.M.; Miller, E.C.; Streicher, J.W.; Walls, R.L. Climate change, extinction, and Sky Island biogeography in a montane lizard. Mol. Ecol. 2019, 28, 2610–2624. [Google Scholar] [CrossRef] [PubMed]
- Coe, S.J.; Finch, D.M.; Friggens, M.M. An assessment of climate change and the vulnerability of wildlife in the Sky Islands of the Southwest. In General Technical Report RMRS-GTR-273. Fort Collins, Colorado; US Department of Agriculture, Forest Service, Rocky Mountain Research Station: Washington, DC, USA, 2012; 208p. [Google Scholar]
- Waser, N.M.; Chittka, L.; Price, M.V.; Williams, N.M.; Ollerton, J. Generalization in pollination systems, and why it matters. Ecology 1996, 77, 1043–1060. [Google Scholar] [CrossRef]
- Johnson, S.D.; Steiner, K.E. Generalization versus specialization in plant pollination systems. Trends Ecol. Evol. 2000, 15, 140–143. [Google Scholar] [CrossRef]
- Ebeling, A.; Klein, A.-M.; Tscharntke, T. Plant–flower visitor interaction webs: Temporal stability and pollinator specialization increases along an experimental plant diversity gradient. Basic Appl. Ecol. 2011, 12, 300–309. [Google Scholar] [CrossRef]
- Lara-Romero, C.; Seguí, J.; Pérez-Delgado, A.; Nogales, M.; Traveset, A. Beta diversity and specialization in plant–pollinator networks along an elevational gradient. J. Biogeogr. 2019, 46, 1598–1610. [Google Scholar] [CrossRef]
- Spehn, E.M.; Körner, C. A global assessment of mountain biodiversity and its function. In Global Change and Mountain Regions; Springer: Berlin/Heidelberg, Germany, 2005; pp. 393–400. [Google Scholar]
- Illán, J.G.; Gutierrez, D.; Diez, S.B.; Wilson, R.J. Elevational trends in butterfly phenology: Implications for species responses to climate change. Ecol. Entomol. 2012, 37, 134–144. [Google Scholar] [CrossRef]
- Lance, R.F.; Bailey, P.; Lindsay, D.L.; Cobb, N.S. Precipitation and the robustness of a plant and flower-visiting insect network in a xeric ecosystem. J. Arid Environ. 2017, 144, 48–59. [Google Scholar] [CrossRef]
- Dáttilo, W.; Guimaraes, P.R., Jr.; Izzo, T.J. Spatial structure of ant–plant mutualistic networks. Oikos 2013, 122, 1643–1648. [Google Scholar] [CrossRef]
- Hoiss, B.; Krauss, J.; Steffan-Dewenter, I. Interactive effects of elevation, species richness and extreme climatic events on plant–pollinator networks. Glob. Chang. Biol. 2015, 21, 4086–4097. [Google Scholar] [CrossRef] [PubMed]
- Marini, L.; Bartomeus, I.; Rader, R.; Lami, F. Species–habitat networks: A tool to improve landscape management for conservation. J. Appl. Ecol. 2019, 56, 923–928. [Google Scholar] [CrossRef]
- Bersier, L.-F.; Banašek-Richter, C.; Cattin, M.-F. Quantitative descriptors of food-web matrices. Ecology 2002, 83, 2394–2407. [Google Scholar] [CrossRef]
- Medan, D.; Montaldo, N.H.; Devoto, M.; Maniese, A.; Vasellati, V.; Roitman, G.G.; Bartoloni, N.H. Plant-pollinator relationships at two altitudes in the Andes of Mendoza, Argentina. Arct. Antarct. Alp. Res. 2002, 34, 233–241. [Google Scholar] [CrossRef]
- Winfree, R.; Williams, N.M.; Dushoff, J.; Kremen, C. Species abundance, not diet breadth, drives the persistence of the most linked pollinators as plant-pollinator networks disassemble. Am. Nat. 2014, 183, 600–611. [Google Scholar] [CrossRef]
- Valido, A.; Rodríguez-Rodríguez, M.C.; Jordano, P. Honeybees disrupt the structure and functionality of plant-pollinator networks. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Bascompte, J.; Jordano, P.; Melián, C.J.; Olesen, J.M. The nested assembly of plant–animal mutualistic networks. Proc. Natl. Acad. Sci. USA 2003, 100, 9383–9387. [Google Scholar] [CrossRef] [PubMed]
- Dalsgaard, B.; Trøjelsgaard, K.; Martín González, A.M.; Nogués-Bravo, D.; Ollerton, J.; Petanidou, T.; Sandel, B.; Schleuning, M.; Wang, Z.; Rahbek, C. Historical climate-change influences modularity and nestedness of pollination networks. Ecography 2013, 36, 1331–1340. [Google Scholar] [CrossRef]
- Ramos-Jiliberto, R.; Domínguez, D.; Espinoza, C.; Lopez, G.; Valdovinos, F.S.; Bustamante, R.O.; Medel, R. Topological change of Andean plant–pollinator networks along an altitudinal gradient. Ecol. Complex. 2010, 7, 86–90. [Google Scholar] [CrossRef]
- Dormann, C.F. Using bipartite to describe and plot two-mode networks in R. R Package Version 2020, 4, 1–28. [Google Scholar]
- Dormann, C.F.; Fründ, J.; Blüthgen, N.; Gruber, B. Indices, graphs and null models: Analyzing bipartite ecological networks. Open Ecol. J. 2009, 2, 7–24. [Google Scholar] [CrossRef]
- McCain, C.M.; Grytnes, J.A. Elevational gradients in species richness. eLS 2010. [Google Scholar] [CrossRef]
- Richman, S.K.; Levine, J.M.; Stefan, L.; Johnson, C.A. Asynchronous range shifts drive alpine plant–pollinator interactions and reduce plant fitness. Glob. Chang. Biol. 2020, 26, 3052–3064. [Google Scholar] [CrossRef]
- McCabe, L.M.; Cobb, N.S.; Butterfield, B.J. Environmental filtering of body size and darker coloration in pollinator communities indicate thermal restrictions on bees, but not flies, at high elevations. PeerJ 2019, 7, e7867. [Google Scholar] [CrossRef] [PubMed]
- Burgos, E.; Ceva, H.; Perazzo, R.P.; Devoto, M.; Medan, D.; Zimmermann, M.; Delbue, A.M. Why nestedness in mutualistic networks? J. Theor. Biol. 2007, 249, 307–313. [Google Scholar] [CrossRef]
- Memmott, J.; Waser, N.M.; Price, M.V. Tolerance of pollination networks to species extinctions. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2004, 271, 2605–2611. [Google Scholar] [CrossRef] [PubMed]
- Benadi, G.; Hovestadt, T.; Poethke, H.J.; Blüthgen, N. Specialization and phenological synchrony of plant–pollinator interactions along an altitudinal gradient. J. Anim. Ecol. 2014, 83, 639–650. [Google Scholar] [CrossRef]
- Carreck, N. Decline of bees and other pollinators. Biol. Environ. Hazards Risks Disasters 2016, 109–118. [Google Scholar] [CrossRef]
- Lois, S.; Cowley, D.E. Conservation of Interacting Species in Network-Constrained Environments; Wiley Online Library: Hoboken, NJ, USA, 2017. [Google Scholar]
- Biella, P.; Akter, A.; Ollerton, J.; Tarrant, S.; Janeček, Š.; Jersáková, J.; Klecka, J. Experimental loss of generalist plants reveals alterations in plant-pollinator interactions and a constrained flexibility of foraging. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- McCabe, L.M.; Chesshire, P.R.; Smith, D.R.; Wolf, A.; Gibbs, J.; Griswold, T.L.; Wright, K.W.; Cobb, N.S. Bee species checklist of the San Francisco Peaks, Arizona. Biodivers. Data J. 2020, 8, e49285. [Google Scholar] [CrossRef]
- Magnacca, K.N. Conservation status of the endemic bees of Hawai ‘i, Hylaeus (Nesoprosopis)(Hymenoptera: Colletidae). Pac. Sci. 2007, 61, 173–190. [Google Scholar] [CrossRef]
- Bowers, J.E.; McLaughlin, S.P. Flora of the Huachuca Mountains, a botanically rich and historically significant sky island in Cochise County, Arizona. J. Ariz.-Nev. Acad. Sci. 1996, 29, 66–107. [Google Scholar]
- Merriam, C.H.; Stejneger, L. Results of a Biological Survey of the San Francisco Mountain Region and Desert of the Little Colorado, Arizona; US Government Printing Office: Washington, DC, USA, 1890; Volume 3.
- LaBerge, W.E. A revision of the bees of the genus Andrena of the Western Hemisphere. Part I. Callandrena.(Hymenoptera: Andrenidae). Trans. Am. Entomol. Soc. 1967, 7, 1–38. [Google Scholar]
- LaBerge, W.E. A revision of the bees of the genus Andrena of the Western Hemisphere. Part XII. Subgenera Leucandrena, Ptilandrena, Scoliandrena and Melandrena. Trans. Am. Entomol. Soc. 1986, 112, 191–248. [Google Scholar]
- LaBerge, W.E. A revision of the bees of the genus Andrena of the Western Hemisphere. Part II. Plastandrena, Aporandrena, Charitandrena. Trans. Am. Entomol. Soc. 1969, 95, 1–47. [Google Scholar]
- Wright, K.W.; Miller, K.B.; Song, H. A molecular phylogeny of the long-horned bees in the genus Melissodes Latreille (Hymenoptera: Apidae: Eucerinae). Insect Syst. Evol. 2020, 1, 1–16. [Google Scholar] [CrossRef]
- Wright, K.W. The Evolution of Diet Breadth in Melissodes Bees (Apidae: Eucerini); The University of New Mexico: Albuquerque, NM, USA, 2018. [Google Scholar]
- Michener, C.D. The Bees of the World; JHU Press: Baltimore, MI, USA, 2000; Volume 1. [Google Scholar]
- Marshall, S.A. Fliesthe Natural History & Diversity of Diptera; Marshall Firefly Press Ltd.: Cardiff, UK, 2012. [Google Scholar]
- Skevington, J.H.; Locke, M.M.; Young, A.D.; Moran, K.; Crins, W.J.; Marshall, S.A. Field Guide to the Flower Flies of Northeastern North America; Princeton University Press: Princeton, NJ, USA, 2019. [Google Scholar]
- Norrbom, A.L.; Sutton, B.D.; Steck, G.J.; Monzon, J. New genera, species and host plant records of Nearctic and Neotropical Tephritidae (Diptera). Zootaxa 2010, 2398, 1–65. [Google Scholar] [CrossRef]
- Brock, J.P.; Kaufman, K. Kaufman Field Guide to Butterflies of North America; Houghton Mifflin Harcourt: Boston, MA, USA, 2006. [Google Scholar]
- Lenth, R.; Singmann, H.; Love, J.; Buerkner, P.; Herve, M. R Package Emmeans; CRAN: Vienna, Austria, 2019. [Google Scholar]
- Dormann, C.F.; Fruend, J.; Gruber, B.; Dormann, M.C.F.; LazyData, T.; ByteCompile, T. Package ‘Bipartite’; CRAN: Vienna, Austria, 2021. [Google Scholar]
- van Altena, C.; Hemerik, L.; de Ruiter, P.C. Food web stability and weighted connectance: The complexity-stability debate revisited. Theor. Ecol. 2016, 9, 49–58. [Google Scholar] [CrossRef]
- Almeida-Neto, M.; Guimaraes, P.; Guimaraes, P.R., Jr.; Loyola, R.D.; Ulrich, W. A consistent metric for nestedness analysis in ecological systems: Reconciling concept and measurement. Oikos 2008, 117, 1227–1239. [Google Scholar] [CrossRef]
- Schwarz, B.; Vázquez, D.P.; CaraDonna, P.J.; Knight, T.M.; Benadi, G.; Dormann, C.F.; Gauzens, B.; Motivans, E.; Resasco, J.; Blüthgen, N. Temporal scale-dependence of plant–pollinator networks. Oikos 2020, 129, 1289–1302. [Google Scholar] [CrossRef]
- Ponisio, L.C.; Gaiarsa, M.P.; Kremen, C. Opportunistic attachment assembles plant–pollinator networks. Ecol. Lett. 2017, 20, 1261–1272. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, W.; Almeida-Neto, M.; Gotelli, N.J. A consumer’s guide to nestedness analysis. Oikos 2009, 118, 3–17. [Google Scholar] [CrossRef]
- Blüthgen, N.; Menzel, F.; Blüthgen, N. Measuring specialization in species interaction networks. BMC Ecol. 2006, 6, 1–12. [Google Scholar] [CrossRef][Green Version]
- Alarcón, R.; Waser, N.M.; Ollerton, J. Year-to-year variation in the topology of a plant–pollinator interaction network. Oikos 2008, 117, 1796–1807. [Google Scholar] [CrossRef]
- Rahbek, C.; Borregaard, M.K.; Colwell, R.K.; Dalsgaard, B.; Holt, B.G.; Morueta-Holme, N.; Nogues-Bravo, D.; Whittaker, R.J.; Fjeldså, J. Humboldt’s enigma: What causes global patterns of mountain biodiversity? Science 2019, 365, 1108–1113. [Google Scholar] [CrossRef]
- Lefebvre, V.; Villemant, C.; Fontaine, C.; Daugeron, C. Altitudinal, temporal and trophic partitioning of flower-visitors in Alpine communities. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Fattorini, S. Disentangling the effects of available area, mid-domain constraints, and species environmental tolerance on the altitudinal distribution of tenebrionid beetles in a Mediterranean area. Biodivers. Conserv. 2014, 23, 2545–2560. [Google Scholar] [CrossRef]
- Maad, J.; Armbruster, W.S.; Fenster, C.B. Floral size variation in Campanula rotundifolia (Campanulaceae) along altitudinal gradients: Patterns and possible selective mechanisms. Nord. J. Bot. 2013, 31, 361–371. [Google Scholar] [CrossRef]
- Biase, L.D.; Fattorini, S.; Cutini, M.; Bricca, A. The Role of Inter-and Intraspecific Variations in Grassland Plant Functional Traits along an Elevational Gradient in a Mediterranean Mountain Area. Plants 2021, 10, 359. [Google Scholar] [CrossRef]
- Kiełtyk, P. Patterns of floral allocation along an elevation gradient: Variation in Senecio subalpinus growing in the Tatra Mountains. Alp. Bot. 2021, 131, 117–124. [Google Scholar] [CrossRef]
- Schemske, D.W.; Mittelbach, G.G.; Cornell, H.V.; Sobel, J.M.; Roy, K. Is there a latitudinal gradient in the importance of biotic interactions? Annu. Rev. Ecol. Evol. Syst. 2009, 40, 245–269. [Google Scholar] [CrossRef]
- Olesen, J.M.; Bascompte, J.; Dupont, Y.L.; Jordano, P. The modularity of pollination networks. Proc. Natl. Acad. Sci. USA 2007, 104, 19891–19896. [Google Scholar] [CrossRef] [PubMed]
- Phillips, B.B.; Shaw, R.F.; Holland, M.J.; Fry, E.L.; Bardgett, R.D.; Bullock, J.M.; Osborne, J.L. Drought reduces floral resources for pollinators. Glob. Chang. Biol. 2018, 24, 3226–3235. [Google Scholar] [CrossRef] [PubMed]
- Liberto, T.D. Record-Breaking June 2021 Heatwave Impacts the U.S. West. Available online: https://www.climate.gov/news-features/event-tracker/record-breaking-june-2021-heatwave-impacts-us-west (accessed on 8 August 2021).
- Biella, P.; Bogliani, G.; Cornalba, M.; Manino, A.; Neumayer, J.; Porporato, M.; Rasmont, P.; Milanesi, P. Distribution patterns of the cold adapted bumblebee Bombus alpinus in the Alps and hints of an uphill shift (Insecta: Hymenoptera: Apidae). J. Insect Conserv. 2017, 21, 357–366. [Google Scholar] [CrossRef]
- Konvicka, M.; Kuras, T.; Liparova, J.; Slezak, V.; Horázná, D.; Klečka, J.; Kleckova, I. Low winter precipitation, but not warm autumns and springs, threatens mountain butterflies in middle-high mountains. PeerJ 2021, 9, e12021. [Google Scholar] [CrossRef]
- McCabe, L.M.A.; Clare, E.; Cobb, N.S. Decreased bee emergence along an elevation gradient: Implications for climate change revealed by a transplant experiment. Ecology 2021, in press. [Google Scholar] [CrossRef]
- Ohlemüller, R.; Anderson, B.J.; Araújo, M.B.; Butchart, S.H.; Kudrna, O.; Ridgely, R.S.; Thomas, C.D. The coincidence of climatic and species rarity: High risk to small-range species from climate change. Biol. Lett. 2008, 4, 568–572. [Google Scholar] [CrossRef] [PubMed]
- Casey, L.; Rebelo, H.; Rotheray, E.; Goulson, D. Evidence for habitat and climatic specializations driving the long-term distribution trends of UK and I rish bumblebees. Divers. Distrib. 2015, 21, 864–875. [Google Scholar] [CrossRef]
| Year | Sampling Dates | PP Sites Visited | MC Sites Visited | SF Sites Visited |
|---|---|---|---|---|
| 2017 | 25–27 July | 2, 4, 5, 6, 7, 8 | 3, 4, 5, 6 | 1, 2, 3, 4, 5, 6 |
| 2017 | 1–3 August | 2, 4, 5, 6, 7, 8 | 1, 2, 3, 4, 5, 6 | 1, 2, 3, 4, 5, 6 |
| 2017 | 14–18 August | 2, 4, 5, 6, 7, 8 | 1, 2, 3, 4, 5, 6 | 1, 2, 3, 4, 5, 6 |
| 2017 | 25–31 August | 2, 4, 5, 6, 7, 8 | 1, 2, 3, 4, 5, 6 | 1, 2, 3, 4, 5, 6 |
| 2018 | 16–19 July | 2, 4, 5, 6, 7, 8 | 1, 2, 3, 4, 5, 6 | 1, 2, 3, 4, 5, 6 |
| 2018 | 30 July–1 August | 2, 4, 6, 7, 8 | 1, 2, 3, 4, 5, 6 | 1, 2, 3, 4, 5, 6 |
| 2018 | 6–8 August | 2, 4, 5, 6, 7, 8 | 1, 2, 3, 4, 5, 6 | 1, 2, 3, 4, 5, 6 |
| 2018 | 13–15 August | 2, 4, 5, 6, 7, 8 | 1, 2, 3, 4, 5, 6 | 1, 2, 3, 4, 5, 6 |
| 2018 | 26–30 August | 2, 4, 5, 6, 7, 8 | 1, 2, 3, 4, 5, 6 | 1, 2, 3, 4, 5, 6 |
| 52 Total | 51 Total | 53 Total |
| Total Individuals | Number of Species | |
|---|---|---|
| Bees | 384 (23% of total) | 59 (38% of total) |
| Flies | 976 (57% of total) | 64 (41% of total) |
| Butterflies/Moths | 343 (20% of total) | 32 (21% of total) |
| Life Zone. | Pollinator Species | Host Plant Species | Unique Interactions | Species by Group | Interactions by Group |
|---|---|---|---|---|---|
| PP (~2200–2500 m) | 73 | 27 | 163 | Bees = 29 (40%) Flies = 35 (48%) Butterflies/moths = 9 (12%) | Bees = 52 (32%) Flies = 85 (52%) Butterflies/moths = 26 (16%) |
| MC (~2550–2700 m) | 102 | 32 | 271 | Bees = 34 (33%) Flies = 42 (41%) Butterflies/moths = 25 (23%) | Bees = 80 (30%) Flies = 131 (48%) Butterflies/moths = 60 (22%) |
| SF (~2750–3100 m) | 72 | 20 | 289 | Bees = 30 (41%) Flies = 24 (33%) Butterflies/moths = 18 (25%) | Bees = 51 (31%) Flies = 72 (38%) Butterflies/moths = 58 (31%) |
| Ponderosa Pine (~2200–2500 m) | ||||
|---|---|---|---|---|
| Species | Group | Gc | Number of Individuals | % of Interactions |
| Anastoechus melanohalteralis | Fly | 1.484 | 11 (3.2%) | 3.0% (5 plants) |
| Apis mellifera | Bee | 1.911 | 25 (7.4%) | 3.6% (6 plants) |
| Ashmeadiella cactorum | Bee | 1.063 | 13 (3.8%) | 2.5% (4 plants) |
| Exoprosopa rostrifera | Fly | 1.911 | 27 (7.9%) | 2.5% (4 plants) |
| Geron 001 | Fly | 1.911 | 25 (7.4%) | 3.0% (5 plants) |
| Hemipenthes sinuosa | Fly | 1.487 | 11 (3.2%) | 3.0% (5 plants) |
| Melissodes confusus | Bee | 1.487 | 6 (1.8%) | 3.6% (6 plants) |
| Fannia 001 | Fly | 1.897 | 12 (3.5%) | 3.0% (5 plants) |
| Muscoidea 001 | Fly | 1.063 | 12 (3.5%) | 3.0% (5 plants) |
| Oarisma garita | Butterfly | 1.487 | 9 (2.6%) | 3.6% (6 plants) |
| Phthiria 003 | Fly | 1.488 | 21 (6.2%) | 1.8% (3 plants) |
| Plebejus melissa | Bee | 4.032 | 17 (5.0%) | 5.5% (9 plants) |
| Ptilodexia 002 | Fly | 3.183 | 21 (6.2%) | 6.1% (10 plants) |
| Generalists comprise: | 61.7% of total individuals | 44.7% of total interactions | ||
| Mixed Conifer (~2550–2700 m) | ||||
| Species | Group | Gc | Number of Individuals | % of Interactions |
| Adejeania vexatrix | Fly | 1.848 | 39 (5.2%) | 2.6% (7 plants) |
| Bombus huntii | Bee | 1.306 | 20 (2.7%) | 2.2% (6 plants) |
| Dialictus sp M2 | Bee | 1.848 | 21(2.8%) | 3.0% (8 plants) |
| Geron 001 | Fly | 1.306 | 15 (2.0%) | 2.6% (7 plants) |
| Hemipenthes sinuosa | Fly | 1.035 | 25 (3.4%) | 2.2% (6 plants) |
| Hylaeus cookii | Bee | 1.306 | 12 (1.6%) | 1.8% (5 plants) |
| Fannia 001 | Fly | 1.014 | 54 (7.3%) | 4.1% (11 plants) |
| Muscoidea 001 | Fly | 1.035 | 16 (2.2%) | 2.2% (6 plants) |
| Oarisma garita | Butterfly | 2.389 | 30 (4.0%) | 3.3% (9 plants) |
| Peleteria (Sphyrimyia) 002 | Fly | 1.577 | 19 (2.6%) | 2.6% (7 plants) |
| Plebejus melissa | Butterfly | 1.577 | 14 (1.9%0 | 3.0% (8 plants) |
| Polydryas arachne | Butterfly | 1.306 | 23 (3.1%) | 2.6% (7 plants) |
| Protandrena (Heterosarus) 001 | Bee | 1.035 | 12 (1.6%) | 1.8% (5 plants) |
| Ptilodexia 002 | Fly | 5.639 | 120 (16.2%) | 6.3% (17 plants) |
| Villa 001 | Fly | 1.848 | 22 (3.0%) | 2.6% (7 plants) |
| Generalists comprise: | 56.6% of total individuals | 42.9% of total interactions | ||
| Spruce Fir (~2750–3100 m) | ||||
| Species | Group | Gc | Number of Individuals | % of Interactions |
| Anthophora terminalis | Bee | 1.203 | 20 (3.2%) | 3.2% (6 plants) |
| Fannia 001 | Fly | 5.663 | 212 (33.7%) | 6.9% (13 plants) |
| Oarisma garita | Butterfly | 3.433 | 60 (9.5%) | 5.3% (10 plants) |
| Plebjeus melissa | Butterfly | 2.746 | 29 (4.6%) | 5.8% (11 plants) |
| Ptilodexia 002 | Fly | 1.203 | 28 (4.5%) | 2.6% (5 plants) |
| Villa 001 | Fly | 1.889 | 49 (7.8%) | 4.2% (8 plants) |
| Generalists comprise: | 63.3% of total individuals | 28.0% of total interactions | ||
| Species | Group | Gc Value | Nested Rank | New Nestedness Value | % Change in Nestedness from Original |
|---|---|---|---|---|---|
| Fannia 001 | Fly | 5.664 | 1 | 20.41 | −17.06% |
| Plebejus melissa | Butterfly | 2.746 | 2 | 23.57 | −4.23% |
| Oarisma garita | Butterfly | 3.432 | 3 | 22.17 | −9.91% |
| All 3 | n/a | n/a | 15.56 | −36.77% |
| Species | Group | Gc Value | Nested Rank | New Nestedness Value | % Change in Nestedness from Original |
|---|---|---|---|---|---|
| Hylaeus cookii | Bee | 0.515 | 11 | 23.93 | −2.7%% |
| Thorybes pylades | Butterfly | −0.172 | 28 | 26.96 | +9.58% |
| Bombus insularis | Bee | −0.515 | 52 | 24.62 | +0.0% |
| All 3 | n/a | n/a | 26.41 | +7.31% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chesshire, P.R.; McCabe, L.M.; Cobb, N.S. Variation in Plant–Pollinator Network Structure along the Elevational Gradient of the San Francisco Peaks, Arizona. Insects 2021, 12, 1060. https://doi.org/10.3390/insects12121060
Chesshire PR, McCabe LM, Cobb NS. Variation in Plant–Pollinator Network Structure along the Elevational Gradient of the San Francisco Peaks, Arizona. Insects. 2021; 12(12):1060. https://doi.org/10.3390/insects12121060
Chicago/Turabian StyleChesshire, Paige R., Lindsie M. McCabe, and Neil S. Cobb. 2021. "Variation in Plant–Pollinator Network Structure along the Elevational Gradient of the San Francisco Peaks, Arizona" Insects 12, no. 12: 1060. https://doi.org/10.3390/insects12121060
APA StyleChesshire, P. R., McCabe, L. M., & Cobb, N. S. (2021). Variation in Plant–Pollinator Network Structure along the Elevational Gradient of the San Francisco Peaks, Arizona. Insects, 12(12), 1060. https://doi.org/10.3390/insects12121060






