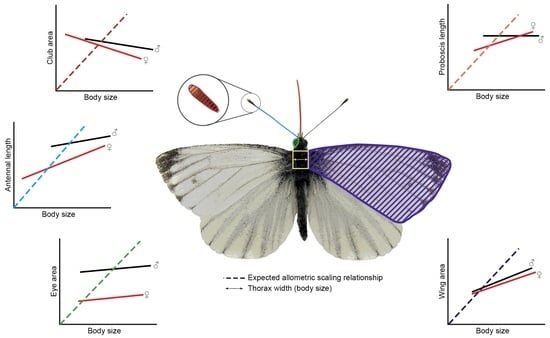
Sensory Organ Investment Varies with Body Size and Sex in the Butterfly Pieris napi
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Animals
2.2. Sample Preparation
2.3. Morphometric Measurements
2.4. Data Analysis
3. Results and Discussion
3.1. The Relationship between Compound Eye Properties and Body Size
3.2. The Relationship between Antennal Properties and Body Size
3.3. The Relationship between Proboscis Length and Body Size
3.4. The Relationship between Wing Area and Body Size
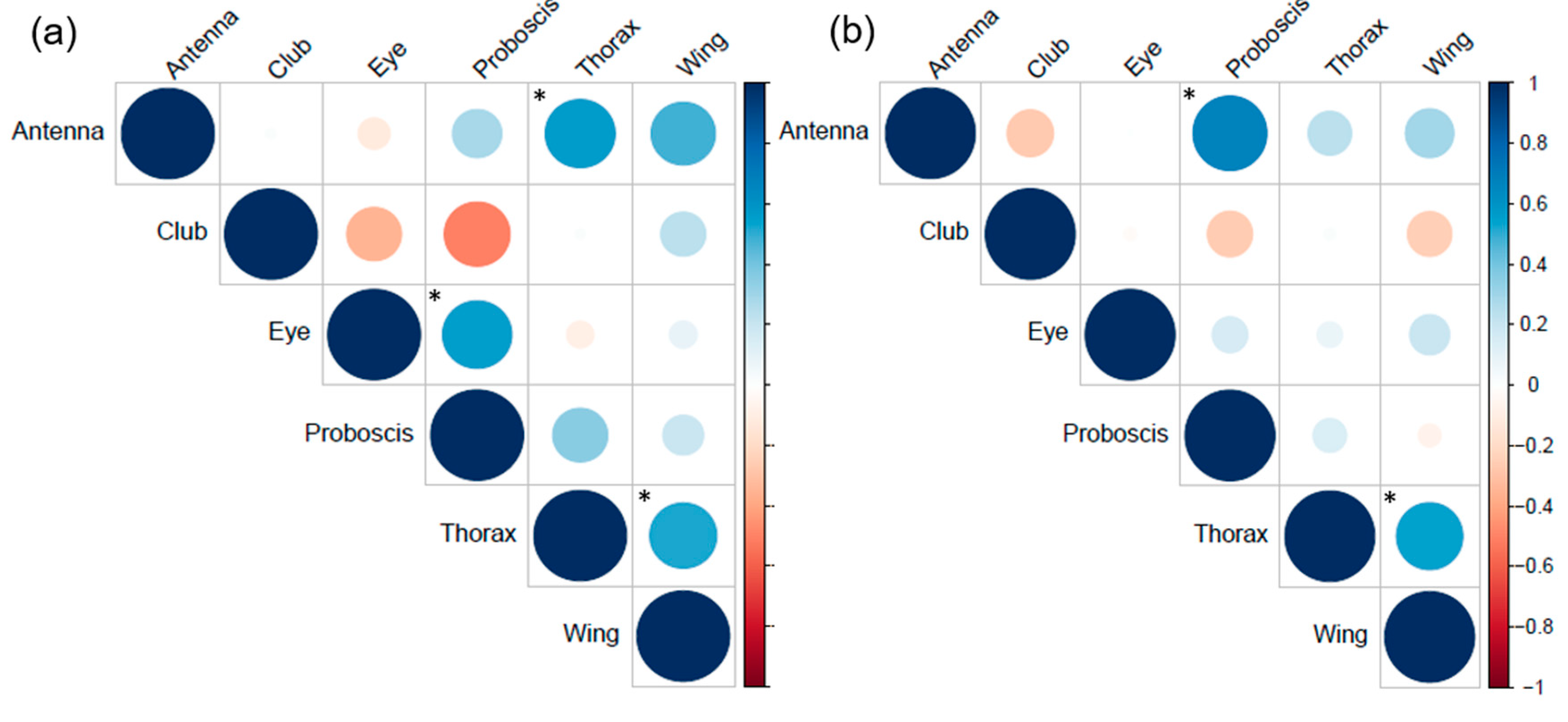
3.5. Correlation between Sensory Traits
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Mark, C.J.; Parsons, S.; Holwell, G.I. Antennal morphology and micro-sensory architecture of the New Zealand magpie moth, Nyctemera annulata (Lepidoptera: Erebidae): Diversity, distribution and dimorphism. Austral Entomol. 2018, 57, 303–323. [Google Scholar] [CrossRef]
- Scheiner, R.; Schnitt, S.; Erber, J. The functions of antennal mechanoreceptors and antennal joints in tactile discrimination of the honeybee (Apis mellifera L.). J. Comp. Physiol. A 2005, 191, 857–864. [Google Scholar] [CrossRef]
- Steinbrecht, R.A.; Lee, J.-K.; Altner, H.; Zimmermann, B. Volume and surface of receptor and auxiliary cells in hygro-/thermoreceptive sensilla of moths (Bombyx mori, Antheraea pernyi, and A. polyphemus). Cell Tissue Res. 1989, 255, 59–67. [Google Scholar] [CrossRef]
- Yuvaraj, J.K.; Andersson, M.N.; Anderbrant, O.; Löfstedt, C. Diversity of olfactory structures: A comparative study of antennal sensilla in Trichoptera and Lepidoptera. Micron 2018, 111, 9–18. [Google Scholar] [CrossRef]
- Kramer, V.R.; Mulvane, C.P.; Brothers, A.; Lehnert, M.S. Allometry among Structures of Proboscises of Vanessa cardui L. (Nymphalidae) and Its Relationship to Fluid Uptake. J. Lepid. Soc. 2015, 69, 183–191. [Google Scholar] [CrossRef]
- Krenn, H.W.; Zulka, K.P.; Gatschnegg, T. Proboscis morphology and food preferences in nymphalid butterflies (Lepidoptera: Nymphalidae). J. Zool. 2001, 254, 17–26. [Google Scholar] [CrossRef]
- Molleman, F.; Krenn, H.W.; Van Alphen, M.E.; Brakefield, P.M.; Devries, P.J.; Zwaan, B.J. Food intake of fruit-feeding butterflies: Evidence for adaptive variation in proboscis morphology. Biol. J. Linn. Soc. 2005, 86, 333–343. [Google Scholar] [CrossRef]
- Ma, L.; Hu, K.; Li, P.; Liu, J.; Yuan, X. Ultrastructure of the proboscis sensilla of ten species of butterflies (Insecta: Lepidoptera). PLoS ONE 2019, 14, e0214658. [Google Scholar] [CrossRef]
- Krenn, H.W. Proboscis assembly in butterflies (Lepidoptera)—A once in a lifetime sequence of events. Eur. J. Entomol. 1997, 94, 495–501. [Google Scholar]
- Krenn, H.W. Feeding Mechanisms of Adult Lepidoptera: Structure, Function, and Evolution of the Mouthparts. Annu. Rev. Entomol. 2010, 55, 307–327. [Google Scholar] [CrossRef] [Green Version]
- Tsai, C.-C.; Childers, R.A.; Shi, N.N.; Ren, C.; Pelaez, J.N.; Bernard, G.D.; Pierce, N.E.; Yu, N. Physical and behavioral adaptations to prevent overheating of the living wings of butterflies. Nat. Commun. 2020, 11, 551. [Google Scholar] [CrossRef] [Green Version]
- Ai, H. Sensors and Sensory Processing for Airborne Vibrations in Silk Moths and Honeybees. Sensors 2013, 13, 9344–9363. [Google Scholar] [CrossRef] [Green Version]
- Ai, H.; Yoshida, A.; Yokohari, F. Vibration receptive sensilla on the wing margins of the silkworm moth Bombyx mori. J. Insect Physiol. 2010, 56, 236–246. [Google Scholar] [CrossRef]
- Yoshida, A.; Noda, A.; Emoto, J. Bristle Distribution along the Wing Margin of the Small White Cabbage Butterfly (Lepidoptera: Pieridae). Ann. Entomol. Soc. Am. 2001, 94, 467–470. [Google Scholar] [CrossRef] [Green Version]
- Rausher, M.D. Search Image for Leaf Shape in a Butterfly. Science 1978, 200, 1071–1073. [Google Scholar] [CrossRef]
- Prokopy, R.J.; Owens, E.D. Visual Detection of Plants by Herbivorous Insects. Annu. Rev. Entomol. 1983, 28, 337–364. [Google Scholar] [CrossRef]
- Rutowski, R.L. The Evolution of Male Mate-Locating Behavior in Butterflies. Am. Nat. 1991, 138, 1121–1139. [Google Scholar] [CrossRef]
- Allen, C.E.; Zwaan, B.J.; Brakefield, P.M. Evolution of Sexual Dimorphism in the Lepidoptera. Annu. Rev. Entomol. 2011, 56, 445–464. [Google Scholar] [CrossRef]
- Ziemba, K.S.; Rutowski, R.L. Sexual Dimorphism in Eye Morphology in a Butterfly (Asterocampa leilia; Lepidoptera, Nymphalidae). Psyche A J. Entomol. 2000, 103, 25–36. [Google Scholar] [CrossRef] [Green Version]
- Collett, T.S.; Land, M.F. Visual control of flight behaviour in the hoverfly Syritta pipiens L. J. Comp. Physiol. 1975, 99, 1–66. [Google Scholar] [CrossRef]
- Straw, A.D.; Warrant, E.J.; O’Carroll, D.C. A ‘bright zone’ in male hoverfly (Eristalis tenax) eyes and associated faster motion detection and increased contrast sensitivity. J. Exp. Biol. 2006, 209, 4339–4354. [Google Scholar] [CrossRef] [Green Version]
- Koontz, M.A.; Schneider, D. Sexual dimorphism in neuronal projections from the antennae of silk moths (Bombyx mori, Antheraea polyphemus) and the gypsy moth (Lymantria dispar). Cell Tissue Res. 1987, 249, 39–50. [Google Scholar] [CrossRef]
- Stevens, M. Sensory Ecology, Behaviour, and Evolution; Oxford University Press: Oxford, UK, 2015; pp. 3–17. [Google Scholar]
- Agrawal, A.A. A scale-dependent framework for trade-offs, syndromes, and specialization in organismal biology. Ecology 2020, 101, e02924. [Google Scholar] [CrossRef]
- Frederiksen, R.; Warrant, E.J. Visual sensitivity in the crepuscular owl butterfly Caligo memnon and the diurnal blue morpho Morpho peleides: A clue to explain the evolution of nocturnal apposition eyes? J. Exp. Biol. 2008, 211, 844–851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jander, U.; Jander, R. Allometry and resolution of bee eyes (Apoidea). Arthropod Struct. Dev. 2002, 30, 179–193. [Google Scholar] [CrossRef]
- Balkenius, A.; Rosén, W.; Kelber, A. The relative importance of olfaction and vision in a diurnal and a nocturnal hawkmoth. J. Comp. Physiol. A 2006, 192, 431–437. [Google Scholar] [CrossRef]
- Ômura, H.; Honda, K.; Hayashi, N. Chemical and Chromatic Bases for Preferential Visiting By the Cabbage Butterfly, Pieris rapae, to Rape Flowers. J. Chem. Ecol. 1999, 25, 1895–1906. [Google Scholar] [CrossRef]
- Ikeura, H.; Kobayashi, F.; Hayata, Y. How do Pieris rapae search for Brassicaceae host plants? Biochem. Syst. Ecol. 2010, 38, 1199–1203. [Google Scholar] [CrossRef]
- Kunte, K. Allometry and functional constraints on proboscis lengths in butterflies. Funct. Ecol. 2007, 21, 982–987. [Google Scholar] [CrossRef]
- Szigeti, V.; Vajna, F.; Kőrösi, Á.; Kis, J. Are all butterflies equal? Population-wise proboscis length variation predicts flower choice in a butterfly. Anim. Behav. 2020, 163, 135–143. [Google Scholar] [CrossRef]
- Forsberg, J.; Wiklund, C. Protandry in the Green-Veined White Butterfly, Pieris napi L. (Lepidoptera; Pieridae). Funct. Ecol. 1988, 2, 81–88. [Google Scholar] [CrossRef]
- Wiklund, C.; Forsberg, J. Sexual Size Dimorphism in Relation to Female Polygamy and Protandry in Butterflies: A Comparative Study of Swedish Pieridae and Satyridae. Oikos 1991, 60, 373–381. [Google Scholar] [CrossRef]
- Taylor, G.J.; Tichit, P.; Schmidt, M.D.; Bodey, A.J.; Rau, C.; Baird, E. Bumblebee visual allometry results in locally improved resolution and globally improved sensitivity. eLife 2019, 8, e40613. [Google Scholar] [CrossRef]
- Smith, D.B.; Bernhardt, G.; Raine, N.E.; Abel, R.L.; Sykes, D.; Ahmed, F.; Pedroso, I.; Gill, R.J. Exploring miniature insect brains using micro-CT scanning techniques. Sci. Rep. 2016, 6, 21768. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Limaye, A. Drishti: A volume exploration and presentation tool. In Developments in X-ray Tomography VIII; International Society for Optics and Photonics: Bellingham, WA, USA, 2012; Volume 8506, p. 85060X. [Google Scholar] [CrossRef] [Green Version]
- Huxley, J.S.; Teissier, G. Terminology of Relative Growth. Nature 1936, 137, 780–781. [Google Scholar] [CrossRef]
- Mirth, C.K.; Frankino, W.A.; Shingleton, A.W. Allometry and size control: What can studies of body size regulation teach us about the evolution of morphological scaling relationships? Curr. Opin. Insect Sci. 2016, 13, 93–98. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing [Internet]. R Foundation for Statistical Computing. 2018. Available online: http://www.R-project.org/ (accessed on 25 October 2021).
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D. Nlme: Linear and Nonlinear Mixed Effects Models. R Package Version 3.1-108. 2017. Available online: http://ftp.uni-bayreuth.de/math/statlib/R/CRAN/doc/packages/nlme.pdf/ (accessed on 25 October 2021).
- Turlure, C.; Schtickzelle, N.; Van Dyck, H.; Seymoure, B.; Rutowski, R. Flight Morphology, Compound Eye Structure and Dispersal in the Bog and the Cranberry Fritillary Butterflies: An Inter- and Intraspecific Comparison. PLoS ONE 2016, 11, e0158073. [Google Scholar] [CrossRef]
- Merry, J.W.; Morehouse, N.I.; Yturralde, K.; Rutowski, R.L. The eyes of a patrolling butterfly: Visual field and eye structure in the Orange Sulphur, Colias eurytheme (Lepidoptera, Pieridae). J. Insect Physiol. 2006, 52, 240–248. [Google Scholar] [CrossRef]
- Lund, N.M.; Cwengros, E.E.; Rutowski, R.L. Sexual dimorphism in eye morphology in Eucheira socialis (Peridae). J. Lepid. Soc. 2001, 55, 74–77. [Google Scholar]
- Tuomaala, M.; Kaitala, A.; Rutowski, R.L. Females show greater changes in wing colour with latitude than males in the green-veined white butterfly, Pieris napi (Lepidoptera: Pieridae). Biol. J. Linn. Soc. 2012, 107, 899–909. [Google Scholar] [CrossRef]
- Rutowski, R.L. Variation of eye size in butterflies: Inter- and intraspecific patterns. J. Zool. 2000, 252, 187–195. [Google Scholar] [CrossRef]
- Yagi, N.; Koyama, N. The Compound Eye of Lepidoptera; Shinkyo Press: Tokyo, Japan, 1963. [Google Scholar]
- Schäpers, A.; Carlsson, M.A.; Gamberale-Stille, G.; Janz, N. The Role of Olfactory Cues for the Search Behavior of a Specialist and Generalist Butterfly. J. Insect Behav. 2015, 28, 77–87. [Google Scholar] [CrossRef]
- Thomas, R.C.; Schultz, C.B. Resource selection in an endangered butterfly: Females select native nectar species. J. Wildl. Manag. 2015, 80, 171–180. [Google Scholar] [CrossRef]
- Corbet, S.A. Butterfly nectaring flowers: Butterfly morphology and flower form. Entomol. Exp. Appl. 2000, 96, 289–298. [Google Scholar] [CrossRef]
- Bauder, J.A.-S.; Warren, A.D.; Krenn, H.W. Evolution of extreme proboscis lengths in Neotropical Hesperiidae (Lepidoptera). J. Res. Lepid. 2014, 47, 65–71. [Google Scholar]
- Le Roy, C.; Cornette, R.; Llaurens, V.; Debat, V. Effects of natural wing damage on flight performance in Morpho butterflies: What can it tell us about wing shape evolution? J. Exp. Biol. 2019, 222, jeb204057. [Google Scholar] [CrossRef] [Green Version]
- Berwaerts, K.; Van Dyck, H.; Aerts, P. Does flight morphology relate to flight performance? An experimental test with the butterfly Pararge aegeria. Funct. Ecol. 2002, 16, 484–491. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, A.; Emoto, J. Variations in the Arrangement of Sensory Bristles along Butterfly Wing Margins. Zool. Sci. 2011, 28, 430–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friberg, M.; Vongvanich, N.; Borg-Karlson, A.-K.; Kemp, D.J.; Merilaita, S.; Wiklund, C. Female mate choice determines reproductive isolation between sympatric butterflies. Behav. Ecol. Sociobiol. 2008, 62, 873–886. [Google Scholar] [CrossRef]
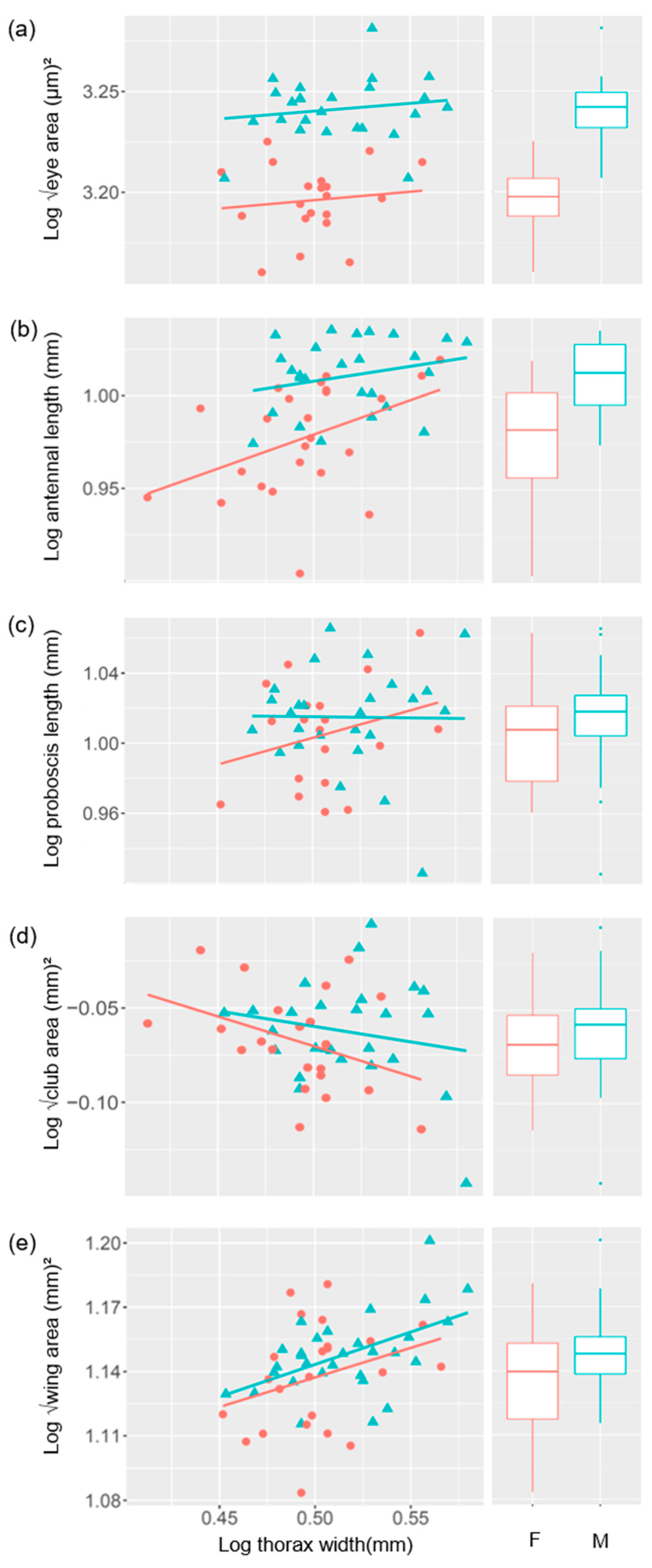
| Sensory Traits | Sex | Sample Size | Slope | y-Intercept | R2 |
|---|---|---|---|---|---|
| Eye | Female | 20 | 0.084 | 3.154 | −0.041 |
| Male | 25 | 0.077 | 3.201 | −0.018 | |
| Antenna | Female | 24 | 0.367 | 0.795 | 0.141 |
| Male | 26 | 0.159 | 0.928 | 0.021 | |
| Club | Female | 22 | −0.315 | 0.087 | 0.101 |
| Male | 26 | −0.163 | 0.021 | −0.003 | |
| Proboscis | Female | 19 | 0.306 | 0.849 | 0.022 |
| Male | 27 | −0.012 | 1.021 | −0.039 | |
| Wing | Female | 23 | 0.276 | 0.999 | 0.045 |
| Male | 30 | 0.302 | 0.992 | 0.247 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moradinour, Z.; Wiklund, C.; Jie, V.W.; Restrepo, C.E.; Gotthard, K.; Miettinen, A.; Perl, C.D.; Baird, E. Sensory Organ Investment Varies with Body Size and Sex in the Butterfly Pieris napi. Insects 2021, 12, 1064. https://doi.org/10.3390/insects12121064
Moradinour Z, Wiklund C, Jie VW, Restrepo CE, Gotthard K, Miettinen A, Perl CD, Baird E. Sensory Organ Investment Varies with Body Size and Sex in the Butterfly Pieris napi. Insects. 2021; 12(12):1064. https://doi.org/10.3390/insects12121064
Chicago/Turabian StyleMoradinour, Zahra, Christer Wiklund, Vun Wen Jie, Carlos E. Restrepo, Karl Gotthard, Arttu Miettinen, Craig D. Perl, and Emily Baird. 2021. "Sensory Organ Investment Varies with Body Size and Sex in the Butterfly Pieris napi" Insects 12, no. 12: 1064. https://doi.org/10.3390/insects12121064
APA StyleMoradinour, Z., Wiklund, C., Jie, V. W., Restrepo, C. E., Gotthard, K., Miettinen, A., Perl, C. D., & Baird, E. (2021). Sensory Organ Investment Varies with Body Size and Sex in the Butterfly Pieris napi. Insects, 12(12), 1064. https://doi.org/10.3390/insects12121064