Abstract
Integrated pest management (IPM) is today a widely accepted pest management strategy to select and use the most efficient control tactics and at the same time reduce over-dependence on chemical insecticides and their potentially negative environmental effects. One of the main pillars of IPM is biological control. While biological control programs of pest insects commonly rely on natural enemies such as predatory insects, parasitoids and microbial pathogens, there is increasing evidence that plant, soil and insect microbiomes can also be exploited to enhance plant defense against herbivores. In this mini-review, we illustrate how microorganisms from diverse origins can contribute to plant fitness, functional traits and indirect defense responses against pest insects, and therefore be indirectly used to improve biological pest control practices. Microorganisms in the rhizosphere, phyllosphere and endosphere have not only been shown to enhance plant growth and plant strength, but also promote plant defense against herbivores both above- and belowground by providing feeding deterrence or antibiosis. Also, herbivore associated molecular patterns may be induced by microorganisms that come from oral phytophagous insect secretions and elicit plant-specific responses to herbivore attacks. Furthermore, microorganisms that inhabit floral nectar and insect honeydew produce volatile organic compounds that attract beneficial insects like natural enemies, thereby providing indirect pest control. Given the multiple benefits of microorganisms to plants, we argue that future IPMs should consider and exploit the whole range of possibilities that microorganisms offer to enhance plant defense and increase attraction, fecundity and performance of natural enemies.
Keywords:
endophyte; honeydew; microbiome; nectar; phyllosphere; plant-insect interactions; rhizosphere 1. Introduction
With a growing intensification of agricultural practices as a means to feed the mounting human population, pests have become a serious burden for crop production [1,2,3,4,5,6,7]. Traditional pest control relies heavily on chemical pesticides and their use has increased dramatically since the 1960s [8]. Although these pesticides were initially very successful, it has become clear that intensive, traditional pesticide use comes with serious economic, social and ecological costs [9], making it socially and ecologically unsustainable.
To meet the various negative effects of chemical pesticides (resistance cases in pests, toxicity on non-target organisms including humans, pollution in environment, …) more and more biopesticides (i.e., a form of pesticide based on microorganisms or natural products) [10,11] and entomophagous arthropods such as predators and parasitoids (i.e., insects laying their eggs on or in the bodies of other arthropods, eventually causing the death of these hosts) are being used to control pest species as part of an integrated pest management (IPM) approach. In general, IPM is defined as a systematic approach to pest management where all possible tactics, including biological control, are implemented to prevent, monitor and control diseases and pests, ensuring that crop damage remains under defined economic thresholds. Chemical pesticides are only used as a last resort when all other tactics fail or are ineffective with care taken to minimize damage to the ecosystem [12,13]. Since January 2014, all European Union (EU) professional growers are obligated to apply IPM tactics according to EU Directive Sustainable Use (Directive 2009/128/EC), indicating that the use of biological control will only increase. Most of these biological control agents are mass-reared arthropods that are released once (referred to as classical biological control, commonly implemented by releasing an exotic natural enemy to control an invasive, non-native pest that has settled in a new geographical area) or multiple times in greenhouses and other crop growth facilities [14]. The latter is known as augmentative biological control, which includes all activities in which natural enemies are periodically released to control native or non-native pest populations. A list of the most commonly used commercial natural enemies deployed in augmentative biological control has been provided by van Lenteren [12]. Hymenopteran parasitoids have been used most extensively in the past because, in comparison to predators, they are more specific, resulting in a more restricted host range and preventing unwanted non-target effects. However, more recently, generalist biocontrol agents such as predatory mites and bugs are increasingly implemented for effective control measures. Despite the obvious benefits of IPM and the increased number of biological control agents commercially available [14], biological control of insect pests is still applied on a very limited scale. In open fields in particular, adoption of biological control agents is limited, and most control practices relate to the stimulation of naturally occurring pest enemies by improving their habitat quality throughout or along arable fields, e.g., by changing small-scale landscape structures and community composition of non-crop plants (e.g., sowing of flower strips, planting of hedge rows, intercropping, …). However, this so-called conservation biological control has recently been shown to have variable success [15] and it remains unclear which structural measures need to be taken to improve pest management in open landscapes [16]. Furthermore, the use of classical biological control is increasingly considered controversial, due to non-target impacts of introducing non-native natural enemies, hindering further implementation of this type of biological control [17]. Introducing non-native species can cause damage to native plants, vector harmful pathogens, cause biodiversity loss, and even replace or interfere with potential native natural enemies [18]. Therefore, there is an increasing demand for effective biocontrol agents that could, alone or in combination with arthropod natural enemies, be used to combat insect pests.
Microorganisms like entomopathogenic fungi, bacteria, nematodes and viruses have been used for the management of insect pests in diverse ecosystems [19]. Entomopathogenic fungi are typically applied as contact insecticides with the expectation of short-term pest control, but long term persistence is also observed depending on habitat conditions. However, despite successful applications, there are several drawbacks associated with the use of such microbial control agents, e.g., susceptibility to environmental stresses, temperature extremes, desiccation and solar radiation [20,21]. To overcome these problems, a general strategy has been to obtain entomopathogens from different geographic regions, or to obtain microbial variants by genetic engineering [22,23]. Another opportunity involves the use of microorganisms for indirect pest control, i.e., by enhancing plant pest resistance and/or by attracting pest natural enemies. Such approach can lead to long-term biological control, especially when combined with other biocontrol agents such as predators or parasitoids [24].
2. Microorganisms: Key Elements in Indirect Biological Pest Control
Microorganisms can contribute to plant fitness and plant functional traits in diverse ways [25]. They do not only have the capacity to promote plant growth and reduce the impact of various biotic and abiotic stresses, but may also induce plant defense, deter pest insects, and/or attract natural enemies of pest species [25,26,27,28,29]. Beneficial plant-associated microorganisms can prime the plant defense process, particularly induced systemic resistance (ISR), through different molecular pathways (jasmonic and salicylic acids and/or ethylene) leading to effects on insect pests [30]. Microbe-mediated ISR includes hypersensitive reactions inducing oxidative stress responses that are essential elements of plant defense against herbivores [31]. However, although jasmonic acid signaling is the main ISR pathway against herbivores triggered by root-associated microorganisms, little is known about the tri-trophic level interaction between plants, insects, and microbes [26]. Additionally, although microbes are known to be connected to plants through diverse molecular pathways, their use for indirect biological pest control is still limited and a better understanding of these microbes, their habitat and how they mediate plant–insect interactions is needed (Figure 1). The outcome of plant–microbe–insect interactions can be dependent on the degree of specialization of the insect. Generalist insect herbivores are generally negatively affected by the toxic and deterrent metabolites of a certain plant species, whereas specialist insects are usually not affected, and can even use such compounds to recognize host plants. Studies on Arabidopsis thaliana have shown that the specialist caterpillar Pieris rapae was not affected by ISR induced by Pseudomonas fluorescens, whereas the generalist Spodoptera exigua was negatively affected [32]. Below, we discuss a diversity of microorganisms associated to different sources and illustrate how they can affect insect targets, both pests and beneficials, through indirect effects. For direct effects of microorganisms as insecticides the reader is referred to other review articles [13,33].
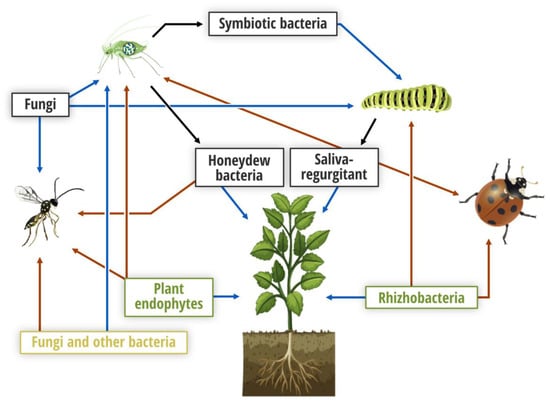
Figure 1.
Illustration of multi-trophic plant–pest interactions mediated by microorganisms focusing on plants, herbivores and entomophagous arthropods. Black, green and orange colors refer to insect, plant (rhizo-, phyllo- and endospheres) and herbivore sources, respectively. Blue and red arrows indicate direct and indirect (through plants) effects on target organisms, respectively, while black arrows illustrate insect origin.
2.1. Rhizosphere Microorganisms
Soil microbiomes are generally complex and contain thousands of interacting microorganisms. A gram of rhizosphere soil might contain around 109 microbial units and 106 distinct taxa [34,35]. Soil microbial mutualists of plants, including mycorrhizal (such as arbuscular myccorhizal or ectomycorrhizal fungi) and non-mycorrhizal fungi (such as Trichoderma species) [36,37], and plant growth promoting rhizobacteria (PGPR) (such as Pseudomonas fluorescens [38,39]) have been shown to promote plant defense against herbivores both above and below ground [13,40]. Although several soilborne microorganisms have been identified to enhance plant growth or plant strength, or to protect plants against pests and pathogens, so far only relatively few formulated products are commercially available, most probably due to some difficulties to ensure efficacy in the field. Currently, the Organic Materials Review Institute (OMRI) lists 174 products under the category of ‘microbial inoculants’ either as crop fertilizers or as biological agents in crop management tools [41]. Beneficial soil microbes could act in plant–insect interactions in different ways. Firstly, they contribute to plant growing, increasing plant size and vigor resulting in an enhanced food supply for herbivores. Improved nutrient composition also increases the nutritional value of the plants, supporting insect biological performance [42]. Secondly, from a positive point of view soil microbes may induce the production of secondary metabolites and enzymes which confer direct or indirect pest resistance in plants by production of toxic compounds and inducing systemic defense responses, respectively [13]. The final impact on insect performance will depend on the interplay between the benefits derived from the enhanced plant growth and the negative effects derived from the induced resistance. Thirdly, soil microbes can modify the volatile organic compounds (VOCs) release from plants that interfere with plant signaling [40]. Reduced release of aromatics including methyl salicylate and terpenes were observed in Arabidopsis after Pseudomonas application. An indirect plant defense against the attacking herbivores was associated to the attraction of higher numbers of parasitoids [43]. Additionally, mycorrhizal fungi have been shown to change plant volatiles to attract spider mite enemies [44].
2.2. Phyllosphere Microorganisms
The term phyllosphere refers to the total above-ground portions of plants as habitat for microorganisms, particularly leaves, stems, buds and flowers. These microorganisms live both on the surfaces of plant organs or inside plant tissues (endosphere, see below). The phyllosphere microbial community is mainly composed of bacteria, but other microorganisms like fungi, viruses, archaea and algae are also common [45]. Bacteria surpass by far other groups, both in cell numbers and diversity [46]. Most of the bacterial groups are scarcely known or are undescribed species as revealed by recent metagenomics studies [47,48]. Aerial surfaces of plants are often an inhospitable environment for microorganisms, as they are highly influenced by fluctuating abiotic conditions and poor nutrient availability. Nevertheless, several microorganisms have managed to colonize this environment. Successful interactions play a crucial role in the homeostasis of plants and offer benefits like growth promotion, defense against pathogens and in general driving plant performance to cope with different stresses [49,50]. Early studies of phyllosphere microorganisms primarily focused on plant pathogens [51]. However, since most microorganisms are commensal on their host plants, more widespread and deeper studies have been performed [52]. New technologies based on next-generation sequencing have allowed culture-independent analyses to be performed that raised great opportunities for characterizing the phyllosphere microbial diversity and ecological properties. These findings have opened new areas of study that integrate not only plant–microbe relationships, but also their interaction with insects. In the context of suppressing diseases and pests, microbial communities in the phyllosphere have received less attention than those in the rhizosphere. Nevertheless, there is growing evidence that phyllosphere microbial communities can contribute to disease suppression in plants [53,54,55]. There is also some evidence that microorganisms inhabiting leaf surfaces can affect insects. For example, the European corn borer (Ostrinia nubilalis) was deterred from ovipositing on maize leaves when Sporobolomyces roseus, a common leaf colonizing yeast, was experimentally sprayed on leaf surfaces [56]. The authors concluded that the change in egg laying behavior was probably the result of removal of nutrients by the yeast that are important for the induction of oviposition, chemical deterrence, or interference with adequate access to the leaf surface. Recent studies have shown that leaf and soil microbiomes are linked [57]. Inoculation of distinct microbiomes collected from soils with different plant species altered the leaf microbiome of Arabidopsis and resistance of the plants against the caterpillar Trichoplusia ni [58]. Further, entomopathogenic fungi like Beauveria bassiana and Metarhizium anisopliae are not only common in the soil, but some strains also exhibit an endophytic phase that can promote plant growth and insect resistance (see below). Additionally, fungi like Trichoderma that were historically considered to be limited to soils are now known to colonize leaves where they can suppress insect pests [59]. More research is needed to uncover how phyllosphere microbiota mediate plant-insect interactions, and how they can be manipulated to protect plants against herbivores.
2.3. Endophytes
Endophytes, i.e., fungi or bacteria that occur inside asymptomatic plant tissues, are ubiquitous and form mutualistic associations with diverse plant species, including wild plant species and crop plants. Several entomopathogenic fungi like B. bassiana, M. anisopliae and Lecanicillium lecanii have been shown to colonize plant tissues as endophytes and have beneficial effects on plant fitness, including resistance against herbivores [60,61]. Increased herbivore resistance is most likely caused by feeding deterrence or antibiosis resulting from the production of endophyte-induced metabolites [61]. Remarkably, these fungi not only provide a benefit to plants by killing their herbivores, but can also translocate nitrogen from aboveground insect cadavers to the plant through fungal mycelia [62]. Endophytic entomopathogenic fungi also protect plants against pathogens, enhance plant growth, increase distribution of soil nutrients within plants, and improve tolerance against abiotic stress and drought [63,64]. These benefits make them highly attractive to be used in agriculture, especially if they can be combined with other biocontrol agents such as parasitoids or predators [65]. However, there is only very little information on the effects of endophytic entomopathogens on higher trophic levels such as parasitoids and secondary parasitoids (i.e., parasitoids of the primary parasitoids; hereafter “hyperparasitoids”), and the few studies performed so far yielded varying results, ranging from no effect to a negative effect on parasitoid life-history traits [66]. Additionally, endophytes have been shown to induce changes in plant odor that led to increased abundance of natural enemies on endophyte-colonized plants, thereby indirectly affecting plant defense [67]. There is also some evidence that endophytic entomopathogens affect the performance and host-selection behavior of hyperparasitoids. For example, parasitized aphid hosts reared on an endophyte-infected model grass (Lolium perenne) reduced the lifespan of hyperparasitoids, and hyperparasitoids were able to perceive the disadvantage for their developing offspring in parasitoids from the endophyte environment and can learn to discriminate against them [68].
2.4. Insect Microbes
In plants, successful defense relies on fast and specific response to biotic attack. In plant–microbe and plant–insect interactions several elicitors and effectors are recognized by specific and unspecific receptors that trigger signal cascades eventually leading to gene expression and plant responses. Following a review on effector biology [69], effectors can be defined as ‘all pathogen/pest proteins and small molecules that alter plant host-cell structure and function’. The plant defense responses corresponding to the production of herbivore induced plant volatiles (HIPVs) are induced by diverse elicitors such as microbial-, pathogen- and herbivore-associated molecular patterns (MAMPs, PAMPs and HAMPs). HIPVs are highly specific and useful in providing reliable pest information to associated natural enemies [70]. Damage-associated molecular patterns (DAMPs) are plant-derived substances and breakdown products, which indicate tissue damage, whereas HAMPs are molecules from herbivores that come into contact with the plant during feeding [71]. Oral secretions such as regurgitant and saliva play an important role in eliciting plant-specific responses to herbivore attacks. Specific signatures by pests are considered as a combination of particular feeding style (among a diversity of sucking and grinding alimentary behaviour) of the herbivore and the presence of particular HAMPs and as effectors found in their oral secretions [72].
Several HAMPs and other effectors have been found in Lepidoptera saliva, including glucosidase, glucose oxidase (GOX) and various ATPases [73]. The origin of some of them was determined to be not from the insect host itself but from associated microbial symbionts. For example, the saliva and its main component GOX from Heliothis zea caterpillars injected with the gut-associated bacterium Enterobacter ludwigii played an important role in elevating tomato anti-herbivore defenses. The caterpillar gut-associated bacteria indirectly mediate plant–insect interactions by triggering salivary elicitors [74]. In addition to HAMPs in Lepidoptera saliva, effectors have also been investigated in detail in aphid saliva. Sitobion avenae pectinase was found to induce volatile emissions in wheat and attracted parasitoids [75]. Polyphenol oxidase (PPO) from S. avenae saliva led to increased expression of genes related to plant defense signaling [76]. Glucose dehydrogenase and oxidase were found in Myzus persicae saliva [77]. Several proteins from Chinese gall aphid saliva were found with binding functions and putative effector activity in gall induction [78]. The role of aphid-associated microbes in the regulation of plant defenses seems to be closely related to insect oral secretions. A range of plant defense effectors was identified in aphids even if functional names and origin, insect host or symbionts were still unknown [79,80,81]. Several salivary proteins between 3 and 10 kDa of the green peach aphid can elicit plant defense responses in Arabidopsis thaliana [82] but were not fully identified. A saliva comparative analysis from three aphid species revealed that proteins with predicted oxidoreductase and peptidase activities accounted for 43–50% and up to 40% of the total proteins identified in M. persicae, Acyrthosiphon pisum and Megoura viciae saliva. From 5% to 20% of these identifications were related to aphid endosymbionts such as Buchnera aphidicola and Serratia symbiotica [83].
2.5. Nectar and Honeydew Microbes
Many adult parasitoids fulfil their energy requirements by feeding on a broad range of accessible sugar sources such as floral nectar, extrafloral nectar and honeydew. Although these sugar-rich substances have traditionally been regarded as simple food sources for insects, they are also an ideal habitat for particular microbes, thereby driving important ecological interactions between plants, microbes and insects. Floral nectar is commonly inhabited by yeasts and bacteria, whose growth largely depends on their capacity to use scarce nutrients in nectar, withstand high osmotic pressures, and cope with unbalanced carbon-to-nitrogen ratios [84,85]. While most nectar-inhabiting microorganisms have evolved specific adaptations that allow them to survive and proliferate in this harsh environment [86], they are dependent on insect vectors to disperse from one flower to another [86,87]. Nectar microbes have been shown to alter sugar and amino acid composition and concentration, acidity, ethanol concentration, and the amount of secondary metabolites [67]. Furthermore, the metabolic activity of nectar-inhabiting microorganisms also affects nectar temperature [88], and leads to the production of volatiles or fermentation products that contribute to the flavor and scent of nectar [89,90]. These microbe-induced changes in nectar chemistry may in turn affect plant–insect interactions. In general, nectar yeasts have a neutral or positive effect on pollinator preference [91,92,93], and in at least one example, the effect on pollinator preference appears to increase male plant fitness [92]. In contrast, nectar bacteria seem to have negative effects on pollinator behaviour, although only few bacterial species have been investigated so far [94], and bacterial density may play an important role in determining the strength of this interaction [95]. In addition to affecting plant–pollinator interactions, changes in nectar chemistry also affect other ecological interactions. A recent study has shown that specialist nectar yeasts influence the volatile composition of nectar, which in turn led to enhanced attractiveness to nectar-feeding parasitoids [90]. Moreover, it has been shown that nectar foraging by parasitoids can be enhanced by associative learning of nectar yeast VOCs [96]. Furthermore, nectar bacteria have been shown to enhance survival of Aphidius parasitoids [97].
Honeydew, i.e., the sugar-rich sticky liquid secreted by aphids and some scale insects when feeding on plant sap, is an important carbohydrate source for parasitoids, increasing parasitoid longevity and fecundity, especially in agro-ecosystems that often lack alternative sugar sources [98,99]. Honeydew also serves as a host-finding cue for parasitoids and predators of honeydew producers [100,101]. In this regard, honeydew-inhabiting microorganisms have been found to play an important role in determining prey location and ovipositional preferences [102]. Both crude and Staphylococcus sciuri-inoculated honeydews strongly stimulated oviposition of hoverflies, important enemies of aphids, and gave an identical number of eggs than obtained with the positive control (plants infested with aphids). By contrast, Goelen and colleagues [103] showed a negative relationship between honeydew bacteria and attraction of the aphid parasitoid Aphidius colemani, suggesting that VOC composition and parasitoid response is context-dependent. While aphid honeydew is primarily considered to comprise sugars and amino acids, its protein composition has only recently been investigated and found to be highly diverse. This huge diversity in proteins originates from several organisms, including the aphid host, endosymbiotic bacteria and gut flora, and is composed of several proteins that might act as mediators in the plant–aphid interaction [104].
It is generally believed that insects respond to microbe-mediated VOCs in nectar and honeydew as they serve reliable signals for a suitable habitat or sugar-rich food source for the insects [105]. Additionally, the VOC producing microbes may also provide the insects other benefits such as aid in food digestion or improvement of insect health, e.g., by protection against diseases [106]. Furthermore, consumption of microbes may provide the insects with essential nutrients improving overall insect fitness. Although the precise mechanisms are not fully understood, enhanced attraction of insect vectors may allow the otherwise immotile microorganisms to be dispersed to new habitats [107]. Likewise, some microbial pathogens have been found to alter the host plant’s volatile profile to attract the pathogen’s insect vector [108,109]. Further research is needed to unravel the precise role of these microbes in plant–insect interactions, and how they can be applied efficiently to suppress pest insects.
3. Production and Formulation
Whereas many microbes from diverse origins may have potential for indirect biocontrol of pest insects (see Table 1 for some case studies), several aspects should be considered before implementation and broad use. One important challenge is the up-scaling of microbial production based on adapted technologies to improve production efficiency to be easier and less expensive. Efforts are being made to optimize microbial growth on grain residues (such as for Metarhizium, Beauveria, and Aspergillus fungi) associated to innovative biofilm techniques or in bioreactors with best adapted media and conditions (such as for Bacillus, Pseudomonas and other bacteria) [110]. New strategies for pest biocontrol may come from the microorganisms from the insects themselves (associated to endosymbionts or gut microbes). Therefore, innovative methods for microbial isolation and culturing should be developed to increase the understanding of these microbiota globally, but also individually at the level of microbial species. The dependence on in vivo insect cells due to the closed interactions and the way to provide similar conditions to grow in bioreactors are the main characteristics to elucidate for further efficient and larger production. Microbes in the digestive tract of insects that significantly affect host physiology, ecology and evolution should be considered as a key player in shaping insect-plant interactions [111] and seem a highly promising source to control pests. Further improvements should also target the kinds of formulations to be adapted to microbial insecticides. Depending on the active material, either the microorganism itself (or particular resistance forms such as spores) or molecules/secondary metabolites produced in the growing medium, different formulations should be investigated and adapted: either as liquid or solid after drying leading to emulsion, encapsulation or granule shapes. The broader availability of microbial biopesticides for innovative pest control is directly related to the capacity to adapt and propose optimal formulations. For example, some endophytes have to be applied on roots and some others on leaves to be fully active and will then orient different formulations. Also, plant nectar and insect honeydew microbes have to be dispersed on plant aerial parts, leaves and flowers, and therefore need to be sprayed in liquid applications. Further concerns to include the survival and stability of the microorganisms to ensure long term effects, mainly when VOCs are the active compounds from living microorganisms. Moreover, even if one particular pest is targeted when using a microbial formulation, another consideration will be to consider non-target organisms constituting the entomofauna including beneficials such as entomophagous predators/parasitoids and pollinators. It may be expected that by overcoming these challenges a new range of ecofriendly products will become available to combat insect pests and become integrated in IPM programs.

Table 1.
Examples of case studies of tri-trophic interactions between microorganisms, herbivores and their natural enemies pointing out an indirect biocontrol effect of microbes.
Author Contributions
All authors (F.F., H.J., F.D., B.L.) contributed to the draft preparation, writing, and review. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
Authors would like to thank collaborators generating data contributing to some statements referred to in this review.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Oerke, E.C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nature Ecol. Evolut. 2019, 3, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.K.M.; Hovmøller, M.S. Aerial dispersal of pathogens on the global and continental scales and its impact on plant disease. Science 2002, 297, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.K.; Cunningham, A.A.; Patel, N.G.; Morales, F.J.; Epstein, P.R.; Daszak, P. Emerging infectious diseases of plants: Pathogen pollution, climate change and agrotechnology drivers. Trends Ecol. Evol. 2004, 19, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Stukenbrock, E.H.; McDonald, B.A. The origins of plant pathogens in agro-ecosystems. Annu. Rev. Phytopathol. 2008, 46, 75–100. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.C.; Henk, D.A.; Briggs, C.J.; Brownstein, J.S.; Madoff, L.C.; McCraw, S.L.; Gurr, S.J. Emerging fungal threats to animal, plant and ecosystem health. Nature 2012, 484, 186–194. [Google Scholar] [CrossRef]
- Lehmann, P.; Ammunet, T.; Barton, M.; Battisti, A.; Eigenbrode, S.D.; Jepsen, J.U.; Kalinkat, G.; Neuvonen, S.; Niemela, P.; Terblanche, J.S.; et al. Complex responses of global insect pests to climate warming. Front. Ecol. Environ. 2020, 18, 141–150. [Google Scholar] [CrossRef]
- Pimentel, D. Environmental and economic costs of the application pesticides primarily in the United States. Environ. Dev. Sustain. 2005, 7, 229–252. [Google Scholar] [CrossRef]
- Benbrook, C.M.; Groth, E.; Halloran, J.M.; Hansen, M.K.; Marquardt, S. Pest Management at the Crossroads; Consumers Union: New York, NY, USA, 1996. [Google Scholar]
- Arthurs, S.; Dara, S.K. Microbial biopesticides for invertebrate pests and their markets in the United States. J. Invertebr. Pathol. 2019, 165, 13–21. [Google Scholar] [CrossRef]
- Hatting, J.L.; Moore, S.D.; Malan, A.P. Microbial control of phytophagous invertebrate pests in South Africa: Current status and future prospects. J. Invertebr. Pathol. 2019, 165, 54–66. [Google Scholar] [CrossRef]
- van Lenteren, J.C. The state of commercial augmentative biological control: Plenty of natural enemies, but a frustrating lack of uptake. BioControl 2012, 57, 1–20. [Google Scholar] [CrossRef]
- van Lenteren, J.; Bolckmans, K.; Köhl, J.; Ravensberg, W.J.; Urbaneja, A. Biological control using invertebrates and microorganisms: Plenty of new opportunities. BioControl 2017, 63, 39–59. [Google Scholar] [CrossRef]
- Perović, D.J.; Gámez-Virués, S.; Landis, D.A.; Wäckers, F.; Gurr, G.M.; Wratten, S.D.; You, M.S.; Desneux, N. Managing biological control services through multi-trophic trait interactions: Review and guidelines for implementation at local and landscape scales. Biol. Rev. 2018, 93, 306–321. [Google Scholar] [CrossRef] [PubMed]
- Karp, D.S.; Chaplin-Kramer, R.; Meehan, T.D.; Martin, E.A.; DeClerck, F.; Grab, H.; Gratton, C.; Hunt, L.; Larsen, A.E.; Martínez-Salinas, A.; et al. Crop pests and predators exhibit inconsistent responses to surrounding landscape composition. Proc. Natl. Acad. Sci. USA 2018, 115, E7863–E7870. [Google Scholar] [CrossRef]
- Egli, L.; Meyer, C.; Scherber, C.; Kreft, H.; Tscharntke, T. Winners and Losers of National and Global Efforts to Reconcile Agricultural Intensification and Biodiversity Conservation. Global Chang. Biol. 2018, 24, 2212–2228. [Google Scholar] [CrossRef]
- Myers, J.H.; Cory, J.S. Biological control agents: Invasive species or valuable solutions? In Impact of Biological Invasions on Ecosystem Services; Vilà, M., Hulme, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; Volume 12, pp. 191–202. [Google Scholar]
- De Clercq, P.; Mason, P.G.; Babendreier, D. Benefits and risks of exotic biological control agents. BioControl 2011, 56, 681–698. [Google Scholar] [CrossRef]
- Ruiu, L. Microbial biopesticides in agroecosystems. Agronomy 2018, 8, 235. [Google Scholar] [CrossRef]
- Lacey, L.L.A.; Frutos, R.; Kaya, H.K. Insect Pathogens as Biological Control Agents: Do They Have a Future? Biol. Control 2001, 21, 230–248. [Google Scholar] [CrossRef]
- de Faria, M.R.; Wraight, S.P. Mycoinsecticides and mycoacaricides: A comprehensive list with worldwide coverage and international classification of formulation types. Biol. Control 2007, 43, 237–256. [Google Scholar] [CrossRef]
- Rangel, D.E. Mutants and isolates of Metarhizium anisopliae are diverse in their relationships between conidial pigmentation and stress tolerance. J. Invertebr. Pathol. 2006, 93, 170–182. [Google Scholar] [CrossRef]
- Rangel, D.E.N.; Braga, G.U.L.; Anderson, A.J.; Roberts, D.W. Variability in conidial thermotolerance of Metarhizium anisopliae isolates from different geographic origins. J. Invertebr. Pathol. 2005, 88, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Jaber, L.R.; Araj, S.E. Interactions among endophytic fungal entomopathogens (Ascomycota: Hypocreales), the green peach aphid Myzus persicae Sulzer (Homoptera: Aphididae), and the aphid endoparasitoid Aphidius colemani Viereck (Hymenoptera: Braconidae). Biol. Control 2018, 116, 53–61. [Google Scholar] [CrossRef]
- Friesen, M.L. Microbially mediated plant functional traits. In Molecular Microbial Ecology of the Rhizosphere, Two Volume Set; De Bruijn, F.J., Ed.; John Wiley and Sons: Hoboken, NJ, USA, 2013; pp. 87–102. [Google Scholar]
- Pineda, A.; Zheng, S.J.; van Loon, J.J.A.; Pieterse, C.M.J.; Dicke, M. Helping plants to deal with insects: The role of beneficial soil-borne microbes. Trends Plant Sci. 2010, 15, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, L.; De Moraes, C.M.; Stephenson, A.G.; Mescher, M.C. Pathogen effects on vegetative and floral odours mediate vector attraction and host exposure in a complex pathosystem. Ecol. Lett. 2012, 15, 1430–1438. [Google Scholar] [CrossRef]
- Beck, J.J.; Vannette, R.L. Harnessing insect-microbe chemical communications to control insect pests of agricultural systems. J. Agric. Food Chem. 2017, 65, 23–28. [Google Scholar] [CrossRef]
- Jaber, L.R.; Ownley, B.H. Can we use entomopathogenic fungi as endophytes for dual biological control of insect pests and plant pathogens? Biol. Control 2017, 107, 50–59. [Google Scholar] [CrossRef]
- Pieterse, C.M.; Van der Does, D.; Zamioudis, C.; Leon-Reyes, A.; Van Wees, S.C. Hormonal modulation of plant immunity. Annu. Rev. Cell Dev. Biol. 2012, 28, 489–521. [Google Scholar] [CrossRef]
- Rachid, M.H.; Chung, Y.R. Induction of Systemic Resistance against Insect Herbivores in Plants by Beneficial Soil Microbes. Front. Plant Sci. 2017, 8, 1816. [Google Scholar]
- Van Oosten, V.R.; Bodenhausen, N.; Reymond, P.; Van Pelt, J.A.; Van Loon, L.L.; Dicke, M.; Pieterse, C.M.J. Differential Effectiveness of Microbially Induced Resistance Against Herbivorous Insects in Arabidopsis. Mol. Plant Microbe Interact. 2008, 7, 919–930. [Google Scholar] [CrossRef]
- Lacey, L.A.; Grzywacz, D.; Shapiro-Ilan, D.I.; Frutos, R.; Brownbridge, M.; Goettel, M.S. Insect pathogens as biological control agents: Back to the future. J. Invertebr. Pathol. 2015, 132, 1–41. [Google Scholar] [CrossRef]
- Curtis, T.P.; Sloan, W.T.; Scannell, J.W. Estimating prokaryotic diversity and its limits. Proc. Natl. Acad. Sci. USA 2002, 99, 10494–10499. [Google Scholar] [CrossRef] [PubMed]
- Curtis, T.P.; Sloan, W.T. Exploring microbial diversity: A vast below. Science 2005, 309, 1331–1333. [Google Scholar] [CrossRef] [PubMed]
- Koide, R.T.; Mosse, B. A History of Research on Arbuscular Mycorrhiza. Mycorrhiza 2004, 14, 145–163. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, D.; Bossi, S.; Cascone, P.; Digilio, M.C.; Prieto, J.D.; Fanti, P.; Guerrieri, E.; Iodice, L.; Lingua, G.; Lorito, M.; et al. Tomato below ground-above ground interactions: Trichoderma longibrachiatum affects the performance of Macrosiphum euphorbiae and its natural antagonists. Mol. Plant Microbe Interact. 2013, 26, 1249–1256. [Google Scholar] [CrossRef] [PubMed]
- Pangesti, N.; Weldegergis, B.T.; Langendorf, B.; van Loon, J.J.; Dicke, M.; Pineda, A. Rhizobacterial colonization of roots modulates plant volatile emission and enhances the attraction of a parasitoid wasp to host-infested plants. Oecologia 2015, 178, 1169–1180. [Google Scholar] [CrossRef]
- Aloo, B.N.; Makumba, B.A.; Mbega, E.R. The potential of Bacilli rhizobacteria for sustainable crop production and environmental sustainability. Microbiol. Res. 2019, 219, 26–39. [Google Scholar] [CrossRef]
- Rasmann, S.; Bennett, A.; Biere, A.; Karley, A.; Guerrieri, E. Root symbionts: Powerful drivers of plant above- and belowground indirect defenses. Insect. Sci. 2017, 24, 947–960. [Google Scholar] [CrossRef]
- Finkel, O.M.; Castrillo, G.; Herrera Paredes, S.; Salas González, I.; Dangl, J.L. Understanding and exploiting plant beneficial microbes. Current Opinion Plant Biol. 2017, 38, 155–163. [Google Scholar] [CrossRef]
- Bukovinszky, T.; van Veen, F.F.; Jongema, Y.; Dicke, M. Direct and indirect effects of resource quality on food web structure. Science 2008, 319, 804–807. [Google Scholar] [CrossRef]
- Kessler, A.; Heil, M. The multiple faces of indirect defences and their agents of natural selection. Funct. Ecol. 2011, 25, 348–357. [Google Scholar] [CrossRef]
- Schausberger, P.; Peneder, S.; Juerschik, S.; Hoffmann, D. Mycorrhiza changes plant volatiles to attract spider mite enemies. Funct. Ecol. 2012, 26, 441–449. [Google Scholar] [CrossRef]
- Vorholt, J.A. Microbial life in the phyllosphere. Nat. Rev. Microbiol. 2012, 10, 828–840. [Google Scholar] [CrossRef] [PubMed]
- Andrews, J.H.; Harris, R.F. The ecology and biogeography of microorganisms on plant surfaces. Annu. Rev. Phytopathol. 2000, 38, 145–180. [Google Scholar] [CrossRef] [PubMed]
- Lambais, M.R. Bacterial diversity in tree canopies of the atlantic forest. Science 2006, 312, 1917. [Google Scholar] [CrossRef] [PubMed]
- Knief, C.; Delmotte, N.; Chaffron, S.; Stark, M.; Innerebner, G.; Wassmann, R. Metaproteogenomic analysis of microbial communities in the phyllosphere and rhizosphere of rice. ISMEJ 2012, 6, 1378–1390. [Google Scholar] [CrossRef] [PubMed]
- Venkatachalam, S.; Ranjan, K.; Prasanna, R.; Ramakrishnan, B.; Thapa, S.; Kanchan, A. Diversity and functional traits of culturable microbiome members, including cyanobacteria in the rice phyllosphere. Plant Biol. 2016, 18, 627–637. [Google Scholar] [CrossRef]
- Saleem, M.; Meckes, N.; Pervaiz, Z.H.; Traw, M.B. Microbial interactions in the phyllosphere increase plant performance under herbivore biotic stress. Front. Microbiol. 2017, 8, 41. [Google Scholar] [CrossRef]
- Montarry, J.; Cartolaro, P.; Delmotte, F.; Jolivet, J.; Willocquet, L. Genetic structure and aggressiveness of Erysiphe necator populations during grapevine powdery mildew epidemics. Appl. Environ. Microbiol. 2008, 74, 6327–6332. [Google Scholar] [CrossRef]
- Rastogi, G.; Coaker, G.L.; Leveau, J.H.J. New insights into the structure and function of phyllosphere microbiota through high-throughput molecular approaches. FEMS Microbiol. Lett. 2013, 348, 1–10. [Google Scholar] [CrossRef]
- Berg, M.; Koskella, B. Nutrient- and dose-dependent microbiome-mediated protection against a plant pathogen. Curr. Biol. 2018, 28, 2487–2492. [Google Scholar] [CrossRef]
- Innerebner, G.; Knief, C.; Vorholt, J.A. Protection of Arabidopsis thaliana against leaf-pathogenic Pseudomonas syringae by Sphingomonas strains in a controlled model system. Appl. Environ. Microbiol. 2011, 77, 3202–3210. [Google Scholar] [CrossRef] [PubMed]
- Ritpitakphong, U.; Falquet, L.; Vimoltust, A.; Berger, A.; Metraux, J.P.; L’Haridon, F. The microbiome of the leaf surface of Arabidopsis protects against a fungal pathogen. New Phytol. 2016, 210, 1033–1043. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.S.; Jones, C.P.; Yao, K.F.; Lee, L.E. An epiphytic yeast (Sporobolomyces roseus) influencing in oviposition preference of the European corn borer (Ostrinia nubilalis) on maize. Acta Oecol. 1993, 14, 563–574. [Google Scholar]
- Bai, Y. Functional overlap of the Arabidopsis leaf and root microbiota. Nature 2015, 528, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Badri, D.V. Potential impact of soil microbiomes on the leaf metabolome and on herbivore feeding behavior. New Phytol. 2013, 198, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Muvea, A.M. Colonization of onions by endophytic fungi and their impacts on the biology of Thrips tabaci. PLoS ONE 2014, 9, e108242. [Google Scholar] [CrossRef]
- Saikkonen, K.; Lehtonen, P.; Helander, M.; Koricheva, J.; Faeth, S.H. Model systems in ecology: Dissecting the endophyte-grass literature. Trends Plant Sci. 2006, 11, 428–433. [Google Scholar] [CrossRef]
- Vega, F.E. Insect pathology and fungal endophytes. J. Invertebr. Pathol. 2008, 98, 277–279. [Google Scholar] [CrossRef]
- Behie, S.W. Endophytic insect-parasitic fungi trans-locate nitrogen directly from insects to plants. Science 2012, 336, 1576–1577. [Google Scholar] [CrossRef]
- Bamisile, B.S.; Dash, C.K.; Akutse, K.S.; Keppanan, R.; Wang, L. Fungal Endophytes: Beyond Herbivore Management. Front. Microbiol. 2018, 9, 544. [Google Scholar] [CrossRef]
- Jaber, L.R.; Alananbeh, K.M. Fungal entomopathogens as endophytes reduce several species of Fusarium causing crown and root rot in sweet pepper (Capsicum annuum L.). Biol. Control 2018, 126, 117–126. [Google Scholar] [CrossRef]
- Barker, G.M.; Addison, P.J. Influence of clavicipitaceous endophyte infection in ryegrass on development of the parasitoid Microctonus hyperodae Loan (Hymenoptera: Braconidae) in Listronotus bonariensis (Kuschel) (Coleoptera: Curculionidae). Biol. Control 1996, 7, 281–287. [Google Scholar] [CrossRef]
- Urrutia, C.M.A.; Wade, M.R.; Phillips, C.B.; Wratten, S.D. Influence of host diet on parasitoid fitness: Unravelling the complexity of a temperate pastoral agroecosystem. Entomol. Exp. Appl. 2007, 123, 63–71. [Google Scholar] [CrossRef]
- Fuchs, B.; Krauss, J. Can Epichloë endophytes enhance direct and indirect plant defence? Fungal Ecol. 2019, 38, 98–103. [Google Scholar] [CrossRef]
- Harri, S.A.; Krauss, J.; Muller, C.B. Fungal endosymbionts of plants reduce lifespan of an aphid secondary parasitoid and influence host selection. Proc. Biol. Sci. 2018, 275, 2627–2632. [Google Scholar] [CrossRef]
- Hogenhout, S.A.; Bos, J.I.B. Effector proteins that modulate plant–insect interactions. Curr. Opin. Plant Biol. 2011, 14, 422–428. [Google Scholar] [CrossRef]
- Dicke, M.; Baldwin, I.T. The evolutionary context for herbivore-induced plant volatiles: Beyond the ‘cry for help’. Trends Plant Sci. 2010, 15, 167–175. [Google Scholar] [CrossRef]
- Turlings, T.C.; Erb, M. Tritrophic interactions mediated by herbivore-induced plant volatiles: Mechanisms, ecological relevance, and application potential. Annu. Rev. Entomol. 2018, 63, 433–452. [Google Scholar] [CrossRef]
- Felton, G.W.; Tumlinson, J.H. Plant-insect dialogs: Complex interactions at the plant-insect interface. Curr. Opin. Plant Biol. 2008, 11, 457–463. [Google Scholar] [CrossRef]
- Tian, D.; Peiffer, M.; Shoemaker, E.; Tooker, J.; Haubruge, E.; Francis, F.; Luthe, D.S.; Felton, G.W. Salivary glucose oxidase from caterpillars mediates the induction of rapid and delayed-induced defenses in the tomato plant. PLoS ONE 2012, 7, e36168. [Google Scholar] [CrossRef]
- Wang, J.; Peiffer, M.; Hoover, K.; Rosa, C.; Zeng, R.; Felton, G.W. Helicoverpa zea gut-associated bacteria indirectly induce defenses in tomato by triggering a salivary elicitor(s). New Phytol. 2017, 214, 1294–1306. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, W.L.; Guo, G.X.; Ji, X.L. Volatile emission in wheat and parasitism by Aphidius avenae after exogenous application of salivary enzymes of Sitobion avenae. Entomol. Exp. Appl. 2009, 130, 215–221. [Google Scholar] [CrossRef]
- Ma, R.; Chen, J.L.; Cheng, D.F.; Sun, J.R. Activation of defense mechanism in wheat by polyphenol oxidase from aphid saliva. J. Agric. Food Chem. 2010, 58, 2410–2418. [Google Scholar] [CrossRef] [PubMed]
- Harmel, N.; Letocart, E.; Cherqui, A.; Giordanengo, P.; Mazzucchelli, G.; Guillonneau, F.; De Pauw, E.; Haubruge, E.; Francis, F. Identification of aphid salivary proteins: A proteomic investigation of Myzus persicae. Insect. Mol. Biol. 2008, 17, 165–174. [Google Scholar] [CrossRef]
- Yang, Z.; Ma, L.; Francis, F.; Yang, Y.; Chen, H.; Wu, H.; Chen, X. Proteins identified from saliva and salivary glands of the Chinese gall aphid Schlechtendalia chinensis. Proteomics 2018, 18, e1700378. [Google Scholar] [CrossRef]
- Bos, J.I.; Prince, D.; Pitino, M.; Maffei, M.E.; Win, J.; Hogenhout, S.A. A functional genomics approach identifies candidate effectors from the aphid species Myzus persicae (green peach aphid). PLoS Genet. 2010, 6, e1001216. [Google Scholar] [CrossRef]
- Atamian, H.S.; Chaudhary, R.; Cin, V.D.; Bao, E.; Girke, T.; Kaloshian, I. In planta expression or delivery of potato aphid Macrosiphum euphorbiae effectors Me10 and Me23 enhances aphid fecundity. Mol. Plant Microbe. Interact. 2013, 26, 67–74. [Google Scholar] [CrossRef]
- Rodriguez, P.A.; Stam, R.; Warbroek, T.; Bos, J.I. Mp10 and Mp42 from the aphid species Myzus persicae trigger plant defenses in Nicotiana benthamiana through different activities. Mol. Plant Microbe. Interact. 2014, 27, 30–39. [Google Scholar] [CrossRef]
- De Vos, M.; Jander, G. Myzus persicae (green peach aphid) salivary components induce defence responses in Arabidopsis thaliana. Plant Cell Environ. 2009, 32, 1548–1560. [Google Scholar] [CrossRef]
- Vandermoten, S.; Harmel, N.; Mazzucchelli, G.; De Pauw, E.; Haubruge, E.; Francis, F. Comparative analyses of salivary proteins from three aphid species. Insect. Mol. Biol. 2014, 23, 67–77. [Google Scholar] [CrossRef]
- Pozo, M.; Lievens, B.; Jacquemyn, H. Impact of microorganisms on nectar chemistry, pollinator attraction and plant fitness. In Nectar: Production, Chemical Composition and Benefits to Animals and Plants; Nova Science Publishers: Hauppauge, NY, USA, 2015; Volume 41. [Google Scholar]
- Lievens, B.; Hallsworth, J.E.; Pozo, M.I.; Belgacem, Z.; Stevenson, A.; Willems, K.; Jacquemyn, H. Microbiology of sugar-rich environments: Diversity, ecology, and system constraints. Environ. Microbiol. 2015, 17, 1–49. [Google Scholar] [CrossRef] [PubMed]
- Vannette, R.L.; Fukami, T. Dispersal enhances beta diversity in nectar microbes. Ecol. Lett. 2017, 20, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Brysch-Herzberg, M. Ecology of yeasts in plant-bumblebee mutualism in Central Europe. FEMS Microbiol. Ecol. 2004, 50, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Herrera, C.M.; Pozo, M.I. Nectar yeasts warm the flowers of a winter-blooming plant. Proc. R. Soc. B 2010, 277, 1827–1834. [Google Scholar] [CrossRef] [PubMed]
- Rering, C.C.; Beck, J.J.; Hall, G.W.; McCartney, M.M.; Vannette, R.L. Nectar-inhabiting microorganisms influence nectar volatile composition and attractiveness to a generalist pollinator. New Phytol. 2018, 220, 750–759. [Google Scholar] [CrossRef]
- Sobhy, I.S.; Baets, D.; Goelen, T.; Herrera-Malaver, B.; Bosmans, L.; Van den Ende, W.; Verstrepen, K.J.; Wäckers, F.; Jacquemyn, H.; Lievens, B. Sweet scents: Nectar specialist yeasts enhance nectar attraction of a generalist aphid parasitoid without affecting survival. Front. Plant Sci. 2018, 9, 1–13. [Google Scholar] [CrossRef]
- Herrera, C.M.; Pozo, M.I.; Medrano, M. Yeasts in nectar of an early-blooming herb: Sought by bumble bees, detrimental to plant fecundity. Ecology 2013, 94, 273–279. [Google Scholar] [CrossRef]
- Schaeffer, R.N.; Irwin, R.E. Yeasts in nectar enhance male fitness in a montane perennial herb. Ecology 2014, 95, 1792–1798. [Google Scholar] [CrossRef]
- Schaeffer, R.N.; Phillips, C.R.; Duryea, M.C.; Andicoechea, J.; Irwin, R.E. Nectar yeasts in the tall Larkspur Delphinium barbeyi (Ranunculaceae) and effects on components of pollinator foraging behavior. PLoS ONE 2014, 9, e108214. [Google Scholar] [CrossRef]
- Good, A.P.; Gauthier, M.-P.L.; Vannette, R.L.; Fukami, T. Honey bees avoid nectar colonized by three bacterial species, but not by a yeast species, isolated from the bee gut. PLoS ONE 2014, 9, e86494. [Google Scholar] [CrossRef]
- Junker, R.R.; Romeike, T.; Keller, A.; Langen, D. Density-dependent negative responses by bumblebees to bacteria isolated from flowers. Apidologie 2014, 45, 467–477. [Google Scholar] [CrossRef]
- Sobhy, I.S.; Goelen, T.; Herrera-Malaver, B.; Verstrepen, K.J.; Wäckers, F.; Jacquemyn, H.; Lievens, B. Associative learning and memory retention of nectar yeast volatiles in a generalist parasitoid. Anim. Behav. 2019, 153, 137–146. [Google Scholar] [CrossRef]
- Lenaerts, M.; Goelen, T.; Paulussen, C.; Herrera-Malaver, B.; Steensels, J.; Van den Ende, W.; Verstrepen, K.J.; Wäckers, F.; Jacquemyn, H.; Lievens, B. Nectar bacteria affect life history of a generalist aphid parasitoid by altering nectar chemistry. Funct. Ecol. 2017, 31, 2061–2069. [Google Scholar] [CrossRef]
- Hogervorst, P.A.; Wäckers, F.L.; Romeis, J. Effects of honeydew sugar composition on the longevity of Aphidius ervi. Entomol. Exp. Appl. 2007, 122, 223–232. [Google Scholar] [CrossRef]
- Wäckers, F.L.; Van Rijn, P.C.; Heimpel, G.E. Honeydew as a food source for natural enemies: Making the best of a bad meal? Biol. Control 2008, 45, 176–184. [Google Scholar] [CrossRef]
- Bouchard, Y.; Cloutier, C. Honeydew as a source of host-searching kairomones for the aphid parasitoid Aphidius nigripes (Hymenoptera: Aphidiidae). Can. J. Zool. 1984, 62, 1513–1520. [Google Scholar] [CrossRef]
- Bargen, H.; Saudhof, K.; Poehling, H.M. Prey finding by larvae and adult females of Episyrphus balteatus. Entomol. Exp. Appl. 1998, 87, 245–254. [Google Scholar] [CrossRef]
- Leroy, P.; Sabri, A.; Heuskin, S.; Thonart, P.; Lognay, G.; Verheggen, F.; Francis, F.; Brostaux, Y.; Felton, G.; Haubruge, E. Microorganisms from Aphid Honeydew Attract and Enhance the Efficacy of Natural Enemies. Nat. Commun. 2011, 2, 1–7. [Google Scholar] [CrossRef]
- Goelen, T.; Sobhy, I.S.; Vanderaa, C.; de Boer, J.G.; Delvigne, F.; Francis, F.; Wäckers, F.; Rediers, H.; Verstrepen, K.J.; Wenseleers, T.; et al. Volatiles of bacteria associated with parasitoid habitats elicit distinct olfactory responses in an aphid parasitoid and its hyperparasitoid. Funct. Ecol. 2020, 34, 507–520. [Google Scholar] [CrossRef]
- Sabri, A.; Vandermoten, S.; Leroy, P.; Haubruge, E.; Hance, T.; Thonart, P.; De Pauw, E.; Francis, F. Proteomic Investigation of Aphid Honeydew Reveals an Unexpected Diversity of Proteins. PLoS ONE 2013, 8, e74656. [Google Scholar] [CrossRef]
- Davis, T.S.; Crippen, T.L.; Hofstetter, R.W.; Tomberlin, J.S. Microbial Volatile Emissions as Insect Semiochemicals. J. Chem. Ecol. 2013, 39, 840–859. [Google Scholar] [CrossRef] [PubMed]
- Vasquez, A.; Forsgren, E.; Fries, I.; Paxton, R.J.; Flaberg, E.; Szekely, L.; Olofsson, T.C. Symbionts as major modulators of insect health: Lactic acid bacteria and honeybees. PLoS ONE 2012, 7, e33188. [Google Scholar] [CrossRef]
- Christiaens, J.F.; Franco, L.M.; Cools, T.L.; De Meester, L.; Michiels, J.; Wenseleers, T.; Hassan, B.A.; Yaksi, E.; Verstrepen, K.J. The fungal aroma gene ATF1 promotes dispersal of yeast cells through insect vectors. Cell Rep. 2014, 9, 425–432. [Google Scholar] [CrossRef]
- Jimenez-Martınez, E.S.; Bosque-Perez, N.A.; Berger, P.H.; Zemetra, R.S.; Ding, H.; Eigenbrode, S.D. Volatile cues influence the response of Rhopalosiphum padi (Homoptera: Aphididae) to Barley yellow dwarf virus-infected transgenic and untransformed wheat. Environ. Entomol. 2004, 33, 1207–1216. [Google Scholar] [CrossRef]
- Mann, R.S.; Ali, J.G.; Hermann, S.L.; Tiwari, S.; Pelz-Stelinski, K.S.; Alborn, H.T.; Stelinski, L.L. Induced release of a plant-defense volatile ‘deceptively’ attracts insect vectors to plants infected with a bacterial pathogen. PLoS Pathog. 2012, 8, e1002610. [Google Scholar] [CrossRef] [PubMed]
- Francis, F.; Druart, F.; Diana Di Mavungu, J.; De Boevre, M.; De Saeger, S.; Delvigne, F. Biofilm mode of cultivation leads to an improvement of the entomotoxic patterns of two Aspergillus species. Microorganisms 2020, 8, 705. [Google Scholar] [CrossRef] [PubMed]
- Hammer, T.J.; Bowers, M.D. Gut microbes may facilitate insect herbivory of chemically defended plants. Oecologia 2015, 179, 1–14. [Google Scholar] [CrossRef]
- Pineda, A.; Soler, R.; Weldegergis, B.T.; Shimwela, M.M.; Van Loon, J.J.A.; Dicke, M. Non-pathogenic rhizobacteria interfere with the attraction of parasitoids to aphid-induced plant volatiles via jasmonic acid signalling. Plant Cell Environ. 2013, 36, 393–404. [Google Scholar] [CrossRef]
- D’Alessandro, M.; Erb, M.; Ton, J.; Brandenburg, A.; Karlen, D.; Zopfi, J. Volatiles produced by soil-borne endophytic bacteria increase plant pathogen resistance and affect tritrophic interactions. Plant Cell Environ. 2014, 37, 813–826. [Google Scholar] [CrossRef]
- Song, G.C.; Ryu, C.M. Two volatile organic compounds trigger plant self- defense against a bacterial pathogen and a sucking insect in cucumber under open field conditions. Int. J. Mol. Sci. 2013, 14, 9803–9819. [Google Scholar] [CrossRef]
- Kaur, T.; Singh, B.; Kaur, A.; Kaur, S. Endophyte-mediated interactions between cauliflower, the herbivore Spodoptera litura, and the ectoparasitoid Bracon hebetor. Oecologia 2015, 179, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Bixby-Brosi, A.; Potter, D. Endophyte-mediated tritrophic interactions between a grass-feeding caterpillar and two parasitoid species with different life histories. Arthropod Plant Interact. 2012, 6, 27–34. [Google Scholar] [CrossRef]
- González-Mas, N.; Cuenca-Medina, M.; Gutiérrez-Sánchez, F.; Quesada-Moraga, E. Bottom-up effects of endophytic Beauveria bassiana on multitrophic interactions between the cotton aphid, Aphis gossypii, and its natural enemies in melon. J. Pest Sci. 2019, 92, 1271–1281. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).