Limited Effect of Management on Apple Pollination: A Case Study from an Oceanic Island
Abstract
1. Introduction
2. Materials and Methods
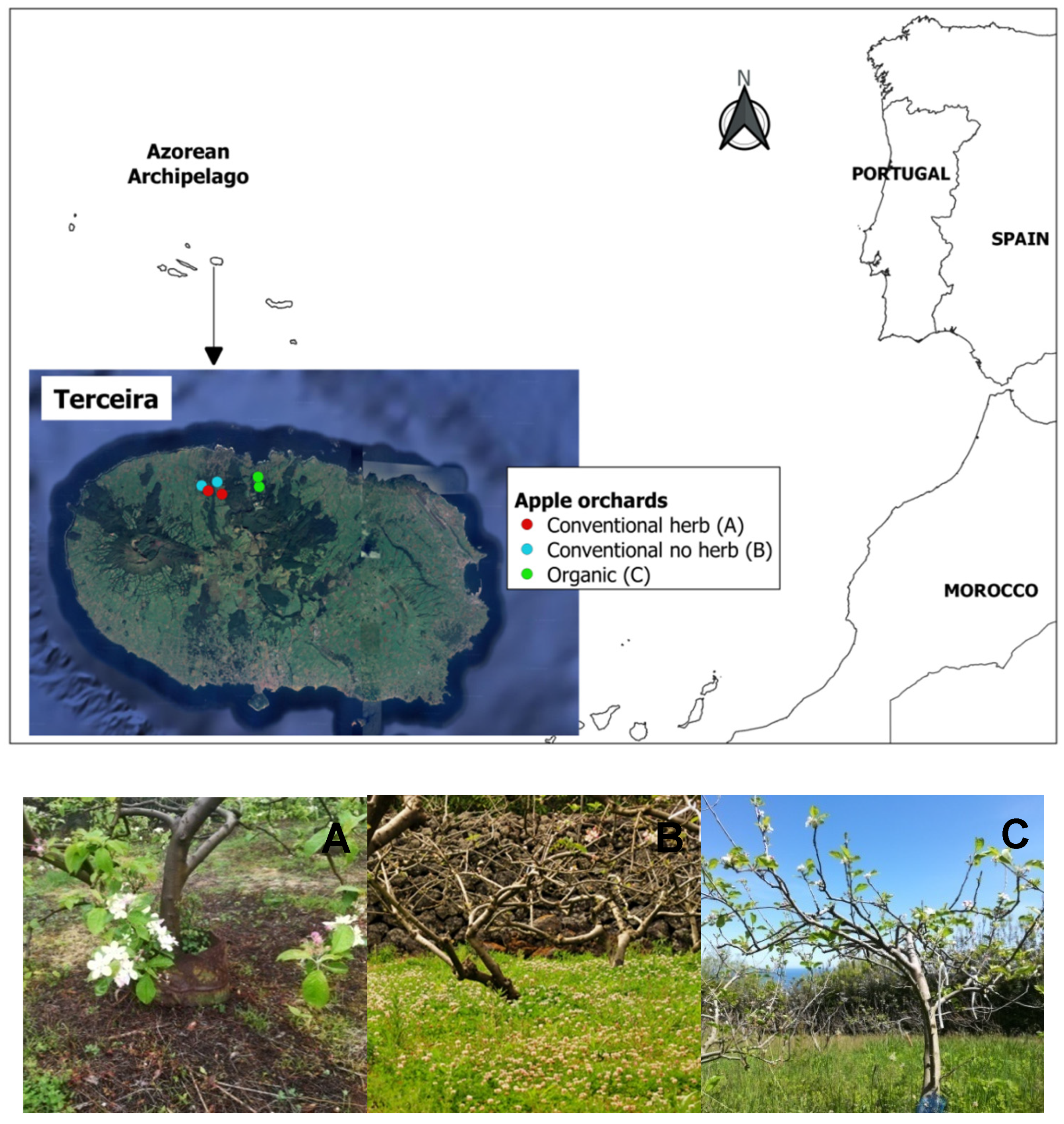
2.1. Study Sites
2.2. Pollinator Sampling
2.3. Pollination Service Provision
2.4. Data Analysis
3. Results
3.1. Species Composition
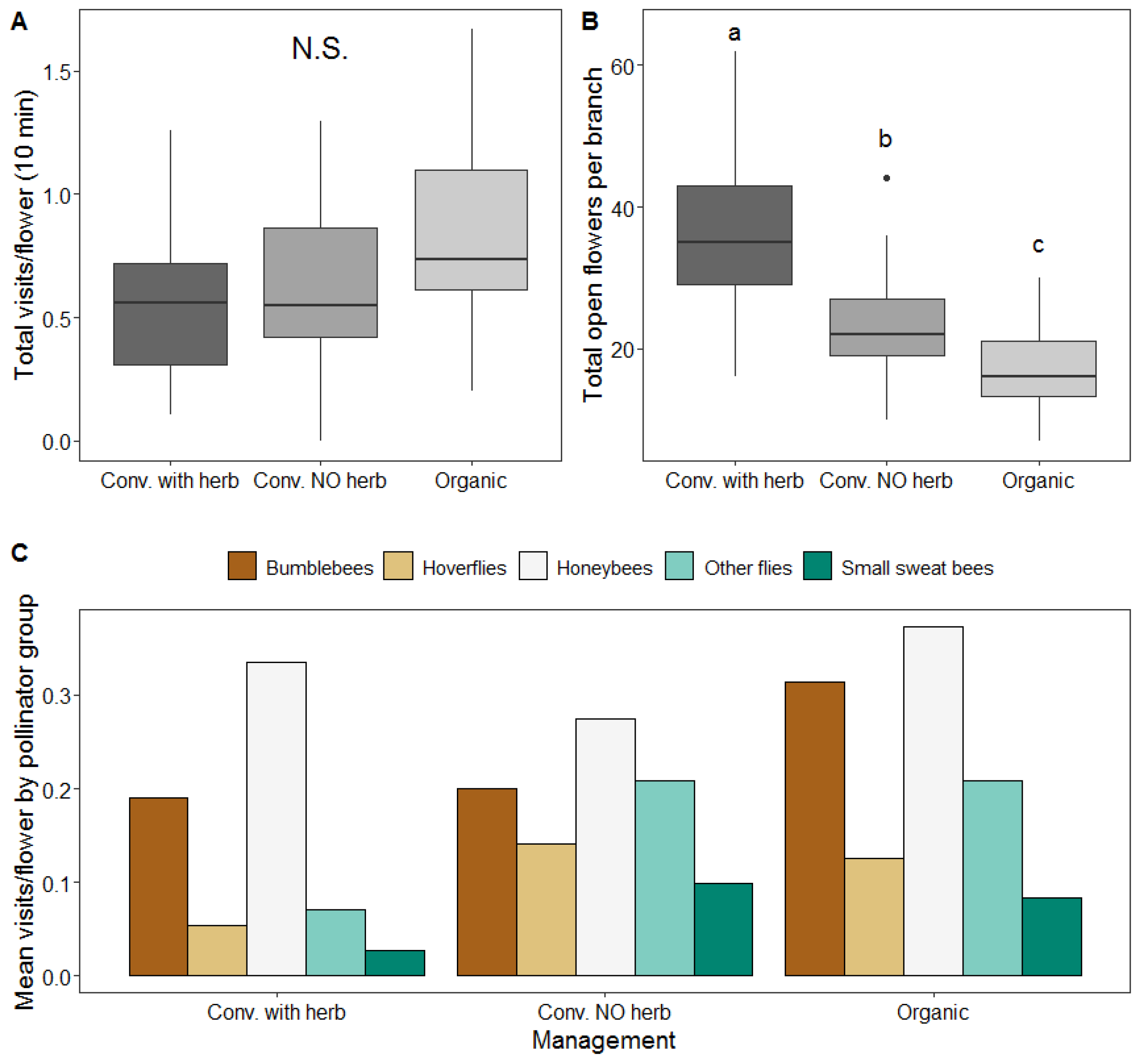
3.2. Effect of Management on Insect Visitation Rates
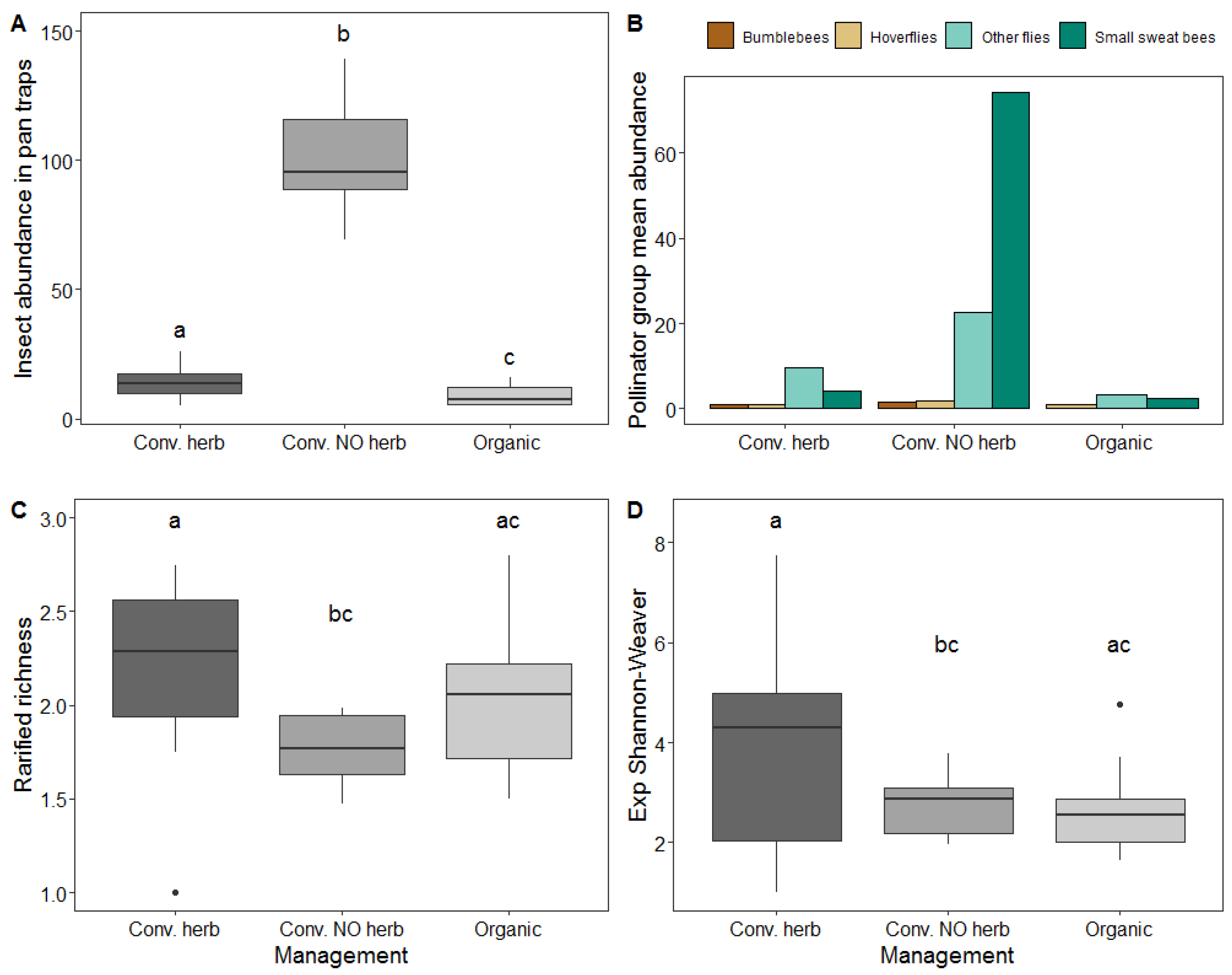
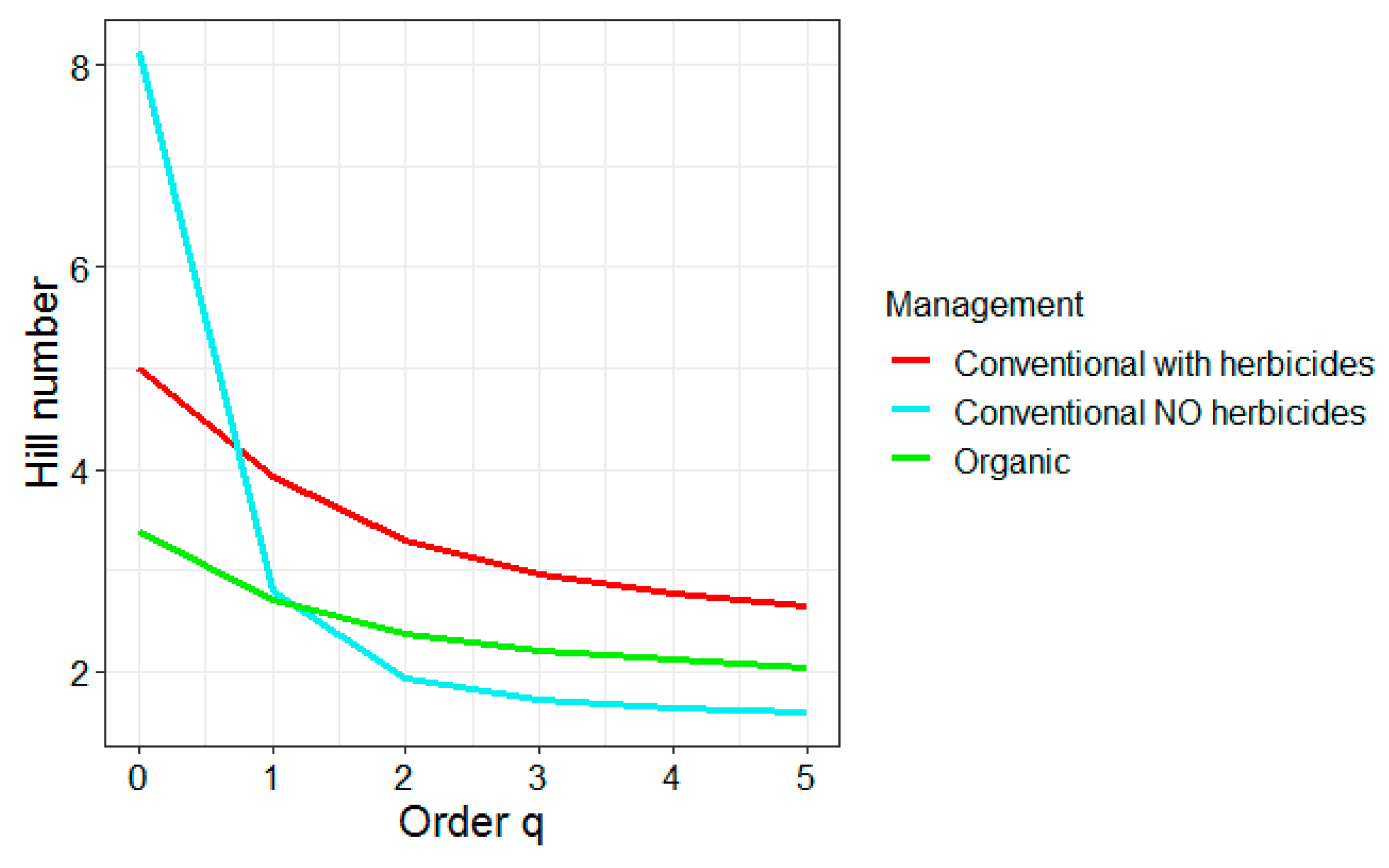
3.3. Effect of Management on Insect Abundance and Diversity
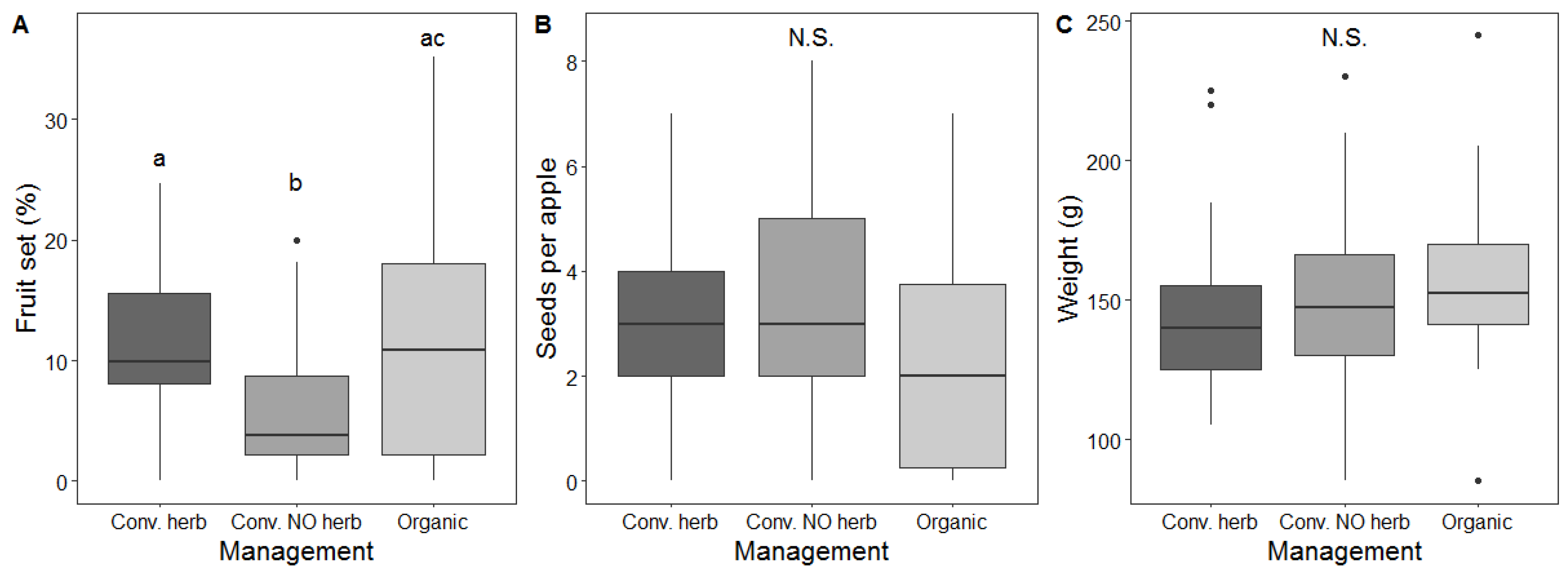
3.4. Effect of Management on Pollination Services
4. Discussion
4.1. Species Composition in Apple Orchards
4.2. Effect of Management on Apple Pollination
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Klein, A.-M.; Vaissière, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. Biol. Sci. 2007, 274, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Aizen, M.A.; Garibaldi, L.A.; Cunningham, S.A.; Klein, A.M. How much does agriculture depend on pollinators? Lessons from long-term trends in crop production. Ann. Bot. 2009, 103, 1579–1588. [Google Scholar] [CrossRef]
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Potts, S.G.; Imperatriz-Fonseca, V.; Ngo, H.T.; Aizen, M.A.; Biesmeijer, J.C.; Breeze, T.D.; Dicks, L.V.; Garibaldi, L.A.; Hill, R.; Settele, J.; et al. Safeguarding pollinators and their values to human well-being. Nature 2016, 540, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Powney, G.D.; Carvell, C.; Edwards, M.; Morris, R.K.A.; Roy, H.E.; Woodcock, B.A.; Isaac, N.J.B. Widespread losses of pollinating insects in Britain. Nat. Commun. 2019, 10, 1–6. [Google Scholar] [CrossRef]
- Cameron, S.A.; Lozier, J.D.; Strange, J.P.; Koch, J.B.; Cordes, N.; Solter, L.F.; Griswold, T.L. Patterns of widespread decline in North American bumble bees. Proc. Natl. Acad. Sci. USA 2011, 108, 662–667. [Google Scholar] [CrossRef]
- Biesmeijer, J.C.; Roberts, S.P.M.; Reemer, M.; Ohlemüller, R.; Edwards, M.; Peeters, T.; Schaffers, A.P.; Potts, S.G.; Kleukers, R.; Thomas, C.D.; et al. Parallel declines in pollinators and insect-pollinated plants in Britain and The Netherlands. Science 2006, 313, 351–354. [Google Scholar] [CrossRef]
- Wagner, D.L. Insect declines in the anthropocene. Annu. Rev. Entomol. 2020, 65, 457–480. [Google Scholar] [CrossRef]
- Vanbergen, A.J. Threats to an ecosystem service: Pressures on pollinators. Front. Ecol. Environ. 2013, 11, 251–259. [Google Scholar] [CrossRef]
- Kennedy, C.M.; Lonsdorf, E.; Neel, M.C.; Williams, N.M.; Ricketts, T.H.; Winfree, R.; Bommarco, R.; Brittain, C.; Burley, A.L.; Cariveau, D.; et al. A global quantitative synthesis of local and landscape effects on wild bee pollinators in agroecosystems. Ecol. Lett. 2013, 16, 584–599. [Google Scholar] [CrossRef]
- Harvey, J.A.; Heinen, R.; Armbrecht, I.; Basset, Y.; Baxter-Gilbert, J.H.; Bezemer, T.M.; Böhm, M.; Bommarco, R.; Borges, P.A.V.; Cardoso, P.; et al. International scientists formulate a roadmap for insect conservation and recovery. Nat. Ecol. Evol. 2020, 4, 174–176. [Google Scholar] [CrossRef] [PubMed]
- Kremen, C.; Williams, N.M.; Aizen, M.A.; Gemmill-Herren, B.; LeBuhn, G.; Minckley, R.; Packer, L.; Potts, S.G.; Roulston, T.; Steffan-Dewenter, I.; et al. Pollination and other ecosystem services produced by mobile organisms: A conceptual framework for the effects of land-use change. Ecol. Lett. 2007, 10, 299–314. [Google Scholar] [CrossRef] [PubMed]
- Williams, N.M.; Kremen, C. Resource distributions among habitats determine solitary bee. Ecol. Appl. 2007, 17, 910–921. [Google Scholar] [CrossRef] [PubMed]
- Holzschuh, A.; Steffan-Dewenter, I.; Tscharntke, T. Agricultural landscapes with organic crops support higher pollinator diversity. Oikos 2008, 117, 354–361. [Google Scholar] [CrossRef]
- Batáry, P.; Báldi, A.; Kleijn, D.; Tscharntke, T. Landscape-moderated biodiversity effects of agri-environmental management: A meta-analysis. Proc. R. Soc. B Biol. Sci. 2011, 278, 1894–1902. [Google Scholar] [CrossRef]
- Tscharntke, T.; Klein, A.M.; Kruess, A.; Steffan-Dewenter, I.; Thies, C. Landscape perspectives on agricultural intensification and biodiversity - Ecosystem service management. Ecol. Lett. 2005, 8, 857–874. [Google Scholar] [CrossRef]
- Tscharntke, T.; Tylianakis, J.M.; Rand, T.A.; Didham, R.K.; Fahrig, L.; Batáry, P.; Bengtsson, J.; Clough, Y.; Crist, T.O.; Dormann, C.F.; et al. Landscape moderation of biodiversity patterns and processes - eight hypotheses. Biol. Rev. 2012, 87, 661–685. [Google Scholar] [CrossRef]
- Brittain, C.; Bommarco, R.; Vighi, M.; Settele, J.; Potts, S.G. Organic farming in isolated landscapes does not benefit flower-visiting insects and pollination. Biol. Conserv. 2010, 143, 1860–1867. [Google Scholar] [CrossRef]
- Porcel, M.; Andersson, G.K.S.; Pålsson, J.; Tasin, M. Organic management in apple orchards: Higher impacts on biological control than on pollination. J. Appl. Ecol. 2018, 55, 2779–2789. [Google Scholar] [CrossRef]
- Joshi, N.K.; Leslie, T.; Rajotte, E.G.; Biddinger, D.J. Environmental impacts of reduced-risk and conventional pesticide programs differ in commercial apple orchards, but similarly influence pollinator community. Chemosphere 2020, 240, 124926. [Google Scholar] [CrossRef]
- FAO. FAOSTAT Food and Agriculture Organization of the United Nations (FAO) 2019; FAO: Rome, Italy, 2019. [Google Scholar]
- Free, J.B. Comparison of the importance of insect and wind pollination of apple trees. Nature 1964, 201, 726–727. [Google Scholar] [CrossRef]
- Delaplane, K.; Mayer, D. Crop Pollination by Bees; CABI Publishing: Wallingford, UK, 2000. [Google Scholar]
- Garratt, M.P.D.; Breeze, T.D.; Jenner, N.; Polce, C.; Biesmeijer, J.C.; Potts, S.G. Avoiding a bad apple: Insect pollination enhances fruit quality and economic value. Agric. Ecosyst. Environ. 2014, 184, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Cross, J.; Fountain, M.; Markó, V.; Nagy, C. Arthropod ecosystem services in apple orchards and their economic benefits. Ecol. Entomol. 2015, 40, 82–96. [Google Scholar] [CrossRef]
- Samnegård, U.; Alins, G.; Boreux, V.; Bosch, J.; García, D.; Happe, A.K.; Klein, A.M.; Miñarro, M.; Mody, K.; Porcel, M.; et al. Management trade-offs on ecosystem services in apple orchards across Europe: Direct and indirect effects of organic production. J. Appl. Ecol. 2019, 56, 802–811. [Google Scholar] [CrossRef]
- Sheffield, C.S.; Kevan, P.G.; Westby, S.M.; Smith, R.F. Diversity of cavity-nesting bees (Hymenoptera: Apoidea) within apple orchards and wild habitats in the Annapolis Valley, Nova Scotia, Canada. Can. Entomol. 2008, 140, 235–249. [Google Scholar] [CrossRef]
- Sheffield, C.S.; Kevan, P.G.; Pindar, A.; Packer, L. Bee (Hymenoptera: Apoidea) diversity within apple orchards and old fields in the Annapolis Valley, Nova Scotia, Canada. Can. Entomol. 2013, 145, 94–114. [Google Scholar] [CrossRef]
- Picanço, A.; Gil, A.; Rigal, F.; Borges, P.A.V. Pollination services mapping and economic valuation from insect communities: A case study in the Azores (Terceira Island). Nat. Conserv. 2017, 18, 1–25. [Google Scholar] [CrossRef]
- Olesen, J.M.; Eskildsen, L.I.; Venkatasamy, S. Invasion of pollination networks on oceanic islands: Importance of invader complexes and endemic super generalists. Divers. Distrib. 2002, 8, 181–192. [Google Scholar] [CrossRef]
- Traveset, A.; Tur, C.; Trøjelsgaard, K.; Heleno, R.; Castro-Urgal, R.; Olesen, J.M.; Santos, A. Global patterns of mainland and insular pollination networks. Glob. Ecol. Biogeogr. 2016, 25, 880–890. [Google Scholar] [CrossRef]
- Picanço, A.; Rigal, F.; Matthews, T.J.; Cardoso, P.; Borges, P.A.V. Impact of land-use change on flower-visiting insect communities on an oceanic island. Insect Conserv. Divers. 2017, 10, 211–223. [Google Scholar] [CrossRef]
- Connor, S.E.; Van Leeuwen, J.F.N.; Rittenour, T.M.; Van Der Knaap, W.O.; Ammann, B.; Björck, S. The ecological impact of oceanic island colonization—a palaeoecological perspective from the Azores. J. Biogeogr. 2012, 39, 1007–1023. [Google Scholar] [CrossRef]
- Triantis, K.A.; Borges, P.A.V.; Ladle, R.J.; Hortal, J.; Cardoso, P.; Gaspar, C.; Dinis, F.; Mendonça, E.; Silveira, L.M.A.; Gabriel, R.; et al. Extinction debt on oceanic Islands. Ecography (Cop.) 2010, 33, 285–294. [Google Scholar] [CrossRef]
- Gathmann, A.; Tscharntke, T. Foraging ranges of solitary bees. J. Anim. Ecol. 2002, 71, 757–764. [Google Scholar] [CrossRef]
- Zurbuchen, A.; Landert, L.; Klaiber, J.; Müller, A.; Hein, S.; Dorn, S. Maximum foraging ranges in solitary bees: Only few individuals have the capability to cover long foraging distances. Biol. Conserv. 2010, 143, 669–676. [Google Scholar] [CrossRef]
- DROTRH Carta de ocupação do solo da Região Autónoma dos Açores. Secretaria Regional do Ambiente; Direcção Regional do Ordenamento do Território e dos Recursos Hídricos: Ponta Delgada, Açores, Portugal, 2008. [Google Scholar]
- Carvell, C.; Isaac, N.J.B.; Jitlal, M.; Peyton, J.; Powney, G.D.; Roy, D.B.; Vanbergen, A.J.; O’Connor, R.S.; Jones, C.M.; Kunin, W.E.; et al. Design and Testing of a National Pollinator and Pollination Monitoring Framework; Final summary report to the Department for Environment, Food and Rural Affairs, Scottish Government and Welsh Government: Project WC1101; Centre for Ecology & Hydrology: Wallingford, UK, 2016. [Google Scholar]
- Borges, P.A.V.; Costa, A.; Cunha, R.; Gabriel, R.; Gonçalves, V.; Martins, A.F.; Melo, I.; Parente, M.; Raposeiro, P.; Rodrigues, P.; et al. A List of the Terrestrial and Marine Biota from the Azores; Princípia: Oeiras, Portugal, 2010. [Google Scholar]
- Campbell, A.J.; Wilby, A.; Sutton, P.; Wäckers, F.L. Do sown flower strips boost wild pollinator abundance and pollination services in a spring-flowering crop? A case study from UK cider apple orchards. Agric. Ecosyst. Environ. 2017, 239, 20–29. [Google Scholar] [CrossRef]
- Chao, A.; Chiu, C.-H.; Jost, L. Unifying species diversity, phylogenetic diversity, functional diversity, and related similarity and differentiation measures through hill numbers. Annu. Rev. Ecol. Evol. Syst. 2014, 45, 297–324. [Google Scholar] [CrossRef]
- Gotelli, N.J.; Colwell, R.K. Quantifying biodiversity: Procedures and pitfalls in the measurement and comparison of species richness. Ecol. Lett. 2001, 4, 379–391. [Google Scholar] [CrossRef]
- Chao, A.; Ricotta, C. Quantifying evenness and linking it to diversity, beta diversity, and similarity. Ecology 2019, 100, e02852. [Google Scholar] [CrossRef]
- Zuur, A.; Ieno, E.; Walker, N.; Savaliev, A.; Smith, G. Mixed Effects Models and Extensions in Ecology With R; Springer Science & Business Media: New York, NY, USA, 2009. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: http://www.r-project.org/ (accessed on 10 January 2020).
- Hofmann, S.; Everaars, J.; Schweiger, O.; Frenzel, M.; Bannehr, L.; Cord, A.F. Modelling patterns of pollinator species richness and diversity using satellite image texture. PLoS One 2017, 12, 1–17. [Google Scholar] [CrossRef]
- Borges, P.A.V.; Cardoso, P.; Kreft, H.; Whittaker, R.J.; Fattorini, S.; Emerson, B.C.; Gil, A.; Gillespie, R.G.; Matthews, T.J.; Santos, A.M.C.; et al. Global Island Monitoring Scheme (GIMS): A proposal for the long-term coordinated survey and monitoring of native island forest biota. Biodivers. Conserv. 2018, 27, 2567–2586. [Google Scholar] [CrossRef]
- Pardo, A.; Borges, P.A.V. Worldwide importance of insect pollination in apple orchards: A review. Agric. Ecosyst. Environ. 2020, 293, 106839. [Google Scholar] [CrossRef]
- Garibaldi, L.A.; Steffan-Dewenter, I.; Winfree, R.; Aizen, M.A.; Bommarco, R.; Cunningham, S.A.; Kremen, C.; Carvalheiro, L.G.; Harder, L.D.; Afik, O.; et al. Wild pollinators enhance fruit set of crops regardless of honey bee abundance. Science 2013, 339, 1608–1611. [Google Scholar] [CrossRef] [PubMed]
- Martins, K.T.; Gonzalez, A.; Lechowicz, M.J. Pollination services are mediated by bee functional diversity and landscape context. Agric. Ecosyst. Environ. 2015, 200, 12–20. [Google Scholar] [CrossRef]
- Rader, R.; Bartomeus, I.; Garibaldi, L.A.; Garratt, M.P.D.; Howlett, B.G.; Winfree, R.; Cunningham, S.A.; Mayfield, M.M.; Arthur, A.D.; Andersson, G.K.S.; et al. Non-bee insects are important contributors to global crop pollination. Proc. Natl. Acad. Sci. USA 2016, 113, 146–151. [Google Scholar] [CrossRef]
- Campbell, A.J.; Biesmeijer, J.C.; Varma, V.; Wäckers, F.L. Realising multiple ecosystem services based on the response of three beneficial insect groups to floral traits and trait diversity. Basic Appl. Ecol. 2012, 13, 363–370. [Google Scholar] [CrossRef]
- García, R.R.; Miñarro, M. Role of floral resources in the conservation of pollinator communities in cider-apple orchards. Agric. Ecosyst. Environ. 2014, 183, 118–126. [Google Scholar] [CrossRef]
- Watson, J.C.; Wolf, A.T.; Ascher, J.S. Forested landscapes promote richness and abundance of native bees (hymenoptera: Apoidea: Anthophila) in Wisconsin apple orchards. Environ. Entomol. 2011, 40, 621–632. [Google Scholar] [CrossRef]
- Marini, L.; Quaranta, M.; Fontana, P.; Biesmeijer, J.C.; Bommarco, R. Landscape context and elevation affect pollinator communities in intensive apple orchards. Basic Appl. Ecol. 2012, 13, 681–689. [Google Scholar] [CrossRef]
- Proesmans, W.; Smagghe, G.; Meeus, I.; Bonte, D.; Verheyen, K. The effect of mass-flowering orchards and semi-natural habitat on bumblebee colony performance. Landsc. Ecol. 2019, 34, 1033–1044. [Google Scholar] [CrossRef]
- Wu, P.; Axmacher, J.C.; Li, X.; Song, X.; Yu, Z.; Xu, H.; Tscharntke, T.; Westphal, C.; Liu, Y. Contrasting effects of natural shrubland and plantation forests on bee assemblages at neighboring apple orchards in Beijing, China. Biol. Conserv. 2019, 237, 456–462. [Google Scholar] [CrossRef]
- Mallinger, R.E.; Gibbs, J.; Gratton, C. Diverse landscapes have a higher abundance and species richness of spring wild bees by providing complementary floral resources over bees’ foraging periods. Landsc. Ecol. 2016, 31, 1523–1535. [Google Scholar] [CrossRef]
- Wittebolle, L.; Marzorati, M.; Clement, L.; Balloi, A.; Daffonchio, D.; Heylen, K.; De Vos, P.; Verstraete, W.; Boon, N. Initial community evenness favours functionality under selective stress. Nature 2009, 458, 623–626. [Google Scholar] [CrossRef] [PubMed]
- Matthews, T.J.; Whittaker, R.J. On the species abundance distribution in applied ecology and biodiversity management. J. Appl. Ecol. 2015, 52, 443–454. [Google Scholar] [CrossRef]
- Andersson, G.K.S.; Rundlöf, M.; Smith, H.G. Organic farming improves pollination success in Strawberries. PLoS ONE 2012, 7, 1–4. [Google Scholar] [CrossRef]
| Management | Insect Visitation | Pan Traps | ||||||
|---|---|---|---|---|---|---|---|---|
| Native/Endemic | Introduced | Native/Endemic | Introduced | |||||
| N | S | N | S | N | S | N | S | |
| Conventional with herbicides | 93 | 6 | 457 | 6 | 84 | 11 | 24 | 5 |
| Conventional NO herbicides | 162 | 11 | 216 | 5 | 737 | 15 | 36 | 5 |
| Organic | 87 | 11 | 274 | 4 | 62 | 8 | 5 | 4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pardo, A.; Lopes, D.H.; Fierro, N.; Borges, P.A.V. Limited Effect of Management on Apple Pollination: A Case Study from an Oceanic Island. Insects 2020, 11, 351. https://doi.org/10.3390/insects11060351
Pardo A, Lopes DH, Fierro N, Borges PAV. Limited Effect of Management on Apple Pollination: A Case Study from an Oceanic Island. Insects. 2020; 11(6):351. https://doi.org/10.3390/insects11060351
Chicago/Turabian StylePardo, Adara, David H. Lopes, Natalia Fierro, and Paulo A. V. Borges. 2020. "Limited Effect of Management on Apple Pollination: A Case Study from an Oceanic Island" Insects 11, no. 6: 351. https://doi.org/10.3390/insects11060351
APA StylePardo, A., Lopes, D. H., Fierro, N., & Borges, P. A. V. (2020). Limited Effect of Management on Apple Pollination: A Case Study from an Oceanic Island. Insects, 11(6), 351. https://doi.org/10.3390/insects11060351






