Minimum Winter Temperature as a Limiting Factor of the Potential Spread of Agrilus planipennis, an Alien Pest of Ash Trees, in Europe
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Temperatures below −30 °C in the Native Range of A. planipennis in Asia
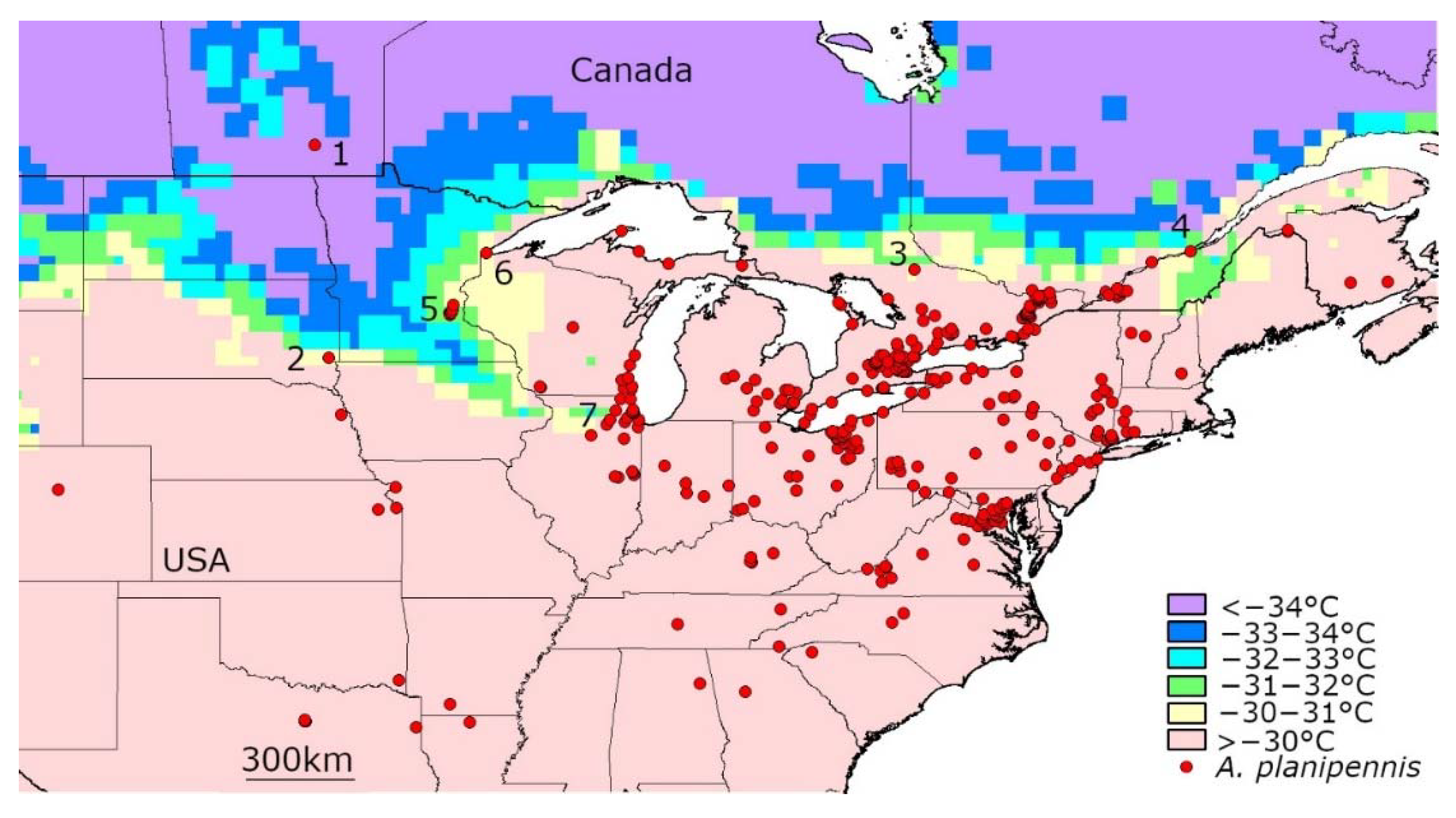
3.2. Temperatures below −30 °C in the Current Range of A. planipennis in North America
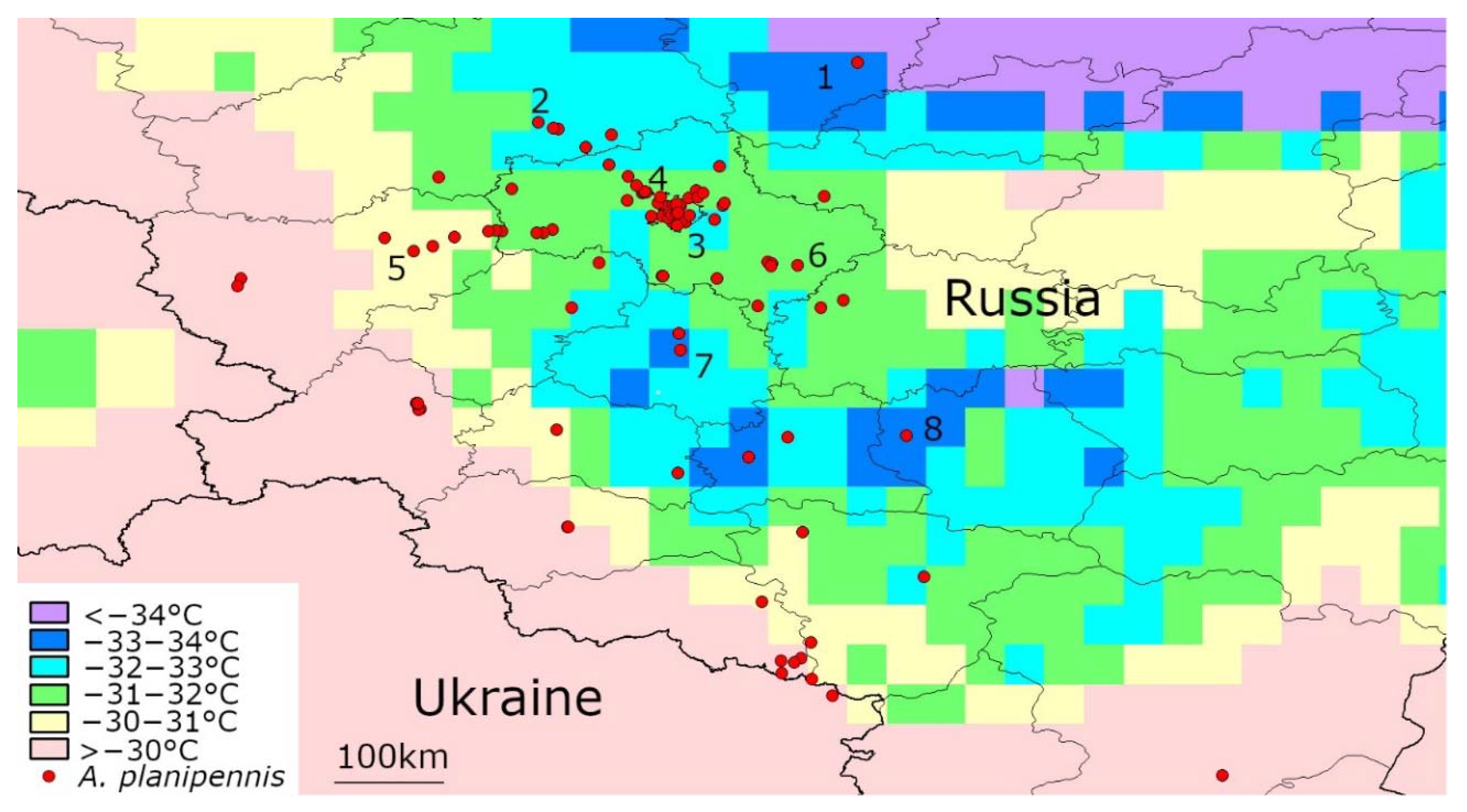
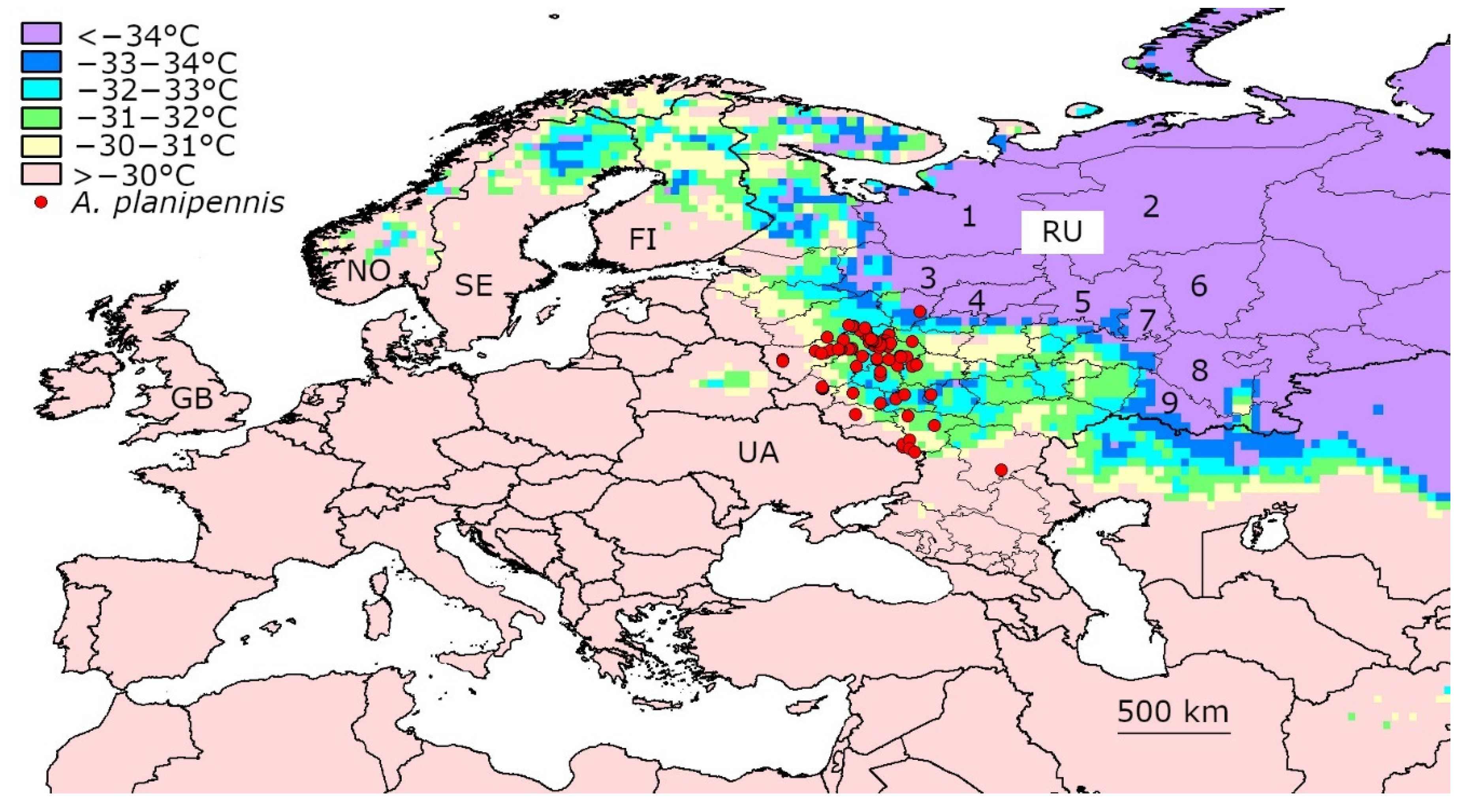
3.3. Temperatures below −30 °C in the Current Range of A. planipennis in European Russia
4. Discussion
5. Conclusions
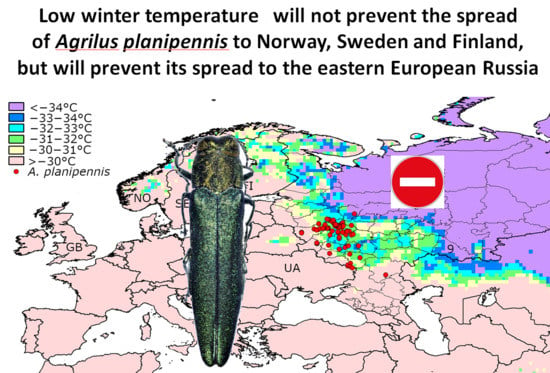
- Populations of A. planipennis survive in regions where the minimum mean daily temperature is −30 to −33 °C.
- No established populations of A. planipennis have been recorded in regions where the minimum mean daily temperature falls below −34 °C.
- It is unlikely that winter temperature would become a limiting factor preventing the spread of A. planipennis in Norway, Sweden, Finland or other countries of Western Europe.
- Low winter temperature could become the limiting factor that prevents the spread of A. planipennis to eastern European Russia, where daily average temperatures below −34 °C are not rare.
- Fraxinus pennsylvanica and F. excelsior occur in eastern European Russia, where the winters are too cold for A. planipennis. Therefore, eastern European Russia could potentially become a refuge for these ash species.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References and Notes
- Baranchikov, Y.N.; Mozolevskaya, E.G.; Yurchenko, G.I.; Kenis, M. Occurrence of the emerald ash borer, Agrilus planipennis in Russia and its potential impact on European forestry. EPPO Bull. 2008, 38, 233–238. [Google Scholar] [CrossRef]
- Herms, D.A.; McCullough, D.G. Emerald ash borer invasion of North America: History, biology, ecology, impacts, and management. Annu. Rev. Entomol. 2014, 59, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Haack, R.A.; Baranchikov, Y.N.; Bauer, L.S.; Poland, T.M. Emerald ash borer biology and invasion history. In Biology and Control of Emerald Ash Borer; Van Driesche, R.G., Reardon, R.C., Eds.; U.S. Department of Agriculture, Forest Service, Forest Health Technology Enterprise Team: Morgantown, WV, USA, 2015; pp. 1–13. [Google Scholar]
- Orlova–Bienkowskaja, M.J.; Volkovitsh, M.G. Are native ranges of the most destructive invasive pests well known? A case study of the native range of the emerald ash borer, Agrilus planipennis (Coleoptera: Buprestidae). Biol. Invasions 2018, 20, 1275–1286. [Google Scholar] [CrossRef]
- Emerald Ash Borer Info. Available online: http://www.emeraldashborer.info/ (accessed on 25 February 2020).
- Drogvalenko, A.N.; Orlova-Bienkowskaja, M.J.; Bieńkowski, A.O. Record of the Emerald Ash Borer (Agrilus planipennis) in Ukraine is confirmed. Insects 2019, 10, 338. [Google Scholar] [CrossRef]
- Orlova–Bienkowskaja, M.J.; Drogvalenko, A.N.; Zabaluev, I.A.; Sazhnev, A.S.; Peregudova, E.Y.; Mazurov, S.G.; Komarov, E.V.; Struchaev, V.V.; Martynov, V.V.; Nikulina, T.V.; et al. Current range of Agrilus planipennis Fairmaire, an alien pest of ash trees, in European Russia and Ukraine. Ann. For. Sci. 2020, 77, 1–14. [Google Scholar] [CrossRef]
- Baranchikov, Y.N. Preparing for protection of European Forests from invasive species of buprestids. Sibirskij Lesnoj Zhurnal 2018, 6, 126–131. [Google Scholar] [CrossRef]
- Orlova–Bienkowskaja, M.J.; Bieńkowski, A.O. Modeling long–distance dispersal of emerald ash borer in European Russia and prognosis of spread of this pest to neighboring countries within next 5 years. Ecol. Evol. 2018, 8, 9295–9304. [Google Scholar] [CrossRef]
- Valenta, V.; Moser, D.; Kapeller, S.; Essl, F. A new forest pest in Europe: A review of Emerald ash borer (Agrilus planipennis) invasion. J. Appl. Entomol. 2017, 141, 507–526. [Google Scholar] [CrossRef]
- EU Commission Delegated Regulation (EU) 2019/1702 of 1 August 2019 Supplementing Regulation (EU) 2016/2031 of the European Parliament and of the Council by Establishing the List of Priority Pests. (2019) OJ L 260, 8–10. Available online: http://data.europa.eu/eli/reg_del/2019/1702/oj (accessed on 27 February 2020).
- DeSantis, R.D.; Moser, W.K.; Gormanson, D.D.; Bartlett, M.G.; Vermunt, B. Effects of climate on emerald ash borer mortality and the potential for ash survival in North America. Agric. For. Meteorol. 2013, 178, 120–128. [Google Scholar] [CrossRef]
- Anulewicz, A.C.; Mccullough, D.G.; Cappaert, D.L.; Poland, T.M. Host range of the emerald ash borer (Agrilus planipennis Fairmaire) (Coleoptera: Buprestidae) in North America: Results of multiple–choice field experiments. Environ. Entomol. 2014, 37, 230–241. [Google Scholar] [CrossRef]
- Majorov, S.R.; Bochkin, V.D.; Nasimovich, Y.A.; Shcherbakov, A.V. Alien Flora of Moscow and Moscow REGION; KMK Publishing house: Moscow, Russia, 2012. [Google Scholar]
- Valenta, V.; Moser, D.; Kuttner, M.; Peterseil, J.; Essl, F. A high-resolution map of emerald ash borer invasion risk for southern central Europe. Forests 2015, 6, 3075–3086. [Google Scholar] [CrossRef]
- Orlova-Bienkowskaja, M.J.; Belokobylskij, S.A. Discovery of the first European parasitoid of the emerald ash borer Agrilus planipennis Fairmaire (Coleoptera: Buprestidae). Eur. J. Entomol. 2014, 111, 594–596. [Google Scholar] [CrossRef]
- Flø, D.; Krokene, P.; Økland, B. Invasion potential of Agrilus planipennis and other Agrilus beetles in Europe: Import pathways of deciduous wood chips and MaxEnt analyses of potential distribution areas. EPPO Bull. 2015, 45, 259–268. [Google Scholar] [CrossRef]
- Orlova–Bienkowskaja, M.J. Ashes in Europe are in danger: The invasive range of Agrilus planipennis in European Russia is expanding. Biol. Invasions 2014, 16, 1345–1349. [Google Scholar] [CrossRef]
- Bale, J.S. Insects and low temperatures: From molecular biology to distributions and abundance. Philos. Trans. R. Soc. Lond. 2002, 357, 849–861. [Google Scholar] [CrossRef]
- Venette, R.; Christianson, L.; Abrahamson, M. Cold Snap Is No Snow Day for Emerald Ash Borer Management. Available online: https://www.nrs.fs.fed.us/disturbance/invasive_species/eab/control_management/cold_hardiness/bp-EAB-and-extreme-cold.pdf (accessed on 27 February 2020).
- Christianson, L.D.; Venette, R.C. Modest effects of host on the cold hardiness of emerald ash borer. Forests 2018, 9, 346. [Google Scholar] [CrossRef]
- Wang, X.Y.; Yang, Z.Q.; Gould, J.R.; Zhang, Y.N.; Liu, G.J.; Liu, E.S. The biology and ecology of the emerald ash borer, Agrilus planipennis, in China. J. Insect Sci. 2010, 10, 128. [Google Scholar] [CrossRef]
- Orlova–Bienkowskaja, M.J.; Bieńkowski, A.O. The life cycle of the emerald ash borer Agrilus planipennis in European Russia and comparisons with its life cycles in Asia and North America. Agric. For. Entomol. 2016, 18, 182–188. [Google Scholar] [CrossRef]
- Jones, M.I.; Gould, J.R.; Mahon, H.J.; Fierke, M.K. Phenology of Emerald Ash Borer (Coleoptera: Buprestidae) and its introduced larval parasitoids in the Northeastern United States. J. Econ. Entomol. 2020, 113, 622–632. [Google Scholar] [CrossRef]
- Crosthwaite, J.C.; Sobek, S.; Lyons, D.B.; Bernards, M.A.; Sinclair, B.J. The overwintering physiology of the emerald ash borer, Agrilus planipennis Fairmaire (Coleoptera: Buprestidae). J. Insect Physiol. 2011, 57, 166–173. [Google Scholar] [CrossRef]
- Jones, M.I.; Gould, J.R.; Fierke, M.K. Mortality of overwintering emerald ash borer (Coleoptera: Buprestidae) associated with an extreme cold event in New York, United States of America. Can. Entomol. 2017, 149, 482–486. [Google Scholar] [CrossRef]
- GBIF.org GBIF Occurrence Download. Available online: https://doi.org/10.15468/dl.dldfcr (accessed on 27 January 2020).
- Copernicus Climate Change Service (C3S): C3S ERA5-Land Reanalysis. Copernicus Climate Change Service. Available online: https://cds.climate.copernicus.eu/cdsapp#!/home (accessed on 27 January 2020).
- Hijmans, R.J.; Guarino, L.; Cruz, M.; Rojas, E. Computer tools for spatial analysis of plant genetic resources data: 1. DIVA-GIS. Plant. Gen. Res. Newsl. 2001, 127, 15–19. [Google Scholar]
- Bornstein, R.D. Observations of the Urban Heat Island Effect in New York City. J. Appl. Meteorol. 1968, 7, 575–582. [Google Scholar] [CrossRef]
- Yurchenko, G.I. Emerald ash borer in Russian Far East. In Emerald Ash Borer—Occurrence and Protection Operations in the USA and Russia; Gninenko Yu, I., Ed.; VNIILM: Pushkino, Russia, 2016; pp. 5–10. [Google Scholar]
- Liu, H.; Bauer, L.S.; Gao, R.; Zhao, T.; Petrice, T.R.; Haack, R.A. Exploratory survey for the emerald ash borer, Agrilus planipennis (Coleoptera: Buprestidae), and its natural enemies in China. Great Lakes Entomol. 2003, 36, 191–204. [Google Scholar]
- Wei, X.; Wu, Y.U.N.; Reardon, R.; Sun, T.H.; Lu, M.; Sun, J.H. Biology and damage traits of emerald ash borer (Agrilus planipennis Fairmaire) in China. J. Insect Sci. 2007, 14, 367–373. [Google Scholar] [CrossRef]
- Bray, M.; Bauer, L.S.; Poland, T.M.; Haack, R.A.; Cognato, A.I.; Smith, J.J. Genetic analysis of emerald ash borer (Agrilus planipennis Fairmaire) populations in Asia and North America. Biol. Invasions 2011, 13, 2869–2887. [Google Scholar] [CrossRef]
- Sobek-Swant, S.; Kluza, D.A.; Cuddington, K.; Lyons, D.B. Potential distribution of emerald ash borer: What can we learn from ecological niche models using Maxent and GARP? For. Ecol. Manag. 2012, 281, 23–31. [Google Scholar] [CrossRef]
- Wei, X.; Reardon, D.; Wu, Y.; Sun, J.H. Emerald ash borer, Agrilus planipennis Fairmaire (Coleoptera: Buprestidae), in China: A review and distribution survey. Acta Entomol. Sin. 2004, 47, 679–685. [Google Scholar]
- Yurchenko, G.I.; Williams, D.W.; Kuzmin, E.A. Monitoring of populations of Emerald Ash Borer (Agrilus planipennis Fairmaire) and its parasitoids in the south part of Russian Far East. In Far Eastern forests condition and actual problems of forest management. In Proceedings of the All-Russian Conference with International Participants, Dal’NIILKh, Khabarovsk, Russia, 10–11 October 2013; pp. 440–444. [Google Scholar]
- Keever, C.C.; Nieman, C.; Ramsay, L.; Ritland, C.E.; Bauer, L.S.; Lyons, D.B.; Cory, J.S. Microsatellite population genetics of the emerald ash borer (Agrilus planipennis Fairmaire): Comparisons between Asian and North American populations. Biol. Invasions 2013, 15, 1537–1559. [Google Scholar] [CrossRef]
- Bray, A.M.; Bauer, L.S.; Fuester, R.W.; Choo, H.Y.; Lee, D.W.; Kamata, N.; Smith, J.J. Expanded explorations for emerald ash borer in Asia and implications for genetic analysis. In Proceedings of the Emerald Ash Borer and Asian Longhorhed Beetle Research and Technology Development Meeting, Cincinnati, OH, USA, 25 October–2 November 2006. [Google Scholar]
- Yurchenko, G.I.; Turova, G.I.; Kuzmin, E.A. To distribution and ecology of emerald ash borer (Agrilus planipennis Fairmaire) in the Russian Far East. In Proceedings of the A.I. Kurentsov’s Annual Memorial Meetings 18, Vladivostok, Russia, 6 March 2007; pp. 94–98. [Google Scholar]
- Wang, X.Y.; Cao, L.M.; Yang, Z.Q.; Duan, J.J.; Gould, J.R.; Bauer, L.S. Natural enemies of emerald ash borer (Coleoptera: Buprestidae) in northeast China, with notes on two species of parasitic Coleoptera. Can. Entomol. 2016, 148, 329–342. [Google Scholar] [CrossRef]
- Canadan Food Inspection Agency. Emerald ash Borer Confirmed in Winnipeg. Available online: https://www.canada.ca/en/food-inspection-agency/news/2017/12/emerald_ash_borerconfirmedinwinnipeg.html (accessed on 2 March 2020).
- Ross, F. Forestry Branch Manitoba Agriculture and Resource Development, Manitoba Canada, City, State. Message by e-mail on 3 March 2020.
- Nikitskij, N.B. New and interesting finds of xylophilous and some other beetles the Moscow region and in Moscow. Bull. Soc. Nat. Mosc. 2009, 114, 50–58. [Google Scholar]
- Baranchikov, Y.N.; Gninenko, Y.I.; Yurchenko, G.I. Emerald ash borer in Russia: 2009 situation update. In Proceedings of the 21st USDA Interagency Research Forum on Invasive Species, Morgantown, WV, USA, 21 January 2010. [Google Scholar]
- Baranchikov, Y.N.; Kurteev, V.V. Invasive area of the emerald ash borer in Europe. In Ecological and economic consequences of invasions of dendrophilous insects. In Proceedings of the All-Russian Conference with International Participants, Krasnoyarsk, Russia, 25–27 September 2012; pp. 91–94. [Google Scholar]
- Vlasov, D.V. Yaroslavl State Historical-Architectural and Art Museum-Reserve, Yaroslavl, Russia. Affiliation, City, State. Message by e-mail on 6 February 2020.
- Venette, R.C.; Abrahamson, M. Cold Hardiness of Emerald Ash Borer, Agrilus planipennis: A New Perspective. Available online: https://www.fs.usda.gov/Internet/FSE_DOCUMENTS/stelprdb5191794.pdf (accessed on 27 February 2020).
- Morozova, O.V. Fraxinus pennsylvanica. In The most Dangerous Invasive Species of Russia (Top 100); Dgebuadze, Y.Y., Petrosyan, V.G., Khlyap, L.A., Eds.; KMK: Moscow, Russia, 2018; pp. 142–145. [Google Scholar]
- Gufranova, I.B. Oleaceae. In Guide for Identification of Higher Plants of Bashkirskaja SSR; Kucherov, E.V., Muldashev, A.A., Eds.; Nauka: Moscow, Russia, 1989; p. 191. [Google Scholar]
- Orlova, N.I. Guide for Identification of Higher Plants of the Vologda Region; Rus: Vologda, Russia, 1997; p. 262. [Google Scholar]
- Chuhina, I.G.; Bagmet, L.V. Fraxinus excelsior. Interactive Agricultural Ecological Atlas of Russia and Neighboring Countries. Afonin, A.N., Greene, S.L., Dzyubenko, N.I., Frolov, A.N., Eds.; 2008. Available online: http://www.agroatlas.ru/en/content/related/Lonicera_edulis/ (accessed on 1 March 2020).
- Ryabinina, Z.N.; Knyazev, M.S. Guide for Identification of Vascular Plants of the Orenburg REGION; KMK: Moscow, Russia, 2009; p. 858. [Google Scholar]
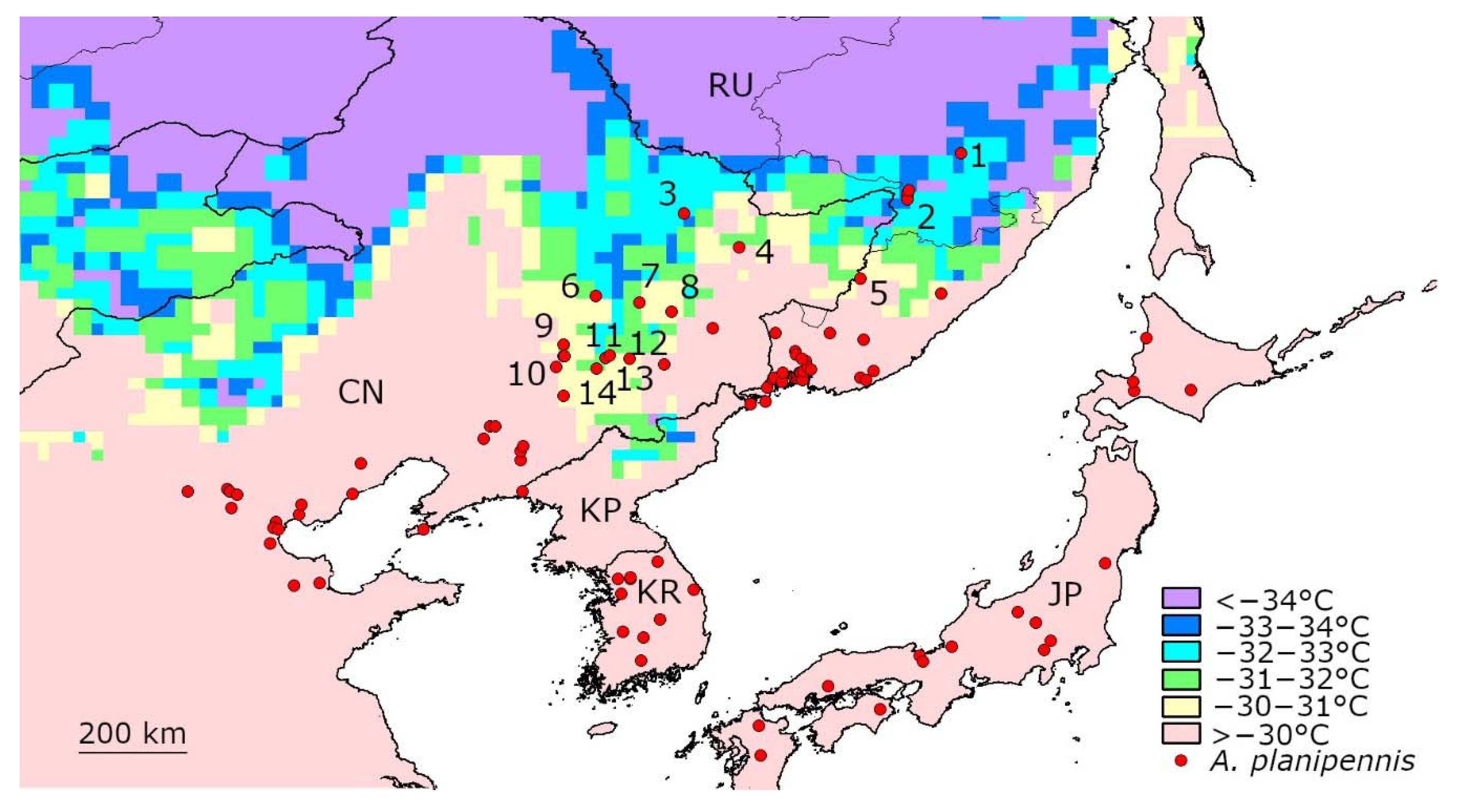
| Country | Locality (Numbers Correspond to the Numbers in Figure 1) | Year of A. planipennis Detection | Number of Days with the Mean t° below −30 °C | Years When the Days with the Mean t° below −30 °C Occurred | Mean Temperature of the Coldest Day (°C) |
|---|---|---|---|---|---|
| Russia | Troitskoe 1 | 2003–2008 | 28 | 2005, 2006, 2010, 2011, 2012, 2013, 2014, 2017 | −33.86 |
| China | Yichun 3 | 2003 | 17 | 2006, 2010, 2011, 2012, 2013, 2014, 2018 | −33.19 |
| Russia | Khabarovsk 2 | 2004–2006, 2016 | 15 | 2010, 2011, 2012, 2013, 2014 | −33.12 |
| China | Harbin 6 | 1964, 2003–2008 | 11 | 2010, 2011, 2012, 2013, 2018 | −32.28 |
| China | Laoshan 7 | 2003–2008 | 4 | 2011, 2018 | −31.91 |
| China | Jiang Nan Forest 11 | 2006 | 4 | 2009, 2010, 2011, 2018 | −31.77 |
| China | Songhuahu Nature Reserve 14 | 2003 | 2 | 2010, 2011 | −31.69 |
| China | Jilin City 13 | 2003–2008 | 2 | 2010, 2011 | −31.69 |
| China | Jiutai 9 | 2003–2008 | 2 | 2011, 2012 | −31.41 |
| China | Hejiang 4 | 2003 | 4 | 2011, 2013 | −31.35 |
| Russia | Dalnerechensk 5 | 2004–2006 | 4 | 2011, 2012, 2013 | −31.23 |
| China | Jiaohe Experimental Forest 12 | 2003 | 3 | 2011, 2018 | −30.75 |
| China | Shangzhi 8 | 2003, 2012 | 1 | 2018 | −30.67 |
| China | Changchun 10 | 2003, 2008 | 2 | 2011, 2012 | −30.16 |
| Country | Locality (Numbers Correspond to the Numbers in Figure 2) | Year of A. planipennis Detection | Number of Days with the Mean t° below −30 °C | Years When the Days with the Mean t° below −30 °C Occurred | Mean Temperature of the Coldest Day (°C) |
|---|---|---|---|---|---|
| Canada | Manitoba, Winnipeg 1 | 2017 | 35 | 2004, 2005, 2007, 2008, 2009, 2013, 2014, 2015, 2016, 2019 | −37.75 |
| Canada | Quebec, L’Islet 4 | 2018 | 1 | 2004 | −31.15 |
| USA | Minnesota, St. Paul 5 | 2008 | 2 | 2019 | −31.10 |
| USA | Illinois, Kane 7 | 2006 | 1 | 2019 | −31.10 |
| USA | South Dakota, Minnehaha 2 | 2018 | 1 | 2009 | −30.36 |
| USA | Wisconsin, Douglas 6 | 2013 | 1 | 2019 | −30.15 |
| Canada | Ontario, Renfrew 3 | 2013 | 1 | 2004 | −30.03 |
| Region | Locality (Numbers Correspond to the Numbers in Figure 3) | Year of A. planipennis Detection | Number of Days with the Mean t° below −30 °C | Years When the Days with the Mean t° below −30 °C Occurred | Mean Temperature of the Coldest Day (°C) |
|---|---|---|---|---|---|
| Yaroslavl | Yaroslavl 1 | 2013–2019 | 7 | 2006, 2012, 2017 | −33.76 |
| Tambov | Michurinsk 8 | 2013, 2017 | 5 | 2006, 2010 | −33.64 |
| Tula | Tula 7 | 2013, 2019 | 3 | 2006 | −33.14 |
| Tver | Tver 2 | 2015–2019 | 4 | 2006, 2017 | −32.03 |
| Moscow | Moscow 3 | 2003–2019 | 2 | 2006 | −31.94 |
| Moscow | Zelenograd 4 | 2011–2019 | 3 | 2006, 2017 | −31.86 |
| Moscow | Kolomna 6 | 2012, 2017 | 1 | 2006 | −31.54 |
| Smolensk | Vyazma 5 | 2012, 2019 | 2 | 2006 | −30.95 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orlova-Bienkowskaja, M.J.; Bieńkowski, A.O. Minimum Winter Temperature as a Limiting Factor of the Potential Spread of Agrilus planipennis, an Alien Pest of Ash Trees, in Europe. Insects 2020, 11, 258. https://doi.org/10.3390/insects11040258
Orlova-Bienkowskaja MJ, Bieńkowski AO. Minimum Winter Temperature as a Limiting Factor of the Potential Spread of Agrilus planipennis, an Alien Pest of Ash Trees, in Europe. Insects. 2020; 11(4):258. https://doi.org/10.3390/insects11040258
Chicago/Turabian StyleOrlova-Bienkowskaja, Marina J., and Andrzej O. Bieńkowski. 2020. "Minimum Winter Temperature as a Limiting Factor of the Potential Spread of Agrilus planipennis, an Alien Pest of Ash Trees, in Europe" Insects 11, no. 4: 258. https://doi.org/10.3390/insects11040258
APA StyleOrlova-Bienkowskaja, M. J., & Bieńkowski, A. O. (2020). Minimum Winter Temperature as a Limiting Factor of the Potential Spread of Agrilus planipennis, an Alien Pest of Ash Trees, in Europe. Insects, 11(4), 258. https://doi.org/10.3390/insects11040258