Optical Modelling and Phylogenetic Analysis Provide Clues to the Likely Function of Corneal Nipple Arrays in Butterflies and Moths
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Material
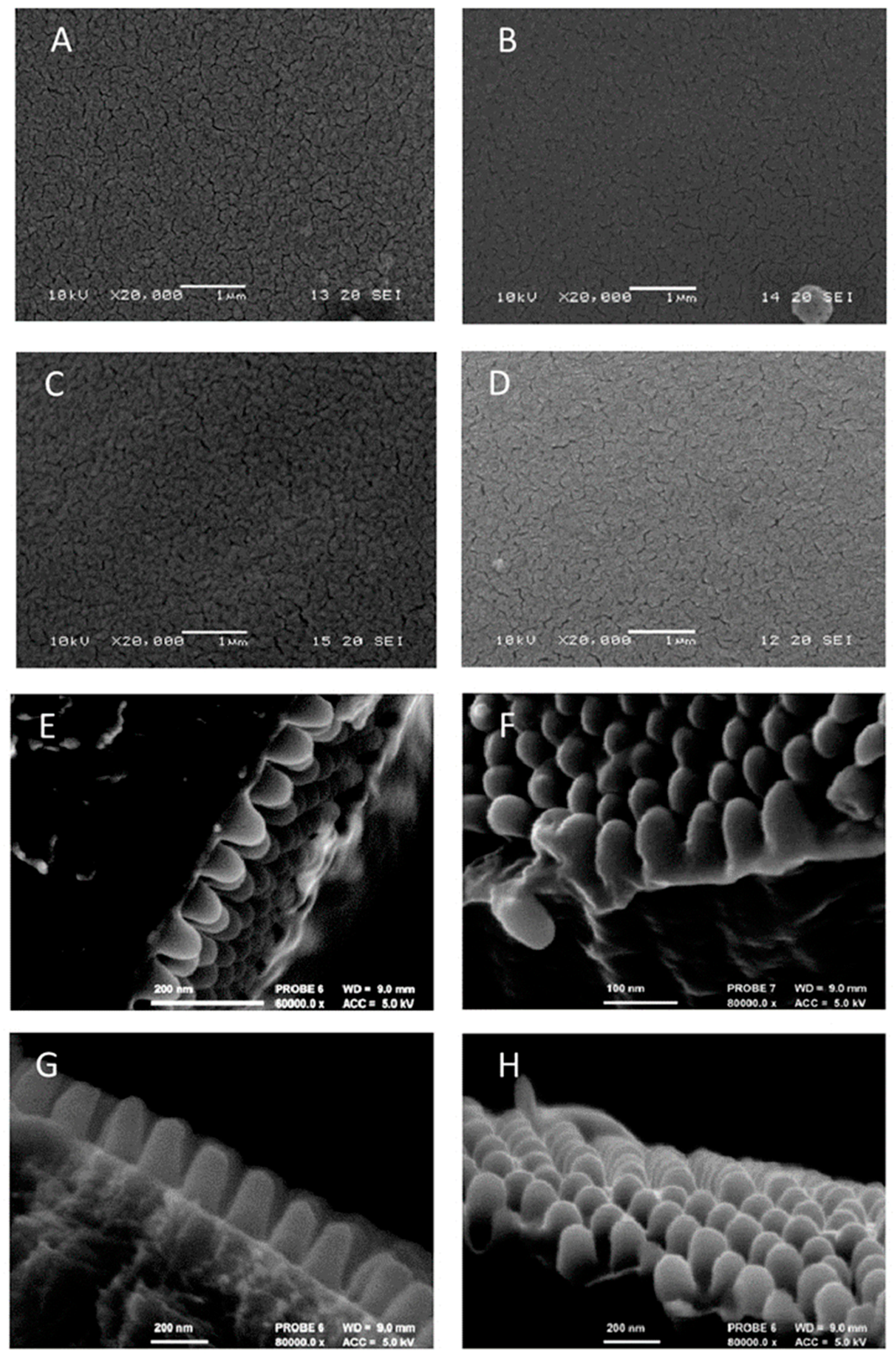
2.2. Electron Microscopy
2.3. Optical Modelling
2.4. Statistical Modelling
3. Results
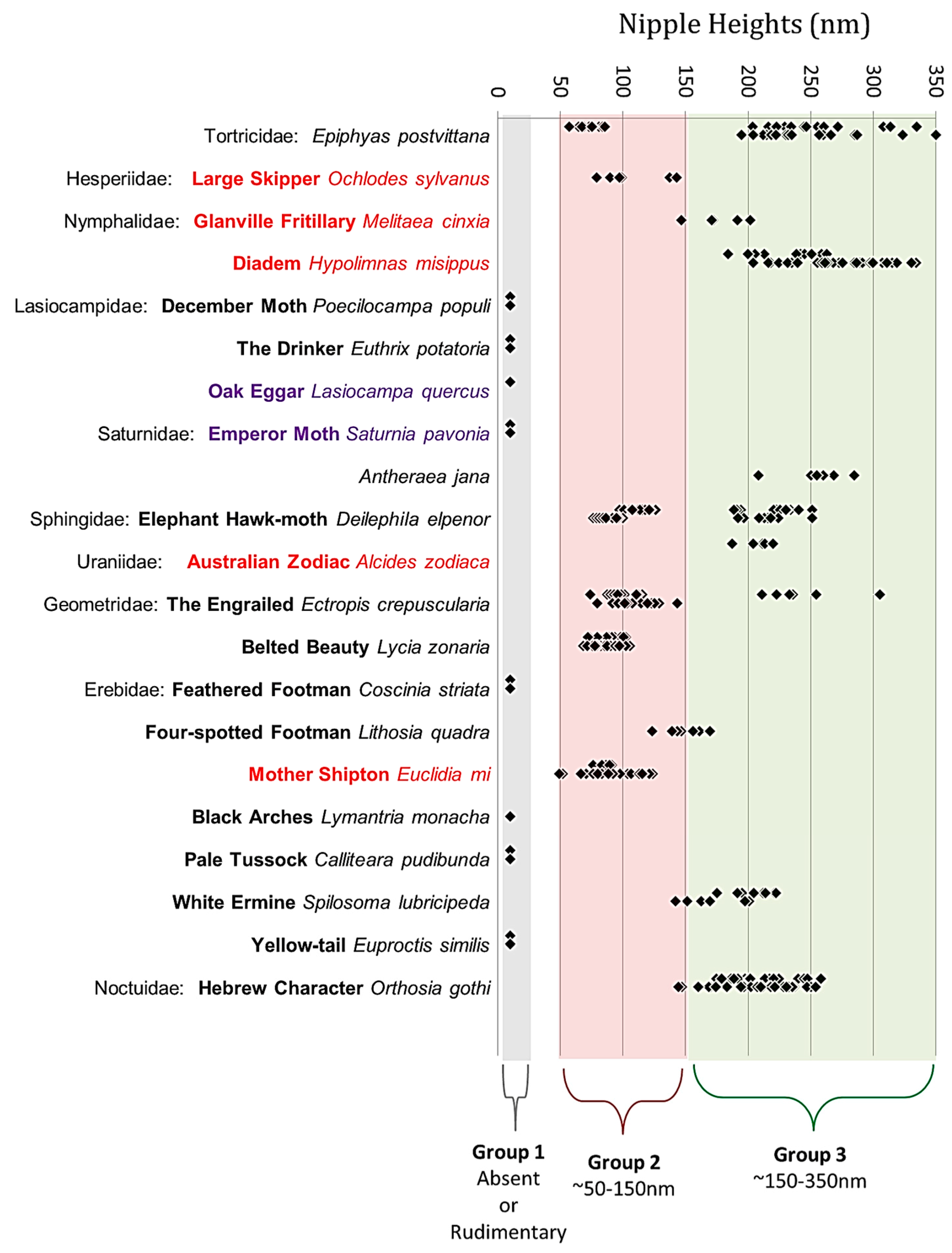
3.1. Nipple Height Classes
3.2. Taxonomic Order
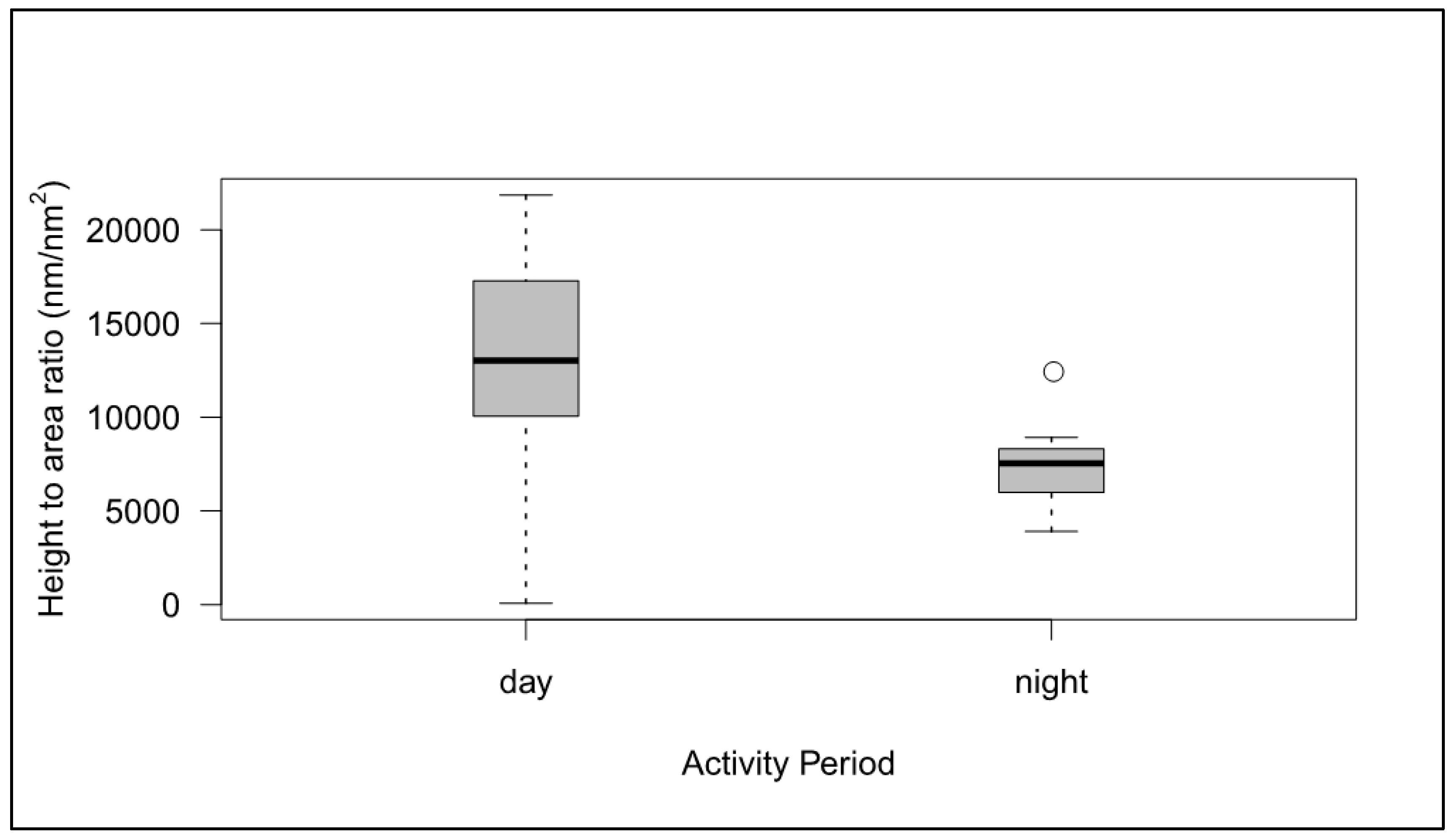
3.3. Diurnal and Nocturnal Species
3.4. Male–Female Differences
3.5. Reflectance
3.6. The Refractive Index
4. Discussion
4.1. Is the Loss of Nipple Arrays Associated with Evolutionary Development in the Lepidoptera?
4.2. The Length of Corneal Nipples Varies within Familes
4.3. Corneal Nipples are Shorter, More Rounded and with Larger Top Areas in Nocturnal Species
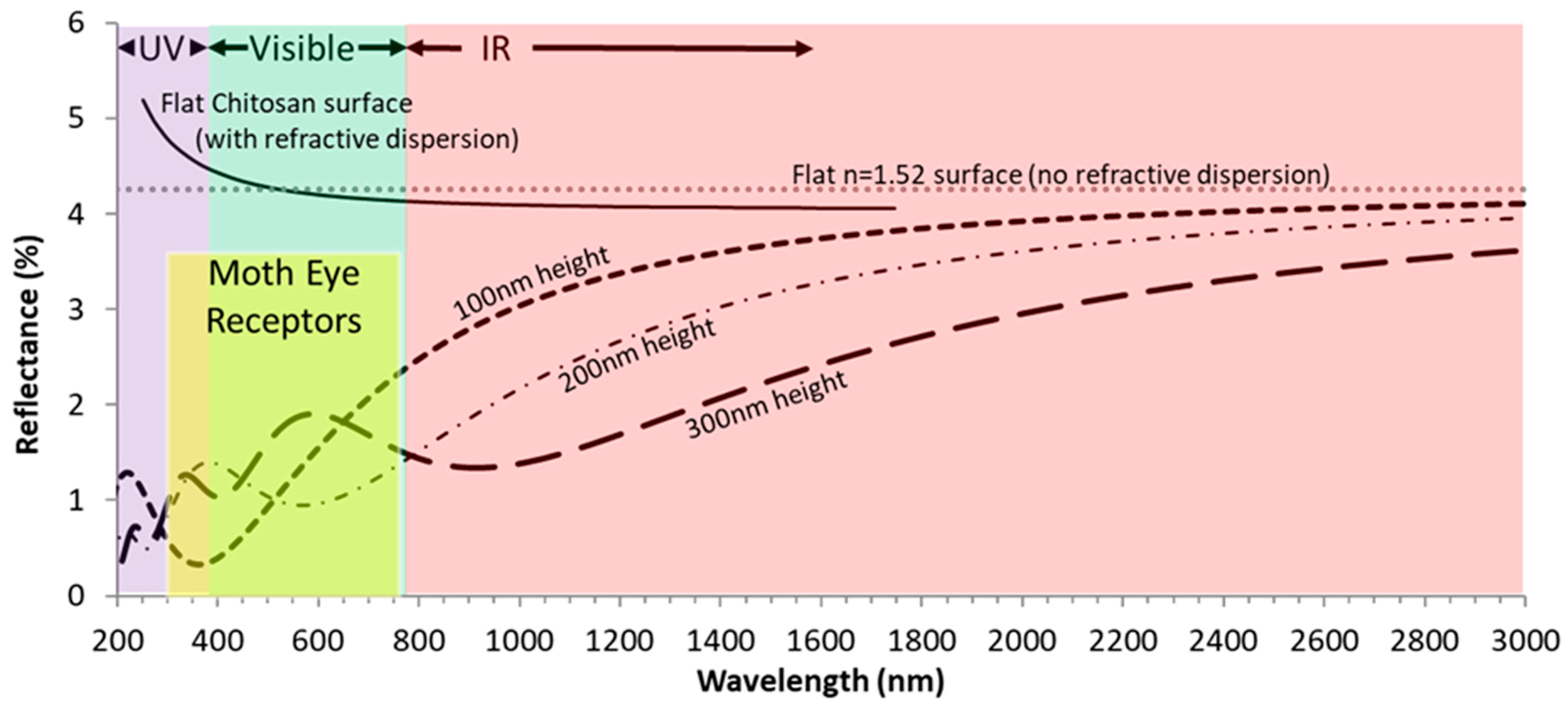
4.4. There is Less Reflectance and More Light Absorption Where Nipples are Longer
4.5. Light Absortion is Greatest at the UV Range of the Spectrum (< 380 nm)
4.6. Is the Closeness of the Effective Refractive Index at the Nipple Top to the Refractive Index of Water a Chance Feature?
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Espeland, M.; Breinholt, J.; Willmott, K.R.; Warren, A.D.; Vila, R.; Toussaint, E.F.; Maunsell, S.C.; Aduse-Poku, K.; Talavera, G.; Eastwood, R.; et al. A Comprehensive and Dated Phylogenomic Analysis of Butterflies. Curr. Biol. 2018, 28, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Regier, J.C.; Mitter, C.; Zwick, A.; Bazinet, A.L.; Cummings, M.P.; Kawahara, A.Y.; Sohn, J.C.; Zwickl, D.J.; Cho, S.; Donald, R.; et al. A Large-Scale, Higher-Level, Molecular Phylogenetic Study of the Insect Order Lepidoptera (Moths and Butterflies). PLoS ONE 2013, 8, e58568. [Google Scholar] [CrossRef] [PubMed]
- Mutanen, M.; Wahlberg, N.; Kaila, L. Comprehensive gene and taxon coverage elucidates radiation patterns in moths and butterflies. Proc. R Soc. B. 2010, 277, 2839–2848. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Zwick, A.; Regier, J.C.; Mitter, C.; Cummings, M.P.; Yao, J.; Du, Z.; Zhao, H.; Kawahara, A.K.; Weller, S. Can deliberately incomplete gene sample augmentation improve a phylogeny estimate for ditrysian Lepidoptera (Hexapoda)? Syst. Biol. 2011, 60, 782–796. [Google Scholar] [CrossRef] [PubMed]
- Agassiz, D.J.L.; Beavan, S.D.; Heckford, R.J. Checklist of the Lepidoptera of the British Isles. In Handbooks for Identification of British Insects; Royal Entomological Society: London, UK, 2013. [Google Scholar]
- Somers-Yeates, R.; Hodgson, D.; McGregor, P.K.; Spalding, A.; Ffrench-Constant, R.H. Shedding light on moths: Shorter wavelengths attract noctuids more than geometrids. Biol. Lett. 2013, 9, 20130376. [Google Scholar] [CrossRef] [PubMed]
- Bernhard, C.G.; Miller, W.H. A corneal nipple pattern in insect compound eyes. Acta Physiol. Scand. 1962, 56, 385–386. [Google Scholar] [CrossRef] [PubMed]
- Bernhard, C.G.; Miller, W.H.; Møller, A.R. The insect corneal nipple array. (Suppl. 243). Acta Physiol. Scand. 1965, 63, 1–25. [Google Scholar]
- Miller, W.H. Ocular Optical Filtering. In Handbook of Sensory Physiology; Autrum, H., Ed.; Springer: Berlin, Germany, 1981; Volume VII/6A, pp. 69–143. [Google Scholar]
- Lee, K.C.; Yui, Q.; Erb, U. Mesostructure of Ordered Corneal Nano-nipple Arrays: The Role of 5–7 Coordination Defects. Sci. Rep. 2016, 6, 28342. [Google Scholar] [CrossRef]
- Bernhard, C.G.; Gemne, G.; SällströmJ, J. Comparative ultrastructure of corneal surface topography in insects with aspects on phylogenesis and function. Z. Vgl. Physiol. 1970, 67, 1–25. [Google Scholar] [CrossRef]
- Razowski, J.; Wojtusiak, J. Cornea of Ommatidia in Lepidoptera (Insects)—A Scanning Electron Microscope Study. Folia Biol. 2006, 54, 49–53. [Google Scholar] [CrossRef][Green Version]
- Meyer-Rochow, V.B. Compound eyes of insects and crustaceans: Some examples that show there is still a lot of work left to be done. Insect Sci. 2015, 22, 461–481. [Google Scholar] [CrossRef] [PubMed]
- Morse, J.C. Phylogeny of Trichoptera. Annu. Rev. Entomol. 1997, 42, 427–450. [Google Scholar] [CrossRef] [PubMed]
- Blagodatski, A.; Sergeev, A.; Kryuchkov, M.; Lopatina, Y.; Katanaev, V.L. Diverse set of Turing nanopatterns coat corneae across insect lineages. PNAS 2015, 112, 10750–10755. [Google Scholar] [CrossRef] [PubMed]
- Parker, A.R.; Hegedus, Z.; Watts, R.A. Solar–absorber antireflector on the eye of an Eocene fly (45 Ma). Proc. R. Soc. Lond. B 1998, 265, 811–815. [Google Scholar] [CrossRef]
- Han, Z.; Jiao, Z.; Shichao Nium, S.; Ren, L. Ascendant bioinspired antireflective materials: Opportunities and challenges coexist. Prog. Mater. Sci. 2019, 103, 1–68. [Google Scholar] [CrossRef]
- Stavenga, D.G.; Foletti, S.; Palasantzas, G.; Arikawa, K. Light on the moth-eye corneal nipple array of butterflies. Proc. R. Soc. Lond. B 2005, 273, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Peisker, H.; Gorb, S.N. Always on the bright side of life: Anti-adhesive properties of insect ommatidia grating. J. Exp. Biol. 2013, 213, 3457–3462. [Google Scholar] [CrossRef] [PubMed]
- Vogt, K. Optische Untersuchungen an der Cornea der MehlmotteEphestia kohniella. J. Comp. Physiol. 1974, 88, 201–216. [Google Scholar] [CrossRef]
- Azofeifa, D.E.; Arguedas, H.J.; Vargas, W.E. Optical properties of chitin and chitosan biopolymers with application to structural color analysis. Opt. Mater.. 2013, 35, 175–183. [Google Scholar] [CrossRef]
- Elieh-Ali-Komi, D.; Hamblin, M.R. Chitin and Chitosan: Production and Application of Versatile Biomedical Nanomaterials. Int. J. Adv. Res. 2016, 4, 411–427. [Google Scholar]
- Kaya, M.; Sargin, I.; Al-jaf, I.; Erdogan, S.; Arslan, G. Characteristics of corneal lens chitin in dragonfly compound eyes. Int. J. Biol. Macromol. 2016, 89, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Breault Research Organization, ASAP Technical Guide: Scattering in ASAP. 2012. Available online: http://www.breault.com/sites/default/files/knowledge_base/brotg0922_scatter_1.pdf (accessed on 12 October 2015).
- Perez-Higueras, J.; Fernández, E.F. High Concentrator Photovoltaics: Fundamentals, Engineering and Power Plants, 1st ed.; Springer International Publishing: Basel, Switzerland, 2015. [Google Scholar]
- Yu, K.T.; Fan, T.; Lou, S.; Zhang, D. Biomimetic optical materials: Integration of nature’s design for manipulation of light. Prog. Mater. Sci. 2013, 58, 825–873. [Google Scholar] [CrossRef]
- Siddique, R.H.; Gomard, G.; Hölscher, H. The role of random nanostructures for the omnidirectional anti-reflection properties of the glasswing butterfly. Nat. Commun. 2015, 6, 6909. [Google Scholar] [CrossRef] [PubMed]
- Knight, M.W.; King, H.S.; Liu, L.; Everitt, H.O.; Nordlander, P.; Halas, N.J. Aluminum for Plasmonics. ACS Nano 2014, 8, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Battie, Y.; En Naciri, A.; Chamorro, W.; Horwat, D. Generalized Effective Medium Theory to Extract the Optical Properties of Two-Dimensional Nonspherical Metallic Nanoparticle Layers. J. Phys. Chem. C 2014, 118, 4899–4905. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, Y. Effective medium theory for anisotropic metamaterials. Sci. Rep. 2015, 5, 7892. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, Y.; Ortiz, D.; Alcaraz De La Osa, R.; Sanz, J.M.; Saiz, J.M.; Gonzalez, F.; Moreno, F. Evaluation of effective medium theories for spherical nano-shells. physics. Optics. 2018, 1705, 02248. [Google Scholar]
- Khardani, K.; Bouaïcha, M.; Bessaïs, B. Bruggeman effective medium approach for modelling optical properties of porous silicon: Comparison with experiment. Phys. Status Solidi 2007, 4, 1986–1990. [Google Scholar] [CrossRef]
- Niklasson, G.A.; Granqvist, C.G.; Hunderi, O. Effective medium models for the optical properties of inhomogeneous materials. Appl. Opt. 1981, 20, 26. [Google Scholar] [CrossRef]
- Bortchagovsky, E.G.; Dejneka, A.; Jastrabik, L.; Lozovski, V.Z.; Mishakova, T.O. Model of the effective-medium approximation for nanostructured layers with the account of interparticle interactions and its ellipsometric registration. AAPP Atti della Accademia Peloritana dei Pericolanti, Classe di Scienze Fisiche, Matematiche e Naturali 2011, 89. [Google Scholar] [CrossRef]
- García-Vidal, F.J.; Pitarke, J.M.; Pendry, J.B. Effective Medium Theory of the Optical Properties of Aligned Carbon Nanotubes. Phys. Rev. Lett. 1997, 78, 4289. [Google Scholar] [CrossRef]
- Gehr, R.J.; Boyd, R.W. Optical Properties of Nanostructured Optical Materials. Chem. Mater. 1996, 8, 1807–1819. [Google Scholar] [CrossRef]
- Su, Z.F.; Sun, S.G.; Wu, C.X.; Cai, Z.P. Study of anomalous infrared properties of nanomaterials through effective medium theory. J. Chem. Phys. 2008, 129. [Google Scholar] [CrossRef] [PubMed]
- Rainford, J.L.; Hofreiter, M.; Nicholson, D.B.; Mayhew, P.J. Phylogenetic Distribution of Extant Richness Suggests Metamorphosis Is a Key Innovation Driving Diversification in Insects. PLoS ONE 2014, 9, e109085. [Google Scholar] [CrossRef] [PubMed]
- Paradis, E.; Claude, J.; Strimmer, K. APE: Analyses of phylogenetics and evolution in R language. Bioinformatics 2004, 20, 289–290. [Google Scholar] [CrossRef]
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available online: https://www.R-project.org/ (accessed on 1 May 2018).
- Chen, P.J.; Awata, H.; Matsushita, A.; Yang, E.C.; Arikawa, K. Extreme Spectral Richness in the Eye of the Common Bluebottle Butterfly, Graphium sarpedon. Front. Ecol. Evol. 2016, 4, 18. [Google Scholar] [CrossRef]
- Pirih, P.; Ilić, M.; Rudolf, J.; Arikawa, K.; Stavenga, D.G.; Belušič, G. The giant butterfly-moth Paysandisia archon has spectrally rich apposition eyes with unique light-dependent photoreceptor dynamics. J. Comp. Physiol. A 2018, 204, 639–651. [Google Scholar] [CrossRef]
- Yack, J.E.; Johnson, S.E.; Brown, S.G.; Warrant, E.J. The eyes of Macrosoma sp. (Lepidoptera: Hedyloidea): A nocturnal butterfly with superposition optics. Arthropod Struct. Dev. 2007, 36, 11–22. [Google Scholar] [CrossRef]
- Fischer, S.; Meyer-Rochow, V.B.; Müller, C.H.G. Challenging limits: Ultrastructure and size-related functional constraints of the compound eye of Stigmella microtheriella (Lepidoptera: Nepticulidae). J. Morphol. 2012, 273, 1064–1078. [Google Scholar] [CrossRef]
- Heath, J.; Pelham-Clinton, E.C. The Moths and Butterflies of Great Britain and Ireland. (Micropterigidae to Heliozelidae); Heath, J., Ed.; Blackwell Scientific Publications: Oxford, UK; London, UK; Curwen Books: New York, NY, USA, 1976; Volume 1. [Google Scholar]
- Fischer, S.; Meyer-Rochow, V.B.; Müller, C.H.G. Compound Eye. Miniaturization in Lepidoptera: A comparative morphological analysis. Acta Zool. 2013. [Google Scholar] [CrossRef]
- Labandeira, C.C.; Sepkoski, J.J. Insect diversity in the fossil record. Science 1993, 261, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Sohn, J.C.; Labandeira, C.; Davis, D.R.; Mitter, C. An annotated catalog of fossil and subfossil Lepidoptera (Insecta: Holometabola) of the world. Zootaxa 2012, 3286, 1–132. [Google Scholar] [CrossRef]
- Popper, K. The Logic of Scientific Discovery; Hutchinson: London, UK, 1959. [Google Scholar]
- Lau, T.F.S.; Meyer-Rochow, V.B. The compound eye of Orgyia antiqua L. (Lepidoptera: Lymantriidae): Sexual dimorphism and light/dark adaptation changes. Eur. J. Entomol. 2007, 104, 247–258. [Google Scholar] [CrossRef]
- Lau, T.F.; Gross, E.M.; Meyer-Rochow, V.B. Sexual dimorphism and light/dark adaptation in the compound eyes of male and female Acentria ephemerella (Lepidoptera: Pyraloidea: Crambidae). Eur. J. Entomol. 2007, 104, 459–470. [Google Scholar] [CrossRef]
- Spalding, A. Loe Bar and the Sandhill Rustic Moth. The Biogeography, Ecology and Cultural History of a Coastal Shingle Bar; Brill: Leiden, The Netherlands, 2015. [Google Scholar]
- Dewan, R.; Fischer, S.; Meyer-Rochow, V.B.; Ozdemir, Y.; Hamraz, S.; Knipp, D. Studying nanostructural nipple arrays of moth eye facets helps to design better thin film solar cells. Bioinspiration Biomim. 2011, 7, 016003. [Google Scholar] [CrossRef] [PubMed]
- Chiadini, F.; Fiumara, V.; Scaglione, A.; Lakhtakia, A. Bioinspired pit texturing of silicon solar cell surfaces. J. Photonics Energy 2013, 3, 034596. [Google Scholar] [CrossRef]
- Cai, J.; Qi, L. Recent advances in antireflective surfaces based on nanostructure arrays. Mater. Horiz. 2015, 2, 37–53. [Google Scholar] [CrossRef]
- Yoshida, A.; Motoyama, M.; Kosaku, A.; Miyamoto, K. Antireflective Nanoprotuberance Array in the Transparent Wing of a Hawkmoth, Cephonodes hylas. Zool. Sci. 1997, 14, 737–741. [Google Scholar] [CrossRef]
- Horridge, G.A.; McLean, M.; Stange, G.; Lillywhite, P.G. A Diurnal Moth Superposition Eye with High Resolution Phalaenoides tristifica (Agaristidae). Proc. R. Soc. Lond. B 1977. [Google Scholar] [CrossRef]
- Möller, R. Insects could exploit UV-green contrast for landmark navigation. J. Theor. Biol. 2002, 214, 619–631. [Google Scholar] [CrossRef]
- Nowinszky, L. The orientation of insects by light—major theories. In The Handbook of Light Trapping; Savaria University Press: Szombathely, Hungary, 2003; pp. 15–18. [Google Scholar]
- Barghini, A.; Souza de Medeiros, B.A. UV Radiation as an Attractor for Insects. Leukos 2012, 9, 47–56. [Google Scholar]
- Lewontin, R. On the anomalous response of Drosophila pseudoobscura to light. Am. Nat. 1959, 93, 321–328. [Google Scholar] [CrossRef]
- Sotthibandhu, S.; Baker, R.R. Celestial orientation by the large yellow underwing moth, Noctua pronuba L. Anim. Behav. 1979, 27, 786–800. [Google Scholar] [CrossRef]
- Hsiao, H.S. Flight paths of night-flying moths to light. J. Insect Physiol. 1973, 19, 1971–1976. [Google Scholar] [CrossRef]
- Spalding, A. Light Pollution and the decline of moths. Br. J. Entomol. Nat. Hist. 2019, 32, 1–18. [Google Scholar]
- Gao, X.; Yan, X.; Yao, X.; Xu, L.; Zhang, K.; Zhang, J.; Yang, B.; Jiang, L. The dry-style antifogging properties of mosquito compound eyes and artificial analogues prepared by soft lithography. Adv. Mater. 2007, 19, 2213–2217. [Google Scholar] [CrossRef]
- Darmanin, T.; Guittard, F. Superhydrophobic and superoleophobic properties in nature. Mater. Today 2015, 18, 273–285. [Google Scholar] [CrossRef]
- Rumble, J.R. CRC Handbook of Chemistry and Physics; CRC Press LLC: Boca Raton, FL, USA, 2018. [Google Scholar]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spalding, A.; Shanks, K.; Bennie, J.; Potter, U.; ffrench-Constant, R. Optical Modelling and Phylogenetic Analysis Provide Clues to the Likely Function of Corneal Nipple Arrays in Butterflies and Moths. Insects 2019, 10, 262. https://doi.org/10.3390/insects10090262
Spalding A, Shanks K, Bennie J, Potter U, ffrench-Constant R. Optical Modelling and Phylogenetic Analysis Provide Clues to the Likely Function of Corneal Nipple Arrays in Butterflies and Moths. Insects. 2019; 10(9):262. https://doi.org/10.3390/insects10090262
Chicago/Turabian StyleSpalding, Adrian, Katie Shanks, Jon Bennie, Ursula Potter, and Richard ffrench-Constant. 2019. "Optical Modelling and Phylogenetic Analysis Provide Clues to the Likely Function of Corneal Nipple Arrays in Butterflies and Moths" Insects 10, no. 9: 262. https://doi.org/10.3390/insects10090262
APA StyleSpalding, A., Shanks, K., Bennie, J., Potter, U., & ffrench-Constant, R. (2019). Optical Modelling and Phylogenetic Analysis Provide Clues to the Likely Function of Corneal Nipple Arrays in Butterflies and Moths. Insects, 10(9), 262. https://doi.org/10.3390/insects10090262






