Reassessment of the Species in the Euwallacea Fornicatus (Coleoptera: Curculionidae: Scolytinae) Complex after the Rediscovery of the “Lost” Type Specimen
Abstract
:1. Introduction
2. Materials and Methods
- California Academy of Sciences (CASC), San Francisco, California
- Zoological Museum, Museum and Institute of Zoology (MIIZ), Polish Academy of Science, Warsaw
- Michigan State University Arthropod Research Collection (MSUC), East Lansing, Michigan
- Natural History Museum (NHMB), Basel
- National Museum of Natural History (NMNH), Washington, D.C.
- Roger A. Beaver collection (RABC), Chiang Mai
- Robert J. Rabaglia collection (RJRC), Annapolis, Maryland
3. Results and Discussion
3.1. Taxonomy
3.1.1. Euwallacea Fornicatior (Eggers)
3.1.2. Euwallacea fornicatus (Eichhoff)
3.1.3. Euwallacea kuroshio Gomez and Hulcr
3.1.4. Euwallacea perbrevis (Schedl) Stat. Res.
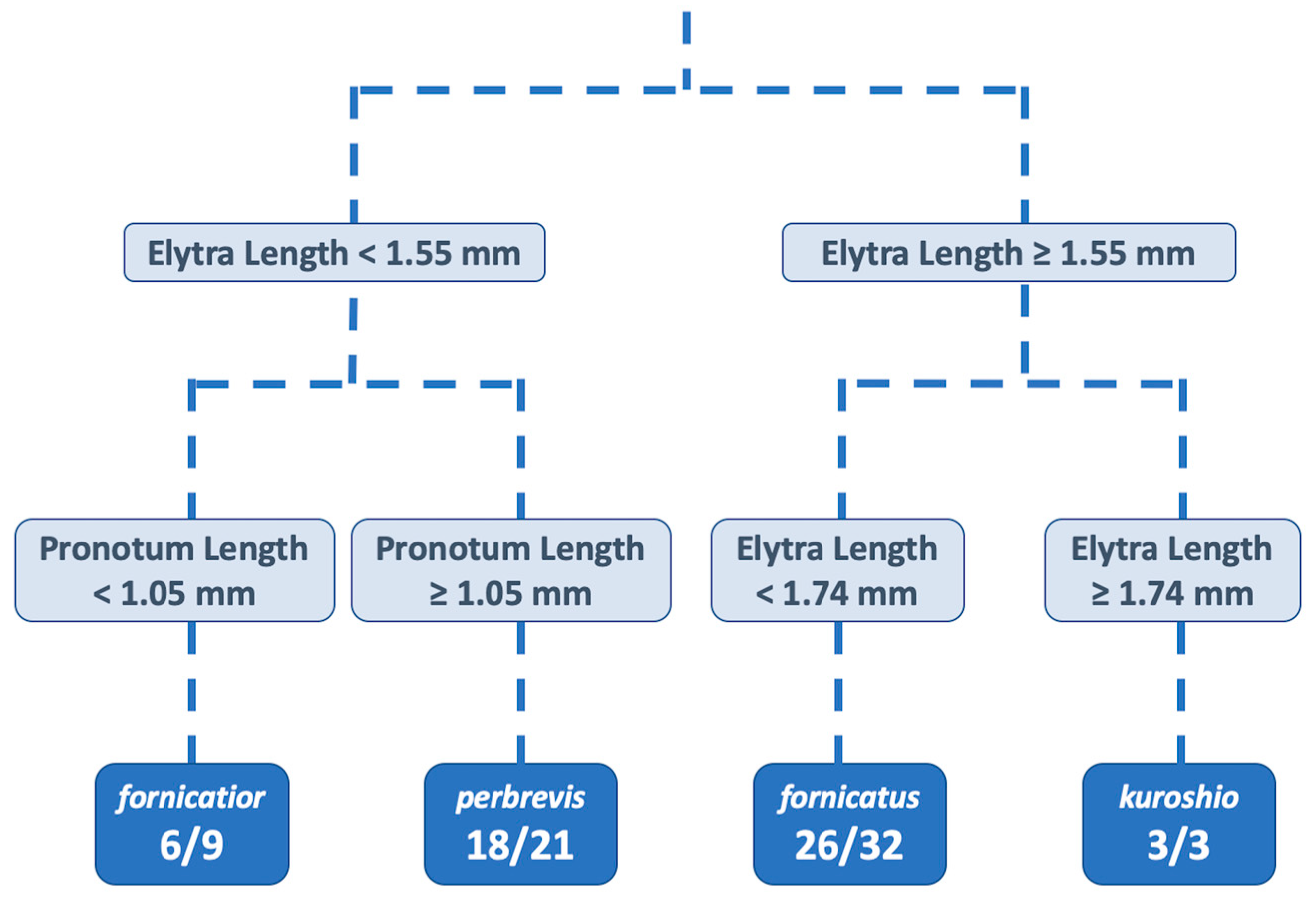
3.2. Identity of the Tea Shot Hole Borer
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kirkendall, L.R.; Biedermann, P.H.W.; Jordal, B.H. Evolution and diversity of bark and ambrosia beetles. In Biology and Ecology of Native and Invasive Species; Vega, F.E., Hofstetter, R.W., Eds.; Academic Press: London, UK, 2015; pp. 85–156. [Google Scholar]
- Hulcr, J.; Atkinson, T.H.; Cognato, A.I.; Jordal, B.H.; McKenna, D.D. Morphology, taxonomy, and phylogenetics of bark beetles. In Biology and Ecology of Native and Invasive Species; Vega, F.E., Hofstetter, R.W., Eds.; Academic Press: London, UK, 2015; pp. 41–84. [Google Scholar]
- Stouthamer, R.; Rugman-Jones, P.; Thu, P.Q.; Eskalen, A.; Thibault, T.; Hulcr, J.; Wang, L.-J.; Jordal, B.H.; Chen, C.-Y.; Cooperband, M.; et al. Tracing the origin of a cryptic invader: Phylogeography of the Euwallacea fornicatus (Coleoptera: Curculionidae: Scolytinae) species complex. Agric. For. Entomol. 2017, 19, 366–375. [Google Scholar] [CrossRef]
- Wood, S.L. Nomenclatural changes and new species of Scolytidae (Coleoptera), part IV. Great Basin Nat. 1989, 49, 167–185. [Google Scholar] [CrossRef]
- Wood, S.L.; Bright, D.E. A catalog of Scolytidae and Platypodidae (Coleoptera), Part 2: Taxonomic index. Great Basin Nat. Mem. 1992, 13, 1–1533. [Google Scholar]
- Eichhoff, W.J. Neue amerikanische Borkenkäfer-Gattungen und Arten. Dtsch. Entomol. Z. 1868, 12, 145–152. [Google Scholar] [CrossRef]
- Węgrzynowicz, P.; Mokrzecki, T. The type material of families Scolytidae and Platypodidae (Coleoptera) in the Museum and Institute of Zoology, PAS, Warsaw. Bull. Mus. Inst. Zool. PAS 1996, 1, 53–65. [Google Scholar]
- Smith, S.M.; Beaver, R.A.; Cognato, A.I. New synonymy, new combinations and taxonomic changes in Japanese xyleborine ambrosia beetles (Coleoptera: Curculionidae: Scolytinae). Zootaxa 2018, 4521, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Cognato, A.I.; Smith, S.M.; Li, Y.; Pham, T.H.; Hulcr, J. Genetic variability among native Xyleborus glabratus Eichhoff populations native to Southeast Asia and the description of two related species. J. Econ. Entomol. 2019, 112, 1274–1284. [Google Scholar] [CrossRef]
- Wood, S.L. The bark and ambrosia beetles of North and Central America (Coleoptera: Scolytidae), a taxonomic monograph. Great Basin Nat. Mem. 1982, 8, 1–1359. [Google Scholar]
- Rabaglia, R.J.; Dole, S.A.; Cognato, A.I. Review of American Xyleborina (Coleoptera: Curculionidae: Scolytinae) occurring North of Mexico, with an illustrated key. Ann. Entomol. Soc. Am. 2006, 99, 1034–1056. [Google Scholar] [CrossRef]
- Mendel, Z.; Protasov, A.; Sharon, M.; Zveibil, A.; Ben Yehuda, S.; O’Donnell, K.; Rabaglia, R.; Wysoki, M.; Freeman, S. An Asian ambrosia beetle Euwallacea fornicatus and its novel symbiotic fungus Fusarium sp. pose a serious threat to the Israeli avocado industry. Phytoparasitica 2012, 40, 235–238. [Google Scholar] [CrossRef]
- Paap, T.; de Beer, W.; Migliorini, D.; Nel, W.J.; Wingfield, M.J. The polyphagous shot hole borer (PSHB) and its fungal symbiont Fusarium euwallaceae: A new invasion in South Africa. Australas. Plant Pathol. 2018, 47, 231–237. [Google Scholar] [CrossRef]
- Gomez, D.F.; Skelton, J.; Steininger, M.S.; Stouthamer, R.; Rugman-Jones, P.; Sittichaya, W.; Rabaglia, R.J.; Hulcr, J. Species within the Euwallacea fornicatus (Coleoptera: Curculionidae) complex revealed by morphometric and phylogenetic analyses. Insect Syst. Divers. 2018, 2, 1–11. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; ISBN 3-900051-07-0. Available online: http://www.R-project.org (accessed on 15 December 2018).
- Gomez, D.F.; Lin, W.; Gao, L.; Li, Y. New host plant records for the Euwallacea fornicatus (Eichhoff) species complex (Coleoptera: Curculionidae: Scolytinae) across its natural and introduced distribution. J. Asia Pac. Entomol. 2019, 22, 338–340. [Google Scholar] [CrossRef]
- Beeson, C.F.C. The biology of the genus Xyleborus, with more new species. Indian For. Rec. 1930, 14, 209–272. [Google Scholar]
- Schedl, K.E. Notes on the genus Xyleborus Eichh. Ann. Mag. Nat. Hist. 1931, 8, 339–347. [Google Scholar] [CrossRef]
- Eggers, H. Neue Borkenkäfer (Scolytidae, Col) aus Indien. Ann. Mag. Nat. Hist. 1936, 17, 626–636. [Google Scholar] [CrossRef]
- Beaver, R.A. The biology of Samoan bark and ambrosia beetles (Coleoptera, Scolytidae and Platypodidae). Bull. Entomol. Res. 1976, 65, 531–548. [Google Scholar] [CrossRef]
- O’Donnell, K.; Sink, S.; Libeskind-Hadas, R.; Hulcr, J.; Kasson, M.T.; Ploetz, R.C.; Konkol, J.L.; Ploetz, J.N.; Carrillo, D.; Campbell, A.; et al. Discordant phylogenies suggest repeated host shifts in the Fusarium–Euwallacea ambrosia beetle mutualism. Fungal Genet. Biol. 2015, 82, 277–290. [Google Scholar] [CrossRef]
- Mendel, Z.; Protasov, A.; Maoz, Y.; Maymon, M.; Miller, G.; Elazar, M.; Freeman, S. The role of Euwallacea nr. fornicatus (Coleoptera: Scolytinae) in the wilt syndrome of avocado trees in Israel. Phytoparasitica 2017, 45, 341–359. [Google Scholar] [CrossRef]
- Haack, R. Exotic bark- and wood-boring Coleoptera in the United States: Recent establishments and interceptions. Can. J. For. Res. 2006, 36, 269–288. [Google Scholar] [CrossRef]
- Coleman, T.W.; Poloni, A.L.; Chen, Y.; Thu, P.Q.; Li, Q.; Sun, J.; Rabaglia, R.J.; Man, G.; Seybold, S.J. Hardwood injury and mortality associated with two shot hole borers, Euwallacea spp., in the invaded region of southern California, USA, and the native region of Southeast Asia. Ann. For. Sci. 2019, 76, 61. [Google Scholar] [CrossRef]
- García-Avila, C.D.J.; Trujillo-Arriaga, F.J.; López-Buenfil, J.A.; González-Gómez, R.; Carrillo, D.; Cruz, L.F.; Ruiz-Galván, I.; Quezada-Salinas, A.; Acevedo-Reyes, N. First report of Euwallacea nr. fornicatus (Coleoptera: Curculionidae) in Mexico. Fla. Entomol. 2016, 99, 555–556. [Google Scholar] [CrossRef]
- Boland, J.M. The impact of an invasive ambrosia beetle on the riparian habitats of the Tijuana River Valley, California. Peer J. 2016, 4, e2141. [Google Scholar] [CrossRef] [PubMed]
- Na, F.; Carrillo, J.D.; Mayorquin, J.S.; Ndinga-Muniania, C.; Stajich, J.E.; Stouthamer, R.; Huang, Y.T.; Lin, Y.T.; Chen, C.Y.; Eskalen, A. Two novel fungal symbionts Fusarium kuroshium sp. nov. and Graphium kuroshium sp. nov. of Kuroshio shot hole borer (Euwallacea sp. nr. fornicatus) cause Fusarium dieback on woody host species in California. Plant Dis. 2018, 102, 1154–1164. [Google Scholar] [CrossRef]
- Kirkendall, L.R.; Ødegaard, F. Ongoing invasions of old-growth tropical forests: Establishment of three incestuous beetle species in Central America (Curculionidae, Scolytinae). Zootaxa 2007, 1588, 53–62. [Google Scholar] [CrossRef]
- Owens, D.; Cruz, L.F.; Montgomery, W.S.; Narvaez, T.I.; Schnell, E.Q.; Tabanca, N.; Duncan, R.E.; Carrillo, D.; Kendra, P.E. Host range expansion and increasing damage potential of Euwallacea nr. fornicatus (Coleoptera: Curculionidae) in Florida. Fla. Entomol. 2018, 101, 229–237. [Google Scholar] [CrossRef]
- Speyer, E.R. Shot-hole borer investigations. Trop. Agric. 1917, 48, 152–155. [Google Scholar]
- Browne, F.G. The biology of Malayan Scolytidae and Platypodidae. Malay. For. Rec. 1961, 22, 1–255. [Google Scholar]
- Walgama, R.S. Ecology and integrated pest management of Xyleborus fornicatus (Coleoptera: Scolytidae) in Sri Lanka. J. Integr. Pest Manag. 2012, 3, A1–A8. [Google Scholar] [CrossRef]
- Gadd, C.H.; Loos, C.A. The ambrosia fungus of Xyleborus fornicatus Eich. Trans. Br. Mycol. Soc. 1947, 31, 13–18. [Google Scholar] [CrossRef]

| GenBank Accession Number | Clade | Species | Country | Locality |
|---|---|---|---|---|
| KU727004 | KSHB | E. kuroshio | Japan | Okinawa |
| KU727005 | KSHB | E. kuroshio | Japan | Okinawa |
| KU727008 | KSHB | E. kuroshio | Taiwan | Not specified |
| MH276944 | KSHB | E. kuroshio | Indonesia | East Java |
| MH276945 | KSHB | E. kuroshio | Indonesia | East Java |
| MH276946 | KSHB | E. kuroshio | USA | California |
| MH276947 | KSHB | E. kuroshio | USA | California |
| MH276948 | KSHB | E. kuroshio | USA | California |
| MH276949 | KSHB | E. kuroshio | USA | California |
| MH276950 | KSHB | E. kuroshio | USA | California |
| JX912724 | PSHB | E. fornicatus | USA | California |
| KU727012 | PSHB | E. fornicatus | Vietnam | Phu Yen |
| KU727014 | PSHB | E. fornicatus | Vietnam | Gia Lai |
| KU727016 | PSHB | E. fornicatus | Thailand | Chiang Mai |
| KU727019 | PSHB | E. fornicatus | Vietnam | Yen Bai |
| KU727020 | PSHB | E. fornicatus | Vietnam | Not specified |
| KU727021 | PSHB | E. fornicatus | USA | California |
| KU727023 | PSHB | E. fornicatus | USA | California |
| KU727026 | PSHB | E. fornicatus | Vietnam | Yen Bai |
| KU727027 | PSHB | E. fornicatus | Japan | Okinawa |
| MH276936 | PSHB | E. fornicatus | China | Guizhou |
| MH276937 | PSHB | E. fornicatus | China | Guizhou |
| MH276938 | PSHB | E. fornicatus | Thailand | Phetchabun |
| MH276939 | PSHB | E. fornicatus | Japan | Okinawa |
| MH276940 | PSHB | E. fornicatus | Vietnam | Hoan Kiem |
| MH276941 | PSHB | E. fornicatus | China | Hong Kong |
| MH276942 | PSHB | E. fornicatus | USA | California |
| MH276943 | PSHB | E. fornicatus | Thailand | Chiang Mai |
| MN266860 | PSHB | E. fornicatus | China | Hong Kong |
| MN266859 | PSHB | E. fornicatus | Vietnam | Thua Thien-Hue |
| MN266861 | PSHB | E. fornicatus | Vietnam | Ninh Binh |
| MN266858 | PSHB | E. fornicatus | Vietnam | Cao Bang |
| KU726992 | TSHBa | E. perbrevis | Australia | Sunshine Coast |
| KU726995 | TSHBa | E. perbrevis | Thailand | Not specified |
| KU726996 | TSHBa | E. perbrevis | USA | Florida |
| KU726997 | TSHBa | E. perbrevis | Thailand | Not specified |
| KU726998 | TSHBa | E. perbrevis | Thailand | Not specified |
| KU726999 | TSHBa | E. perbrevis | Thailand | Chiang Mai |
| KU727001 | TSHBa | E. perbrevis | Thailand | Chumphon |
| KU727003 | TSHBa | E. perbrevis | Thailand | Surat Thani |
| MH276907 | TSHBa | E. perbrevis | Indonesia | East Java |
| MH276908 | TSHBa | E. perbrevis | Indonesia | East Java |
| MH276909 | TSHBa | E. perbrevis | Indonesia | East Java |
| MH276910 | TSHBa | E. perbrevis | Indonesia | East Java |
| MH276911 | TSHBa | E. perbrevis | Indonesia | East Java |
| MH276912 | TSHBa | E. perbrevis | Indonesia | East Java |
| MH276913 | TSHBa | E. perbrevis | Papua New Guinea | Eastern Highlands |
| MH276914 | TSHBa | E. perbrevis | USA | Florida |
| MH276915 | TSHBa | E. perbrevis | USA | Florida |
| MH276916 | TSHBa | E. perbrevis | USA | Florida |
| MH276917 | TSHBa | E. perbrevis | Thailand | Nakhon Si Thammarat |
| MH276918 | TSHBa | E. perbrevis | Thailand | Nakhon Si Thammarat |
| MH276919 | TSHBa | E. perbrevis | Thailand | Nakhon Si Thammarat |
| MH276920 | TSHBa | E. perbrevis | Thailand | Trat |
| MH276921 | TSHBa | E. perbrevis | Thailand | Trat |
| MH276922 | TSHBa | E. perbrevis | Thailand | Chanthaburi |
| MH276923 | TSHBa | E. perbrevis | Thailand | Chanthaburi |
| MH276924 | TSHBa | E. perbrevis | Thailand | Trat |
| MH276925 | TSHBa | E. perbrevis | China | Hainan |
| MH276926 | TSHBa | E. perbrevis | Thailand | Chiang Mai |
| MH276927 | TSHBa | E. perbrevis | Thailand | Chiang Mai |
| MH276928 | TSHBa | E. perbrevis | American Samoa | Aoa |
| MH276929 | TSHBa | E. perbrevis | Papua New Guinea | Madang |
| KU726991 | TSHBb | E. fornicatior | Singapore | Not specified |
| MH276930 | TSHBb | E. fornicatior | Papua New Guinea | Madang |
| MH276931 | TSHBb | E. fornicatior | Papua New Guinea | Madang |
| MH276932 | TSHBb | E. fornicatior | Papua New Guinea | Madang |
| MH276933 | TSHBb | E. fornicatior | Papua New Guinea | Oro |
| MH276934 | TSHBb | E. fornicatior | Papua New Guinea | Oro |
| MH276935 | TSHBb | E. fornicatior | Papua New Guinea | Oro |
| Species | Total Length (Dorsal) | Length/Width Ratio (Dorsal) | Elytral Length (Lateral; Diagonal) | Pronotal Length (Lateral; Diagonal) | Elytral Width (Dorsal) | Pronotal Width (Dorsal) | Protibial Denticle Number |
|---|---|---|---|---|---|---|---|
| fornicatior | 2.20–2.37 | 2.15−2.35 | 1.40–1.46 | 1.02–1.06 | 0.48–0.52 | 1.00–1.06 | 6–7 |
| fornicatus | 2.60−2.70 | 2.25−2.36 | 1.44–1.72 | 1.02–1.16 | 0.48–0.62 | 1.00–1.14 | 8–9 |
| kuroshio | 2.40−2.80 | 2.17−2.40 | 1.50–1.82 | 1.08–1.16 | 0.52–0.56 | 1.06–1.16 | 8–11 |
| perbrevis | 2.30−2.50 | 2.46−2.55 | 1.42–1.68 | 1.04–1.16 | 0.48–0.56 | 1.02–1.14 | 7–10 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
M. Smith, S.; F. Gomez, D.; A. Beaver, R.; Hulcr, J.; I. Cognato, A. Reassessment of the Species in the Euwallacea Fornicatus (Coleoptera: Curculionidae: Scolytinae) Complex after the Rediscovery of the “Lost” Type Specimen. Insects 2019, 10, 261. https://doi.org/10.3390/insects10090261
M. Smith S, F. Gomez D, A. Beaver R, Hulcr J, I. Cognato A. Reassessment of the Species in the Euwallacea Fornicatus (Coleoptera: Curculionidae: Scolytinae) Complex after the Rediscovery of the “Lost” Type Specimen. Insects. 2019; 10(9):261. https://doi.org/10.3390/insects10090261
Chicago/Turabian StyleM. Smith, Sarah, Demian F. Gomez, Roger A. Beaver, Jiri Hulcr, and Anthony I. Cognato. 2019. "Reassessment of the Species in the Euwallacea Fornicatus (Coleoptera: Curculionidae: Scolytinae) Complex after the Rediscovery of the “Lost” Type Specimen" Insects 10, no. 9: 261. https://doi.org/10.3390/insects10090261
APA StyleM. Smith, S., F. Gomez, D., A. Beaver, R., Hulcr, J., & I. Cognato, A. (2019). Reassessment of the Species in the Euwallacea Fornicatus (Coleoptera: Curculionidae: Scolytinae) Complex after the Rediscovery of the “Lost” Type Specimen. Insects, 10(9), 261. https://doi.org/10.3390/insects10090261





