In Vivo Effects of A Pro-PO System Inhibitor on the Phagocytosis of Xenorhabdus Nematophila in Galleria Mellonella Larvae
Abstract
:1. Introduction
2. Material and Methods
2.1. Insect Host and Bacterial Strains
2.2. Bleeding of Insects, Hemocyte Cultures and Separation of Cell-Free Plasma
2.3. Fluorescence Labelling and Cell Surface Biochemical Modifications of Bacteria
2.4. Phagocytosis Assays
2.5. Evaluation of Phagocytosis by Fluorescence Microscopy and by Flow Cytometry
2.6. Densitometric Analysis of Larvae Color Change
2.7. Nodule Count
2.8. Cell Cultures of the Human Promyelocytic Cell Line THP1 and Phagocytosis Assay
2.9. Data Analysis
3. Results
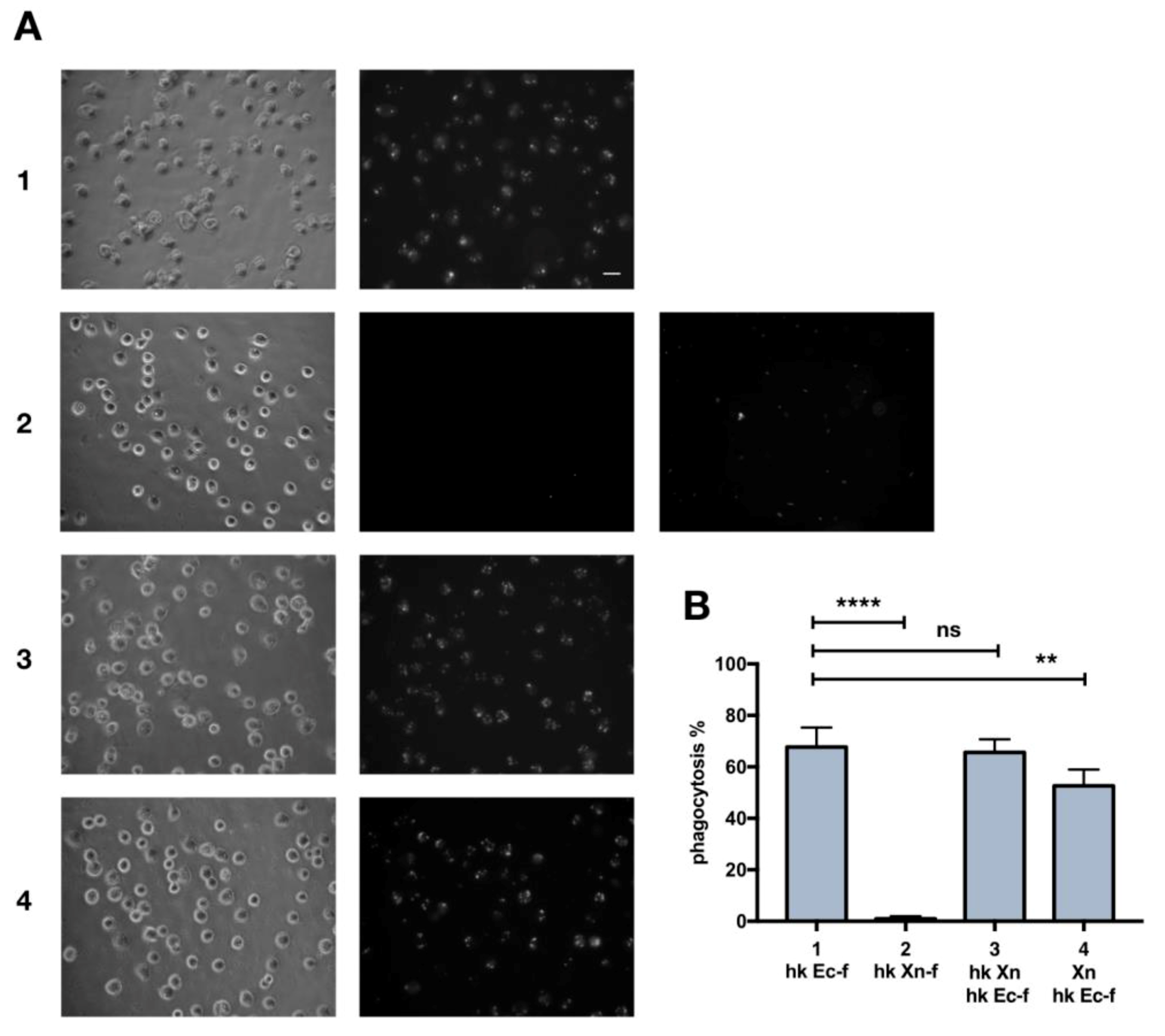
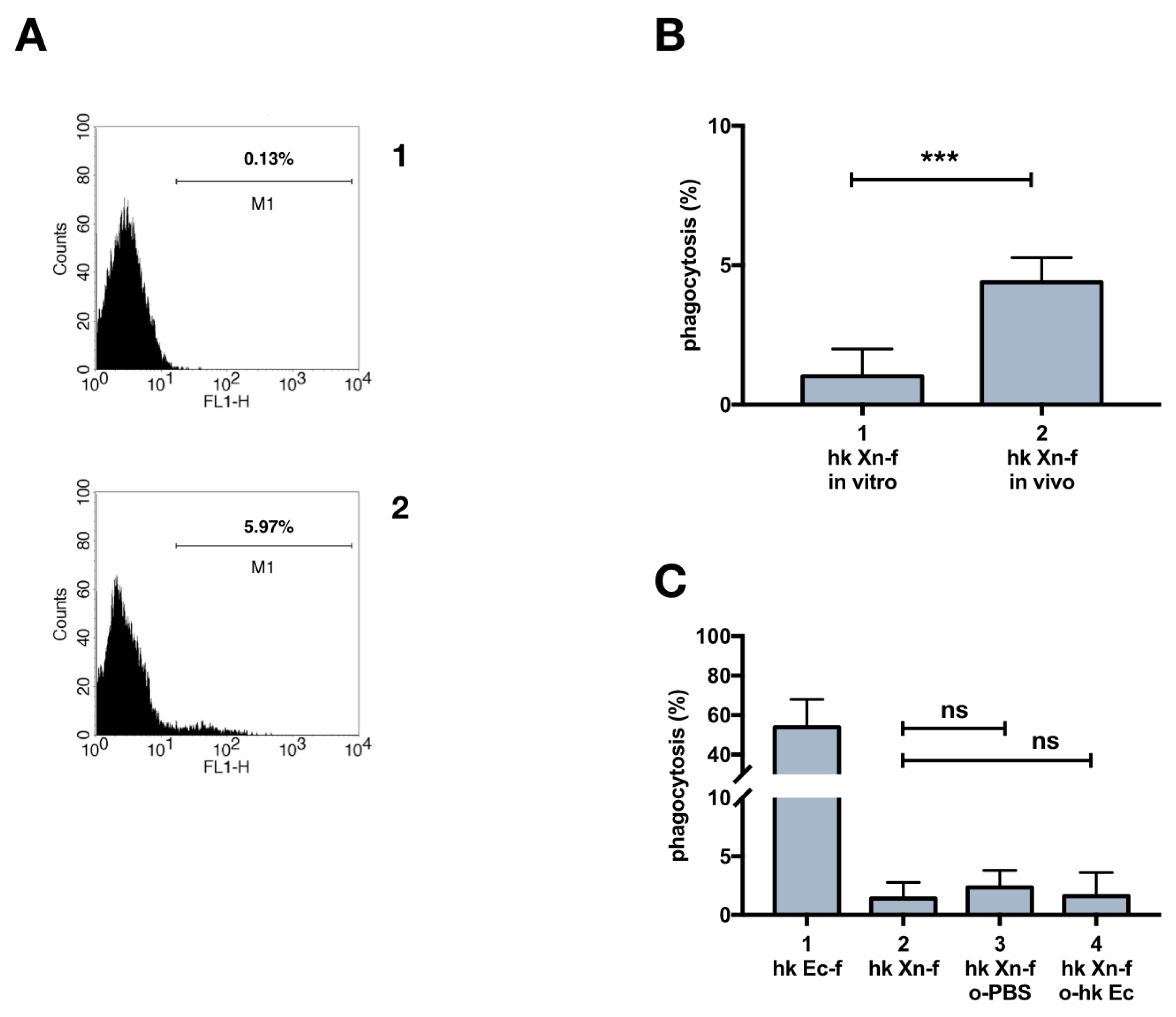
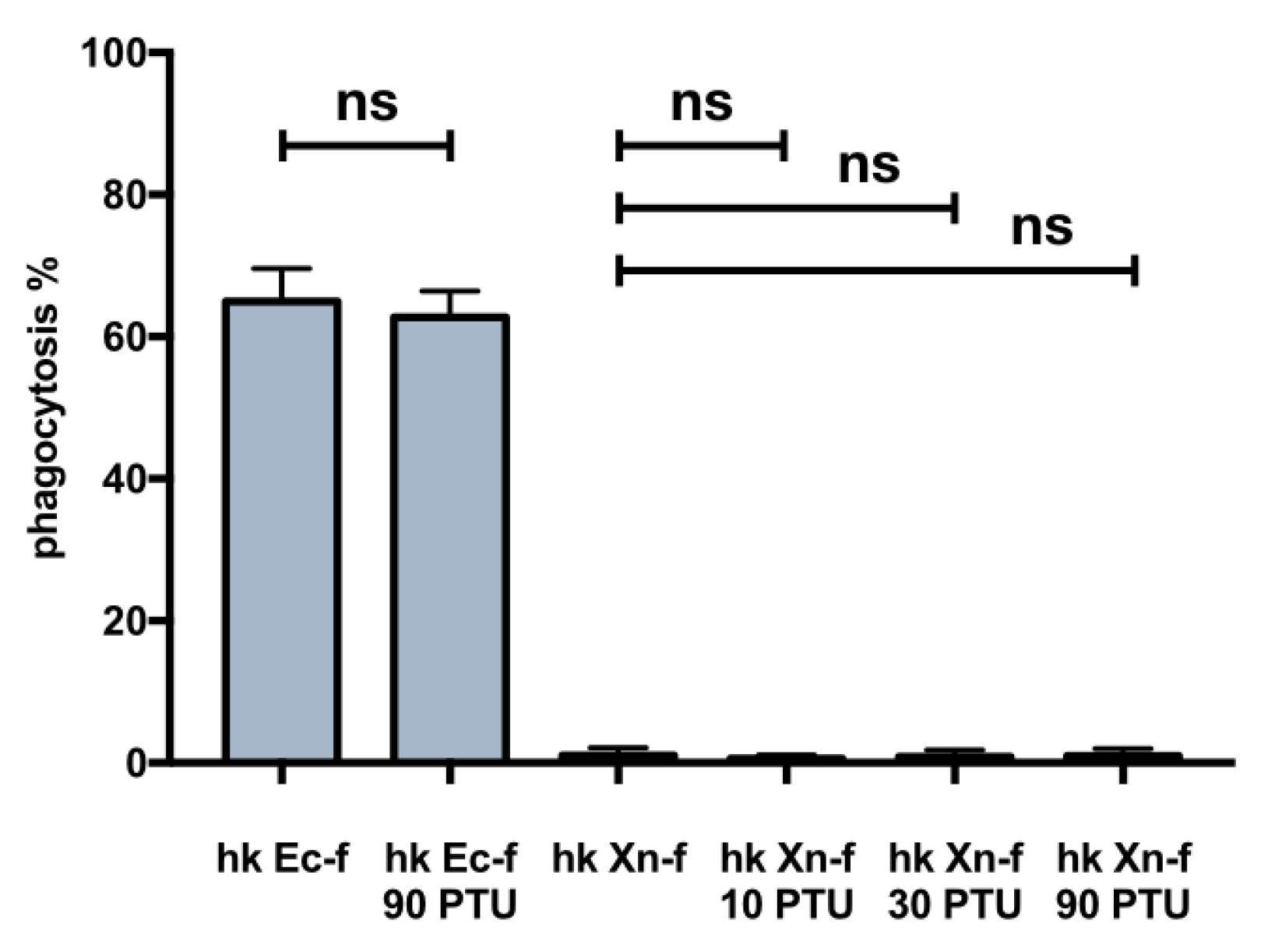
3.1. Heat-Killed X. Nematophila Escapes In Vitro Uptake by Hemocytes without Directly Inhibiting In Vitro or In Vivo the Cellular Mechanisms of Phagocytosis
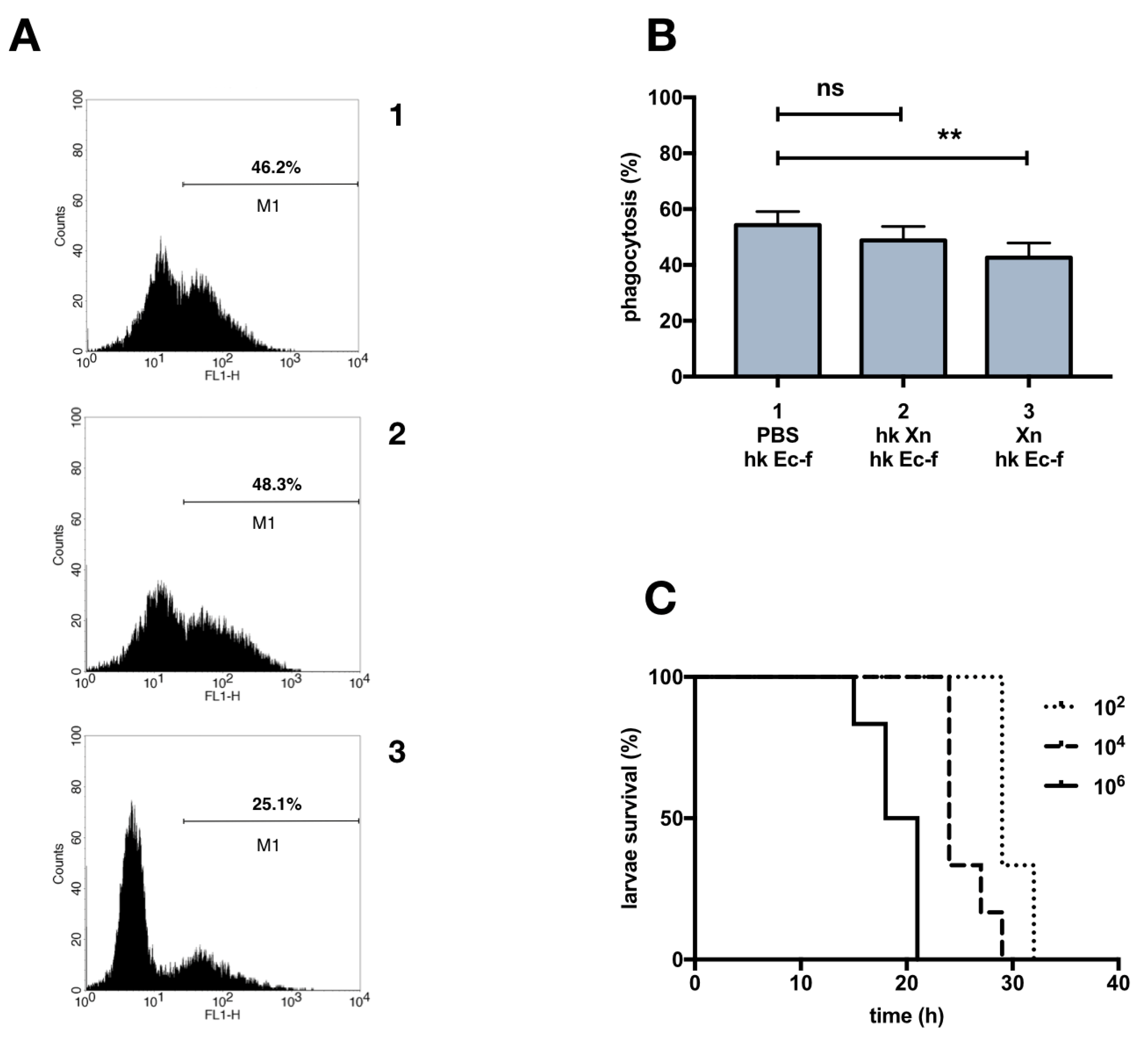
3.2. Phagocytosis of X. Nematophila In Vivo
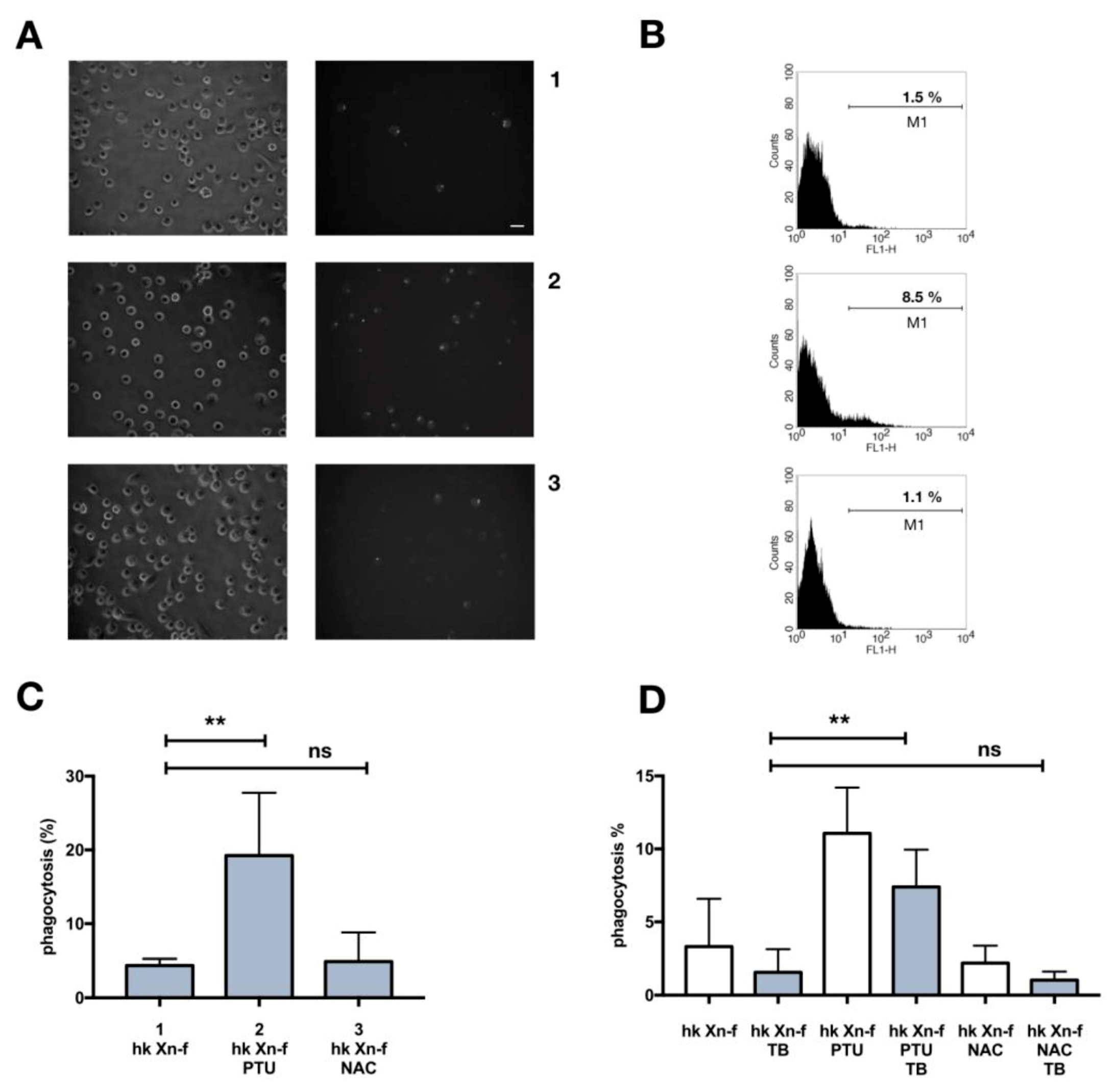
3.3. Immune System Priming with Heat-Killed E. Coli and Effects on X. Nematophila Phagocytosis
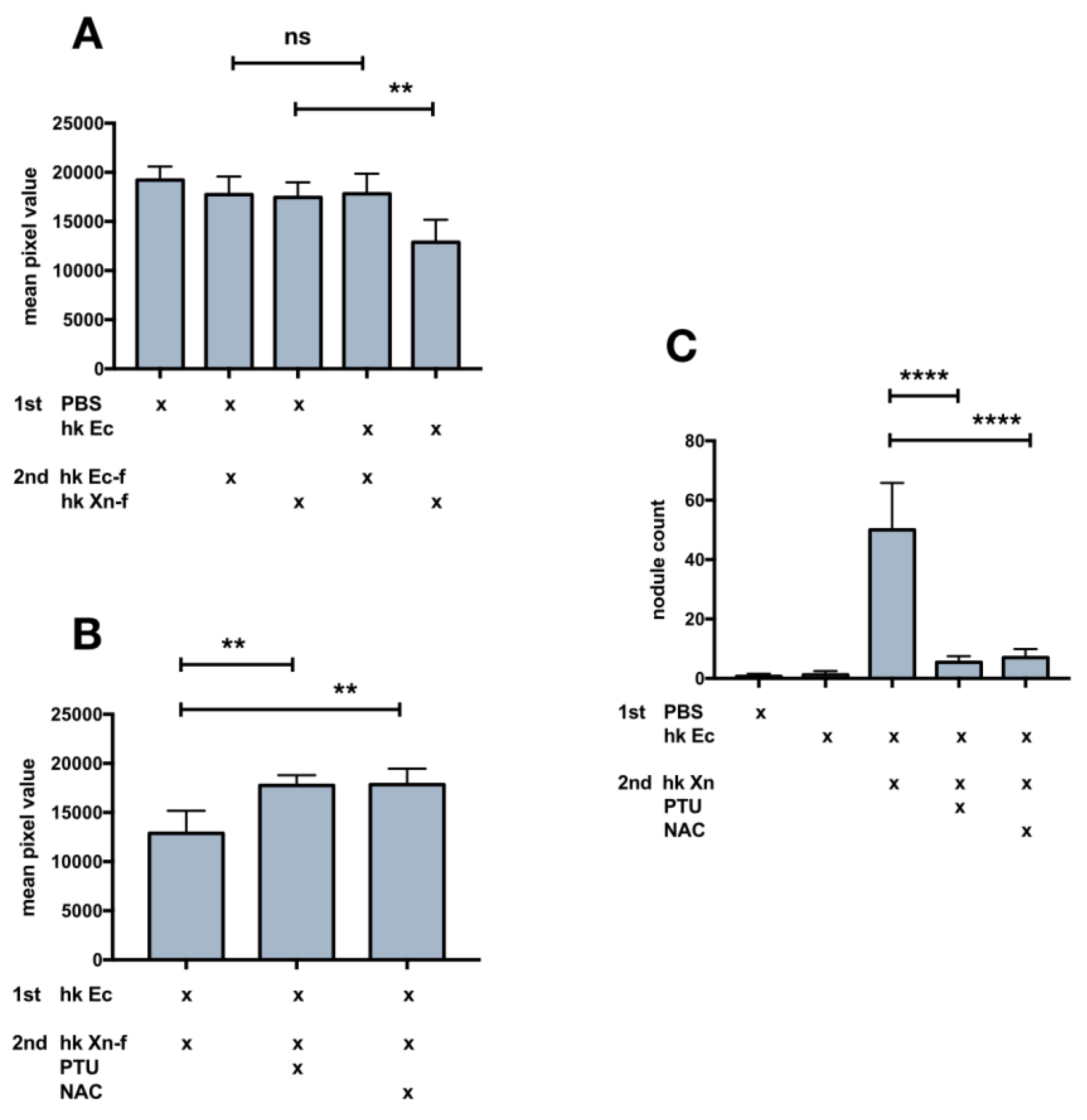
3.4. Changes in the Appearance (Darkening) of the Caterpillar Body after X. Nematophila Infection, Antagonistic Effect of Immunomodulatory Drugs
3.5. Effects on Host Physiology of Immunomodulatory Drugs
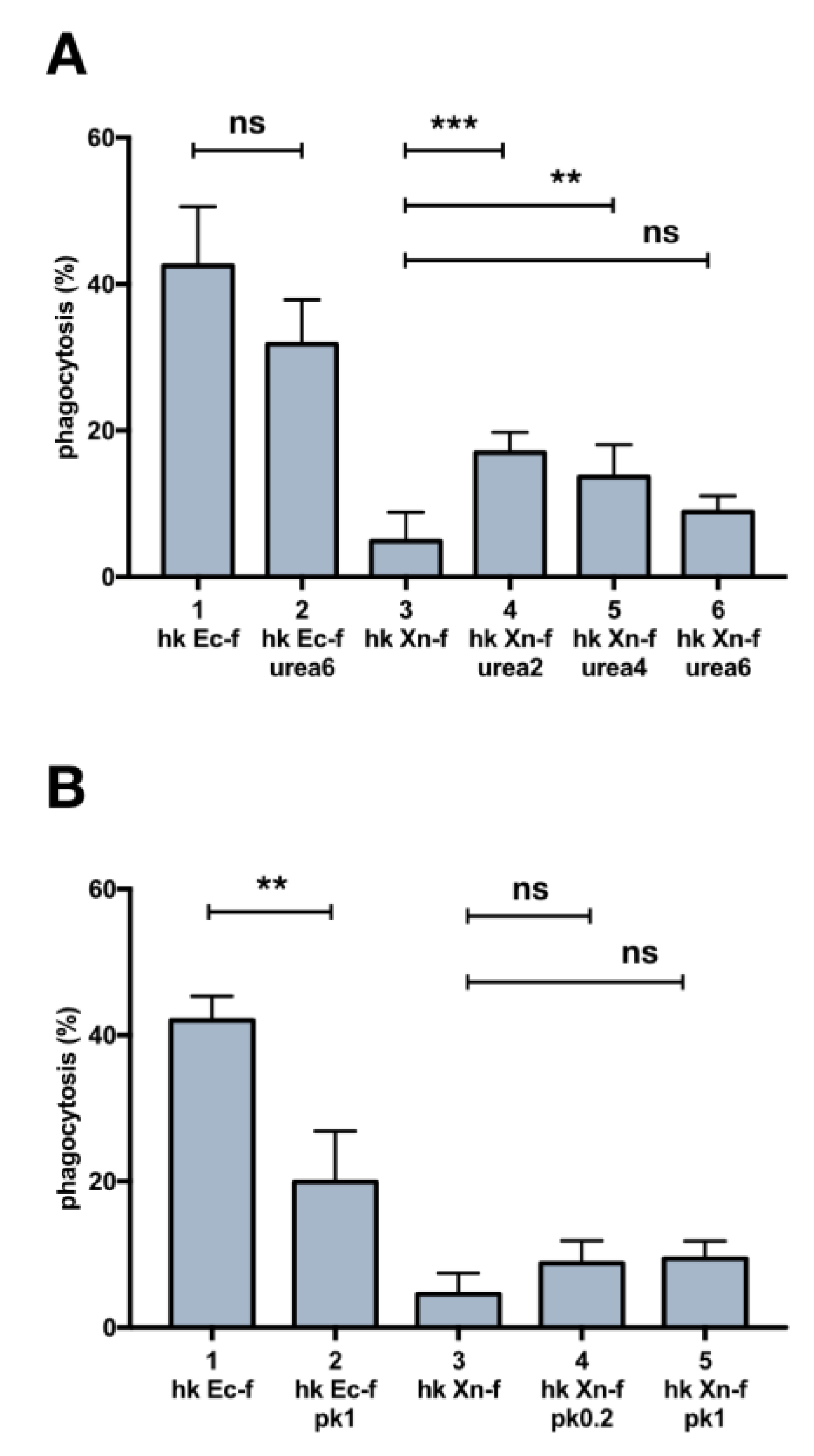
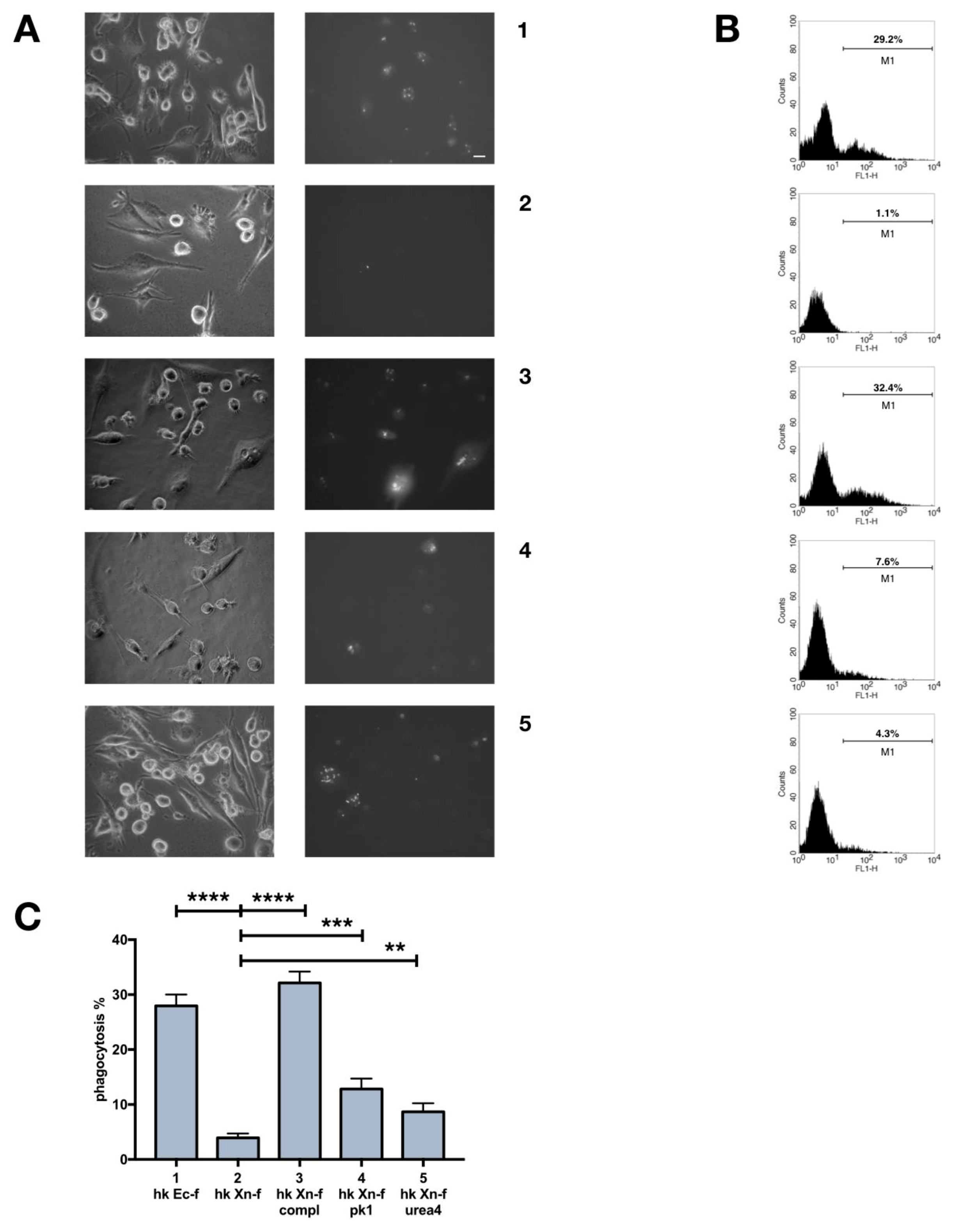
3.6. Biochemical Modification of X. Nematophila Cell Surface, Effects on In Vitro and In Vivo Phagocytosis
3.7. Dead X. Nematophila Resists Phagocytosis by a Mammalian Macrophage Cell Line
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lavine, M.D.; Strand, M.R. Insect hemocytes and their role in immunity. Insect Biochem. Mol. Biol. 2002, 32, 1295–1309. [Google Scholar] [CrossRef]
- Jiang, H.; Vilcinskas, A.; Kanost, M.R. Immunity in lepidopteran insects. Adv. Exp. Med. Biol. 2010, 708, 181–204. [Google Scholar] [PubMed]
- Flannagan, R.S.; Jaumouillé, V.; Grinstein, S. The cell biology of phagocytosis. Annu. Rev. Pathol. 2012, 7, 61–98. [Google Scholar] [CrossRef] [PubMed]
- Rosales, C. Phagocytosis, a cellular immune response in insects. Invertebr. Surviv. J. 2011, 8, 109–131. [Google Scholar]
- Tojo, S.; Naganuma, F.; Arakawa, K.; Yokoo, S. Involvement of both granular cells and plasmatocytes in phagocytic reactions in the greater wax moth, Galleria mellonella. J. Insect Physiol. 2000, 46, 1129–1135. [Google Scholar] [CrossRef]
- Satyavathi, V.V.; Minz, A.; Nagaraju, J. Nodulation: An unexplored cellular defense mechanism in insects. Cell. Signal. 2014, 226, 1753–1763. [Google Scholar] [CrossRef] [PubMed]
- Kanost, M.R.; Gorman, M.J. Phenoloxidase in insect immunity. In Insect Immunology, 1st ed.; Beckage, N.E., Ed.; Academic Press: Cambridge, MA, USA, 2008; pp. 69–96. [Google Scholar] [CrossRef]
- Nappi, A.J.; Christensen, B.M. Melanogenesis and associated cytotoxic reactions: Applications to insect innate immunity. Insect Biochem. Mol. Biol. 2005, 35, 443–459. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Ahmed, S.; Stanley, D.; An, C. Eicosanoid-mediated immunity in insects. Dev. Comp. Immunol. 2018, 83, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Hyrsl, P.; Dobes, P.; Wang, Z.; Hauling, T.; Wilhelmsson, C.; Theopold, U. Clotting factors and eicosanoids protect against nematode infections. J. Innate Immun. 2011, 3, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Ulvila, J.; Vanha-Aho, L.M.; Rämet, M. Drosophila phagocytosis—Still many unknowns under the surface. APMIS 2011, 119, 651–662. [Google Scholar] [CrossRef]
- Gotz, P.; Weise, C.; Kopacek, P.; Losen, S.; Weisner, A. Isolated Apolipophorin III from Galleria mellonella stimulates the immune reactions of this insect. J. Insect Physiol. 1997, 43, 383–391. [Google Scholar] [PubMed]
- Kim, C.H.; Shin, Y.P.; Noh, M.Y.; Jo, Y.H.; Han, Y.S.; Seong, Y.S.; Lee, I.H. An insect multiligand recognition protein functions as an opsonin for the phagocytosis of microorganisms. J. Biol. Chem. 2010, 285, 25243–25250. [Google Scholar] [CrossRef] [PubMed]
- Sarantis, H.; Grinstein, S. Subversion of phagocytosis for pathogen survival. Cell Host Microbe 2012, 12, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Celli, J.; Finlay, B.B. Bacterial avoidance of phagocytosis. Trends Microbiol. 2002, 10, 232–237. [Google Scholar] [CrossRef]
- Herbert, E.E.; Goodrich-Blair, H. Friend and foe: The two faces of Xenorhabdus nematophila. Nat. Rev. Microbiol. 2007, 5, 634–646. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Herbert, E.E.; Cowles, C.E.; Cowles, K.N.; Menard, M.L.; Orchard, S.S.; Goodrich-Blair, H. Clonal variation in Xenorhabdus nematophila virulence and suppression of Manduca sexta immunity. Cell. Microbiol. 2007, 9, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Kim, Y. Eicosanoids rescue Spodoptera exigua infected with Xenorhabdus nematophilus, the symbiotic bacteria to the entomopathogenic nematode Steinernema carpocapsae. J. Insect Physiol. 2000, 46, 1469–1476. [Google Scholar] [CrossRef]
- Park, Y.; Kim, Y.; Putnam, S.M.; Stanley, D.W. The bacterium Xenorhabdus nematophilus depresses nodulation reaction to infection by inhibiting eicosanoid biosynthesis in tobacco hornworms, Manduca sexta. Arch. Insect Biochem. Physiol. 2003, 52, 71–80. [Google Scholar] [CrossRef]
- Vigneux, F.; Zumbihl, R.; Jubelin, G.; Ribeiro, C.; Poncet, J.; Baghdiguian, S.; Givaudan, A.; Brehélin, M. The xaxAB genes encoding a new apoptotic toxin from the insect pathogen Xenorhabdus nematophila are present in plant and human pathogens. J. Biol. Chem. 2007, 282, 9571–9580. [Google Scholar] [CrossRef]
- Banerjee, J.; Singh, J.; Joshi, M.C.; Ghosh, S.; Banerjee, N. The cytotoxic fimbrial structural subunit of Xenorhabdus nematophila is a pore-forming toxin. J. Bacteriol. 2006, 188, 7957–7962. [Google Scholar] [CrossRef]
- Brown, S.E.; Cao, A.T.; Hines, E.R.; Akhurst, R.J.; East, P.D. A novel secreted protein toxin from the insect pathogenic bacterium Xenorhabdus nematophila. J. Biol. Chem. 2004, 279, 14595–14601. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Park, Y.; Kim, Y.; Hwang, J.; Lee, D. An entomopathogenic bacterium, Xenorhabdus nematophila, suppresses expression of antimicrobial peptides controlled by Toll and Imd pathways by blocking eicosanoid biosynthesis. Arch. Insect Biochem. Physiol. 2013, 83, 151–169. [Google Scholar] [CrossRef] [PubMed]
- Ji, D.; Kim, Y. An entomopathogenic bacterium, Xenorhabdus nematophila, inhibits the expression of an antibacterial peptide, cecropin, of the beet armyworm, Spodoptera exigua. J. Insect Physiol. 2004, 50, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Mastore, M.; Brivio, M.F. Cuticular surface lipids are responsible for disguise properties of an entomoparasite against host cellular responses. Dev. Comp. Immunol. 2008, 32, 1050–1062. [Google Scholar] [CrossRef] [PubMed]
- Brivio, M.F.; Moro, M.; Mastore, M. Down-regulation of antibacterial peptide synthesis in an insect model induced by the body-surface of an entomoparasite (Steinernema feltiae). Dev. Comp. Immunol. 2006, 30, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Kim, Y. An entomopathogenic bacterium, Xenorhabdus nematophila, inhibits hemocyte phagocytosis of Spodoptera exigua by inhibiting phospholipase A2. J. Invertebr. Pathol. 2007, 96, 64–70. [Google Scholar] [CrossRef]
- Ramarao, N.; Nielsen-Leroux, C.; Lereclus, D. The insect Galleria mellonella as a powerful infection model to investigate bacterial pathogenesis. J. Vis. Exp. 2012, 70, e4392. [Google Scholar] [CrossRef]
- Rahman, A.; Bharali, P.; Borah, L.; Bathari, M.; Taye, R.R. Post embryonic development of Galleria mellonella L. and its management strategy. J. Entomol. Zool. Stud. 2017, 5, 1523–1526. [Google Scholar]
- Brivio, M.F.; Toscano, A.; De Pasquale, S.M.; De Lerma Barbaro, A.; Giovannardi, S.; Finzi, G.; Mastore, M. Surface protein components from entomopathogenic nematodes and their symbiotic bacteria: Effects on immune responses of the greater wax moth, Galleria mellonella (Lepidoptera: Pyralidae). Pest Manag. Sci. 2018. [Google Scholar] [CrossRef]
- Boemare, N.; Akhurst, R. The genera Photorhabdus and Xenorhabdus. In The Prokaryotes, 3rd ed.; Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, K.H., Stackebrandt, E., Eds.; Springer: New York, NY, USA, 2006; pp. 451–494. [Google Scholar]
- Drevets, D.A.; Campbell, P.A. Macrophage phagocytosis: Use of fluorescence microscopy to distinguish between extracellular and intracellular bacteria. J. Immunol. Methods 1991, 142, 31–38. [Google Scholar] [CrossRef]
- Cooper, D.; Eleftherianos, I. Memory and specificity in the insect immune system: Current perspectives and future challenges. Front. Immunol. 2017, 8, 539. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Xu, L.; Yi, Y. Galleria mellonella larvae are capable of sensing the extent of priming agent and mounting proportionatal cellular and humoral immune responses. Immunol. Lett. 2016, 174, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Rämet, M.; Manfruelli, P.; Pearson, A.; Mathey-Prevot, B.; Ezekowitz, R.A. Functional genomic analysis of phagocytosis and identification of a Drosophila receptor for E. coli. Nature 2002, 416, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Busetto, S.; Trevisan, E.; Patriarca, P.; Menegazzi, R. A single-step, sensitive flow cytofluorometric assay for the simultaneous assessment of membrane-bound and ingested Candida albicans in phagocytosing neutrophils. Cytometry 2004, 58, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to Image: 25 years of image analysis. Nature Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Schwende, H.; Fitzke, E.; Ambs, P.; Dieter, P. Differences in the state of differentiation of THP-1 cells induced by phorbol ester and 1,25-dihydroxyvitamin D3. J. Leukoc. Biol. 1996, 59, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Au, C.; Dean, P.; Reynolds, S.E.; Ffrench-Constant, R.H. Effect of the insect pathogenic bacterium Photorhabdus on insect phagocytes. Cell. Microbiol. 2004, 6, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Ernst, J.D. Bacterial inhibition of phagocytosis. Cell. Microbiol. 2000, 2, 379–386. [Google Scholar] [CrossRef]
- Goosney, D.L.; Celli, J.; Kenny, B.; Finlay, B.B. Enteropathogenic Escherichia coli inhibits phagocytosis. Infect. Immun. 1999, 67, 490–495. [Google Scholar]
- Kline, K.A.; Fälker, S.; Dahlberg, S.; Normark, S.; Henriques-Normark, B. Bacterial adhesins in host-microbe interactions. Cell Host Microbe 2009, 5, 580–592. [Google Scholar] [CrossRef]
- Wheeler, R.T.; Kombe, D.; Agarwala, S.D.; Fink, G.R. Dynamic, morphotype specific Candida albicans beta-glucan exposure during infection and drug treatment. PLoS Pathog. 2008. [Google Scholar] [CrossRef] [PubMed]
- Aimanianda, V.; Bayry, J.; Bozza, S.; Kniemeyer, O.; Perruccio, K.; Elluru, S.R. Surface hydrophobin prevents immune recognition of airborne fungal spores. Nature 2009, 460, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Okagaki, L.H.; Strain, A.K.; Nielsen, J.N.; Charlier, C.; Baltes, N.J.; Chrétien, F.; Heitman, J.; Dromer, F.; Nielsen, K. Cryptococcal cell morphology affects host cell interactions and pathogenicity. PLoS Pathog. 2010. [Google Scholar] [CrossRef]
- Vilcinskas, A.; Matha, V.; Gotz, P. Inhibition of phagocytic activity of plasmatocytes isolated from Galleria mellonella by entomogenous fungi and their secondary metabolites. J. Insect Phys. 1997, 43, 475–483. [Google Scholar] [CrossRef]
- Chun, C.D.; Brown, J.C.S.; Madhani, H.D. A major role for capsule-independent phagocytosis-inhibitory mechanisms in mammalian infection by Cryptococcus neoformans. Cell Host Microbe 2011, 9, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.; Chen, C.; Ratcliffe, N.A. Innate immunity in insects: The role of multiple, endogenous serum lectins in the recognition of foreign invaders in the cockroach, Blaberus discoidalis. J. Immunol. 1999, 162, 1590–1596. [Google Scholar] [PubMed]
- Sideri, M.; Tsakas, S.; Markoutsa, E.; Lampropoulou, M.; Marmaras, V.J. Innate immunity in insects: Surface-associated dopa decarboxylase-dependent pathways regulate phagocytosis, nodulation and melanization in medfly hemocytes. Immunology 2008, 123, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Tang, H. Regulation and function of the melanization reaction in Drosophila. Fly (Austin) 2009, 3, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Sułek, M.; Vertyporokh, L.; Waleczko, P.; Wojda, I. Immune priming of Galleria mellonella larvae with Bacillus thuringiensis affects coagulation and phenoloxidase activity upon subsequent infection. Invertebr. Surv. J. 2019, 16, 66–71. [Google Scholar]
- Zafarullah, M.; Li, W.Q.; Sylvester, J.; Ahmad, M. Molecular mechanisms of N-acetylcysteine actions. Cell Mol. Life Sci. 2003, 60, 6–20. [Google Scholar] [CrossRef]
- Shelby, K.S.; Popham, H.J. Plasma phenoloxidase of the larval tobacco budworm, Heliothis virescens, is virucidal. J. Insect Sci. 2006, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Clark, K.D.; Lu, Z.; Strand, M.R. Regulation of melanization by glutathione in the moth Pseudoplusia includens. Insect Biochem. Mol. Biol. 2010, 40, 460–467. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Lerma Barbaro, A.; Gariboldi, M.B.; Mastore, M.; Brivio, M.F.; Giovannardi, S. In Vivo Effects of A Pro-PO System Inhibitor on the Phagocytosis of Xenorhabdus Nematophila in Galleria Mellonella Larvae. Insects 2019, 10, 263. https://doi.org/10.3390/insects10090263
De Lerma Barbaro A, Gariboldi MB, Mastore M, Brivio MF, Giovannardi S. In Vivo Effects of A Pro-PO System Inhibitor on the Phagocytosis of Xenorhabdus Nematophila in Galleria Mellonella Larvae. Insects. 2019; 10(9):263. https://doi.org/10.3390/insects10090263
Chicago/Turabian StyleDe Lerma Barbaro, Andrea, Marzia B. Gariboldi, Maristella Mastore, Maurizio F. Brivio, and Stefano Giovannardi. 2019. "In Vivo Effects of A Pro-PO System Inhibitor on the Phagocytosis of Xenorhabdus Nematophila in Galleria Mellonella Larvae" Insects 10, no. 9: 263. https://doi.org/10.3390/insects10090263
APA StyleDe Lerma Barbaro, A., Gariboldi, M. B., Mastore, M., Brivio, M. F., & Giovannardi, S. (2019). In Vivo Effects of A Pro-PO System Inhibitor on the Phagocytosis of Xenorhabdus Nematophila in Galleria Mellonella Larvae. Insects, 10(9), 263. https://doi.org/10.3390/insects10090263








