In Vitro Analyses of the Toxicity, Immunological, and Gene Expression Effects of Cobalt-Chromium Alloy Wear Debris and Co Ions Derived from Metal-on-Metal Hip Implants
Abstract
:1. Introduction
2. Wear Particle Generation and Metal Ion Release
2.1. Chromium
| Author | Implant | Body fluid | Follow up | Mean Concentration (µg/L) | ||
|---|---|---|---|---|---|---|
| Co | Cr | |||||
| Daniel, et al. [55] | MoM resurfacing | Whole blood | Up to 4 years | Pre-op | 0.2 | 0.3 |
| 1 year | 1.3 | 2.4 | ||||
| 4 years | 1.2 | 1.1 | ||||
| Ziaee, et al. [56] | MoM resurfacing | Whole blood | Mean of 53 months | Control | 0.3 | 0.2 |
| Patients | 1.4 | 1.9 | ||||
| Antoniou, et al. [57] | MoM (THA and resurfacing), MoP (THA) | Whole blood | 1 year | Control | 1.8 | 0.1 |
| MoM THA | 2.6 | 0.6 | ||||
| MoM resurfacing | 2.4 | 0.5 | ||||
| MoP THA | 1.7 | 0.1 | ||||
| non-steep (component abduction <55°) | 2.4 | 3.6 | ||||
| Wretenberg [58] | MoM THA (Case report) | Whole blood | 37 years | - | 22.9 | 19.4 |
| Hart, et al. [59] | Painful MoM resurfacings | Whole blood | median of 27 months | Unilateral | 4.5 * | 3.0 * |
| Bilateral | 10.6 * | 7.9 * | ||||
| Hart, et al. [60] | Failed MoM resurfacing | Whole blood | Mean 51 months | - | 112.6 | 61.7 |
| Langton, et al. [61] | MoM resurfacing (ASR, BHR) | Whole blood | minimum of 12 months | ASR | 2.7 * | 4.2 * |
| BHR | 1.8 * | 4.2 * | ||||
| Adverse reactions | 69.0 | 29.3 | ||||
| Davda, et al. [62] | Symptomatic MoM, THA and resurfacing | Synovial fluid | Mean of 36 months | Unexplained pain | 1127.0 * | 1337.0 * |
| Defined cause of failure | 1014.0 * | 1512.0 * | ||||
| Hart, et al. [63] | MoM, THA and resurfacing | Whole blood | 39–42 months | Failed | 6.9 * | 5.0 * |
| Well-functioning | 1.7 * | 2.3 * | ||||
| Non-pseudotumor | 1.9 * | 2.1 * | ||||
| Pseudotumor | 9.2 * | 12.0 * | ||||
| Malviya, et al. [64] | MoM, MoP, THA | Whole blood | 2 years | MoM | 5.2 | 2.8 |
| MoP | 1.6 | 0.8 | ||||
| Fritzsche, et al. [65] | bilateral MoM resurfacing followed by unilateral MoM THA (Case report) | Whole blood, aspirate of pseudotumor | 3 months after revision surgery | Blood | 138.0 | 39.0 |
| Aspirate of pseudotumor | 258.0 | 1011.0 | ||||
| Well-functioning | 2.3 | 1.6 | ||||
| Matthies, et al. [66] | MoM, THA and resurfacing | Whole blood | Median of 39 months | No pseudotumor | 2.9 | 3.2 |
| Pseudotumor | 11.0 | 6.7 | ||||
| Lass, et al. [67] | MoM, THA | Synovial fluid | minimum of 18 years | - | 113.4 * | 54.0 * |
2.2. Cobalt
3. Biological Effects
3.1. Toxicity
3.2. Immunological
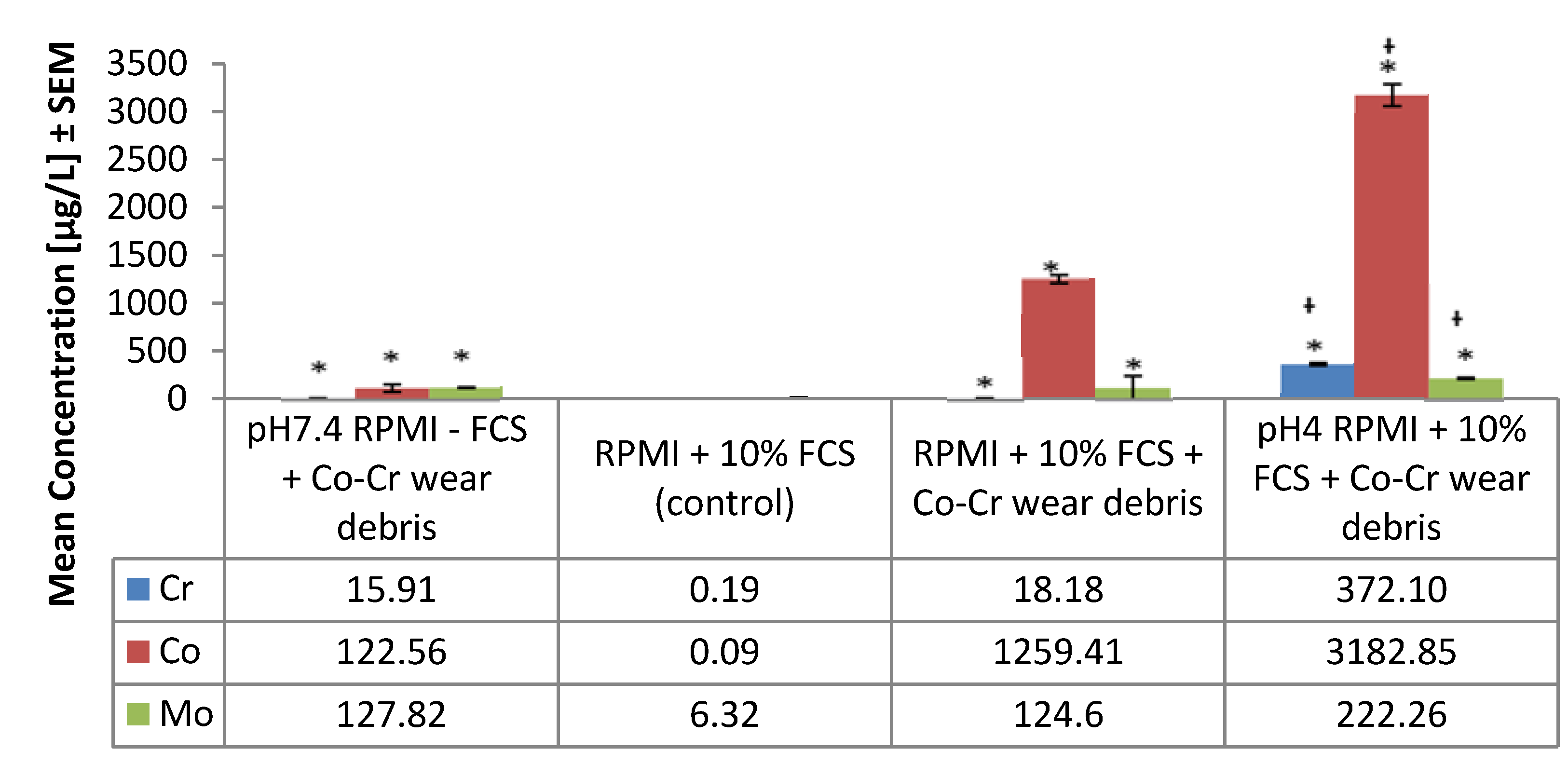
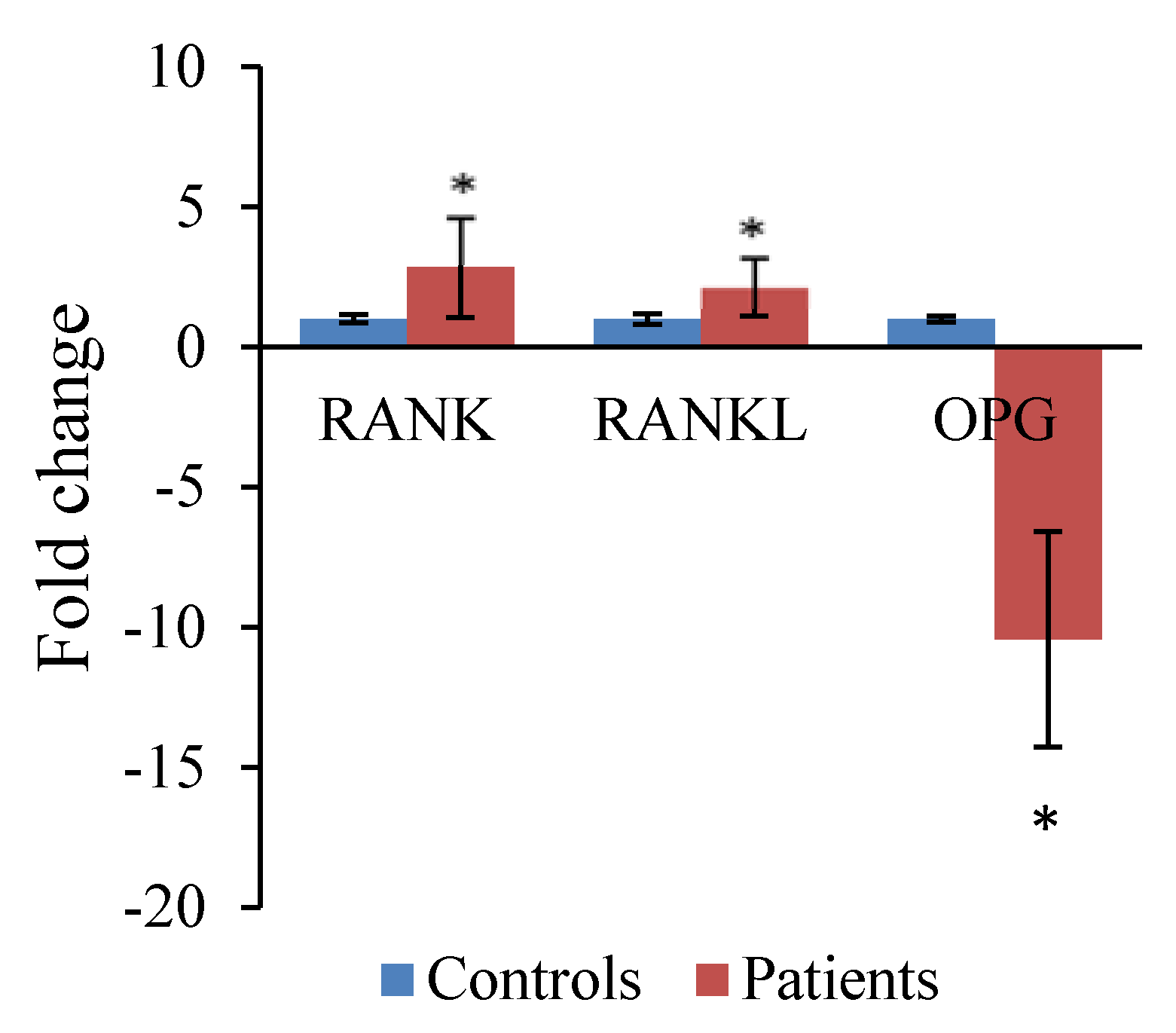
3.3. Gene Expression
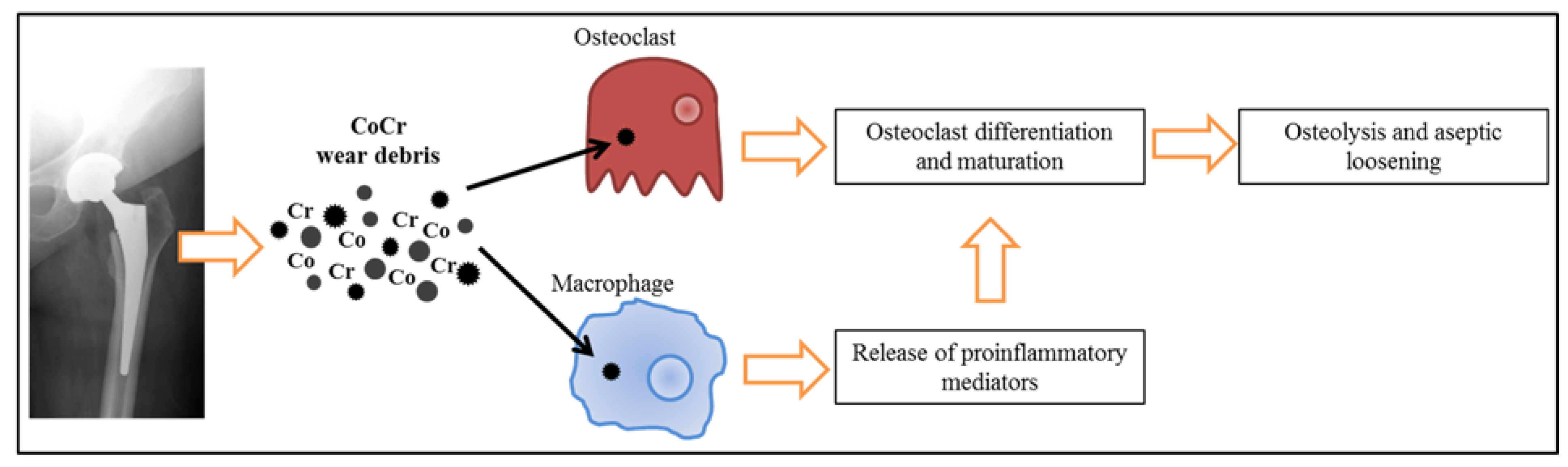
4. Discussion
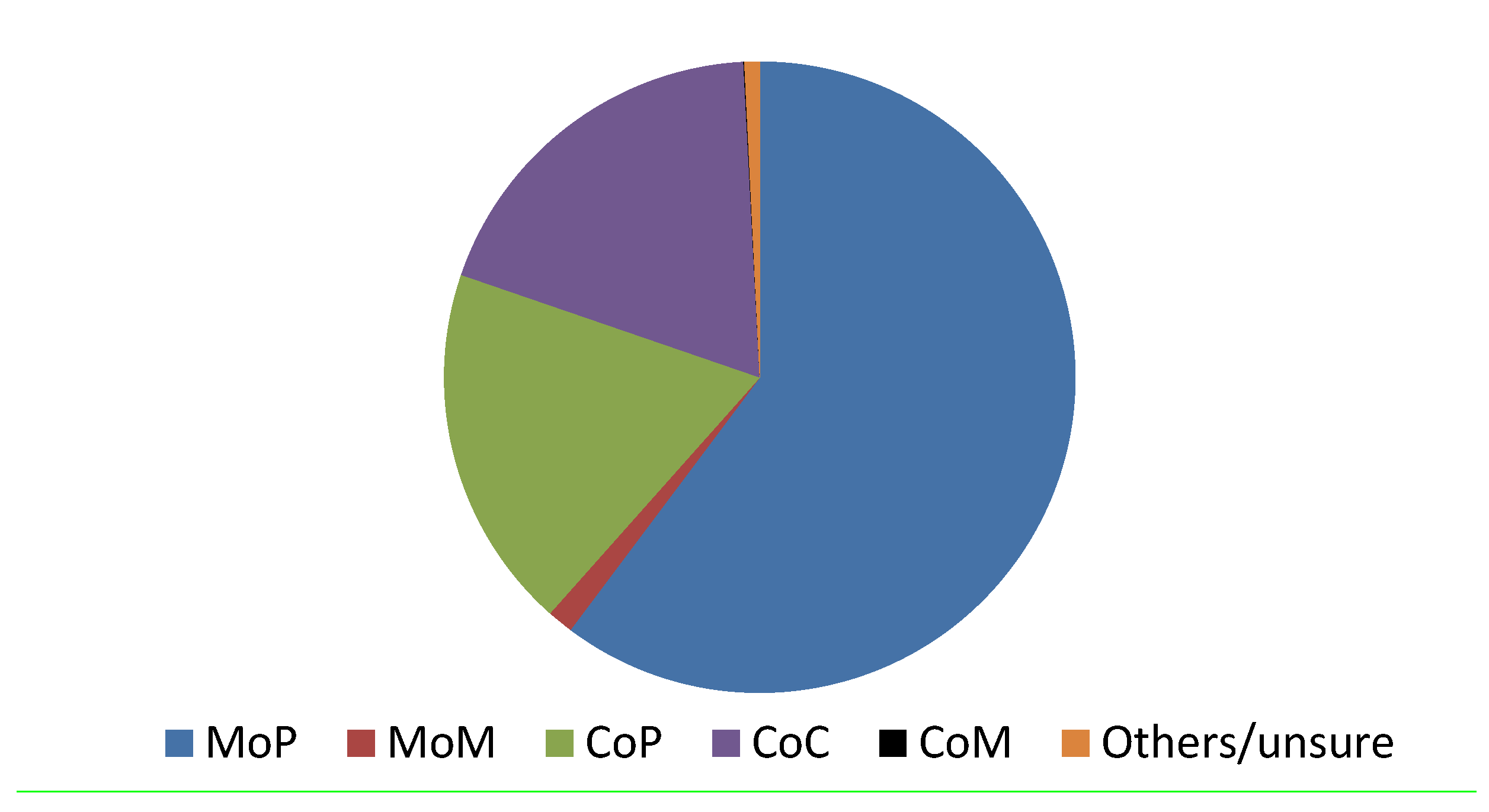
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cobelli, N.; Scharf, B.; Crisi, G.M.; Hardin, J.; Santambrogio, L. Mediators of the inflammatory response to joint replacement devices. Nat. Rev. Rheumatol. 2011, 7, 600–608. [Google Scholar]
- Australian Orthopaedic Association National Joint Replacement Registry. Annual Report 2014. Available online: https://aoanjrr.dmac.adelaide.edu.au/annual-reports-2014 (accessed on 20 January 2015).
- National Joint Registry. 11th Annual Report. Available online: http://www.njrcentre.org.uk/njrcentre/Reports,PublicationsandMinutes/Annualreports/tabid/86/Default.aspx. (accessed on 20 January 2015).
- Furnes, O.; Paxton, E.; Cafri, G.; Graves, S.; Bordini, B.; Comfort, T.; Rivas, M.C.; Banerjee, S.; Sedrakyan, A. Distributed analysis of hip implants using six national and regional registries: Comparing metal-on-metal with metal-on-highly cross-linked polyethylene bearings in cementless total hip arthroplasty in young patients. J. Bone Joint Surg. Am. 2014, 96A, 25–33. [Google Scholar]
- Kanda, A.; Kaneko, K.; Obayashi, O.; Mogami, A. A 42-year-old patient presenting with femoral head migration after hemiarthroplasty performed 22 years earlier: A case report. J. Med. Case Rep. 2015, 9, 17. [Google Scholar]
- Sedrakyan, A.; Romero, L.; Graves, S.; Davidson, D.; de Steiger, R.; Lewis, P.; Solomon, M.; Vial, R.; Lorimer, M. Survivorship of hip and knee implants in pediatric and young adult populations. J. Bone Joint Surg. Am. 2014, 96A, 73–78. [Google Scholar]
- Eikmans, M.; Rekers, N.V.; Anholts, J.D.H.; Heidt, S.; Claas, F.H.J. Blood cell mRNAs and microRNAs: Optimized protocols for extraction and preservation. Blood 2013, 121, E81–E89. [Google Scholar]
- Malchau, H.; Herberts, P.; Eisler, T.; Garellick, G.; Soderman, P. The swedish total hip replacement register. J. Bone Joint Surg. Am. 2002, 84A, 2–20. [Google Scholar]
- Prokopovich, P. Interactions between mammalian cells and nano- or micro-sized wear particles: Physico-chemical views against biological approaches. Adv. Colloid Interface Sci. 2014, 213, 36–47. [Google Scholar]
- Reito, A.; Puolakka, T.; Elo, P.; Pajamaki, J.; Eskelinen, A. High prevalence of adverse reactions to metal debris in small-headed ASR™ hips. Clin. Orthop. Relat. Res. 2013, 471, 2954–2961. [Google Scholar]
- U.S. Food and Drug Administration (FDA). Medical devices. Available online: http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/ImplantsandProsthetics/MetalonMetalHipImplants/ucm241770.htm (accessed on 20 January 2015).
- Wynn-Jones, H.; Macnair, R.; Wimhurst, J.; Chirodian, N.; Derbyshire, B.; Toms, A.; Cahir, J. Silent soft tissue pathology is common with a modern metal-on-metal hip arthroplasty. Acta Orthop. 2011, 82, 301–307. [Google Scholar]
- Langton, D.J.; Jameson, S.S.; Joyce, T.J.; Gandhi, J.N.; Sidaginamale, R.; Mereddy, P.; Lord, J.; Nargol, A.V.F. Accelerating failure rate of the asr total hip replacement. J. Bone Joint Surg. Br. 2011, 93B, 1011–1016. [Google Scholar]
- Hayter, C.L.; Gold, S.L.; Koff, M.F.; Perino, G.; Nawabi, D.H.; Miller, T.T.; Potter, H.G. MRI findings in painful metal-on-metal hip arthroplasty. Am. J. Roentgenol. 2012, 199, 884–893. [Google Scholar]
- Hart, A.J.; Hester, T.; Sinclair, K.; Powell, J.J.; Goodship, A.E.; Pele, L.; Fersht, N.L.; Skinner, J. The association between metal ions from hip resurfacing and reduced T-cell counts. J. Bone Joint Surg. Br. 2006, 88, 449–454. [Google Scholar]
- Keegan, G.M.; Learmonth, I.D.; Case, C.P. Orthopaedic metals and their potential toxicity in the arthroplasty patient—Review of current knowledge and future strategies. J. Bone Joint Surg. Br. 2007, 89B, 567–573. [Google Scholar]
- Moroni, A.; Savarino, L.; Hoque, M.; Cadossi, M.; Baldini, N. Do ion levels in hip resurfacing differ from metal-on-metal tha at midterm? Clin. Orthop. Relat. Res. 2011, 469, 180–187. [Google Scholar]
- Hallab, N.J.; Jacobs, J.J. Biologic effects of implant debris. Bull. NYU Hospital Joint Dis. 2009, 67, 182–188. [Google Scholar]
- Mathew, M.T.; Nagelli, C.; Pourzal, R.; Fischer, A.; Laurent, M.P.; Jacobs, J.J.; Wimmer, M.A. Tribolayer formation in a metal-on-metal (MoM) hip joint: An electrochemical investigation. J. Mech. Behav. Biomed. Mater. 2014, 29, 199–212. [Google Scholar]
- Clayton, R.A.E.; Beggs, I.; Salter, D.M.; Grant, M.H.; Patton, J.T.; Porter, D.E. Inflammatory pseudotumor associated with femoral nerve palsy following metal-on-metal resurfacing of the hip. J. Bone Joint Surg. Am. 2008, 90A, 1988–1993. [Google Scholar]
- Pandit, H.; Glyn-Jones, S.; McLardy-Smith, P.; Gundle, R.; Whitwell, D.; Gibbons, C.L.M.; Ostlere, S.; Athanasou, N.; Gill, H.S.; Murray, D.W. Pseudotumours associated with metal-on-metal hip resurfacings. J. Bone Joint Surg. Br. 2008, 90B, 847–851. [Google Scholar]
- Dowson, D. Tribological principles in metal-on-metal hip joint design. Proc. Inst. Mech. Eng. H J. Eng. Med. 2006, 220, 161–171. [Google Scholar]
- Yan, Y.; Neville, A.; Dowson, D. Biotribocorrosion—An appraisal of the time dependence of wear and corrosion interactions: I. The role of corrosion. J. Phys. D Appl. Phys. 2006, 39, 3200–3205. [Google Scholar]
- Konttinen, Y.T.; Pajarinen, J. Surgery adverse reactions to metal-on-metal implants. Nat. Rev. Rheumatol. 2013, 9, 5–6. [Google Scholar]
- Teoh, S.H. Fatigue of biomaterials: A review. Int. J. Fatigue 2000, 22, 825–837. [Google Scholar]
- Ryu, J.J.; Shrotriya, P. Synergistic Mechanisms of Bio-Tribocorrosion in Medical Implants. In Bio-Tribocorrosion in Biomaterials and Medical Implants; Yan, Y., Ed.; Elsevier: Sawston, Cambridge, UK, 2013; pp. 25–44. [Google Scholar]
- Hallab, N.J.; Jacobs, J.J. Orthopedic implant fretting corrosion. Corros. Rev. 2003, 21, 183–213. [Google Scholar]
- Sargeant, A.; Goswami, T. Hip implants—Paper VI—Ion concentrations. Mater. Des. 2007, 28, 155–171. [Google Scholar]
- Singh, R.; Dahotre, N.B. Corrosion degradation and prevention by surface modification of biometallic materials. J. Mater. Sci. Mater. Med. 2007, 18, 725–751. [Google Scholar]
- Cadosch, D.; Chan, E.; Gautschi, O.P.; Filgueira, L. Metal is not inert: Role of metal ions released by biocorrosion in aseptic loosening-current concepts. J. Biomed. Mater. Res. A 2009, 91A, 1252–1262. [Google Scholar]
- Doni, Z.; Alves, A.C.; Toptan, F.; Gomes, J.R.; Ramalho, A.; Buciumeanu, M.; Palaghian, L.; Silva, F.S. Dry sliding and tribocorrosion behaviour of hot pressed cocrmo biomedical alloy as compared with the cast CoCrMo and Ti6Al4V alloys. Mater. Des. 2013, 52, 47–57. [Google Scholar]
- Zeng, Y.; Feng, W. Metal allergy in patients with total hip replacement: A review. J. Int. Med. Res. 2013, 41, 247–252. [Google Scholar]
- Steinemann, S.G. Metal implants and surface reactions. Inj. Int. J. Care Inj. 1996, 27, 16–22. [Google Scholar]
- Hanawa, T. Metal ion release from metal implants. Mater. Sci. Eng. C Biomim. Supramol. Syst. 2004, 24, 745–752. [Google Scholar]
- Friesenbichler, J.; Maurer-Ertl, W.; Sadoghi, P.; Lovse, T.; Windhager, R.; Leithner, A. Serum metal ion levels after rotating-hinge knee arthroplasty: Comparison between a standard device and a megaprosthesis. Int. Orthop. 2012, 36, 539–544. [Google Scholar]
- Penny, J.O.; Varmarken, J.E.; Ovesen, O.; Nielsen, C.; Overgaard, S. Metal ion levels and lymphocyte counts: ASR hip resurfacing prosthesis vs. standard THA 2-year results from a randomized study. Acta Orthop. 2013, 84, 130–137. [Google Scholar]
- Gilbert, J.L.; Sivan, S.; Liu, Y.; Kocagoez, S.B.; Arnholt, C.M.; Kurtz, S.M. Direct in vivo inflammatory cell-induced corrosion of cocrmo alloy orthopedic implant surfaces. J. Biomed. Mater. Res. A 2015, 103, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Hallab, N.; Merritt, K.; Jacobs, J.J. Metal sensitivity in patients with orthopaedic implants. J. Bone Joint Surg. Am. 2001, 83A, 428–436. [Google Scholar]
- Yang, J.; Black, J. Competitive-binding of chromium, cobalt and nickel to serum-proteins. Biomaterials 1994, 15, 262–268. [Google Scholar]
- Sethi, R.K.; Neavyn, M.J.; Rubash, H.E.; Shanbhag, A.S. Macrophage response to cross-linked and conventional UHMWPE. Biomaterials 2003, 24, 2561–2573. [Google Scholar]
- Rajamaki, K.; Nordstrom, T.; Nurmi, K.; Akerman, K.E.O.; Kovanen, P.T.; Oorni, K.; Eklund, K.K. Extracellular acidosis is a novel danger signal alerting innate immunity via the NLRP3 inflammasome. J. Biol. Chem. 2013, 288, 13410–13419. [Google Scholar]
- Steen, K.H.; Steen, A.E.; Reeh, P.W. A dominant role of acid ph in inflammatory excitation and sensitization of nociceptors in rat skin, in-vitro. J. Neurosci. 1995, 15, 3982–3989. [Google Scholar]
- Posada, O.M.; Gilmour, D.; Tate, R.J.; Grant, M.H. CoCr wear particles generated from cocr alloy metal-on-metal hip replacements, and cobalt ions stimulate apoptosis and expression of general toxicology-related genes in monocyte-like U937 cells. Toxicol. Appl. Pharmacol. 2014, 281, 125–135. [Google Scholar]
- Geborek, P.; Saxne, T.; Pettersson, H.; Wollheim, F.A. Synovial-fluid acidosis correlates with radiological joint destruction in rheumatoid-arthritis knee joints. J. Rheumatol. 1989, 16, 468–472. [Google Scholar]
- Mansson, B.; Geborek, P.; Saxne, T.; Bjornsson, S. Cytidine deaminase activity in synovial-fluid of patients with rheumatoid-arthritis—Relation to lactoferrin, acidosis, and cartilage proteoglycan release. Ann. Rheum. Dis. 1990, 49, 594–597. [Google Scholar]
- Bagchi, D.; Stohs, S.J.; Downs, B.W.; Bagchi, M.; Preuss, H.G. Cytotoxicity and oxidative mechanisms of different forms of chromium. Toxicology 2002, 180, 5–22. [Google Scholar]
- Gray, S.J.; Sterling, K. The tagging of red cells and plasma proteins with radioactive chromium. J. Clin. Invest. 1950, 29, 1604–1613. [Google Scholar]
- Dillon, C.T.; Lay, P.A.; Bonin, A.M.; Cholewa, M.; Legge, G.J.F. Permeability, cytotoxicity, and genotoxicity of Cr(III) complexes and some Cr(V) analogues in V79 chinese hamster lung cells. Chem. Res. Toxicol. 2000, 13, 742–748. [Google Scholar]
- Biedermann, K.A.; Landolph, J.R. Role of valence state and solubility of chromium compounds on induction of cytotoxicity, mutagenesis, and anchorage independence in diploid human fibroblasts. Cancer Res. 1990, 50, 7835–7842. [Google Scholar]
- Tkaczyk, C.; Huk, O.L.; Mwale, F.; Antoniou, J.; Zukor, D.J.; Petit, A.; Tabrizian, M. The molecular structure of complexes formed by chromium or cobalt ions in simulated physiological fluids. Biomaterials 2009, 30, 460–467. [Google Scholar]
- Raja, N.S.; Sankaranarayanan, K.; Dhathathreyan, A.; Nair, B.U. Interaction of chromium(III) complexes with model lipid bilayers: Implications on cellular uptake. Biochim. Biophys. Acta 2011, 1808, 332–340. [Google Scholar]
- Fornsaglio, J.L.; O’Brien, T.J.; Patierno, S.R. Differential impact of ionic and coordinate covalent chromium (Cr)-DNA binding on DNA replication. Mol. Cell Biochem. 2005, 279, 149–155. [Google Scholar]
- Shrivastava, H.Y.; Ravikumar, T.; Shanmugasundaram, N.; Babu, M.; Nair, B.U. Cytotoxicity studies of chromium(III) complexes on human dermal fibroblasts. Free Radic. Biol. Med. 2005, 38, 58–69. [Google Scholar]
- Afolaranmi, G.A.; Tettey, J.; Meek, R.M.D.; Grant, M.H. Release of chromium from orthopaedic arthroplasties. Open Orthop. J. 2008, 2, 10–18. [Google Scholar]
- Daniel, J.; Ziaee, H.; Pradhan, C.; Pynsent, P.B.; McMinn, D.J.W. Blood and urine metal ion levels in young and active patients after Birmingham hip resurfacing arthroplasty—Four-year results of a prospective longitudinal study. J. Bone Joint Surg. Br. 2007, 89B, 169–173. [Google Scholar]
- Ziaee, H.; Daniel, J.; Datta, A.K.; Blunt, S.; McMinn, D.J.W. Transplacental transfer of cobalt and chromium in patients with metal-on-metal hip arthroplasty—A controlled study. J. Bone Joint Surg. Br. 2007, 89B, 301–305. [Google Scholar]
- Antoniou, J.; Zukor, D.J.; Mwale, F.; Minarik, W.; Petit, A.; Huk, O.L. Metal ion levels in the blood of patients after hip resurfacing: A comparison between twenty-eight and thirty-six-millimeter-head metal-on-metal prostheses. J. Bone Joint Surg. Am. 2008, 90A, 142–148. [Google Scholar]
- Wretenberg, P. Good function but very high concentrations of cobalt and chromium ions in blood 37 years after metal-on-metal total hip arthroplasy. Med. Devices (Auckland, N.Z.) 2008, 1, 31–32. [Google Scholar]
- Hart, A.J.; Sabah, S.; Henckel, J.; Lewis, A.; Cobb, J.; Sampson, B.; Mitchell, A.; Skinner, J.A. The painful metal-on-metal hip resurfacing. J. Bone Joint Surg. Br. 2009, 91B, 738–744. [Google Scholar]
- Hart, A.J.; Quinn, P.D.; Sampson, B.; Sandison, A.; Atkinson, K.D.; Skinner, J.A.; Powell, J.J.; Mosselmans, J.F.W. The chemical form of metallic debris in tissues surrounding metal-on-metal hips with unexplained failure. Acta Biomater. 2010, 6, 4439–4446. [Google Scholar]
- Langton, D.J.; Jameson, S.S.; Joyce, T.J.; Hallab, N.J.; Natu, S.; Nargol, A.V.F. Early failure of metal-on-metal bearings in hip resurfacing and large-diameter total hip replacement a consequence of excess wear. J. Bone Joint Surg. Br. 2010, 92B, 38–46. [Google Scholar]
- Davda, K.; Lali, F.V.; Sampson, B.; Skinner, J.A.; Hart, A.J. An analysis of metal ion levels in the joint fluid of symptomatic patients with metal-onmetal hip replacements. J. Bone Joint Surg. Br. 2011, 93B, 738–745. [Google Scholar]
- Hart, A.J.; Sabah, S.A.; Bandi, A.S.; Maggiore, P.; Tarassoli, P.; Sampson, B.; Skinner, J.A. Sensitivity and specificity of blood cobalt and chromium metal ions for predicting failure of metal-on-metal hip replacement. J. Bone Joint Surg. Br. 2011, 93B, 1308–1313. [Google Scholar]
- Malviya, A.; Ramaskandhan, J.R.; Bowman, R.; Kometa, S.; Hashmi, M.; Lingard, E.; Holland, J.P. What advantage is there to be gained using large modular metal-on-metal bearings in routine primary hip replacement? A preliminary report of a prospective randomised controlled trial. J. Bone Joint Surg. Br. 2011, 93B, 1602–1609. [Google Scholar]
- Fritzsche, J.; Borisch, C.; Schaefer, C. Case report: High chromium and cobalt levels in a pregnant patient with bilateral metal-on-metal hip arthroplasties. Clin. Orthop. Relat. Res. 2012, 470, 2325–2331. [Google Scholar]
- Matthies, A.K.; Skinner, J.A.; Osmani, H.; Henckel, J.; Hart, A.J. Pseudotumors are common in well-positioned low-wearing metal-on-metal hips. Clin. Orthop. Relat. Res. 2012, 470, 1895–1906. [Google Scholar]
- Lass, R.; Grubl, A.; Kolb, A.; Stelzeneder, D.; Pilger, A.; Kubista, B.; Giurea, A.; Windhager, R. Comparison of synovial fluid, urine, and serum ion levels in metal-on-metal total hip arthroplasty at a minimum follow-up of 18 years. J. Orthop. Res. 2014, 32, 1234–1240. [Google Scholar]
- Valko, M.; Morris, H.; Cronin, M.T.D. Metals, toxicity and oxidative stress. Curr. Med. Chem. 2005, 12, 1161–1208. [Google Scholar]
- De Flora, S. Threshold mechanisms and site specificity in chromium(VI) carcinogenesis. Carcinogenesis 2000, 21, 533–541. [Google Scholar]
- Merritt, K.; Brown, S.A. Release of hexavalent chromium from corrosion of stainless-steel and cobalt-chromium alloys. J. Biomed. Mater. Res. 1995, 29, 627–633. [Google Scholar]
- MacDonald, S.J. Can a safe level for metal ions in patients with metal-on-metal total hip arthroplasties be determined? J. Arthroplast. 2004, 19, 71–77. [Google Scholar]
- Nickens, K.P.; Patierno, S.R.; Ceryak, S. Chromium genotoxicity: A double-edged sword. Chem. Biol. Interact. 2010, 188, 276–288. [Google Scholar]
- Codd, R.; Dillon, C.T.; Levina, A.; Lay, P.A. Studies on the genotoxicity of chromium: From the test tube to the cell. Coord. Chem. Rev. 2001, 216, 537–582. [Google Scholar]
- Shettlemore, M.G.; Bundy, K.J. Examination of in vivo influences on bioluminescent microbial assessment of corrosion product toxicity. Biomaterials 2001, 22, 2215–2228. [Google Scholar]
- De Smet, K.; de Haan, R.; Calistri, A.; Campbell, P.A.; Ebramzadeh, E.; Pattyn, C.; Gill, H.S. Metal ion measurement as a diagnostic tool to identify problems with metal-on-metal hip resurfacing. J. Bone Joint Surg. Am. 2008, 90A, 202–208. [Google Scholar]
- Catalani, S.; Rizzetti, M.C.; Padovani, A.; Apostoli, P. Neurotoxicity of cobalt. Hum. Exp. Toxicol. 2012, 31, 421–437. [Google Scholar]
- Kravenskaya, Y.V.; Fedirko, N.V. Mechanisms underlying interaction of zinc, lead, and cobalt with nonspecific permeability pores in the mitochondrial membranes. Neurophysiology 2011, 43, 163–172. [Google Scholar]
- Bleackley, M.R.; MacGillivray, R.T.A. Transition metal homeostasis: From yeast to human disease. Biometals 2011, 24, 785–809. [Google Scholar]
- Virginio, C.; Church, D.; North, R.A.; Surprenant, A. Effects of divalent cations, protons and calmidazolium at the rat P2X7 receptor. Neuropharmacology 1997, 36, 1285–1294. [Google Scholar]
- Park, J.D.; Cherrington, N.J.; Klaassen, C.D. Intestinal absorption of cadmium is associated with divalent metal transporter 1 in rats. Toxicol. Sci. 2002, 68, 288–294. [Google Scholar]
- Griffin, K.P.; Ward, D.T.; Liu, W.; Stewart, G.; Morris, I.D.; Smith, C.P. Differential expression of divalent metal transporter DMT1 (Slc11a2) in the spermatogenic epithelium of the developing and adult rat testis. Am. J. Physiol. Cell Physiol. 2005, 288, C176–C184. [Google Scholar]
- Forbes, J.R.; Gros, P. Iron, manganese, and cobalt transport by Nramp1 (Slc11a1) and Nramp2 (Slc11a2) expressed at the plasma membrane. Blood 2003, 102, 1884–1892. [Google Scholar]
- De Boeck, M.; Kirsch-Volders, M.; Lison, D. Cobalt and antimony: Genotoxicity and carcinogenicity. Mutat. Res. 2003, 533, 135–152. [Google Scholar]
- Catelas, I.; Wimmer, M.A. New insights into wear and biological effects of metal-on-metal bearings. J. Bone Joint Surg. Am. 2011, 93A, 76–83. [Google Scholar]
- Lucarelli, M.; Gatti, A.M.; Savarino, G.; Quattroni, P.; Martinelli, L.; Monari, E.; Boraschi, D. Innate defence functions of macrophages can be biased by nano-sized ceramic and metallic particles. Eur. Cytokine Netw. 2004, 15, 339–346. [Google Scholar]
- Papageorgiou, I.; Brown, C.; Schins, R.; Singh, S.; Newson, R.; Davis, S.; Fisher, J.; Ingham, E.; Case, C.P. The effect of nano- and micron-sized particles of cobalt-chromium alloy on human fibroblasts in vitro. Biomaterials 2007, 28, 2946–2958. [Google Scholar]
- Dalal, A.; Pawar, V.; McAllister, K.; Weaver, C.; Hallab, N.J. Orthopedic implant cobalt-alloy particles produce greater toxicity and inflammatory cytokines than titanium alloy and zirconium alloy-based particles in vitro, in human osteoblasts, fibroblasts, and macrophages. J. Biomed. Mater. Res. A 2012, 100A, 2147–2158. [Google Scholar]
- Posada, O.M.; Tate, R.J.; Grant, M.H. Effects of CoCr metal wear debris generated from metal-on-metal hip implants and co ions on human monocyte-like U937 cells. Toxicol. Vitro 2014, 29, 271–280. [Google Scholar]
- Simonsen, L.O.; Harbak, H.; Bennekou, P. Cobalt metabolism and toxicology-A brief update. Sci. Total Environ. 2012, 432, 210–215. [Google Scholar]
- VanOs, R.; Lildhar, L.L.; Lehoux, E.A.; Beaule, P.E.; Catelas, I. In vitro macrophage response to nanometer-size chromium oxide particles. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 149–159. [Google Scholar]
- Tsaousi, A.; Jones, E.; Case, C.P. The in vitro genotoxicity of orthopaedic ceramic (Al2O3) and metal (CoCr alloy) particles. Mutat. Res. 2010, 697, 1–9. [Google Scholar]
- Araya, J.; Maruyama, M.; Inoue, A.; Fujita, T.; Kawahara, J.; Sassa, K.; Hayashi, R.; Kawagishi, Y.; Yamashita, N.; Sugiyama, E.; et al. Inhibition of proteasome activity is involved in cobalt-induced apoptosis of human alveolar macrophages. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002, 283, L849–L858. [Google Scholar]
- Zou, W.G.; Yan, M.D.; Xu, W.J.; Huo, H.R.; Sun, L.Y.; Zheng, Z.C.; Liu, X.Y. Cobalt chloride induces PC12 cells apoptosis through reactive oxygen species and accompanied by AP-1 activation. J. Neurosci. Res. 2001, 64, 646–653. [Google Scholar]
- Akbar, M.; Brewer, J.M.; Grant, M.H. Effect of chromium and cobalt ions on primary human lymphocytes in vitro. J. Immunotoxicol. 2011, 8, 140–149. [Google Scholar]
- Catelas, I.; Petit, A.; Vali, H.; Fragiskatos, C.; Meilleur, R.; Zukor, D.J.; Antoniou, J.; Huk, O.L. Quantitative analysis of macrophage apoptosis vs. Necrosis induced by cobalt and chromium ions in vitro. Biomaterials 2005, 26, 2441–2453. [Google Scholar]
- Catelas, I.; Petit, A.; Zukor, D.J.; Huk, O.L. Cytotoxic and apoptotic effects of cobalt and chromium ions on J774 macrophages—Implication of caspase-3 in the apoptotic pathway. J. Mater. Sci. Mater. Med. 2001, 12, 949–953. [Google Scholar]
- Catelas, I.; Petit, A.; Zukor, D.J.; Antoniou, J.; Huk, O.L. TNF-alpha secretion and macrophage mortality induced by cobalt and chromium ions in vitro—Qualitative analysis of apoptosis. Biomaterials 2003, 24, 383–391. [Google Scholar]
- Caicedo, M.; Jacobs, J.J.; Reddy, A.; Hallab, N.J. Analysis of metal ion-induced DNA damage, apoptosis, and necrosis in human (Jurkat) T-cells demonstrates Ni2+, and V3+ are more toxic than other metals: Al3+, Be2+, Co2+, Cr3+, Cu2+, Fe3+, Mo5+, Nb5+, Zr2+. J. Biomed. Mater. Res. A 2008, 86A, 905–913. [Google Scholar]
- Huber, M.; Reinisch, G.; Zenz, P.; Zweymueller, K.; Lintner, F. Postmortem study of femoral osteolysis associated with metal-on-metal articulation in total hip replacement an analysis of nine cases. J. Bone Joint Surg. Am. 2010, 92A, 1720–1731. [Google Scholar]
- Langton, D.J.; Joyce, T.J.; Jameson, S.S.; Lord, J.; Van Orsouw, M.; Holland, J.P.; Nargol, A.V.F.; de Smet, K.A. Adverse reaction to metal debris following hip resurfacing the influence of component type, orientation and volumetric wear. J. Bone Joint Surg. Br. 2011, 93B, 164–171. [Google Scholar]
- Case, C.P.; Langkamer, V.G.; James, C.; Palmer, M.R.; Kemp, A.J.; Heap, P.F.; Solomon, L. Widespread dissemination of metal debris from implants. J. Bone Joint Surg. Br. 1994, 76, 701–712. [Google Scholar]
- Tower, S.S. Arthroprosthetic cobaltism: Neurological and cardiac manifestations in two patients with metal-on-metal arthroplasty a case report. J. Bone Joint Surg. Am. 2010, 92, 2847–2851. [Google Scholar]
- Tower, S.S. Metal on metal hip implants arthroprosthetic cobaltism associated with metal on metal hip implants. Br. Med. J. 2012, 344, e430. [Google Scholar]
- Oldenburg, M.; Wegner, R.; Baur, X. Severe cobalt intoxication due to prosthesis wear in repeated total hip arthroplasty. J. Arthroplast. 2009, 24, 825.e815–825.e820. [Google Scholar]
- Steens, W.; von Foerster, G.; Katzer, A. Severe cobalt poisoning with loss of sight after ceramic-metal pairing in a hip—A case report. Acta Orthop. 2006, 77, 830–832. [Google Scholar]
- Ikeda, T.; Takahashi, K.; Kabata, T.; Sakagoshi, D.; Tomita, K.; Yamada, M. Polyneuropathy caused by cobalt-chromium metallosis after total hip replacement. Muscle Nerve 2010, 42, 140–143. [Google Scholar]
- Machado, C.; Appelbe, A.; Wood, R. Arthroprosthetic cobaltism and cardiomyopathy. Heart Lung Circ. 2012, 21, 759–760. [Google Scholar]
- Pelclova, D.; Sklensky, M.; Janicek, P.; Lach, K. Severe cobalt intoxication following hip replacement revision: Clinical features and outcome. Clin. Toxicol. 2012, 50, 262–265. [Google Scholar]
- Rizzetti, M.C.; Liberini, P.; Zarattini, G.; Catalani, S.; Pazzaglia, U.; Apostoli, P.; Padovani, A. Loss of sight and sound. Could it be the hip? Lancet 2009, 373, 1052. [Google Scholar]
- Devlin, J.J.; Pomerleau, A.C.; Brent, J.; Morgan, B.W.; Deitchman, S.; Schwartz, M. Clinical features, testing, and management of patients with suspected prosthetic hip-associated cobalt toxicity: A systematic review of cases. J. Med. Toxicol. 2013, 9, 405–415. [Google Scholar]
- Bradberry, S.M.; Wilkinson, J.M.; Ferner, R.E. Systemic toxicity related to metal hip prostheses. Clin. Toxicol. 2014, 52, 837–847. [Google Scholar]
- Clark, M.J.; Prentice, J.R.; Hoggard, N.; Paley, M.N.; Hadjivassiliou, M.; Wilkinson, J.M. Brain structure and function in patients after metal-on-metal hip resurfacing. Am. J. Neuroradiol. 2014, 35, 1753–1758. [Google Scholar]
- Makela, K.T.; Visuri, T.; Pulkkinen, P.; Eskelinen, A.; Remes, V.; Virolainen, P.; Junnila, M.; Pukkala, E. Risk of cancer with metal-on-metal hip replacements: Population based study. Br. Med. J. 2012, 345, e4646. [Google Scholar]
- Christian, W.V.; Oliver, L.D.; Paustenbach, D.J.; Kreider, M.L.; Finley, B.L. Toxicology-based cancer causation analysis of cocr-containing hip implants: A quantitative assessment of genotoxicity and tumorigenicity studies. J. Appl. Toxicol. 2014, 34, 939–967. [Google Scholar]
- Frigerio, E.; Pigatto, P.D.; Guzzi, G.; Altomare, G. Metal sensitivity in patients with orthopaedic implants: A prospective study. Contact Dermat. 2011, 64, 273–279. [Google Scholar]
- Romesburg, J.W.; Wasserman, P.L.; Schoppe, C.H. Metallosis and metal-induced synovitis following total knee arthroplasty: Review of radiographic and CT findings. J. Radiol. Case Rep. 2010, 4, 7–17. [Google Scholar]
- Desrochers, J.; Amrein, M.W.; Matyas, J.R. Microscale surface friction of articular cartilage in early osteoarthritis. J. Mech. Behav. Biomed. Mater. 2013, 25, 11–22. [Google Scholar]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar]
- Kaufman, A.M.; Alabre, C.I.; Rubash, H.E.; Shanbhag, A.S. Human macrophage response to uhmwpe, tialv, cocr, and alumina particles: Analysis of multiple cytokines using protein arrays. J. Biomed. Mater. Res. A 2008, 84A, 464–474. [Google Scholar]
- Cachinho, S.C.P.; Pu, F.R.; Hunt, J.A. Cytokine secretion from human peripheral blood mononuclear cells cultured in vitro with metal particles. J. Biomed. Mater. Res. A 2013, 101A, 1201–1209. [Google Scholar]
- Devitt, B.M.; Queally, J.M.; Vioreanu, M.; Butler, J.S.; Murray, D.; Doran, P.P.; OʼByrne, J.M. Cobalt ions induce chemokine secretion in a variety of systemic cell lines. Acta Orthop. 2010, 81, 756–764. [Google Scholar]
- Landgraeber, S.; Jaeger, M.; Jacobs, J.J.; Hallab, N.J. The pathology of orthopedic implant failure is mediated by innate immune system cytokines. Mediat. Inflamm. 2014, 2014, 185150. [Google Scholar]
- Valladares, R.D.; Nich, C.; Zwingenberger, S.; Li, C.; Swank, K.R.; Gibon, E.; Rao, A.J.; Yao, Z.; Goodman, S.B. Toll-like receptors-2 and 4 are overexpressed in an experimental model of particle-induced osteolysis. J. Biomed. Mater. Res. A 2014, 102, 3004–3011. [Google Scholar]
- Tyson-Capper, A.J.; Lawrence, H.; Holland, J.P.; Deehan, D.J.; Kirby, J.A. Metal-on-metal hips: Cobalt can induce an endotoxin-like response. Ann. Rheum. Dis. 2013, 72, 460–461. [Google Scholar]
- Potnis, P.A.; Dutta, D.K.; Wood, S.C. Toll-like receptor 4 signaling pathway mediates proinflammatory immune response to cobalt-alloy particles. Cell. Immunol. 2013, 282, 53–65. [Google Scholar]
- Werling, D.; Jungi, T.W. Toll-like receptors linking innate and adaptive immune response. Vet. Immunol. Immunopathol. 2003, 91, 1–12. [Google Scholar]
- Piccinini, A.M.; Midwood, K.S. Dampening inflammation by modulating TLR signalling. Mediat. Inflamm. 2010, 2010, 1. [Google Scholar]
- Minang, J.T.; Arestrom, I.; Troye-Blomberg, M.; Lundeberg, L.; Ahlborg, N. Nickel, cobalt, chromium, palladium and gold induce a mixed Th1- and Th2-type cytokine response in vitro in subjects with contact allergy to the respective metals. Clin. Exp. Immunol. 2006, 146, 417–426. [Google Scholar]
- Hegewald, J.; Uter, W.; Pfahlberg, A.; Geier, J.; Schnuch, A.; IVDK. A multifactorial analysis of concurrent patch-test reactions to nickel, cobalt, and chromate. Allergy 2005, 60, 372–378. [Google Scholar]
- Fors, R.; Stenberg, B.; Stenlund, H.; Persson, M. Nickel allergy in relation to piercing and orthodontic appliances—A population study. Contact Dermatitis 2012, 67, 342–350. [Google Scholar]
- Willert, H.G.; Buchhorn, G.H.; Fayyazi, A.; Flury, R.; Windler, M.; Koster, G.; Lohmann, C.H. Metal-on-metal bearings and hypersensitivity in patients with artificial hip joints—A clinical and histomorphological study. J. Bone Joint Surg. Am. 2005, 87A, 28–36. [Google Scholar]
- Boardman, D.R.; Middleton, F.R.; Kavanagh, T.G. A benign psoas mass following metal-on-metal resurfacing of the hip. J. Bone Joint Surg. Br. 2006, 88B, 402–404. [Google Scholar]
- Moulon, C.; Vollmer, J.; Weltzien, H.U. Characterization of processing requirements acid metal cross-reactivities in T cell clones from patients with allergic contact dermatitis to nickel. Eur. J. Immunol. 1995, 25, 3308–3315. [Google Scholar]
- Villanueva, M.B.G.; Koizumi, S.; Jonai, H. Cytokine production by human peripheral blood mononuclear cells after exposure to heavy metals. J. Health Sci. 2000, 46, 358–362. [Google Scholar]
- Jiranek, W.A.; Machado, M.; Jasty, M.; Jevsevar, D.; Wolfe, H.J.; Goldring, S.R.; Goldberg, M.J.; Harris, W.H. Production of cytokines around loosened cemented acetabular components—Analysis with immunohistochemical techniques and in-situ hybridization. J. Bone Joint Surg. Am. 1993, 75A, 863–879. [Google Scholar]
- Goodman, S.B.; Huie, P.; Song, Y.; Schurman, D.; Maloney, W.; Woolson, S.; Sibley, R. Cellular profile and cytokine production at prosthetic interfaces—Study of tissues retrieved from revised hip and knee replacements. J. Bone Joint Surg. Br. 1998, 80B, 531–539. [Google Scholar]
- Voronov, I.; Santerre, J.P.; Hinek, A.; Callahan, J.W.; Sandhu, J.; Boynton, E.L. Macrophage phagocytosis of polyethylene particulate in vitro. J. Biomed. Mater. Res. 1998, 39, 40–51. [Google Scholar]
- Bainbridge, J.A.; Revell, P.A.; Al-Saffar, N. Costimulatory molecule expression following exposure to orthopaedic implants wear debris. J. Biomed. Mater. Res. 2001, 54, 328–334. [Google Scholar]
- Bohler, M.; Kanz, F.; Schwarz, B.; Steffan, I.; Walter, A.; Plenk, H.; Knahr, K. Adverse tissue reactions to wear particles form co-alloy articulations, increased by alumina-blasting particle contamination from cementless Ti-based total hip implants—A report of seven revisions with early failure. J. Bone Joint Surg. Br. 2002, 84B, 128–136. [Google Scholar]
- Campbell, P.; Ebramzadeh, E.; Nelson, S.; Takamura, K.; de Smet, K.; Amstutz, H.C. Histological features of pseudotumor-like tissues from metal-on-metal hips. Clin. Orthop. Relat. Res. 2010, 468, 2321–2327. [Google Scholar]
- Davies, A.P.; Willert, H.G.; Campbell, P.A.; Learmonth, I.D.; Case, C.P. An unusual lymphocytic perivascular infiltration in tissues around contemporary metal-on-metal joint replacements. J. Bone Joint Surg. Am. 2005, 87A, 18–27. [Google Scholar]
- Korovessis, P.; Petsinis, G.; Repanti, M.; Repantis, T. Metallosis after contemporary metal-on-metal total hip arthroplasty—Five to nine-year follow-up. J. Bone Joint Surg. Am. 2006, 88A, 1183–1191. [Google Scholar]
- Polyzois, I.; Nikolopoulos, D.; Michos, I.; Patsouris, E.; Theocharis, S. Local and systemic toxicity of nanoscale debris particles in total hip arthroplasty. J. Appl. Toxicol. 2012, 32, 255–269. [Google Scholar]
- Thomas, P.; Thomas, M.; Summer, B.; Dietrich, K.; Zauzig, M.; Steinhauser, E.; Krenn, V.; Arnholdt, H.; Flaig, M.J. Impaired wound-healing, local eczema, and chronic inflammation following titanium osteosynthesis in a nickel and cobalt-allergic patient: A case report and review of the literature. J. Bone Joint Surg. Am. 2011, 93, e61. [Google Scholar]
- Gao, X.; He, R.-X.; Yan, S.-G.; Wu, L.-D. Dermatitis associated with chromium following total knee arthroplasty. J. Arthroplast. 2011, 26. [Google Scholar] [CrossRef]
- Kwon, Y.M.; Thomas, P.; Summer, B.; Pandit, H.; Taylor, A.; Beard, D.; Murray, D.W.; Gill, H.S. Lymphocyte proliferation responses in patients with pseudotumors following metal-on-metal hip resurfacing arthroplasty. J. Orthop. Res. 2010, 28, 444–450. [Google Scholar]
- Masui, T.; Sakano, S.; Hasegawa, Y.; Warashina, H.; Ishiguro, N. Expression of inflammatory cytokines, rankl and opg induced by titanium, cobalt-chromium and polyethylene particles. Biomaterials 2005, 26, 1695–1702. [Google Scholar]
- Kanaji, A.; Caicedo, M.S.; Virdi, A.S.; Sumner, D.R.; Hallab, N.J.; Sena, K. Co-Cr-Mo alloy particles induce tumor necrosis factor alpha production in MLO-Y4 osteocytes: A role for osteocytes in particle-induced inflammation. Bone 2009, 45, 528–533. [Google Scholar]
- Jakobsen, S.S.; Larsen, A.; Stoltenberg, M.; Bruun, J.M.; Soballe, K. Effects of as-cast and wrought cobalt-chrome-molybdenum and titanium-aluminium-vanadium alloys on cytokine gene expression and protein secretion in J774a.1 macrophages. Eur. Cells Mater. 2007, 14, 45–54. [Google Scholar]
- Garrigues, G.E.; Cho, D.R.; Rubash, H.E.; Goldring, S.R.; Herndon, J.H.; Shanbhag, A.S. Gene expression clustering using self-organizing maps: Analysis of the macrophage response to particulate biomaterials. Biomaterials 2005, 26, 2933–2945. [Google Scholar]
- Takayama, S.; Sato, T.; Krajewski, S.; Kochel, K.; Irie, S.; Millan, J.A.; Reed, J.C. Cloning and functional-analysis of BAG-1—A novel Bcl-2-binding protein with anti-cell death activity. Cell 1995, 80, 279–284. [Google Scholar]
- Terada, S.; Komatsu, T.; Fujita, T.; Terakawa, A.; Nagamune, T.; Takayama, S.; Reed, J.C.; Suzuki, E. Co-expression of Bcl-2 and BAG-1, apoptosis suppressing genes, prolonged viable culture period of hybridoma and enhanced antibody production. Cytotechnology 1999, 31, 143–151. [Google Scholar]
- Vermes, C.; Chandrasekaran, R.; Jacobs, J.J.; Galante, J.O.; Roebuck, K.A.; Glant, T.T. The effects of particulate wear debris, cytokines, and growth factors on the functions of MG-63 osteoblasts. J. Bone Joint Surg. Am. 2001, 83A, 201–211. [Google Scholar]
- Fujiyoshi, K.; Hunt, J.A. The effect of particulate material on the regulation of chemokine receptor expression in leukocytes. Biomaterials 2006, 27, 3888–3896. [Google Scholar]
- Petit, A.; Mwale, F.; Tkaczyk, C.; Antoniou, J.; Zukor, D.J.; Huk, O.L. Induction of protein oxidation by cobalt and chromium ions in human U937 macrophages. Biomaterials 2005, 26, 4416–4422. [Google Scholar]
- Petit, A.; Mwale, F.; Tkaczyk, C.; Antoniou, J.; Zukor, D.J.; Huk, O.L. Cobalt and chromium ions induce nitration of proteins in human U937 macrophages in vitro. J. Biomed. Mater. Res. A 2006, 79A, 599–605. [Google Scholar]
- Tkaczyk, C.; Huk, O.L.; Mwale, F.; Antoniou, J.; Zukor, D.J.; Petit, A.; Tabrizian, M. Effect of chromium and cobalt ions on the expression of antioxidant enzymes in human U937 macrophage-like cells. J. Biomed. Mater. Res. A 2010, 94A, 419–425. [Google Scholar]
- Rothfuss, A.; Speit, G. Overexpression of heme oxygenase-1 (HO-1) in V79 cells results in increased resistance to hyperbaric oxygen (HBO)-induced DNA damage. Environ. Mol. Mutagen. 2002, 40, 258–265. [Google Scholar]
- Hallab, N.J.; Vermes, C.; Messina, C.; Roebuck, K.A.; Glant, T.T.; Jacobs, J.J. Concentration- and composition-dependent effects of metal ions on human MG-63 osteoblasts. J. Biomed. Mater. Res. 2002, 60, 420–433. [Google Scholar]
- Queally, J.M.; Devitt, B.M.; Butler, J.S.; Malizia, A.P.; Murray, D.; Doran, P.P.; OʼByrne, J.M. Cobalt ions induce chemokine secretion in primary human osteoblasts. J. Orthop. Res. 2009, 27, 855–864. [Google Scholar]
- Luo, L.; Petit, A.; Antoniou, J.; Zukor, D.J.; Huk, O.L.; Liu, R.C.W.; Winnik, F.M.; Mwale, F. Effect of cobalt and chromium ions on MMP-1 TIMP-1, and TNF-alpha gene expression in human U937 macrophages: A role for tyrosine kinases. Biomaterials 2005, 26, 5587–5593. [Google Scholar]
- Takagi, M. Neutral proteinases and their inhibitors in the loosening of total hip prostheses. Acta Orthop. Scand. 1996, 67, 1–29. [Google Scholar]
- Crotti, T.N.; Smith, M.D.; Findlay, D.M.; Zreiqat, H.; Ahern, M.J.; Weedon, H.; Hatzinikolous, G.; Capone, M.; Holding, C.; Haynes, D.R. Factors regulating osteoclast formation in human tissues adjacent to peri-implant bone loss: Expression of receptor activator NF kappaB, RANK ligand and osteoprotegerin. Biomaterials 2004, 25, 565–573. [Google Scholar]
- Haynes, D.R.; Crotti, T.N.; Loric, M.; Bain, G.I.; Atkins, G.J.; Findlay, D.M. Osteoprotegerin and receptor activator of nuclear factor kappaB ligand (RANKL) regulate osteoclast formation by cells in the human rheumatoid arthritic joint. Rheumatology 2001, 40, 623–630. [Google Scholar]
- Holding, C.A.; Findlay, D.M.; Stamenkov, R.; Neale, S.D.; Lucas, H.; Dharmapatni, A.; Callary, S.A.; Shrestha, K.R.; Atkins, G.J.; Howie, D.W.; et al. The correlation of RANK, RANKL and TNF alpha expression with bone loss volume and polyethylene wear debris around hip implants. Biomaterials 2006, 27, 5212–5219. [Google Scholar]
- Mao, X.; Pan, X.Y.; Zhao, S.; Peng, X.C.; Cheng, T.; Zhang, X.L. Protection against titanium particle-induced inflammatory osteolysis by the proteasome inhibitor bortezomib in vivo. Inflammation 2012, 35, 1378–1391. [Google Scholar]
- Chen, D.S.; Zhang, X.L.; Guo, Y.Y.; Shi, S.F.; Mao, X.; Pan, X.Y.; Cheng, T. MMP-9 inhibition suppresses wear debris-induced inflammatory osteolysis through downregulation of RANK/RANKL in a murine osteolysis model. Int. J. Mol. Med. 2012, 30, 1417–1423. [Google Scholar]
- Jiang, Y.; Jia, T.; Gong, W.; Wooley, P.H.; Yang, S.-Y. Effects of Ti, PMMA, UHMWPE, and Co-Cr wear particles on differentiation and functions of bone marrow stromal cells. J. Biomed. Mater. Res. A 2013, 101, 2817–2825. [Google Scholar]
- Pioletti, D.P.; Kottelat, A. The influence of wear particles in the expression of osteoclastogenesis factors by osteoblasts. Biomaterials 2004, 25, 5803–5808. [Google Scholar]
- Zijlstra, W.P.; Bulstra, S.K.; van Raay, J.; van Leeuwen, B.M.; Kuijer, R. Cobalt and chromium ions reduce human osteoblast-like cell activity in vitro, reduce the OPG to RANKL ratio, and induce oxidative stress. J. Orthop. Res. 2012, 30, 740–747. [Google Scholar]
- Perez-Sayans, M.; Manuel Somoza-Marin, J.; Barros-Angueira, F.; Gandara Rey, J.M.; Garcia-Garcia, A. RANK/RANKL/OPG role in distraction osteogenesis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2010, 109, 679–686. [Google Scholar]
- Medicines and Healthcare Products Regulatory agency (MHRA). Medical device alert: All metal-on-metal (MoM) hip replacements (MDA/2012/036). Available online: http://www.mhra.gov.uk/Publications/Safetywarnings/MedicalDeviceAlerts/CON155761. (accessed on 20 January 2015).
- Sampson, B.; Hart, A. Clinical usefulness of blood metal measurements to assess the failure of metal-on-metal hip implants. Ann. Clin. Biochem. 2012, 49, 118–131. [Google Scholar]
- Anderson, J.M. In vitro and in vivo monocyte, macrophage, foreign body giant cell, and lymphocyte interactions with biomaterials. In Biological Interactions on Material Surfaces; Springer US: New York, NY, USA, 2009; pp. 225–244. [Google Scholar]
- Zhang, K.; Kaufman, R.J. From endoplasmic-reticulum stress to the inflammatory response. Nature 2008, 454, 455–462. [Google Scholar]
- Hansson, G.K.; Libby, P. The immune response in atherosclerosis: A double-edged sword. Nat. Rev. Immunol. 2006, 6, 508–519. [Google Scholar]
- Tsai, Y.-Y.; Huang, Y.-H.; Chao, Y.-L.; Hu, K.-Y.; Chin, L.-T.; Chou, S.-H.; Hour, A.-L.; Yao, Y.-D.; Tu, C.-S.; Liang, Y.-J.; et al. Identification of the nanogold particle-induced endoplasmic reticulum stress by omic techniques and systems biology analysis. ACS Nano 2011, 5, 9354–9369. [Google Scholar]
- Zhang, R.; Piao, M.J.; Kim, K.C.; Kim, A.D.; Choi, J.-Y.; Choi, J.; Hyun, J.W. Endoplasmic reticulum stress signaling is involved in silver nanoparticles-induced apoptosis. Int. J. Biochem. Cell Biol. 2012, 44, 224–232. [Google Scholar]
- Martinez-Zamudio, R.; Ha, H.C. Environmental epigenetics in metal exposure. Epigenetics 2011, 6, 820–827. [Google Scholar]
- Salnikow, K.; Zhitkovich, A. Genetic and epigenetic mechanisms in metal carcinogenesis and cocarcinogenesis: Nickel, arsenic, and chromium. Chem. Res. Toxicol. 2008, 21, 28–44. [Google Scholar]
- Stoccoro, A.; Karlsson, H.L.; Coppede, F.; Migliore, L. Epigenetic effects of nano-sized materials. Toxicology 2013, 313, 3–14. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Posada, O.M.; Tate, R.J.; Meek, R.M.D.; Grant, M.H. In Vitro Analyses of the Toxicity, Immunological, and Gene Expression Effects of Cobalt-Chromium Alloy Wear Debris and Co Ions Derived from Metal-on-Metal Hip Implants. Lubricants 2015, 3, 539-568. https://doi.org/10.3390/lubricants3030539
Posada OM, Tate RJ, Meek RMD, Grant MH. In Vitro Analyses of the Toxicity, Immunological, and Gene Expression Effects of Cobalt-Chromium Alloy Wear Debris and Co Ions Derived from Metal-on-Metal Hip Implants. Lubricants. 2015; 3(3):539-568. https://doi.org/10.3390/lubricants3030539
Chicago/Turabian StylePosada, Olga M., Rothwelle J. Tate, R.M. Dominic Meek, and M. Helen Grant. 2015. "In Vitro Analyses of the Toxicity, Immunological, and Gene Expression Effects of Cobalt-Chromium Alloy Wear Debris and Co Ions Derived from Metal-on-Metal Hip Implants" Lubricants 3, no. 3: 539-568. https://doi.org/10.3390/lubricants3030539
APA StylePosada, O. M., Tate, R. J., Meek, R. M. D., & Grant, M. H. (2015). In Vitro Analyses of the Toxicity, Immunological, and Gene Expression Effects of Cobalt-Chromium Alloy Wear Debris and Co Ions Derived from Metal-on-Metal Hip Implants. Lubricants, 3(3), 539-568. https://doi.org/10.3390/lubricants3030539





