Abstract
The physicochemical processes that occur during selective transfer in the contact area of a bronze/steel friction pair lubricated with glycerin are experimentally studied by the polarization method to observe how they influence the tribological properties (friction and wear) of the pair. The proposed method allows for the study of tribochemical transformations of glycerin and the friction pair materials during the work process with selective transfer. The analysis of the experimental results allows for the establishment of the conditions for a stable and stationary selective transfer during the operation of the bronze/steel pair, by friction, at which the friction coefficient (COF) values and wear are low. This was achieved by implementing continuous lubrication with fresh glycerin in the contact area, choosing the optimal flow rate, and maintaining an optimal ratio between glycerin and the chemical transformation products, within well-established limits, to avoid undesirable consequences. Acrolein, as a product of chemical transformation (resulting from the catalytic dehydration of glycerin), is the most important for the initiation and stability of the selective transfer, and as the main reaction product, also represents a pathway of regeneration. Thus, it was found that the friction relative moments and the acrolein concentration presented conclusive/specific results at loads of 4–15 MPa and a sliding speed of 0.3 m/s. The optimum lubricant entry speed is 15–30 mg/min, for a minimum COF and reduced wear (about 0.028–0.03 at relatively high operating temperatures (45 and 60 °C)), and at low temperatures (30 °C) the minimum COF is about 0.038, but the lubricant inlet entry speed increases considerably, by around 1000 mg/min. Therefore, this paper aims to demonstrate the possibility of moving to another stage of practical use of a friction pair (with greatly improved tribological properties) that operates with selective transfer, much different from the ones still present, using a lubricant with special properties (glycerin). The research method used (polarization) highlights the physicochemical properties, tribochemical transformations of the lubricant, and the friction pair materials present in the contact area, for the understanding, maintenance, and stability of selective transfer, based on experiments, as a novelty compared to other studies.
1. Introduction
A new stage for obtaining experimental results related to the functional performance of friction pairs regarding contact pressure, sliding speed, lubrication requirements and conditions, friction and wear behavior, etc., is constituted by tribosystems, which operate by selective transfer, to implement them in the design and execution of machine elements [1,2]. The selective transfer assumes material transfer from one component element of the friction pair to another in the presence of a lubricant, forming a layer–tribolayer–superficial anti-friction effect of the order of a few microns, which behaves very well in friction and wear. This process/phenomenon is possible, as a result of the trend to use lubricants in friction pairs that accelerate oxidation processes once they are heated, which leads to their mechanical destruction, with a catalytic and electrochemical effect on the friction surfaces.
Experimental tests have shown that in this friction, the beginning stage is useful to quickly realize thermodynamically unstable processes in the lubricant and on the friction pair’s contact surfaces. At the same time, to unfold physiochemical processes favorable to friction in the operating conditions, such as polymerization, the formation of metal–organic compounds, colloids, and tensio-active substances at the contact surfaces have the effect of transporting metal particles in the contact area and the friction surface’s area, which inevitably leads to a reduction in friction and wear.
Thus, friction under such conditions is called selective transfer and is used where the friction of mixed and adhesion layers is not safe enough or the duration of use of the friction pairs is not ensured [2,3,4].
The conditions for a selective transfer are characterized by the complexity of physicochemical processes in the contact areas, as well as by factors acting on the processes, such as the thermodynamic instability of the lubricant, pressure, sliding speed, temperature, collisions between the asperities of the friction surfaces, and tribodestruction. Tribodestruction of a lubricant (i.e., the catalytic effect of oxide layers and the material on the lubricant) leads to the beginning of friction under selective transfer conditions, both to solve the lubricant oxidation problem and to reduce the friction and wear of the material [2,3].
Research on the physicochemical mechanism of tribodestruction of a lubricant and electrochemical reactions that occur in friction pairs during selective transfer led to the conclusion that the reduction in friction and wear is a result of the action of self-regulating phenomena of equilibrium processes disturbed in the friction process and the occurrence of wear [2,3,4]. Thus, in practice, those lubricants that have a self-regulating capacity should be used, as they work not only under the conditions of selective transfer, but also under the conditions of friction in the adhesion and mixed layers, such as polymerization in the contact areas. For this reason, selective transfer represents a complex process that consists of the following: reducing the real pressure in the contact area, compensation for deformation and reducing shear resistance in the superficial areas, the return of particles removed from the friction surface areas back to the contact area, and the formation of a polymerized protective layer.
The use of such friction pairs (tribosystems) implies using those pairs of materials and lubricants that will surely reduce friction and wear, thereby extending the working life of machines, installations, and equipment [5]. To highlight the utility and novelty of selective transfer, a brief analysis will be presented in order to see how the use of tribosystems/friction pairs, which operate with selective transfer, was achieved. Thus, initially, the main direction of action in the fight against wear and friction reduction was to increase the hardness of the friction surfaces [6,7], proving to be a great help in increasing the reliability, service life, and wear resistance of the friction pairs. In addition, conventional, as well as non-conventional materials, manufacturing, and lubrication technologies, etc., should be used. There are also other methods of obtaining and depositing layers by coating friction surfaces, so-called traditional methods (thermal spray deposition, thermal spraying with gases, electric arc, plasma, high-velocity thermal evaporation, sputtering of materials, pulsed magnetron, chemical and physical vapor deposition, etc.). However, traditional methods are insufficient to cope with increasing demands, high speeds, and deteriorating lubrication conditions.
Thus, thermal spray is a continuous melt-spray process in which particles (up to 50 μm in diameter) of virtually any material are melted and accelerated to high velocities, through either a combustion flame or non-transferred thermal-plasma arc [7]. Different thermal spray variants for coatings exist, but the current state-of-the-art route is usually atmospheric plasma spraying (for ceramic coatings) and spraying by high-velocity oxy-fuel methods (for metallic and intermetallic coatings) [8]. In addition, through computer simulation, Xu et al. [9] show the use of thermal spray for polymer coatings without the need for prolonged drying. Also, sputtering of materials is an effective technique for producing ultrathin films with varied compositions, structures, and properties for diverse applications [10]. Then, Ramade et al. [11] present the thin-layer deposition technique by thermal evaporation on a paper substrate, and Koskinen [12] applies this technique to polymeric coatings.
The physical vapor deposition method used in the nanostructures’ synthesis, and described by Hamid et al. [13], and the variant, the technique of high-power pulsed magnetron sputtering, make possible the deposition of dense and smooth coatings on complex-shaped substrates by the generation of ultra-dense plasmas with unique properties, applied by Sarakinos et al. [14]. Laser ablation has a variety of applications, including the generation of hot plasma and thin film deposition on solid substrates appropriately positioned in vacuum or air [15]. Finally, chemical vapor deposition is a powerful technology, still used for producing high-quality solid thin films and coatings, as shown by Sun et al. [16].
Another reason, if reference is made to the increase in the hardness of the friction surfaces, is that the effective contact surfaces are small, due to the high hardness of the asperities, and thus, in the contact areas, the pressures are very high, leading to intensive wear [17,18,19].
Unlike these traditional methods, many of which are still used, but with improved variants to meet current requirements, the global trend in machine construction is to replace classic friction pairs with new types to reduce the consumption of expensive and deficient/scarce materials [2,20]. In addition, there has been a need to obtain good-quality experimental results with a high functional (tribological) performance for friction pairs (tribosystems) widely used in machine construction. This is the case for friction pairs made of classical (conventional) materials, and especially unconventional ones, made of alloys or pseudo-alloys in thin layers, deposited by spraying (metallization), or electrochemical deposition. It turns out that these manufacturing technologies require specific equipment, extended production time, additional production costs, etc. As a result, it was necessary to find possibilities and techniques to reduce manufacturing time, production cost, increase the reliability of the friction pairs, improve lubrication conditions, etc. [21]. One way/possibility to solve this problem is to use friction pairs that operate under selective transfer conditions, having tribological performances comparable to those of fluid friction.
In this case, a thin layer of copper is applied to a hard steel surface, on which a thin layer of polymer is present, as a result of operating in contact with a bronze surface, which also has a thin layer of copper, thus allowing the realization of low-wear friction pairs [22,23,24]. Thus, selective transfer is also a contact interaction by friction, characterized by special molecular exchange interactions.
This occurs as a result of various physicochemical reactions, which lead to an independent decrease in friction and wear; the formation of a thin metallic film is typical, in which a typical mechanism of movement of defects (dislocations) by diffusion occurs [25]. The film appears in the initial phase, through the selective dissolution of the anodic components in the surface layers of the metal or alloy, upon the appearance of the selective transfer from the lubricant that determines the metallic coating. It is a protective polymer film, which originates from tribodestruction products and the hydrocarbons of the lubricant in the contact area through the chemisorption of these products to the anodic components of the alloys of the respective pair and favors the occurrence of selective transfer [2,3,26,27]. Additionally, it is specified that tribodestruction products have a relatively high thermal stability, contributing to the natural selection of thermodynamically unstable compounds, which, after an oxidation, lead to more stable substances.
Compared to friction under selective transfer conditions, dry or mixed friction results in thermal energy dissipation, which ultimately leads to destruction as a result of structural changes and fatigue. The thin oxide layer and the lubricant layer adsorbed by it, as well as the mixture of moisture and oxygen, do not protect the superficial layer from deformations, hardening, and destruction. The cause of these deficiencies is the lack of regeneration in the event of mechanical damage in the areas of the friction surfaces, but also the lack of equilibrium in the processes leading to destruction. These deficiencies, which occur in the friction of adhesion layers and dry friction, are not only a consequence of the thermodynamic instability of the lubricant, but also a consequence of the instability of the materials (metals), except for some noble metals, especially during the friction process, respectively, glass.
A lubricant that achieves metallic coatings is the one that determines selective transfer in the friction pairs made of materials that allow this phenomenon to occur favorably. An example is the pair bronze/steel lubricated with glycerin, etc., used in this paper.
In the base material, there are also surface-active elements, or they are formed by the decomposition of additives. Ionic lubrication is based on the discharge of metal ions that arise through electrochemical processes in the friction area. As a result, a so-called “servowitt film” appears, i.e., a life-saving film, after the principle of friction pairs from living nature [2,3].
Servowitt film is a polymerized protective layer/film, porous and “life-saving”, formed as a result of the flow of energy that arises in the friction process and ensures ionic lubrication (lubrication based on the discharge of metal ions, due to the electrochemical processes in the friction area).
In search of new ways to increase wear resistance, scientists have indicated orientation after examples from nature, for the adaptation and application of examples from nature in physical–mechanical systems. In this regard, it is necessary to research how friction pairs in living nature work, especially those that are intensely stressed. Thus, the working mode of friction pairs with selective transfer is similar to that of animal joints. In this case, a thin layer of copper is applied/formed to the hard steel surface, on which a thin layer of polymer is present, due to the operation in contact with a bronze surface, which also has a thin layer of copper, allowing the creation of friction pairs with reduced wear. Thus, through selective transfer, wear can be reduced, and the COF can be of the order of magnitude of fluid friction. The causes that lead to wear and COFs with low values are as follows:
- -
- Lowering the real contact pressure by leveling the microscopic irregularities and forming a metallic film;
- -
- Compensating for the deformations and decreasing the movement resistance in the friction areas by reducing the number of accidents in the surface layers;
- -
- Reflux of wear particles or metal ions in the contact area and the formation of the film through the development of electrokinetic potentials in dispersed media.
Through the presence of an electrically charged double layer, they cause electrophoretic movement of the particles in the field of direct contact and a displacement of the ions corresponding to the direction of the field. Copper layer formation and polymeric film development at the interface, as a result of the electrostatic discharge of wear particles and metal ions, which are birthed through electrochemical processes in the friction/contact area, and by the development of electrokinetic potentials in the lubricating medium. The wear particles and metal ions are mostly made of copper and have an electric charge, which keeps them in the interstitium, and under the action of the electric field are able to move in the direction of the field. Recent results presented by Galembeck et al. [28] show that triboelectricity in polymers is triggered by mechanochemical and wear or mass selective transfer phenomena. Experimental results obtained by the same authors [28] support the following hypothesis: charge-bearing particles are ionic polymer fragments formed through the mechanical action of charge exchange at the liquid–solid interface. Tribochemical reactions, which are involved in friction and wear processes, are crucial to solving the tribological problems of the bronze/steel friction pair (tribosystem).
The origin of chemical reactions in the mechanical contact of this pair depends on the reaction areas: inside the contact area, near, or in the vicinity of the contact area (especially in the gap of the beginning of movement by friction and penetration of the lubricant). Each reaction area has a significant role depending on the tribosystem, the friction conditions, and the time.
The reaction areas can operate simultaneously or consecutively to cause chemical reactions, leading to effective or poor lubrication of the tribosystem. Thus, inside the contact area, the chemisorbed lubricant molecules are suddenly pressurized, sheared under high-shear stresses, and possibly heated up by the combined effect of the normal and tangential loads to cause chemical reactions combined with acid–base reactions [29] and can occur together with the wear process in the contact area.
In the gap just outside of the contact area, extremely intense electric fields are generated due to the charging in the friction process and surface potential. By these intense electric fields, discharging of the lubricant (causing tribochemical reactions in the vicinity of the contact) takes place to generate an enormous number of electrons, and positive ions are generated through the electron avalanche process, due to the collisions of high-energy electrons accelerated during the charging in the friction process.
Outside of the contact area, no dynamic physical process occurs to cause substantial tribochemical reactions except the traditional static reactions [29], generally declining with time. Burlakova et al. [30] have determined the influence of the nature of the lubricating medium on the qualitative composition of wear products during long-term frictional interaction of a brass–steel friction pair in model media, which can also work with selective transfer.
Copper nanoparticles in the form of a tribo-film deposited on the steel surface are formed in the lubricant composition. Quantum chemical calculations demonstrated that carboxylic acids form complexes with copper ions and clusters. The concentration of iron ions decreases during the transition from formic to hexanoic acid, from the series of monobasic carboxylic acids. Additionally, the authors of Ref. [30] show that with an increase in the time of frictional interaction of the friction pair (tribosystem), the particles of wear products agglomerate in the lubricating medium.
Preventing metal oxidation, by forming a solid adsorption layer of active substances at the surface, ensures the plasticity and deformation stability of the protective polymer film, reduces contact pressure, and creates additional sliding areas with low displacement resistance [24].
Therefore, for the study of these physicochemical processes that take place in the contact area of the friction pairs and that operate with selective transfer, polarization is used, applicable after some experiments on the mode of influence on friction and wear, by changing the lubricant entry speed (here glycerin).
Hence, at a well-determined amount of chemical transformation products in the lubricant/glycerin, conditions are created for a stable and stationary selective transfer during the operation of the bronze/steel pair, by friction.
The stability of selective transfer is ensured by regulating the rate of entry of the fresh glycerin into the contact area to achieve an optimal ratio between glycerin and the chemical transformation products, especially acrolein, in well-established limits. Acrolein, as a product of the catalytic dehydration of glycerin, is important for the initiation and stability of the selective transfer, as the main reaction product, and a regeneration pathway. Therefore, this study aims to implement continuous lubrication with glycerin based on the polarization method and the analysis of experimental results. The goal is to avoid unwanted consequences, choosing the optimal glycerin flow rate, to obtain stable conditions and a stationary state of the selective transfer, at which the COF values and wear are low.
2. Materials and Methods
Experiments were carried out with the help of polarization to combination/association by friction of a pair from copper alloy (bronze) with steel lubricated with glycerin, for research by friction under the conditions of a selective transfer of the processes of mutual action and by the entry method of the lubricant into the contact areas. With the help of this method, the quantitative and qualitative transformations of the lubricant (here glycerin) can be studied very easily, as well as the determination/establishment of the properties of the selective solution, respectively and the damage to materials due to friction, even after frictional stress.
This combination of materials was thus chosen because the elementary processes of the selective transfer phenomenon can be explained through the special properties of glycerin [2,3,26,31,32].
This is possible when, due to the variations in the contact conditions, a series of bonds are formed in the glycerin, which change the lubricant’s operating properties and implicitly influence the selective transfer. These bonds formed in the glycerin are related to oxidation and other chemical transformations of the glycerin, as a result of the increase in temperature and of the catalytic activity of the metals during frictional association [33,34]. In the case of the friction pair of bronze/steel lubricated with glycerin, the bronze friction surface is dissolved in the first period of operation when the glycerin acts in the friction process as a weak acid. Then, the atoms of the component elements of the bronze (Sn, Al, Zn, Fe, etc.) are transferred in glycerin, and the bronze’s friction surface will be enriched with the copper atoms [27,31,35].
After this, the bronze surface, in the friction process, provokes a diffusion flow by deformation [36] of new atoms (Sn, Al, Zn, Fe, etc.) of the bronze towards the surface, and thus they end up in the glycerin, and the bronze surface becomes predominantly rich in copper. Thus, a large number of vacancies are created in the bronze layer from the surface; some of them join together to form pores, in which glycerin molecules will enter.
Because glycerin restores copper oxides, and the copper layer’s friction surface is released by the oxide films, it becomes active and able to adhere to the layer from the steel surface because it has free valences. As a result, a thin layer of copper gradually covers the steel surface. The copper layer formed at the bronze surface thins out, as a result of its transfer to the steel surface, and the process is repeated/resumes. This process takes place until a copper layer is formed on the bronze and steel surfaces in contact, with an optimal thickness of 1–3 μm on average. After the copper film covers the bronze and steel surfaces in contact with relative motion, the glycerin molecules no longer react with the bronze, but they attract the atoms of the bronze component elements; the process of copper enrichment of the bronze surface stops, and thus the selective transfer begins to unfold [2,3].
In the case of a reduced amount of lubricant material, copper particles (micelles) can form in it, which are surrounded by a dense ring of lubricant molecules. The particles have an electric charge, which keeps them in the interstitium, and under the action of the electric field, they move into the cracks of the surfaces. As a result of the formation of the servowitt film between the anode and cathode areas on the bronze surface, the process of copper enrichment of the bronze surface can stop completely, and thus, the friction regime starts to become stable. If, for some reason, the copper layer is destroyed, then the process will resume, accompanied by some catalytic transformations, and the bronze surface will become enriched in copper until it no longer passes into the passive state.
It is mentioned that chemical transformations of glycerin come from glycerin’s thermodynamic instability and the material, due to the catalytic effect of the oxide layers and the material on glycerin. As a result of oxidation, a series of products are derived from glycerin (aldehyde, glyceric acid, acrolein, formic acid aldehyde, etc.). This was possible by mass spectroscopy and chemical analysis [37,38].
As shown above, due to the variation in the contact conditions, a series of bonds are formed in the glycerin, which change the operating properties of the glycerin and at the same time influence the selective transfer (stability of the selective transfer over time). One such bond (but not the only one) is the aldehyde of acrylic acid, called acrolein, whose concentration in glycerin changes for the bronze/steel pair. Acrolein is produced by the catalytic dehydration of glycerin; it is important as the main reaction product, and represents a regeneration pathway [39].
The tests were carried out on a test machine that works on the pin-disc tribometer principle with a steel disc (OLC 45 (AISI/SAE 1045)) and a pin made of a copper alloy (bronze CuSn12T (UNS-C90800)) with a sliding speed of 0.3 m/s. It is mentioned that the disc has a diameter of 100 mm, and the pin diameter is 10 mm, without having the certainty that the contact surface is 100%. Tests were carried out on this testing machine to determine the moments and coefficients of friction and the concentration of products derived from the friction process.
Thus, the experiments for determining the friction moments and the acrolein concentration were performed at loads of 4, 5.5, and 15 MPa (represented by the contact pressure), for 6 h, and for each load, with a minimum of 5 trials. In the case of COFs, the experiments were performed on an installation that has the possibility of changing the temperature at a speed of 0.33 m/s and a pressure of 10.5 MPa, under conditions of a continuous fresh lubricant and temperatures of 30, 45, and 60 °C. And in this case, the number of trials was a minimum of 5 trials for each temperature at 5 lubricant entry speeds (30, 60, 100, 700, and 1500 mg/min). In total, the number of attempts is on the order of hundreds. Additionally, the method of controlling the lubricant flow rate is by the quantity of lubricant used, expressed in mg, to operate the friction pair with selective transfer for one minute, that is, by the lubricant inlet speed in mg/min.
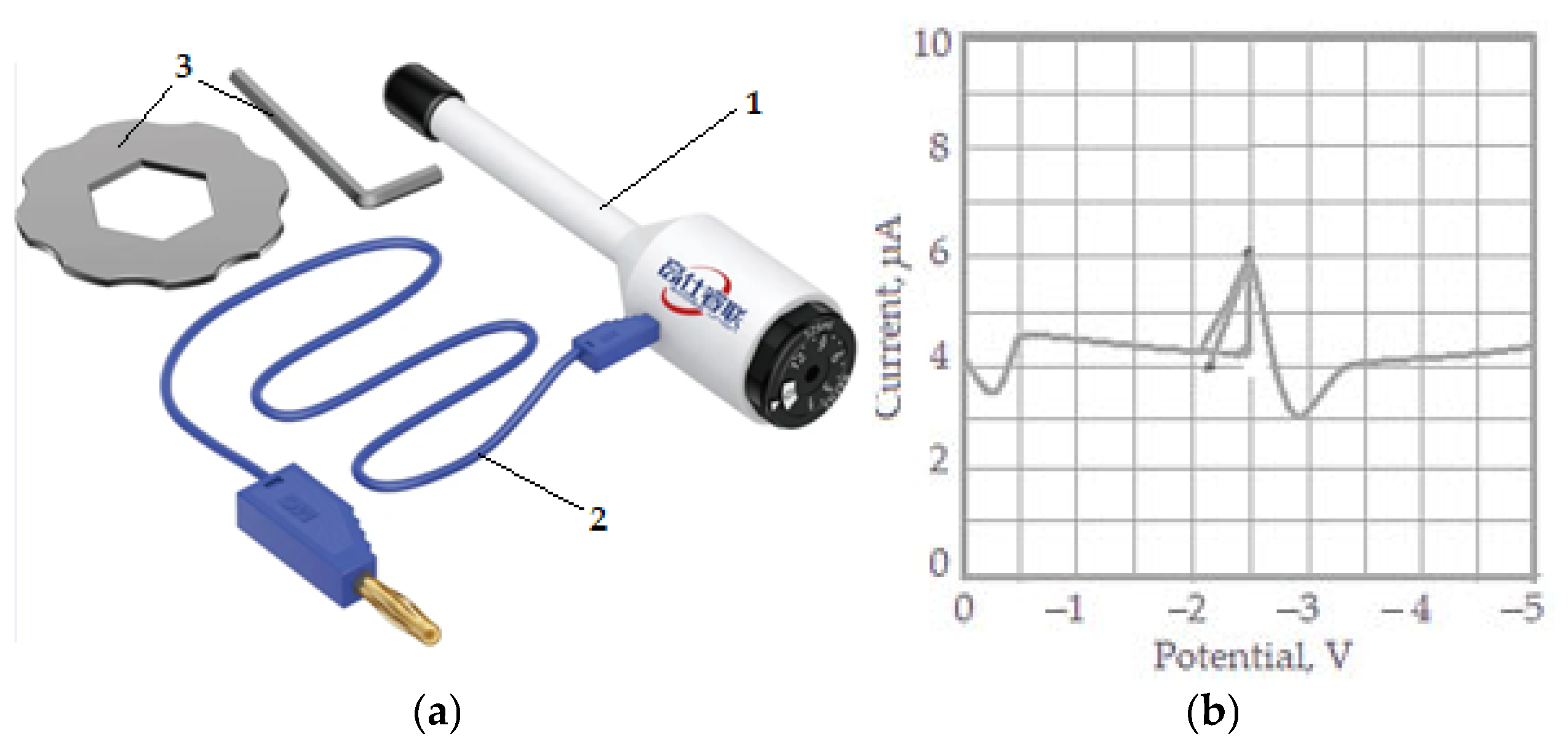
The measurements were performed in continuous current using a mercury drop electrode as presented in Figure 1a. This is an electrochemical analytical tool for the qualitative and quantitative analysis of the content of different ions, atoms, and molecules in an electrolyte solution based on the calibration curve (current–voltage) obtained when the substance is electrolyzed with controlled potential. The test result is a polarography curve or polar-gram (Figure 1b). The half-wave potential of each substance on the polar plot is the basis for qualitative analysis, while the wave height represents the limiting diffusion current for quantitative analysis.
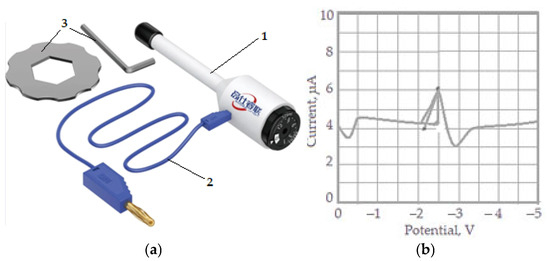
Figure 1.
Mercury drop electrode and accessory (a): 1—mercury drop electrode (work electrode); 2—power supply cable with electrical current; 3—accessory; polarography curve (b).
The drain period was 1 to 3 s, and the recording was performed in a time of 0.7 s. A silver chloride electrode was used as a control electrode. The concentration of the researched material (mixture of glycerin, zinc, and copper) was determined with the help of a calibration curve (see Figure 1b).
The content of zinc and copper in the lubricant (glycerin) was determined after the test in a closed circuit of the lubricant. This procedure makes it possible to determine the content of wear particles in the lubricant.
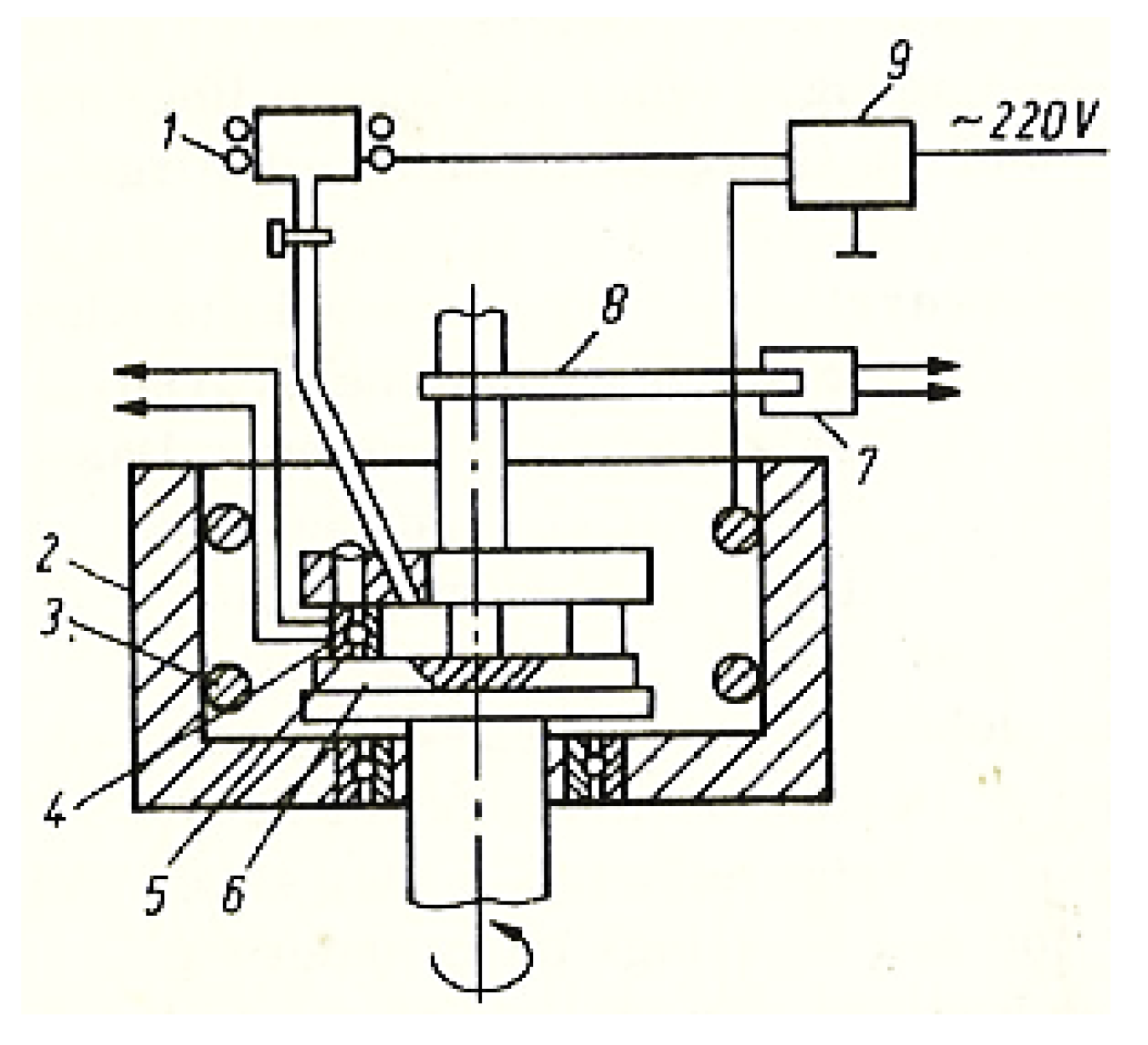
In this case, no losses of materials were reported, as happens with the use of liquid lubricants. The absolute measurement was determined experimentally with the help of a calibrating solution. To examine the influence of the lubricant’s entry speed in the friction contact area, the installation in Figure 2 was used with the possibility of changing the temperature at a speed of 0.33 m/s and a pressure of 10.5 MPa, under the conditions of continuous assurance with fresh lubricant (glycerin).
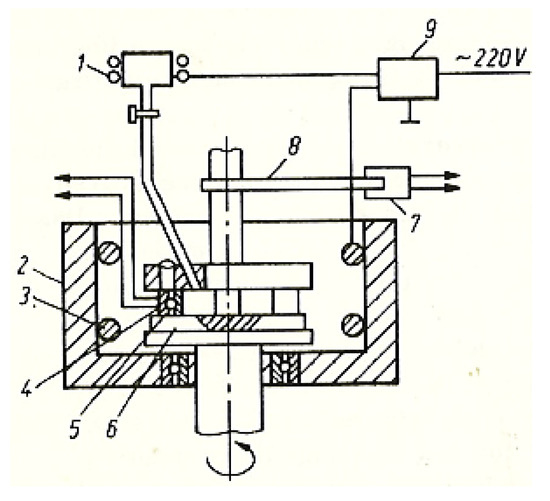
Figure 2.
Block diagram of the installation for examining the lubricant at a constant temperature: 1—tank with lubricant (glycerin); 2—bathtub; 3—heating element; 4—temperature sensor; 5—upper sample, immovable; 6—lower rotating disc; 7—dynamometric measuring head; 8—rod; 9—potentiometer.
A lubricant tank was used to investigate the possibility of continuous glycerin entry into the contact area of the friction pair, allowing comparative research on the evolution of the COF (implicit and wear) under the conditions of a selective transfer.
Also, to confirm tribopolymer presence or chemical bonding states and to understand various phenomena that occur during the selective transfer process, including surface reactions, material properties, and the effectiveness of surface layers, a JXA-5A electronic microprobe, JEOL Ltd., Tokyo, Japan, was used. The electronic microprobe utilizes surface-sensitive techniques that focus on the outermost layers of a material, providing detailed information on the chemical composition, electronic structure, and surface morphology.
Then, a scanning electron transmission microscopy (STEM) type Quantax EDS for TEM, Bruker, Kharlsruhe, Germany, was used to obtain images that show evidence of an ultra-nanocrystalline thin layer from the contact area of the friction pair. To obtain an image with useful information about the failure/wear/delamination observed after the friction and wear of the steel disc in contact with the bronze pin, in the presence of glycerin, a thermographic camera was used, utilizing infrared radiation (IR), type RT630 Expert Thermal Camera, Raythink, Yantai, China. Additionally, to highlight the wear, an analytical balance was needed (ACA620, 620 g/0.001 g, TELECOMED SRL, Valea Adanca, Iasi, Romania), alongside a high-precision digital micrometer (0–25/0.0002 mm IP65 INSIZE Czech s.r.o, Ivančice, Cehia).
3. Results and Discussion
For the stability of the selective transfer over time, when choosing glycerin, one must consider not only the output properties but also those of the changes occurring in the process of stress during frictional operation. This condition is directly related to the problem of stability to carry out a selective transfer in time [40,41]. Such a bond is constituted by the aldehyde of acrylic acid called acrolein [42].
The operating properties of glycerin are changed from the very beginning of friction due to the mechanical–chemical processes on the friction surfaces between bronze and steel lubricated with glycerin. As a result of oxidation, acrolein is formed, together with a series of products derived from glycerin (aldehyde, glyceric acid, formic acid aldehyde, etc.), but through triboactivation, they can also directly polymerize the hydrocarbons, also being sources of free radicals. These were shown/demonstrated following a chemical analysis and mass spectroscopy [2]. Under conditions of selective transfer, the polymerization film contains surface-active substances that adhere to contact surfaces by transforming glycerin into value-added molecules.
Glycerin molecules, together with the copper particles, the mixture of heterometallic acids with many bases, polyamides, metal ions, and other simple electrically charged particles from glycerin, polymerize and create an additional protective layer on the friction surfaces, preventing their oxidation (oxide layer reduction at the tribo-interface) and their direct contact. The polymerization film is formed from the organic substances’ free radicals that appear in the tribodestruction process of the lubricating material. They come from lubricant thermodynamic instability due to the catalytic effect of the oxide layers and the materials on the lubricant; as a result, the contact pressure decreases. Therefore, all these are related to the stability of selective transfer, which is mainly influenced by acrolein.
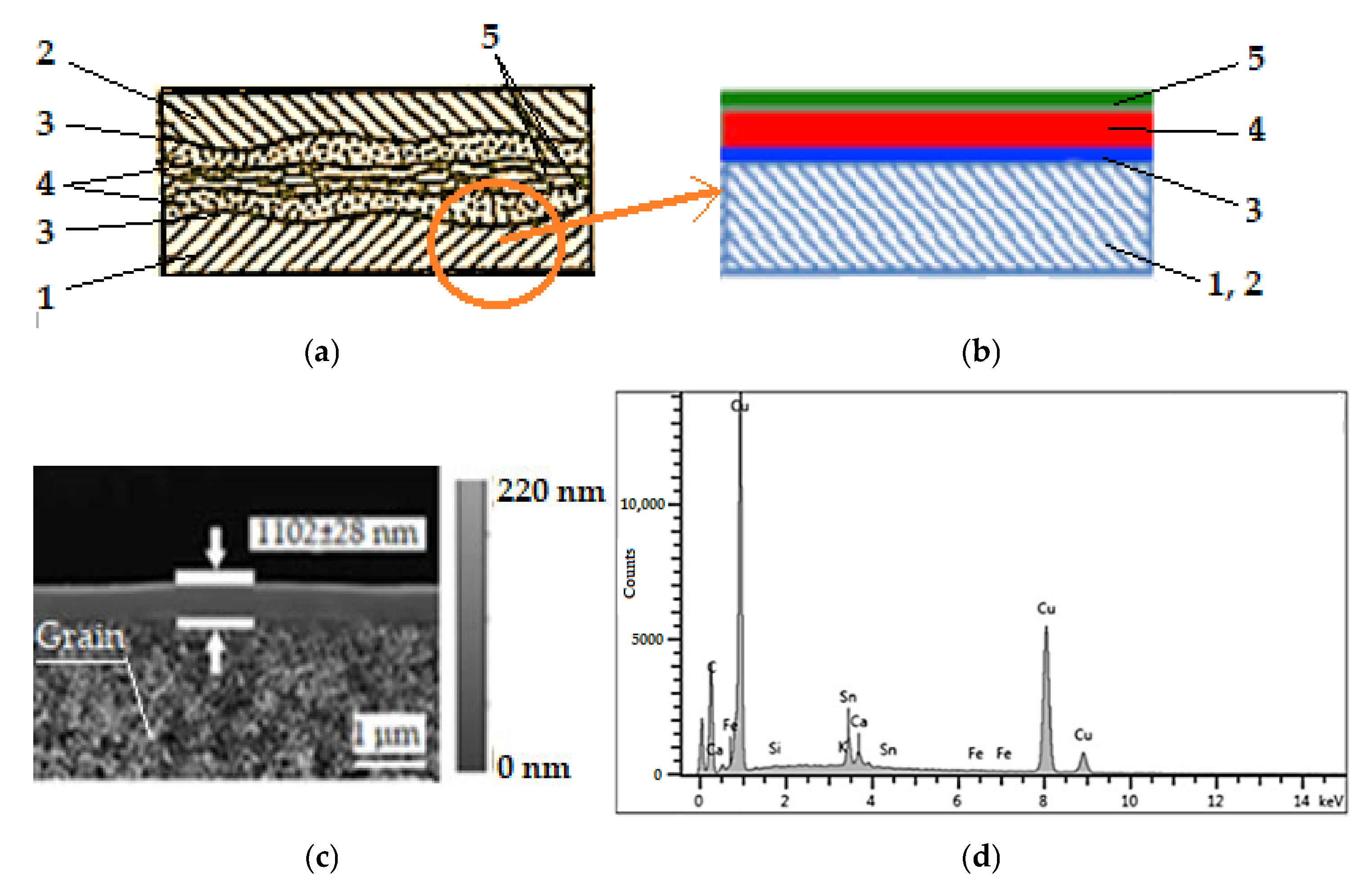
To justify the selective copper layer formation was presented in Figure 3, its structure is shown schematically in Figure 3a, the typical structure of a selective layer composite is shown in Figure 3b, an SEM image is shown Figure 3c with a resolution of 1 μm (512 × 512 pixel), and an EDX spectrum is shown in Figure 3d to validate the copper layer composition. The SEM image in Figure 3c has a magnification of the order of 1000× and it can be seen that the grains are quite small. A careful analysis of the structure of the selective layer in Figure 3a,b leads to the statement that the selective layer is a composite self-lubricating selective layer, with four elements. The friction and wear characteristics of this layer provide information about the effective lubrication intervals and general patterns of tribological behavior.
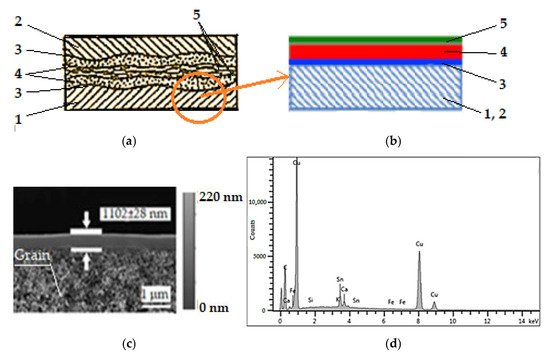
Figure 3.
Scheme of contact surfaces with selective transfer (a): 1—steel, 2—bronze, 3—bonding layer (intermediate layer), 4—servowitt film, 5—polymer film [2,3]; typical structure of a selective layer composite (b): 1, 2—material support of steel or bronze, 3—bonding layer (intermediate layer), 4—servowitt film, 5—polymer film [22]; SEM image of the selective layer obtained in the friction process, by the interfacial polymerization (c) [22]; EDX spectrum: composition of the selective layer (d).
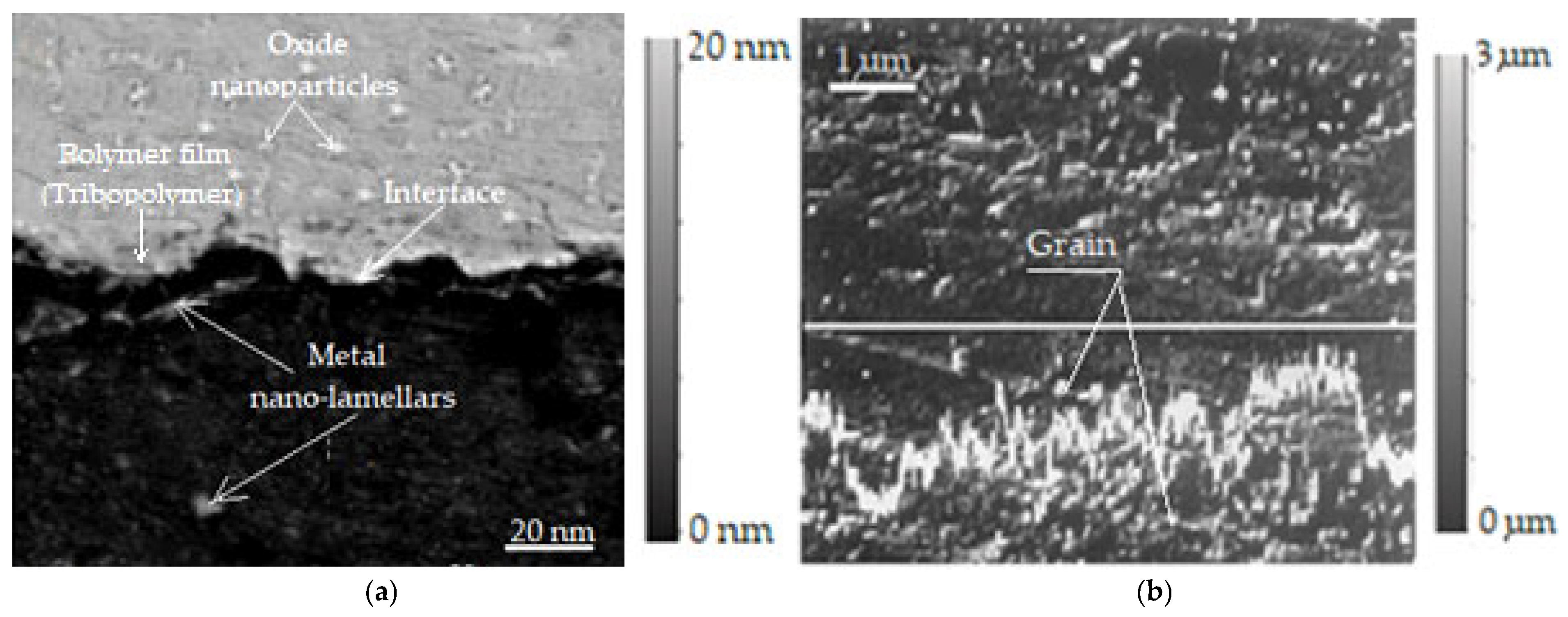
Additionally, to confirm the presence of tribopolymers and chemical bonding states, the bright field image in Figure 4a shows that the metallic nanoparticles (nano lamellae) and oxide nanoparticle regions have an obvious interface, on which a polymer film (tribopolymer) was formed. The resolution of this image is 20 nm and is presented magnified by 1000×. Instead, the study of the composition of the thin surface layers obtained under selective transfer conditions, as well as the presence of tribopolymers, required investigations using the JXA-5A electron microprobe, which is a capable piece of equipment for such investigations. Thus, in Figure 4b, a secondary electron image magnified by 5000× with a resolution of 1 μm is presented, where the variation profile of the copper and iron concentration on the surface of the areas on which the transfer of a thin superficial layer was performed under selective transfer conditions is observed, following the direction marked by a white line.
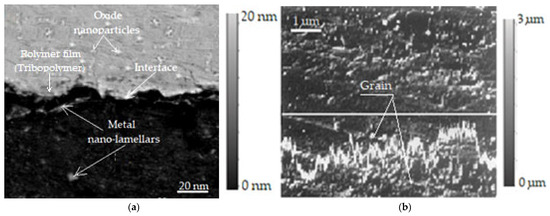
Figure 4.
Chemical bond state with an interface between metal nanoparticles and oxide nanoparticles, on which a polymer film (tribopolymer) was formed (a) [43,44]; secondary electron images of a bronze/steel friction pair lubricated with glycerin at a speed of 0.33 m/s (b) [2].
It is worth mentioning that tribopolymers (Figure 4a) are formed by friction-induced chemical reactions and can exhibit diverse chemical bonding states. These states, including covalent, ionic, and various secondary bonds, are crucial in understanding tribological behavior and material properties under stress. The type of chemical bonding present in tribopolymers significantly impacts their strength, durability, and overall performance in friction and wear.
The secondary electron image (Figure 4b) shows the relief of the steel friction surface on which the thin superficial layer of copper from bronze was transferred, which functioned by friction under selective transfer conditions, and based on which the uniformity of the start can be appreciated. The lower part below the profile shown in Figure 4b represents the variation in the iron concentration in the base material (steel), from the area where the superficial layer was deposited by selective transfer in the friction process, and the upper part (above the profile) represents the variation in the copper concentration in the layer that was obtained by selective transfer. These images (see Figure 4) were obtained using the photo camera included in the electronic microprobe and were taken on the bronze/steel pair, lubricated with glycerin, after a 2 h friction stress, at the same load of 10.5 MPa and sliding speed of 0.33 m/s.
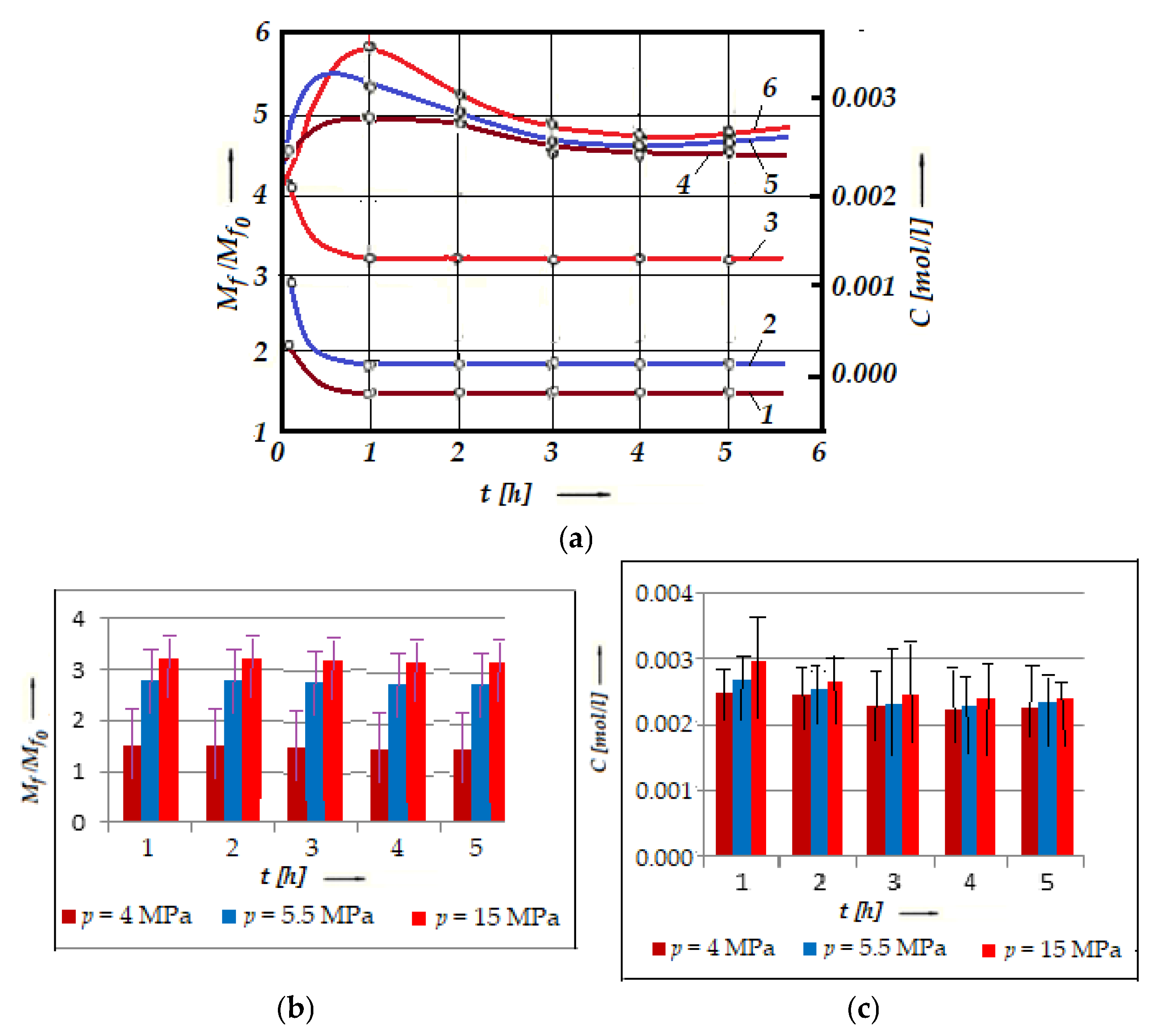
As mentioned above, the conditions in the contact zone of a friction pair operating with selective transfer vary, due to which a series of bonds are formed in the glycerin, which modify the operating properties (oxidation and reduction) of the glycerin. Valorization of the oxidation–reduction properties of the glycerin makes it possible to operate the friction pair under the conditions of selective transfer, for a known contact pressure and sliding speed. At different frictional stress and sliding speeds, the conditions in the contact area change (the pressure in the contact area and the sliding speed are known but have different values). This was justified by the observations during the experiments carried out on the installation presented in Figure 2, using calibration solutions and the experimental results from Figure 5a, presented in the form of histograms (with error bars and statistical data for friction relative moment, Mf/Mfo, Figure 5b, and for acrolein concentration, C, Figure 5c).
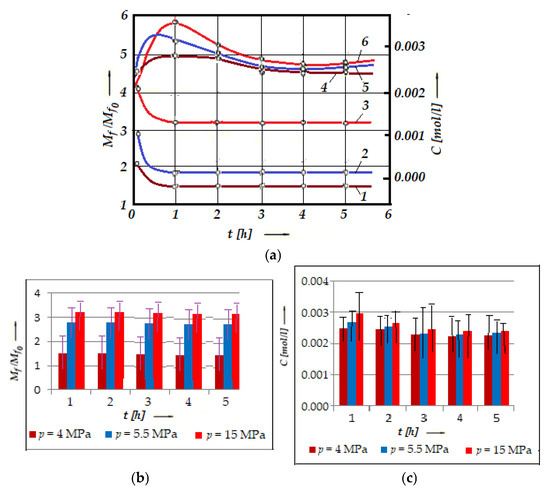
Figure 5.
Variation in the friction relative moment, Mf/Mfo (curves 1, 2, and 3), and of the concentration C of acrolein in glycerin (curves 4, 5, and 6) with time, for bronze/steel (the material pair UNS-C90800/AISI/SAE 1045) at different contact pressures: 4 MPa (curves 1 and 6), 5.5 MPa (curves 2 and 5), and 15 MPa (curves 3 and 4) (a), histogram for the friction relative moment, Mf/Mfo (b), and histogram for the concentration, C of acrolein in glycerin (c) versus time, t, at the same loads.
The experimental results presented in Figure 5 pointed out the modification of the acrolein concentration, C, in glycerin for a bronze/steel pair in association with the relative friction moments, Mf/Mfo (and implicit wear mechanism), discussed below.
Considering that acrolein is a product of the chemical transformations of glycerin and constitutes one of the bonds formed in glycerin, which modifies its functional properties, it plays an important role associated with its catalytic action in selective transfer.
The starting points, in this functional figure (Figure 5), of the relative moments of friction Mf/Mfo (Mfo is the starting moment of friction, and Mf is the moment during the friction process) correspond to the time of entry into the function under the action of different contact pressures. After about an hour, a pronounced selective transfer can be observed, which precedes the very low value of the relative moment (see Figure 5a, curves 1, 2, and 3), as well as the presence of a copper layer on the friction areas. At the same moment (see Figure 5a, curves 4, 5, and 6), the concentration, C, reaches its maximum value.
Also, we notice that Mf/Mfo increases, and C decreases with increasing load, at any moment, which can be explained by the fact that when the load increases, the friction surfaces tend to get closer to each other. It is important to note that the Mf/Mfo decreases rapidly, and the C increases relatively quickly with the test time (after approximately one hour of operation). Then, Mf/Mfo stabilizes at a certain value, and the concentration, C, decreases relatively slowly until 3 h of operation, after which it tends to stabilize with very small variations.
These results prove that the friction pair operates in conditions of selective transfer, and therefore, with minimal friction and wear, a relatively low operating temperature, and an optimal lubricant entry speed. This is due to their remarkable properties of the copper layer, which include very good thermal and electrical conductivity, corrosion resistance, wear resistance, superior mechanical strength, and self-lubricating [45].
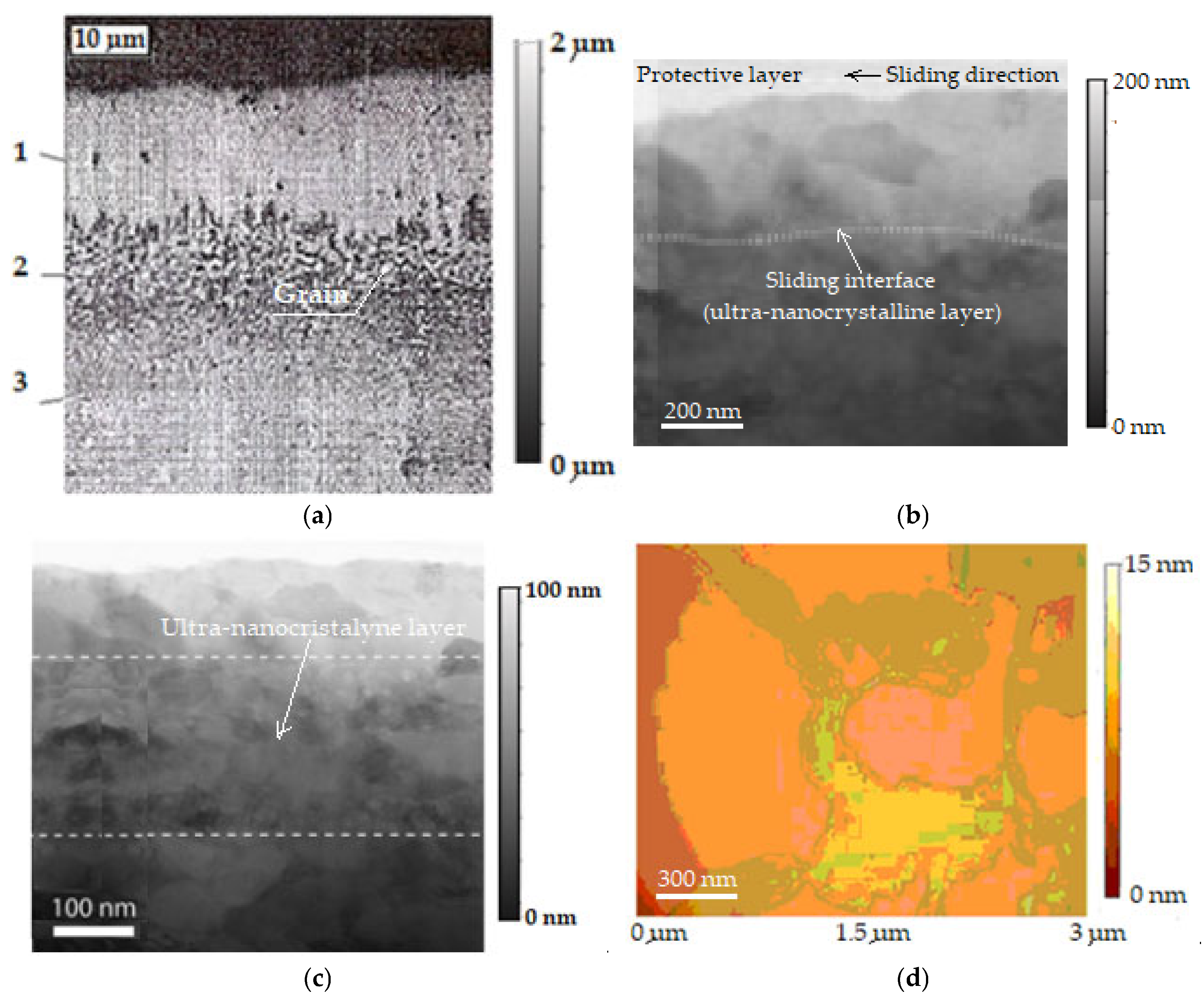
The presence of the copper layer in the friction areas is proven in Figure 6a, where the microstructure of the selective layer can be observed in depth with the selective layer transfer (1) in which copper dominates (over 85%), the interface (2), and the basic material (steel) (3), having a resolution of 10 μm and magnified by 20,000× [32,46]. Additionally, by scanning electron transmission microscopy (STEM), the images in Figure 6b,c are evidence of an ultra-nanocrystalline thin layer in the stationary friction regime. The images are magnified views to highlight the ultra-nanocrystalline grain sliding (shear) interface, the protective layer, and the sliding direction (see Figure 6b), respectively, and the ultra-nanocrystalline grain sizes (see Figure 6c), and correspond to the wear track by friction. The image resolution in Figure 6b is 200 nm and is presented magnified by 6000×, and the image in Figure 6c has a resolution of 100 nm and presents a magnification of 3000×. The image in Figure 6d has a resolution of 300 nm and presents a magnification of 15,000×; further explanations are presented below.
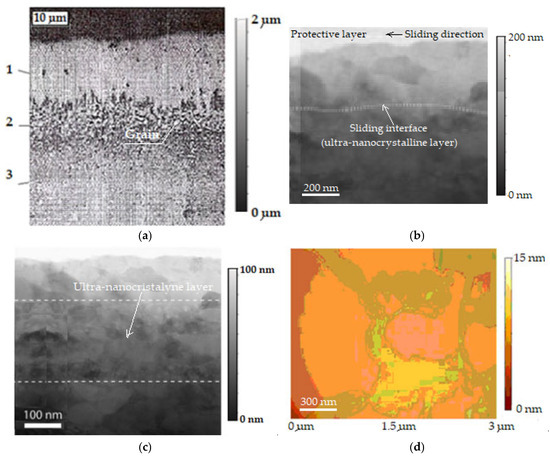
Figure 6.
Microstructure of the selective layer in depth: 1—selective layer transferred, 2—interface, 3—basic material (steel) (a) [2], bimodal steady-state friction regime with ultra-nanocrystalline grain sliding (shear) interface (b) [44], evidence of ultra-nanocrystalline thin layer and the ultra-nanocrystalline gran sizes (c) [44], failure/delamination, observed after the friction and wear of the steel disc in contact with the bronze pin, in the presence of glycerin (d) [2].
Analysis of the observed microstructural features (see Figure 3c, Figure 4b and Figure 6a) of the bronze/steel friction pair under selective transfer conditions shows that friction and wear are low. This is due to the formation of a quasi-stable ultra-nanocrystalline thin layer (grains with sizes nano-metric) with reduced shear strength. This is related to the formation of a thin, ultra-nanocrystalline surface film generated by shear and demonstrates a direct correlation between the surface grain size and the COF [44]. At the same time, this is possible because it makes the connection between friction and the transition from a dislocation-mediated plasticity state in the sliding region at the grain boundary, when the average surface grain size is sufficiently refined, as well as due to the grain refinement in a regime where sliding at the grain boundary dominates [44].
Thus, sliding at the grain boundary is the dominant deformation mechanism at the interface of the contact areas. For paired metallic contacts (the case of the studied friction pair), in the absence of oxides and hydrocarbons, friction is effectively a measure of the resistance required to shear the metallic junctions. The steady-state grain size is defined by the rate of grain refinement through deformation and stress-induced growth and it affects shear strength (by reducing it) [44].
Hence, the shear strength is grain-size-dependent on the metals in the nanocrystalline regime, such that by applying contact mechanics models, it is possible to predict the shear strength of a sliding metallic junction. Then, by extension, we can predict the COF—based on microstructure (material properties and contact parameters), where the formation of the low-friction interface layer appears to be dependent on the location of the wear mark. This implies that the low friction and wear are related to the reduction in shear strength at the interface, associated with the formation of an ultra-nanocrystalline surface thin layer, shown in Figure 6b, where the wear path at the end of the experiment is visualized, showing the grain size of the ultra-nanocrystalline, thin shear layer, by scanning electron transmission microscopy (STEM).
Physicochemical research on the structure of the servowitt film [3] has demonstrated that its material is in a state similar to a melt, resulting from the decomposition of the copper melt at low temperatures [2,3] because the lubricating material eases deformation by shearing/displacement in the diffusion process. The film is not able to absorb, is porous, exhibits low sliding forces, has an oxide-free upper layer, and transfers from one surface to another, i.e., adheres without damage and without increasing the friction force.
Porosity is explained by the creation of a large number of vacancies in the copper layer on the surfaces, which unite, forming pores, and which are filled with glycerin molecules. The lack of oxide films causes the formation of chemisorption processes, which give additional wear resistance, and the servowitt film does not change its properties; it can be deformed multiple times, without being destroyed, and dislocations pass easily into the film. During selective transfer, the servowitt film is a very strong polymerization catalyst. The wear products are represented through the copper particles and by the presence of the servowitt film on the friction surfaces. These particles are electrically charged, and they concentrate in the interstitium under the action of the electric charges, because then, under the action of the electric field, they are moved into the cracks of the surfaces.
As explained above, the increase in concentration, C (see Figure 5), at the beginning of the rubbing period is related to oxidation and other chemical transformations of glycerin, especially the formation of acrolein [47], but also of metal–organic compounds, colloids, and tensio-active substances [2]. The cause is the increase in temperature when the catalytic action of the metals is activated during the association by friction. Support for these statements comes even from the in-depth analysis of Figure 5. Thus, from the beginning of the friction period, it was observed that the concentration, C, increases until it reaches its maximum, after about an hour, and the relative friction moments Mf/Mf0 stabilize. Then, C decreases relatively easily until a certain time, when it stabilizes proportionally (after about 4 h).
The effect is the transport of metallic particles into the contact area until the equilibrium is established between the contact area and the friction surface area [2,3], corresponding to the concentration stabilization zones, C, respectively, of the relative friction moments, Mf/Mf0 (see Figure 5). After this period, the acrolein concentration, C, no longer depends, except to a very small extent, on the contact pressure. It even decreases with time, as a result of acrolein’s superficiality and its ability to dissolve through oxidation and dissolution reactions. To see more clearly the variation in the acrolein concentration, C, with time and load, the average values are presented in Table 1.

Table 1.
The values of acrolein concentration, C, with time, for loads of 4, 5.5, and 15 MPa.
The mechanisms underlying this behavior involve the formation of a protective transfer layer during sliding and the effective lubrication provided which reduces direct contact and facilitates smoother interactions between the contact/friction surfaces [45].
Friction-induced electrochemical reactions resulted in the development of oxides at the contact interface, substantially influencing tribological characteristics and wear mechanisms. Thus, delamination, tribo-oxidation wear, adhesive wear, and abrasive wear were the main wear mechanisms observed in the self-lubricating composite selective layer [48]. Failure/delamination/wear assumes the separation of layered material into its layers. This separation can happen due to various factors like stress, moisture, or improper adhesion between layers. It is a form of material failure, particularly in structures where the adhesion between layers is compromised.
The phenomenon of frictional damage as a function of time, load, and sliding speed (as shown in Figure 6c) was observed both at the edge of the disc and in the center of the disc wear track. Thus, several zones/regions can be seen on the image; the lighter zones (yellow and pink) are delaminated, where glycerin predominates. The darker areas (mustard and olive) represent the polymer film, followed by the extended orange color area, which is a mixture of glycerin with a lot of copper particles. The central pink color area is considered to be a mixture of glycerin with a smaller number of copper particles, and the brick-colored area on the left side of the image may represent a hybrid area with iron oxide particles.
As shown before, it is found that acrolein plays an important role in the beginning period of friction, for the achievement/unfolding of the selective transfer, due to the activation of the catalytic action of metals to association by friction under load, as a result of the increase in temperature.
After this period, the concentration of acrolein no longer depends on the charge in contact and decreases with time. Therefore, its reaction activity decreases, i.e., its dissolution ability through the oxidation and reduction reactions. It becomes superficial but with the possibility of regeneration after the entry of fresh glycerin [26,47].
In addition, it is specified that acrolein is the simplest unsaturated aldehyde and exhibits high reactivity due to the presence of a C=C double bond conjugated with the carbonyl group. The catalytic dehydration of glycerin has become important because it can produce acrolein (2-propenal) as the main reaction product and represents a route for regeneration [49].
It should also be noted that too high a quantity of chemical products, which appear in the transformations of glycerin, deregulates the stability of the selective transfer.
Due to the friction, in the friction pair’s contact area, the temperature increases and determines the acceleration of metal oxidation reactions. Thus, the wear of metal surfaces at the beginning of friction takes place through the friction of the limit and adherent layers, and it is formed by the wear of the oxide layers [26]. If the friction pair works at high pressures, the wear increases, as a result of the oxide layer wear and the formation of some local welding bridges. In time, the friction pair elements’ temperature continues to increase due to friction (through improper heat removal or external heating) and an increase in the oxide layer’s thickness (therefore also quantitatively) and, implicitly, an increase in wear, an inevitable phenomenon in the mixed or adhesion friction of layers [3,26]. Therefore, in this stage/phase, the stability of the selective transfer is deregulated. Thus, the wear of metal surfaces at the beginning of friction takes place through the friction of the limit and adherent layers, and it is realized by the wear of the oxide layers [26].
From here, it follows that glycerin is a simple product but active in adjusting the selective transfer and still indicated for this. Stable conditions are found only in the established ratio of the oxidation and reduction properties of the glycerin, and they change as a result of the aging of glycerin in the friction process.
It emerges that the regulation of the stability of selective transfer consists especially in maintaining an optimal ratio of the lubricant and the aging products, within well-established limits. They also ensure a minimum friction force and take place, for example, by adjusting the speed of the fresh glycerin entry.
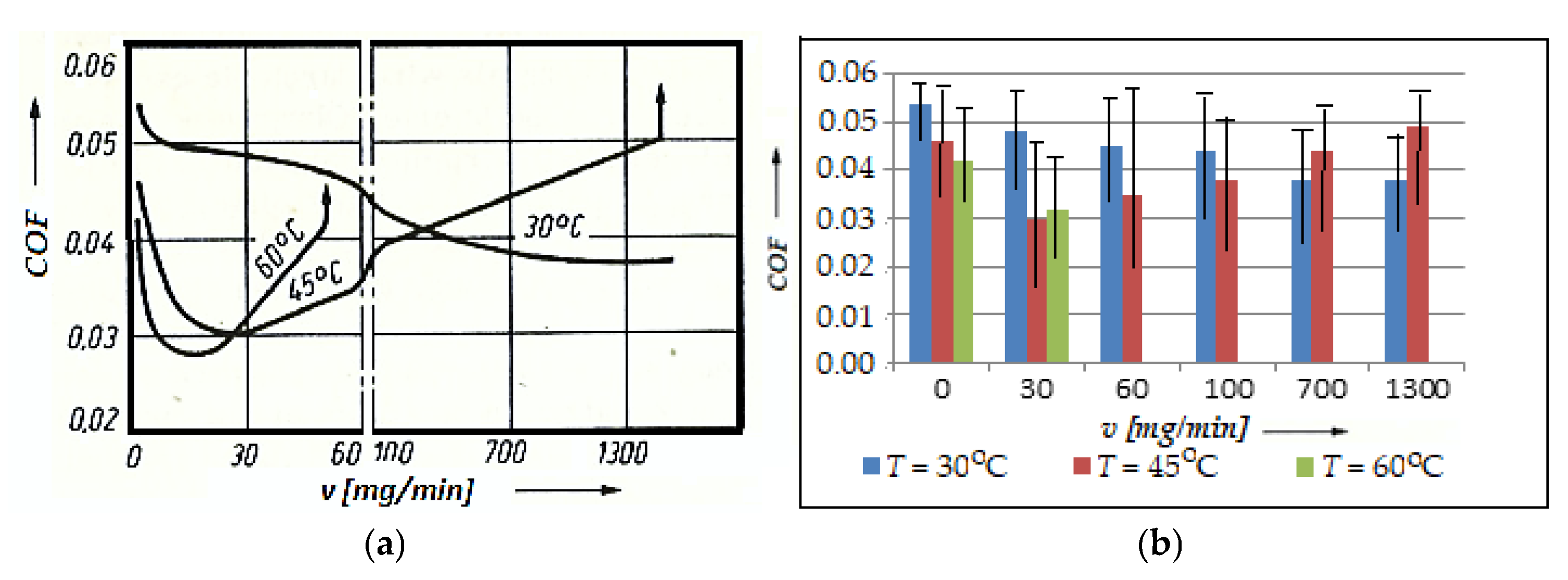
To examine such aspects, the variation in the COF with the speed of the glycerin entering the friction contact area was plotted in Figure 7a, whose histogram is shown in Figure 6b, with the error bars and statistical data, for the considered pair (bronze/steel), at temperatures of 30 °C, 45 °C, and 60 °C.
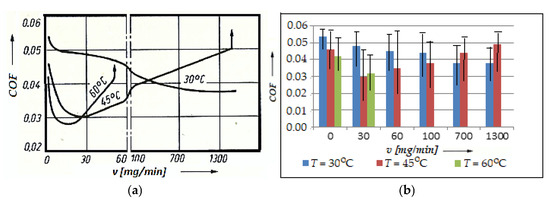
Figure 7.
Variation in the friction coefficient, COF, with the entry speed of the glycerin at temperatures of 30, 45, and 60 °C (a); histogram for the friction coefficient, COF, versus the entry speed of glycerin, v, at the same temperatures (30, 45, and 60 °C) (b).
The experiment was carried out on an installation provided with the possibility of temperature changing at a speed of 0.33 m/s and a pressure of 10.5 MPa, under the conditions of a continuous fresh lubricant (see Figure 2). From the obtained results (see Figure 7), it is visible that for the chosen friction conditions, there is an optimal speed of entry of the fresh glycerin into the contact area, which also ensures a minimum value of the COF, respectively, of wear. This occurs especially at high temperatures and low glycerin entry speeds.
At high glycerin entry speeds, the COF has minimum values at low temperatures, after which it tends to stabilize and a selective transfer can no longer unfold under these conditions. At high temperatures, the range of different glycerin entry speeds shrinks, at which point the COF has a very small value and highlights a selective transfer, and at the same time causes the chemical transformation process into glycerin. Comparative research on the COF in the conditions of selective transfer to the continuous entry of the lubricant from the lubricant tank, it is observed from the beginning of the friction that there is a very large exchange of lubricant in the tank.
After a long period of attempts due to friction, the contact conditions in the tank are still the same, which also include the reduced amount of glycerin. The test time, the minimum COF, and wear depend on the amount of lubricant for the respective friction conditions. An increase in the value of the COF at a higher glycerin entry speed proves that glycerin no longer has the same properties that are valid for selective transfer. Only at a well-determined amount of the chemical transformation products in the lubricant are conditions created for stable and stationary selective transfer at the operating temperature by friction.
The analysis of the experimental results showed that both in the case of the lack of aged products as well as in those in surplus, the stability conditions for selective transfer are damaged. Making lubrication with a continuous supply of glycerin gives the possibility of avoiding such unintended consequences and an optimal choice of the speed of flow of the glycerin. This must be ensured after a certain time to obtain stable conditions for selective transfer; the values of the COF and wear are low [3,27,31].
In order to analyze the repeatability of the results, a minimum of five tests were performed under the same conditions, obtaining similar results. The statistical interpretation of the experimental results considers the following parameters: mean and coefficient of variation (cv). The cv is defined as the ratio between the standard deviation and the mean. The points indicated on the graphs (Mf/Mf0 and C as a function of time (see Figure 4), respectively; COF as a function of the lubricant entry speed (see Figure 6)) represent the average of five experimental results, performed under the same conditions. Thus, for Mf/Mf0, the result was cv = 0.92; for C, cv = 0.86; for COF, cv = 0.83.
This possibility is established following the experiments carried out and their results, in which the friction pair was kept in an oil tank with an unchanged amount of glycerin. In this case, the COF values become stable, which is typical for selective transfer, after long-term tests. It is possible that no copper servowitt layer forms [2,21], but apart from this, one can observe the dark layer on the friction area as well as the traces of destruction, but also the change in the color of the glycerin.
Thus, the stability of selective transfer is closely related to the aging of the glycerin, and it can be achieved by introducing surplus lubricant, which then changes the oxidizing capacity of the glycerin and protects the friction areas from micro-wear and, therefore, avoids excessive wear and, at the same time, also regulates the temperature in the friction area.
Therefore, the stability of selective transfer is affected both by the aging of glycerin and by the existence of glycerin in surplus. But, too many chemical products that occur during the chemical transformation of glycerin disrupt the stability of selective transfer. It follows that glycerin, as a simple but active product, is indicated for the regulation of selective transfer. Thus, only in a set ratio of the oxidation–reduction properties of glycerin, are stable conditions for selective transfer found. However, the oxidation and reduction properties change, resulting from the aging of the glycerin during the friction process. This results in maintaining an optimal ratio of glycerin and aging products within well-established limits, which is possible by adjusting the entry speed of fresh glycerin while ensuring minimal friction and wear. To analyze these aspects, the variation in COF with entry speed was plotted for glycerin in the friction contact area (see Figure 7). Therefore, on the one hand, the stability of selective transfer is affected by glycerin aging, because it degrades, and the ratio between it and aging products becomes unstable; on the other hand, the existence of excess glycerin causes an amount of increase in chemical products and thus disrupts the stability of selective transfer.
Lubrication with a continuous glycerin supply creates the possibility of avoiding such an undesirable consequence, as well as establishing the optimal flow speed of glycerin after a certain time. The goal is to obtain stable conditions for selective transfer when friction and wear are low. The tribodestruction of the glycerin, as one of the factors acting on the physicochemical processes in the contact areas, leads to the onset of friction in conditions of selective transfer, mainly to solve the oxidation problem. It was seen that, in the case of a friction pair of bronze/steel lubricated with glycerin, in the first period of operation, dissolution of the bronze friction surface takes place, where the glycerin acts as a weak acid. Then, the component elemental atoms of the bronze (Sn, Al, Zn, Fe, etc.) are transferred into glycerin; thus, the bronze friction surface is enriched with copper atoms [3,50], and the phenomenon is repeated in the friction process time.
The process of bronze dissolution continues until a copper layer is formed on the bronze and steel surfaces in contact, with an optimal thickness (on average of 1–3 μm) [2]. In the case of dry friction, and at the limit, the surfaces of the friction pair elements are always covered with films of oxides, avoiding the metal surfaces’ direct contact and their micro-welding. The oxide films are fragile, and, in the friction process, they are the first to be destroyed. In the conditions of selective transfer, friction occurs without oxidation of the contact surfaces [3,26]. The compact layers of positively charged substances, active and adsorbing on the surface and formed in the friction process, protect the surfaces against oxidation and thus avoid the penetration of oxygen into the servowitt film [2,3]. The lack of an oxide film leads to the formation of chemisorption processes, which give additional wear resistance.
In conditions of selective transfer, the adsorbed layer, which also contains surface-active substances, assumes the function of protection against oxidation and of the micro-weld and regulates the temperature in the friction area [2,3,5].
Both the kinematics of the wear process and the particularities of obtaining selective transfer depend on the physicochemical properties of the glycerin.
Thus, at the start, friction takes place on the contact area of the copper alloy (bronze), influenced by the separate dislocations of the structure, corresponding to a correlation ratio of copper and zinc concentration in glycerin, with the bronze pin, which rubs with the disc of steel. Then, the surface dispersion of the bronze follows, without changes in its composition. This is possible by using the polarization method, using circular light polarization over the lens disc. Figure 8 presents the circular light guides that are popular for uniform and diffuse illumination.
Figure 8.
Circular light guides (a) and the elements of the illuminated surface (b).
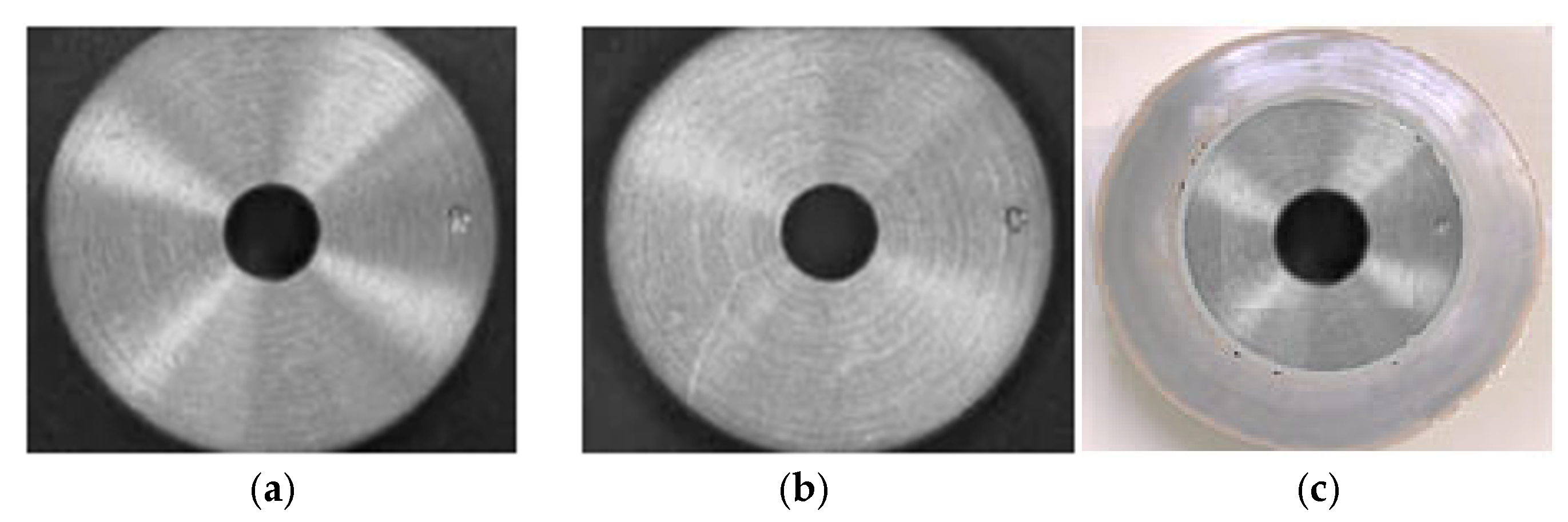
However, glare or reflection of the disk itself can occur. Separate polarization of the disc output and the lens can reduce these effects and highlight surface details. Thus, in Figure 9, the disc surface is presented both without (Figure 9a) and with polarization (Figure 9b). Additionally, the image of the worn disc (Figure 9c) has been introduced in order to observe/visualize the difference.
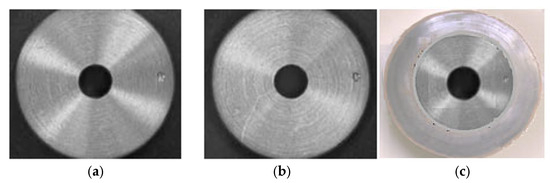
Figure 9.
Disc surface without polarization (a), with polarization over disk and lens (b), and image of the worn disc surface (c).
By placing a polarizer over the lens, glare from highly reflective surfaces or optical windows is removed. Due to the partial polarization of the light, the light source may or may not require a polarization filter in this scenario.
When the static conditions are concealed, another ratio appears, namely that of the concentration between copper and zinc in the lubricant.
This certifies the beginning of a selective transfer during the wear of copper alloy (bronze), when stable organo-metallic bonds are formed, for example, complex bonds, in which zinc is no longer catalytic, a process that can be observed in tests with loads of 1.72 and 5.8 MPa. The results of the tests are presented in Table 2, which contains the values for taking up zinc from glycerin for the pair of bronze/steel.

Table 2.
Values of the zinc loss coefficient over time.
The proportion of the zinc loss is a sensitive recognition quantity for the kinematics and the wear characteristics of bronze at a load of 1.72 MPa, where the zinc loss coefficient is already 1.6 after 0.5 h (30 min) from the beginning of the frictional stress; following that, under the conditions corresponding to the beginning of wear, it approaches 1 (see Table 2). This proves the fact that we have no wear process. At a contact load of 5.8 MPa, part of the selective wear changes in the start period through the leakage effect, and at the same time, with this the friction of the copper alloy (bronze) in the area of separate dislocations of the structure.
In this case, the copper alloy is passed without any change in composition, in the friction area of the steel, which hardens in the cold. When transforming the glycerin, products appear that act as antioxidant substances in the processes that take place in the friction areas of the surfaces. An analysis of the change in zinc loss coefficients during frictional stress gives the possibility of obtaining an important control factor for the wear process in the presence of an active lubricant (here glycerin). The change in glycerin activity is influenced by kinetic processes, and it favors or disfavors the stable positioning of friction conditions.
The results of experimental research in the presence of a lubricant with zinc and copper content are important because, through a glycerin exchange, the wear products from the lubricant must be removed, and the start of a new leakage process will start with a new glycerin. Here, it must be remembered that the formation of the real contact surface after a change in glycerin follows again, even if not with the same intensity as in the previous process or as at the beginning of the tests, i.e., with friction areas not yet polished. These results correspond very well with the results obtained by the concentration of the products of the chemical transformations from glycerin, and in researching the particularities of obtaining the conditions for selective transfer.
The increase in zinc loss coefficients, as well as the increase in the respective ionization speed for zinc, is probably caused by taking over the active products of transformation from glycerin, as well as through the intensity of the electrochemical corrosion processes on the friction areas under the conditions of selective transfer [18,51,52].
To the extent that the properties of glycerin are established in stationary conditions, this also decreases the ionization speed, taking into account the balance, destruction, and formation of wear particles in the friction areas. This balance determines the low values of wear intensity and COF.
To estimate the wear under selective transfer conditions (which is very low), it was quantitatively established by analytical calculation based on some data obtained by measurement during experimental tests. During experimental tests, quantitative data on wear were collected by measuring the mass loss, Δm (in grams, by weighing), concurrently with the thickness of the worn layer, Δh (in mm), for both the pin and the disc. The mass loss, Δm, in grams, was measured by weighing with the help of an analytical balance at 620 g/0.001 g and measuring the average thickness of the worn layer, Δh, in mm, with a high-precision digital micrometer at 0–25/0.0002 mm.
The measurements were made every 15 min, within a 2 h interval (120 min), for the case of the disc with Ra = 2 μm, rotational speed, v = 0.33 m/s, and load, F, corresponding to the contact pressure of 10.5 MPa. It is mentioned that the thickness of the worn layer, Δh, was measured in marked and well-established points (four points at 90 degrees, for the pin, and eight points at 45 degrees, for the disc), considering as a value the average of the values from these points. Thus, the volumetric wear coefficient, K (volumetric wear intensity), was calculated with Archard’s general law, of the form K = ΔV/F·Lf, where ΔV—volume loss, in mm3; F—load in contact, in N; and Lf—friction length, in m. In addition, ΔV = Ac·Δh (Ac—contact area, in mm2, Ac = Lf·ds, and Lf = πdm·n, with dm = 95 mm—average diameter of the wear track, in mm; n—rotational number of the disc, in rpm; ds = 10 mm—pin diameter, respectively, Δh—average thin of layer removed by wear, in mm, measured value during experimental). The load in contact, F, results from contact pressure, p = 10.5 MPa, i.e., F = p/Ac.
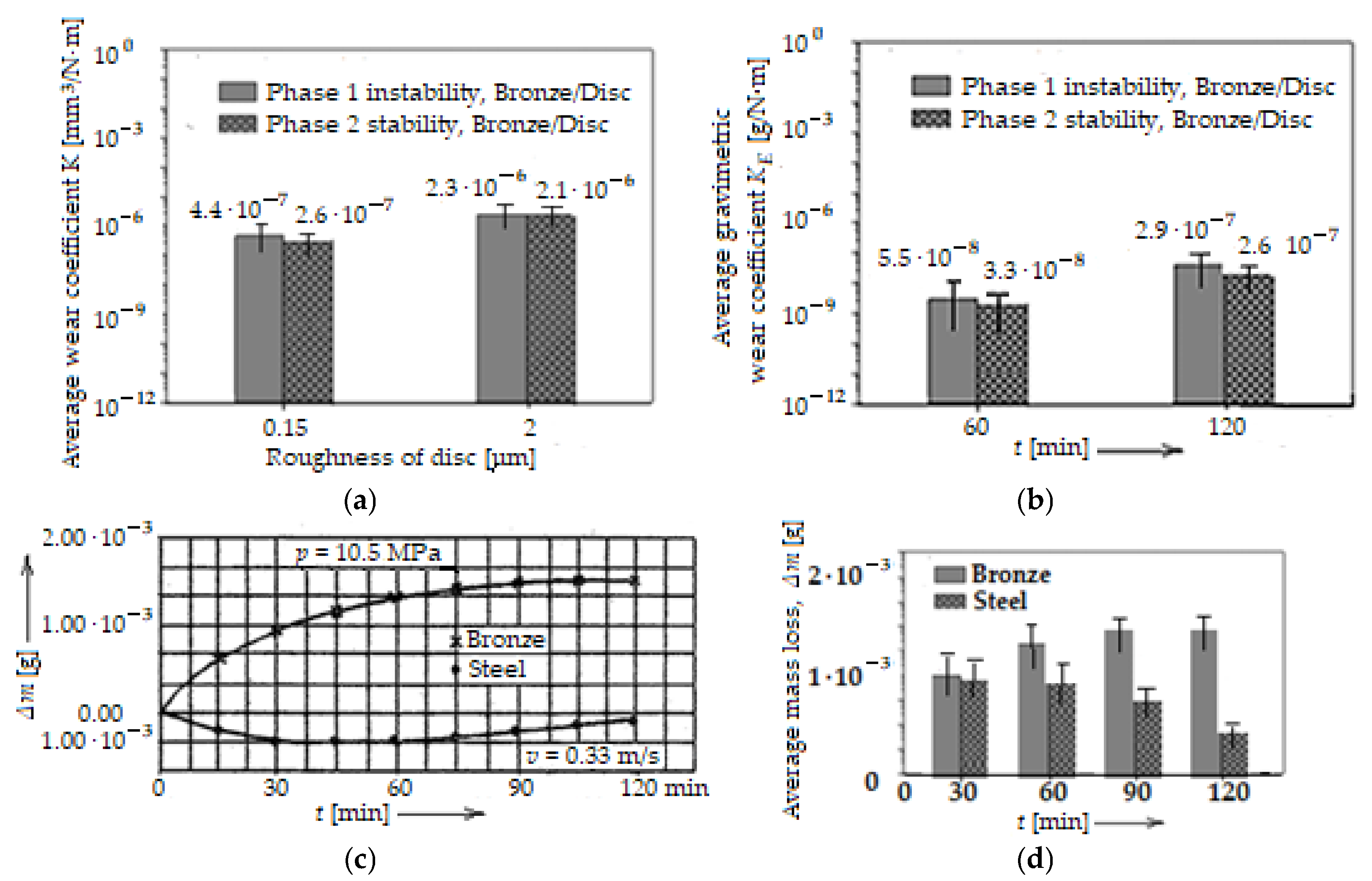
The data, thus collected and calculated, allowed the determination of the volumetric wear coefficient, K (volumetric wear intensity), which was calculated with Archard’s general law, respectively, of the gravimetric wear intensity, KE (gravimetric wear coefficient), with the relation KE = Δm/ρ·F·Lf, which also derives from Archard’s general law, and ρ is the density of the material. The results are presented in the histogram of Figure 10a, as average values of the coefficient K, for two roughness values Ra (0.15 μm and 2 μm), and KE in the histogram of Figure 10b, using its average values (based on experimental data) as a function of time (after 1 h and 2 h of operation).
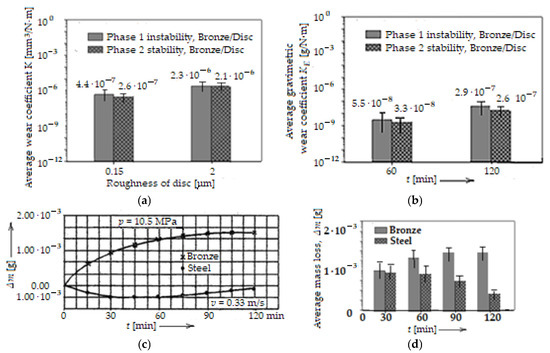
Figure 10.
Average wear coefficient, K, of bronze pins versus disc surface roughness, Ra (a), average wear coefficient, KE of bronze pins versus time, t (b), comparative variation in mass loss, Δm (c), and histogram of average mass loss, Δm (d), through wear of the pin and disc, versus time, t, for a particular load and sliding speed.
Additionally, as specified above, the data collected on the mass loss, Δm, during the experimental tests also allowed for the quantitative estimation of the wear, the evolution of which in time is shown in Figure 10c, for both the bronze pin and the steel disc, with the disc’s roughness also being Ra = 2 μm. The results with values and bar errors are presented comparatively (with those in Figure 10c) in the histogram in Figure 10d, after 1 h and 2 h of operation under selective transfer conditions.
Two phases are distinguished that can be easily identified in Figure 10a. The first phase is one of instability and corresponds to the running-in, and the second phase is one of stability. It is observed that in the first phase, the coefficient K is higher than that in the second phase, at Ra = 0.15 μm, while at Ra = 2 μm the coefficient K becomes relatively constant, because the running-in time is very short/rapid. Also, at Ra = 2 μm the coefficient K is higher than at Ra = 0.15 μm, and implicitly, so is wear, because the asperities of the disc surface are higher, and the amount of material removed in the friction process is greater, at least in the instability phase and even at the beginning of the second phase (of stability). Regarding the mass loss, Δm, we note that it is much reduced (with the mention that it is higher for the bronze pin than for the steel disc), with a tendency to stabilize over time, because it takes place until the equilibrium/stability conditions are established in the contact areas. Next, the process proceeds in the opposite direction (the surplus material transferred to the steel disc passes back to the bronze pin).
If the coefficient K increases relatively slowly with the increasing roughness, it is observed that the coefficient KE also evolves similarly, but in time. Comparing the values of KE with those of K (see Figure 10a,b), for Ra = 2 μm, they are much lower, which shows that there is a significant difference between the analytical and experimental results.
The analysis of experimental results in Figure 7a and Figure 10b obtained on the bronze pin/steel disc tribometer shows a normal evolution of the COF and wear, with very low values, which proves that under the selective transfer conditions, the durability of the friction pairs increases.
Under the conditions in which the copper alloy (bronze) is rubbed before testing on the steel surface, and both rubbing areas are consistent with the copper concentration, they will be selectively correlated, and the coefficient of zinc losses in the initial stage is equal to 1. It then grows during friction. This has a great influence on the selective transfer at the wear of the copper alloy (bronze) for the specific friction conditions.
The probable increase in wear and, simultaneously, shredding through a strong pressing caused by pressure and high temperatures can favor the appearance of layers from the copper alloy at frictional stress. In this way, a stratification of the bronze friction is produced. This process is subordinate to the lubricant and results from the possibility of the quality regulation of these friction layers by choosing an appropriate glycerin.
By plasticization of the friction areas during friction stress, as a result of the action of the active materials on the surface, which are in glycerin, and through the minimal hardness of the formed surfaces, the real contact area increases during friction. The real pressure and temperature in the contact areas decrease, and the friction process is reduced.
Therefore, similar research on the wear products of copper–zinc alloys, as well as the transformation products of glycerin in the friction contact area, gives the possibility of the dependence of the wear process on the physicochemical transformations of glycerin.
4. Conclusions
- The friction pair, bronze/steel lubricated with glycerin, was chosen because the elementary processes of the selective transfer phenomenon can be explained through the special properties of glycerin and sure works with selective transfer.
- The physicochemical processes that take place in the contact area were experimentally studied by the polarization method because it allows the study of the tribochemical transformations of the glycerin and the friction pair materials, from the contact area, in the friction process.
- By the catalytic dehydration of glycerin, derived products are obtained, of which acrolein is the most important, as the main reaction product, and at the same time represents a route for regeneration (for the initiation/triggering and stability of the selective transfer).
- It was found that selective transfer stability is affected both by the aging lubricant with its aged products, and those in surplus in the rubbing process.
- The realization of lubrication with a continuous and fresh supply of glycerin ensures selective transfer stability, in correlation with the load and operating temperature at which the values of the COF and wear are low, and the temperature is regulated in the friction area.
- The results of experimental research in the presence of glycerin with a zinc and copper content have highlighted the possibility of establishing the content of wear particles in the lubricant, and by changing the glycerin, the wear products from the glycerin are removed/leaked.
- In addition, the wear products of copper–zinc alloys, and the tribochemical transformation products of glycerin in the friction contact area, show that the friction and wear process is dependent on the physicochemical transformations of the lubricant.
- This study demonstrates that it is possible to move to another level of use of friction pairs (modern friction pairs with greatly improved tribological properties), which operate by selective transfer.
- Based on experimental tests, the physicochemical properties and the tribochemical transformations of the glycerin and the materials of the friction pair from the contact area were presented to understand and prove the practical utility, maintenance, and stability of the selective transfer.
- These aspects complete the deepening of the selective transfer phenomenon, as a novelty for practical applications and compared to previous studies in the field.
- For the future, it is recommended to continue research using other methods to improve and maintain the stability of selective transfer. If the chosen pair of materials is suitable for selective transfer, other lubricants can be experimented with that have self-regulating capacity and work both under the conditions of selective transfer and the conditions of friction between the adhesion and mixed layers, which also allow polymerization in the contact areas.
Author Contributions
Conceptualization, F.I.; methodology, F.I., D.C.C. and A.-F.H.; software, D.C.C.; validation, F.I., D.C.C. and A.-F.H.; formal analysis, F.I., D.C.C. and A.-F.H.; investigation, F.I., D.C.C. and A.-F.H.; resources, F.I., D.C.C. and A.-F.H.; data curation, F.I., D.C.C. and A.-F.H.; writing—original draft preparation, F.I.; writing—review and editing, F.I.; visualization, F.I., D.C.C. and A.-F.H.; supervision, F.I., D.C.C. and A.-F.H.; project administration, F.I.; funding acquisition, F.I., D.C.C. and A.-F.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mott, R.L.; Vavrek, E.M.; Wang, J. Machine Elements in Mechanical Design, 6th ed.; Pearson Education: New York, NY, USA, 2018; ISBN 10: 0-13-444118-4/13: 978-0-13-444118-4. [Google Scholar]
- Ilie, F. Tribological Study of the Thin Superficial Layers Formed in the Friction Couples by Selective Transfer; Technical Publishing House: Bucharest, Romania, 2002. [Google Scholar]
- Garkunov, D.N. Erhöhung der Verschleissfestigkeit auf der Grundlage der Selektiven Übertragung; UEB Verlag Technik: Berlin, Germany, 1981. [Google Scholar]
- Polzer, G.; Meissner, F. Grundlagen zu Reibung und Verschleiss; VEB Deutscher Verlag fur Grundstoffindustrie: Leipzig, Germany, 1983. [Google Scholar]
- Available online: https://plantman.com.au/10-maintenance-tips-to-extend-the-life-of-machinery-and-equipment/ (accessed on 15 April 2025).
- Reid, J.V.; Schey, J.A. The effect of surface hardness on friction. Wear 1987, 118, 113–125. [Google Scholar] [CrossRef]
- Herman, H.; Sampath, S.; McCune, R. Thermal Spray: Current Status and Future Trends. MRS Bull. 2000, 25, 17–25. [Google Scholar] [CrossRef]
- Joshi, S. Special Issue: Advances in Thermal Spray Technology. Materials 2020, 13, 3521. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Bao, Y.; Gawne, D.T.; Zhang, T. Process control for thermal-spray deposition of thermoset coatings using computer simulation. Prog. Org. Coat. 2016, 101, 407–415. [Google Scholar] [CrossRef][Green Version]
- Garg, R.; Gonuguntla, S.; Sk, S.; Iqbal, M.S.; Dada, A.O.; Pal, U.; Ahmadipour, M. Sputtering thin films: Materials, applications, challenges and future directions. Adv. Colloid Interface Sci. 2024, 330, 103203. [Google Scholar] [CrossRef] [PubMed]
- Ramade, C.; Silvestre, S.; Delannoy, F.P.; Sorli, B. Thin film HF RFID tag deposited on paper by thermal evaporation. Int. J. Radio Freq. Identif. Technol. Appl. 2012, 4, 49–66. [Google Scholar] [CrossRef]
- Koskinen, J. 4.02-Cathodic-Arc and Thermal-Evaporation Deposition. Compr. Mater. Process. 2014, 4, 3–55. [Google Scholar] [CrossRef]
- Hamid, N.; Suhaimi, S.; Othman, M.Z.; Ismail, W.Z.W. A Review on Thermal Evaporation Method to Synthesis Zinc Oxide as Photocatalytic Material. Nano Hybrids Compos. 2021, 31, 55–63. [Google Scholar] [CrossRef]
- Sarakinos, K.; Alami, J.; Konstantinidis, S. High power pulsed magnetron sputtering: A review on scientific and engineering state of the art. Surf. Coat. Technol. 2010, 204, 1661–1684. [Google Scholar] [CrossRef]
- Cutroneo, M.; Havranek, V.; Mackova, A.; Malinsky, P.; Silipigni, L.; Slepicka, P.; Fajstavr, D.; Torrisi, L. Laser ablation for material processing. Radiat. Eff. Defects Solids 2022, 177, 71–84. [Google Scholar] [CrossRef]
- Sun, L.; Yuan, G.; Gao, L.; Yang, J.; Chhowalla, M.; Gharahcheshmeh, M.H.; Karen, K.; Gleason, K.K.; Choi, Y.S.; Hong, B.H.; et al. Nature Reviews Methods Primers. Nat. Rev. Methods Primers 2021, 1, 5. [Google Scholar] [CrossRef]
- Bellemare, S.C.; Dao, M.; Suresh, S. Effects of mechanical properties and surface friction on elasto-plastic sliding contact. Mech. Mater. 2008, 40, 206–219. [Google Scholar] [CrossRef]
- Kalin, M.; Zugelj, B.; Lamut, M.; Hamouda, K. Elastic and plastic deformation of surface asperities and their load-carrying mechanisms during the formation of a real contact area. Tribol. Int. 2023, 178 Pt A, 108067. [Google Scholar] [CrossRef]
- Ciavarella, M. A New Plasticity Index including Size-Effects in the Contact of Rough Surfaces. Lubricants 2024, 12, 83. [Google Scholar] [CrossRef]
- Polyakov, P.A.; Litvinov, A.E.; Polyakova, E.A.; Fedotov, E.S.; Tagiev, R.S. Design of surface profile of pairs of friction unit. IOP Conf. Ser. Mater. Sci. Eng. 2020, 843, 012001. [Google Scholar] [CrossRef]
- Padgurskas, J.; Rukuiza, R.; Vötter, M.; Wollesen, V. New tribotechnical materials for the friction pair radial lip seal/shaft. Ind. Lubr. Tribol. 1999, 51, 233–238. [Google Scholar] [CrossRef]
- Ilie, F.; Cotici, C.-D.; Juganaru, A. Physiochemical Processes to Reduce Friction and Wear Under Selective Transfer Conditions—A Review. Lubricants 2025, 13, 135. [Google Scholar] [CrossRef]
- Popov, V.L.; Li, Q.; Lyashenko, I.A.; Pohrt, R. Adhesion and friction in hard and soft contacts: Theory and experiment. Friction 2021, 9, 1688–1706. [Google Scholar] [CrossRef]
- Parkatzidis, K.; Wang, H.S.; Truong, N.P.; Anastasaki, A. Recent Developments and Future Challenges in Controlled Radical Polymerization: A 2020 Update. Chem 2020, 6, 1575–1588. [Google Scholar] [CrossRef]
- Ilie, F. Diffusion and mass transfer mechanisms during frictional selective transfer. Int. J. Heat Mass Transf. 2018, 116, 1260–1265. [Google Scholar] [CrossRef]
- Garkunov, D.N. Triboengineering. Design, Production and Operation of Machines; Izd. MSKhA: Moskau, Russia, 2002. (In Russian) [Google Scholar]
- Chen, X.; Han, Z.; Li, X.; Lu, K. Lowering coefficient of friction in Cu alloys with stable gradient nanostructures. Sci. Adv. 2016, 2, e1601942. [Google Scholar] [CrossRef]
- Galembeck, F.; Burgo, T.; Balestrin, L.; Gouveia, R.; Silva, C.; Galembeck, A. Friction, Tribochemistry and Triboelectricity: Recent Progress and Perspectives. RSC Adv. 2014, 4, 64280–64298. [Google Scholar] [CrossRef]
- Nakayama, K.; Martin, J.-M. Tribochemical reactions at and in the vicinity of a sliding contact. Wear 2006, 261, 235–240. [Google Scholar] [CrossRef]
- Burlakova, V.; Ekaterina, D.; Igor, U.; Milov, A.; Lukyanov, B. Wear products and tribochemical reactions during friction of a brass-steel pair. Wear 2020, 462–463, 203517. [Google Scholar] [CrossRef]
- Cao, H.; Zhou, X.; Li, X.; Lu, K. Friction mechanism in the running-in stage of copper: From plastic deformation to delamination and oxidation. Tribol. Int. 2017, 115, 3–7. [Google Scholar] [CrossRef]
- Ilie, F. Tribological behavior of a friction couple functioning with selective mass transfer. Heat Mass Transf. 2017, 52, 625–633. [Google Scholar] [CrossRef]
- Yu, H.; Chen, H.; Zheng, Z.; Ba, Z.; Qiao, D.; Feng, D.; Gong, Z.; Dong, G. Transformation mechanism between the frictional interface under dioctyl sebacate lubrication. Tribol. Int. 2021, 155, 106745. [Google Scholar] [CrossRef]
- Bezerra, A.C.d.M.; Coelho, N.M.d.A.; Bertelli, F.; Pacheco, M.T.T.; Silveira, L. Temperature-Induced Chemical Changes in Lubricant Automotive Oils Evaluated Using Raman Spectroscopy. Appl. Spectrosc. 2021, 75, 145–155. [Google Scholar] [CrossRef]
- Nunez, E.E.; Polycarpou, A.A. The effect of surface roughness on the transfer of polymer films under unlubricated testing conditions. Wear 2015, 326–327, 74–83. [Google Scholar] [CrossRef]
- Sirés, I.; Brillas, E.; Oturan, M.A.; Rodrigo, M.A.; Panizza, M. Electrochemical Advanced Oxidation Processes: Today and Tomorrow. A Review. Environ. Sci. Pollut. Res. 2014, 21, 8336–8367. [Google Scholar] [CrossRef]
- Snyder, D.T.; Pulliam, C.J.; Ouyang, Z.; Cooks, R.G. Miniature and Fieldable Mass Spectrometers: Recent Advances. Anal. Chem. 2015, 88, 2–29. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Khajeh, A.; Martini, A.; Kim, S.H. Chemical and physical origins of friction on surfaces with atomic steps. Sci. Adv. 2019, 5, eaaw0513. [Google Scholar] [CrossRef] [PubMed]
- Rosas, I.P.; Larios, J.L.C.; Zeifert, B.; Blásquez, J.S. Catalytic Dehydration of Glycerine to Acrolein. In Glycerine Production and Transformation—An Innovative Platform for Sustainable Biorefinery and Energy; Frediani, M., Ed.; University of Florence: Firenze, Italy, 2019; Chapter 2. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Yu, K.; Qian, M. A two-dimensional thin film structure with spectral selective emission capability suitable for high-temperature environments. Case Stud. Therm. Eng. 2024, 63, 105261. [Google Scholar] [CrossRef]
- Zayed, E.M.; Shazly, M.; El-Sabbagh, A.; El-Mahallawy, N.A. Deformation behavior and properties of severe plastic deformation techniques for bulk materials: A review. Heliyon 2023, 9, e16700. [Google Scholar] [CrossRef]
- Zadkhast, S.; Jatskevich, J.; Vaahedi, E. A Multi-Decomposition Approach for Accelerated Time-Domain Simulation of Transient Stability Problems. IEEE Trans. Power Syst. 2015, 30, 2301–2311. [Google Scholar] [CrossRef]
- Yin, C.; Li, N.; Wu, Y.; Liang, Y.; Yang, C.; Wu, J. Frictional shear-induced nanolamellar oxidation and transformation to oxide nanoparticles during pearlitic steel sliding. Mater. Des. 2023, 233, 112299. [Google Scholar] [CrossRef]
- Chandross, M.; Curry, J.F.; Babuska, T.F.; Lu, P.; Furnish, T.A.; Kustas, A.B.; Nation, B.L.; Staats, W.L.; Argibay, N. Shear-induced softening of nanocrystalline metal interfaces at cryogenic temperatures. Scr. Mater. 2018, 143, 54–58. [Google Scholar] [CrossRef]
- Shekhar, C.; Wani, M.F.; Sehgal, R.; Saleem, S.S.; Ziyamukhamedova, U.; Tursunov, N. Recent Progress in Particulate Reinforced Copper-Based Composites: Fabrication, Microstructure, Mechanical, and Tribological Properties—A Review. Adv. Eng. Mater. 2024, 27, 2401748. [Google Scholar] [CrossRef]
- Ilie, F.; Ipate, G. A Modelling Study of the Correlation between the Layer Obtained by Selective Transfer and the Dislocations Movement at the Friction Surfaces Limit. Metals 2022, 12, 180. [Google Scholar] [CrossRef]
- Available online: https://www.chembk.com/en/chem/Acrolein (accessed on 29 April 2025).
- Shekhar, C.; Wani, M.F.; Sehgal, R.; Ziyamukhamedova, U.; Tursunov, N. A Novel Ceramic Reinforced Metal Matrix Composite (Cu–Ni/TiC–CaF2): Fabrication, Microstructure, Mechanical and Tribological Characterization. Metall. Mater. Trans. B 2025, 56, 1289–1315. [Google Scholar] [CrossRef]
- Banu, I.; Guta, G.; Bildea, C.S.; Bozga, G. Design and performance evaluation of a plant for glycerol conversion to acrolein. Environ. Eng. Manag. J. 2015, 14, 509–517. [Google Scholar] [CrossRef]
- Ilie, F.; Laurian, T. Investigation into the Effect of Concentration of Benzotriazole on the Selective Layer Surface in the Chemical Mechanical Planarization Process. J. Mater. Eng. Perform. 2015, 24, 4919–4927. [Google Scholar] [CrossRef]
- Ilie, F. Modelling of the contact processes in a friction pair with selective-transfer. J. Mater. Res. Technol. 2021, 12, 2453–2461. [Google Scholar] [CrossRef]
- Juozas Padgurskas, J.; Snitka, V.; Jankauskas, V.; Andriušis, A. Selective transfer phenomenon in lubricated sliding surfaces with copper and its alloy coatings made by electro-pulse. Wear 2006, 260, 652–661. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).