The Effect of Kappa Phases on Tribocorrosion Behaviour of Nickel Aluminum Bronze (NAB) and Manganese Aluminum Bronze (MAB)
Abstract
1. Introduction
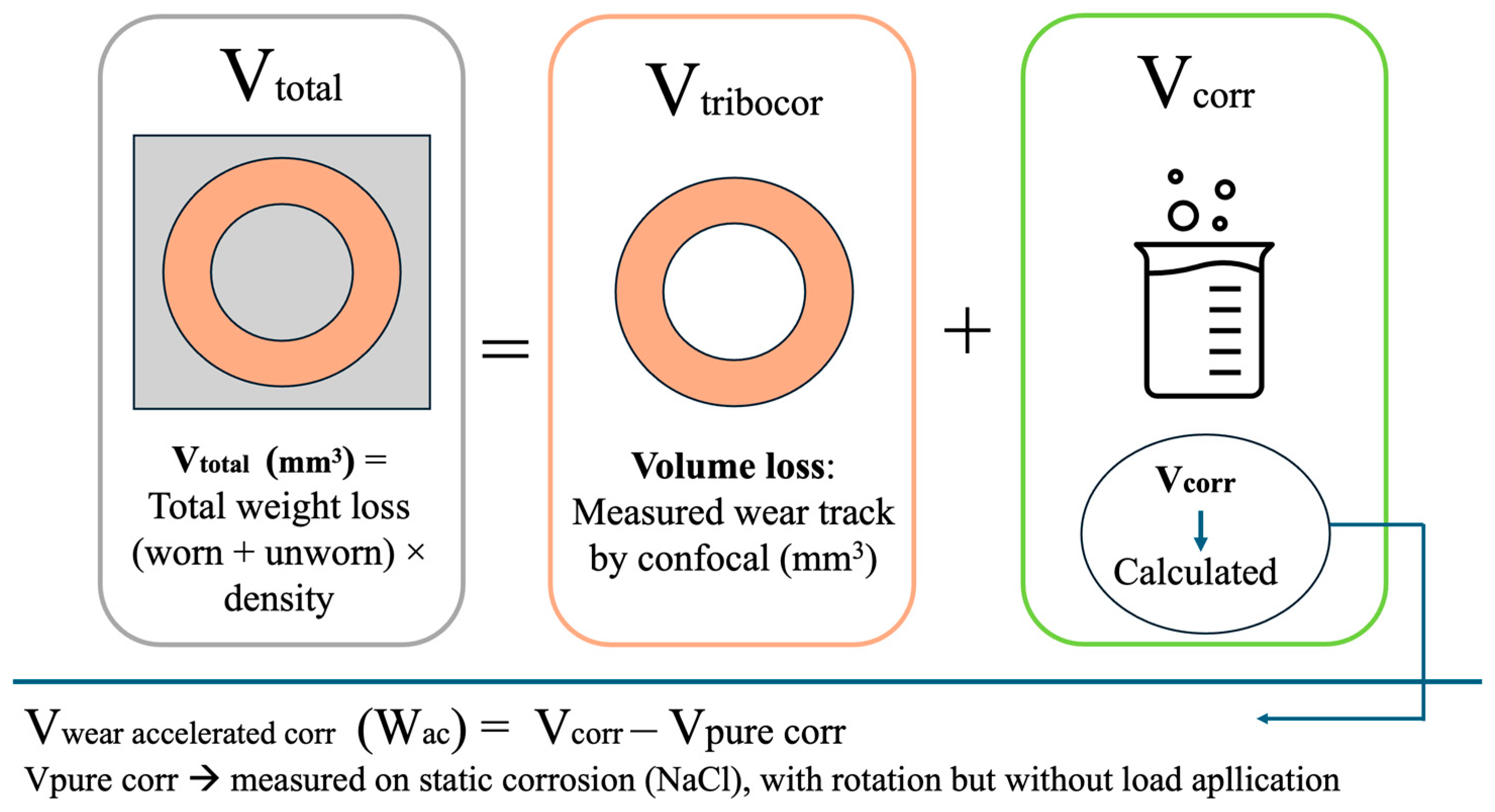
2. Materials and Methods
3. Results and Discussion
3.1. Chemical and Structural Characterization
3.2. Mechanical Properties
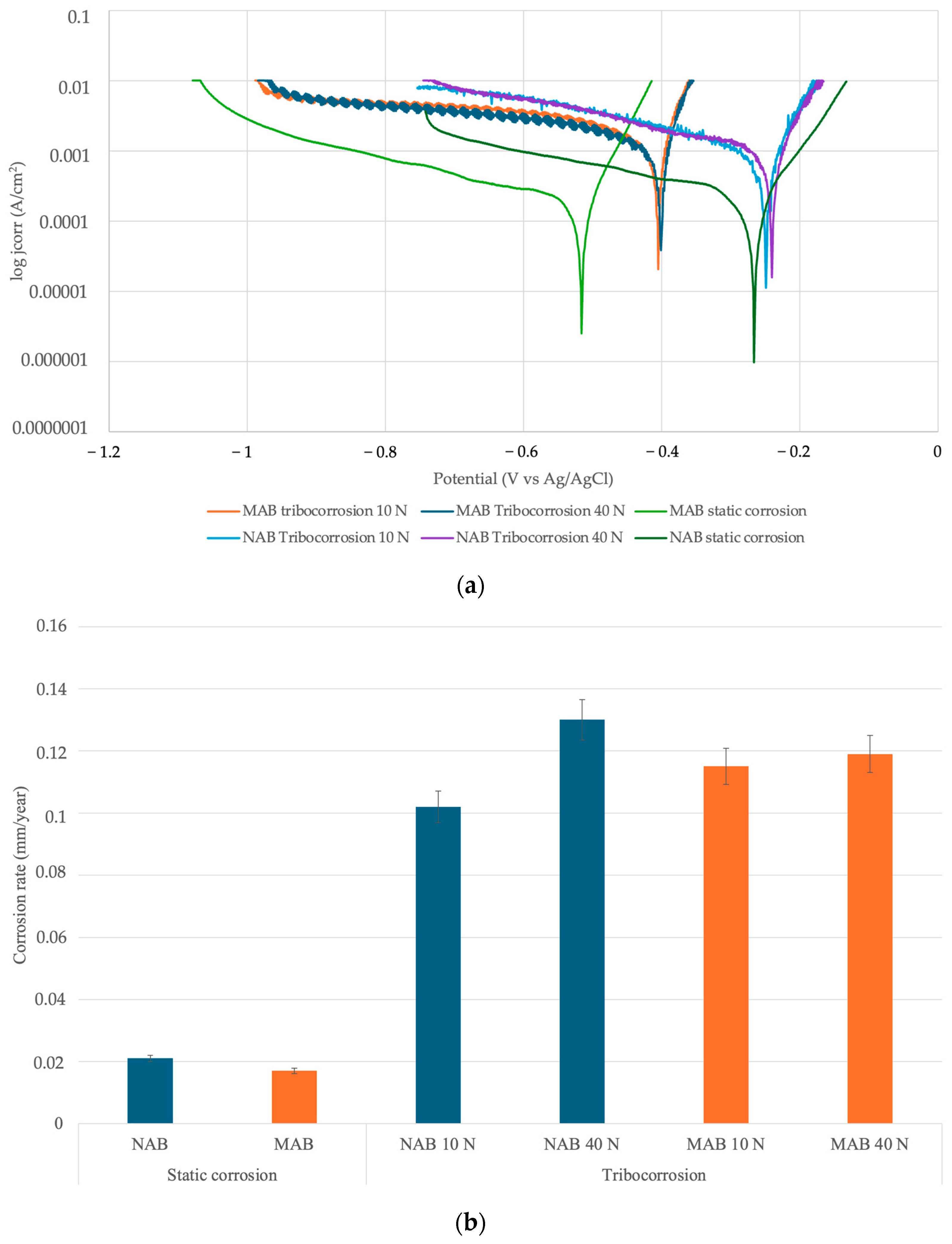
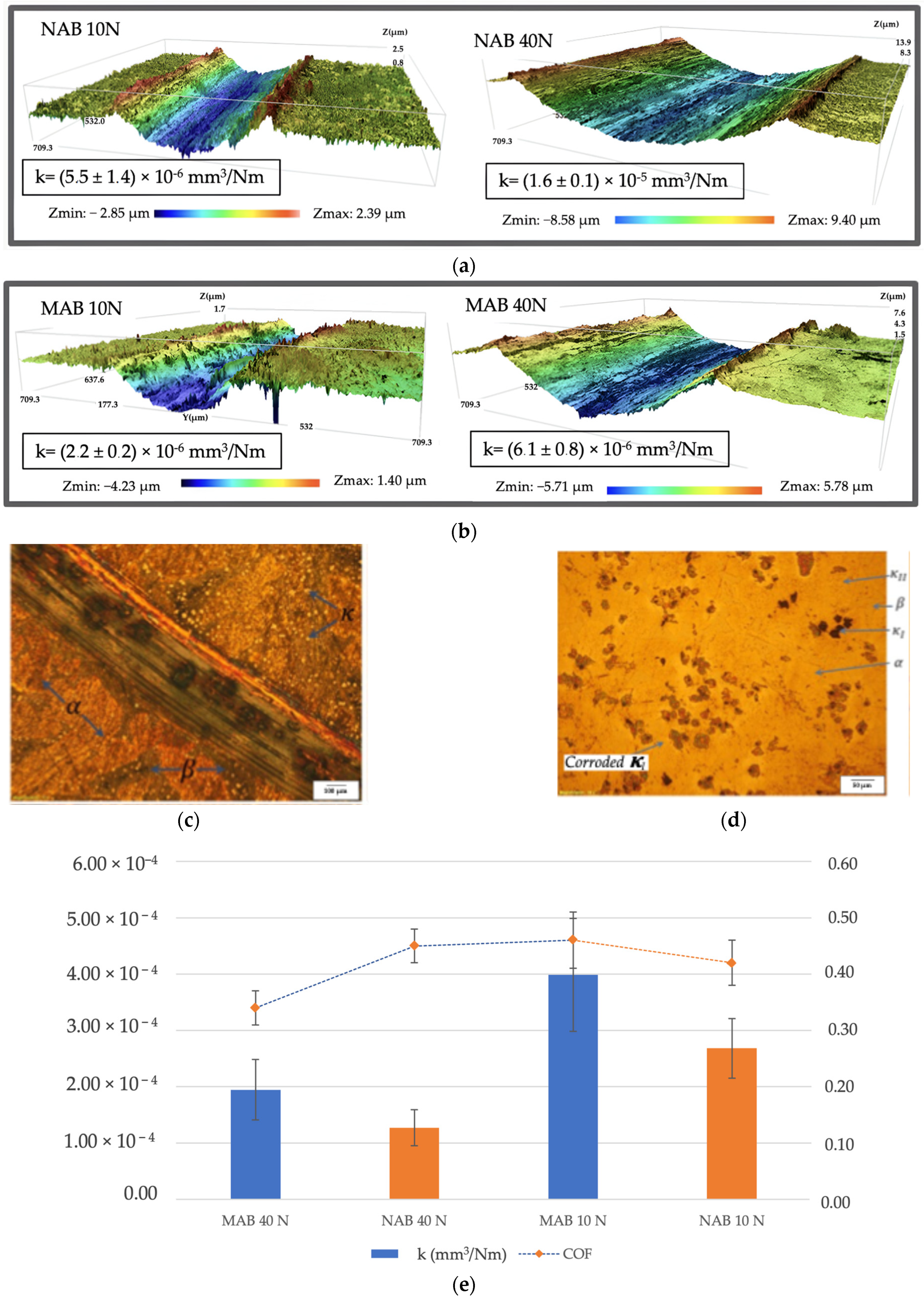
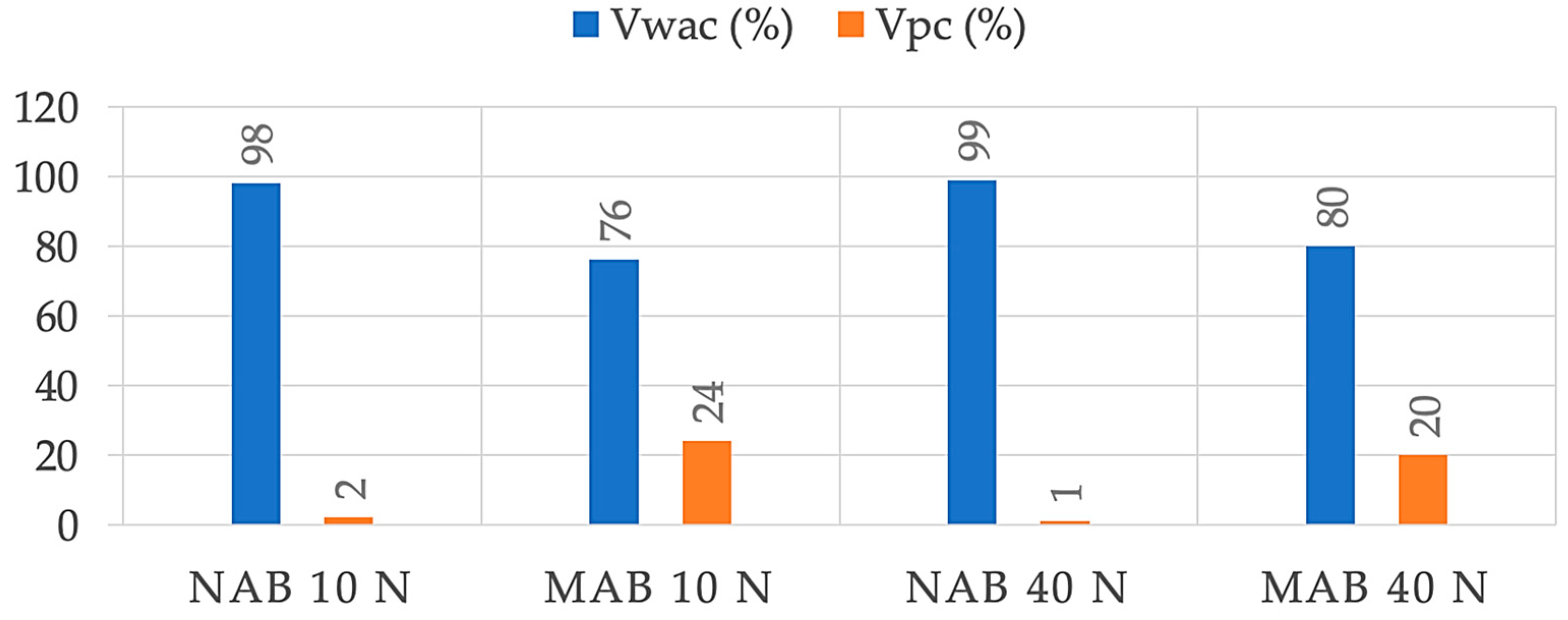
3.3. Corrosion and Tribocorrosion Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MAB | Manganese-Aluminum Bronze |
| NAB | Nickel-Aluminum Bronze |
| SEM | Scanning Electron Microscopy (SEM) |
| XRD | X-ray diffraction (COF) |
| COF | coefficient Of Friction |
| OCP | Open Circuit Potential |
References
- Ponthiaux, P.; Wenger, F.; Drees, D.; Celis, J.P. Electrochemical techniques for studying tribocorrosion processes. Wear 2004, 256, 459–468. [Google Scholar] [CrossRef]
- Stack, M.M. Mapping tribo-corrosion processes in dry and in aqueous conditions: Some new directions for the new millennium. Tribol. Int. 2002, 35, 681–689. [Google Scholar] [CrossRef]
- De Stefano, M.; Ruggiero, A. Tribocorrosion of couplings in seawater environment: An investigation on the positive-negative role of synergy. Tribol. Int. 2024, 200, 110143. [Google Scholar] [CrossRef]
- Munoz, A.I.; Espallargas, N.; Mischler, S. Tribocorrosion; Springer International Publishing: Cham, Switzerland, 2020; pp. 93–94. [Google Scholar]
- Mischler, S.; Debaud, S.; Landolt, D. Wear-accelerated corrosion of passive metals in tribocorrosion systems. J. Electrochem. Soc. 1998, 145, 750. [Google Scholar] [CrossRef]
- Wood, R.J. Marine wear and tribocorrosion. Wear 2017, 376, 893–910. [Google Scholar] [CrossRef]
- Anaee, R.A.M.; Abdulmajeed, M.H. Tribocorrosion. In Advances in Tribology; Darji, P.H., Ed.; IntechOpen: London, UK, 2016; pp. 89–110. [Google Scholar] [CrossRef]
- Pei, W.; Pei, X.; Xie, Z.; Wang, J. Research progress of marine anti-corrosion and wear-resistant coating. Tribol. Int. 2024, 198, 109864. [Google Scholar] [CrossRef]
- Min, J.; Wang, X.-Z.; Wang, Y.; Bai, Y.; Sabola, S.A.; Gong, W.; Wang, L.; Li, J.; Li, Z. Tribocorrosion in nitric acid of Zr alloy, Ti alloy, and 310 SS used for reprocessing of spent nuclear fuel. Tribol. Int. 2024, 201, 110184. [Google Scholar] [CrossRef]
- He, X.; Kumara, C.; Sulejmanovic, D.; Keiser, J.R.; Gallego, N.; Qu, J. Tribocorrosion of stainless steel sliding against graphite in FLiNaK molten salt. Wear 2023, 522, 204706. [Google Scholar] [CrossRef]
- Okoani, A.O.; Nand, A.; Jiang, C.P.; Ramezani, M. Investigating the Tribocorrosion Behaviour of NiTiNOL60 Alloy in Engineering and Biomedical Applications—An Overview. Metals 2004, 14, 1334. [Google Scholar] [CrossRef]
- Bueno, A.H.S.; Solis, J.; Zhao, H.; Wang, C.; Simoes, T.A.; Bryant, M.; Neville, A. Tribocorrosion evaluation of hydrogenated and silicon DLC coatings on carbon steel for use in valves, pistons and pumps in oil and gas industry. Wear 2018, 394–395, 60–70. [Google Scholar] [CrossRef]
- Li, Z.; Yu, H.; Sun, D. The tribocorrosion mechanism of aluminum alloy 7075-T6 in the deep ocean. Corros. Sci. 2021, 183, 109306. [Google Scholar] [CrossRef]
- Silva, R.C.C.; Nogueira, R.P.; Bastos, I.N. Tribocorrosion of UNS S32750 in chloride medium: Effect of the load level. Electrochim. Acta 2011, 56, 8839–8845. [Google Scholar] [CrossRef]
- Sousa, L.; Basilio, L.; Alves, A.C.; Toptan, F. Tribocorrosion-resistant biofunctionalized Ti-Al2O3 composites. Surf. Coat. Technol. 2021, 420, 127329. [Google Scholar] [CrossRef]
- Mégret, A.; Prince, L.; Olivier, M.G.; Vitry, V. Tribo-and tribocorrosion properties of magnesium AZ31 alloy. Coatings 2023, 13, 448. [Google Scholar] [CrossRef]
- Akinribide, O.J.; Akinwamide, S.O.; Obadele, B.A.; Ogundare, O.D.; Ayeleru, O.O.; Olubambi, P.A. Tribological behaviour of ductile and austempered grey cast iron under dry environment. Mater. Today Proc. 2021, 38, 1174–1182. [Google Scholar] [CrossRef]
- Possoli, F.A.A.; Souza, A.P.N.; Bernardelli, E.A.; Borges, P.C. Tribocorrosion assessment of low-temperature plasma nitrided super duplex stainless steel. Surf. Coat. Technol. 2024, 479, 130572. [Google Scholar] [CrossRef]
- Bidiville, A.; Favero, M.; Stadelmann, P.; Mischler, S. Effect of surface chemistry on the mechanical response of metals in sliding tribocorrosion systems. Wear 2007, 263, 207–217. [Google Scholar] [CrossRef]
- Aslan, N.S.; Rajih, A.K.; Haleem, A.H. Effect of Alloying Elements on the Erosion–Corrosion Behaviour of Cu-Based Alloys. IOP Conf. Ser. Mater. Sci. Eng. 2020, 987, 012018. [Google Scholar] [CrossRef]
- Wang, K.; Tian, X.; Liu, X.; Wang, G.; Deng, J. Study on the erosion-corrosion behavior of copper-nickel alloys in high temperature and high humidity marine environment. J. Phys. Conf. Ser. 2025, 2951, 012103. [Google Scholar] [CrossRef]
- Wu, L.; Xu, Y.; Ma, A.; Zhang, L.; Zheng, Y. Influence of pre-immersion aeration conditions on corrosion product films and erosion-corrosion resistance of 90/10 and 70/30 copper-nickel tubes in 1 wt% NaCl solution. Corros. Sci. 2024, 228, 111817. [Google Scholar] [CrossRef]
- Lin, S.; Li, D.; Zhou, Q.; Chu, M.; Sun, Y.; Liu, M.; Zheng, K.; Qiao, S.; Zhao, L.; Zhao, L.; et al. Study on corrosion perforation behavior of copper nickel alloy pipe during service in marine environment. Eng. Fail. Anal. 2023, 153, 107628. [Google Scholar] [CrossRef]
- Wu, L.; Ma, A.; Zhang, L.; Zheng, Y. Intergranular erosion corrosion of pure copper tube in flowing NaCl solution. Corros. Sci. 2022, 201, 110304. [Google Scholar] [CrossRef]
- Kosec, T.; Ćurković, H.O.; Legat, A. Investigation of the corrosion protection of chemically and electrochemically formed patinas on recent bronze. Electrochim. Acta 2010, 56, 722–731. [Google Scholar] [CrossRef]
- Chiavari, C.; Rahmouni, K.; Takenouti, H.; Joiret, S.; Vermaut, P.; Robbiola, L. Composition and electrochemical properties of natural patinas of outdoor bronze monuments. Electrochim. Acta 2007, 52, 7760–7769. [Google Scholar] [CrossRef]
- Zahner, L.W. Copper, Brass, and Bronze Surfaces: A Guide to Alloys, Finishes, Fabrication and Maintenance in Architecture and Art; John Wiley and Sons: Hoboken, NJ, USA, 2020. [Google Scholar]
- Culpan, E.A.; Rose, G.R.F. Microstructural characterization of cast nickel aluminium bronze. J. Mater. Sci. 1978, 13, 1647–1657. [Google Scholar] [CrossRef]
- Lloyd, D.; Lorimer, G.W.; Ridley, N. Characterization of phases in a nickel-aluminium bronze. Metals Technol. 1980, 7, 114–119. [Google Scholar] [CrossRef]
- Poojary, S.; Marakini, V.; Rao, R.N.; Vijayan, V. Enhancing microstructure and mechanical properties of nickel aluminium bronze alloy through tin addition. Sci. Rep. 2023, 13, 16907. [Google Scholar] [CrossRef]
- Küçükömeroğlu, T.; Şentürk, E.; Kara, L.; İpekoğlu, G.; Çam, G. Microstructural and mechanical properties of friction stir welded nickel-aluminum bronze (NAB) alloy. J. Mater. Eng. Perform. 2016, 25, 320–326. [Google Scholar] [CrossRef]
- Orzolek, S.M.; Semple, J.K.; Fisher, C.R. Influence of processing on the microstructure of nickel aluminum bronze (NAB). Addit. Manuf. 2022, 56, 102859. [Google Scholar] [CrossRef]
- Luca, R.; Nascimento, P.H.T.; Santos, V.T.; Silva, M.R.; Lobo, F.G.; Teram, R.; Nascimento, M.S.; Couto, A.A.; Filho, A.M.; dos Santos, G.A. Correlation of Solidification Thermal Variables with Microstructure and Hardness in CuMn11Al8Fe3Ni3 Manganese–Aluminum–Bronze Alloy. Materials 2025, 18, 234. [Google Scholar] [CrossRef]
- Böhm, J.; Linhardt, P.; Strobl, S.; Haubner, R.; Biezma, M.V. Microstructure of a heat treated nickel-aluminum bronze and its corrosion behavior in simulated fresh and sea water. Mater. Perform. Charact. 2016, 5, 689–700. [Google Scholar] [CrossRef]
- Haubner, R.; Strobl, S.; Ball, G.; Linhardt, P.; Biezma, M.V. Effects of heat treatment on the microstructure and corrosion behavior of manganese aluminum bronzes. Pract. Metallogr. 2024, 61, 769–782. [Google Scholar] [CrossRef]
- Wharton, J.A.; Barik, R.C.; Kear, G.; Wood, R.J.K.; Stokes, K.R.; Walsh, F.C. The corrosion of nickel–aluminium bronze in seawater. Corros. Sci. 2005, 47, 3336–3367. [Google Scholar] [CrossRef]
- Huttunen-Saarivirta, E.; Isotahdon, E.; Metsäjoki, J.; Salminen, T.; Carpén, L.; Ronkainen, H. Tribocorrosion behaviour of aluminium bronze in 3.5 wt.% NaCl solution. Corros. Sci. 2018, 144, 207–223. [Google Scholar] [CrossRef]
- Zhang, B.B.; Wang, J.Z.; Yuan, J.Y.; Yan, F.Y. Tribocorrosion behavior of nickel aluminum bronze in seawater: Identification of corrosion-wear components and effect of pH. Mater. Corros. 2018, 69, 106–114. [Google Scholar] [CrossRef]
- Song, Q.N.; Li, H.L.; Nan, X.U.; Jiang, Z.Y.; Zhang, G.Y.; Bao, Y.F.; Jiang, Y.-F.; Zhao, L.-J.; Ji, C.-C.; Zhao, J.-H.; et al. Selective phase corrosion and cavitation erosion behaviors of various copper alloys in 3.5% NaCl solutions with different pH values. Trans. Nonferrous Met. Soc. China 2023, 33, 3039–3053. [Google Scholar] [CrossRef]
- Tini, S.C.; Zeren, A.; Avcu, Y.Y.; Abakay, E.; Guney, M.; Avcu, E. Cavitation erosion behaviour of MAB-CU4 alloy: Influences of cavitation number, attack angle, time, and stand-off distance. Mater. Res. Express 2024, 11, 116506. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An Improved technique for determining hardness and Elastic Modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Yan, Q.; Zhang, H.; Man, C.; Pang, K.; Wang, X.; Cui, Z.; Cui, H. The Effect of Intermetallic Compounds on Corrosion and Tribocorrosion Behavior of 7075 Aluminum Alloy. J. Mater. Eng. Perform. 2024, 33, 11494–11509. [Google Scholar] [CrossRef]
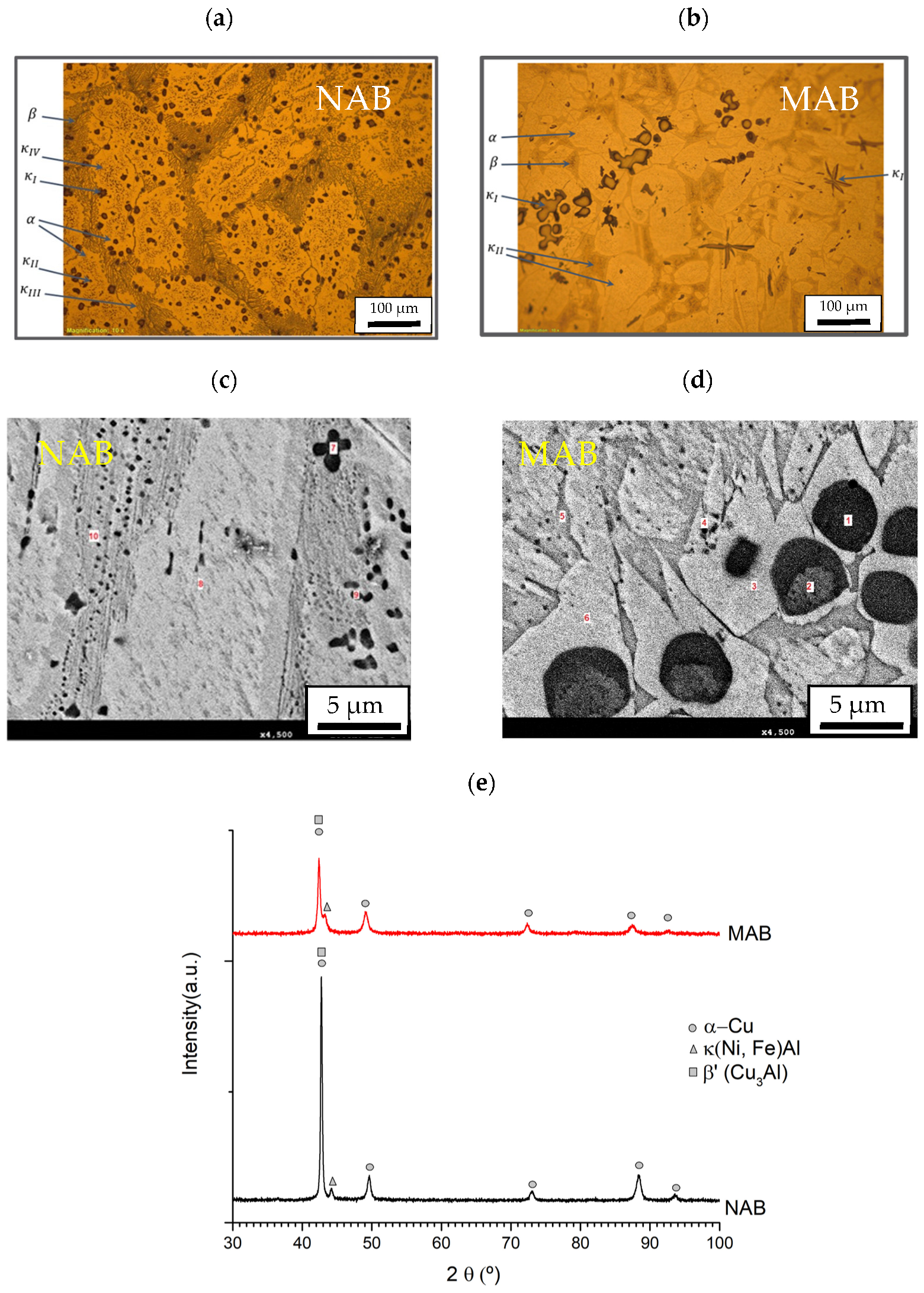




| α Phase (%) | β Phase (%) | Kappa (%) | Mean Size Kappa (µm2) | |
|---|---|---|---|---|
| NAB | 52.9 | 32.3 | 14.3 | 253 |
| MAB | 57.7 | 30.4 | 11.8 | 753 |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Al (%) | 6.1 | 4.8 | 5.5 | 7.0 | 8.4 | 6.5 | 11.7 | 7.7 | 11.1 | 10.0 |
| Ni (%) | 2.2 | 2.0 | 1.7 | 1.0 | 3.0 | 1.7 | 9.8 | 6.3 | 7.8 | 6.3 |
| Mn (%) | 17.8 | 16.8 | 12.0 | 19.9 | 13.2 | 11.0 | 1.7 | 1.3 | 1.7 | 1.3 |
| Fe (%) | 62.0 | 57.6 | 7.8 | 14.4 | 2.5 | 9.8 | 52.9 | 3.7 | 24.3 | 7.0 |
| Cu (%) | 11.5 | 18.3 | 71.0 | 54.9 | 70.7 | 69.0 | 22.9 | 84.2 | 54.5 | 75.0 |
| Cu (mg/L) | Ni (mg/L) | Al (mg/L) | Fe (mg/L) | Mn (mg/L) | |
|---|---|---|---|---|---|
| NAB | 29.66 ± 1.32 | 0.72 ± 0.05 | 3.24 ± 0.18 | 1.36 ± 0.06 | 0.57 ± 0.02 |
| MAB | 1.19 ± 0.06 | 0.25 ± 0.02 | 1.71 ± 0.13 | 13.18 ± 0.45 | 6.98 ± 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berlanga-Labari, C.; Claver, A.; Biezma-Moraleda, M.V.; Fernández-Palacio, J. The Effect of Kappa Phases on Tribocorrosion Behaviour of Nickel Aluminum Bronze (NAB) and Manganese Aluminum Bronze (MAB). Lubricants 2025, 13, 290. https://doi.org/10.3390/lubricants13070290
Berlanga-Labari C, Claver A, Biezma-Moraleda MV, Fernández-Palacio J. The Effect of Kappa Phases on Tribocorrosion Behaviour of Nickel Aluminum Bronze (NAB) and Manganese Aluminum Bronze (MAB). Lubricants. 2025; 13(7):290. https://doi.org/10.3390/lubricants13070290
Chicago/Turabian StyleBerlanga-Labari, Carlos, Adrián Claver, María Victoria Biezma-Moraleda, and José Fernández-Palacio. 2025. "The Effect of Kappa Phases on Tribocorrosion Behaviour of Nickel Aluminum Bronze (NAB) and Manganese Aluminum Bronze (MAB)" Lubricants 13, no. 7: 290. https://doi.org/10.3390/lubricants13070290
APA StyleBerlanga-Labari, C., Claver, A., Biezma-Moraleda, M. V., & Fernández-Palacio, J. (2025). The Effect of Kappa Phases on Tribocorrosion Behaviour of Nickel Aluminum Bronze (NAB) and Manganese Aluminum Bronze (MAB). Lubricants, 13(7), 290. https://doi.org/10.3390/lubricants13070290








