Abstract
This study investigates the tribocorrosion behavior of 304 stainless steel (304SS), S31254 super austenitic stainless steel (S31254 SASS), and a medium-entropy austenitic stainless steel (MEASS) in 3.5 wt.% NaCl solution under sliding conditions. The objective is to clarify the performance differences among these alloys when exposed to simultaneous mechanical wear and corrosion. Electrochemical techniques, including potentiodynamic polarization and potentiostatic sliding tests, were used to evaluate corrosion resistance and repassivation behavior. Quantitative analysis based on ASTM G119 revealed that MEASS showed a 68% lower total material loss compared to 304SS and a 55% lower loss compared to S31254. MEASS also exhibited the lowest corrosion current density (1.46 μA/cm2) under tribocorrosion conditions, representing an 83% reduction compared to 304SS. These improvements are attributed to the higher chromium and nickel contents of MEASS, which enhance passive film stability and reduce susceptibility to localized corrosion. The results demonstrate that MEASS offers superior resistance to combined mechanical and corrosive degradation in chloride-containing environments.
1. Introduction
Materials used in marine engineering are frequently exposed to aggressive environments where corrosion serves as a primary form of degradation. This concern is especially critical in offshore structures, particularly in the splash zone [1,2,3], where alternating exposure to seawater and air accelerates corrosion due to continuous access to oxygen and moisture. When structural components are further subjected to suspended particles in seawater flow, the relative motion between particles and material surfaces accelerates surface degradation [4]. This results in the simultaneous occurrence of mechanical wear and electrochemical corrosion. Although wear and corrosion are often treated as independent degradation mechanisms—mechanical and electrochemical, respectively—their combination can lead to damage more severe than the sum of each individual process [5,6,7,8]. This synergistic phenomenon, known as tribocorrosion, arises from the complex interaction between mechanical and chemical processes and often results in accelerated material loss. Understanding tribocorrosion is therefore essential for optimizing material design and protection strategies in marine environments.
Austenitic stainless steels are widely employed in structural and industrial applications owing to their excellent corrosion resistance and mechanical properties. Their corrosion resistance is primarily attributed to the spontaneous formation of a stable passive film, composed predominantly of chromium-rich oxides [9]. This film serves as a protective barrier, isolating the underlying metal from corrosive environments. However, under tribocorrosion conditions—where mechanical and electrochemical processes act concurrently—the passive film can be repeatedly disrupted by sliding or rubbing, exposing fresh metal and locally compromising passivity. This exposure significantly increases corrosion rates within the wear track [10,11]. Hence, a comprehensive understanding of the tribocorrosion behavior of austenitic stainless steels is crucial for their application in marine environments, where both corrosion and wear stresses are simultaneously present.
Over the past decade, the tribocorrosion behavior of stainless steels has attracted considerable research attention [10,11,12,13,14,15,16,17]. Obadele et al. [10] investigated the tribocorrosion performance of 310SS and 316SS, reporting that 316SS exhibited superior resistance compared to 310SS. This improvement was attributed to the rapid re-passivation capability of 316SS following passive film disruption. Sun and Rana [11] examined 304SS in 0.5 M NaCl solution and observed that both sliding load and velocity significantly influenced the cathodic shift of open-circuit potential (OCP) during sliding. Similarly, Zhang et al. [18] studied the tribocorrosion behavior of S31254 SASS in seawater, finding that corrosion pits acted as preferential sites for mechanical damage. This interaction between localized corrosion and wear produced a strong synergistic effect, accelerating material degradation. In their study, abrasive wear, adhesion, delamination, and plastic deformation were identified as the dominant wear mechanisms. Iwabuchi et al. [19] also explored the sliding wear behavior of stainless steels in seawater and concluded that the observed increase in mass loss resulted from wear-induced corrosion, which differs from the findings reported by other researchers [20,21]. It is worth noting that several tribocorrosion studies on stainless steels have been carried out in acidic media, such as H2SO4 solutions [22,23,24], as stainless steel components in industrial applications are often subjected to aggressive acidic environments that simultaneously promote chemical corrosion and mechanical wear.
In recent years, the need for reliable and affordable materials with strong corrosion resistance and mechanical strength has grown, particularly for use in harsh service environments. SASSs have become a practical alternative to conventional austenitic stainless steels due to their superior performance. With higher levels of chromium (20–24 wt.%), nickel (17–22 wt.%), molybdenum (4–8 wt.%), and nitrogen (0.15–0.5 wt.%), SASSs provide excellent strength, improved toughness, and significantly better resistance to pitting and crevice corrosion compared to common grades such as AISI 304 and 316 [25,26,27,28]. These properties make SASS suitable for a variety of applications in the marine, chemical, mining, and nuclear industries [29,30,31].
Although SASS offers strong resistance to corrosion, components exposed to aggressive environments still face simultaneous wear and corrosion. This combined effect, known as tribocorrosion, can cause more severe material damage than the individual contributions of wear and corrosion alone. To ensure the stable performance of SASS over time, it is important to clarify how these materials respond under such combined conditions. While many studies have investigated the corrosion and tribocorrosion behavior of conventional stainless steels, including S31254 [18,32,33,34], there is still limited information regarding the behavior of advanced stainless steels in similarly harsh environments. Our previous study presented the first comprehensive comparison of the tribocorrosion behavior between a novel MEASS and conventional S31254 alloy in sulfuric acid solution. The results demonstrated that material degradation in MEASS was predominantly caused by mechanical wear. Its improved resistance to material loss, relative to S31254, was attributed to its higher hardness arising from enhanced strain-hardening behavior [35]. Despite these promising results, the tribocorrosion characteristics of MEASS in chloride-containing environments have not yet been investigated. The present work addresses this gap by examining the tribocorrosion responses of three stainless steel alloys in chloride-rich media, aiming to identify the controlling degradation mechanisms and provide insights for the design of more durable alloys for harsh marine environments.
2. Materials and Methodology
2.1. Materials and Sample Preparation
The chemical compositions of the three alloys are listed in Table 1. The MEASS samples were subjected to heat treatment at 700 °C for 4 h, following the procedure established in our previous study [36]. Each specimen was cut into dimensions of 15 mm × 15 mm × 1 mm and ground using SiC abrasive papers up to 4000 grit. Specimens were subsequently cleaned in alcohol using an ultrasonic cleaner for 10 min. Prior to testing, a 1 cm2 test area was defined by masking the remaining surface with electrical insulation tape.

Table 1.
Chemical composition (wt.%) of 304SS, S31254, and MEASS.
2.2. Corrosion and Tribocorrosion Tests
To investigate the corrosion and tribocorrosion behavior of the three alloys, electrochemical techniques were employed, including OCP measurements, potentiodynamic polarization, and potentiostatic sliding tests. All tests were conducted in 3.5 wt.% NaCl solution using a potentiostat (Ref 600, Gamry Instruments, Warminster, PA, USA). A standard three-electrode cell was used, consisting of a saturated calomel electrode (SCE) as the reference, a carbon rod as the counter electrode, and the sample as the working electrode. In the OCP measurements, OCP measurements were conducted without sliding in the first 1200 s and subsequently with sliding for a time period of 3600 s,. and then the potential was recorded continuously for the last 1200 s when the sliding was suspended. Potentiodynamic polarization tests were conducted from −500 mV to +1500 mV at a scan rate of 1 mV/s under both static (pure corrosion) and dynamic (tribocorrosion) conditions. The related corrosion potential (Ecorr) and corrosion current density (icorr) were determined using the Tafel extrapolation method. Potentiostatic sliding tests were conducted at fixed anodic potentials: +100 mVSCE for 304SS and +500 mVSCE for both S31254 and MEASS, based on their respective passive ranges. Current was first measured for 500 s without sliding, followed by 2000 s of sliding, and an additional 500 s without sliding. All tribocorrosion tests were performed using a pin-on-disc tribometer integrated with the potentiostat. A zirconia ball (10 mm diameter) served as the counter body, sliding at 120 rpm under a normal load of 4.9 N in 3.5 wt.% NaCl.
2.3. Microstructural Characterization and Hardness Measurements
Post-test surface morphologies were analyzed using scanning electron microscopy (SEM, JEOL JSM-6510, Tokyo, Japan). Cross-sectional wear profiles were characterized using white-light interferometry (Profilm3D optical profiler, Filmetrics, San Diego, CA, USA). Hardness measurements were performed using a Vickers hardness tester (FM-700e, Future-Tech Corporation, Tokyo, Japan) under a load of 0.5 kg for 10 s. For each specimen, the reported hardness values represent the average of 10 individual tests. The measured hardness values were 220 ± 7 HV for 304SS, 237 ± 3 HV for S31254, and 259 ± 6 HV for MEASS.
2.4. Material Loss Rate Analysis
Tribocorrosion performance was evaluated according to the ASTM G119 standard by calculating the individual contributions of corrosion, wear, and their synergistic effects [37]. Total material loss (T) is expressed as:
T = C0 + W0 + S
In this equation, C0 denotes the corrosion rate in the absence of wear (i.e., pure corrosion), which was calculated from the corrosion current density derived from potentiodynamic polarization curves under static corrosion conditions. W0 represents the wear rate in the absence of corrosion (i.e., pure mechanical wear), determined by measuring the mass difference of the specimen before and after potentiostatic sliding tests conducted at −500 mVSCE for 24 h. The two current density values were subsequently converted to mass loss through Faraday’s law, by normalizing with the exposed surface area and multiplying by the total exposure time of 24 h in the corrosive solution. T value was determined by weighing the specimen before and after the tribocorrosion test conducted at OCP for 24 h. Prior to post-test weighing, the specimen was ultrasonically cleaned in ethanol for 10 min to ensure the complete removal of corrosion products from the surface.
Furthermore, the wear–corrosion synergistic factor (S) can be separated into two components, as defined in Equation (2):
where ∆CW represents the increase in corrosion caused by wear and ∆WC corresponds to the increase in wear resulting from corrosion. These parameters can be determined using Equations (3) and (4), respectively:
S = ΔCW + ΔWC
ΔCW = C − C0
ΔWC = T − W0 − C
The value of C is calculated from the corrosion current density obtained from potentiodynamic polarization curves measured during simultaneous mechanical wear, reflecting the corrosion rate under tribological conditions.
All electrochemical and tribocorrosion tests were repeated at least three times under identical conditions. The data presented in the tables represent the average values with corresponding standard deviations to ensure the reliability and reproducibility of the results.
3. Results
3.1. Intermittent OCP Measurements
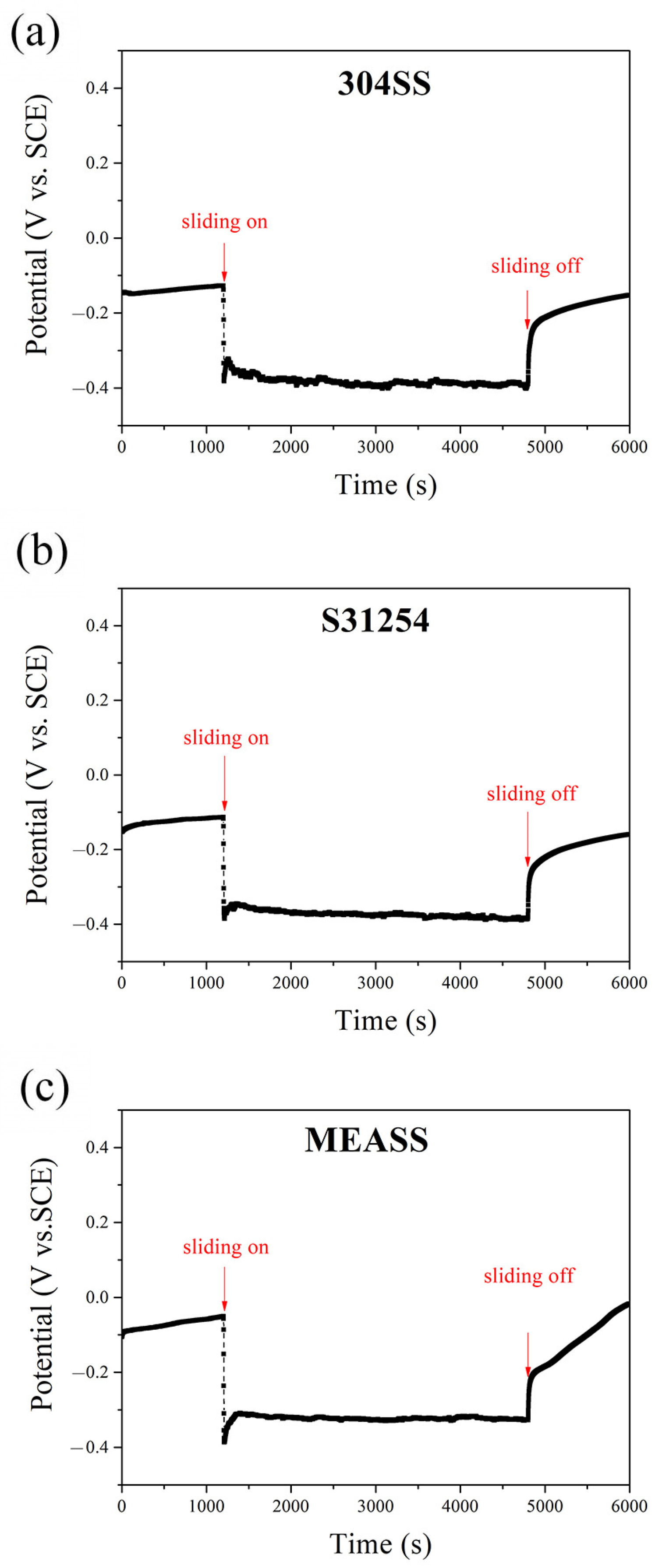
The evolution of OCP for the three alloys—304SS, S31254, and MEASS—before, during, and after sliding in 3.5 wt.% NaCl solution is illustrated in Figure 1. During the initial immersion stage (first 1200 s), the OCP of each alloy gradually stabilized, suggesting the spontaneous formation of protective passive films on their surfaces [38]. Among the three alloys, MEASS showed the most noble potential (−50 mVSCE), followed by S31254 (−110 mVSCE) and 304SS (−130 mVSCE), indicating a more corrosion-resistant passive film on MEASS prior to mechanical disturbance. When sliding was initiated, all alloys exhibited a sudden drop in OCP to more negative values, which is attributed to the mechanical disruption of passive films during sliding, resulting in the exposure of active regions within the wear track [39,40]. This decline was most severe for 304SS and least for MEASS. Throughout the sliding period (3600 s), the OCP values fluctuated significantly for 304SS and S31254, while MEASS maintained a relatively stable and higher potential, suggesting better resistance to continuous passivity breakdown and reformation cycles. Upon termination of sliding, all three alloys began to repassivate, as evidenced by the recovery of OCP values toward their pre-sliding levels. MEASS demonstrated the most rapid and complete recovery, indicating a strong self-repassivation capability. In contrast, 304SS exhibited a slower potential recovery, implying limited ability to re-establish its passive film. These observations support the conclusion that MEASS possesses superior electrochemical stability and repassivation behavior under tribological stress in chloride environments.
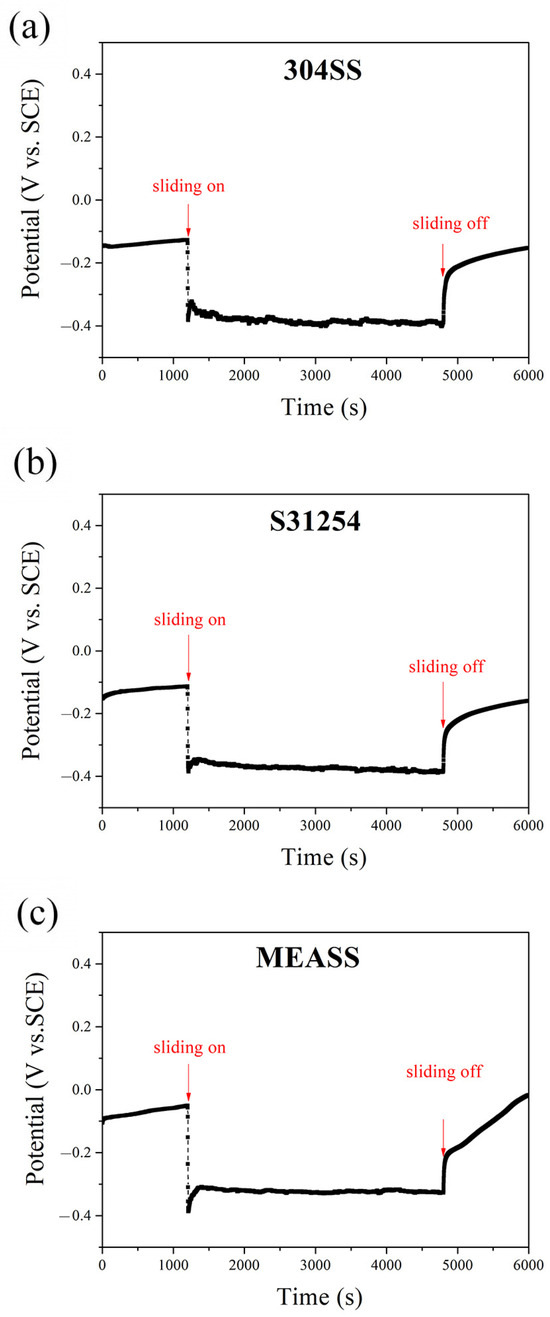
Figure 1.
OCP evolution before, during, and after sliding in NaCl solution: (a) 304SS; (b) S31254; (c) MEASS.
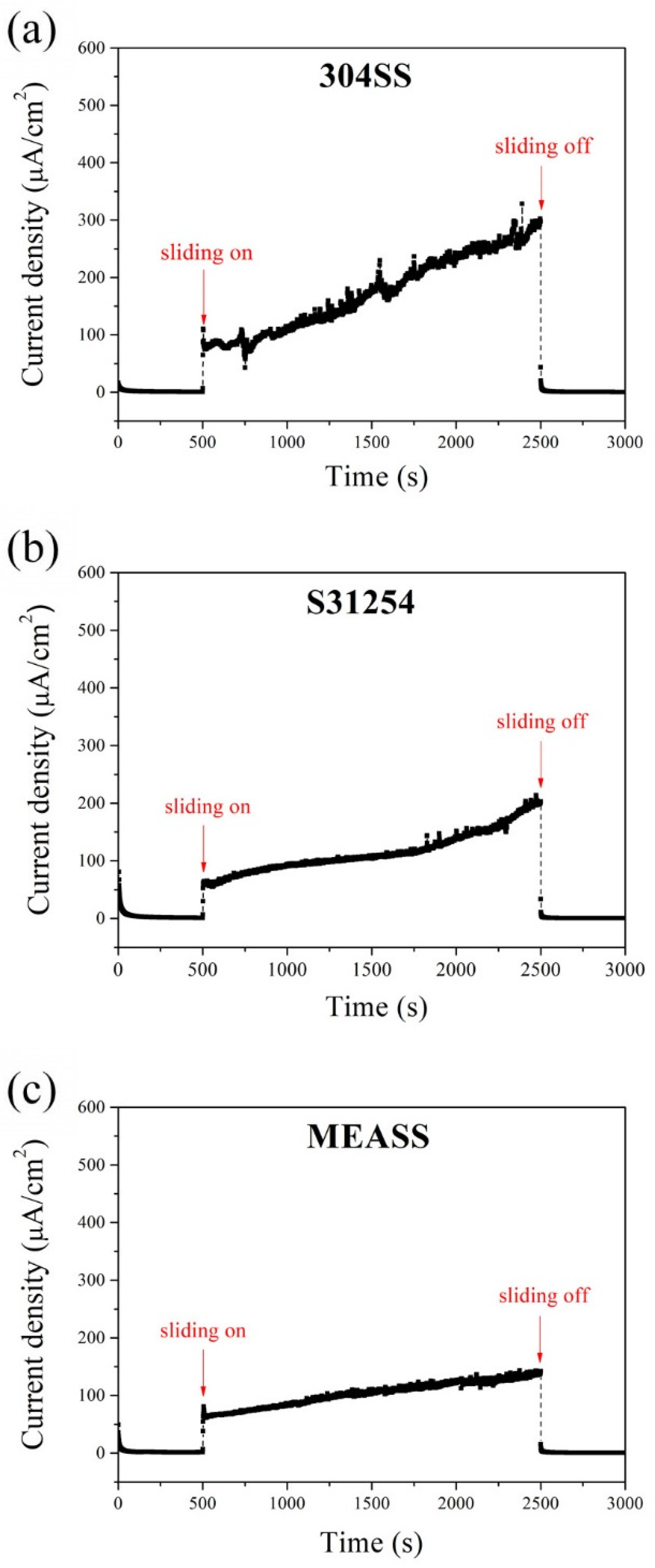
Figure 2 presents the potentiostatic current transients of the three alloys under potentiostatic conditions at their respective passive potentials. During the initial 500 s without sliding, all specimens maintained low and stable current densities, indicating the presence of a stable passive film and minimal electrochemical activity. Once sliding began, the current densities increased significantly due to mechanical removal of the passive film, exposing fresh metal to the electrolyte. These increases were accompanied by current fluctuations throughout the sliding period, reflecting repeated cycles of film breakdown and recovery. Among the three alloys, 304SS showed the highest current rise and the most pronounced fluctuations, reaching 301 μA/cm2 by the end of the sliding stage. S31254 exhibited moderate values, while MEASS maintained the lowest current density (140 μA/cm2) with relatively stable behavior. These results indicate that MEASS offers improved electrochemical stability and repassivation capability when exposed to mechanical wear under potentiostatic conditions.
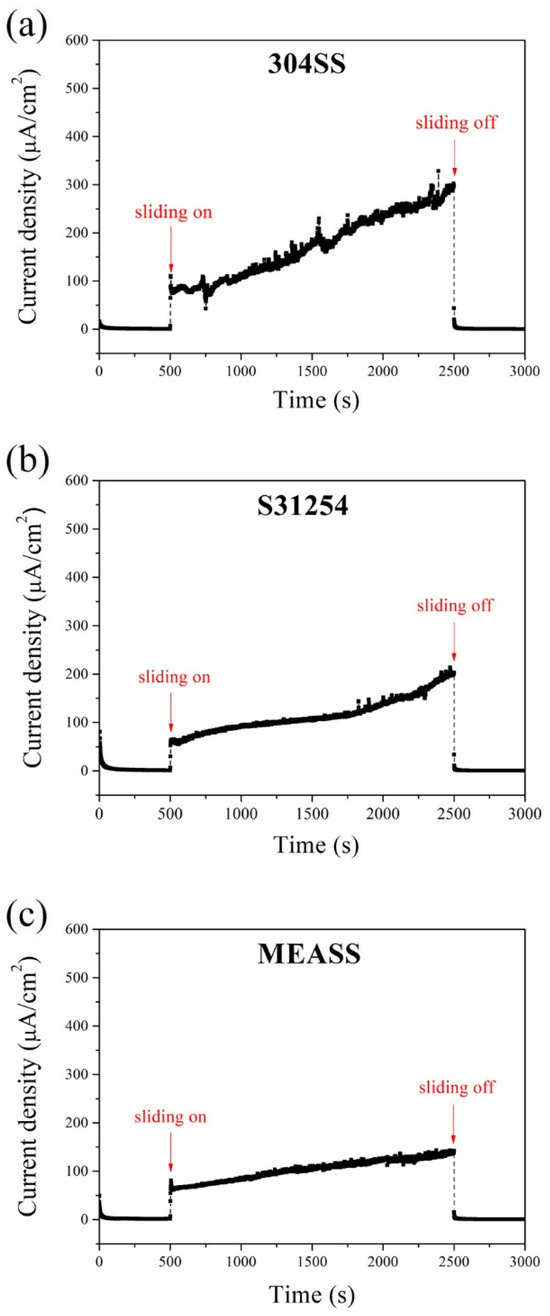
Figure 2.
Potentiostatic current transients at anodic potential: (a) 304SS; (b) S31254; (c) MEASS.
3.2. Potentiodynamic Polarization Test
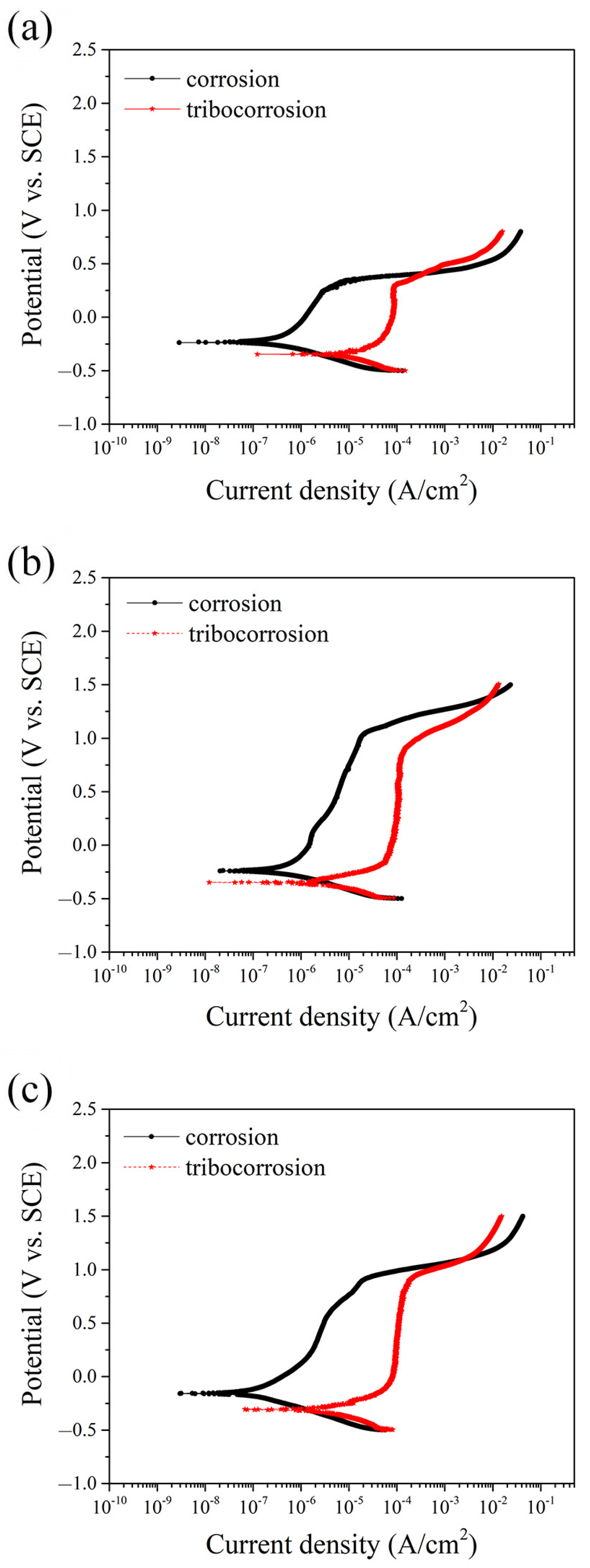
To further evaluate the corrosion and tribocorrosion behavior of the tested alloys, potentiodynamic polarization curves were obtained under both static (immersion) and dynamic (sliding) conditions, as shown in Figure 3. The relevant electrochemical parameters, including corrosion potential (Ecorr), corrosion current density (icorr) and passive current density (ipass), are summarized in Table 2. Under static conditions, as illustrated in Figure 3, all three alloys exhibited distinct active-passive transitions, which are characteristic of stainless steels in chloride environments. Among them, MEASS showed the highest Ecorr and the lowest icorr values, indicating superior corrosion resistance and passive film stability. In contrast, 304SS had the lowest Ecorr and the highest icorr, suggesting its greater susceptibility to corrosion in NaCl solution. When sliding was introduced, simulating tribocorrosion conditions, each alloy experienced a significant negative shift in Ecorr and a marked increase in icorr, demonstrating the combined effect of mechanical disruption and electrochemical attack. As shown in Table 2, the increase in icorr for 304SS was particularly pronounced, rising by more than an order of magnitude, which reflects severe damage to its passive film and reduced repassivation capability. S31254 exhibited moderate deterioration in its electrochemical parameters, while MEASS maintained the most stable performance, with the highest Ecorr and the lowest icorr under sliding conditions. This indicates that MEASS is more resistant to tribocorrosion-induced degradation and capable of sustaining protective film integrity during mechanical wear.
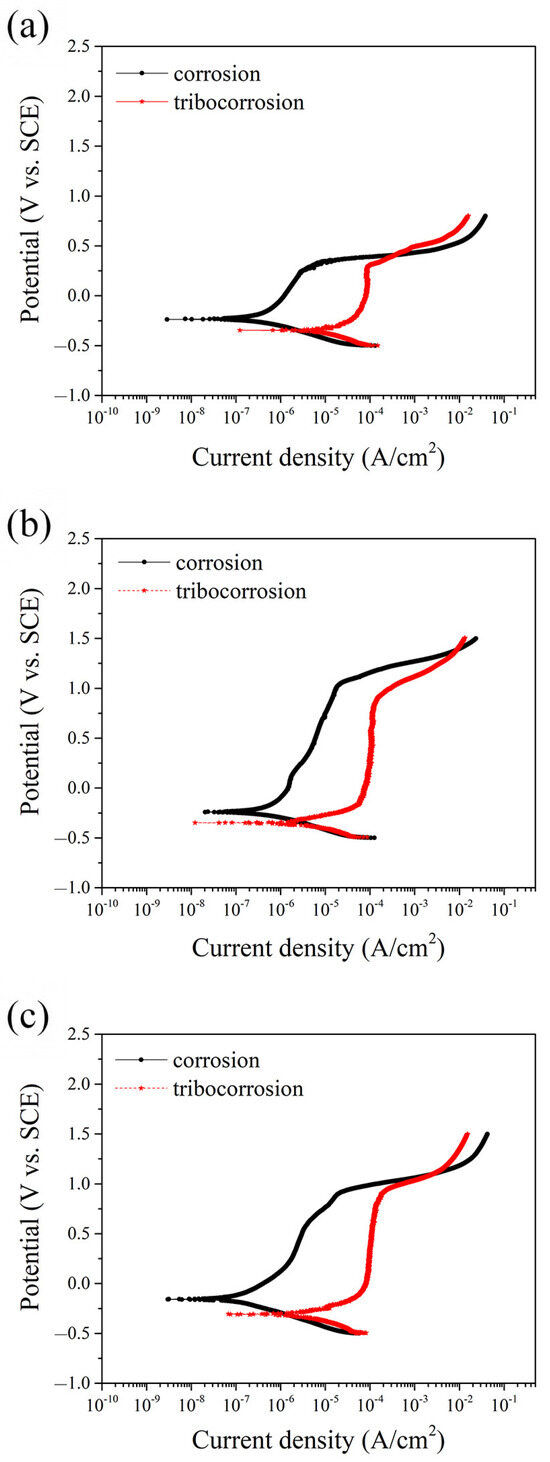
Figure 3.
Potentiodynamic polarization curves of the three alloys in 3.5 wt.% NaCl solution under static and sliding conditions: (a) 304SS; (b) S31254; (c) MEASS.

Table 2.
Electrochemical parameters from the polarization curves of the three alloys under static and sliding conditions.
3.3. Wear Morphology and Material Loss
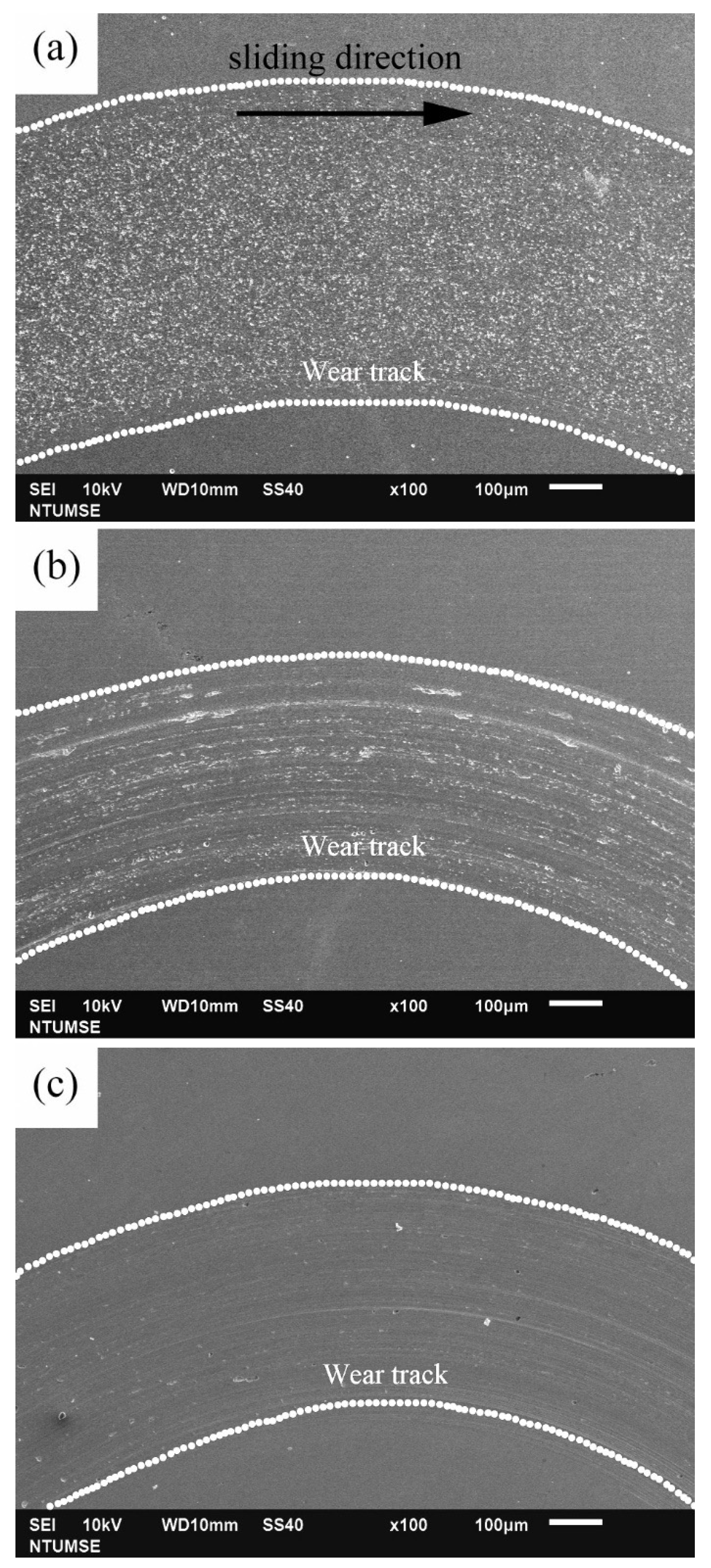
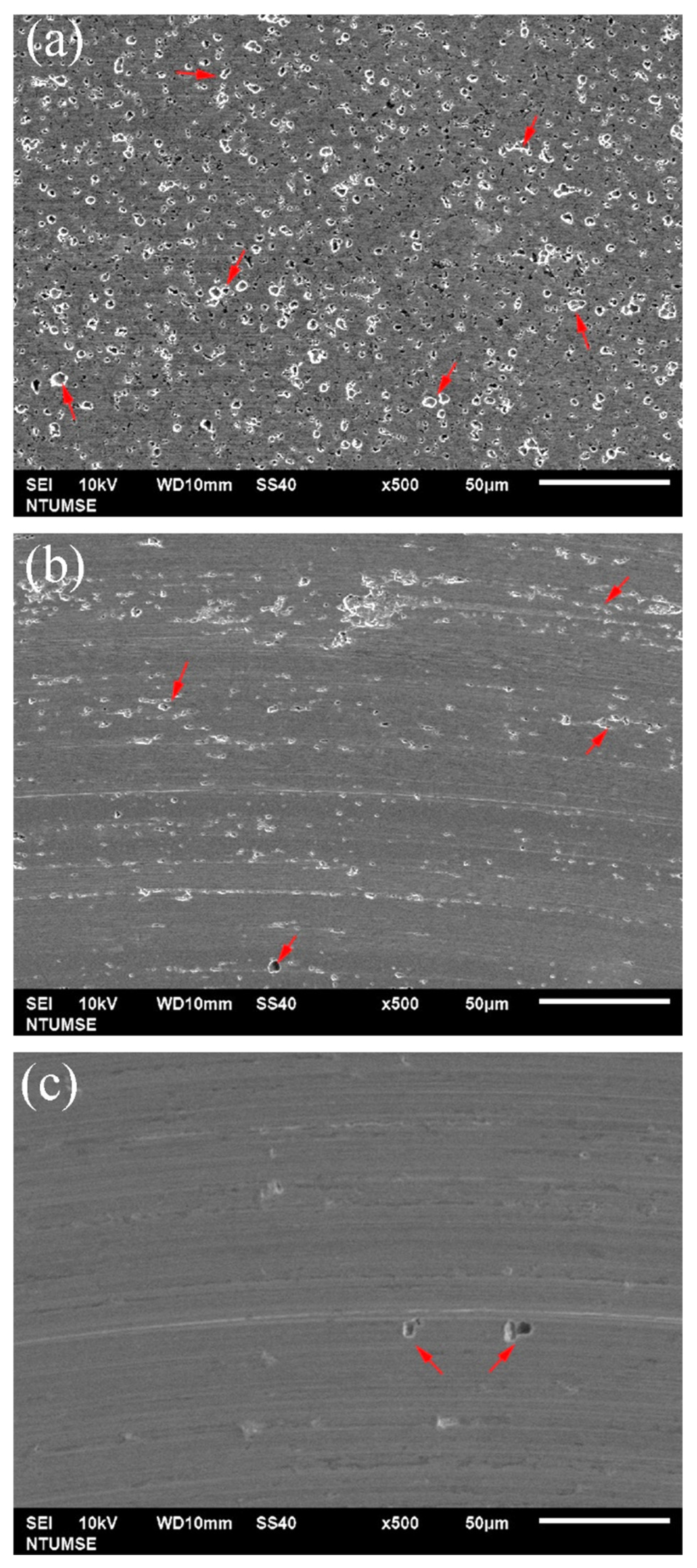
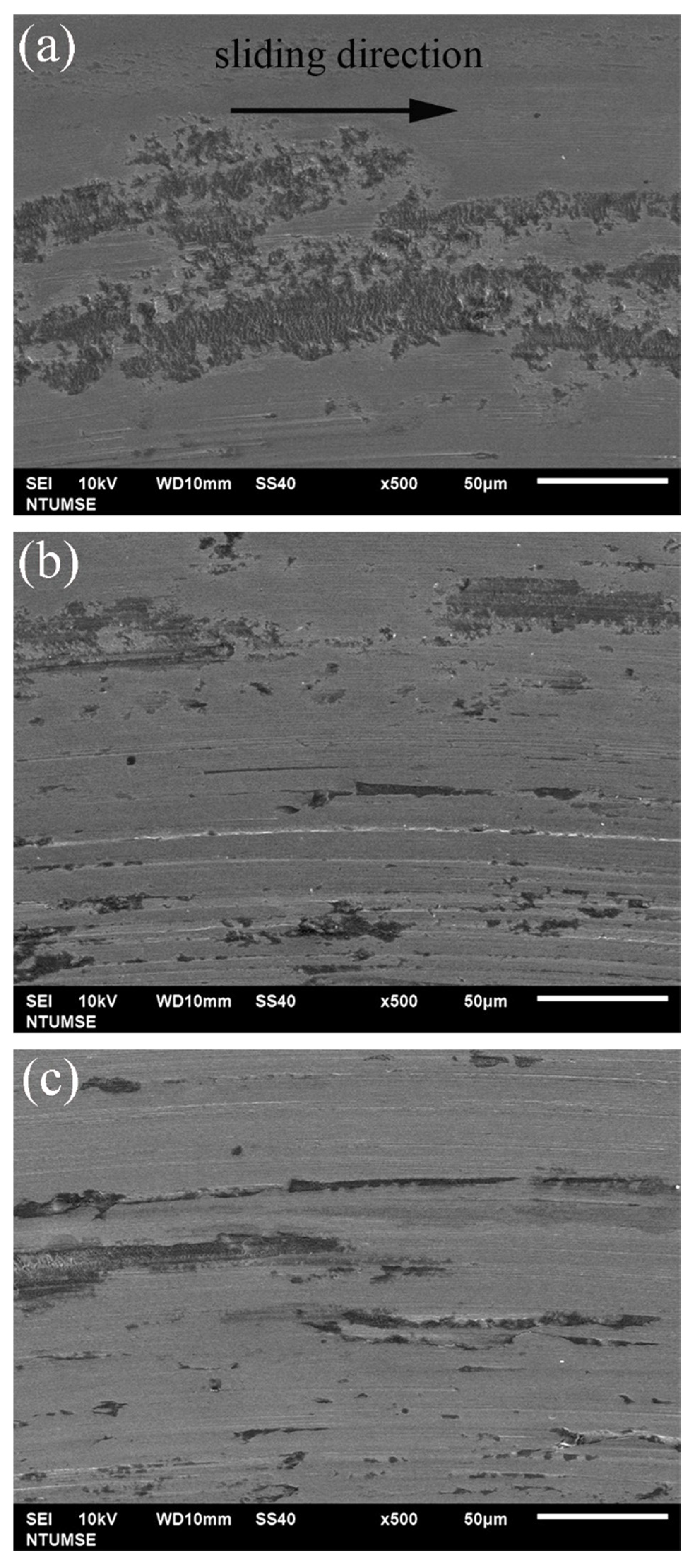
To better illustrate the morphological changes of the three alloys under tribocorrosion and pure mechanical wear conditions, SEM images of the worn surfaces obtained after potentiodynamic polarization measurements with sliding are presented in Figure 4. Figure 5 shows high-magnification views of the central regions of the wear tracks shown in Figure 4. Sliding directions are indicated by arrows in the images. Figure 6 presents SEM images of the worn surfaces obtained after potentiostatic sliding tests conducted at −500 mVSCE for 24 h. Significant differences in wear morphology can be observed among the alloys. As shown in Figure 4, the wear track of 304SS after tribocorrosion testing is notably broader compared to those of S31254 and MEASS. Moreover, Figure 5a,b reveal numerous corrosion pits on the worn surfaces of the 304SS and S31254 specimens. These corrosion pits are indicated by red arrows in Figure 5. The surface of 304SS is densely populated with deep corrosion pits. The surface of S31254 shows relatively fewer pits, and their depth appears shallower than those observed on 304SS, suggesting a more protective passive film and improved resistance to chloride-induced attack. MEASS displays the most favorable surface conditions, with only isolated shallow pits and minimal surface disruption, as shown in Figure 5c. Its surface remains largely preserved following the tribocorrosion test. This observation highlights the alloy’s ability to maintain passivity and resist localized attack in a chloride environment. These observations indicate that MEASS possesses superior resistance to tribocorrosion compared to 304SS and S31254.
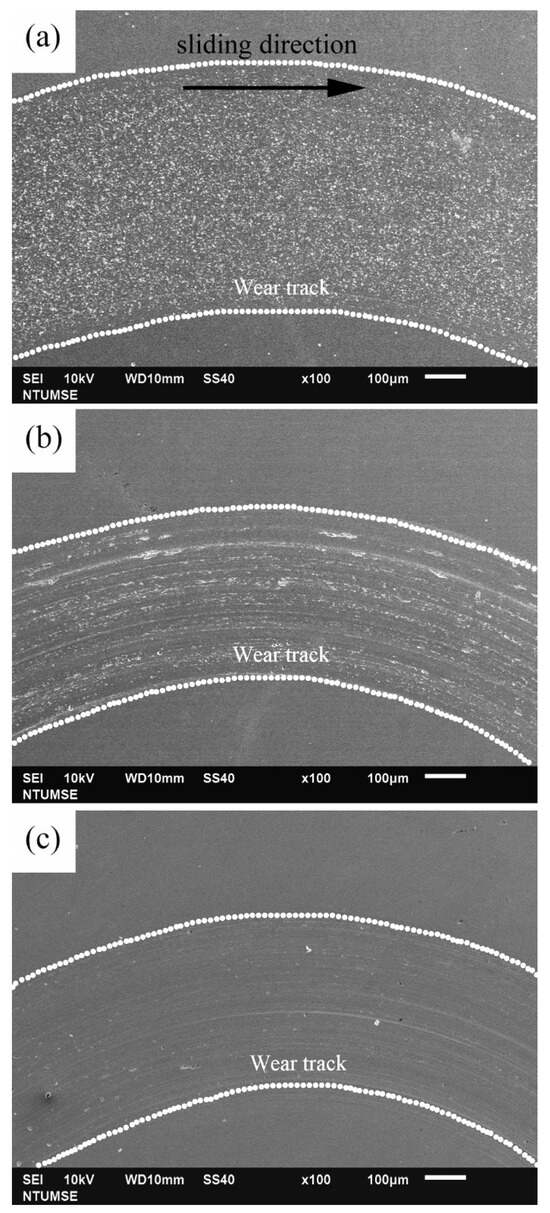
Figure 4.
SEM images of wear tracks after potentiodynamic polarization measurements under sliding conditions: (a) 304SS; (b) S31254; (c) MEASS. Arrows indicate sliding direction.
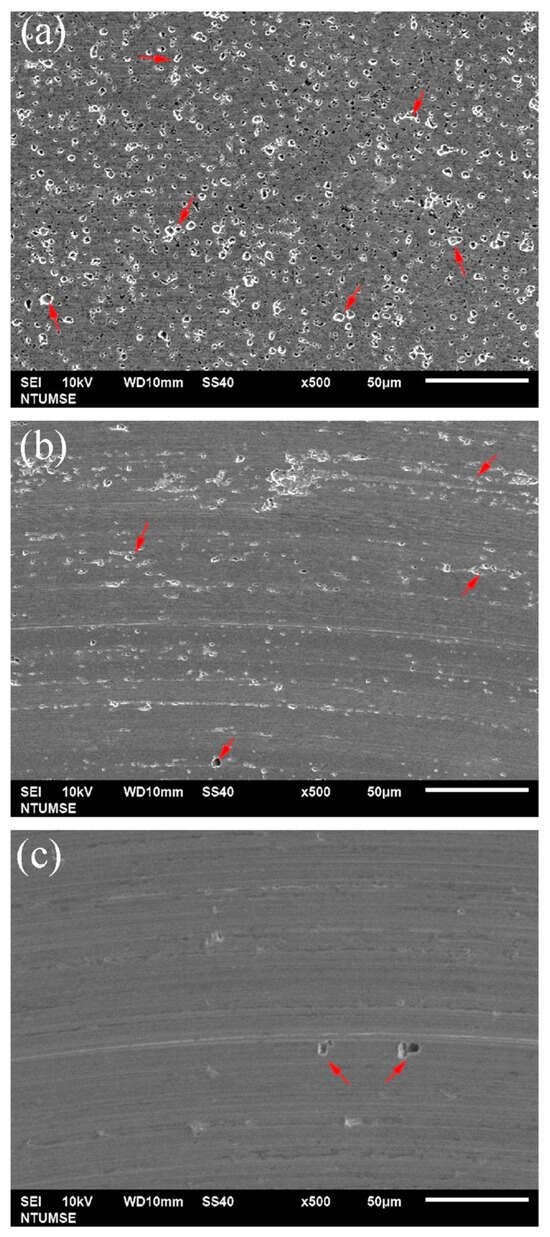
Figure 5.
High-magnification SEM images of the central regions of the wear tracks on (a) 304SS, (b) S31254, and (c) MEASS after potentiodynamic polarization measurements under sliding conditions. Red arrows indicate corrosion pits formed within the wear tracks.
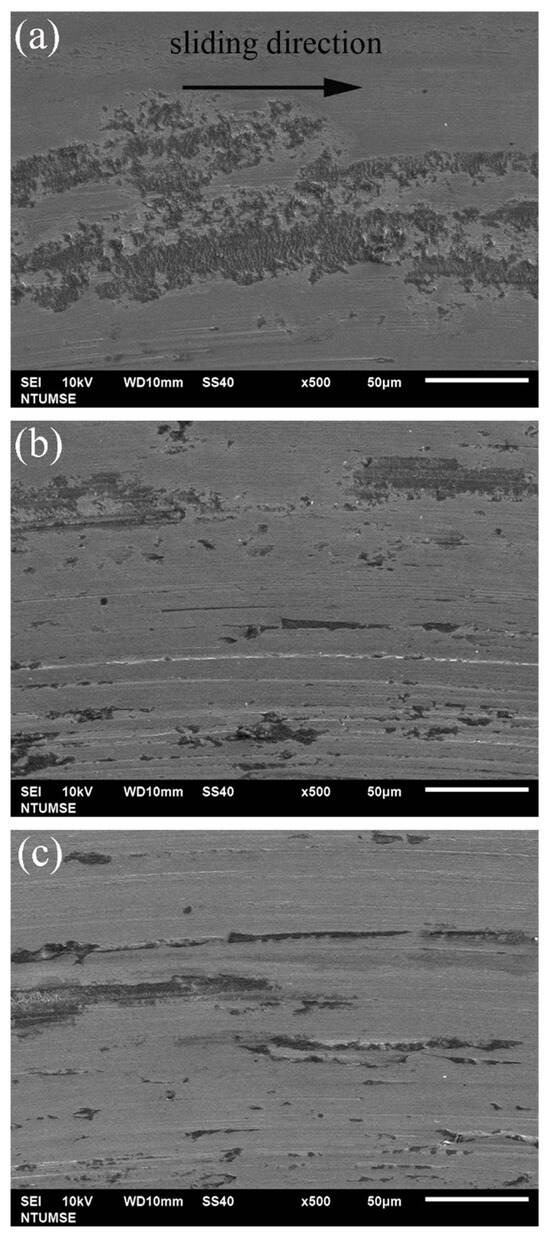
Figure 6.
High-magnification SEM images of the central regions of the wear tracks on (a) 304SS, (b) S31254, and (c) MEASS after tribocorrosion tests conducted under cathodic polarization conditions. Arrows indicate sliding direction.
In addition to the tribocorrosion tests conducted at OCP, the wear track morphologies of the three alloys were also examined under cathodic polarization conditions, corresponding to the pure mechanical wear condition (W0). As shown in Figure 6, the surface features observed under cathodic conditions differ markedly from those under tribocorrosion at OCP (Figure 4 and Figure 5). Since corrosion was effectively suppressed under cathodic polarization, no corrosion pits were observed. Instead, the wear tracks were primarily characterized by peeling marks resulting from repeated mechanical contact. Among the three materials, 304SS exhibited the most evident peeling features, whereas MEASS showed the least, consistent with their relative hardness. This trend will be further supported by the quantitative analysis of W0 presented later in the discussion.
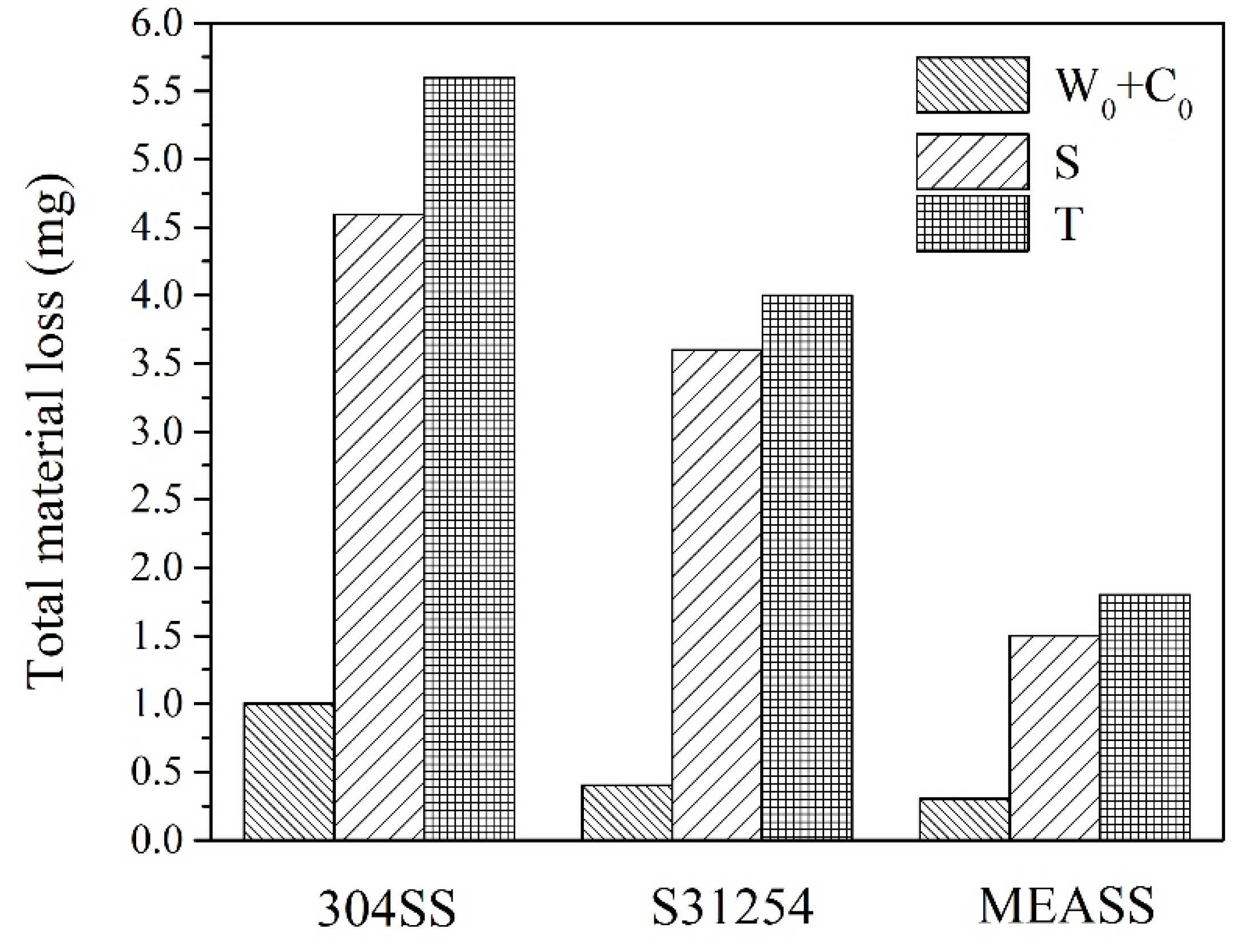
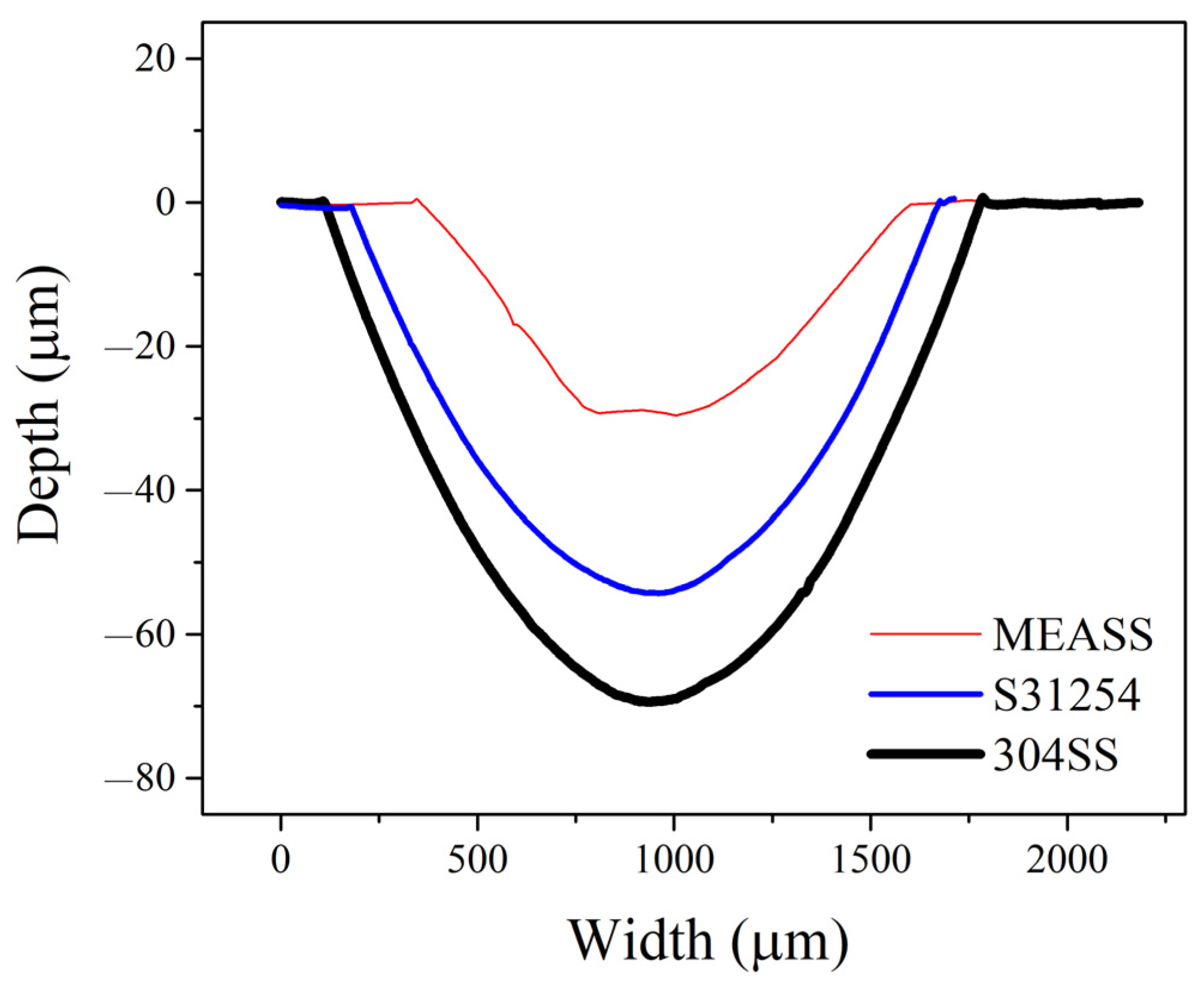
Total material loss (T) from tribocorrosion at OCP after 24 h is shown in Figure 7. Among the three alloys, 304SS exhibited the highest material loss, while S31254 and MEASS demonstrated comparatively lower values. Notably, MEASS showed the smallest total material loss, indicating enhanced ability to withstand tribocorrosion damage. This trend aligns well with the observed electrochemical results and surface morphologies. To further illustrate the extent of material degradation, cross-sectional profiles of the wear tracks for each alloy after 24 h of tribocorrosion testing at OCP are provided in Figure 8. As shown, 304SS exhibited the widest and deepest wear track, with a maximum depth of 72.8 μm. The wear track on S31254 was narrower and shallower, measuring approximately 54.3 μm in depth. In contrast, MEASS had the narrowest and most shallow wear track, reaching only 27.1 μm in depth. These dimensional differences, summarized in Table 3, clearly reflect the relative resistance of each alloy to the combined effects of mechanical wear and corrosion. The minimal wear track dimensions observed for MEASS are consistent with its enhanced electrochemical stability, improved passive film integrity, and reduced susceptibility to localized corrosion in chloride environments. These findings strongly support the conclusion that MEASS possesses the highest durability and tribocorrosion resistance among the evaluated stainless steels under the current test conditions.
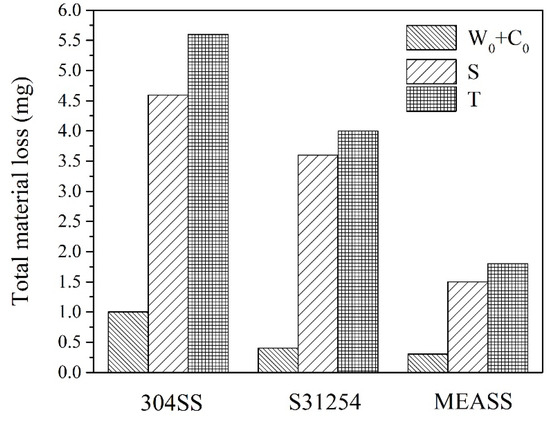
Figure 7.
Total material loss after 24 h tribocorrosion tests conducted at OCP.
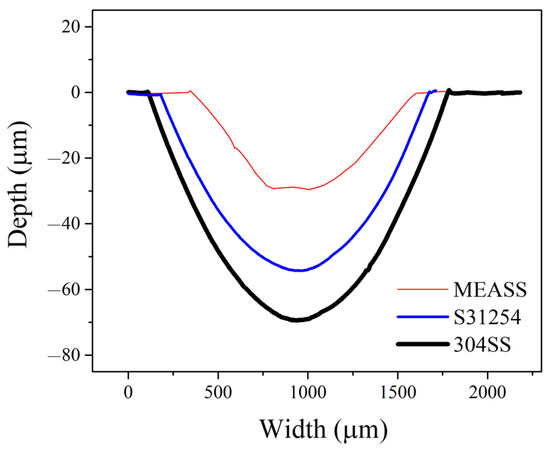
Figure 8.
Cross-sectional wear profiles of 304SS, S31254, and MEASS after 24 h tribocorrosion tests conducted at OCP.

Table 3.
Wear track width and depth after 24 h tribocorrosion tests conducted at OCP.
4. Discussion
Tribocorrosion represents a coupled degradation process in which mechanical wear and electrochemical corrosion interact synergistically, particularly under sliding contact in chloride-containing environments. In marine and industrial applications, this combined effect often results in material loss exceeding the sum of wear and corrosion acting independently. The mechanisms can generally be categorized into wear-induced corrosion, where sliding disrupts passive films and enhances electrochemical activity, and corrosion-induced wear, where corrosive attack weakens the material and facilitates mechanical damage. In this study, the tribocorrosion performance of 304SS, S31254, and a MEASS was investigated in 3.5 wt.% NaCl solution. Quantitative decomposition of total material loss (T) into pure mechanical wear (W0), pure corrosion (C0), and synergistic components—wear-induced corrosion (ΔCW) and corrosion-induced wear (ΔWC)—was carried out according to ASTM G119, and these components are summarized in the Table 4, Table 5 and Table 6. It is observed that the W0 values appear to correlate with the material hardness, where MEASS, having the highest hardness, shows the lowest W0, while 304SS, with the lowest hardness, exhibits the highest W0. This suggests that higher hardness may help mitigate pure mechanical wear under tribological conditions. However, the contribution of W0 to T remains relatively low for all three alloys. Among these components, the ΔWc was found to be the most significant contributor to T, for all three alloys, where it accounted for more than 80% of the total material loss. This result highlights the dominant role of localized corrosion in promoting additional material degradation through wear when stainless steels are exposed to chloride environments under sliding conditions. This behavior contrasts significantly with the results obtained in our previous study conducted in H2SO4 solution, where tribocorrosion was primarily governed by mechanical wear rather than corrosion-related processes. In the acidic environment, both C0 and ΔWC contributed minimally to the total material loss—accounting for only 1.24% and 10.6% in S31254 and 3.27% and 7.9% in MEASS, respectively [35]. This indicates that under sulfuric acid conditions, the wear mechanisms are less influenced by electrochemical reactions, and material degradation is mainly due to abrasive and adhesive wear. By contrast, in the current NaCl environment, the role of ΔWC has become dominant, responsible for over 82% of the total loss for both S31254 and MEASS. This dramatic shift is attributed to the strong tendency of chloride ions to penetrate and locally break down the passive oxide film on stainless steels, initiating pitting corrosion. Once localized corrosion occurs, the formation of corrosion pits on the wear surface markedly accelerates material degradation. This is because pit formation significantly alters the surface characteristics and wear behavior of materials. The presence of pits increases surface roughness, resulting in more irregular contact during sliding and promoting abrasive wear through intensified mechanical interactions at the surface. In addition, the formation of pits reduces the actual contact area within the wear track, leading to an increase in local contact pressure under a constant applied load. The resulting high contact stresses promote plastic deformation, facilitate microcrack initiation and propagation, and accelerate the detachment of surface material, thereby contributing to the overall increase in wear. Based on the above discussion, it can be inferred that the corrosion pits shown in Figure 4a and Figure 5a are expected to increase surface roughness and friction by enhancing mechanical interlocking [10], thereby further intensifying corrosion-induced material loss. SEM analysis of the worn surfaces supports these trends. For 304SS, numerous large and deep pits were observed along the wear track, leading to the highest material loss among the tested alloys. These pits likely intensified local contact pressures and friction during sliding, accelerating crack propagation and material detachment. S31254 exhibited fewer pits, with moderate surface damage. In contrast, MEASS showed minimal pitting and maintained a relatively smooth surface after testing, which correlates with its lower total material loss. The superior tribocorrosion performance of MEASS can be ascribed to its elevated chromium (Cr) and nickel (Ni) contents, both of which are known to play essential roles in enhancing corrosion resistance and film durability. Chromium is the key element responsible for forming a dense, adherent Cr2O3-based passive film that protects stainless steels in aggressive environments, particularly in the presence of chloride ions. The resistance to pitting corrosion is commonly assessed using the pitting resistance equivalent number (PREN), defined as PREN = %Cr + 3.3 × %Mo + 1.6 × %W + 16 × %N [41,42]. An increased Cr content, typically above 12 wt.%, improves the durability and self-repairability of the passive film under corrosive conditions. Nickel, on the other hand, though not directly part of the oxide film composition, stabilizes the austenitic phase and contributes to improved repassivation kinetics. Therefore, the combined effects of Cr and Ni in MEASS enable it to resist chloride-induced passive film breakdown and delay the onset of localized corrosion under tribological loading. As a result, MEASS demonstrates a lower extent of corrosion-induced wear and thus a reduced total material loss. These findings suggest that when corrosion-induced wear dominates tribocorrosion, such as in chloride-containing solutions, the ability to resist localized corrosion becomes the decisive factor. Therefore, enhancing pitting resistance through alloy design is critical to improving tribocorrosion performance in aggressive environments.

Table 4.
Quantitative decomposition of total material loss for 304SS in 3.5 wt.% NaCl solution according to ASTM G119.

Table 5.
Quantitative decomposition of total material loss for S31254 in 3.5 wt.% NaCl solution according to ASTM G119.

Table 6.
Quantitative decomposition of total material loss for MEASS in 3.5 wt.% NaCl solution according to ASTM G119.
Overall, the results confirm that MEASS exhibits the most favorable tribocorrosion resistance among the alloys tested. Its enhanced performance is driven not only by its high corrosion resistance but also by its ability to suppress pit formation and thereby minimize mechanical wear induced by corrosion-related defects. These findings highlight the importance of alloy composition in controlling tribocorrosion and offers valuable insights for designing stainless steels for demanding marine applications.
5. Conclusions
This study comparatively evaluated the tribocorrosion behavior of 304SS, S31254, and MEASS in 3.5 wt.% NaCl solution. The main findings are summarized as follows:
- Among the tested alloys, MEASS exhibited the lowest total material loss under tribocorrosion conditions. SEM analysis further confirmed minimal surface damage and pit formation in MEASS, highlighting its ability to effectively resist localized corrosion and mechanical degradation.
- Quantitative analysis revealed that corrosion-induced wear (ΔWc) was the primary contributor to total material loss, accounting for more than 80% in chloride-containing environments.
- Electrochemical tests demonstrated superior electrochemical stability and repassivation ability for MEASS, which exhibited the lowest corrosion current density (1.46 μA/cm2), representing an 83% reduction compared to 304SS under tribocorrosion conditions.
- Enhanced tribocorrosion resistance of MEASS is primarily attributed to its higher Cr and Ni contents, which strengthen the passive film, thus minimizing pit formation and subsequent mechanical degradation.
Future Work: Future studies should examine the long-term tribocorrosion behavior of MEASS under varying mechanical loads, temperatures, and real seawater compositions, to further establish its practical application potential in marine engineering.
Author Contributions
Conceptualization, Y.-R.C. and Y.-L.L.; Methodology, C.-H.W.; Validation, S.-Y.H. and P.-S.H.; Formal analysis, C.-H.W. and Y.-R.C.; Investigation, C.-H.W.; Resources, H.-W.Y.; Writing—original draft, C.-H.W. and Y.-L.L.; Writing—review & editing, H.-W.Y., I.-C.C., P.-W.C. and Y.-L.L.; Supervision, Y.-L.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Ministry of Science and Technology, R.O.C by MOST 108-2221-E-002-055-MY2 and MOST 109-2224-E-002-002.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, Y.-L.L., upon reasonable request.
Acknowledgments
This work was sponsored by the Ministry of Science and Technology, R.O.C, under Grant No. MOST 108-2221-E-002-055-MY2 and MOST 109-2224-E-002-002. We especially thank I-Chung Cheng and Peng-Wei Chu for providing technical assistance and valuable contributions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Melchers, R.E.; Moan, T.; Gao, Z. Corrosion of working chains continuously immersed in seawater. J. Mar. Sci. Technol. 2007, 12, 102–110. [Google Scholar] [CrossRef]
- Zou, D.; Luo, W.; Chen, Q.; He, X.; Liu, T. Corrosion evolution and quantitative corrosion monitoring of Q355 steel for offshore wind turbines in multiple marine corrosion zones. Ocean Eng. 2024, 311, 119044. [Google Scholar] [CrossRef]
- Syrek-Gerstenkorn, B.; Paul, S. Metallic coatings in offshore wind sector—A mini review. NPJ Mater. Degrad. 2024, 8, 86. [Google Scholar] [CrossRef]
- Vázquez-Hernández, A.; Ellwanger, G.; Sagrilo, L. Long-term response analysis of FPSO mooring systems. Appl. Ocean Res. 2011, 33, 375–383. [Google Scholar] [CrossRef]
- Landolt, D.; Mischler, S.; Stemp, M. Electrochemical methods in tribocorrosion: A critical appraisal. Electrochim. Acta 2001, 46, 3913–3929. [Google Scholar] [CrossRef]
- Landolt, D.; Mischler, S. Tribocorrosion of Passive Metals and Coatings; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Wu, Y.; Wang, Z.; Chen, J.; Ma, Y.; Yan, Y.; Qiao, L. Effect of frictional frequency on the subsurface evolution of 316L stainless steel in tribocorrosion and its influence on the synergistic effect between corrosion and wear. Tribol. Int. 2023, 178, 108026. [Google Scholar] [CrossRef]
- Ma, X.; Tan, W.; Bonzom, R.; Mi, X.; Zhu, G. Impact-sliding fretting tribocorrosion behavior of 316L stainless steel in solution with different halide concentrations. Friction 2023, 11, 2310–2328. [Google Scholar] [CrossRef]
- Mischler, S.; Spiegel, A.; Landolt, D. The role of passive oxide films on the degradation of steel in tribocorrosion systems. Wear 1999, 225, 1078–1087. [Google Scholar] [CrossRef]
- Obadele, B.A.; Andrews, A.; Shongwe, M.B.; Olubambi, P.A. Tribocorrosion behaviours of AISI 310 and AISI 316 austenitic stainless steels in 3.5% NaCl solution. Mater. Chem. Phys. 2016, 171, 239–246. [Google Scholar] [CrossRef]
- Sun, Y.; Rana, V. Tribocorrosion behaviour of AISI 304 stainless steel in 0.5 M NaCl solution. Mater. Chem. Phys. 2011, 129, 138–147. [Google Scholar] [CrossRef]
- Jun, C. Tribocorrosion behaviors of Ti–6Al–4V and Monel K500 alloys sliding against 316 stainless steel in artificial seawater. Trans. Nonferrous Met. Soc. China 2012, 22, 1356–1365. [Google Scholar]
- Jun, C.; Zhang, Q.; Li, Q.-A.; Fu, S.-L.; Wang, J.-Z. Corrosion and tribocorrosion behaviors of AISI 316 stainless steel and Ti6Al4V alloys in artificial seawater. Trans. Nonferrous Met. Soc. China 2014, 24, 1022–1031. [Google Scholar]
- Dalmau, A.; Richard, C.; Igual-Muñoz, A. Degradation mechanisms in martensitic stainless steels: Wear, corrosion and tribocorrosion appraisal. Tribol. Int. 2018, 121, 167–179. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, X.; An, Y.; Hou, G.; Li, S.; Deng, W.; Zhou, H.; Chen, J. Effects of loads on corrosion-wear synergism of NiCoCrAlYTa coating in artificial seawater. Tribol. Int. 2018, 118, 421–431. [Google Scholar] [CrossRef]
- Huttunen-Saarivirta, E.; Kilpi, L.; Hakala, T.J.; Carpen, L.; Ronkainen, H. Tribocorrosion study of martensitic and austenitic stainless steels in 0.01 M NaCl solution. Tribol. Int. 2016, 95, 358–371. [Google Scholar] [CrossRef]
- Dai, Z.; Liu, M.; Jiang, S.; Li, M.; Li, S.; Duan, D. Effects of different cathodic reactions on tribocorrosion behavior of AISI 430 in 0.5 mol/L sulfuric acid. J. Mater. Eng. Perform. 2022, 31, 2708–2714. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, X.-Y.; Yan, F.-Y. Tribocorrosion behaviour of type S31254 steel in seawater: Identification of corrosion–wear components and effect of potential. Mater. Chem. Phys. 2016, 179, 273–281. [Google Scholar] [CrossRef]
- Iwabuchi, A.; Sasaki, T.; Hori, K.; Tatsuyanagi, Y. Tribological Properties of SUS304 Steel in Seawater: Electrochemical Approach to the Wear Behaviour. JSME Int. J. Ser. 1 Solid Mech. Strength Mater. 1992, 35, 117–122. [Google Scholar] [CrossRef]
- Jiang, X.; Li, S.; Tao, D.; Yang, J. Accelerative effect of wear on corrosion of high-alloy stainless steel. Corrosion 1993, 49, 836–841. [Google Scholar] [CrossRef]
- Zhang, T.; Jiang, X.; Li, S.; Lu, X. A quantitative estimation of the synergy between corrosion and abrasion. Corros. Sci. 1994, 36, 1953–1962. [Google Scholar] [CrossRef]
- Liu, M.; Duan, D.-L.; Jiang, S.-L.; Li, M.-Y.; Li, S. Tribocorrosion behavior of 304 stainless steel in 0.5 mol/L sulfuric acid. Acta Metall. Sin. Engl. Lett. 2018, 31, 1049–1058. [Google Scholar] [CrossRef]
- Ouknin, M.; Boumezzourh, A.; Lakbaibi, Z.; Ponthiaux, P.; Costa, J.; Majidi, L. Tribological behavior of stainless steel in sulfuric acid in the presence of Thymus zygis subsp. gracilis essential oil: Experimental and quantum chemical studies. Corros. Rev. 2021, 39, 279–295. [Google Scholar] [CrossRef]
- Stachowiak, A.; Zwierzycki, W. Analysis of the tribocorrosion mechanisms in a pin-on-plate combination on the example of AISI304 steel. Wear 2012, 294, 277–285. [Google Scholar] [CrossRef]
- Anburaj, J.; Nazirudeen, S.M.; Narayanan, R.; Anandavel, B.; Chandrasekar, A. Ageing of forged superaustenitic stainless steel: Precipitate phases and mechanical properties. Mater. Sci. Eng. A 2012, 535, 99–107. [Google Scholar] [CrossRef]
- Hänninen, H.; Romu, J.; Ilola, R.; Tervo, J.; Laitinen, A. Effects of processing and manufacturing of high nitrogen-containing stainless steels on their mechanical, corrosion and wear properties. J. Mater. Process. Technol. 2001, 117, 424–430. [Google Scholar] [CrossRef]
- Plaut, R.L.; Herrera, C.; Escriba, D.M.; Rios, P.R.; Padilha, A.F. A short review on wrought austenitic stainless steels at high temperatures: Processing, microstructure, properties and performance. Mater. Res. 2007, 10, 453–460. [Google Scholar] [CrossRef]
- Lewis, A.; Bingert, J.; Rowenhorst, D.; Gupta, A.; Geltmacher, A.; Spanos, G. Two-and three-dimensional microstructural characterization of a super-austenitic stainless steel. Mater. Sci. Eng. A 2006, 418, 11–18. [Google Scholar] [CrossRef]
- Wang, S.-H.; Wu, C.-C.; Chen, C.-Y.; Yang, J.-R.; Chiu, P.-K.; Fang, J. Cyclic deformation and phase transformation of 6Mo superaustenitic stainless steel. Met. Mater. Int. 2007, 13, 275–283. [Google Scholar] [CrossRef]
- Li, H.; Zhang, B.; Jiang, Z.; Zhang, S.; Feng, H.; Han, P.; Dong, N.; Zhang, W.; Li, G.; Fan, G. A new insight into high-temperature oxidation mechanism of super-austenitic stainless steel S32654 in air. J. Alloys Compd. 2016, 686, 326–338. [Google Scholar] [CrossRef]
- Malandruccolo, A. Properties of Superaustenitic Stainless Steels. In Superaustenitic Stainless Steels: A Comprehensive Overview; Springer: Berlin/Heidelberg, Germany, 2024; pp. 155–211. [Google Scholar]
- Jinyao, M.; Nan, D.; Zhensen, G.; Peide, H. Effect of B and Ce Micro-alloying on Secondary Phase Precipitation and Corrosion Resistance of S31254 Super Austenitic Stainless Steel. J. Chin. Soc. Corros. Prot. 2024, 44, 1610–1616. [Google Scholar]
- Shockley, J.M.; Horton, D.J.; Wahl, K.J. Effect of aging of 2507 super duplex stainless steel on sliding tribocorrosion in chloride solution. Wear 2017, 380, 251–259. [Google Scholar] [CrossRef]
- Haruman, E.; Sun, Y.; Adenan, M. A comparative study of the tribocorrosion behaviour of low temperature nitrided austenitic and duplex stainless steels in NaCl solution. Tribol. Int. 2020, 151, 106412. [Google Scholar] [CrossRef]
- Liu, C.-C.; Huang, S.-Y.; Chu, Y.-R.; Yang, T.-H.; Yen, H.-W.; Cheng, I.-C.; Chu, P.-W.; Lee, Y.-L. Tribocorrosion Behavior of Medium-Entropy Super Austenitic Stainless Steel in Acidic Environments. Lubricants 2025, 13, 125. [Google Scholar] [CrossRef]
- Chen, T.-E.; Huang, S.-Y.; Chu, Y.-R.; Chen, S.-C.; Tseng, M.-Y.; Yen, H.-W.; Lee, Y.-L. Investigating the effects of aging time on corrosion resistance and antibacterial property of newly designed medium-entropy super austenitic stainless steels. Materialia 2023, 27, 101687. [Google Scholar] [CrossRef]
- ASTM G119-09; Standard Guide for Determining Amount of Synergism Between Wear and Corrosion. ASTM: West Conshohocken, PA, USA, 2009.
- Zhu, L.; Yang, P.; Lyu, W.; Wang, Q.; Wang, K. Electrochemical corrosion characteristics of 316L stainless steel in simulated coastal gold mine wastewater under different pH. Int. J. Electrochem. Sci. 2022, 17, 22099. [Google Scholar] [CrossRef]
- Bailey, R. Tribocorrosion response of surface-modified Ti in a 0.9% NaCl solution. Lubricants 2018, 6, 86. [Google Scholar] [CrossRef]
- Du, J.; Hu, L.; Chen, M.; Sun, J.; Song, Q.; Xiao, J. Tribocorrosion behavior of aluminum alloys in 3.5 wt% NaCl solution. J. Mater. Res. Technol. 2025, 36, 985–997. [Google Scholar] [CrossRef]
- Torbati-Sarraf, H.; Shabani, M.; Jablonski, P.D.; Pataky, G.J.; Poursaee, A. The influence of incorporation of Mn on the pitting corrosion performance of CrFeCoNi High Entropy Alloy at different temperatures. Mater. Des. 2019, 184, 108170. [Google Scholar] [CrossRef]
- Li, T.; Swanson, O.J.; Frankel, G.S.; Gerard, A.Y.; Lu, P.; Saal, J.E.; Scully, J. Localized corrosion behavior of a single-phase non-equimolar high entropy alloy. Electrochim. Acta 2019, 306, 71–84. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).