1. Introduction
Tribocorrosion is an irreversible degradation process that affects materials due to the combined chemical and mechanical interactions at their surfaces when exposed to corrosive solutions [
1]. This synergy between corrosion and wear has a significant impact on the performance and safety of engineering materials and can be broadly classified into two mechanisms: wear-induced corrosion and corrosion-induced wear. In wear-induced corrosion, the wear process raises the surface temperature of the material, accelerating chemical reactions and increasing the corrosion rate. Mechanical wear causes plastic deformation on the material’s surface, which raises its energy state and predisposes it to further corrosion. Moreover, the wear process can damage the protective passivation film, significantly enhancing corrosion. Unlike wear-induced corrosion, corrosion-induced wear originates from chemical reactions that deteriorate the surface before any significant mechanical loading occurs. When a material is exposed to a corrosive environment, chemical reactions can lead to the formation of surface defects such as pits, cracks, or the deposition of corrosion products (e.g., oxides). These defects weaken the surface by reducing its hardness and altering its microstructure. For example, if the corrosion products formed are relatively soft, they may provide a lubricating effect that alters wear behavior. On the other hand, if corrosion renders the surface more brittle, the material is more susceptible to removal by wear. The dislodged corrosion products or oxides, once scattered in the solution, can participate in three-body wear with the substrate, further increasing material loss. These intertwined processes highlight the inherent complexity of tribocorrosion, making its management an inevitable challenge in engineering applications. López-Ortega et al. [
2] investigated the tribocorrosion behavior of two types of materials: those that form a passive film and those that actively dissolve. For passivating materials, immersion in an electrolyte leads to the formation of a protective passive film on the surface. However, during sliding contact, this film is disrupted, exposing the unprotected substrate to the electrolyte and initiating galvanic corrosion. In contrast, for actively dissolving materials, immersion results in the formation of a rust layer, which offers poor protection. When sliding occurs, this rust layer is readily removed, thereby diminishing the galvanic effect.
Stainless steels are widely utilized in marine engineering due to their excellent corrosion resistance and mechanical strength. Their inherent ability to form a passive film helps protect against the corrosive effects of chloride ions present in seawater. However, in marine applications, components often experience not only corrosive attack but also mechanical wear. There are numerous studies that have examined tribocorrosion behavior of stainless steels in sodium chloride environments [
3,
4,
5,
6,
7,
8,
9]. For example, Zhang et al. [
3] investigated the tribocorrosion of S31254 steel in artificial seawater and found that sliding contact increases the corrosion current density by three orders of magnitude. This marked deterioration in corrosion resistance is attributed to the damage inflicted on the passivation film during sliding. Similarly, Obadele et al. [
6] evaluated the tribocorrosion behavior of AISI 310 and AISI 316 austenitic stainless steels in a 3.5% NaCl solution. They observed that the corrosion potential (E
corr) decreased during tribocorrosion as a result of the removal or destruction of the oxide films, which in turn promoted wear-induced corrosion. The tribocorrosion performance of stainless steels is also strongly influenced by applied load and rotating speed [
4,
5,
10]. Silva et al. [
4] examined the effect of load on the tribocorrosion behavior of S32750 steel in a 3.5 wt.% sodium chloride environment. Their results indicated that higher applied loads lead to more unstable polarization curves, with both the corrosion current density and the current density within the passivation interval increasing. Additionally, a larger area of damaged passivation film was observed on the material’s surface under higher loads. Complementarily, Zhang et al. [
10] conducted intermittent OCP measurements under varying loads. They reported that heavier loads cause a more significant drop in the OCP, and although the potential recovers immediately once sliding ceases—indicating the reformation of the passivation film—the recovery takes longer when the load is higher due to more severe film damage. The pH value of the environment also affects tribocorrosion behavior. Zhang et al. [
11] studied the tribocorrosion of 304 stainless steel (304SS) in solutions with different pH values and found that higher pH leads to lower corrosion currents and an increased pitting potential. This phenomenon is explained by the role of chloride ions, which react with metal ions in the passive film to form chloride salts that compromise film integrity, whereas hydroxide ions form protective hydroxides due to their superior metal ion bonding properties. Consequently, elevated pH conditions tend to inhibit corrosion. Finally, the viscosity of the test solution is another factor influencing tribocorrosion performance. Cao et al. [
12] demonstrated that increasing the solution’s viscosity hinders the wear process, thereby reducing the material loss rate.
On the other hand, stainless steel components used in industrial applications are frequently exposed to highly aggressive acidic environments that promote both chemical corrosion and mechanical wear. Consequently, the corrosion and tribocorrosion behavior of stainless steels in acidic media has attracted considerable attention [
13,
14,
15,
16,
17,
18,
19,
20]. For instance, Wang et al. [
15] investigated the abrasion–corrosion behavior of 304 stainless steel (304SS) in a 0.5 mol/L H
2SO
4 solution and observed that stirring significantly accelerates hydrogen evolution. Similarly, Zhang et al. [
16] examined the tribocorrosion wear of duplex stainless steels in sulfuric acid solutions containing chloride ions, demonstrating that the presence of NaCl increases the overall mass loss rate and intensifies the synergistic effects between wear and corrosion. Therefore, understanding the underlying degradation mechanisms in acidic media is essential for ensuring the structural safety of stainless steel components.
In recent years, the demand for cost-effective materials that offer superior corrosion resistance and excellent mechanical properties has increased, especially for applications in harsh environments. SASS have garnered attention as a viable substitute for traditional austenitic stainless steels. These advanced materials are formulated with higher amounts of critical alloying elements—specifically, chromium (20–24 wt.%), nickel (17–22 wt.%), molybdenum (4–8 wt.%), and nitrogen (0.15–0.5 wt.%)—which together deliver exceptional strength, enhanced toughness, and significantly superior resistance to both pitting and crevice corrosion when compared to standard grades such as AISI 304 and 316 [
21,
22,
23,
24]. Consequently, SASS is extensively utilized in a wide range of engineering applications, including those in the marine, chemical, mining, and nuclear industries [
25,
26]. Nonetheless, in highly corrosive environments, SASS components experience simultaneous corrosion and wear, leading to material degradation that far exceeds the simple additive effects of each process. Therefore, a comprehensive understanding of the tribocorrosion behavior of SASS is important to ensure its long-term reliability and performance. While extensive studies have examined the corrosion and tribocorrosion behaviors of conventional stainless steel [
3,
8,
27,
28], such as S31254 [
3], our understanding of how advanced alloys perform under these harsh conditions remains limited. In our previous work, we introduced a novel MEASS that demonstrated comparable corrosion resistance in NaCl solutions and superior mechanical properties relative to the commercial S31254 [
24]. Despite the promising potential of MEASS for extreme service environments, there is limited understanding of their tribocorrosion behavior under aggressive environments. This study provides the first comprehensive investigation of the tribocorrosion behavior of MEASS compared to S31254 in a H
2SO
4 solution, contributing to the development of advanced materials for aggressive, high-wear applications. Wear track microstructures that formed after the tests were characterized using SEM and TEM, and the study systematically discusses the corrosion, wear, and synergistic interactions between these degradation processes for both steels. This research aims to clarify the key mechanisms influencing the tribocorrosion performance of two steels in acidic environments, thereby supporting the development of more durable materials for demanding industrial applications.
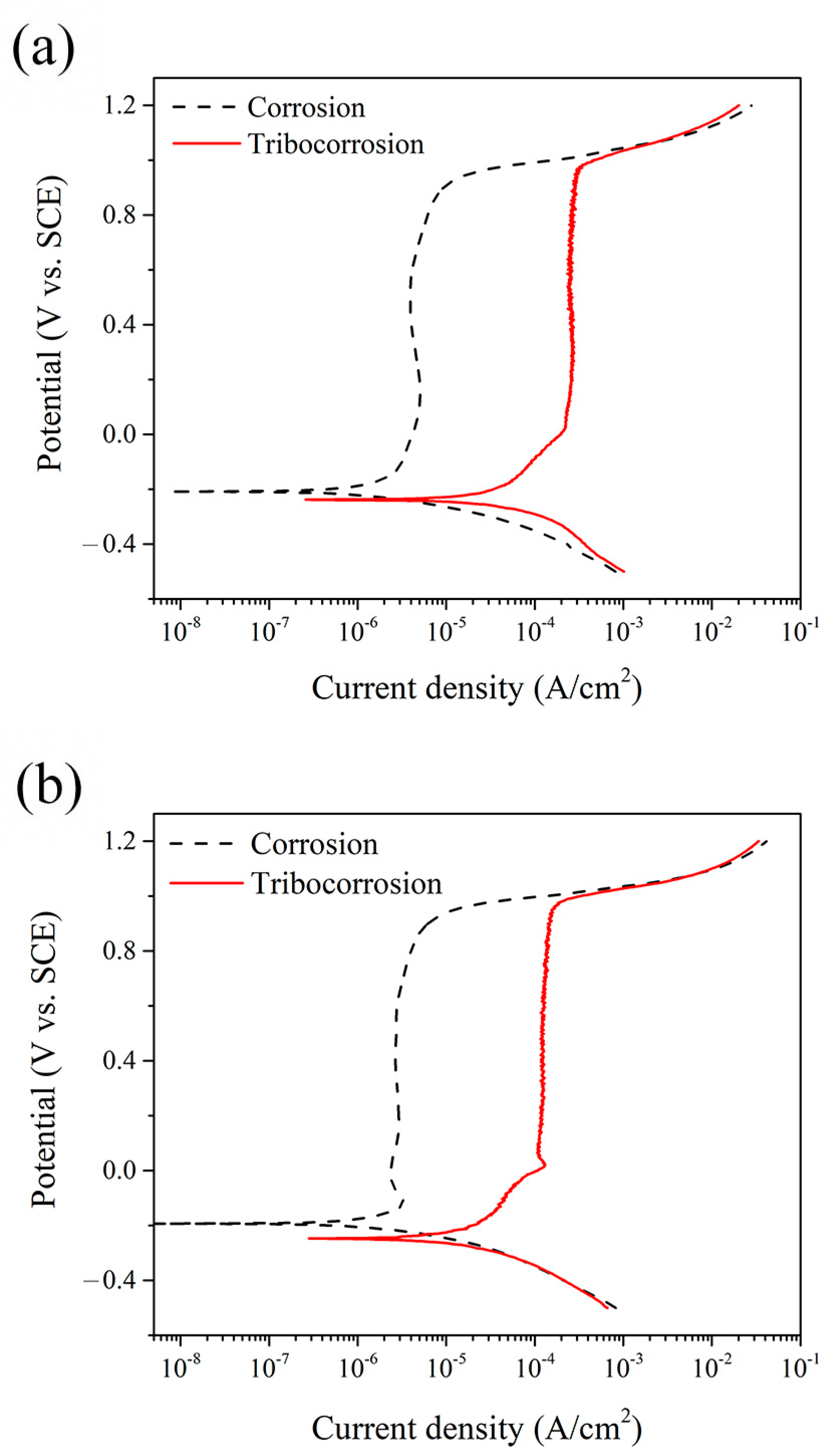
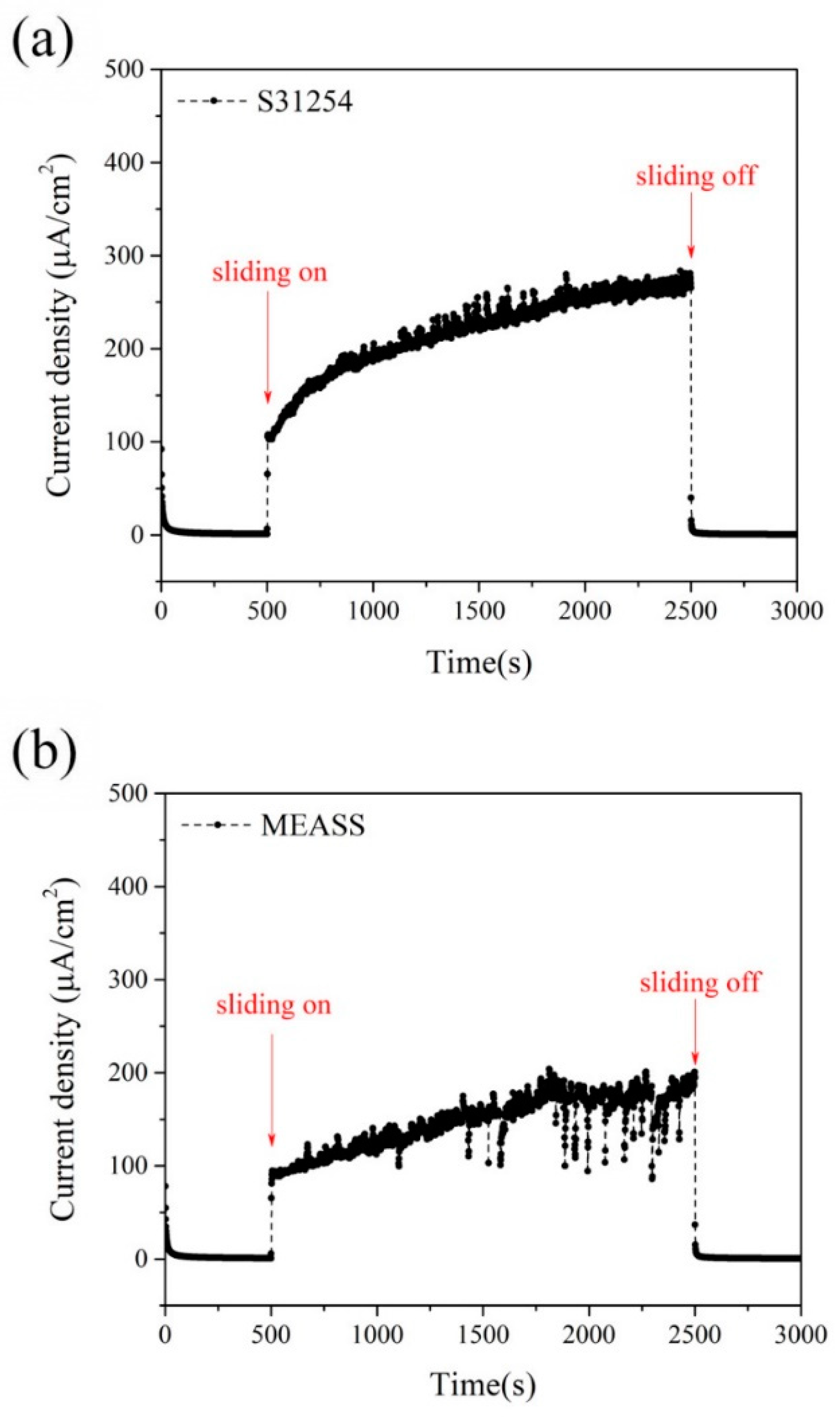
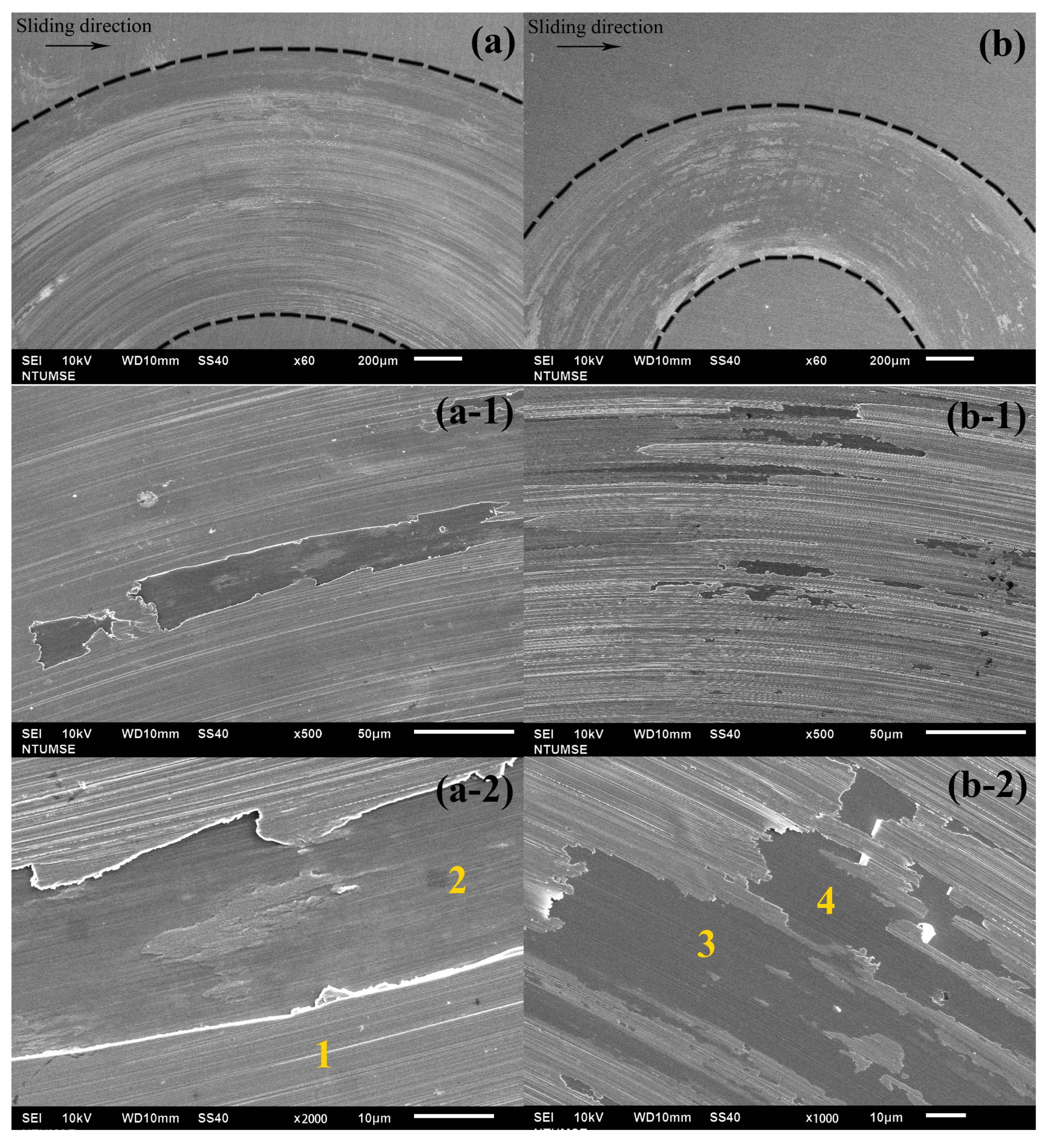
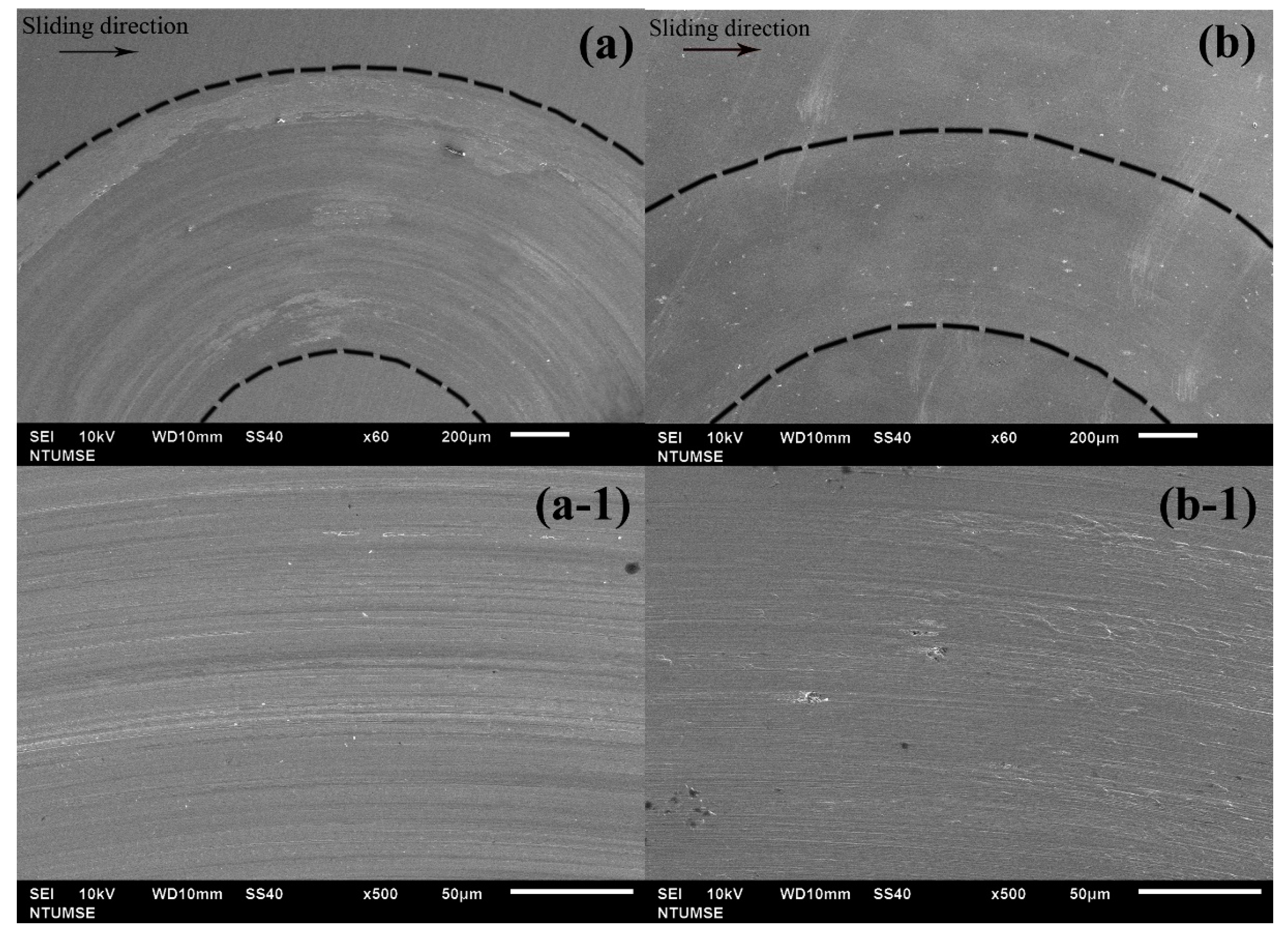
4. Discussion
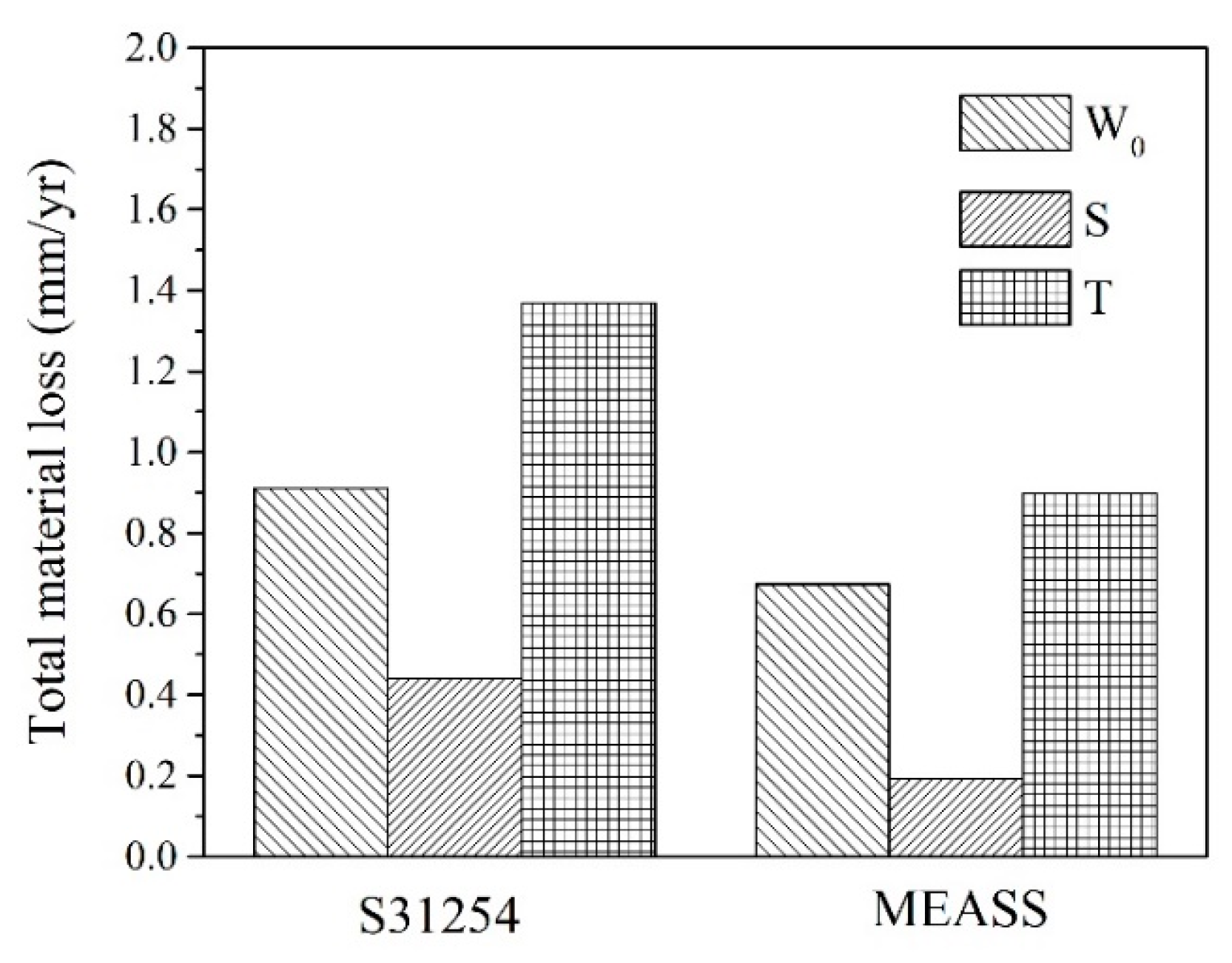
Tribocorrosion is the surface reaction behavior resulting from the combined effects of mechanical, chemical, and electrochemical processes during sliding contact friction in corrosive environments. In addition to the individual effects of wear and corrosion, their interaction plays a crucial role in determining the performance and safety of engineering materials and can be classified into two mechanisms: wear-induced corrosion and corrosion-induced wear. In wear-induced corrosion, mechanical wear elevates the surface temperature, accelerates chemical reactions, and damages the protective film, thereby promoting corrosion. The other mechanism, corrosion-induced wear, occurs when chemical reactions create surface defects—such as pits and cracks—that weaken the material and modify its wear behavior by either lubricating or embrittling the surface. The continuous interaction between these processes accelerates material degradation and leads to premature failure. Therefore, in this study, we not only measure the total material loss by weighing samples before and after the tribocorrosion tests (see
Figure 7) but also quantify the contributions of pure mechanical wear and the combined effects of wear and corrosion on steels operating under tribological contact in corrosive environments. Accordingly, the material loss rates attributed to pure mechanical wear, pure corrosion, wear-induced corrosion, and corrosion-induced wear were calculated following the ASTM G119 standard, with the results summarized in
Table 6 and
Table 7. In these tables, the S value represents the combined synergistic effects (ΔC
w + ΔW
c), while C and W denote the total corrosion components (C
0 + ΔC
w) and wear components (W
0 + ΔW
c), respectively. The data in
Table 6 and
Table 7 reveal that, although both steels have similar C
0 values, the MEASS specimen exhibits a lower ΔC
w value compared to S31254. This difference is attributed to the superior repassivation capability of MEASS when its passive film is disrupted by wear. Wear-induced corrosion typically arises from additional corrosion occurring on the freshly exposed metal surface following the mechanical removal of the passive film. The enhanced repassivation ability of MEASS promotes a more rapid reformation of the passive film in the worn areas, thereby reducing the ΔC
w value during the tribocorrosion test. Furthermore, while the corrosion components (C
0 + ΔC
w) account for approximately 20% of the total material loss rate for both steels, their contribution is significantly lower than that of the wear components (W
0 + ΔW
c) under tribocorrosion conditions in 1 M H
2SO
4. Given the negligible contribution of ΔW
c to the overall wear components, it is evident that pure mechanical wear is the dominant factor governing the total material loss rate—contributing about 67% for S31254 and 75% for MEASS during tribocorrosion tests conducted at OCP.
Moreover, the W
0 value of MEASS is approximately 26% lower than that of S31254 under identical tribocorrosion test conditions. This reduction is likely due to the enhanced surface hardness of MEASS relative to S31254 during the tests. The results suggest that the pure mechanical wear rate—closely linked to the contact surface hardness—is the primary factor influencing the tribocorrosion behavior of both steels in H
2SO
4.
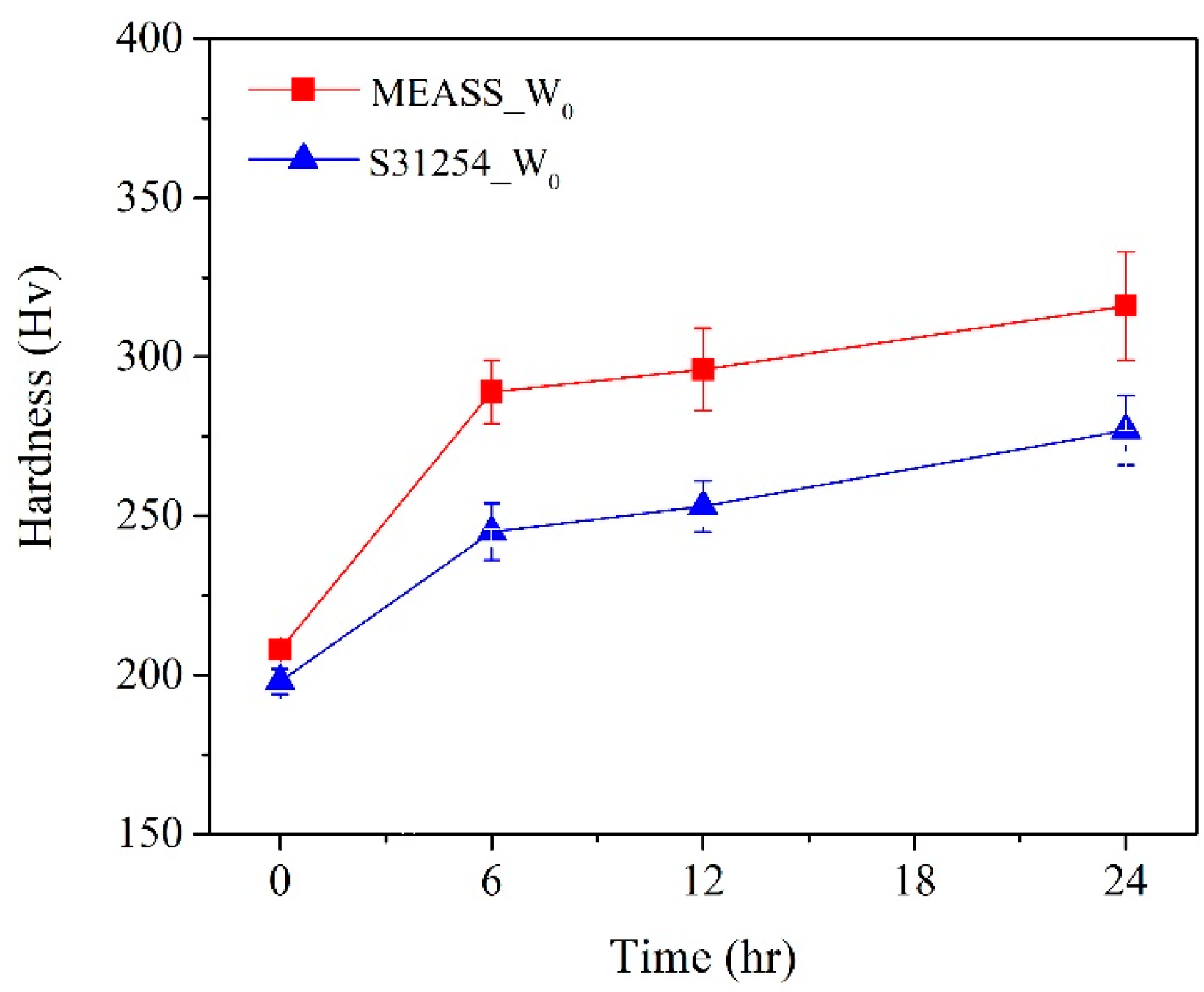
Figure 12 illustrates the evolution of hardness for S31254 and MEASS, measured on the worn surfaces after various durations of the W
0 test in H
2SO
4. As depicted, both steels exhibit a continuous increase in hardness with increasing pure wear time. This enhancement is attributed to strain hardening and the accumulation of deformation in the subsurface region [
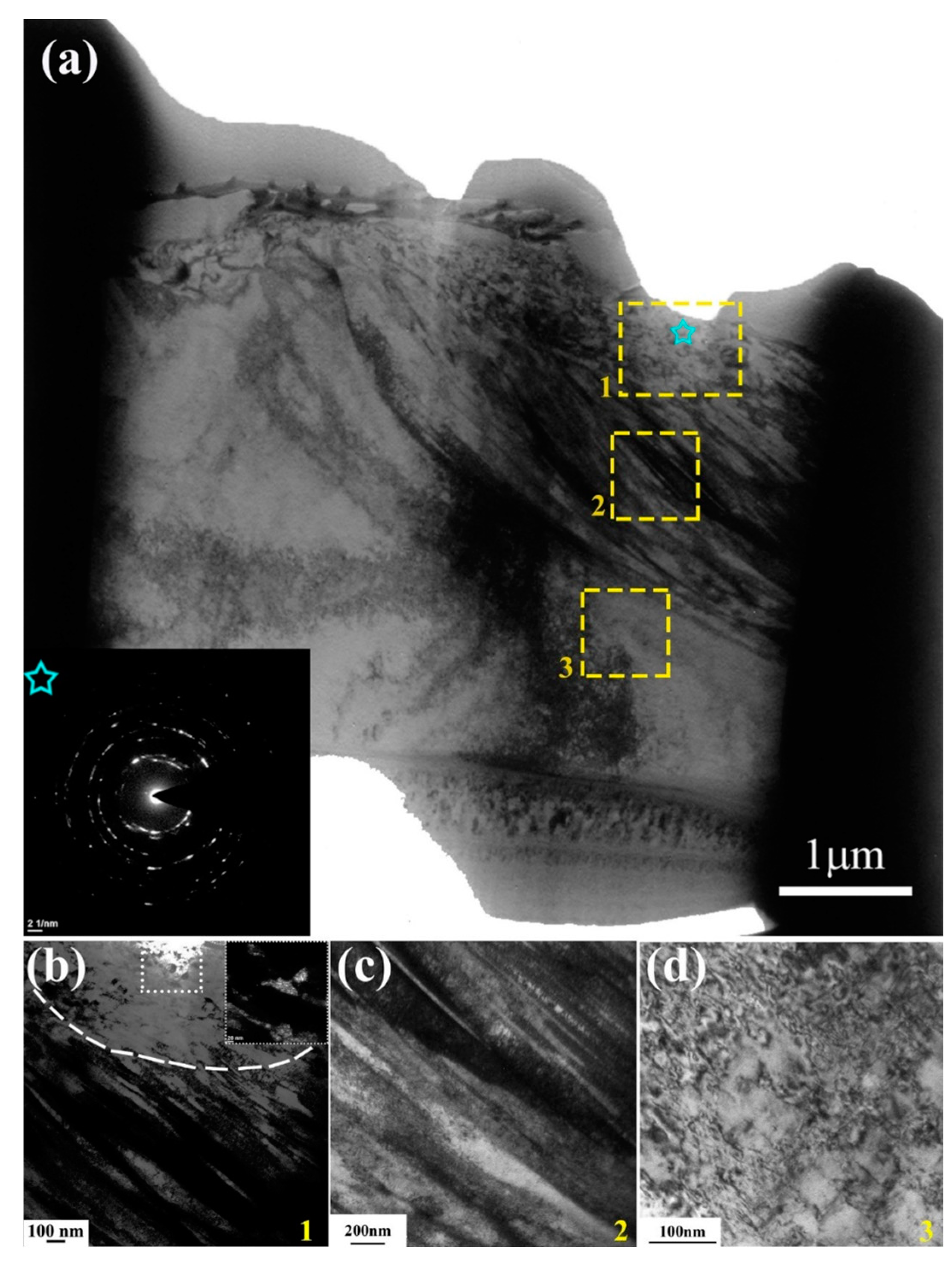
33]. Notably, the surface hardness of MEASS increased significantly—from 208 Hv before testing to 289 Hv after 6 h, representing a 38.9% increase. In contrast, the S31254 specimen showed a more modest improvement of 23.7%, suggesting that strain hardening occurs more prominently in the MEASS specimen. The pronounced strain hardening behavior observed in MEASS can be explained well by the TEM results described previously.
Figure 10 and
Figure 11 demonstrate a gradient worn microstructure from surface to volume. As the strain decreases, the microstructure transitions from UFG/NG to deformation bands or Taylor lattice, and then to dislocation debris. During plastic deformation, an extremely high dislocation density can induce dynamic recrystallization, forming UFGs and NGs in the deformed microstructure [
34]. Hence, MEASS is expected to exhibit a higher capacity for dislocation storage. This is supported by its uniform UFG/NG layer and higher hardness. It is well-known that planar slip and Taylor lattice structures can enhance the capacity for dislocation storage in face-centered cubic materials [
35,
36]. In summary, the planar slip of dislocations in MEASS contributes to a higher dislocation density and latter dynamic recrystallization of UFGs and NGs, resulting in its high hardness. Overall, although both steels follow a similar hardness evolution trend, MEASS consistently exhibits higher hardness than S31254 under all conditions, leading to its superior tribocorrosion performance in the H
2SO
4 acid solution.
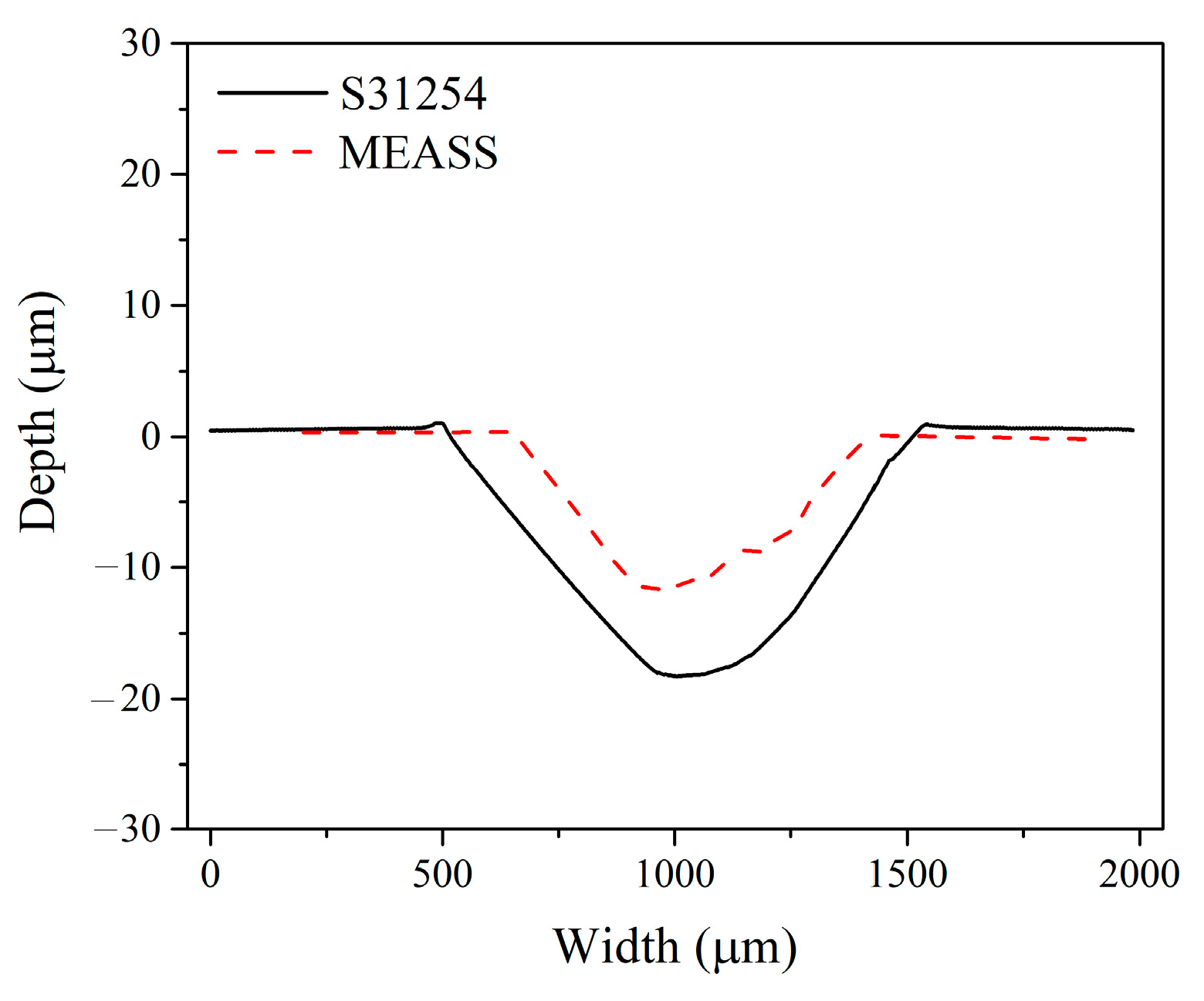
5. Conclusions
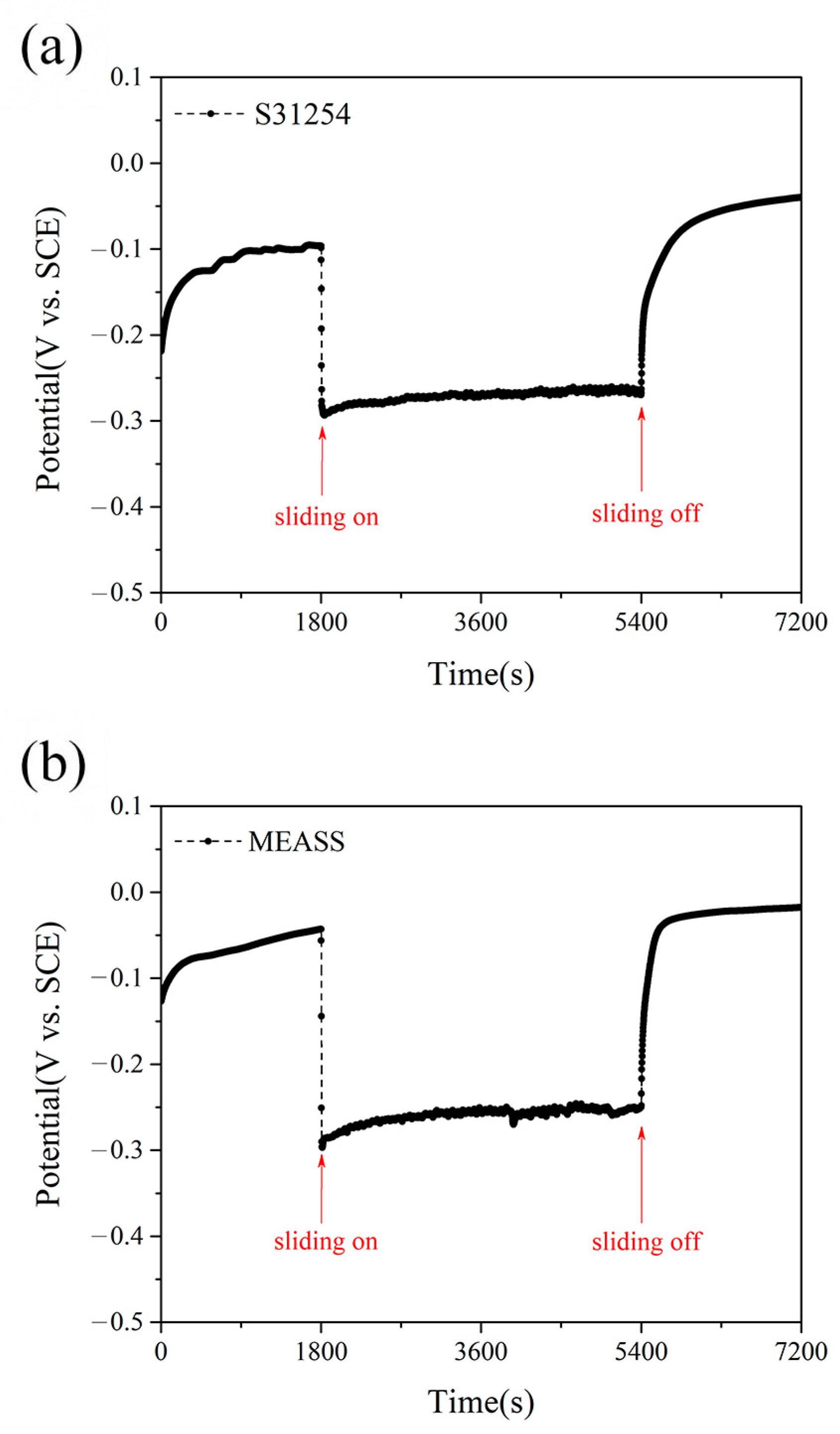
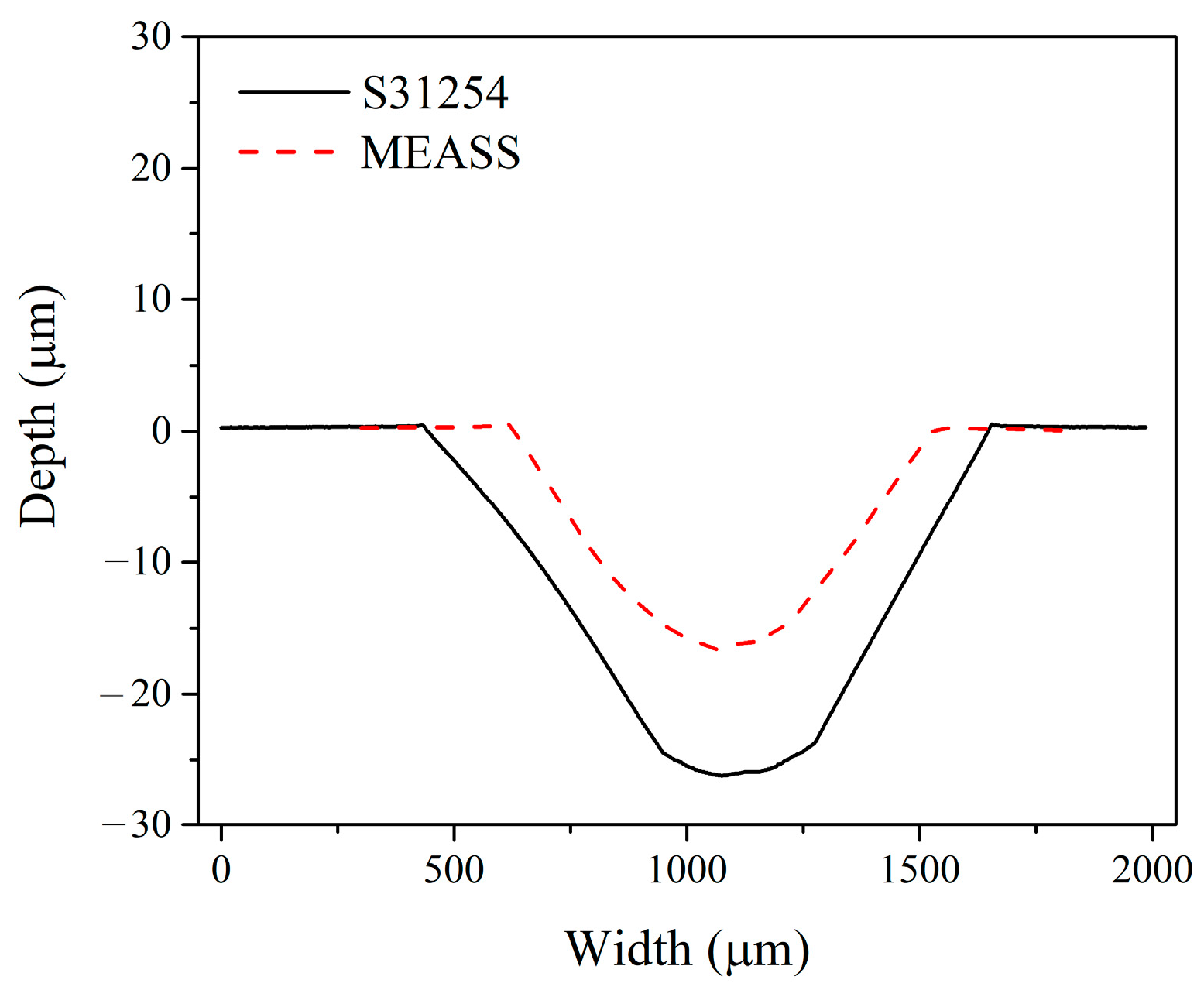
In this study, the tribocorrosion behavior of MEASS in a H2SO4 solution was investigated and compared with that of conventional S31254, with evaluations of material loss resulting from pure corrosion, pure wear, corrosion-accelerated wear, and wear-accelerated corrosion. Based on tribocorrosion measurements and microstructural characterization, it was found that both steels exhibited similar behavior in potentiodynamic polarization tests under sliding and non-sliding conditions; however, OCP measurements revealed that MEASS possesses a higher repassivation capability than S31254. After tribocorrosion tests, the wear track on the S31254 specimen showed a significantly greater width and depth compared to that on the MEASS specimen, and over 24 h, the total material loss for MEASS was lower than that for S31254. For the first time, the tribocorrosion behavior of MEASS in a H2SO4 solution was investigated and found to be predominantly governed by mechanical wear, with the reduced wear in MEASS attributed to its higher hardness arising from superior strain hardening behavior. MEASS exhibits excellent tribocorrosion resistance, making it suitable for demanding applications requiring durability, minimal material loss, and low maintenance. Future research will focus on optimizing alloy composition and heat treatment to further enhance corrosion and wear resistance, as well as extending tests to diverse corrosive environments and long-term industrial conditions.