Corrosion Resistance and Wear Properties of CoCrFeNiMn/TiC High-Entropy Alloy-Based Composite Coatings Prepared by Laser Cladding
Abstract
1. Introduction
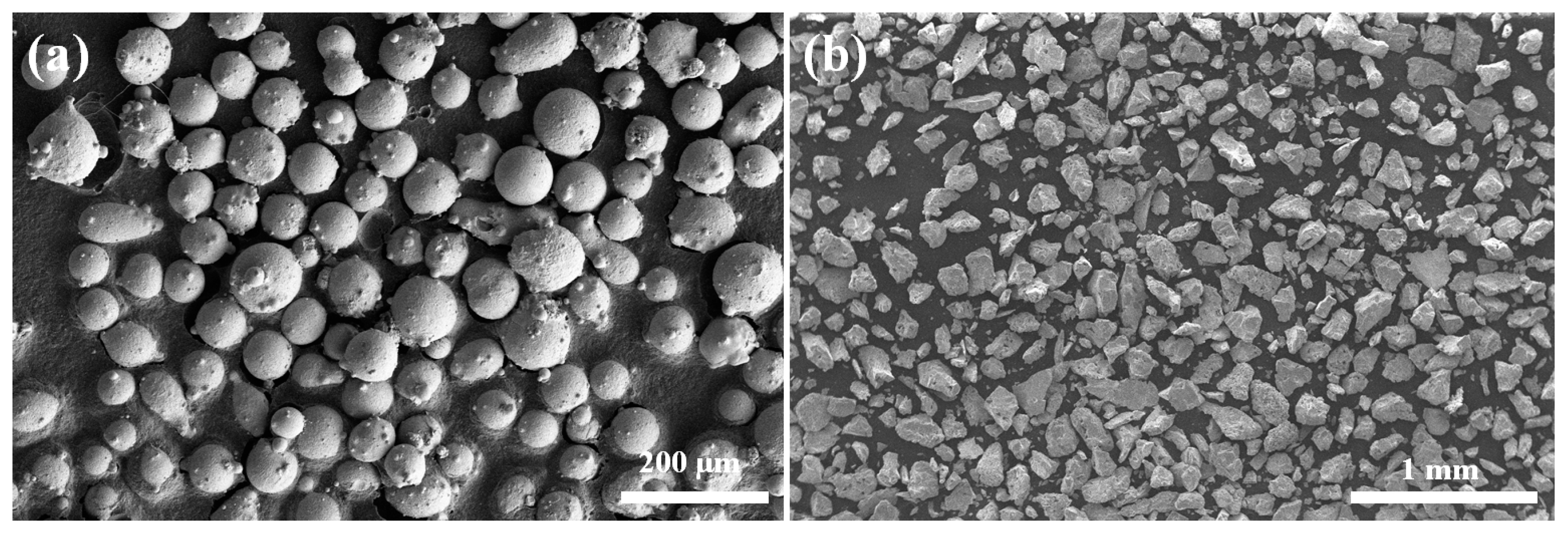
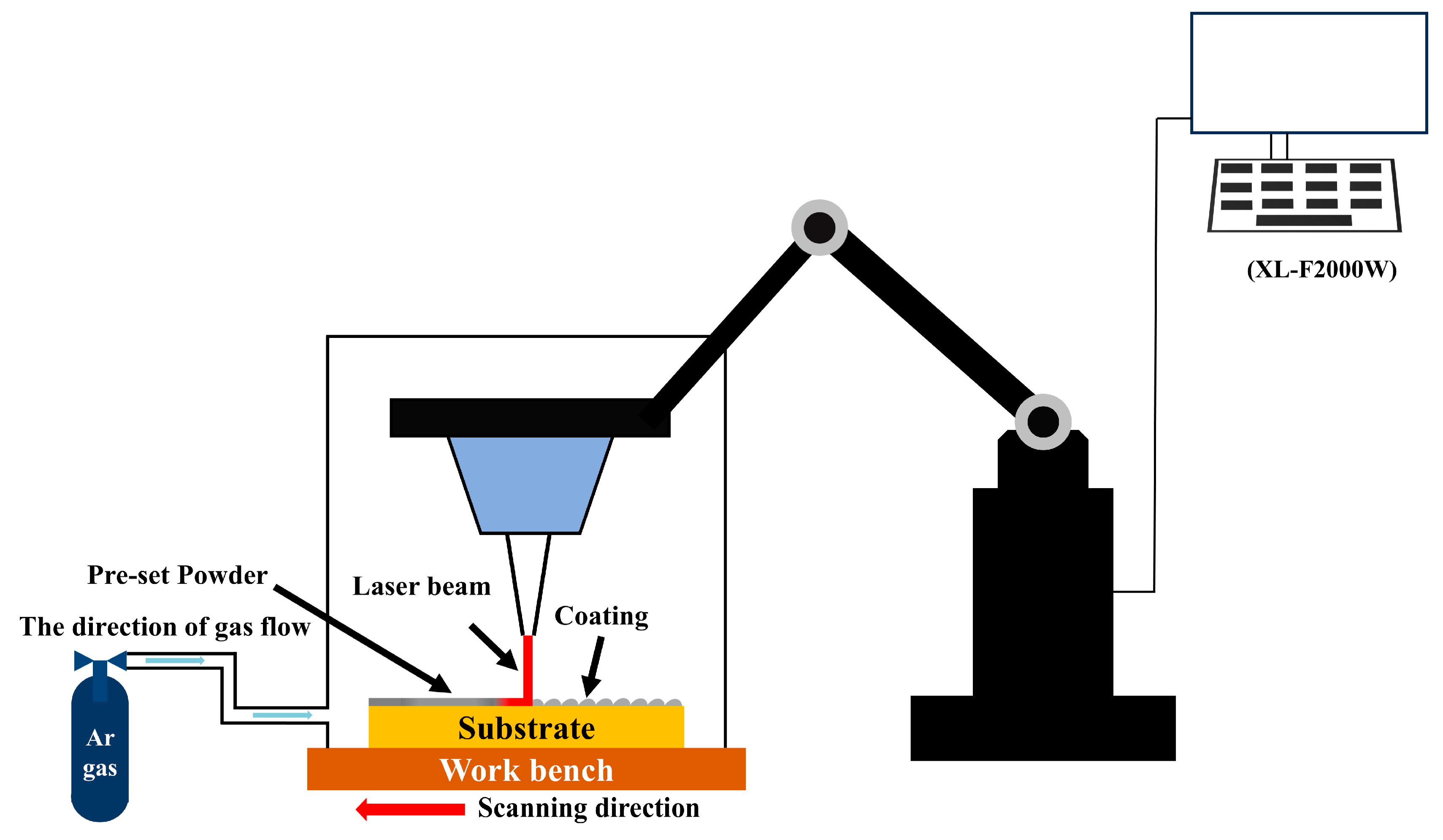
2. Experimental Procedures
3. Results and Analysis
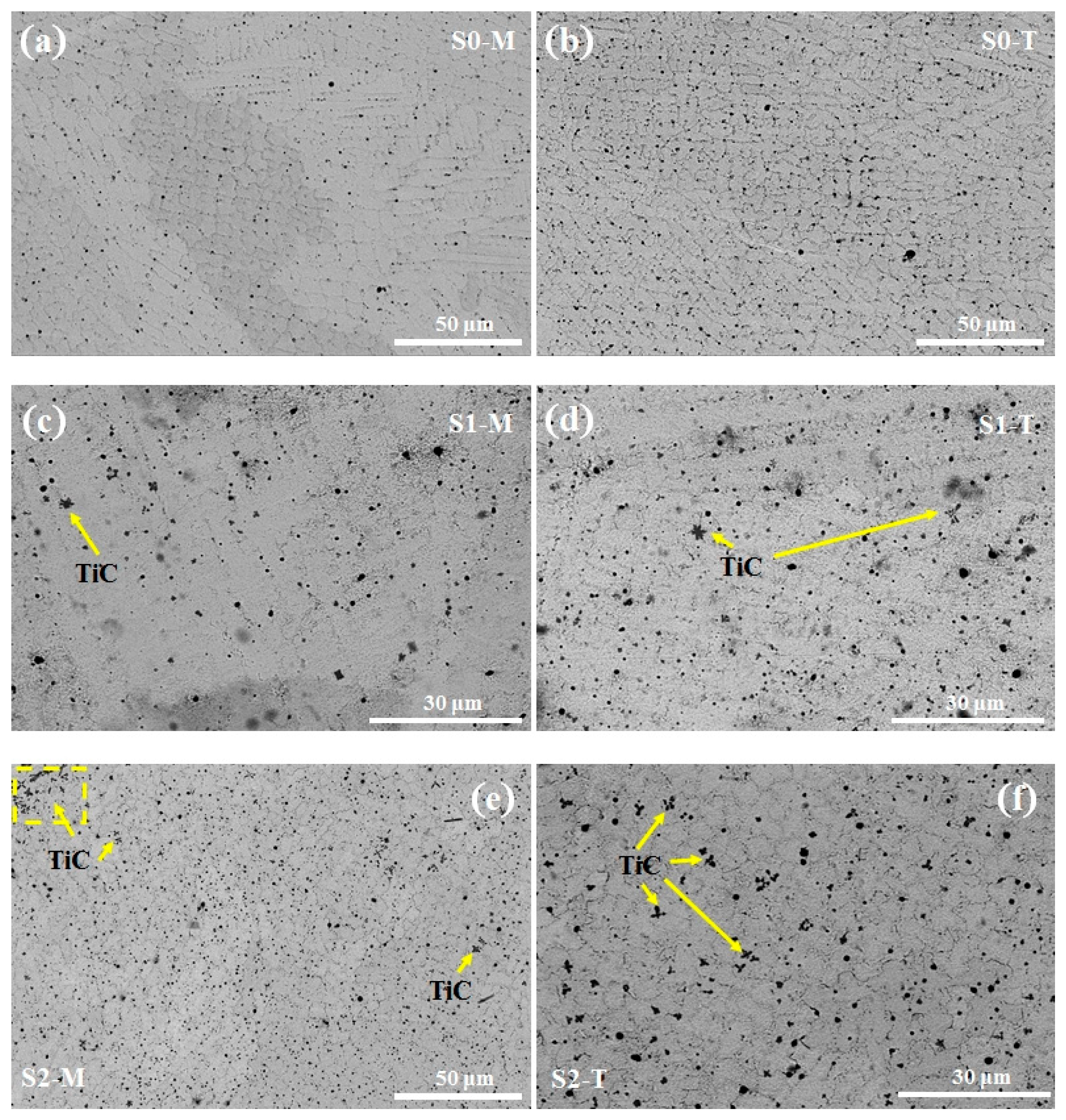
3.1. Metallographic Analysis
3.2. Microscopic Morphology
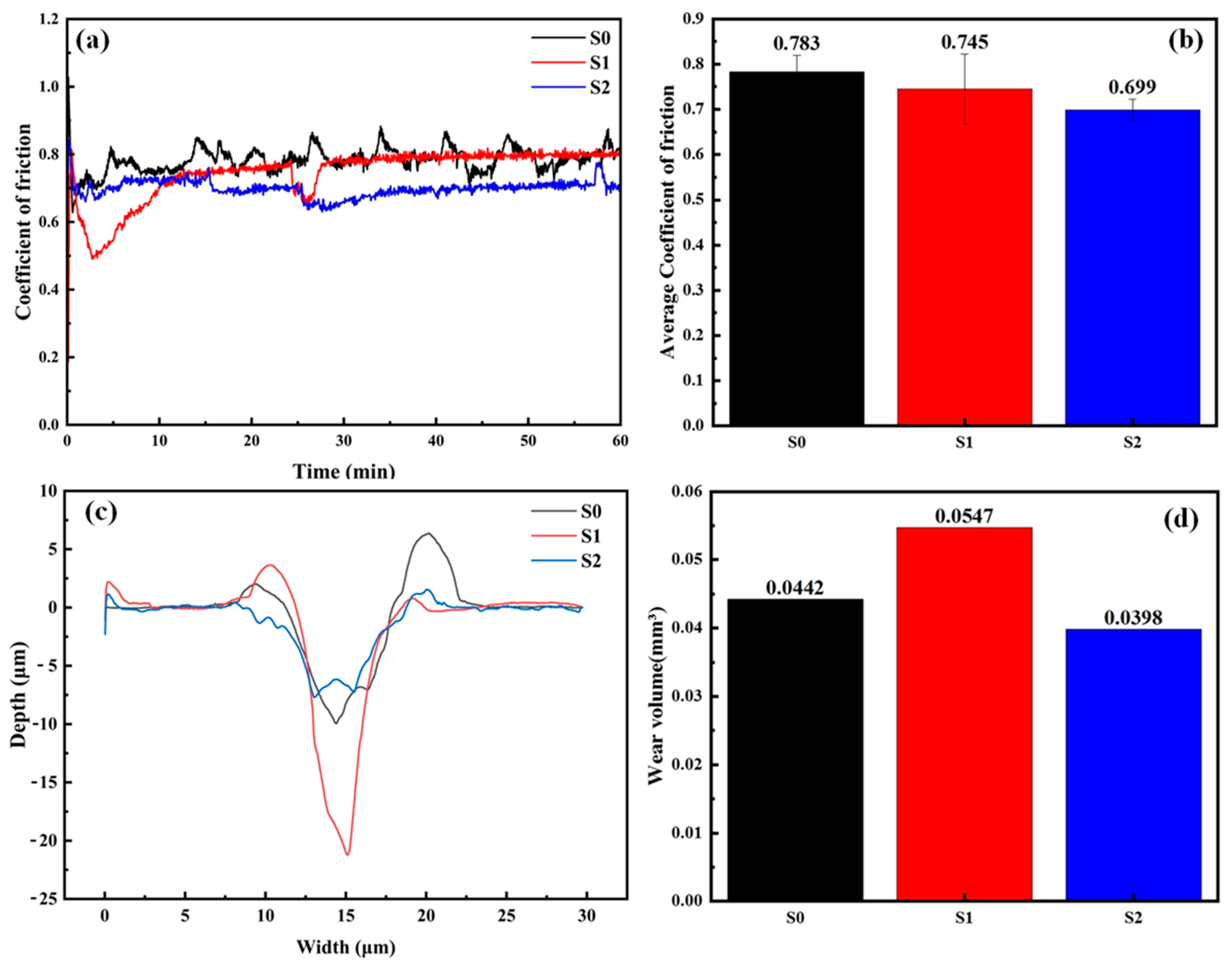
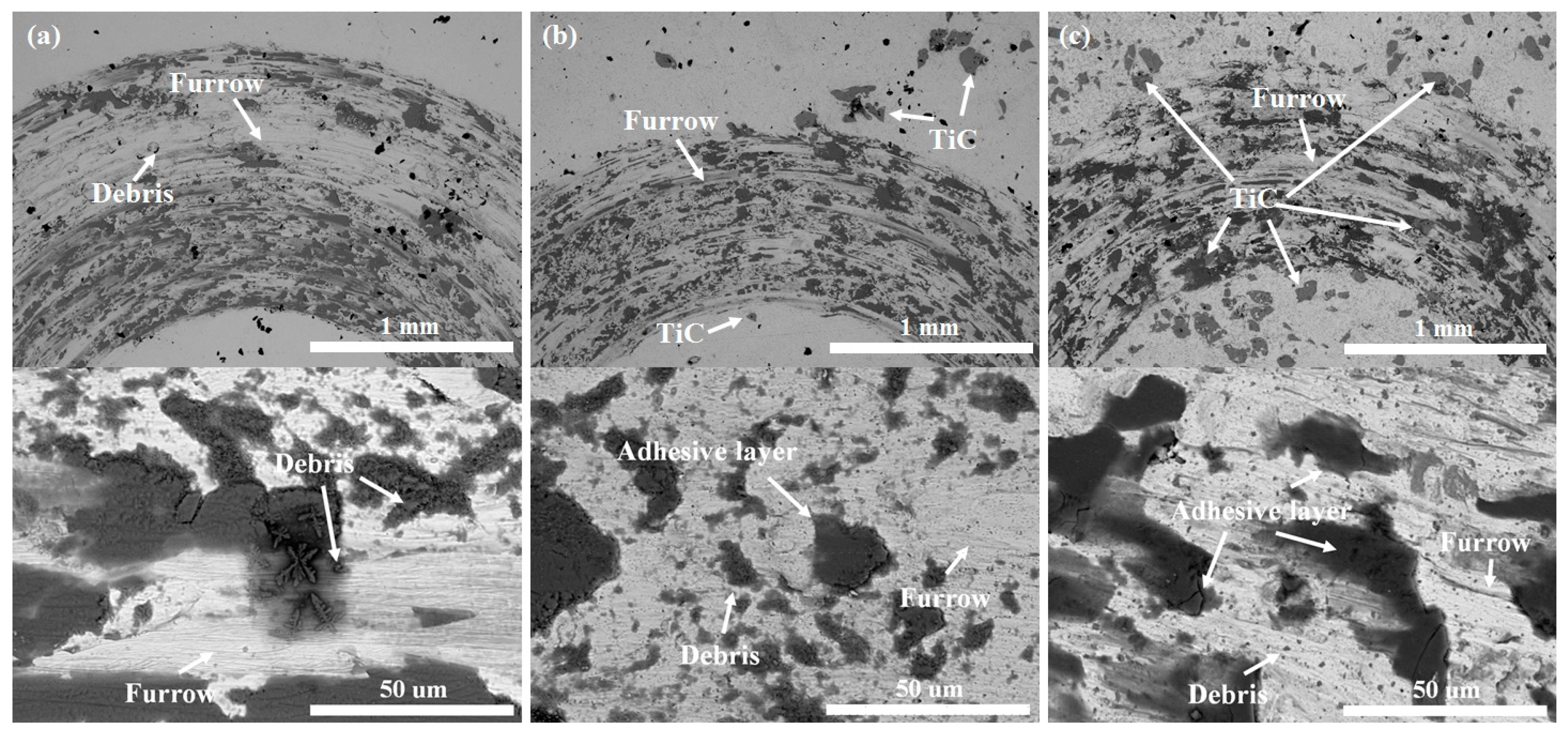
3.3. Wear Resistance
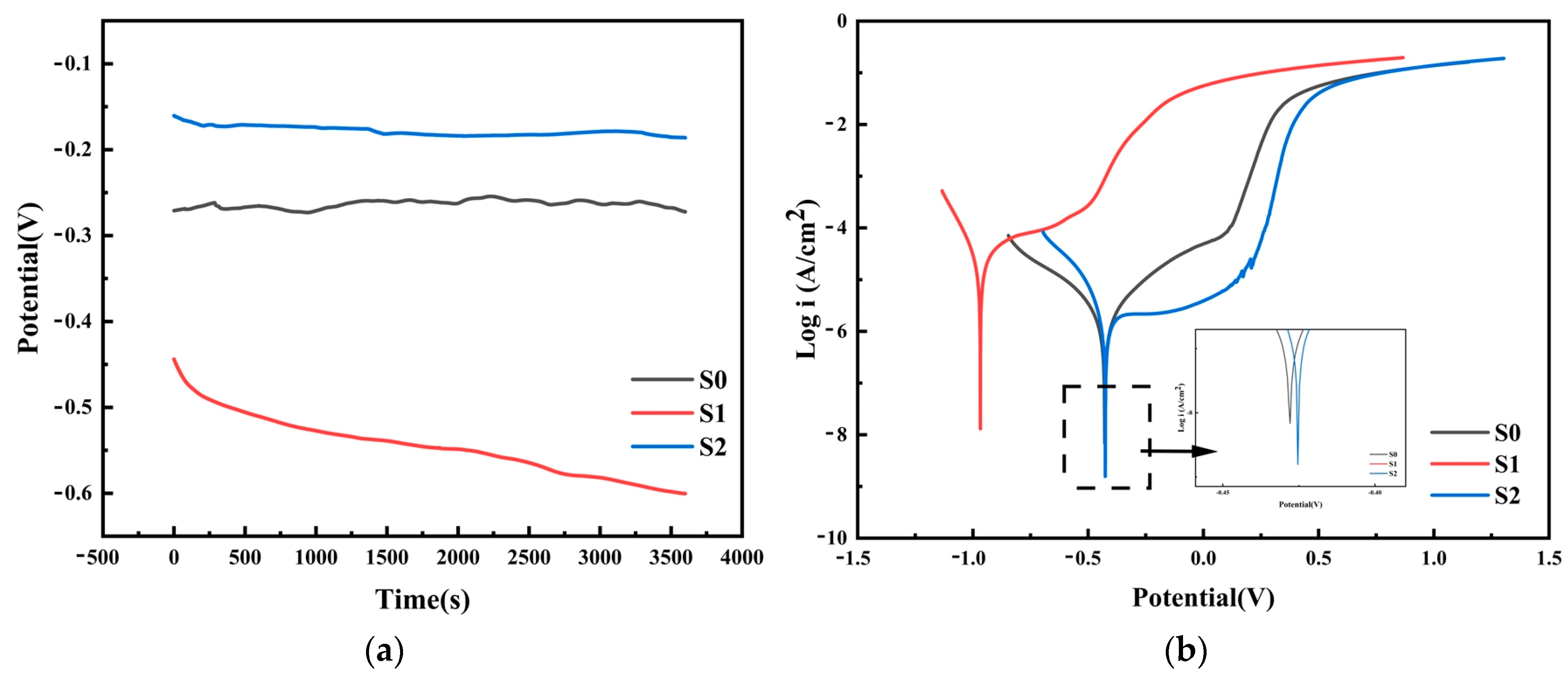
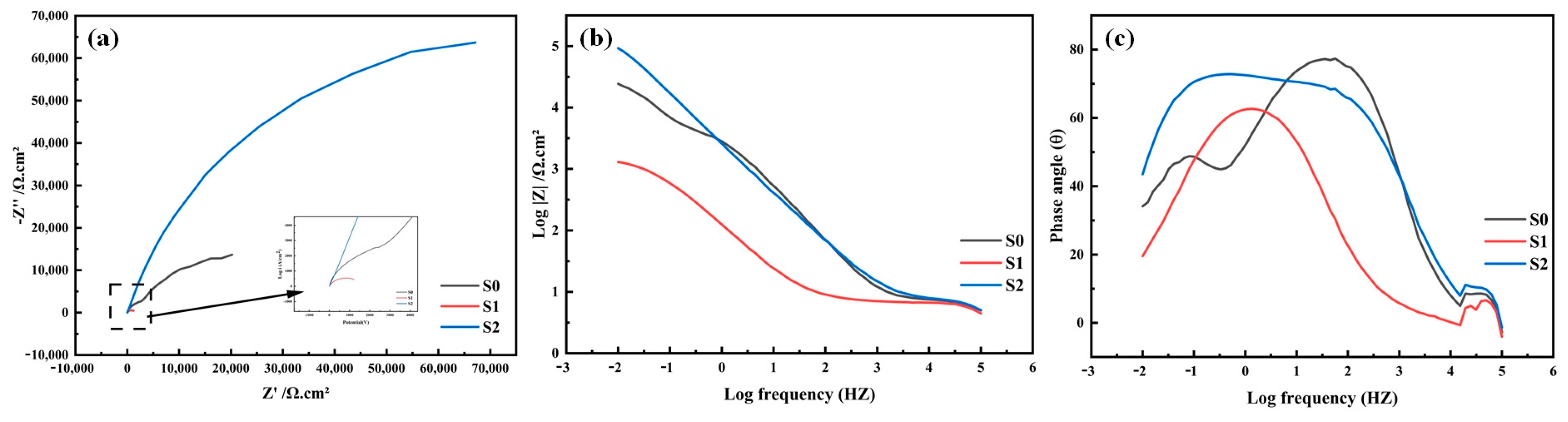
3.4. Corrosion Resistance
4. Conclusions
- (1)
- In the coating (S2) with 20 wt% TiC addition, the TiC and M23C6 phases are uniformly distributed in the high-entropy alloy matrix, and lattice distortion occurs in the coating (broadening and leftward shift of the XRD peaks). In contrast, in the 10 wt% TiC coating (S1), due to gravitational sedimentation and laser decomposition, the reinforcing phase is locally enriched and unevenly distributed.
- (2)
- The S2 coating has the lowest coefficient of friction and wear volume because the uniformly distributed hard phases can disperse the stress. However, in the S1 coating, due to the detachment of TiC and stress concentration, the wear is aggravated.
- (3)
- In a 3.5% NaCl solution, compared with samples S0 and S1, sample S2 showed the most outstanding corrosion resistance because of the protective effect of the dense passivation film.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, P.; Zhang, J.; Yang, T.; Zhang, T.; Zhang, J.; Lin, J.; Yan, Y.; Li, C.; Si, X.; Cao, J.; et al. Characteristics, applications and perspective of high entropy alloys for interfacial joining: A review. J. Manuf. Process. 2024, 110, 303–317. [Google Scholar] [CrossRef]
- Li, D.; Liaw, P.K.; Xie, L.; Zhang, Y.; Wang, W. Advanced high-entropy alloys breaking the property limits of current materials. J. Mater. Sci. Technol. 2024, 186, 219–230. [Google Scholar] [CrossRef]
- Abdullah, M.R.; Peng, Z. Review and perspective on additive manufacturing of refractory high entropy alloys. Mater. Today Adv. 2024, 22, 100497. [Google Scholar] [CrossRef]
- Liang, G.; Jin, G.; Cui, X.; Qiu, Z.; Wang, J. Synthesis and characterization of directional array TiN-reinforced AlCoCrCuNiTi high-entropy alloy coating by magnetic-field-assisted LC. J. Mater. Eng. Perform. 2021, 30, 3568–3576. [Google Scholar] [CrossRef]
- Sun, D.; Zhu, L.S.; Cai, Y.C.; Yan, Y.N.; Ge, F.Y.; Shan, M.D.; Tian, Y.B.; Han, J.; Jiang, Z.Y. Tribology comparison of laser-cladded CrMnFeCoNi coatings reinforced by three types of ceramic (TiC/NbC/B4C). Surf. Coat. Technol. 2022, 450, 129013. [Google Scholar] [CrossRef]
- Sun, D.; Cai, Y.C.; Zhu, L.S.; Gao, F.F.; Shan, M.D.; Manladan, S.M.; Geng, K.P.; Han, J.; Jiang, Z.Y. High-temperature oxidation and wear properties of TiC-reinforced CrMnFeCoNi high entropy alloy composite coatings produced by LC. Surf. Coat. Technol. 2022, 438, 128407. [Google Scholar] [CrossRef]
- Wei, Y.N.; Chen, Y.; Guo, B.B.; Zhu, L.H. The microstructure and mechanical properties of TiC-reinforced W-matrix composites prepared by spark plasma sintering. Int. J. Refract. Met. Hard Mater. 2023, 112, 106158. [Google Scholar] [CrossRef]
- Zhuang, D.D.; Tao, W.W.; Du, B.; Zhang, S.H.; Lian, X.L.; Wang, F. Microstructure and properties of TiC-enhanced CrMnFeCoNi high-entropy alloy coatings prepared by LC. Tribol. Int. 2023, 180, 108246. [Google Scholar] [CrossRef]
- Xiong, J.K.; Sun, D.; Dong, N.; Cai, Y.C.; Wang, D.Y.; Luo, Z. Oxidation mechanism of TiC reinforced CrMnFeCoNi composite coatings by LC at 600 °C/900 °C. Mater. Lett. 2022, 324, 132710. [Google Scholar] [CrossRef]
- Zhou, J.L.; Cheng, Y.H.; He, B.; Wan, Y.X.; Chen, H.; Wang, Y.F.; Yang, J.Y. Enhancement of high-entropy alloy coatings with multi-scale TiC ceramic particles via high-speed LC: Microstructure, wear and corrosion. Appl. Surf. Sci. 2025, 685, 162061. [Google Scholar] [CrossRef]
- Zhao, W.; Kedong, Y.; Ma, Q.I.; Song, C.; Xiao, G.; Zhang, H.; Lv, Y.; Guo, N.; Li, Z. Synergistic effects of Mo and in-situ TiC on the microstructure and wear resistance of AlCoCrFeNi high entropy alloy fabricated by LC. Tribol. Int. 2023, 188, 108827. [Google Scholar] [CrossRef]
- Zhang, X.R.; Cui, X.F.; Qi, M.; Zhang, Q.; Qi, Y.P.; Jin, G. Corrosion and passivation behavior of in-situ TiC reinforced Al 0.1 CrNbSi 0.1 TaTiV refractory high entropy alloy coatings via doping C. Corros. Sci. 2024, 227, 111736. [Google Scholar] [CrossRef]
- Shang, X.; Liu, Q.; Guo, Y.; Ding, K.; Liao, T.; Wang, F. Nano-TiC reinforced [Cr–Fe 4 4 4 ]Cr 3 high-entropy-alloy composite coating fabricated by LC. J. Mater. Res. Technol. 2022, 21, 2076–2088. [Google Scholar] [CrossRef]
- Wu, H.; Wang, L.; Zhang, S.; Wu, C.L.; Zhang, C.H.; Sun, X.Y. Corrosion and cavitation erosion behaviors of laser clad FeNiCoCr high-entropy alloy coatings with different types of TiC reinforcement. Surf. Coat. Technol. 2023, 471, 129910. [Google Scholar] [CrossRef]
- Zhang, J.; Jia, T.; Zhu, H.G.; Xie, Z.H. Microstructure and mechanical properties of in-situ TiC reinforced FeCoNiCu2.0 high entropy alloy matrix composites. Mater. Sci. Eng. A 2021, 822, 141671. [Google Scholar] [CrossRef]
- Wilson, J.M.; Shin, Y.C. Microstructure and wear properties of laser-deposited functionally graded Inconel 690 reinforced with TiC. Surf. Coat. Technol. 2012, 207, 517–522. [Google Scholar] [CrossRef]
- Yu, K.D.; Zhao, W.; Li, Z.; Guo, N.; Xiao, G.C.; Zhang, H. High–temperature oxidation behavior and corrosion resistance of in–situ TiC and Mo reinforced AlCoCrFeNi–based high entropy alloy coatings by LC. Ceram Int. 2023, 49, 10151–10164. [Google Scholar] [CrossRef]
- Wu, C.L.; Xu, T.Z.; Wang, Z.Y.; Zhang, C.H.; Zhang, S.; Ni, C.L. Laser surface alloying of FeCoCrAlNiTi high entropy alloy composite coatings reinforced with TiC on 304 stainless steel to enhance wear behavior. Ceram Int. 2022, 48, 20690–20698. [Google Scholar] [CrossRef]
- Liu, H.; Liu, J.; Chen, P.J.; Yang, H.F. Microstructure and high temperature wear behaviour of in–situ TiC reinforced AlCoCrFeNi–based high–entropy alloy composite coatings fabricated by LC. Opt. Laser Technol. 2019, 118, 140–150. [Google Scholar] [CrossRef]
- Jiang, D.; Hong, C.; Zhong, M.; Alkhayat, M.; Weisheit, A.; Gasser, A.; Zhang, H.; Kelbassa, I.; Poprawe, R. Fabrication of nano-TiCp reinforced Inconel 625 composite coatings by partial dissolution of micro-TiCp through LC energy input control. Surf. Coat. Technol. 2014, 249, 125–131. [Google Scholar] [CrossRef]
- Luo, F.Y.; Wang, S.S.; Shi, W.Q.; Xiong, Z.Y.; Huang, J. Wear behavior of single-layer graphene oxide reinforced CoCrFeNiMn HEA coating by LC. Intermetallics 2024, 175, 108512. [Google Scholar] [CrossRef]
- Yeh, J.; Chen, S.; Lin, S.; Gan, J.; Chin, T.; Shun, T.; Tsau, C.; Chang, S. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2003, 3571, 257–264. [Google Scholar] [CrossRef]
- Wang, T.; Zhu, L.; Song, H.Y.; Wang, H. Effect of WC-17Co content on microstructure and properties of IN718 composites prepared by laser cladding. Opt. Laser Technol. 2022, 148, 107780. [Google Scholar] [CrossRef]
- Luo, F.Y.; Shi, W.Q.; Xiong, Z.Y.; Huang, J. Microstructure and properties analysis of AlCoCrFeNi high-entropy alloy/iron-based amorphous composite coatings prepared by LC. J. Non-Cryst. Solids 2024, 624, 122732. [Google Scholar] [CrossRef]
- Luo, F.Y.; Wang, S.S.; Shi, W.Q.; Zhao, Y.; Huang, J. Wear behavior and corrosion resistance of laser-clad Ni60-1% carbon nanotubes coating. Surf. Coat. Technol. 2024, 482, 130686. [Google Scholar] [CrossRef]
- Gu, Z.; Xi, S.Q.; Sun, C.F. Microstructure and properties of laser cladding and CoCr2.5FeNi2Tix high-entropy alloy composite coatings. J. Alloys Compd. 2020, 819, 152986. [Google Scholar] [CrossRef]
- Li, Y.T.; Fu, H.G.; Ma, T.J.; Wang, K.M.; Yang, X.J.; Lin, J. Microstructure and wear resistance of AlCoCrFeNi-WC/TiC composite coating by laser cladding. Mater. Charact. 2022, 194, 112479. [Google Scholar] [CrossRef]
- Ismail, H.; Vaclav, O.; Kornel, C.; Jeffthm, D. Microstructure and Phase Formation in a Rapidly Solidified Laser-Deposited Ni-Cr-B-Si-C Hardfacing Alloy. Metall. Mater. Trans. A 2014, 45A2, 878–892. [Google Scholar]
- Liu, X.M. Mechanism and control of the nick-based alloy WC laser cladded layer microstructure. Appl. Laser 2006, 5, 299–302. [Google Scholar]
- Li, Y.H.L.J.; Tao, Y.F.; Hu, L.F. High-temperature wear and oxidation behaviors of TiNi/Ti2Ni matrix composite coatings with TaC addition prepared on Ti6Al4V by laser cladding. Appl. Surf. Sci. 2017, 402, 478–494. [Google Scholar]
- Yuan, W.Y.; Li, R.F.; Zhu, Y.Y.; Zhao, Y.; Zhang, X.Q.; Liu, B.; Zhang, B.S. Structure and properties of nickel-plated CNTs/Fe-based amorphous composite coatings fabricated by high-speed LC. Surf. Coat. Technol. 2022, 438, 128363. [Google Scholar] [CrossRef]
- Shahriari, A.; Ghaffari, M.; Khaksar, L.; Nasiri, A.; Hadadzadeh, A.; Amirkhiz, B.S.; Mohammadi, M. Corrosion resistance of 13wt% Cr martensitic stainless steels: Additively manufactured CX versus wrought ni-containing AISI 420. Corros. Sci. 2021, 184, 109362. [Google Scholar] [CrossRef]
- Muangtong, P.; Rodchanarowan, A.; Chaysuwan, D.; Chanlek, N.; Goodall, R. The corrosion behaviour of CoCrFeNix x = Cu, Al, Sn) high entropy alloy systems in chloride solution. Corros. Sci. 2020, 172, 108740. [Google Scholar] [CrossRef]
- Zhou, Y.Q.; Kong, D.C.; Wang, L.; Li, R.X.; Ni, X.Q.; Cheng, M.; Dong, C.F.; Engelberg, D. Pit growth kinetics of additively manufactured MoNi over-alloyed type 316L stainless steel. J. Mater. Res. Technol. 2023, 27, 7532–7547. [Google Scholar] [CrossRef]
- Li, J.X.; Liu, Z.D.; Ma, H.R.; Liu, Q.B.; Mao, J.; Zhang, J.; Kong, Y. Comparative study on the corrosion behaviour of 1.4529 super austenitic stainless steel and laser-cladding 1.4529 coating in simulated desulfurized flue gas condensates. Corros. Sci. 2022, 209, 110794. [Google Scholar] [CrossRef]

| Material | Chemical Composition (Mass. %) | ||||||
|---|---|---|---|---|---|---|---|
| C | Si | Mn | Fe | Cr | Ni | Co | |
| Q235B | 0.17 | 0.16 | 0.38 | Bal. | - | - | - |
| CoCrFeNiMn | 0.025 | - | 19.36 | 19.76 | 18.71 | 20.81 | 21.24 |
| Name of Coatings | Mass Fractions/(Mass.%) |
|---|---|
| S0 | CoCrFeNiMn |
| S1 | CoCrFeNiMn + 10 wt% TiC |
| S2 | CoCrFeNiMn + 20 wt% TiC |
| Parameter | Ecorr (V) | Icorr (A/cm2) | |
|---|---|---|---|
| Sample | |||
| S0 | −0.428 | ||
| S1 | −0.968 | ||
| S2 | −0.425 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhan, Q.; Luo, F.; Huang, J.; Wang, Z.; Ma, B.; Liu, C. Corrosion Resistance and Wear Properties of CoCrFeNiMn/TiC High-Entropy Alloy-Based Composite Coatings Prepared by Laser Cladding. Lubricants 2025, 13, 210. https://doi.org/10.3390/lubricants13050210
Zhan Q, Luo F, Huang J, Wang Z, Ma B, Liu C. Corrosion Resistance and Wear Properties of CoCrFeNiMn/TiC High-Entropy Alloy-Based Composite Coatings Prepared by Laser Cladding. Lubricants. 2025; 13(5):210. https://doi.org/10.3390/lubricants13050210
Chicago/Turabian StyleZhan, Qiang, Fangyan Luo, Jiang Huang, Zhanshan Wang, Bin Ma, and Chengpu Liu. 2025. "Corrosion Resistance and Wear Properties of CoCrFeNiMn/TiC High-Entropy Alloy-Based Composite Coatings Prepared by Laser Cladding" Lubricants 13, no. 5: 210. https://doi.org/10.3390/lubricants13050210
APA StyleZhan, Q., Luo, F., Huang, J., Wang, Z., Ma, B., & Liu, C. (2025). Corrosion Resistance and Wear Properties of CoCrFeNiMn/TiC High-Entropy Alloy-Based Composite Coatings Prepared by Laser Cladding. Lubricants, 13(5), 210. https://doi.org/10.3390/lubricants13050210






