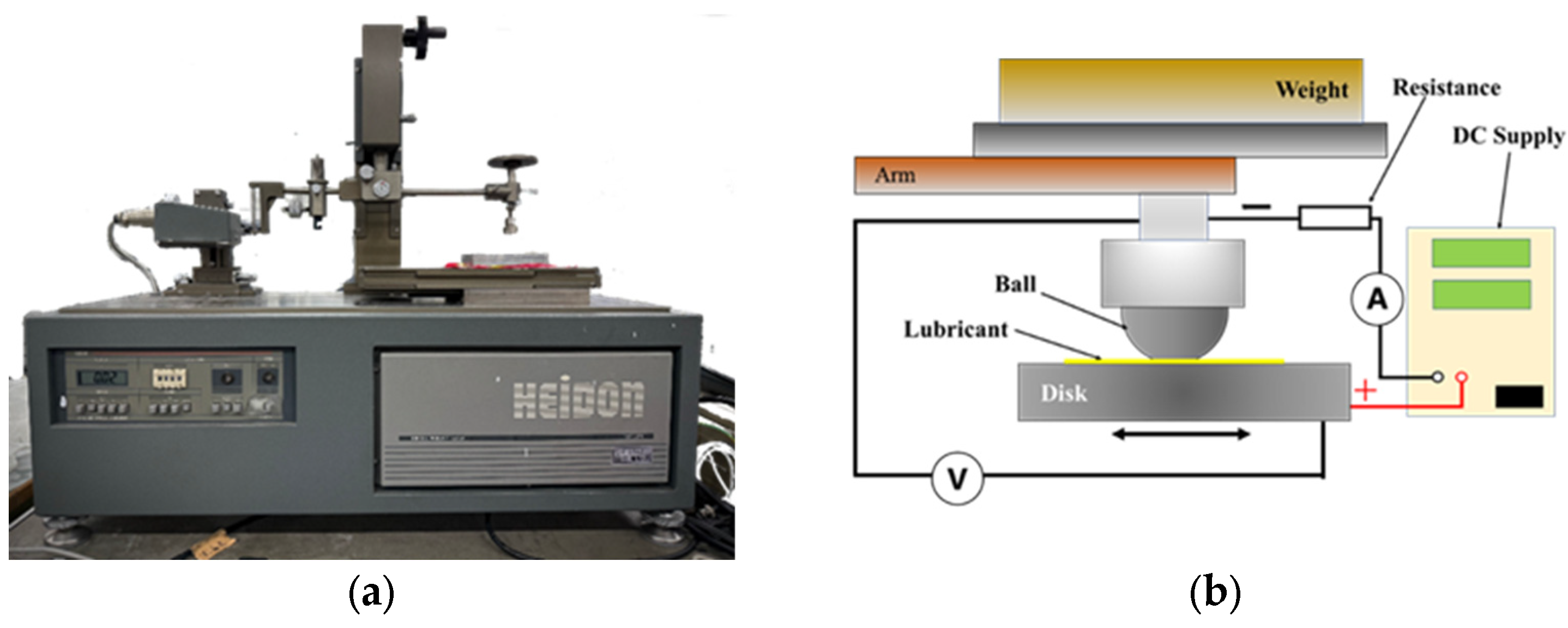
3.1. Friction Test Results
As shown in
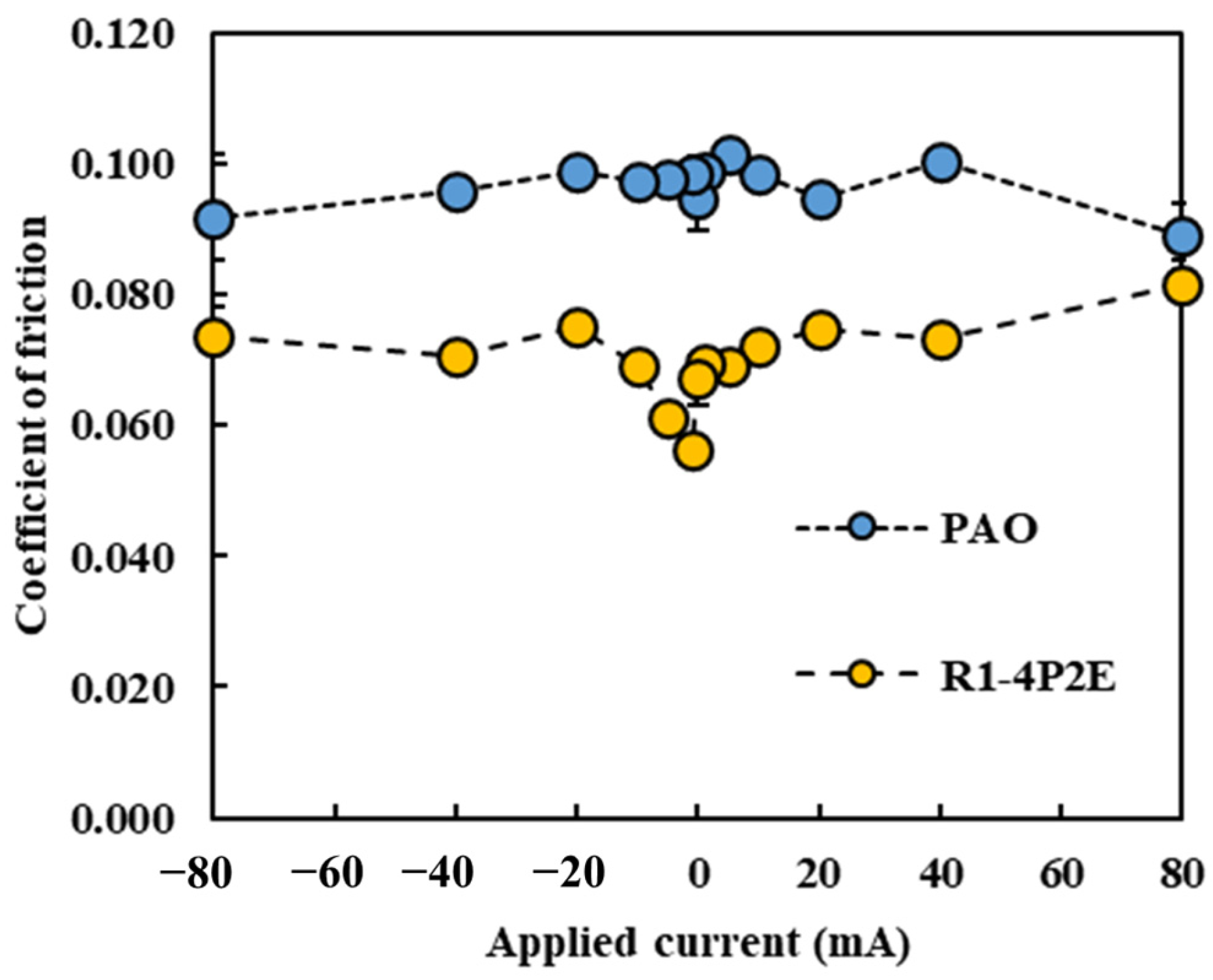
Figure 3, the effect of current direction and magnitude on the average COF was evaluated. The COF values reported are steady-state values, obtained by averaging the data from the last 10 min of each test (after the running-in period). In all cases, the sign of the applied current is defined from the perspective of the ball (i.e., a “+” current means the ball is the positive electrode).
Under all the tested current conditions, the PPE-based lubricant (R1-4P2E) exhibited a lower COF than the PAO. This trend is consistent with previous findings [
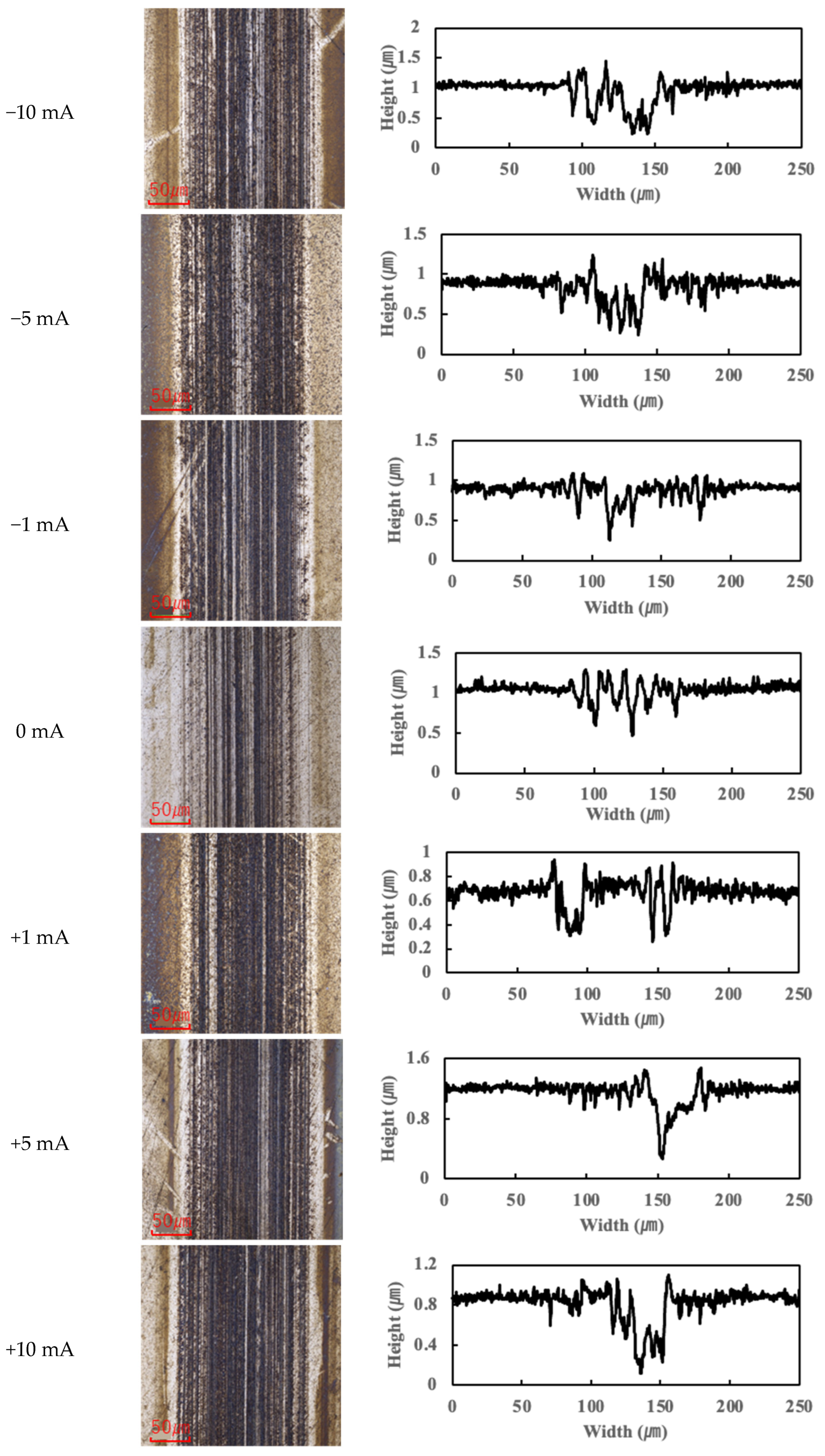
25] reporting that PPE-based lubricants significantly reduce friction compared to PAO. Within the current range of approximately −10 mA to +10 mA, R1-4P2E maintained a COF of about 0.06–0.07, which was 30–40% lower than that of the PAO (~0.10). However, for current values exceeding 10 mA, a notable increase in COF was observed for R1-4P2E. For example, at +20 mA the COF increased by approximately 10% relative to the 0 mA (non-electrified) condition, and at +80 mA it increased by roughly 25%. Furthermore, the rise in COF was more pronounced when the ball was the positive electrode. At +20 mA, the COF with the ball being positive was higher than the COF with the ball being negative, suggesting that current polarity has a significant influence on friction behavior.
These effects became evident at around a 10 mA threshold. Below ~10 mA, R1-4P2E retained its low-friction performance, whereas above ~10 mA the influence of current on friction became significant. By contrast, the PAO lubricant showed minimal variation in COF across all tested current conditions, maintaining a stable COF of approximately 0.10 regardless of whether a current was applied. These observations suggest that PAO’s frictional behavior was essentially unaffected by electrical current, whereas R1-4P2E’s frictional behavior changed significantly with the magnitude and direction of the current. It is evident that PPE-based lubricants are more susceptible to applied electrical current than PAO. Still, within the ±10 mA range, R1-4P2E consistently showed low friction coefficients.
Previous studies have suggested that PPE molecules can form polymeric tribofilms during friction, which helps to reduce friction [
25]. The findings of this study support this hypothesis: under moderate current conditions, a thin tribofilm derived from PPE forms on the contact surfaces, which contributes to lubrication and helps maintain a low friction coefficient. However, once the applied current exceeded roughly 10 mA, the excessive formation and accumulation of tribofilm began to adversely affect friction, causing the COF to rise beyond the baseline. (This aspect is further discussed in
Section 3.3.)
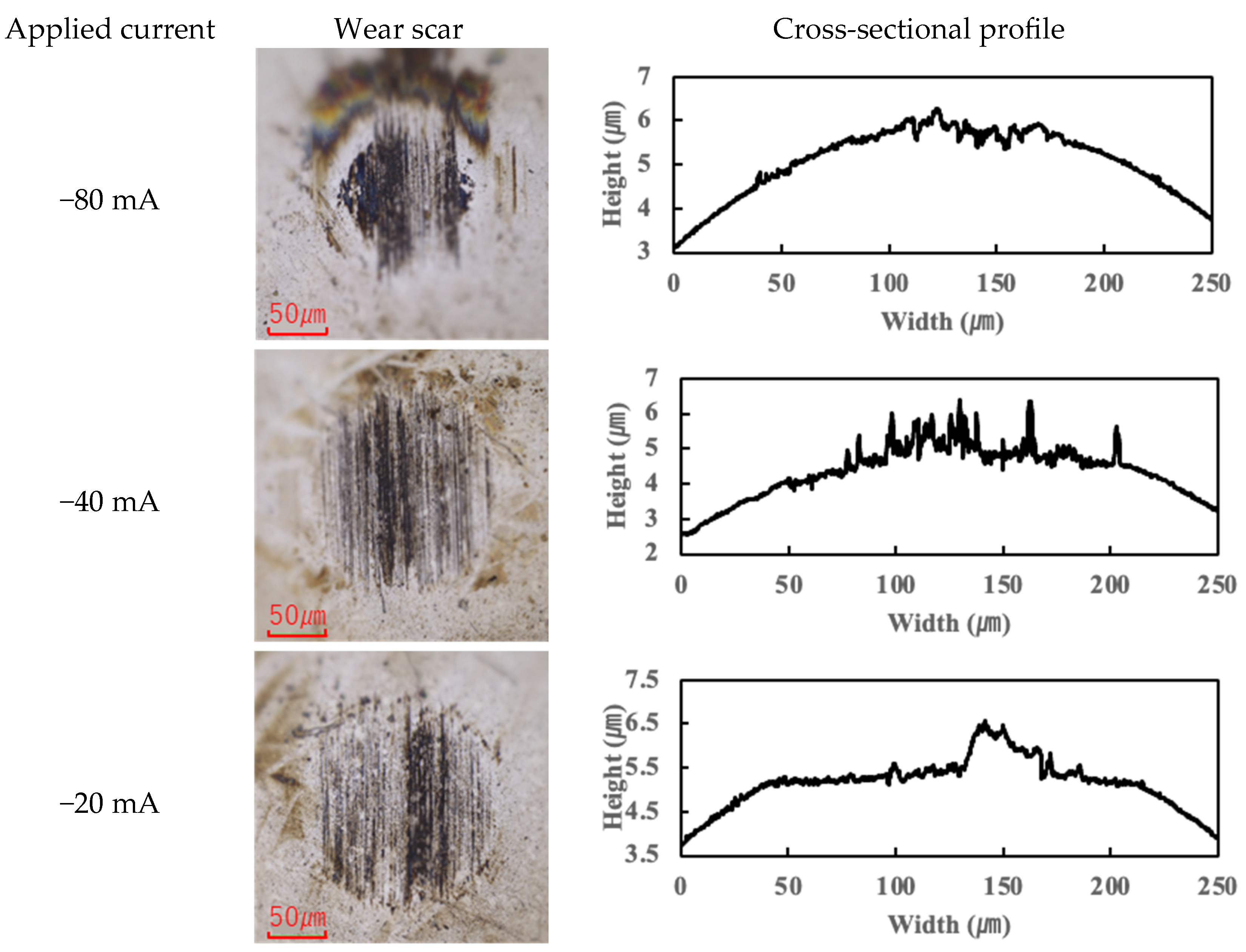
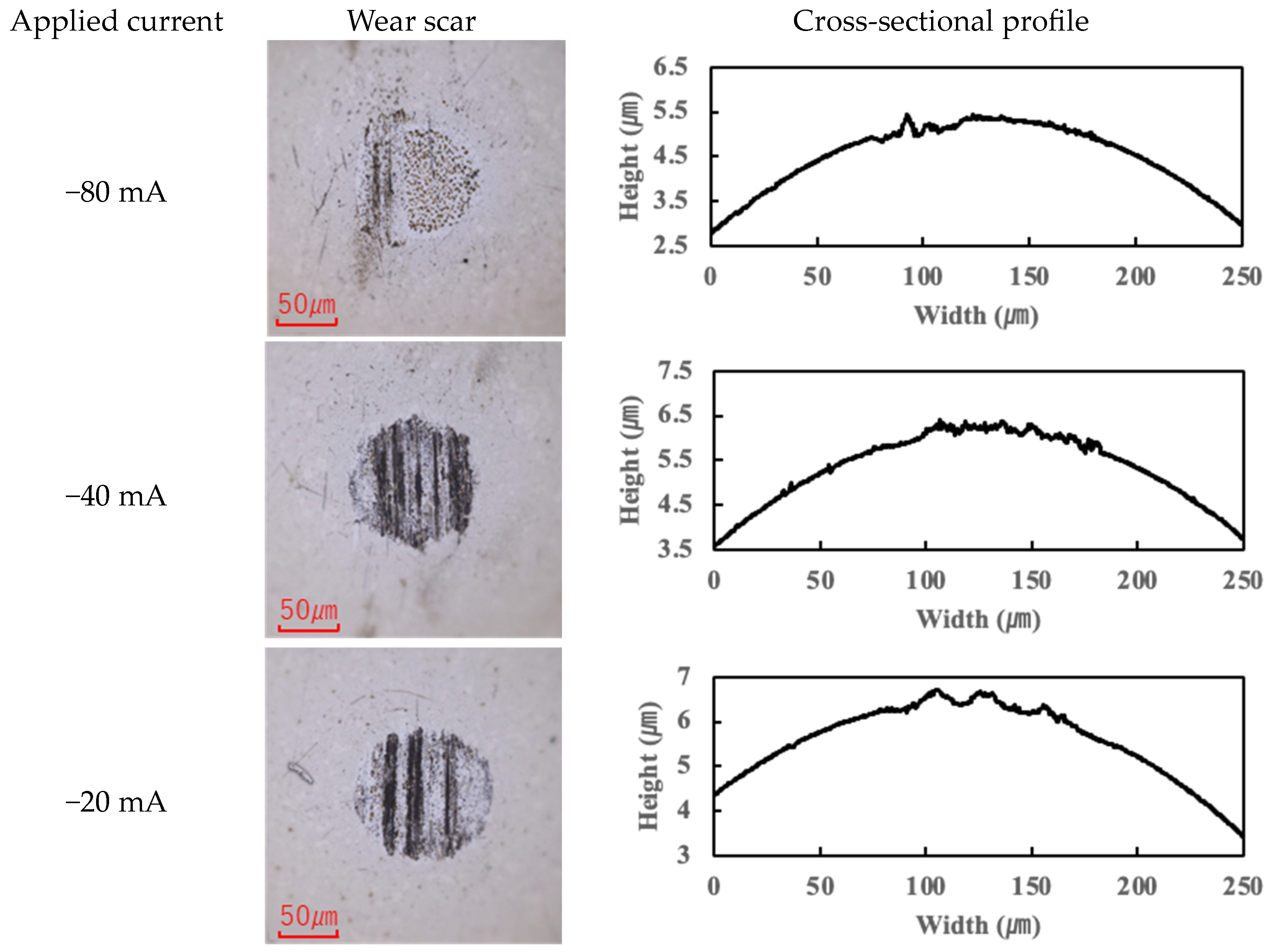
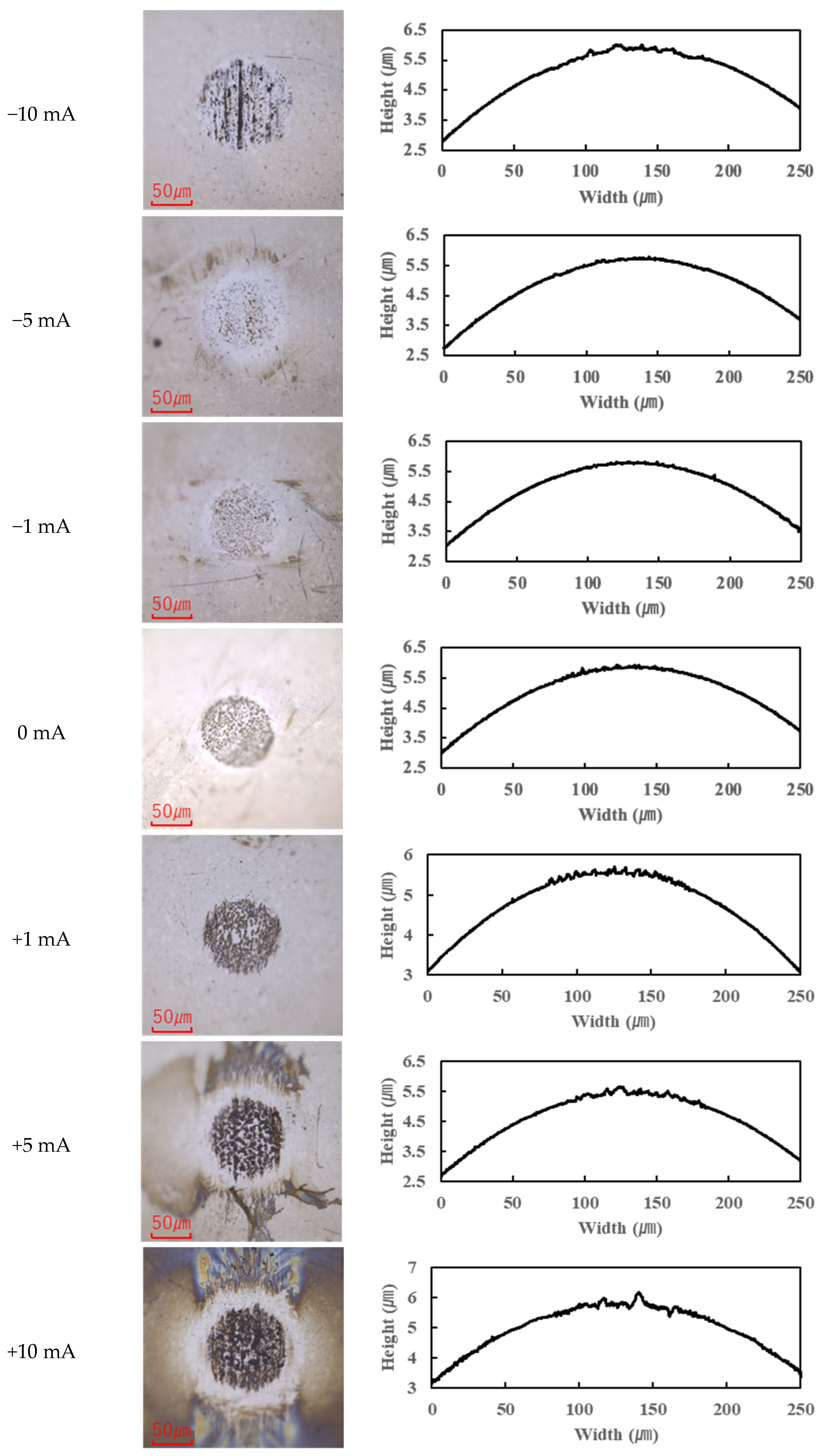
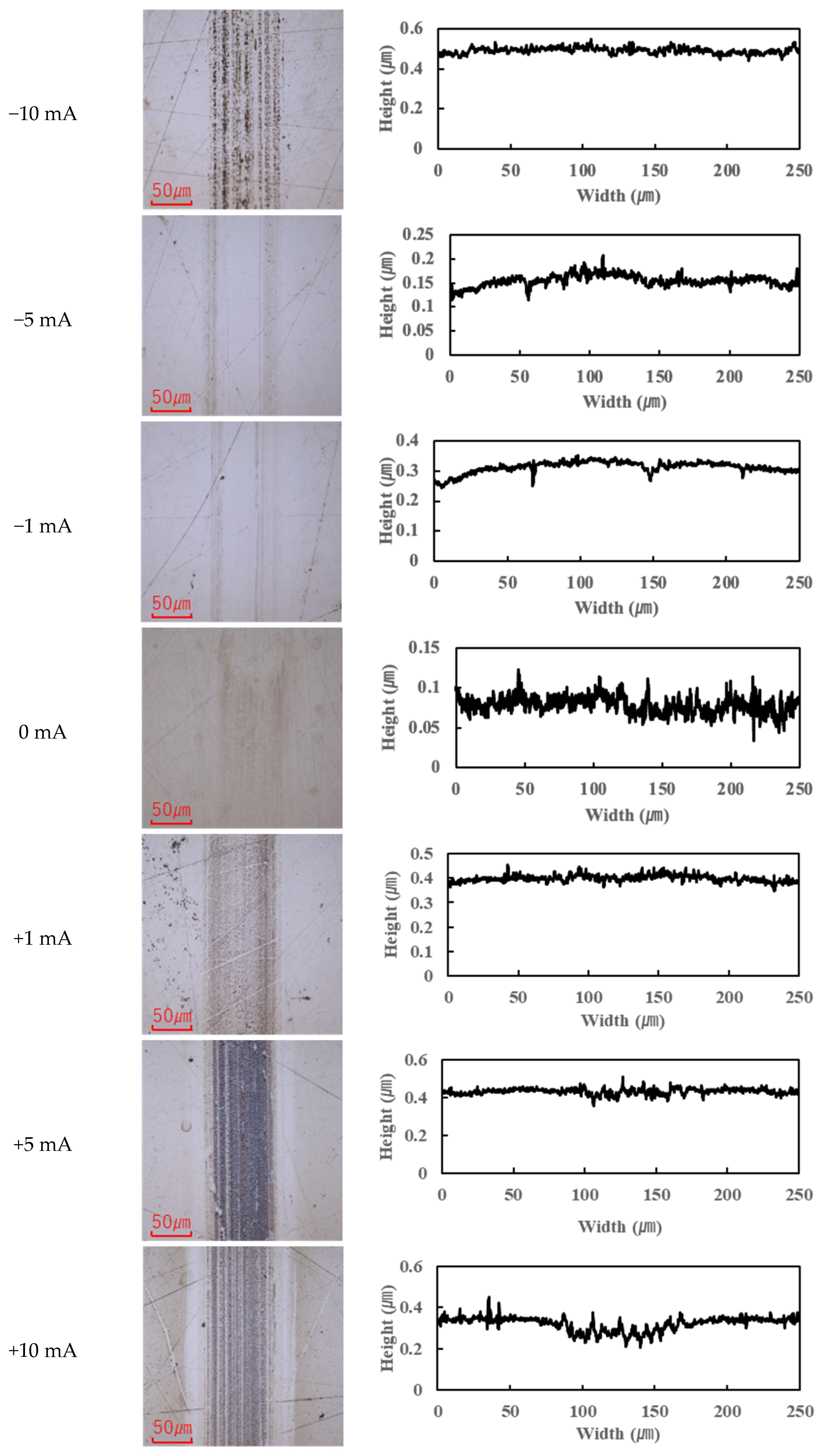
The application of current also affected wear.
Figure 4,
Figure 5,
Figure 6 and
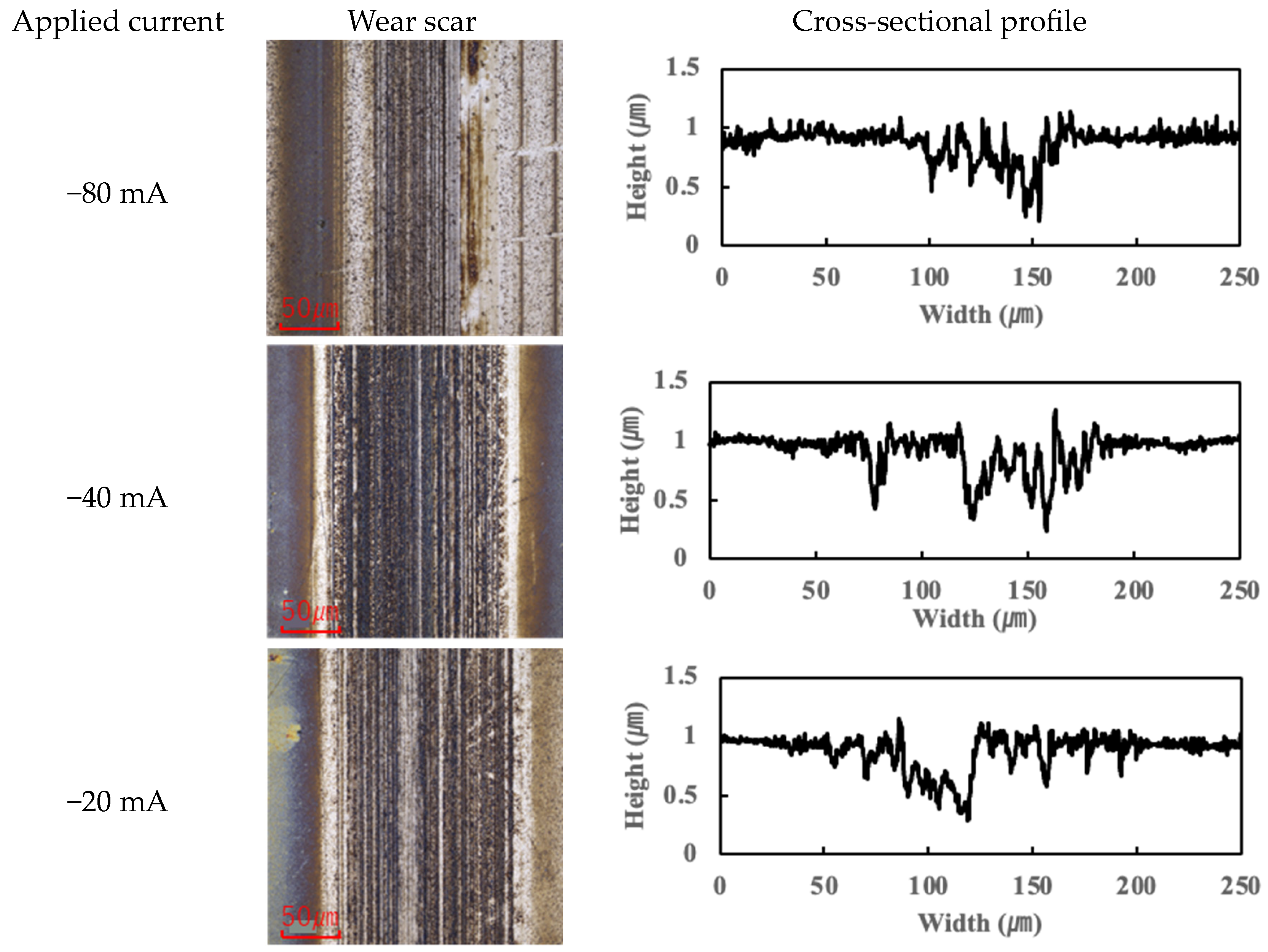
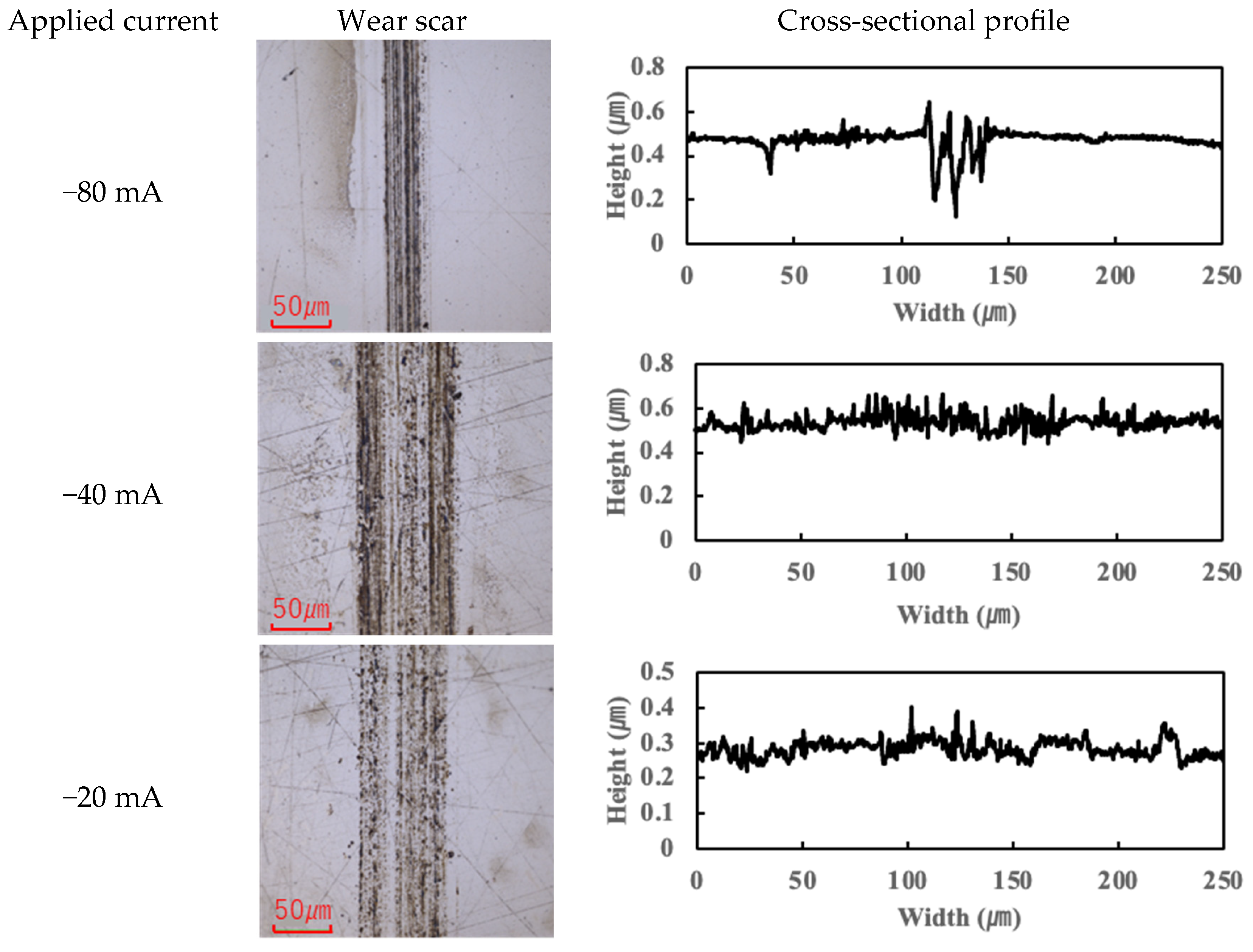
Figure 7 present the wear scars and surface profiles of the ball and disk under different current conditions. The PAO tests showed almost no change in wear scar appearance across the range of currents. The wear scars with PAO lubrication remained bright and metallic in appearance, with only the minor localized attachment of wear debris. As seen in
Figure 4 and
Figure 5 (PAO and ball and disk, respectively), varying the current direction or magnitude did not produce any significant difference in scar color or condition, and no distinct tribofilm layer was evident (apart from a small amount of scattered debris).
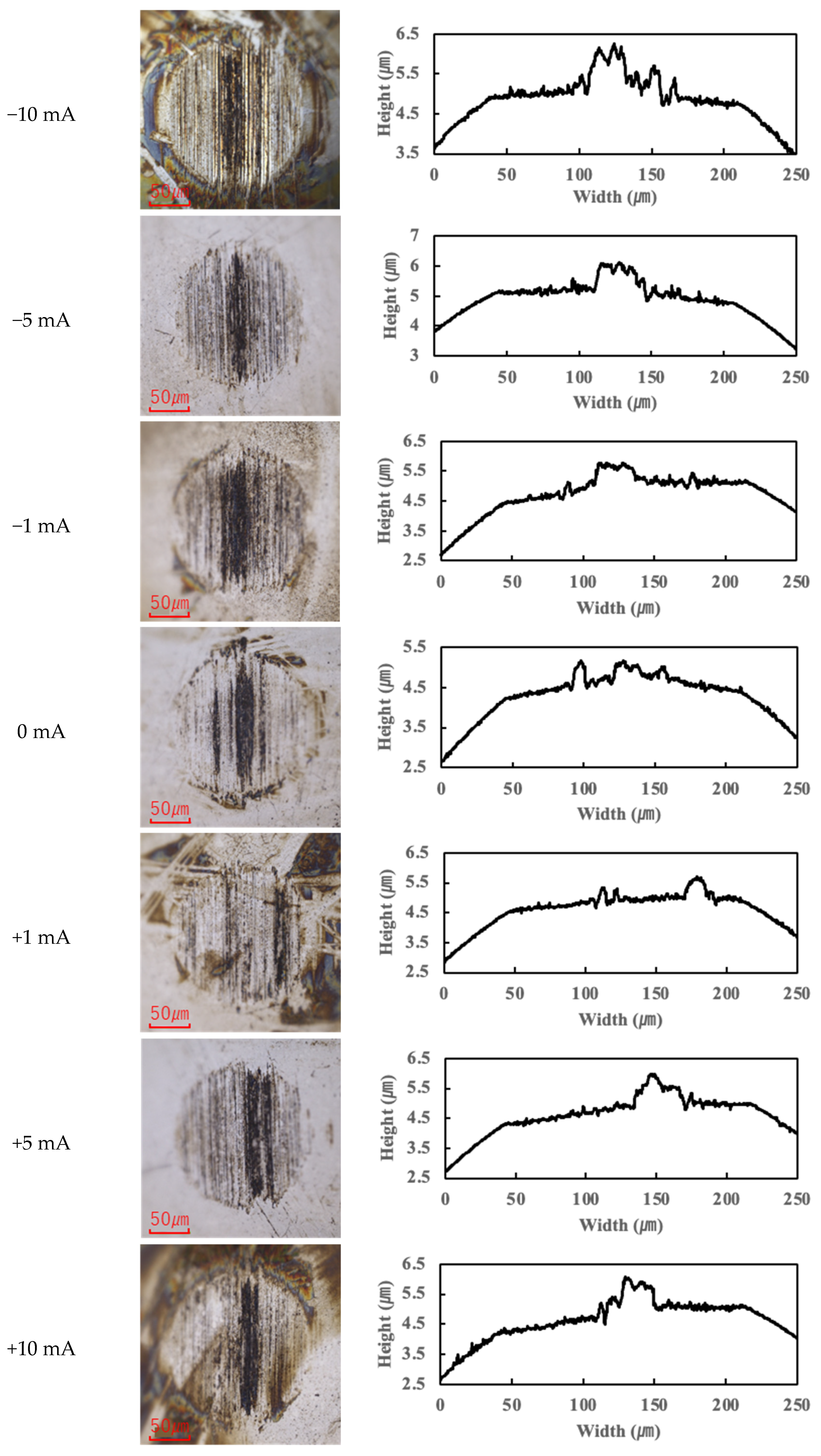
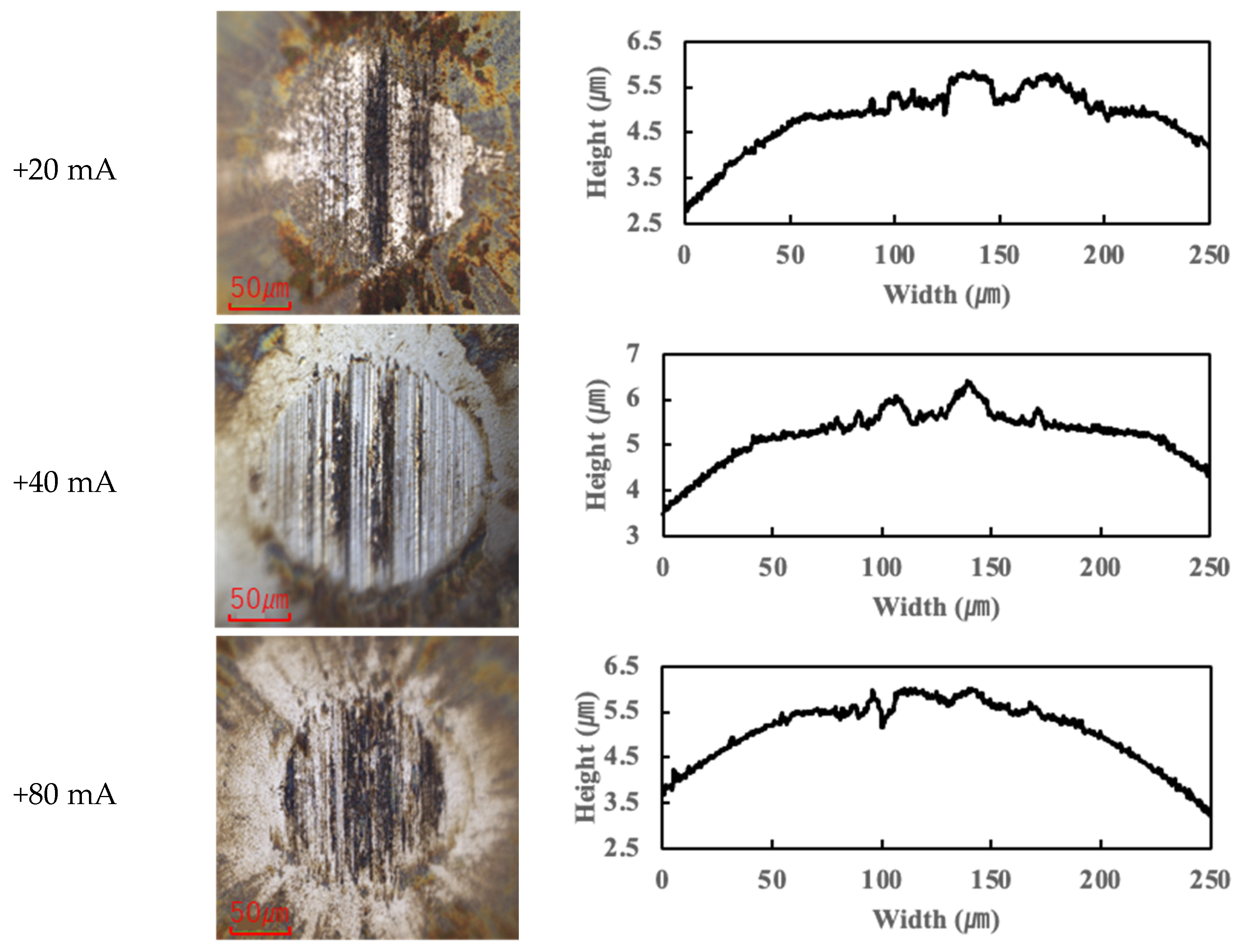
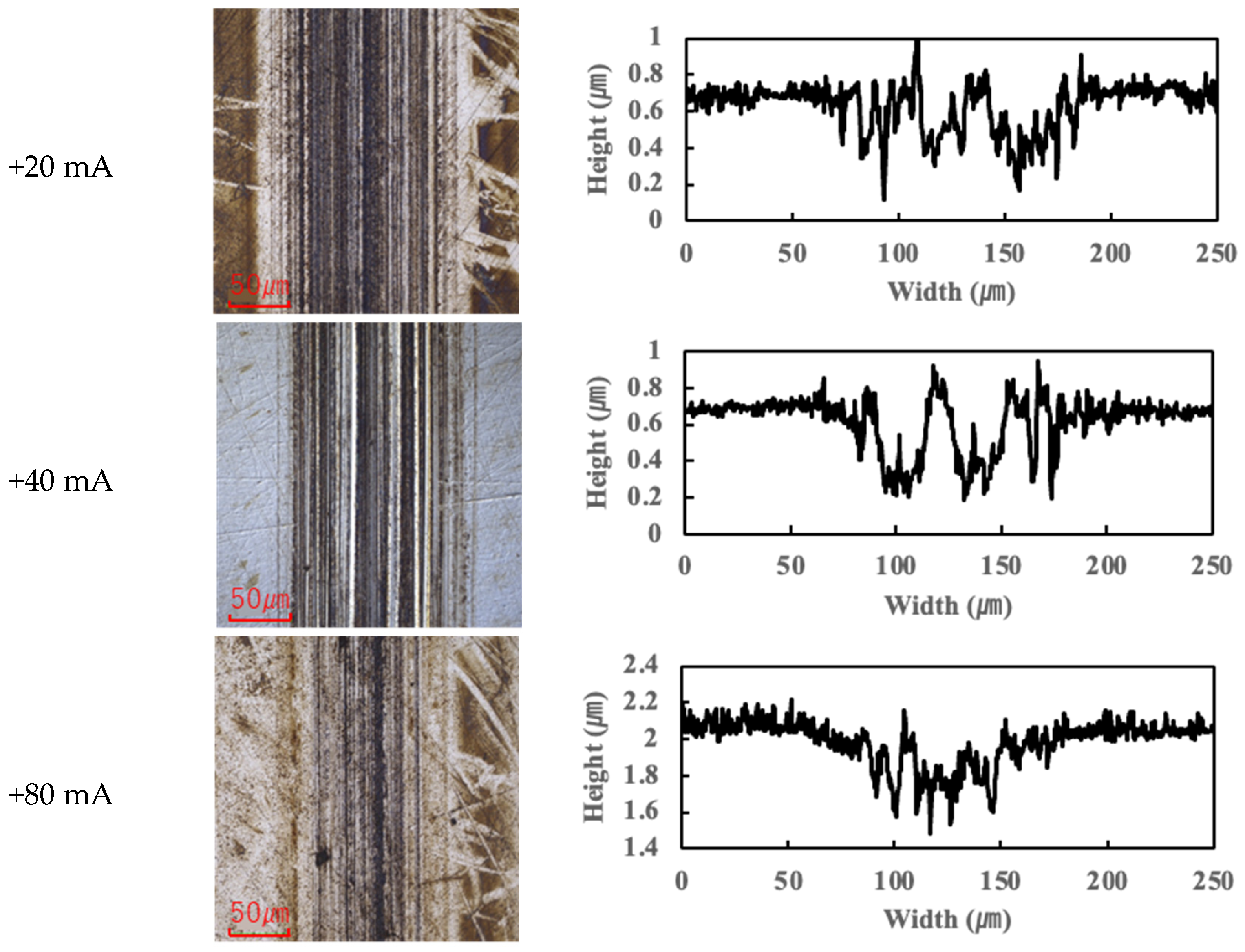
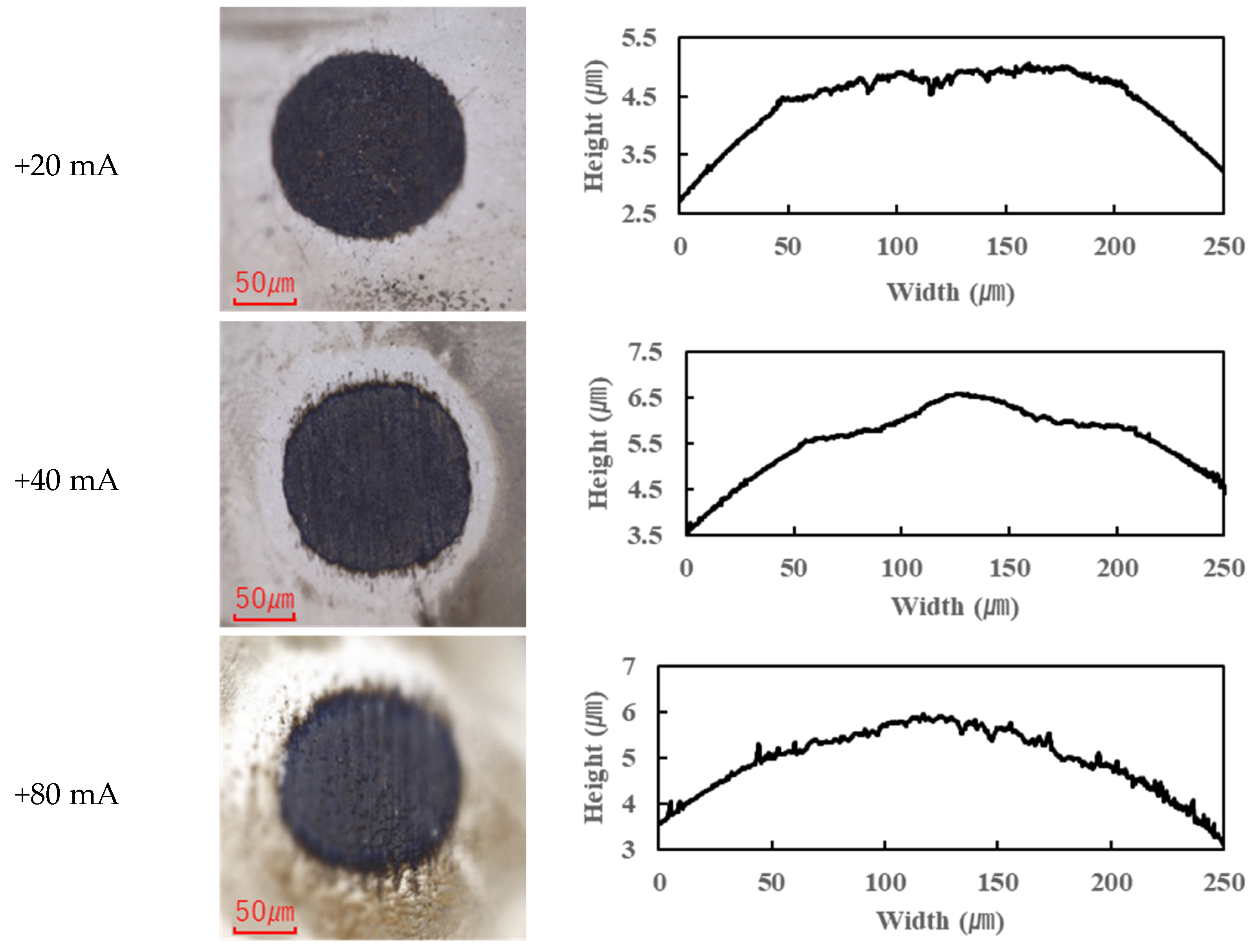
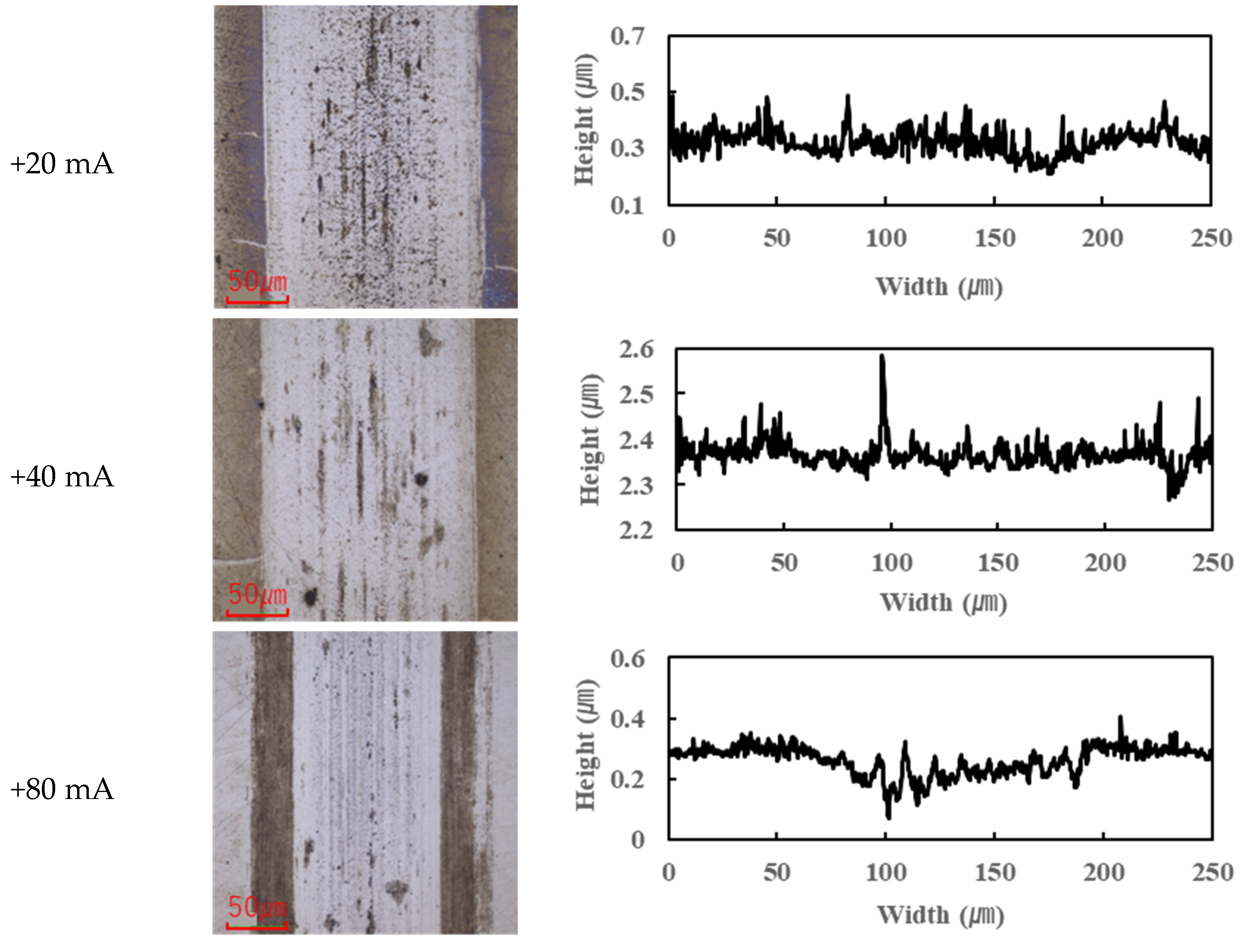
In contrast, the PPE-based lubricant (R1-4P2E) resulted in smaller wear scars than the PAO, confirming its superior wear resistance, but it also produced noticeable black tribofilms on the wear surfaces. The extent of tribofilm adhesion with R1-4P2E varied markedly with the applied current. When using R1-4P2E, a black tribofilm formed on the ball’s wear scar under certain conditions, and its amount and coverage depended on current magnitude and polarity. Notably, tribofilm adhesion was much more pronounced on the positively charged surface. Under high-current conditions (approximately +20 mA to +80 mA) with the ball as the positive electrode, the entire wear scar on the ball was covered by a thick black tribofilm (
Figure 6). This adhesive layer was substantial enough to be visible to the naked eye, forming a coating over the ball’s contact area. In the same +20 to +80 mA tests, the opposing disk (which was negative in this scenario) showed almost no adhesion; its wear track remained shiny and metallic with little to no black film (
Figure 7).
For the reverse polarity (ball negative, disk positive) at high currents, the tribofilm distribution flipped: only a very thin film appeared on the ball’s wear scar (with the ball now being the negative electrode—see
Figure 6), while a much thicker tribofilm adhered to the disk’s wear scar (
Figure 7). These results clearly indicate that tribofilm formation is strongly biased towards the positively charged surface and suppressed on the negatively charged side. Under no-current or low-current (≤10 mA) conditions, any tribofilm formed with R1-4P2E was extremely thin; the wear scars in those cases remained largely clean metal with minimal visible film.
The presence or absence of tribofilm had a direct impact on wear volume.
Figure 8 summarizes the wear scar size as a function of current for both lubricants (here represented by the ball wear scar diameter, which correlates with wear volume). Under no-current and ball-negative conditions, R1-4P2E consistently produced very small wear scars, demonstrating excellent wear resistance. This can be attributed to R1-4P2E’s ability to form a high-viscosity lubricating film that maintains a stable separation between the sliding surfaces, thereby suppressing wear. However, when the ball was positive under high current, the excessive accumulation of black tribofilm led to a significant increase in wear scar diameter—nearly approaching the wear size observed with PAO. In cases where the disk was positive (ball-negative), tribofilm built up preferentially on the disk; since the disk’s contact with the ball is intermittent (as the ball reciprocates), the tribofilm growth in that scenario was less extensive than in the ball-positive case. In general, excessive tribofilm formation created a thick, wide interfacial layer that increased the effective contact area and accelerated wear. This corresponds with the increase in COF observed in the +20 mA to +80 mA range, as a heavier tribofilm can disrupt smooth sliding and contribute to higher friction.
3.2. Surface Analysis
After the tribological tests, various surface analytical techniques were employed to elucidate the tribofilms’ composition and formation mechanisms. The analyses focused on characterizing the chemical species present in the tribofilms for R1-4P2E, especially under electrified conditions.
3.2.1. Raman Analysis
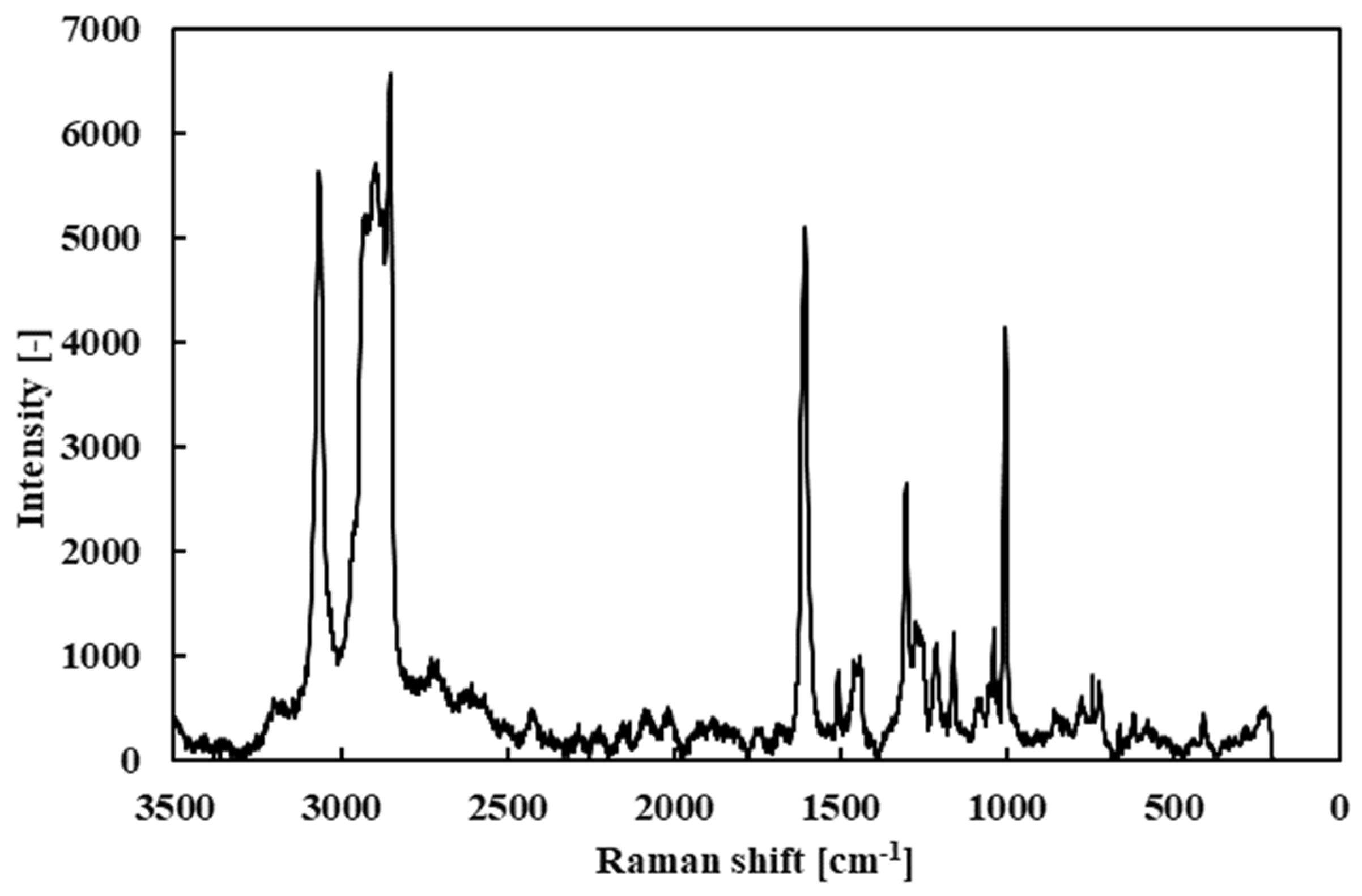
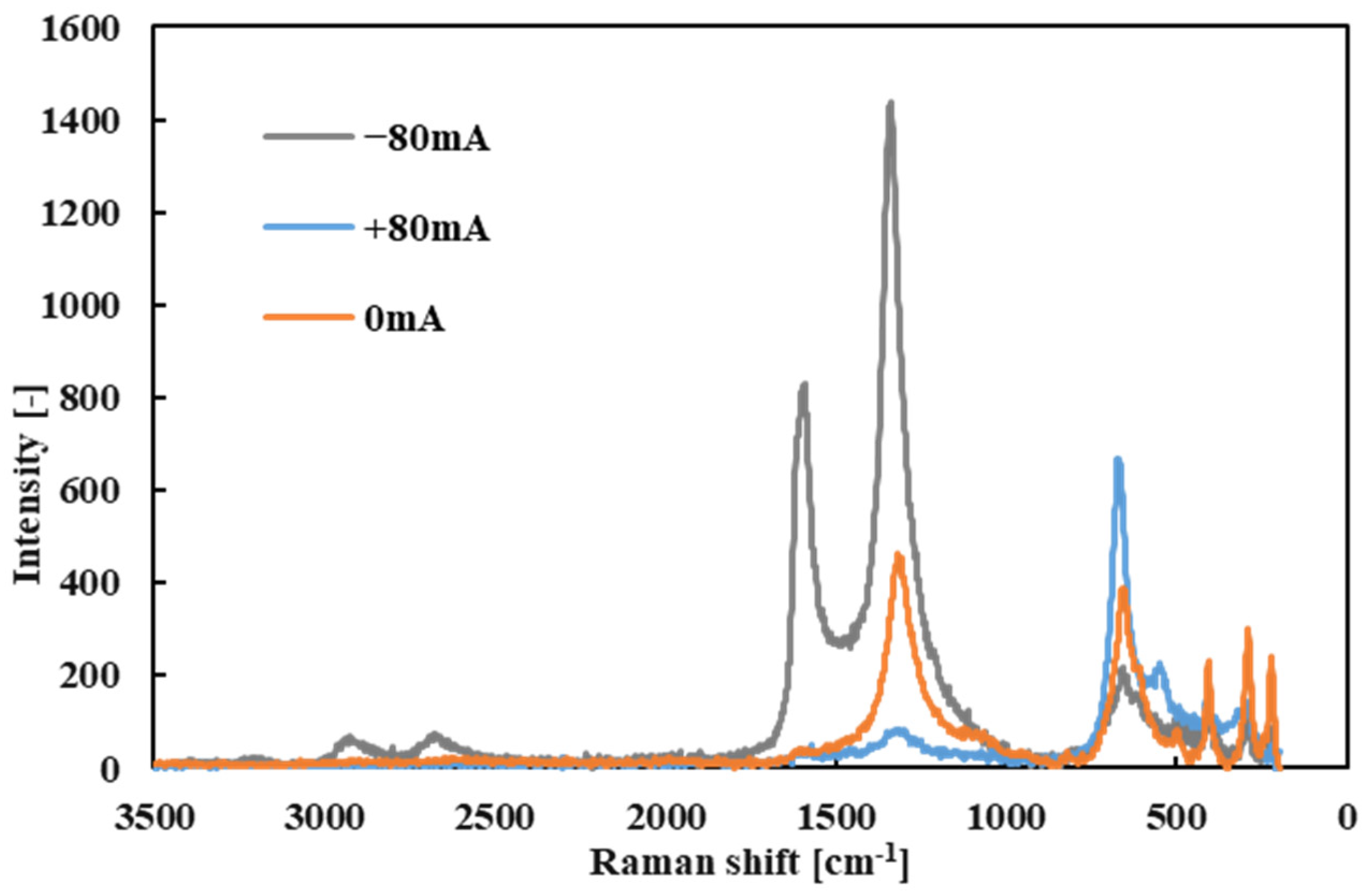
First, the Raman spectrum of the fresh R1-4P2E lubricant (liquid) was measured as a baseline (
Figure 9). The spectrum showed prominent peaks near 3000 cm
−1 (attributed to C–H stretching vibrations of alkyl groups), around 1600 cm
−1 (C=C stretching vibrations from the aromatic rings), and near 1000 cm
−1 (C–H bending vibrations). These peaks confirm the presence of vibrational modes characteristic of PPE’s molecular structure.
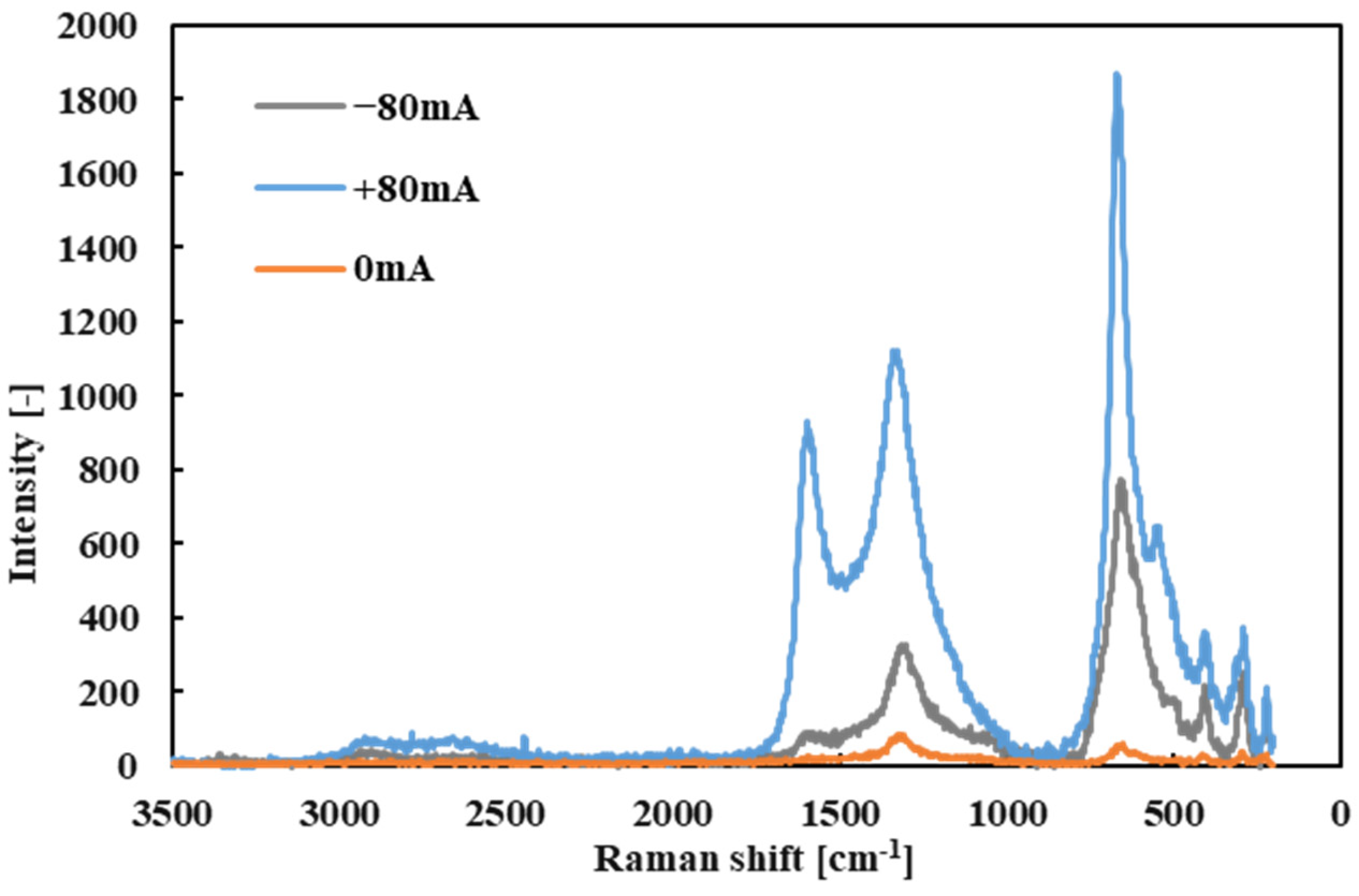
Subsequently, point Raman analyses were performed on the black tribofilms that formed on the wear scars of both the ball and the disk after testing with R1-4P2E (
Figure 10 and
Figure 11). Several distinct Raman bands were detected in the tribofilm spectra, with prominent peaks at approximately 222, 290, 408, 541, 666, and 1330 cm
−1. These peaks correspond to characteristic Raman signals of hematite (α-Fe
2O
3)—an iron oxide—indicating that iron oxide particles are present in the tribofilm. In particular, the peaks at 222, 290, 408, 541, and 666 cm
−1 match known modes of α-Fe
2O
3 [
33,
34]. Additionally, a broad band around 1330 cm
−1 was observed; this was the D-band, characteristic of disordered (amorphous) carbon [
25,
35]. The presence of a strong D-band suggests that a carbonaceous component (from the thermal-/pressure-induced decomposition of the PPE lubricant) is a major part of the tribofilm.
Under certain conditions, an additional peak appeared near 1590 cm
−1 in the tribofilm spectrum. This peak corresponds to the G-band, indicative of graphitic carbon structures [
25,
35]. Notably, the G-band was most evident in tribofilms formed when the electrode was positive on that specimen (for example, the ball at +80 mA vs. disk at −80 mA scenario). The appearance of the G-band implies that, under those high-current conditions, the tribofilm contained a degree of graphitized carbon in addition to amorphous carbon.
These Raman results indicate that the tribofilm formed in PPE lubrication consists of a mixture of iron oxides (hematite) and carbonaceous materials derived from the lubricant (predominantly amorphous carbon, with some graphitic carbon under specific conditions). Moreover, the spatial distribution of Raman intensity revealed a strong polarity effect. During tests with applied current, Raman signals for iron oxide and carbon were significantly stronger on the positively charged specimen surface. This confirms that the tribofilm formed preferentially on the positive electrode surface and accumulated there. This observation is consistent with the visual analysis of the wear scars: thick black tribofilms were observed on the positively charged ball, whereas very little deposition was found on the negatively charged disk (even at +80 mA). In summary, the Raman spectroscopy findings demonstrate a clear polarity dependence of tribofilm formation; in electrified conditions, lubricant decomposition products (carbon) and iron oxide debris preferentially accumulate on the positively charged surface. An important consideration is whether the iron oxide (identified as hematite, α-Fe
2O
3) in the tribofilm might act as a catalyst in PPE’s degradation. Iron oxides and iron wear debris are known to catalyze the oxidative decomposition of organic compounds [
36]. In our context, the presence of Fe
2O
3 on the worn surfaces could accelerate PPE breakdown by providing sites for oxidation reactions, thereby generating more carbonaceous decomposition products that build up the tribofilm. This catalytic effect could explain why thicker tribofilms form at higher currents: once some Fe
2O
3 is produced (from steel surface oxidation/wear), it may promote further lubricant decomposition. On the other hand, if excessive, Fe
2O
3 particles might also increase abrasive wear. Further investigation (for example, adding iron oxide particles in controlled tests) would be needed to isolate and confirm the catalytic influence of Fe
2O
3 on PPE degradation.
3.2.2. ToF-SIMS Analysis
To analyze the detailed composition of the tribofilm, we performed time-of-flight secondary ion mass spectrometry (ToF-SIMS). This analysis focused on detecting carbonaceous fragments that indicate the polymerization or graphitization of the lubricant. In particular, we examined fragments at mass numbers that were multiples of 72 (denoted as I
C6n), which served as indicators of graphite-like carbon clusters. The intensities of these IC6n fragments were normalized to the total ion count (TIC) in order to compare their relative abundance under different conditions. Similarly, hydrocarbon-related fragments of the form I
CnH2n+1 (originating from aliphatic chains) were analyzed, and the results for both types of fragments are presented in
Figure 12 and
Figure 13.
The ToF-SIMS spectra confirmed the presence of IC6n fragments in the tribofilm, indicating the formation of graphite-like (graphitic) structures within the film. Importantly, these graphitic carbon fragments were detected in the tribofilm regardless of whether a current was applied. In both the non-electrified and ±80 mA conditions, IC6n species appeared, suggesting that some degree of carbon clustering/graphitization occurred from the PPE decomposition even in purely mechanical rubbing. This finding aligns with the Raman results, where the ~1590 cm−1 G-band of graphitic carbon was observed, reinforcing that graphitic structures are indeed present in the tribofilm.
However, in contrast to the Raman observations of stronger carbon signals on the positive side, the ToF-SIMS analysis did not show a clear dependence of the IC6n fragment abundance on the presence or direction of current. In other words, the amount of graphitic carbon fragments detected was fairly similar with or without electrical current. This suggests that while graphitic carbon is generated in the tribofilm, its overall concentration is not strongly influenced by the electrical conditions (within the range tested). The formation of graphitic structures appears to occur as part of the tribochemical process in PPE, rather than being significantly driven by the electric field.
On the other hand, the hydrocarbon-related fragments (ICnH2n+1) showed a noticeable increase in intensity in the lower-molecular-weight range under electrified conditions. This implies that an electrified environment enhances the breakdown of hydrocarbon chains in the lubricant, producing more low-mass hydrocarbon fragments. In practical terms, the electric current seems to promote the further decomposition of the lubricant’s alkyl side chains.
Combining the Raman and ToF-SIMS findings, we conclude that the tribofilm contains graphitic carbon materials and that the decomposition of the PPE lubricant is accelerated by electrical current, leading to the increased generation of carbonaceous tribofilm components. A graphitic carbon presence is confirmed, but the distribution of those graphitic components does not drastically change with polarity in the ToF-SIMS results. In contrast, the overall level of decomposition (as evidenced by smaller hydrocarbon fragments) is higher with a high electrical current, pointing to a more aggressive breakdown of the lubricant in electrified tribological environments.
3.2.3. XPS Analysis
To further investigate the chemical states of the elements in the tribofilm, XPS was performed on the disk’s worn surface for different test conditions.
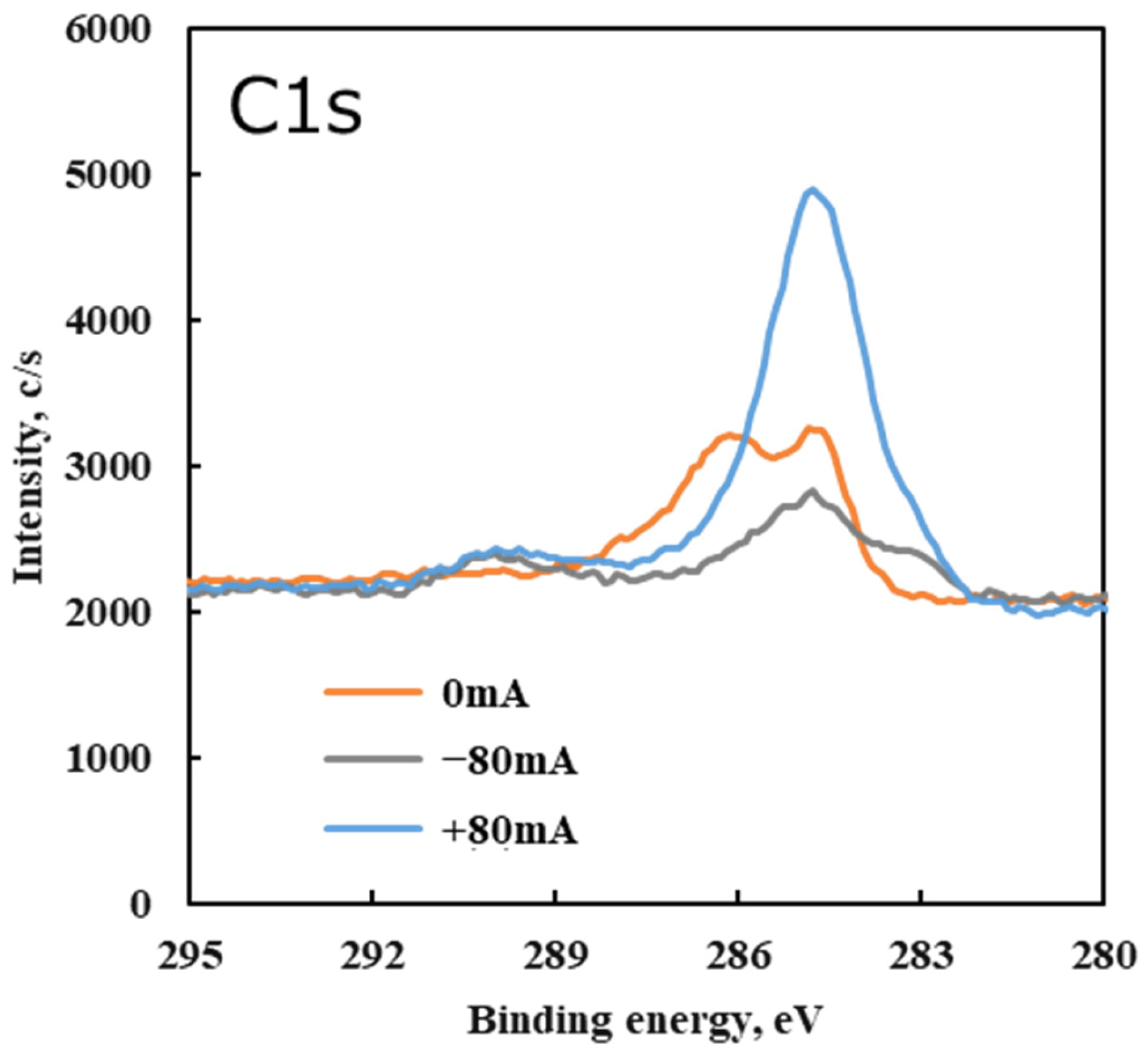
Figure 14 shows the C 1s spectra and
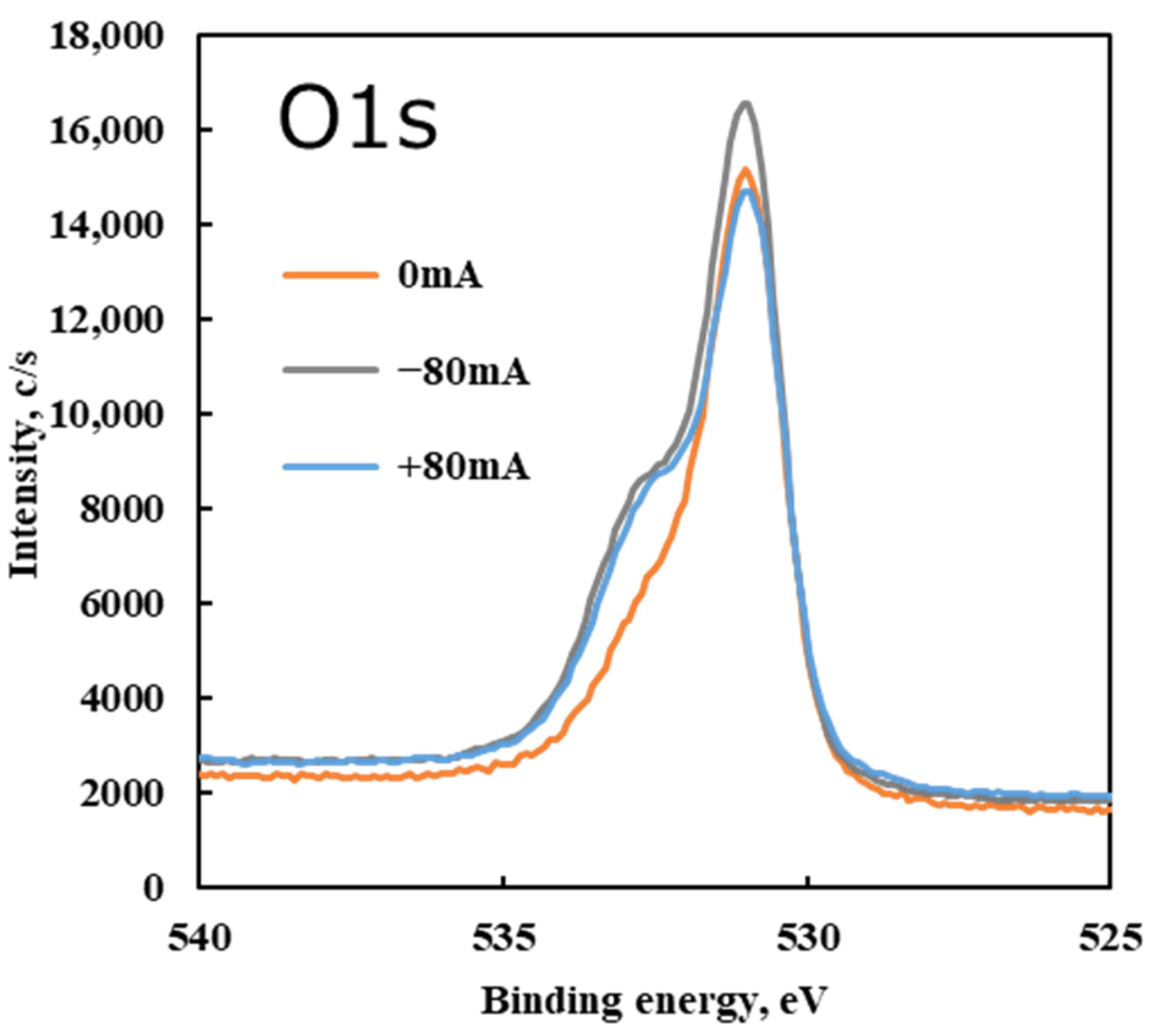
Figure 15 shows the O 1s spectra of the disk surface under non-electrified and electrified conditions.
In the C 1s spectrum, a primary peak was observed at approximately 284.5 eV under all conditions. This peak corresponds to carbon in C–C bonds, which is attributed to the hydrocarbon backbone of the lubricant and carbonaceous tribofilm. Under the non-electrified condition (0 mA), an additional C 1s peak appeared near 286 eV. The 286 eV component is associated with C–O bonds (ethers/alcohols). This indicates that some PPE lubricant residue (likely intact or partially decomposed molecules with ether linkages) remained adsorbed on the surface after the test without current. In other words, without an electrical current, a small amount of lubricant decomposition occurred due to friction, leaving behind species with C–O (ether) groups on the steel surface.
Under electrified conditions (+80 mA or −80 mA tests), the 286 eV C 1s peak (C–O) disappeared, and a new peak emerged around 290 eV in the C 1s spectrum. The 290 eV region corresponds to O=C–O (carboxyl) and related carbonate species [
37]. The appearance of this 290 eV peak under electrical bias suggests that carbon from the PPE lubricant underwent further oxidation when a current was applied. Specifically, the applied electric current likely caused the cleavage of PPE’s ether bonds and facilitated the formation of highly oxidized carbon species (such as carboxylates or carbonates). These species are indicative of a higher oxidation state of carbon than the original lubricant molecules. In essence, friction plus current led to more oxidative reactions on the carbonaceous tribofilm. Moreover, under the ±80 mA conditions a subtle C 1s shoulder became evident at ~283 eV. This low binding energy signal is attributed to carbidic carbon (carbon bonded to metal, e.g., an iron carbide species) [
37], implying that at a high current some decomposed carbon species bonded directly with the steel surface.
The O 1s spectra support this interpretation. Across all conditions, the O 1s spectra had their main peak at ~531 eV, which can be attributed to oxygen in metal oxides, primarily Fe–O (consistent with iron oxides like Fe
2O
3 identified by Raman). Under electrified conditions, a shoulder peak arose around 532.5 eV in the O 1s spectrum. Signals in the 532–533 eV range are generally associated with oxygen in hydroxides (OH
−) and in organic/inorganic carbonates [
37]. The emergence of the 532.5 eV O 1s component with current indicates that oxidation reactions progressed further on the surface, producing hydroxide species and/or carbonate compounds. These could come from moisture traces (hydroxides) or from the oxidation of organic fragments of the lubricant (forming carboxylate/carbonate groups).
Collectively, the XPS findings suggest that the combination of frictional heating/mechanical action and the presence of an electric current greatly promotes the decomposition and oxidation of the PPE lubricant on the steel surface. The PPE’s ether bonds were cleaved, and the carbon in the tribofilm became bonded to oxygen in new functional groups (like carbonyl, carboxyl, hydroxyl). This observation is consistent with the Raman analysis, which indicated a tribofilm composed of carbon (from decomposed lubricant) and iron oxide. XPS adds that, under electrical conditions, those carbonaceous components are more oxidized (forming polar oxygen-containing species). These results reinforce the conclusion that electrical currents accelerate tribochemical reactions, resulting in a tribofilm that contains both carbonaceous material and oxidized compounds (including iron oxides and oxidized carbon fragments).
3.3. Discussion
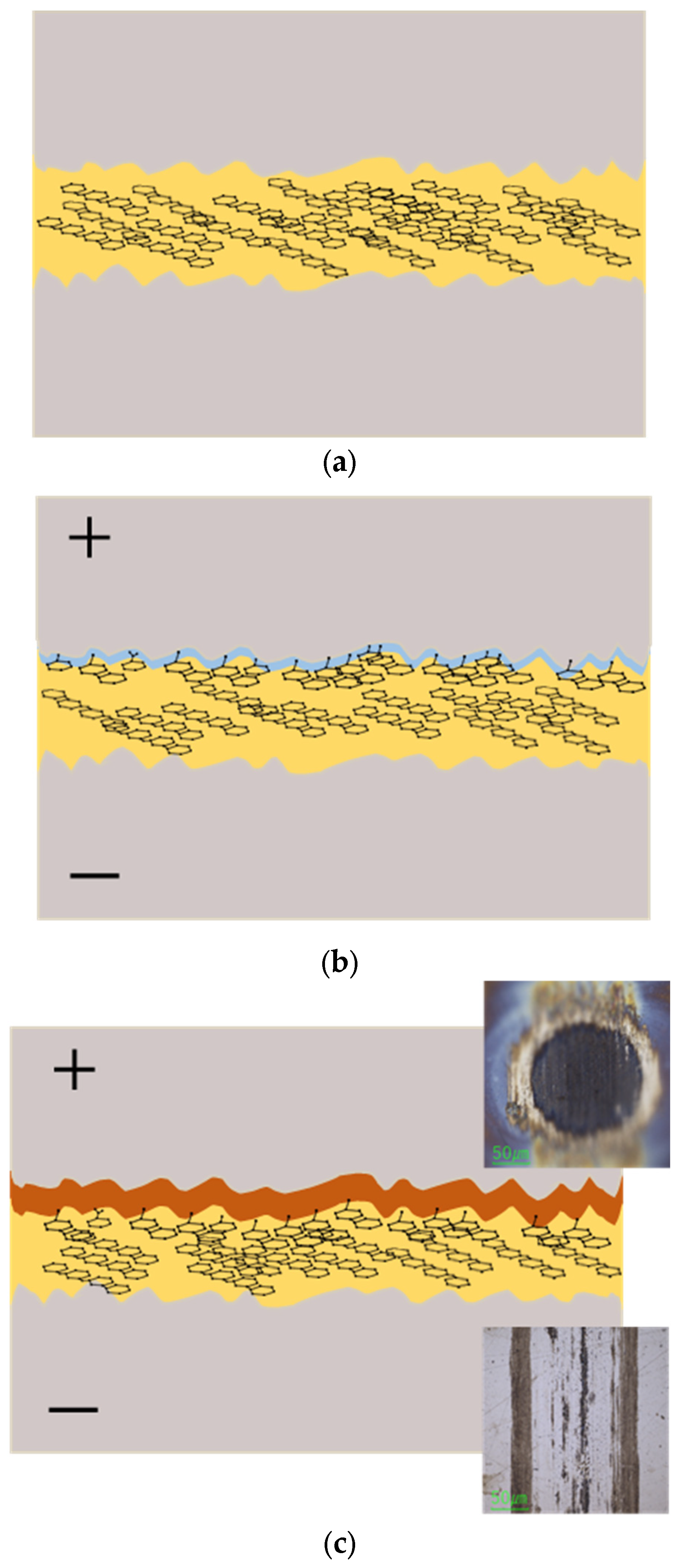
Based on the above results, we propose a mechanism for tribofilm formation in PPE-lubricated systems, illustrated schematically in
Figure 16. Under non-electrified conditions, PPE molecules have a plate-like structure that allows them to adsorb parallel to the steel surface, forming a protective film that reduces friction (
Figure 16a). This adsorption and alignment help minimize direct metal-to-metal contact. However, when friction generates nascent metal surfaces or significantly raises the local temperature, PPE molecules become more susceptible to decomposition (
Figure 17). The breaking of molecular bonds (particularly the ether linkages) can occur due to the high surface energy of freshly exposed metal and the heat of friction.
In an electrified environment, these decomposition reactions are further accelerated by the presence of electrons and electric fields. The electrical current provides additional energy and can drive electrochemical reactions. Our XPS analysis revealed that an applied current leads to the cleavage of PPE’s ether bonds and the oxidation of the resulting fragments into polar functional groups (such as carbonyls and hydroxyls). Originally, PPE molecules are largely non-polar. Once decomposed and oxidized, they generate polar species.
These polar decomposition products are influenced by the electric field in the contact. They tend to migrate toward the positive electrode (the positively charged surface) under the field’s influence. Experimentally, we observed this effect: a thick carbonaceous deposit accumulated on the ball when the ball was the positive electrode, whereas almost no deposit formed on the ball when it was negative. This strongly supports the idea that the tribochemically generated products carry charge or dipoles that make them drift in the electric field, concentrating on the anode side (positive surface).
Through this process, a continuous supply of decomposition products (both carbonaceous fragments from the lubricant and iron oxide wear debris from the steel) is generated by ongoing friction. Simultaneously, the electric field drives these products onto the positive electrode surface and helps them adhere there. The tribofilm thus begins to form on the positive side. Initially, this tribofilm may be an ultrathin layer. As the test continues (and as long as current flows and friction generates more debris), the carbonaceous material and oxides accumulate, and the tribofilm grows progressively thicker (
Figure 16b,c). In our experiments, after approximately 1 h of testing at +80 mA, a visibly thick tribofilm had developed on the positive (ball) surface.
A thin tribofilm is generally beneficial: it can fill in microscopic asperities and smooth the surface, thereby reducing friction. However, if the tribofilm grows too thick or extensive, it can become detrimental. An excessively thick tribofilm may be soft or abrasive and can increase effective contact area, disrupt the lubrication film, or break off in chunks—all of which can increase friction and wear. Indeed, we found that in the low-current range (up to ±10 mA), the tribofilm remained very thin and maintained the low-friction, low-wear benefits of PPE. In the high-current range (>10 mA), however, the tribofilm built up much more, which corresponded with a rise in friction and accelerated wear. Thus, there appears to be a threshold of electrical current (around 10 mA in our setup) below which tribofilm formation is mild and beneficial, and above which tribofilm accumulation becomes excessive and harmful to tribological performance.
Previous studies have reported that PPE lubricants can form polymeric films on surfaces during rubbing, which help reduce friction and also suppress hydrogen generation in steel [
25]. The present study adds nuance to those findings by showing that the behavior of PPE’s tribofilm is strongly influenced by electrical current. We observed that the direction and magnitude of an applied current significantly affect the tribofilm’s growth and distribution (preferentially forming on the positive side). This is a notable new insight: tribofilms predominantly forming on the positively charged surface in an electric field is a phenomenon that needs to be considered in EV drivetrain applications. In summary, our proposed mechanism is that, under electrified conditions, PPE’s decomposition products, being polar, are drawn to the positive electrode, where they accumulate into a tribofilm. While a small amount of such film can be protective, too much leads to higher friction and wear. This understanding can guide the design of lubricants and additives for electrified systems to balance these effects.