Abstract
When exposed to high contact pressure and shear conditions, the sliding and/or rolling contact interfaces of moving mechanical systems can experience significant friction and wear losses, thereby impairing their efficiency, reliability, and environmental sustainability. Traditionally, these losses have been minimized using high-performance solid and liquid lubricants or surface engineering techniques like physical and chemical vapor deposition. However, increasingly harsh operating conditions of more advanced mechanical systems (including wind turbines, space mechanisms, electric vehicle drivetrains, etc.) render such traditional methods less effective or impractical over the long term. Looking ahead, an emerging and complementary solution could be tribocatalysis, a process that spontaneously triggers the formation of nanocarbon-based tribofilms in situ and on demand at lubricated interfaces, significantly reducing friction and wear even without the use of high-performance additives. These films often comprise a wide range of amorphous or disordered carbons, crystalline graphite, graphene, nano-onions, nanotubes, and other carbon nanostructures known for their outstanding friction and wear properties under the most demanding tribological conditions. This review highlights recent advances in understanding the underlying mechanisms involved in forming these carbon-based tribofilms, along with their potential applications in real-world mechanical systems. These examples underscore the scientific significance and industrial potential of tribocatalysis in further enhancing the efficiency, reliability, and environmental sustainability of future mechanical systems.
1. Introduction
Moving mechanical systems are vital for the well-being of our highly mobile and industrialized society, as they empower everything from flawless transportation and manufacturing to safe and efficient energy production and automation, thus fundamentally sustaining our modern lifestyle. However, these systems are also prone to surface and interface-mediated inefficiencies and degradations mainly caused by wear, scuffing, fretting, corrosion, fatigue, oxidation, and plastic deformation. These problems collectively account for over 70% of all machine failures in global industrial operations [1,2,3]. Additionally, friction between moving mechanical assemblies consumes significant energy and thus reduces the efficiency of most mechanical systems.
Accordingly, addressing friction, wear, and other surface-specific issues in moving assemblies has long been a primary focus of tribologists and lubrication engineers [4,5,6,7,8,9]. Through the continuous design, development, and deployment of advanced lubricants, materials, and surface engineering techniques, notable progress has been made in minimizing friction and wear and hence the energy and material losses in such assemblies in recent decades [10,11]. In particular, various anti-friction, anti-wear, and extreme-pressure additives have been developed by chemists and lubrication scientists, and they have been proven highly effective in protecting sliding and/or rolling contact surfaces against wear, scuffing, and fatigue failures. Furthermore, with the advent of Physical Vapor Deposition (PVD) and Chemical Vapor Deposition (CVD) technologies, several superhard and low-friction coatings have been developed and shown to be very effective in reducing surface-specific tribological failures [12,13,14,15,16,17]. These methods produce highly versatile and durable coatings on the surfaces of moving mechanical assemblies, and thus significantly extend their service lives in industrial operations. Some hard coatings, like DLCs, are naturally slick and can provide ultra-low friction and wear even under dry and marginally lubricated sliding conditions.
In mechanical applications, sliding contact interfaces of mechanical systems often undergo cyclic loading and unloading during which normal and shear deformation may occur and thus create nascent or atomically clean metal surfaces. Progressive wear or material removal can also facilitate the creation of atomically clean surfaces, which are often exposed to a large variety of chemical species (such as water molecules, oxygen, airborne hydrocarbon molecules or dust particles, liquid hydrocarbons, chemical additives, etc.) in their surrounding environment. Under sliding contact conditions, these fresh, atomically clean surfaces can easily react with such species to form a chemical boundary film, or “tribofilm,”. In essence, the idea of tribocatalysis is often linked to the development of such tribofilms, especially from such lubricant additives as zinc dialkyl dithiophosphate (ZDDP), tricresyl phosphate (TCP), molybdenum dithiocarbamate (MoDTC), and cyclopropane carboxylic acid (CPCa), among others [11,18,19,20,21,22]. For instance, ZDDP, a well-known anti-wear additive, generally creates a thin, glass-like phosphate-based tribofilm that strongly protects against wear [23,24]. Conversely, friction modifiers like MoDTC promote the formation of a crystalline molybdenum disulfide (MoS2) tribofilm, which shears easily due to its lamellar structure to reduce friction [25,26,27]. However, these traditional anti-friction and anti-wear additives have recently been restricted due to their negative environmental impacts [28]. Specifically, their sulfur and phosphorus contents can adversely affect the performance and efficiency of the after-treatment devices in internal combustion engines. Accordingly, lubrication scientists have actively been searching for more environmentally friendly alternatives to ZDDP and MoDTC additives.
It is essential to understand that ZDDP and MoDTC form chemical tribofilms unique to their molecular structures and chemistry. Despite concerted efforts, modifying these molecules into more environmentally friendly variants has been tricky, as the new tribofilms produced by such variants either did not perform well or provided limited benefits. Instead, recent research has increasingly focused on extracting tribocatalytically driven solid carbon tribofilms directly from the base oil molecules or new carbon-based additive molecules. These tribofilms advantageously result from the hydrocarbon molecules of the oils themselves and hence do not depend on harmful additives. An additional benefit of these solid carbon tribofilms is their compatibility with other functional additives, including nanomaterials, which could further improve friction and wear performance. For instance, some recent studies have explored combining cyclopropane carboxylic acid (CPCa, a carbon-based liquid additive) with materials like NiAl-layered double hydroxide (LDH) nanoparticles. This synergistic approach has led to the conversion of hydrocarbon molecules in lubricating oils into graphitic carbon nanostructures, and thus greatly enhanced friction and wear performance, especially at elevated temperatures [29]. Likewise, catalytically active titanium dioxide nanoadditives and titanium nitride coatings have also been found to promote the formation of such carbon-rich tribofilms from PAO oils blended with MoDTC [30,31].
In previous research, carbon-based thin solid films, including diamond, diamond-like carbon (DLC), carbon nitride, graphene, etc., produced by PVD and CVD, have demonstrated very impressive friction and wear properties. Among others, DLC and graphene have even been shown to enable superlubric sliding regimes (where friction coefficients reach values below 0.01) under both dry and wet sliding conditions [32,33,34,35,36,37]. Most interestingly, some recent studies confirmed that these highly beneficial carbon-based tribofilms can be extracted in situ from hydrocarbon oils and gas molecules through tribocatalysis under ambient air conditions. Specifically, by incorporating catalytically active elements like copper (Cu) or nickel (Ni) into coatings [18,38] or bulk metallic alloys [39], long-chain hydrocarbon molecules in lubricating oils were shown to be catalytically cracked into smaller dimers and trimers. Such smaller units subsequently combined to form solid carbon-based tribolayers on rubbing surfaces [38]. These and other recent developments represent a new and highly promising direction for increasingly demanding tribological applications, including wind turbines, electric vehicles, and space mechanisms. Specifically, with their little or no known adverse environmental impacts compared to traditional ZDDP and MoDTC, such catalytically active additives, coatings, and bulk materials can have a significant positive effect on future lubrication practices, involving hydrocarbon-based lubricants and greases.
The strong influence of catalysis on the thermodynamics and kinetics of chemical reactions is well-established across many industrial and biological systems [40]. This influence primarily hinges on three critical factors: access to catalytically active surfaces that can lower the activation energy for bond formation and/or breakage, the availability of precursor or source materials, and adequate temperature to provide the necessary activation energy. As it happens, most tribological surfaces inherently possess these prerequisites for catalysis to take place. Specifically, frictional energy dissipation can satisfy the activation energy need for tribocatalysis, and the wear can continually create virgin or nascent metallic surfaces where catalytic reactions can favorably occur. Moreover, recent surface engineering advances [18,38,41,42,43,44] demonstrate that tribological surfaces can be intentionally modified or engineered to enhance their catalytic activity further, opening new avenues for controlling the nature and extent of tribochemical processes more effectively and selectively for the most desirable outcomes.
This review highlights recent progress in the design, development, and applications of tribocatalytic systems and surfaces that can offer lower friction and superior wear and scuffing resistance. We also emphasize significant advances made using surface and structural analyses and computational simulations (both reactive and ab initio molecular dynamics), which were crucial for understanding the key underlying mechanisms behind the in situ formation of carbon-based tribolayers and their significantly enhanced friction and wear performance. These catalytically driven tribolayers provide unprecedented flexibility for reducing harmful additives in lubricating oils. More excitingly, they may also facilitate self-healing, leading to fill-for-life functions in future tribological systems, ranging from traditional combustion engines to emerging electric vehicles. By leveraging the new insights gained from these studies, it is hoped that we can strategically design future tribological systems to fully harness the benefits of catalysis in better controlling friction and wear than before, thereby saving energy, ensuring reliability, and protecting the environment.
2. Tribocatalysis Basics
For years, the term tribocatalysis has been associated with a sustainable and cost-effective pathway for applications such as wastewater treatment, carbon dioxide reduction, water splitting, and sterilization [45,46]. Specifically, it was shown that mechanical stimuli achieved by piezoelectric and triboelectric effects can be used to enhance the catalytic degradation of organic pollutants and dyes, even in the absence of light [45,47,48,49]. In these instances, the rubbing of molecules generates triboelectric charges that trigger reactions with adsorbed oxygen or water, thereby accelerating the degradation process [50,51]. Similarly, this same principle can be applied to sterilization technology, where the release of electrons and formation of holes drive reactions with dissolved oxygen and hydroxide in water to generate radicals, effectively killing bacteria [52].
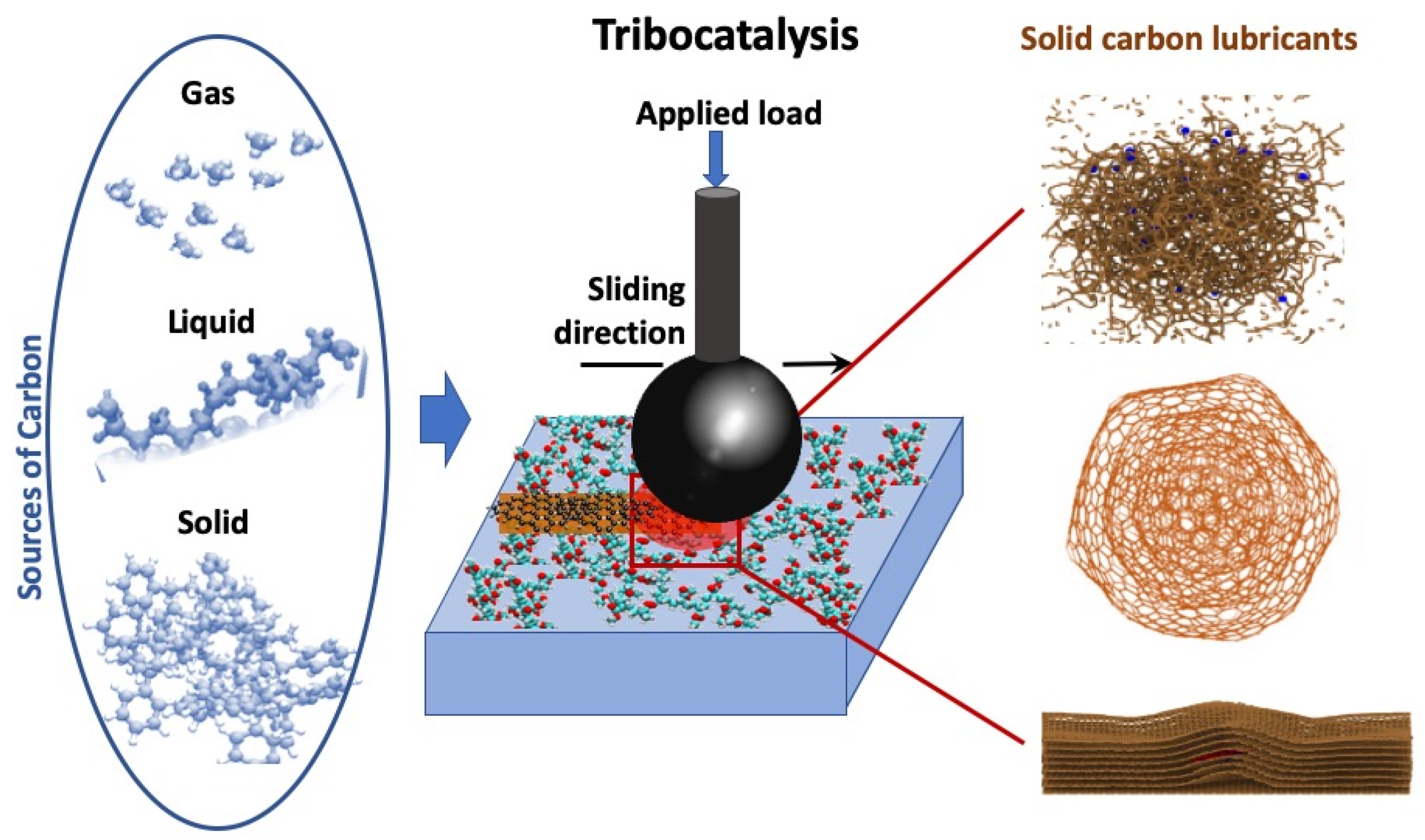
In recent years, tribocatalysis has also gained significant interest from a tribological perspective. Previous research has shown that local asperity temperatures, often called “flash temperatures” due to friction and deformation, can reach nearly 1000 °C during sliding contacts, whether dry or lubricated [53,54]. This transient temperature spike can significantly impact the physical, chemical, and mechanical states of the contacting asperities, especially under high contact pressures and shear conditions. Specifically, frictional heat can provide the activation energy needed for tribocatalysis to proceed on virgin or nascent metallic surfaces created continuously by wear and deformation. Such a catalytic process can further be enhanced, especially when a catalyst metal is present in the bulk or purposely added to the surface, as illustrated in Figure 1 [41]. In general, the role of the catalytic material is to lower the activation energy for C–H bond dissociation and backbone C–C bond scission, thereby facilitating the formation of shorter hydrocarbon fragments [55]. However, the catalysts also influence carbon reconstruction by acting as templates and providing active nucleation sites for carbon growth, thus affecting the final structure of the carbon films.
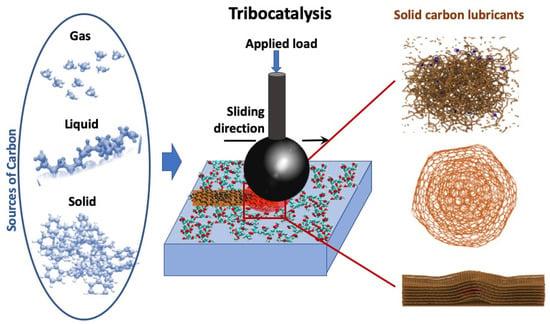
Figure 1.
Summary of the tribocatalytic nanocarbon film generation concept on a catalytically active surface for achieving low friction and wear. The source of carbon, provided in gas, liquid, or solid form, undergoes a catalytically driven transformation during sliding into various forms of carbon-based solid lubricants. The right column highlights the examples of carbon-based structures produced during the tribocatalytically driven process: (from top to bottom) amorphous carbon (DLC), onion-like carbon, and graphitic layers. Reproduced with permission from ref. [41].
Recent advances in computational modeling and simulation techniques have greatly enhanced our understanding of how such catalytically active metals or clusters can help form carbon-based tribofilms. Specifically, these simulations show that when catalyst metals (such as Cu or Ni) contact gaseous or liquid hydrocarbons, such as methane or lubricating oil, they promote the dehydrogenation of these hydrocarbon molecules [38,56,57]. In the case of liquid olefins (or oils) [38], these catalysts also cause the random scission of their carbon-carbon backbones, thus forming smaller fragments or dimers and trimers. These fragmented carbon products then recombine and deposit on sliding surfaces, eventually creating a dense, diamond-like carbon-based tribofilm. It was demonstrated experimentally that the growth rate for such films follows an Arrhenius model, being dependent on the applied contact stress and the surrounding temperature:
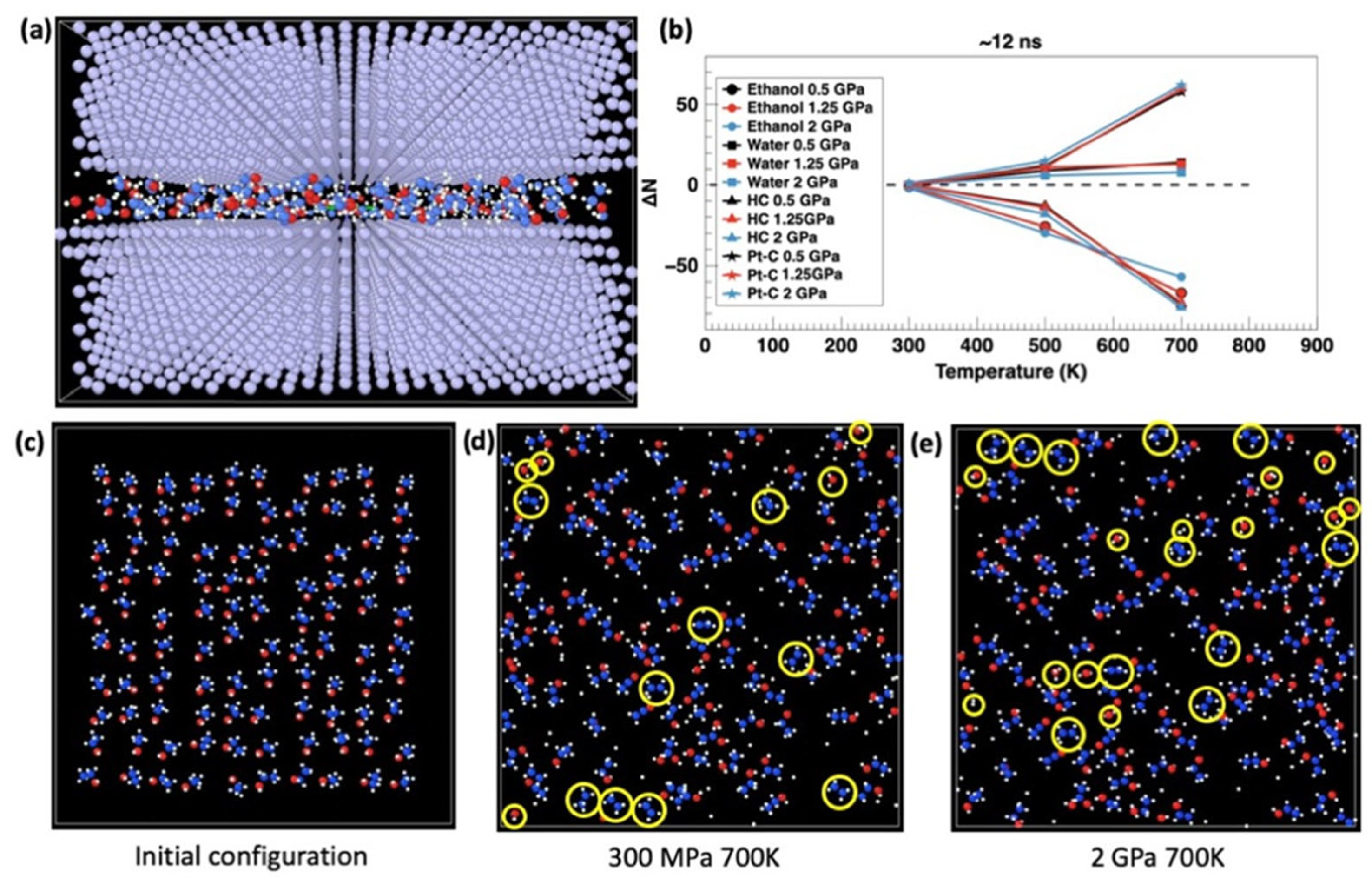
with Γ0 dependent on the nature of the growth species, internal activation of the tribofilm formation energy ΔUact being 0.96 eV, σ equal to the contact pressure, the activation volume ΔVact calculated from the model to be 0.307 Å3, kB being the Boltzmann constant, and T being the absolute temperature. Furthermore, a more detailed theoretical analysis of tribocatalytic activity in a Platinum-Gold (Pt-Au) system unraveled a strong dependence on both temperature and applied load conditions for enhanced tribocatalysis, as shown in Figure 2 [58].
Γ = Γ0 exp(−(ΔUact − σΔVact)/kBT)
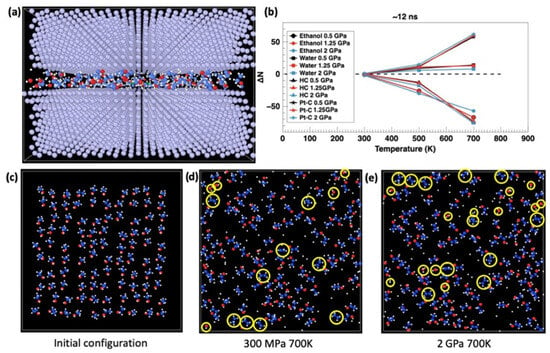
Figure 2.
MD-based observation of the tribocatalysis process. (a) MD model of the studied system. (b) Change in the number of bonds as a function of temperature and pressure after 12ns of simulation time. Snapshots of the simulations in the initial configuration (c) and after ~12 ns of shear at contact pressures of 700K at 300 MPa (d) and 2GPa (e). H-C bonds of ethanol molecules are dissociated with increasing temperature and pressure; an increase in the number of Pt-C bonds is observed upon cooling, transforming into graphene. Temperature has a much stronger effect than the contact pressure. Yellow circles highlight examples of molecular fragments which were counted in the simulations: ethanol, water, H-C bonds, and Pt-C bonds. Reproduced with permission from ref. [58].
All in all, recent advances in computational modeling and simulation have significantly enhanced our understanding of the fundamental mechanisms driving tribochemistry and tribocatalysis [38,59,60]. In particular, Density Functional Theory calculations have been very instrumental in evaluating the various stages of tribofilm formation on a catalytically active surface. This includes everything from assessing the effectiveness of hydrocarbon molecules’ adsorption on the surface to understanding the specific steps involved in the dissociation and subsequent formation of C-C, C-H, and C-O bonds, ultimately resulting in the formation of carbon-based tribofilms on the surface [61,62,63,64]. Concurrently, molecular dynamics simulations have provided invaluable visualizations of these molecular-level transformations, allowing researchers to better probe or appreciate the contributions of external parameters like temperature and load on such tribofilm formation [44,56,65,66]. The major advantage of the tribocatalysis, in contrast to the more traditional approaches using oil lubricants, additives, solid lubricants, and coatings, is that it is a self-regulating process that activates upon reaching high friction (leading to temperature increase) and high wear (release of catalysts from the coating/composites) regimes. At the same time, it provides an approach for repairing and replenishing the damage only selectively, when and where needed. Nevertheless, there are still certain limitations that exist. Controlling tribocatalytic processes can be challenging when operating parameters are dictated by application requirements, leaving little flexibility to adjust them for optimal tribofilm formation. At the same time, the requirement to introduce catalytic particles can make the approach incompatible with certain materials, such as soft polymers, whose properties may be adversely affected.
3. Representative Case Studies
As evident from the foregoing, a substantial volume of research already exists and confirms the potential benefits of tribocatalysis in tribology. Specifically, coatings and bulk materials incorporating catalyst metals have been proven to be highly effective in turning long-chain hydrocarbon molecules of lubricating oils into carbon-based boundary films or tribolayers. In addition to solid catalysts or surfaces, the positive effects of catalytically active lubrication additives have also been well-documented. These additives were able to initiate tribocatalysis, leading to the formation of carbon-rich tribolayers on sliding surfaces. Here, we highlight some of these cases, emphasizing their potential usefulness in significantly improving the friction and wear performance of sliding contacts.
3.1. Bulk Catalytic Alloys and Composites
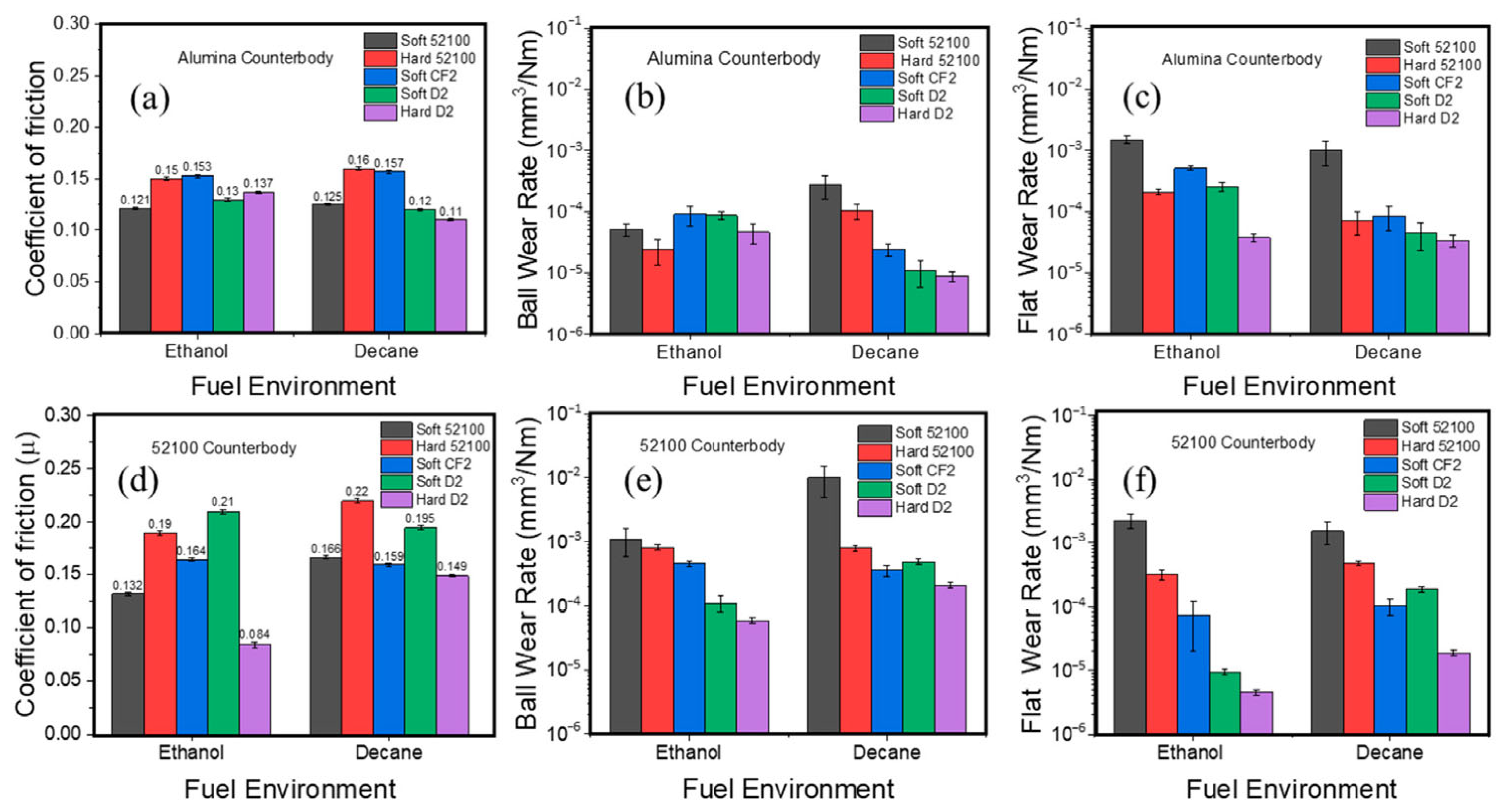
The initial idea of tribocatalysis as a method to tackle friction and wear problems likely comes from using chemical additives specifically designed to chemisorb on surfaces and then react with the surfaces to produce a chemical boundary film that reduces wear. Key examples include TCP and ZDDP, which adsorb onto native iron oxide groups and then react further to form highly durable and protective boundary films against wear and scuffing [25]. Iron is a catalytically active metal; without oxygen, its catalytic reactivity can be pretty high and thus can crack hydrocarbon molecules of oils to generate carbon-rich tribolayers [67,68,69,70]. Many other elements that strengthen steels, such as Ni, Co, W, Mo, V, and Cr, are well-known transition metal catalysts. Therefore, all of them can enhance the catalytic reactivity of such steel alloys and other materials. For example, Khan et al. showed that steels rich in Mo, W, and other catalyst elements can readily form protective carbon-rich tribolayers under lubricated sliding conditions [39]. Steels with high chromium content, which have been previously reported to reduce wear [71], also perform well under lubricated sliding conditions. Specifically, Cr2O3 forming on the sliding surfaces was found to promote the formation of solid oligomers and friction polymers from polyalphaolefin and dodecane [39]. Similar findings were observed on fuel-lubricated sliding tests [72]. Specifically, three types of steels, 52100, D2, and CF2 (soft and hardened), were tested against alumina and 52100 steel counterbodies in ethanol and decane liquids. Figure 3 summarizes these test results, showing similar trends in friction and wear across different environments. D2 steel exhibited the least wear among all materials due to the availability of several catalytic elements in its structure. It also showed a very distinct frictional behavior compared to other steel cases. Again, most of these differences can be attributed to the steels’ catalytic alloying element contents, which help form carbon-based tribofilms in the presence of ethanol or decane, and thus provide extra protection during sliding. Raman analysis of wear tracks revealed the formation of carbon-based tribofilms, with softer steels showing higher D band intensity (greater disorder) and harder D2 steel displaying a stronger G band (more ordered carbon structure). The results underscore alloy composition as the primary factor governing wear resistance, as specific elements can catalyze hydrocarbon-to-polymer transformations during sliding. When using the approach of including catalytic alloying elements, it should be taken into account that the need for specific materials may impact the properties of the bulk structures, such as, for example, hardness. However, the large library of the tribocatalytically active materials makes it possible to optimize the selection close to the needs.
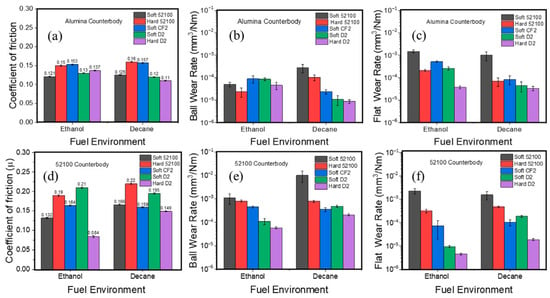
Figure 3.
Steady-state friction values and ball and flat wear rates of soft and hard 52100, soft CF2, and soft and hard D2 steel upon testing in ethanol and decane against alumina (a–c, respectively) and 52100 (d–f, respectively) counter bodies. Reproduced with permission from ref. [72].
3.2. Powders in the Form of Additives or as Mixtures
Studies from Wang et al. and Chang et al. [73,74] showed that magnesium silicate hydroxide (MSH) nanoparticles used as an additive in lubricating oils can form protective carbon-rich tribofilms on steel surfaces. MSH is mainly found in serpentine minerals, and its tribological properties mainly depend on Si-O, Mg-O, and -OH active groups. When these groups become trapped at sliding contact interfaces, under high-pressure and shear conditions, they can help break down oil molecules into diamond-like carbon layers.
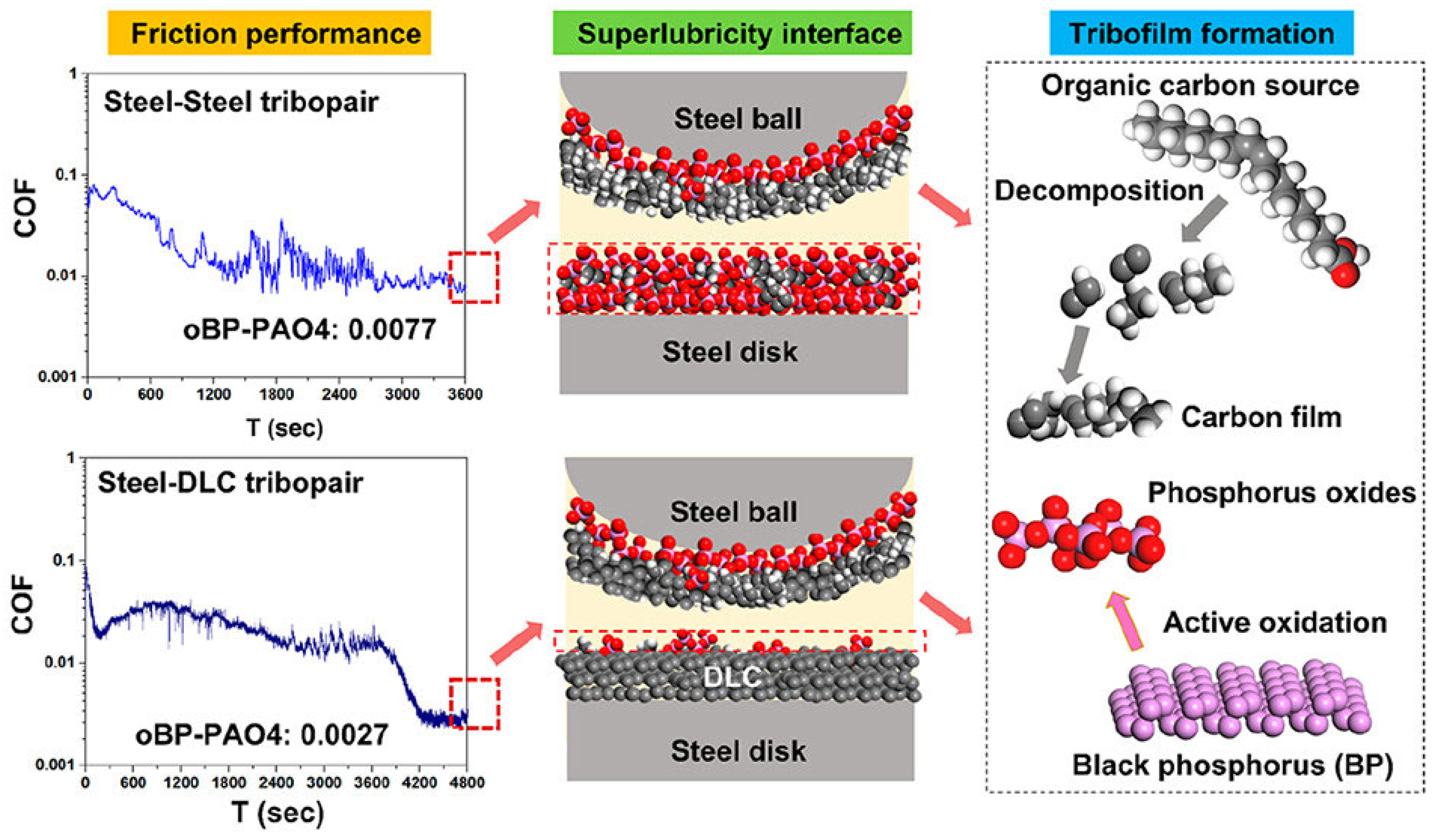
Alternatively, black phosphorus has been used as an additive to not only promote the formation of a protective DLC layer against wear but also to facilitate easy shearing and therefore further lower friction and wear at steel-steel and steel-DLC interfaces due to its layered structure [75]. Here, the oxidized BP surface with P═O and P–OH groups helps the adsorption and subsequent cleavage of oleic acid (OA) molecules, ultimately forming a carbon-rich film at the sliding interface under oil lubrication conditions (Figure 4).
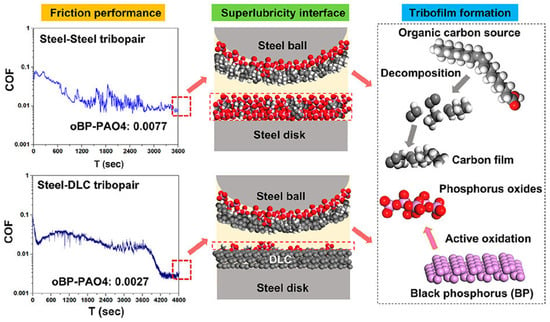
Figure 4.
Reduction in the coefficient of friction in steel-steel and steel-DLC tribopairs observed as a result of oBP-promoted formation of carbon/phosphorus oxide tribofilms. Reproduced with permission from ref. [75]. Red color indicates oxygen atoms, grey indicates carbon atoms, white indicates hydrogen atoms, and pink indicates phosphorus aroms.
3.3. Coatings
The use of hard coatings simplifies both of the previous approaches by providing not only excellent protection against wear but also being able to help with the catalytic decomposition of hydrocarbon molecules of oils into carbon-based tribolayers under lubricated conditions. With advances in PVD and CVD processes, producing such coatings with multiple alloying elements with high catalytic reactivity has become very easy in recent years. In fact, most of the hard nitride coatings nowadays use more than 2 or 3 elements and, in some cases, more than five elements to trigger high-entropy alloying effects [76,77,78]. For example, the in situ formation and continuous repair of protective carbon tribofilms have been significantly enhanced by the use of catalytically reactive metals like Pt [58,79,80], Ni [42,81], Cu [38,82], Fe [83], and Mg in coatings [84]. In one of these cases, researchers compared the lubricated tribological performance of magnetron-sputtered Gold (Au), Silver (Ag), and Copper (Cu) coatings on steel substrates [62]. All three coatings demonstrated remarkable improvements in tribological performance, showing up to a 43% decrease in friction and a 69% decrease in wear width. These impressive results stemmed from forming a carbon-rich polymer-like tribofilm derived from PAO2 oil used in sliding experiments. These catalytic metals can also be successfully incorporated into hard nitride coatings, e.g., MoN-Ag. This integration promotes tribocatalytic activity in oils and alkanes while at the same time maintaining excellent mechanical hardness and robustness during testing [85,86]. Furthermore, with the incorporation of Cu into binary nitride coatings, such as MoVN-Cu and CrCuN, the extraction of carbon-based tribofilms from various hydrocarbon molecules, including alkanes, alcohols, and glycerol, has been increased significantly [61,87,88].
Erdemir et al. [38,81] pioneered synthesizing metal nitride-copper-based coatings to extract highly protective, carbon-rich tribofilms from gaseous and liquid hydrocarbons. The key to this protective tribofilm formation lies in the inclusion of nanoscale catalytically reactive copper grains within the metal nitride matrix, which significantly enhances tribocatalysis by decomposing hydrocarbon molecules during sliding contact. These tribocatalytic coatings illustrate the potential benefits of integrating such reactive species that can continuously replenish worn-away tribolayers at sliding interfaces. More recently, similar coatings, incorporating copper within various metal nitride matrices, significantly improved friction and wear characteristics when tested in alkenes. However, the degree of improvement varied depending on the chain length of alkane molecules [61]. Metal nitride-based hard coatings are widely recognized for their high hardness, toughness, wear resistance, and stability even in highly corrosive and oxidative environments [89,90,91,92]. Consequently, combining them with tribocatalytic elements offers a highly beneficial approach for more enhanced protection against wear in hydrocarbon-based gases (i.e., methane, propane, or natural gas) and oils (i.e., mineral, synthetic, or bio-based oils) [38,81]. Prime examples include MoN-Cu and VN-Cu coatings, produced via Physical Vapor Deposition (PVD) methods. These coatings have been shown to promote the formation of carbon-rich tribofilms in PAO, 5W30 formulated oil (which contains Zinc Dialkyl Dithiophosphate and other additives), and even in an alkyne environment [38,61].
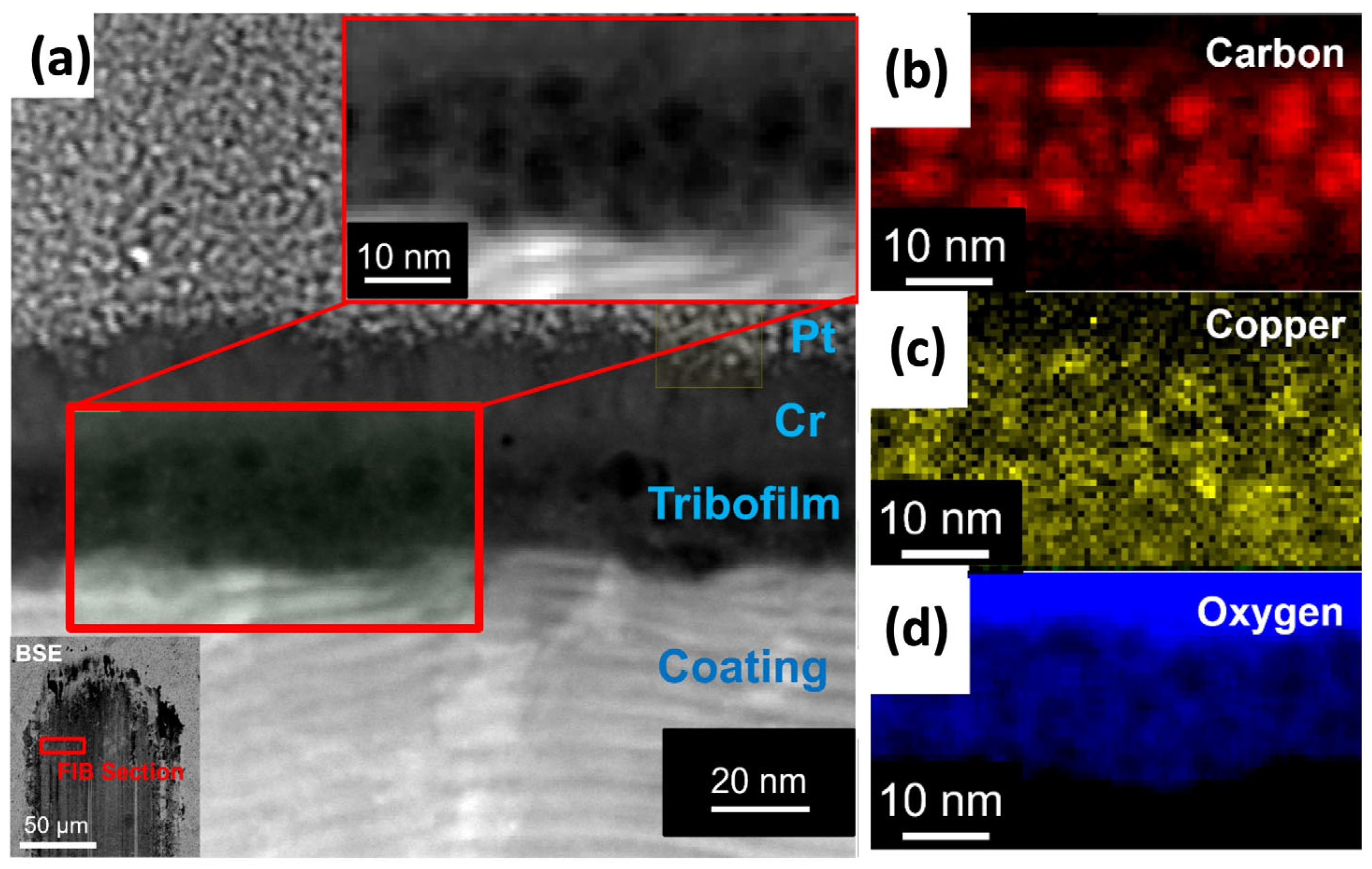
In a similar study involving hydrocarbon fuels [93], a detailed investigation was carried out on the durability of the MoVN-Cu metal nitride nanocomposite coating across different high- and low-viscosity fuels, over a wide range of temperatures, loads, and sliding velocities. The results demonstrated a very robust tribocatalytic effect from the MoVN-Cu coating, leading to superior tribological performance. Post-test analysis of the wear tracks formed during sliding confirmed the in situ formation of carbon-based tribofilms in the presence of both ethanol and dodecane. Additionally, Transmission Electron Microscopy (TEM) of these tribofilms indicated a clear correlation between copper-rich and carbon-rich areas (Figure 5). This observation confirmed that the copper clusters provided the catalytic surface that triggered the formation of such carbon-rich tribofilms.
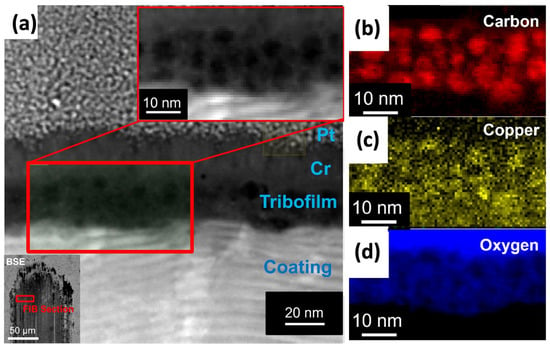
Figure 5.
STEM analysis of tribofilm particles formed on the MoVN-Cu coating surface during tribological experiments; (a) HAADF image of tribofilm produced in dodecane environment and adhered to coating surface with corresponding normalized EELS map of (b) Carbon, XEDS map of (c) Copper, and EELS map of (d) Oxygen. The lower left inset image of (a) is a BSE image of the wear track from which the FIB section was collected for TEM analysis. Reproduced with permission from ref. [93].
In another study by Pan et al. [94], the decomposition of PAO10 oil molecules was catalytically activated by the use of a solid TiB2-MoS2 film. The resultant tribofilm consisted of fullerene-like carbon, which was claimed to be responsible for achieving ultralow-friction and wear. This research expanded the existing library of materials that can trigger tribocatalysis capable of even enabling superlubricity.
3.4. High-Entropy Alloys
The high-entropy alloy (HEA) concept has been around in the materials field for more than two decades and has led to the development of myriad new metallic and ceramic materials with impressive properties. From a tribocatalysis point of view, the same concept can also be considered strategically for designing and developing new alloys, providing significantly enhanced tribocatalytic effects. Their inherent ability to combine several elements, in particular, offers multifunctionality in the resulting alloys, including increased capacity to boost tribocatalytic activity at sliding interfaces [95,96,97]. Beyond their tribocatalytic potentials, high-entropy alloys and their nitrides, carbides, and borides can also be very desirable for their superior physical, chemical, thermal, and mechanical properties [98]. A compelling example comes from the work of Wang et al. [97], where 2D HEA nanoflakes air-spray coated onto a steel substrate demonstrated remarkable friction and wear performance. Specifically, such a coating maintained coefficient of friction (COF) values below 0.1 in ambient air, even under contact pressures reaching 936 MPa. The authors attributed these exceptional results to the tribocatalytically induced formation of an amorphous carbon (a-C) tribofilm derived from the residual ethanol molecules.
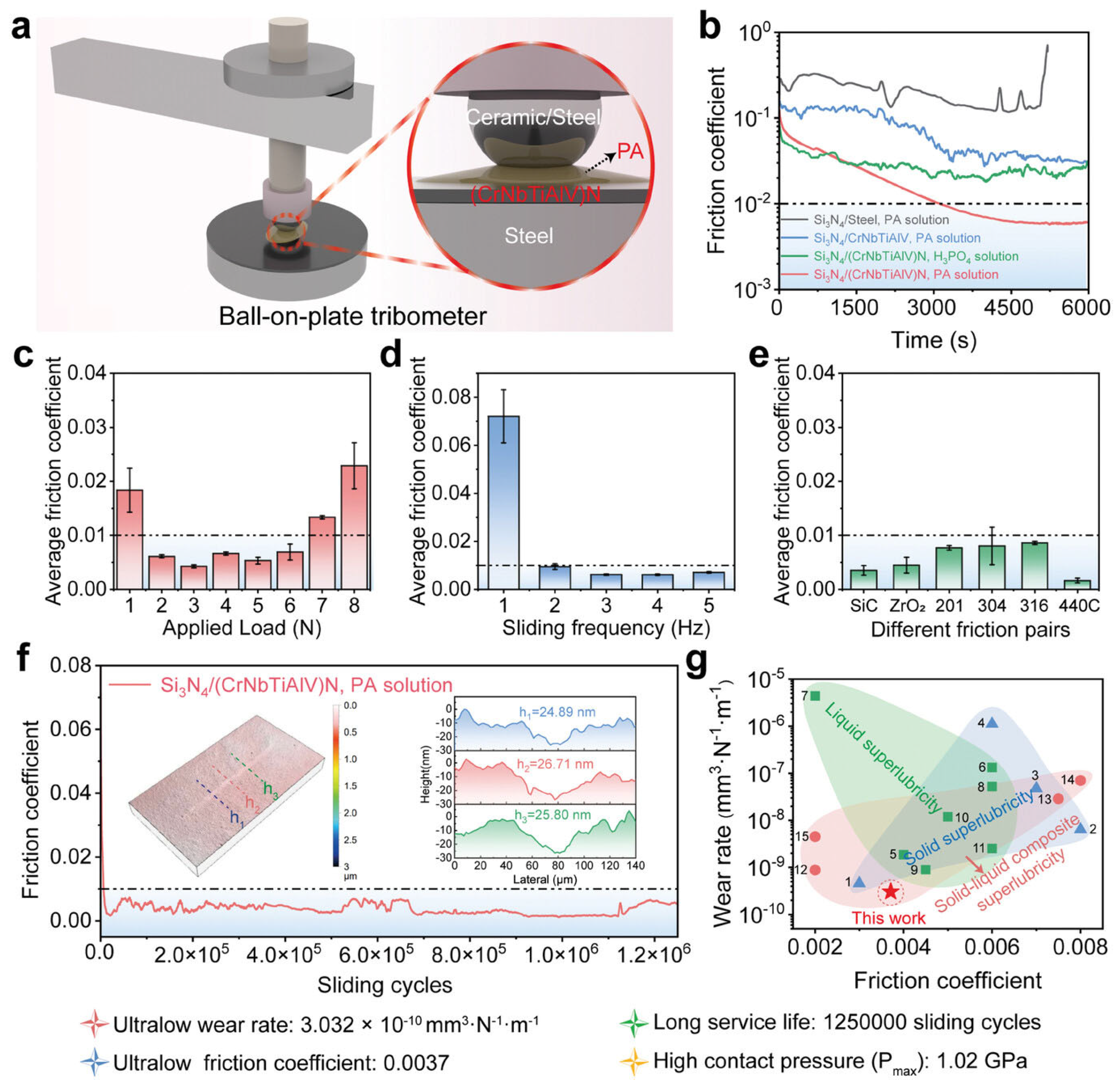
The innovative application of the high-entropy alloy concept has been further extended beyond metals well into the realm of ceramics, thereby transferring their inherent multifunctionality to a new class of materials [99,100,101]. In a groundbreaking study [90], smooth and hard (CrNbTiAlV)N high-entropy ceramic (HEC) films were deposited on steel substrates. When tested in phytic acid, these HEC films achieved and sustained a superlubricity regime with a coefficient of friction (COF) below 0.004 and ultralow wear for at least 1.25 million reciprocating cycles under a 1.47 GPa contact pressure (Figure 6) [102]. The authors attributed such an extraordinary improvement in tribological performance to the HEC nanocrystal-assisted hydrolysis of phytic acid molecules. This process yielded inositol molecules that were arranged and sheared parallel to the HEC surfaces, alongside phosphoric acid molecules that functioned as a boundary lubricant, collectively lowering and mitigating the friction coefficient below 0.01, thanks to the highly catalytic nature of HEC.
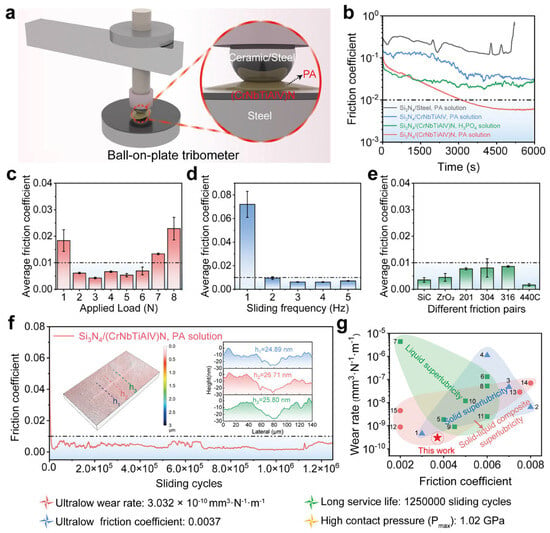
Figure 6.
Tribological performance of HEC coating in PA solution. (a) Schematic diagram of a ball-on-plate tribometer. (b) COF results. (c) The average COF of HEC film lubricated with PA solution under different applied loads. (d) The average COF of HEC film lubricated with PA solution at various sliding frequencies under an external load of 2 N. (e) The average COF when evaluated against different counterbodies. (f) The long-duration COF of the HEC film was lubricated with a PA solution. (g) Comparison of the friction coefficient and wear rate of PA solution lubricated (CrNbTiAlV)N film surface with previous work. Reproduced with permission from ref. [102].
4. Conclusions and Future Perspectives
This review highlighted some of the most recent advances in forming carbon-rich tribofilms on sliding surfaces through tribocatalysis, especially under high contact pressures and shear conditions. Although the concept of tribocatalysis for in situ creation of such tribofilms is still evolving, it has quickly gained significant attention as a promising way to address growing friction and wear problems under harsh tribological conditions. Based on the research outputs presented here, it is clear that tribocatalysis has the potential to overcome some of the drawbacks of traditional methods (Table 1), including those involving environmentally harmful anti-friction and wear and extreme pressure additives. In particular, research so far has shown that tribocatalysis may offer the ability to further reduce or even potentially eliminate the environmentally harmful ZDDP additive from lubricating oils. While ZDDP molecules react with rubbing surfaces to form a protective iron phosphate tribolayer, tribocatalysis creates a unique condition that decomposes hydrocarbon molecules in lubricating oils to form an equally robust and wear-protective carbon-rich tribolayer. Catalytic effects for cracking hydrocarbon molecules can easily originate from the catalytically active alloying elements or dopants integrated into bulk metals or ceramic structures and coatings. It can also come from the catalytically active additives or powder mixtures within a carrier oil. Creating an inert test environment and/or passing electricity through the lubricated contact interfaces has also been shown to enhance tribocatalysis and thus further facilitate the formation of highly protective carbon-based tribofilms on sliding steel surfaces in a more controllable and tunable manner [49,103]. Alternatively, though not yet realized experimentally, there is a potential to control and accelerate tribocatalysis with thermoelectric processes converting thermal gradients into electrical energy and promoting decomposition of the hydrocarbon compounds [104].
These and numerous other studies have demonstrated that the tribocatalysis concept is very effective in achieving impressive friction and wear properties in sliding contacts. Its relatively easy adaptability to diverse operating conditions further underscores its potential for designing more efficient and durable mechanical systems in the future. Through increased adoption of tribocatalysis, the long-sought-after goals of self-repairing sliding surfaces may soon become a reality, as they are well-proven in lab or bench studies. Though most studies focus on sliding contacts, tribocatalysis may emerge as a practical approach for enhancing the performance of machine elements such as full bearing systems, which are typically lubricated with oils and greases, and the inherent sources of hydrocarbons. All in all, tribocatalysis holds great promise for managing friction and wear, thus delivering substantial benefits, including higher efficiency and reliability in future moving mechanical systems. This is especially true for rapidly expanding electric vehicle technology [105,106], where these advancements can be pivotal in further optimizing the performance of their drivetrain components and ultimately attaining fill-for-life lubrication solutions.

Table 1.
Overview of tribocatalysis in comparison to other traditional lubrication approaches.
Table 1.
Overview of tribocatalysis in comparison to other traditional lubrication approaches.
| Lubrication Approach | Examples | Lubrication Mechanism | Advantages | Limitations |
|---|---|---|---|---|
| Liquids | Mineral and synthetic base oils, vegetable or bio-based lubricants, water-based lubricants, fuels, etc. [107,108,109,110,111,112,113,114,115,116] | Wetting surfaces, viscous/hydrodynamic lift, shearing in between liquid layers | Easy replenishment, disposal, and recyclability | Failure upon a lubricant starvation regime, not compatible with vacuum and high temperature |
| Additives in liquid lubricants | TCP, ZDDP, MoDTC, ionic liquids, nano-colloids in oils [117,118,119,120,121,122,123,124,125,126] | Tribofilm formation through surface reactions with additives | The formed tribofilm protects the surface under lubricant starvation | Designed towards specific surfaces, requires certain activation energy, and is not compatible with vacuum or high-temperature environments |
| PVD/CVD Coatings | Carbide (e.g., WC), nitride (e.g., TiN, CrN, AlN), oxide (e.g., Al2O3) coatings [127,128,129,130,131,132,133,134,135,136,137,138,139,140,141] | High mechanical hardness, high resistance to wear and corrosion | Compatible with various environments and high-temperature regimes | Need replenishment or re-application due to finite thickness; strong adhesion to the substrate and mechanical and tribological characteristics may extend their lifetime |
| Solid lubricants | Graphene, BN, boric acid, WS2, and MoS2 flakes [142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161] | Shearing of the lamellar planes | Compatible with various environments and elevated-temperature regimes | Need replenishment (but easier than coatings); in general, weaker adhesion to the substrates than in the case of coatings |
| Tribocatalysis | Catalytic elements in bulk materials, coatings, or powders (e.g., Mo, Ni, Cu, Pt) in the presence of hydrocarbon sources (e.g., liquid: oils, fuels, alcohols; gaseous: methane, ethane, ethanol vapors, and solid: polymers, amorphous carbon | In situ and on-demand formation of carbon-rich protective tribofilms | Continuous replenishment of the protective films through the decomposition of hydrocarbon molecules, compatible with a wide range of ambient air or inert gas environments and high-temperature regimes | Incorporation of catalytic materials, control of the tribocatalysis is possible only with adjustment of the operation conditions, compatibility with surface treatment approaches |
Author Contributions
Authors (D.B. and A.E.) have equally contributed to this manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Support for D.B.’s efforts in this manuscript was provided by the National Science Foundation (NSF) (Award No. 2018132). A.E.’s efforts were provided by the Texas A&M Engineering Experiment Station startup funds and the Governor’s University Research Initiative.
Data Availability Statement
Since this is a review article, the data or results used are available through the original source references. The permissions granted by the publishers secure the reuse of these figures in our manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Szeri, A.Z. Tribology: Friction, Lubrication, and Wear Hemisphere; Hemisphere Publishing Company: New York, NY, USA, 1980; p. 548.
- Booser, E.R. Tribology Data Handbook: An Excellent Friction, Lubrication, and Wear Resource; CRC Press: Boca Raton, FL, USA, 1997. [Google Scholar]
- Noria Corporation. Common Causes of Machine Failures. 2004. Available online: https://www.machinerylubrication.com/Read/29331/machine-failure-causes (accessed on 10 October 2021).
- Medvedovski, E.; Chinski, F.A.; Stewart, J. Wear-and Corrosion-Resistant Boride-Based Coatings Obtained through Thermal Diffusion CVD Processing. Adv. Eng. Mater. 2014, 16, 713–728. [Google Scholar] [CrossRef]
- Chattopadhyay, R. Surface Wear: Analysis, Treatment, and Prevention; ASM International: Detroit, MI, USA, 2001. [Google Scholar]
- Fu, H.; Xiao, Q.; Fu, H. Heat treatment of multi-element low alloy wear-resistant steel. Mater. Sci. Eng. A 2005, 396, 206–212. [Google Scholar] [CrossRef]
- Voevodin, A.; Zabinski, J. Laser surface texturing for adaptive solid lubrication. Wear 2006, 261, 1285–1292. [Google Scholar] [CrossRef]
- Bonse, J.; Kirner, S.V.; Griepentrog, M.; Spaltmann, D.; Krüger, J. Femtosecond laser texturing of surfaces for tribological applications. Materials 2018, 11, 801. [Google Scholar] [CrossRef] [PubMed]
- Korotkov, V.A. Wear resistance of carbon steel with different types of hardening. J. Frict. Wear 2015, 36, 149–152. [Google Scholar] [CrossRef]
- Berman, D.; Rosenkranz, A.; Marian, M. Fundamental and Practical Aspects of Tribology; Taylor & Francis: London, UK, 2024. [Google Scholar]
- Shirani, A.; Berkebile, S.; Berman, D. Promoted high-temperature lubrication and surface activity of polyolester lubricant with added phosphonium ionic liquid. Tribol. Int. 2023, 180, 108287. [Google Scholar] [CrossRef]
- Aouadi, S.M.; Gu, J.; Berman, D. Self-healing ceramic coatings that operate in extreme environments: A review. J. Vac. Sci. Technol. A 2020, 38, 050802. [Google Scholar] [CrossRef]
- Bunshah, R.; Weissmantel, C. Handbook of Hard Coatings: Deposition Technolgies, Properties and Applications; William Andrew: Kansas City, MO, USA, 2000. [Google Scholar]
- Holmberg, K.; Ronkainen, H.; Matthews, A. Tribology of thin coatings. Ceram. Int. 2000, 26, 787–795. [Google Scholar] [CrossRef]
- Padture, N.P.; Gell, M.; Jordan, E.H. Thermal barrier coatings for gas-turbine engine applications. Science 2002, 296, 280–284. [Google Scholar] [CrossRef]
- Sproul, W.D. Physical vapor deposition tool coatings. Surf. Coatings Technol. 1996, 81, 1–7. [Google Scholar] [CrossRef]
- Tamarin, Y. Protective Coatings for Turbine Blades; ASM International: Detroit, MI, USA, 2002. [Google Scholar]
- Huynh, K.K.; Pham, S.T.; Tieu, K.A.; Wan, S. Tribocatalysis Induced Carbon-Based Tribofilms—An Emerging Tribological Approach for Sustainable Lubrications. Lubricants 2023, 11, 327. [Google Scholar] [CrossRef]
- Wong, V.W.; Tung, S.C. Overview of automotive engine friction and reduction trends–Effects of surface, material, and lubricant-additive technologies. Friction 2016, 4, 1–28. [Google Scholar] [CrossRef]
- Wang, J.; Li, Z.; Zhao, H.; Ren, S.; Wang, C.; Huang, X.; Zheng, L.; Ren, T. Synergistic effects between sulfur- and phosphorus-free organic molybdenums and ZDDP as lubricating additives in PAO 6. Tribol. Int. 2022, 165, 107324. [Google Scholar] [CrossRef]
- Johnson, B.; Wu, H.; Desanker, M.; Pickens, D.; Chung, Y.-W.; Wang, Q.J. Direct formation of lubricious and wear-protective carbon films from phosphorus- and sulfur-free oil-soluble additives. Tribol. Lett. 2018, 66, 2. [Google Scholar] [CrossRef]
- Ozimina, D.; Kulczycki, A.; Janas, D.; Desaniuk, T.; Deliś, M. Carbon-Based Functional Nanomaterials as Tools for Controlling the Kinetics of Tribochemical Reactions. Materials 2024, 17, 785. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Luo, H.; Chi, M.; Ma, C.; Blau, P.J.; Dai, S.; Viola, M.B. Comparison of an oil-miscible ionic liquid and ZDDP as a lubricant anti-wear additive. Tribol. Int. 2014, 71, 88–97. [Google Scholar] [CrossRef]
- Zhang, J.; Spikes, H. On the mechanism of ZDDP antiwear film formation. Tribol. Lett. 2016, 63, 24. [Google Scholar] [CrossRef]
- Morina, A.; Neville, A.; Priest, M.; Green, J. ZDDP and MoDTC interactions in boundary lubrication—The effect of temperature and ZDDP/MoDTC ratio. Tribol. Int. 2006, 39, 1545–1557. [Google Scholar] [CrossRef]
- Khaemba, D.N.; Neville, A.; Morina, A. New insights on the decomposition mechanism of Molybdenum DialkyldiThioCarbamate (MoDTC): A Raman spectroscopic study. RSC Adv. 2016, 6, 38637–38646. [Google Scholar] [CrossRef]
- Espejo, C.; Wang, C.; Thiébaut, B.; Charrin, C.; Neville, A.; Morina, A. The role of MoDTC tribochemistry in engine tribology performance. A Raman microscopy investigation. Tribol. Int. 2020, 150, 106366. [Google Scholar] [CrossRef]
- Ta, T.D.; Tieu, A.K.; Tran, B.H. Influences of iron and iron oxides on ultra-thin carbon-based tribofilm lubrication. Tribol. Int. 2022, 173, 107665. [Google Scholar] [CrossRef]
- Huynh, K.K.; Pham, S.T.; Tieu, A.K.; Collins, S.M.; Lu, C.; Wan, S. Tribo-induced catalytically active oxide surfaces enabling the formation of the durable and high-performance carbon-based tribofilms. Tribol. Int. 2023, 184, 108476. [Google Scholar] [CrossRef]
- Fan, Z.; Wang, S.; Xiang, Y.; Liu, Y.; Yu, Z.; Ge, X.; Wang, W. Synergetic lubrication between MoDTC and TiO2 nano-additive: Dynamic evolution of tribofilm induced by tribocatalysis and tribomechanical effect. Tribol. Int. 2025, 207, 110608. [Google Scholar] [CrossRef]
- Fu, X.; Li, Y.; Su, H.; Cao, L.; Wan, Y.; Li, R. Tribological interactions between TiN PVD coating and MoDTC under boundary lubrication conditions. Vacuum 2022, 195, 110646. [Google Scholar] [CrossRef]
- Berman, D.; Deshmukh, S.A.; Sankaranarayanan, S.K.R.S.; Erdemir, A.; Sumant, A.V. Macroscale superlubricity enabled by graphene nanoscroll formation. Science 2015, 348, 1118–1122. [Google Scholar] [CrossRef]
- Ying, P.; Gao, X.; Berman, D.; Hod, O.; Urbakh, M. Scaling-Up of Structural Superlubricity: Challenges and Opportunities. Adv. Funct. Mater. 2025, 35, 2423024. [Google Scholar] [CrossRef]
- Erdemir, A. Diamond-Like Carbon Films and Their Superlubricity, Superlubricity; Elsevier: Amsterdam, The Netherlands, 2021; pp. 215–230. [Google Scholar]
- Jang, S.; Chen, Z.; Kim, S.H. Environmental effects on superlubricity of hydrogenated diamond-like carbon: Understanding tribochemical kinetics in O2 and H2O environments. Appl. Surf. Sci. 2022, 580, 152299. [Google Scholar] [CrossRef]
- Zeng, Q.; Ning, Z. High-temperature tribological properties of diamond-like carbon films: A review. Rev. Adv. Mater. Sci. 2021, 60, 276–292. [Google Scholar] [CrossRef]
- Ayyagari, A.; Alam, K.I.; Berman, D.; Erdemir, A. Progress in Superlubricity Across Different Media and Material Systems—A Review. Front. Mech. Eng. 2022, 8, 908497. [Google Scholar] [CrossRef]
- Erdemir, A.; Ramirez, G.; Eryilmaz, O.L.; Narayanan, B.; Liao, Y.; Kamath, G.; Sankaranarayanan, S.K.R.S. Carbon-based tribofilms from lubricating oils. Nature 2016, 536, 67–71. [Google Scholar] [CrossRef]
- Khan, A.M.; Ahmed, J.; Liu, S.; Martin, T.; Berkebile, S.; Chung, Y.-W.; Wang, Q.J. Formation of wear-protective tribofilms on different steel surfaces during lubricated sliding. Tribol. Lett. 2023, 71, 63. [Google Scholar] [CrossRef]
- Jin, Z.; Jiang, L.; He, Q. Critical learning from industrial catalysis for nanocatalytic medicine. Nat. Commun. 2024, 15, 3857. [Google Scholar] [CrossRef]
- Berman, D.; Erdemir, A. Achieving Ultralow Friction and Wear by Tribocatalysis: Enabled by In-Operando Formation of Nanocarbon Films. ACS Nano 2021, 15, 18865–18879. [Google Scholar] [CrossRef] [PubMed]
- Shirani, A.; Al Sulaimi, R.; Macknojia, A.Z.; Eskandari, M.; Berman, D. Tribocatalytically-active nickel/cobalt phosphorous films for universal protection in a hydrocarbon-rich environment. Sci. Rep. 2023, 13, 10914. [Google Scholar] [CrossRef] [PubMed]
- Shirani, A.; Joy, T.; Rogov, A.; Lin, M.; Yerokhin, A.; Mogonye, J.E.; Korenyi-Both, A.; Aouadi, S.M.; Voevodin, A.A.; Berman, D. PEO-Chameleon as a potential protective coating on cast aluminum alloys for high-temperature applications. Surf. Coat. Technol. 2020, 397, 126016. [Google Scholar] [CrossRef]
- Wu, X.; Wu, T.; Yu, L.; Han, H.; Bian, S.; Jiang, Y.; Li, T.; Zuo, B.; Zhu, D.; Chen, C.; et al. Adaptive TiN-Cu/PAO composite lubrication system: The tribocatalysis-induced PAO6 transferring to amorphous carbon. Tribol. Int. 2024, 196, 109689. [Google Scholar] [CrossRef]
- Xiao, L.; Xu, X.; Wu, Z.; Sun, T.; He, X.; Xu, X.; Qin, L.; Chen, D. Recent progress and prospect of friction-driven-tribocatalysis: From basic principle to material design. Surf. Interfaces 2024, 56, 105557. [Google Scholar] [CrossRef]
- Liu, M.-N.; Liu, J.-H.; Wang, L.-Y.; Yin, F.; Zheng, G.; Li, R.; Zhang, J.; Long, Y.-Z. Strategies for Improving Contact-Electro-Catalytic Efficiency: A Review. Nanomaterials 2025, 15, 386. [Google Scholar] [CrossRef]
- Li, X.; Tong, W.; Shi, J.; Chen, Y.; Zhang, Y.; An, Q. Tribocatalysis mechanisms: Electron transfer and transition. J. Mater. Chem. A 2023, 11, 4458–4472. [Google Scholar] [CrossRef]
- Wu, M.; Xu, Y.; He, Q.; Sun, P.; Weng, X.; Dong, X. Tribocatalysis of homogeneous material with multi-size granular distribution for degradation of organic pollutants. J. Colloid Interface Sci. 2022, 622, 602–611. [Google Scholar] [CrossRef]
- Liu, N.; Wang, R.; Zhao, J.; Jiang, J.; Fan, F.R. Piezoelectricity and triboelectricity enhanced catalysis. Nano Res. Energy 2024, 3, e9120137. [Google Scholar] [CrossRef]
- Li, P.; Wu, J.; Wu, Z.; Jia, Y.; Ma, J.; Chen, W.; Zhang, L.; Yang, J.; Liu, Y. Strong tribocatalytic dye decomposition through utilizing triboelectric energy of barium strontium titanate nanoparticles. Nano Energy 2019, 63, 103832. [Google Scholar] [CrossRef]
- Yang, B.; Chen, H.; Guo, X.; Wang, L.; Xu, T.; Bian, J.; Yang, Y.; Liu, Q.; Du, Y.; Lou, X. Enhanced tribocatalytic degradation using piezoelectric CdS nanowires for efficient water remediation. J. Mater. Chem. C 2020, 8, 14845–14854. [Google Scholar] [CrossRef]
- Jin, Z.; Zheng, X.; Zhu, Z.; Jiang, C.; Wu, S.; Hu, C.; Liu, L.; Fang, L.; Cheng, Z. Tribocatalytic sterilization of BN2/CN Z-type heterojunctions. Nano Energy 2024, 122, 109284. [Google Scholar] [CrossRef]
- Ashby, M.F.; Abulawi, J.; Kong, H.S. Temperature maps for frictional heating in dry sliding. Tribol. Trans. 1991, 34, 577–587. [Google Scholar] [CrossRef]
- Qin, W.; Jin, X.; Kirk, A.; Shipway, P.; Sun, W. Effects of surface roughness on local friction and temperature distributions in a steel-on-steel fretting contact. Tribol. Int. 2018, 120, 350–357. [Google Scholar] [CrossRef]
- Fan, F.R.; Xie, S.; Wang, G.W.; Tian, Z.Q. Tribocatalysis: Challenges and perspectives. Sci. China Chem. 2021, 64, 1609–1613. [Google Scholar] [CrossRef]
- Gershman, I.S.; Gershman, E.I. Catalytic effect during friction. J. Frict. Wear 2011, 32, 431–436. [Google Scholar] [CrossRef]
- Zhang, Y.; Yao, W.; Fang, H.; Hu, A.; Huang, Z. Catalytic alkane dehydrogenations. Sci. Bull. 2015, 60, 1316–1331. [Google Scholar] [CrossRef]
- Shirani, A.; Li, Y.; Smith, J.; Curry, J.; Lu, P.; Wilson, M.; Chandross, M.; Argibay, N.; Berman, D. Mechanochemically driven formation of protective carbon films from ethanol environment. Mater. Today Chem. 2022, 26, 101112. [Google Scholar] [CrossRef]
- Ta, H.T.T.; Tran, N.V.; Tieu, A.K.; Zhu, H.; Yu, H.; Ta, T.D. Computational Tribochemistry: A Review from Classical and Quantum Mechanics Studies. J. Phys. Chem. C 2021, 125, 16875–16891. [Google Scholar] [CrossRef]
- Qi, Y.; Yang, J.; Rappe, A.M. Theoretical modeling of tribochemical reaction on Pt and Au contacts: Mechanical load and catalysis. ACS Appl. Mater. Interfaces 2016, 8, 7529–7535. [Google Scholar] [CrossRef]
- Shirani, A.; Li, Y.; Eryilmaz, O.L.; Berman, D. Tribocatalytically-activated formation of protective friction and wear reducing carbon coatings from alkane environment. Sci. Rep. 2021, 11, 20643. [Google Scholar] [CrossRef]
- Song, W.; Li, J.; Zeng, C.; Ouyang, C.; Sun, S.; Wang, K.; Li, J.; Luo, J. Tribo-catalysis triggered the in-situ formation of amphiphilic molecules to reduce friction and wear. Tribol. Int. 2023, 185, 108541. [Google Scholar] [CrossRef]
- Onodera, T.; Kawasaki, K.; Nakakawaji, T.; Higuchi, Y.; Ozawa, N.; Kurihara, K.; Kubo, M. Tribocatalytic reaction of polytetrafluoroethylene sliding on an aluminum surface. J. Phys. Chem. C 2015, 119, 15954–15962. [Google Scholar] [CrossRef]
- Martini, A.; Kim, S.H. Activation volume in shear-driven chemical reactions. Tribol. Lett. 2021, 69, 150. [Google Scholar] [CrossRef]
- Harrison, J.A.; Brenner, D.W. Simulated tribochemistry: An atomic-scale view of the wear of diamond. J. Am. Chem. Soc. 1994, 116, 10399–10402. [Google Scholar] [CrossRef]
- Dong, R.; Zhang, Y.; Xu, H.-Z.; Bao, L.; Ma, S.; Wang, X.-G.; Yu, Q.; Cai, M.; Zhou, F.; Liu, W. Friction induced mechanochemistry: Self-adaptive lubrication through in-situ tribo-click system. Chem. Eng. J. 2022, 454, 139772. [Google Scholar] [CrossRef]
- Hu, M.Y.; Jensen, J.O.; Zhang, W.; Cleemann, L.N.; Xing, W.; Bjerrum, N.J.; Li, Q. Hollow spheres of iron carbide nanoparticles encased in graphitic layers as oxygen reduction catalysts. Angew. Chem. Int. Ed. 2014, 53, 3675–3679. [Google Scholar] [CrossRef]
- Bost, N.; Ammar, M.; Bouchetou, M.; Poirier, J. The catalytic effect of iron oxides on the formation of nano-carbon by the Boudouard reaction in refractories. J. Eur. Ceram. Soc. 2016, 36, 2133–2142. [Google Scholar] [CrossRef]
- Lu, R.; Nanao, H.; Kobayashi, K.; Kubo, T.; Mori, S. Effect of Lubricant additives on tribochemical decomposition of hydrocarbon oil on nascent steel surfaces. J. Jpn. Pet. Inst. 2010, 53, 55–60. [Google Scholar] [CrossRef]
- Li, K.; Amann, T.; Walter, M.; Moseler, M.; Kailer, A.; Rühe, J. Ultralow friction induced by tribochemical reactions: A novel mechanism of lubrication on steel surfaces. Langmuir 2013, 29, 5207–5213. [Google Scholar] [CrossRef]
- Grejtak, T.; Qu, J. Improving mechanical properties of carbon and tool steels via chromizing. Adv. Appl. Ceram. 2023, 122, 215–225. [Google Scholar] [CrossRef]
- Macknojia, A.Z.; Montoya, V.L.; Cairns, E.; Eskandari, M.; Liu, S.; Chung, Y.-W.; Wang, Q.J.; Berkebile, S.P.; Aouadi, S.M.; Voevodin, A.A.; et al. Tribological Analysis of Steels in Fuel Environments: Impact of Alloy Content and Hardness. Appl. Sci. 2024, 14, 1898. [Google Scholar] [CrossRef]
- Wang, B.; Chang, Q.; Gao, K.; Fang, H.; Qing, T.; Zhou, N. The synthesis of magnesium silicate hydroxide with different morphologies and the comparison of their tribological properties. Tribol. Int. 2018, 119, 672–679. [Google Scholar] [CrossRef]
- Chang, Q.; Rudenko, P.; Miller, D.J.; Wen, J.; Berman, D.; Zhang, Y.; Arey, B.; Zhu, Z.; Erdemir, A. Operando formation of an ultra-low friction boundary film from synthetic magnesium silicon hydroxide additive. Tribol. Int. 2017, 110, 35–40. [Google Scholar] [CrossRef]
- Gao, K.; Bin, W.; Berman, D.; Ren, Y.; Luo, J.; Xie, G. Self-adaptive macroscale superlubricity based on the tribocatalytic properties of partially oxidized black phosphorus. Nano Lett. 2023, 23, 6823–6830. [Google Scholar] [CrossRef]
- Chen, R.; Cai, Z.; Pu, J.; Lu, Z.; Chen, S.; Zheng, S.; Zeng, C. Effects of nitriding on the microstructure and properties of VAlTiCrMo high-entropy alloy coatings by sputtering technique. J. Alloys Compd. 2020, 827, 153836. [Google Scholar] [CrossRef]
- Lo, W.-L.; Hsu, S.-Y.; Lin, Y.-C.; Tsai, S.-Y.; Lai, Y.-T.; Duh, J.-G. Improvement of high entropy alloy nitride coatings (AlCrNbSiTiMo)N on mechanical and high temperature tribological properties by tuning substrate bias. Surf. Coatings Technol. 2020, 401, 126247. [Google Scholar] [CrossRef]
- Hahn, R.; Kirnbauer, A.; Bartosik, M.; Kolozsvári, S.; Mayrhofer, P. Toughness of Si alloyed high-entropy nitride coatings. Mater. Lett. 2019, 251, 238–240. [Google Scholar] [CrossRef]
- Argibay, N.; Babuska, T.; Curry, J.; Dugger, M.; Lu, P.; Adams, D.; Nation, B.; Doyle, B.; Pham, M.; Pimentel, A.; et al. In-situ tribochemical formation of self-lubricating diamond-like carbon films. Carbon 2018, 138, 61–68. [Google Scholar] [CrossRef]
- Curry, J.F.; Babuska, T.F.; Furnish, T.A.; Lu, P.; Adams, D.P.; Kustas, A.B.; Nation, B.L.; Dugger, M.T.; Chandross, M.; Clark, B.G.; et al. Achieving ultralow wear with stable nanocrystalline metals. Adv. Mater. 2018, 30, e1802026. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, G.; Eryilmaz, O.L.; Fatti, G.; Righi, M.C.; Wen, J.; Erdemir, A. Tribochemical conversion of methane to graphene and other carbon nanostructures: Implications for friction and wear. ACS Appl. Nano Mater. 2020, 3, 8060–8067. [Google Scholar] [CrossRef]
- Al Sulaimi, R.; Eskandari, M.; Shirani, A.; Macknojia, A.Z.; Miller, W.; Berman, D. Effect of Cu and Ni Inclusion on Tribological Performance of Tribocatalytically Active Coatings in Hydrocarbon Environments. Coatings 2023, 14, 61. [Google Scholar] [CrossRef]
- Berman, D.; Mutyala, K.C.; Srinivasan, S.; Sankaranarayanan, S.K.R.S.; Erdemir, A.; Shevchenko, E.V.; Sumant, A.V. Iron-Nanoparticle Driven Tribochemistry Leading to Superlubric Sliding Interfaces. Adv. Mater. Interfaces 2019, 6, 1901416. [Google Scholar] [CrossRef]
- Gao, K.; Wang, B.; Shirani, A.; Chang, Q.; Berman, D. Macroscale superlubricity accomplished by Sb2O3-MSH/C under high temperature. Front. Chem. 2021, 9, 667878. [Google Scholar] [CrossRef]
- Jiang, Y.; Wu, X.; Yu, L.; Chen, C.; Han, H.; Bian, S.; Zuo, B.; Zhao, L.; Xu, J. In-situ tribo-induced formation of superb lubricious carbon-based tribofilms on the catalytical active MoN-Ag film surfaces. Tribol. Int. 2024, 196, 109715. [Google Scholar] [CrossRef]
- Xu, X.; Li, Q.; Su, F.; Sun, J.; Li, W. In-situ formation of onion-like carbon film by tribo-induced catalytic degradation of hydrocarbon: Effect of lubrication condition and load. Chem. Eng. J. 2023, 459, 141566. [Google Scholar] [CrossRef]
- Liu, N.; Gao, J.; Li, Y.; Wang, J.; Wan, Y.; Li, R. Different tribological behavior of CrN and CrCuN coatings under glycerol lubrication. Surf. Coatings Technol. 2022, 436, 128262. [Google Scholar] [CrossRef]
- Bisht, A.K.; Vaishya, R.O.; Walia, R.; Singh, G. Nitrides ceramic coatings for tribological applications: A journey from binary to high-entropy compositions. Ceram. Int. 2023, 50, 8553–8585. [Google Scholar] [CrossRef]
- Macknojia, A.; Dockins, M.; Ayyagari, A.; Montoya, V.; Rodriguez, J.; Cairns, E.; Murthy, N.; Berkebile, S.; Voevodin, A.; Aouadi, S.; et al. Tribological Evaluation of Coatings in Fuel Environments. J. Tribol. 2025, 147, p.074501. [Google Scholar] [CrossRef]
- Subramanian, C.; Strafford, K. Review of multicomponent and multilayer coatings for tribological applications. Wear 1993, 165, 85–95. [Google Scholar] [CrossRef]
- Walsh, F.C.; de Leon, C.P. A review of the electrodeposition of metal matrix composite coatings by inclusion of particles in a metal layer: An established and diversifying technology. Trans. IMF 2014, 92, 83–98. [Google Scholar] [CrossRef]
- Aouadi, S.; Luster, B.; Kohli, P.; Muratore, C.; Voevodin, A. Progress in the development of adaptive nitride-based coatings for high temperature tribological applications. Surf. Coatings Technol. 2009, 204, 962–968. [Google Scholar] [CrossRef]
- Jacques, K.; Shirani, A.; Smith, J.; Scharf, T.W.; Walck, S.D.; Berkebile, S.; Eryilmaz, O.L.; Voevodin, A.A.; Aouadi, S.; Berman, D. MoVN-Cu Coatings for In Situ Tribocatalytic Formation of Carbon-Rich Tribofilms in Low-Viscosity Fuels. ACS Appl. Mater. Interfaces 2023, 15, 30070–30082. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Gao, X.; Liu, C.; Zhang, K.; Zheng, W.; Chen, C. Macroscale superdurable superlubricity achieved in lubricant oil via operando tribochemical formation of fullerene-like carbon. Cell Rep. Phys. Sci. 2022, 3, 101130. [Google Scholar] [CrossRef]
- Kasar, A.K.; Scalaro, K.; Menezes, P.L. Tribological properties of high-entropy alloys under dry conditions for a wide temperature range—A review. Materials 2021, 14, 5814. [Google Scholar] [CrossRef]
- George, E.P.; Curtin, W.A.; Tasan, C.C. High entropy alloys: A focused review of mechanical properties and deformation mechanisms. Acta Mater. 2020, 188, 435–474. [Google Scholar] [CrossRef]
- Wang, S.; Sunkara, S.V.; Manna, S.; Ahmadiparidari, A.; Kumar, K.; Yang, T.; Namvar, S.; Seraji, P.; Huang, Z.; Cabana, J.; et al. Self-Lubricating Tribo-Catalytic Activity of 2D High Entropy Alloy Nanoflakes. Small 2025, 21, e2500322. [Google Scholar] [CrossRef]
- Xiao, J.-K.; Tan, H.; Chen, J.; Martini, A.; Zhang, C. Effect of carbon content on microstructure, hardness and wear resistance of CoCrFeMnNiCx high-entropy alloys. J. Alloys Compd. 2020, 847, 156533. [Google Scholar] [CrossRef]
- Oses, C.; Toher, C.; Curtarolo, S. High-entropy ceramics. Nat. Rev. Mater. 2020, 5, 295–309. [Google Scholar] [CrossRef]
- Akrami, S.; Edalati, P.; Fuji, M.; Edalati, K. High-entropy ceramics: Review of principles, production and applications. Mater. Sci. Eng. R Rep. 2021, 146, 100644. [Google Scholar] [CrossRef]
- Zhang, R.-Z.; Reece, M.J. Review of high entropy ceramics: Design, synthesis, structure and properties. J. Mater. Chem. A 2019, 7, 22148–22162. [Google Scholar] [CrossRef]
- Du, C.; Yu, T.; Sui, X.; Zhang, Z.; Cai, R.; Zhang, L.; Feng, Y.; Feng, M.; Zhou, F.; Wang, D. Macro-Superlubricity Induced by Tribocatalysis of High-Entropy Ceramics. Adv. Mater. 2025, 37, 2413781. [Google Scholar] [CrossRef]
- Farfan-Cabrera, L.I.; Lee, S.; Skowron, S.; Erdemir, A. Enhancing lubrication of electrified interfaces by inert gas atmosphere. J. Tribol. 2025, 147, 51104. [Google Scholar] [CrossRef]
- Sharifi, T.; Zhang, X.; Costin, G.; Yazdi, S.; Woellner, C.F.; Liu, Y.; Tiwary, C.S.; Ajayan, P. Thermoelectricity enhanced electrocatalysis. Nano Lett. 2017, 17, 7908–7913. [Google Scholar] [CrossRef] [PubMed]
- Berman, D.; Wyatt, B.C.; Anasori, B.; Farfan-Cabrera, L.I.; Erdemir, A.; Rosenkranz, A. Potential of 2D MXenes in electromobility. Mater. Today 2025. [Google Scholar] [CrossRef]
- Berman, D.; Farfan-Cabrera, L.I.; Rosenkranz, A.; Erdemir, A. 2D materials for durable and sustainable electric vehicles. Nat. Rev. Mater. 2024, 9, 527–529. [Google Scholar] [CrossRef]
- Fox, N.J.; Stachowiak, G.W. Vegetable oil-based lubricants—A review of oxidation. Tribol. Int. 2007, 40, 1035–1046. [Google Scholar] [CrossRef]
- Mang, T.; Dresel, W. Lubricants and Lubrication; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Mortier, R.M.; Orszulik, S.T.; Fox, M.F. Chemistry and Technology of Lubricants; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Rudnick, L.R. Synthetics, Mineral Oils, and Bio-Based Lubricants: Chemistry and Technology; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Torbacke, M.; Rudolphi, Å.K.; Kassfeldt, E. Lubricants: Introduction to Properties and Performance; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Soni, S.; Agarwal, M. Lubricants from renewable energy sources–a review. Green Chem. Lett. Rev. 2014, 7, 359–382. [Google Scholar] [CrossRef]
- Syahir, A.Z.; Zulkifli, N.W.M.; Masjuki, H.H.; Kalam, M.A.; Alabdulkarem, A.; Gulzar, M.; Khuong, L.S.; Harith, M.H. A review on bio-based lubricants and their applications. J. Clean. Prod. 2017, 168, 997–1016. [Google Scholar] [CrossRef]
- Somers, A.E.; Howlett, P.C.; Macfarlane, D.R.; Forsyth, M. A Review of ionic liquid lubricants. Lubricants 2013, 1, 3–21. [Google Scholar] [CrossRef]
- Rahman, M.H.; Warneke, H.; Webbert, H.; Rodriguez, J.; Austin, E.; Tokunaga, K.; Rajak, D.K.; Menezes, P.L. Water-based lubricants: Development, properties, and performances. Lubricants 2021, 9, 73. [Google Scholar] [CrossRef]
- Tomala, A.; Karpinska, A.; Werner, W.; Olver, A.; Störi, H. Tribological properties of additives for water-based lubricants. Wear 2010, 269, 804–810. [Google Scholar] [CrossRef]
- Spikes, H. The history and mechanisms of ZDDP. Tribol. Lett. 2004, 17, 469–489. [Google Scholar] [CrossRef]
- Taylor, L.J.; Spikes, H.A. Friction-enhancing properties of ZDDP antiwear additive: Part I—Friction and morphology of ZDDP reaction films. Tribol. Trans. 2003, 46, 303–309. [Google Scholar] [CrossRef]
- Lin, Y.; So, H. Limitations on use of ZDDP as an antiwear additive in boundary lubrication. Tribol. Int. 2004, 37, 25–33. [Google Scholar] [CrossRef]
- de Barros’BOuchet, M.; Martin, J.; Le-Mogne, T.; Vacher, B. Boundary lubrication mechanisms of carbon coatings by MoDTC and ZDDP additives. Tribol. Int. 2005, 38, 257–264. [Google Scholar] [CrossRef]
- Dorgham, A.; Azam, A.; Parsaeian, P.; Wang, C.; Morina, A.; Neville, A. An assessment of the effect of relative humidity on the decomposition of the ZDDP antiwear additive. Tribol. Lett. 2021, 69, 72. [Google Scholar] [CrossRef]
- Guan, B.; Pochopien, B.A.; Wright, D.S. The chemistry, mechanism and function of tricresyl phosphate (TCP) as an anti-wear lubricant additive. Lubr. Sci. 2015, 28, 257–265. [Google Scholar] [CrossRef]
- Acharya, B.; Avva, K.S.; Thapa, B.; Pardue, T.N.; Krim, J. Synergistic effect of nanodiamond and phosphate ester anti-wear additive blends. Lubricants 2018, 6, 56. [Google Scholar] [CrossRef]
- De Feo, M.; Minfray, C.; Bouchet, M.I.D.B.; Thiebaut, B.; Martin, J.M. MoDTC friction modifier additive degradation: Correlation between tribological performance and chemical changes. RSC Adv. 2015, 5, 93786–93796. [Google Scholar] [CrossRef]
- Shu, J.; Harris, K.; Munavirov, B.; Westbroek, R.; Leckner, J.; Glavatskih, S. Tribology of polypropylene and Li-complex greases with ZDDP and MoDTC additives. Tribol. Int. 2018, 118, 189–195. [Google Scholar] [CrossRef]
- Du, S.; Yue, W.; Wang, Y.; She, D.; Huang, H.; Fu, Z. Synergistic effects between sulfurized-nanocrystallized 316L steel and MoDTC lubricating oil additive for improvement of tribological performances. Tribol. Int. 2016, 94, 530–540. [Google Scholar] [CrossRef]
- Prengel, H.; Pfouts, W.; Santhanam, A. State of the art in hard coatings for carbide cutting tools. Surf. Coat. Technol. 1998, 102, 183–190. [Google Scholar] [CrossRef]
- Govande, A.R.; Chandak, A.; Sunil, B.R.; Dumpala, R. Carbide-based thermal spray coatings: A review on performance characteristics and post-treatment. Int. J. Refract. Met. Hard Mater. 2022, 103, 105772. [Google Scholar] [CrossRef]
- Grzesik, W.; Zalisz, Z.; Nieslony, P. Friction and wear testing of multilayer coatings on carbide substrates for dry machining applications. Surf. Coat. Technol. 2002, 155, 37–45. [Google Scholar] [CrossRef]
- Wilhelmsson, O.; Råsander, M.; Carlsson, M.; Lewin, E.; Sanyal, B.; Wiklund, U.; Eriksson, O.; Jansson, U. Design of Nanocomposite Low-Friction Coatings. Adv. Funct. Mater. 2007, 17, 1611–1616. [Google Scholar] [CrossRef]
- Kato, K.; Umehara, N.; Adachi, K. Friction, wear and N2-lubrication of carbon nitride coatings: A review. Wear 2003, 254, 1062–1069. [Google Scholar] [CrossRef]
- Sue, J.; Chang, T. Friction and wear behavior of titanium nitride, zirconium nitride and chromium nitride coatings at elevated temperatures. Surf. Coat. Technol. 1995, 76, 61–69. [Google Scholar] [CrossRef]
- Liu, A.; Deng, J.; Cui, H.; Chen, Y.; Zhao, J. Friction and wear properties of TiN, TiAlN, AlTiN and CrAlN PVD nitride coatings. Int. J. Refract. Met. Hard Mater. 2012, 31, 82–88. [Google Scholar] [CrossRef]
- Luo, Q. Origin of Friction in Running-in Sliding Wear of Nitride Coatings. Tribol. Lett. 2009, 37, 529–539. [Google Scholar] [CrossRef]
- Aouadi, S.; Gao, H.; Martini, A.; Scharf, T.; Muratore, C. Lubricious oxide coatings for extreme temperature applications: A review. Surf. Coat. Technol. 2014, 257, 266–277. [Google Scholar] [CrossRef]
- Franz, R.; Mitterer, C. Vanadium containing self-adaptive low-friction hard coatings for high-temperature applications: A review. Surf. Coat. Technol. 2013, 228, 1–13. [Google Scholar] [CrossRef]
- Hayward, I.; Singer, I.; Seitzman, L. Effect of roughness on the friction of diamond on CVD diamond coatings. Wear 1992, 157, 215–227. [Google Scholar] [CrossRef]
- You, Q.; Xiong, J.; Li, H.; Guo, Z.; Huo, Y. Study on the microstructure and high temperature friction and wear characteristics of three CVD coated cermets. Int. J. Refract. Met. Hard Mater. 2021, 96, 105495. [Google Scholar] [CrossRef]
- Holmberg, K.; Ronkainen, H.; Laukkanen, A.; Wallin, K. Friction and wear of coated surfaces—scales, modelling and simulation of tribomechanisms. Surf. Coat. Technol. 2007, 202, 1034–1049. [Google Scholar] [CrossRef]
- Hirata, A.; Yoshioka, N. Sliding friction properties of carbon nanotube coatings deposited by microwave plasma chemical vapor deposition. Tribol. Int. 2004, 37, 893–898. [Google Scholar] [CrossRef]
- Miki, H.; Tsutsui, A.; Takeno, T.; Takagi, T. Friction properties of partially polished CVD diamond films at different sliding speeds. Diam. Relat. Mater. 2012, 24, 167–170. [Google Scholar] [CrossRef]
- Guo, Y.; Zhou, X.; Lee, K.; Yoon, H.C.; Xu, Q.; Wang, D. Recent development in friction of 2D materials: From mechanisms to applications. Nanotechnology 2021, 32, 312002. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, M.; Jin, L.; Li, L.; Mo, Y.; Su, G.; Li, X.; Zhu, H.; Tian, Y. Recent advances in friction and lubrication of graphene and other 2D materials: Mechanisms and applications. Friction 2019, 7, 199–216. [Google Scholar] [CrossRef]
- Rejhon, M.; Lavini, F.; Khosravi, A.; Shestopalov, M.; Kunc, J.; Tosatti, E.; Riedo, E. Relation between interfacial shear and friction force in 2D materials. Nat. Nanotechnol. 2022, 17, 1280–1287. [Google Scholar] [CrossRef]
- Wang, J.; Ma, M.; Tosatti, E. Kinetic friction of structurally superlubric 2D material interfaces. J. Mech. Phys. Solids 2023, 180, 105396. [Google Scholar] [CrossRef]
- Snapp, P.; Kim, J.M.; Cho, C.; Leem, J.; Haque, F.; Nam, S. Interaction of 2D materials with liquids: Wettability, electrochemical properties, friction, and emerging directions. NPG Asia Mater. 2020, 12, 22. [Google Scholar] [CrossRef]
- Baboukani, B.S.; Ye, Z.; Reyes, K.G.; Nalam, P.C. Prediction of nanoscale friction for two-dimensional materials using a machine learning approach. Tribol. Lett. 2020, 68, 57. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, Y.; Hu, X.; Fang, L.; Song, Y.; Liu, D.; Luo, J. Influence of elastic property on the friction between atomic force microscope tips and 2D materials. Nanotechnology 2020, 31, 285710. [Google Scholar] [CrossRef]
- Spear, J.C.; Ewers, B.W.; Batteas, J.D. 2D-nanomaterials for controlling friction and wear at interfaces. Nano Today 2015, 10, 301–314. [Google Scholar] [CrossRef]
- Androulidakis, C.; Zhang, K.; Robertson, M.; Tawfick, S.H. Tailoring the mechanical properties of 2D materials and heterostructures. 2D Mater. 2018, 5, 032005. [Google Scholar] [CrossRef]
- Guo, W.; Yin, J.; Qiu, H.; Guo, Y.; Wu, H.; Xue, M. Friction of low-dimensional nanomaterial systems. Friction 2014, 2, 209–225. [Google Scholar] [CrossRef]
- Vazirisereshk, M.R.; Martini, A.; Strubbe, D.A.; Baykara, M.Z. Solid lubrication with MoS2: A review. Lubricants 2019, 7, 57. [Google Scholar] [CrossRef]
- Savan, A.; Pflüger, E.; Voumard, P.; Schröer, A.; Simmonds, M. Modern solid lubrication: Recent developments and applications of MoS2. Lubr. Sci. 2000, 12, 185–203. [Google Scholar] [CrossRef]
- Zabinski, J.; Donley, M.; McDevitt, N. Mechanistic study of the synergism between Sb2O3 and MoS2 lubricant systems using Raman spectroscopy. Wear 1993, 165, 103–108. [Google Scholar] [CrossRef]
- Serles, P.; Gaber, K.; Pajovic, S.; Colas, G.; Filleter, T. High temperature microtribological studies of MoS2 lubrication for low earth orbit. Lubricants 2020, 8, 49. [Google Scholar] [CrossRef]
- Wang, Y.; Du, Y.; Deng, J.; Wang, Z. Friction reduction of water based lubricant with highly dispersed functional MoS2 nanosheets. Colloids Surf. A Physicochem. Eng. Asp. 2019, 562, 321–328. [Google Scholar] [CrossRef]
- Zhang, X.; Luster, B.; Church, A.; Muratore, C.; Voevodin, A.A.; Kohli, P.; Aouadi, S.; Talapatra, S. Carbon nanotube−MoS2 composites as solid lubricants. ACS Appl. Mater. Interfaces 2009, 1, 735–739. [Google Scholar] [CrossRef] [PubMed]
- Dallavalle, M.; Sändig, N.; Zerbetto, F. Stability, dynamics, and lubrication of MoS2 platelets and nanotubes. Langmuir 2012, 28, 7393–7400. [Google Scholar] [CrossRef] [PubMed]
- Chkhartishvili, L.; Tabatadze, G.; Nackebia, D.; Bzhalava, T.; Kalandadze, I. Hexagonal boron nitride as a solid lubricant additive (an overview). Nano Stud. 2016, 14, 2016. [Google Scholar]
- Martin, J.M.; Le Mogne, T.; Chassagnette, C.; Gardos, M.N. Friction of hexagonal boron nitride in various environments. Tribol. Trans. 1992, 35, 462–472. [Google Scholar] [CrossRef]
- Mandelli, D.; Ouyang, W.; Hod, O.; Urbakh, M. Negative friction coefficients in superlubric graphite–hexagonal boron nitride heterojunctions. Phys. Rev. Lett. 2019, 122, 076102. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).