Lubrication-Enhanced Mechanisms of Bentonite Grease Using 2D MoS2 with Narrow Lateral Size and Thickness Distributions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
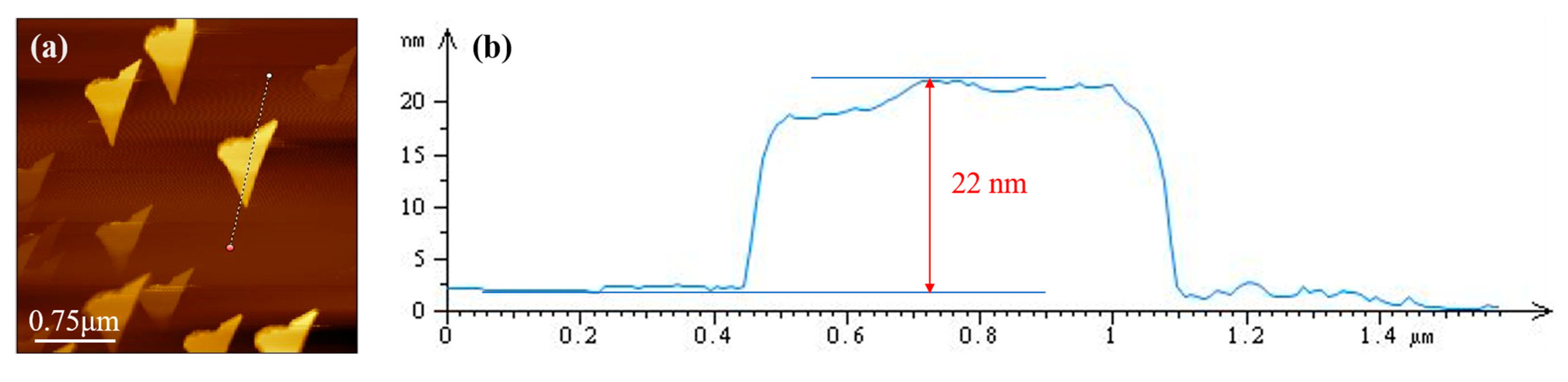
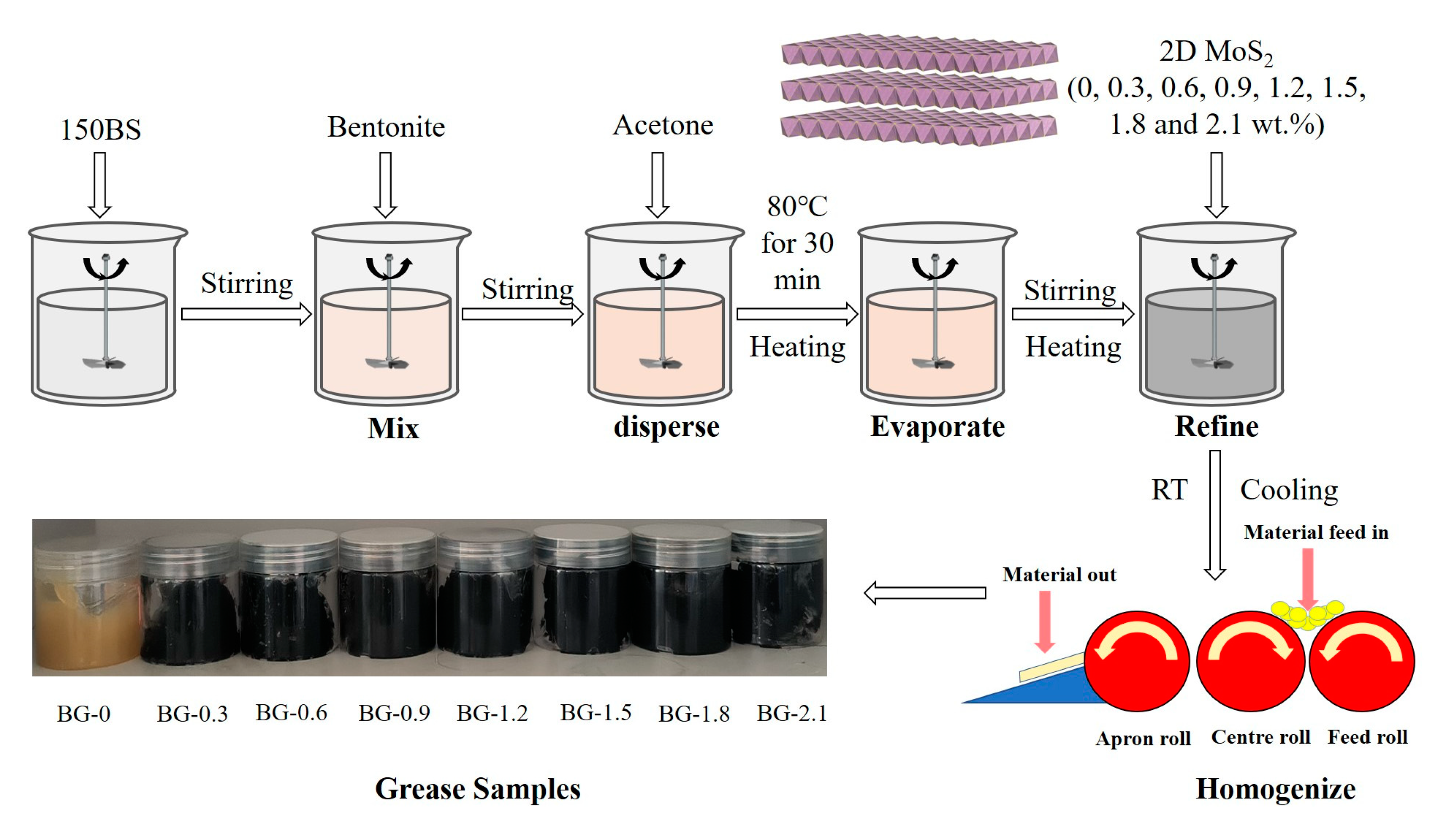
2.2. Preparation and Characterization of Bentonite Grease with 2D MoS2
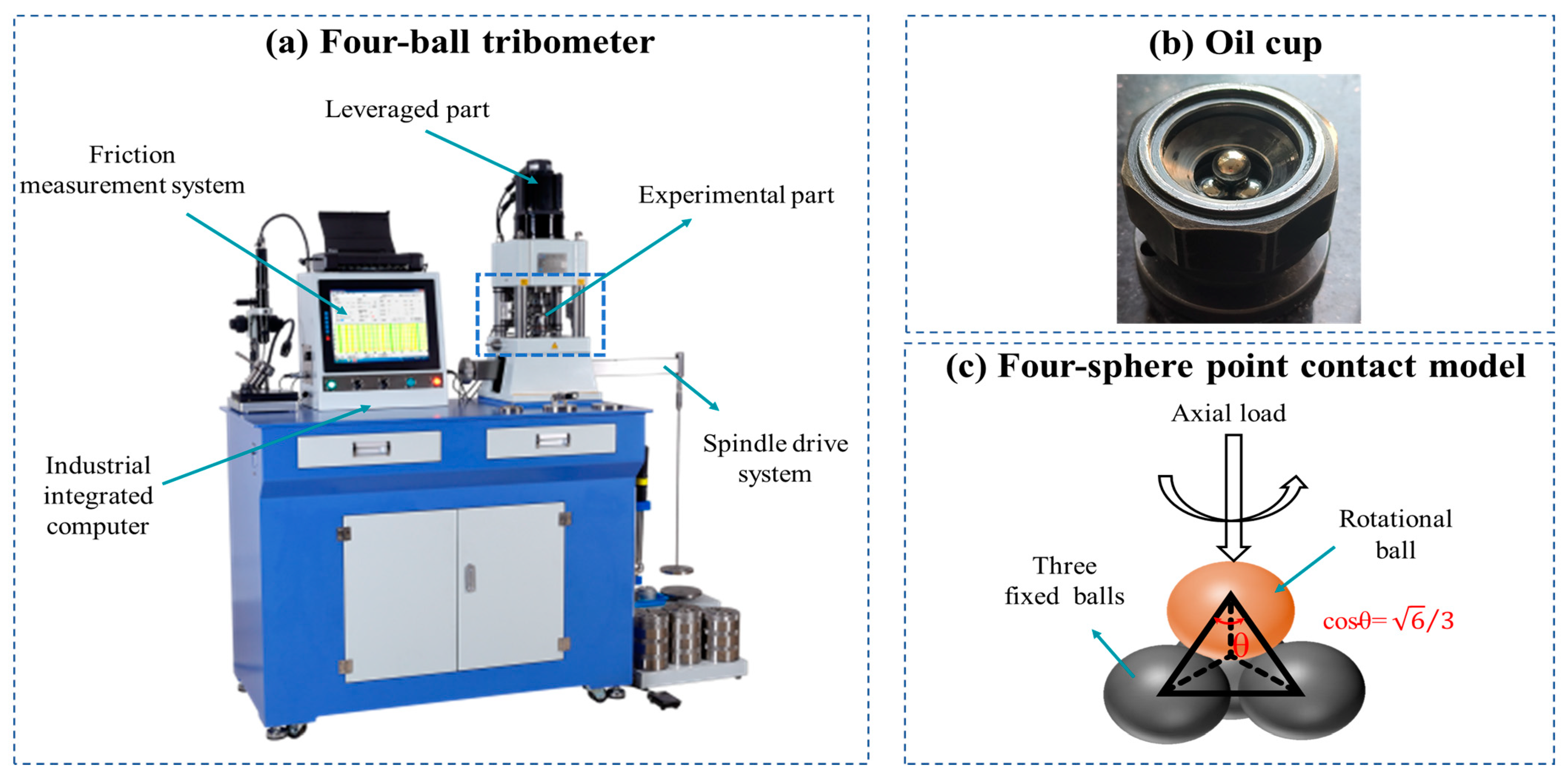
2.3. Tribology Tests and Analysis
3. Results and Discussion
3.1. Physico-Chemical Properties
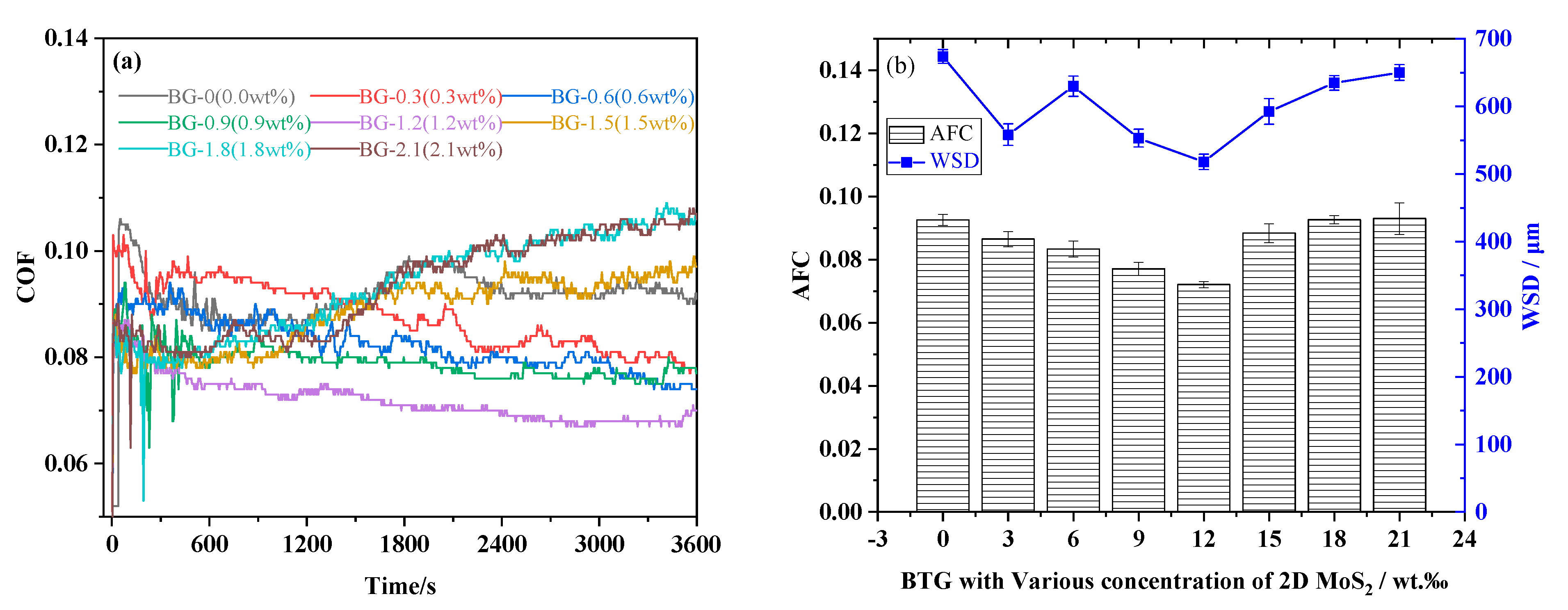
3.2. Friction and Wear Performance
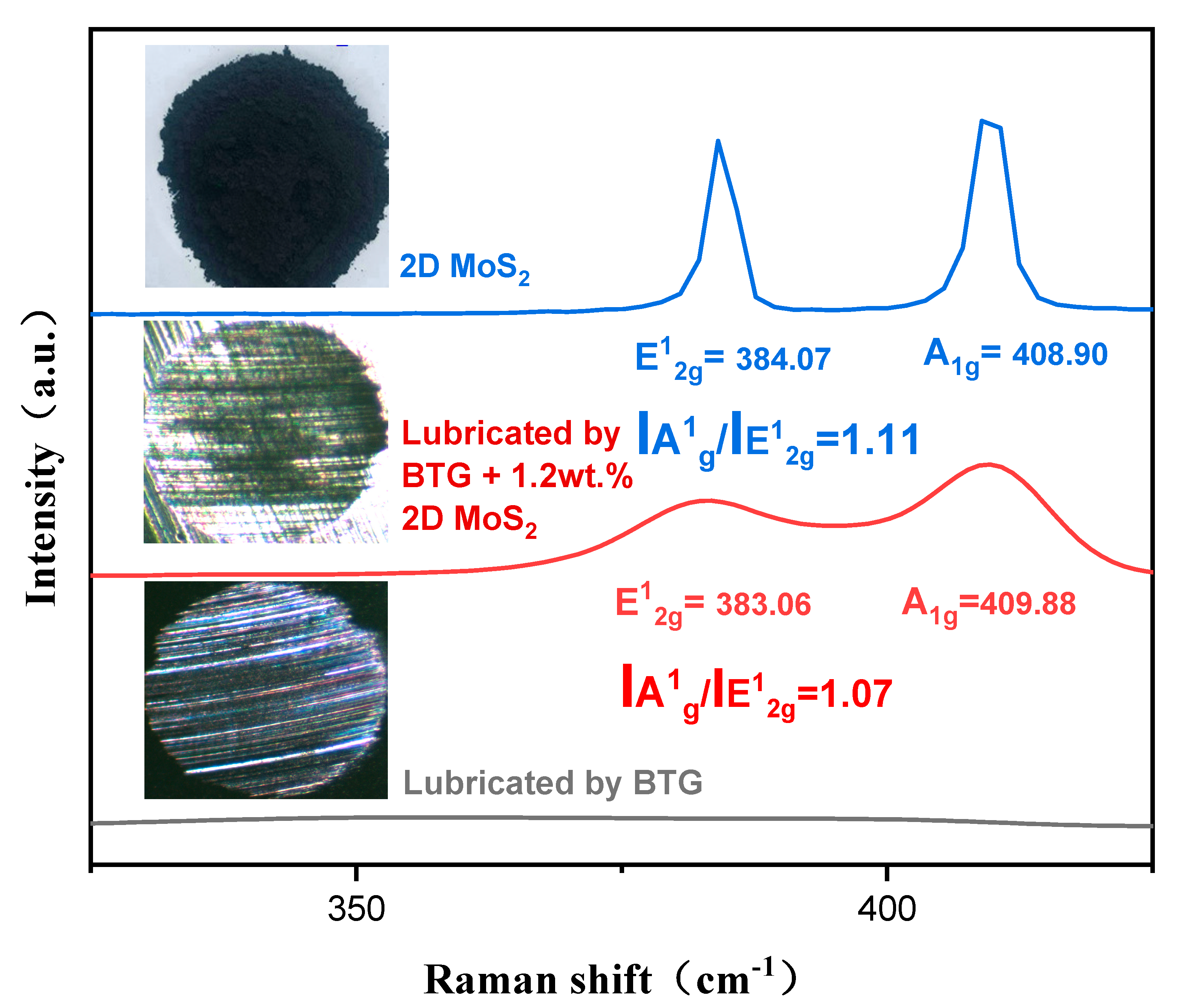
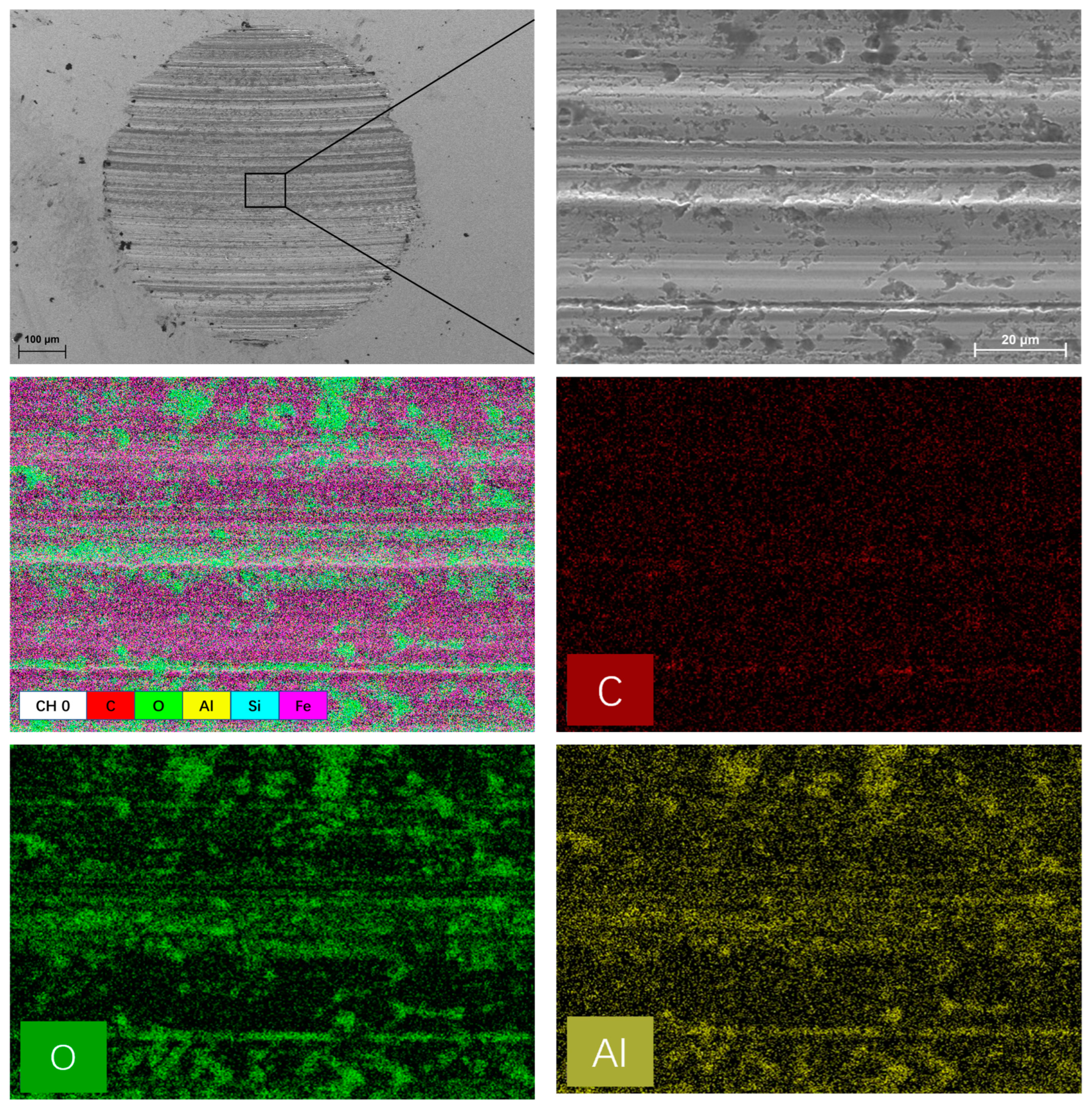
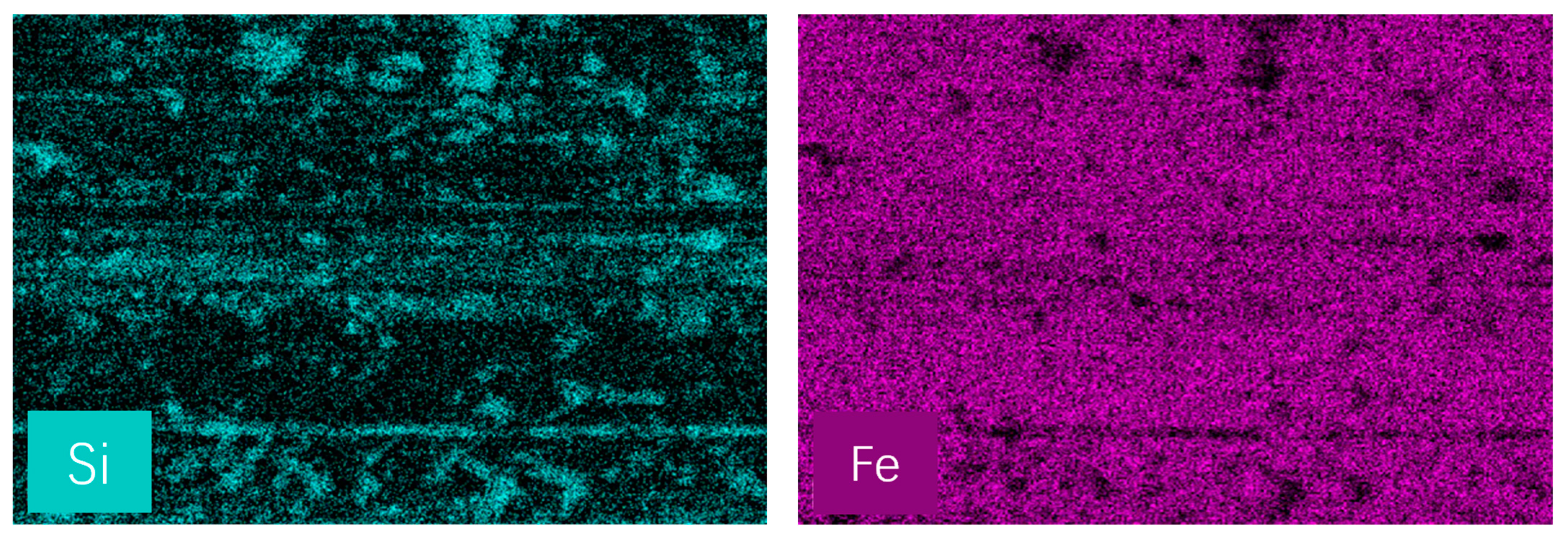
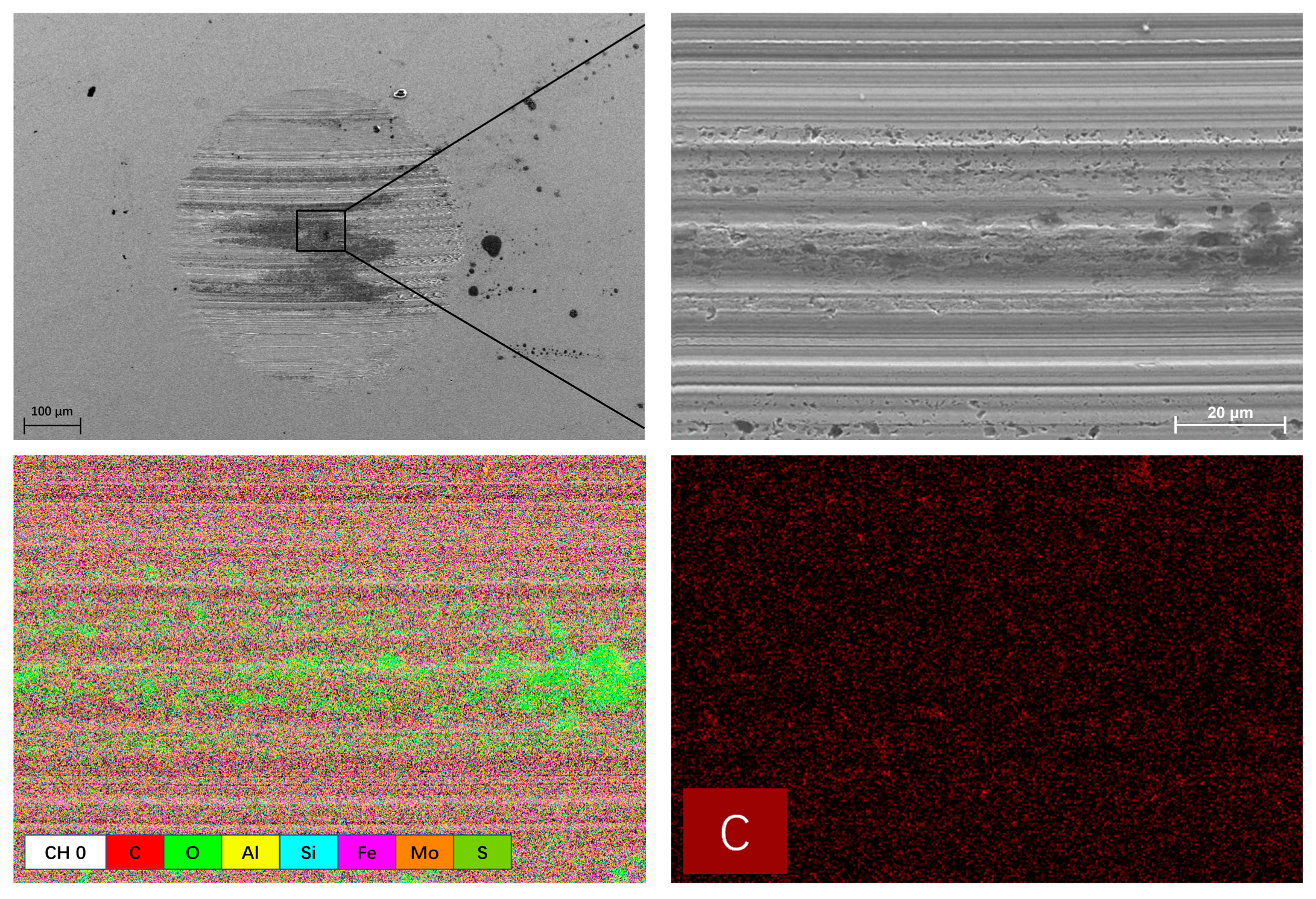
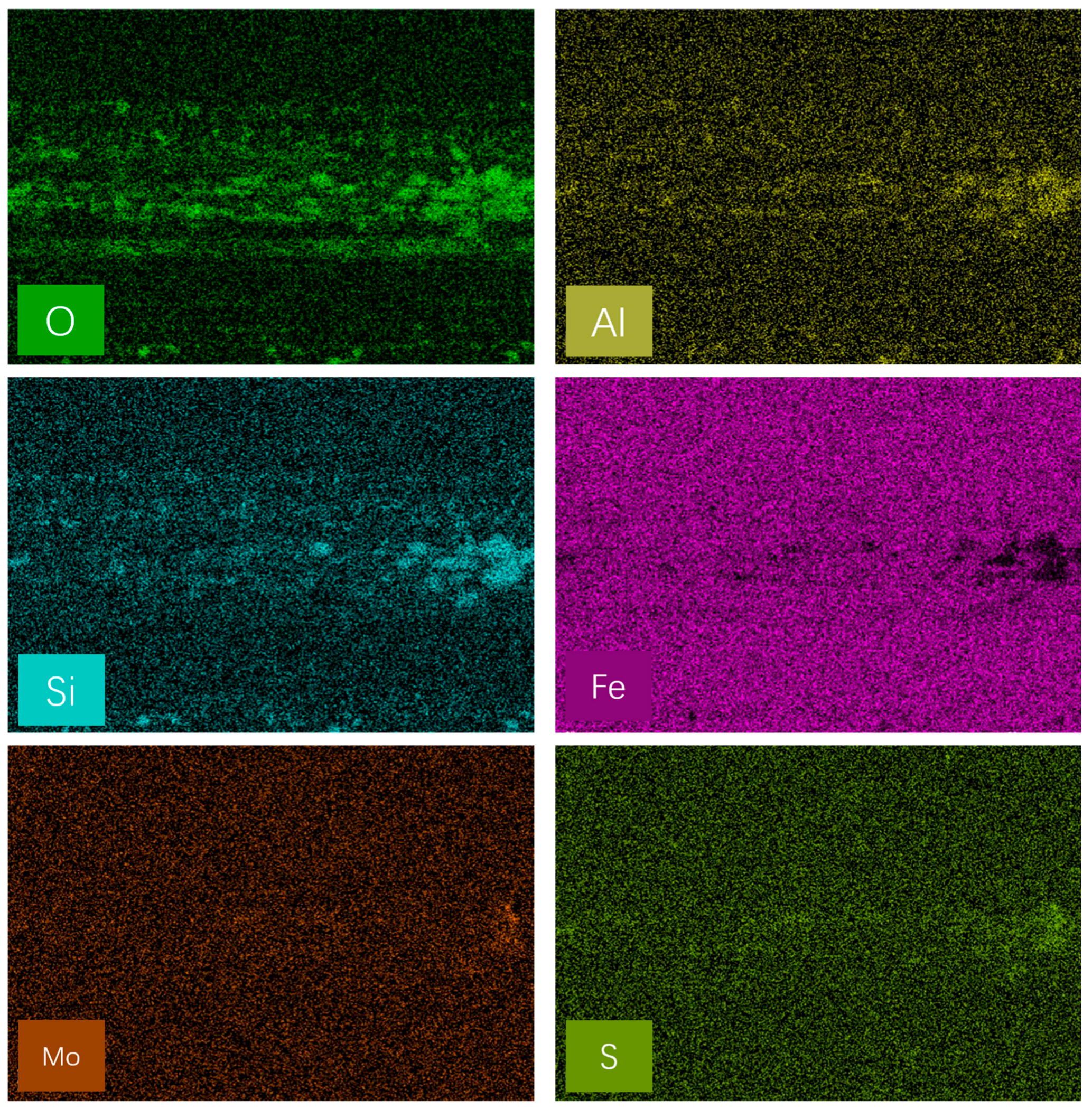
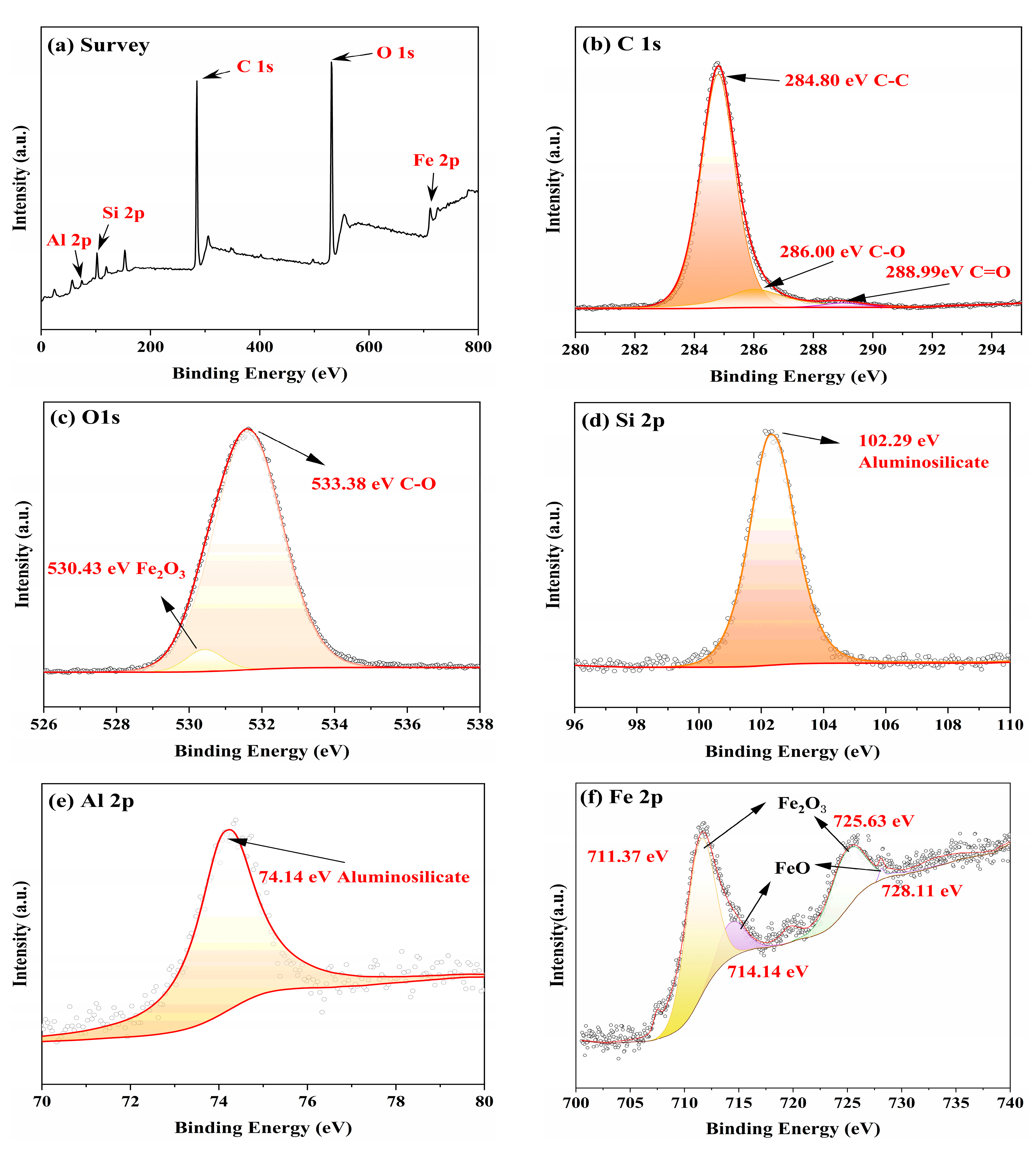
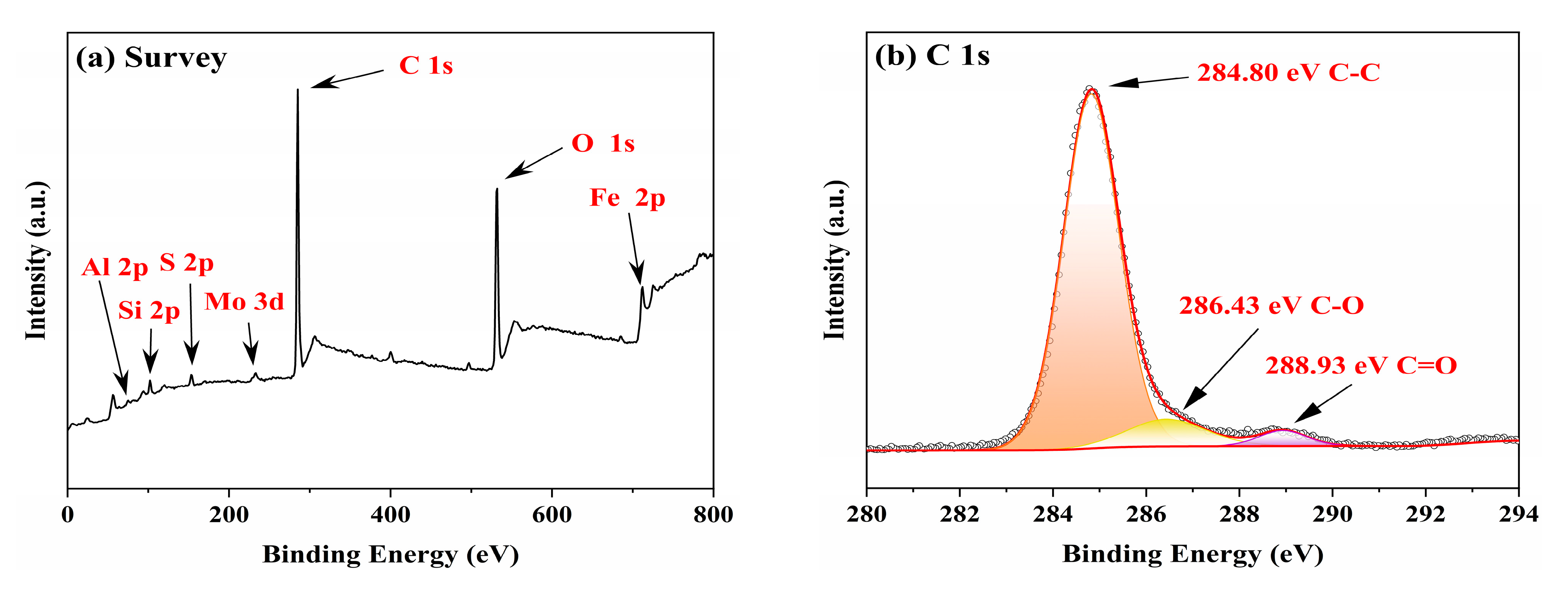
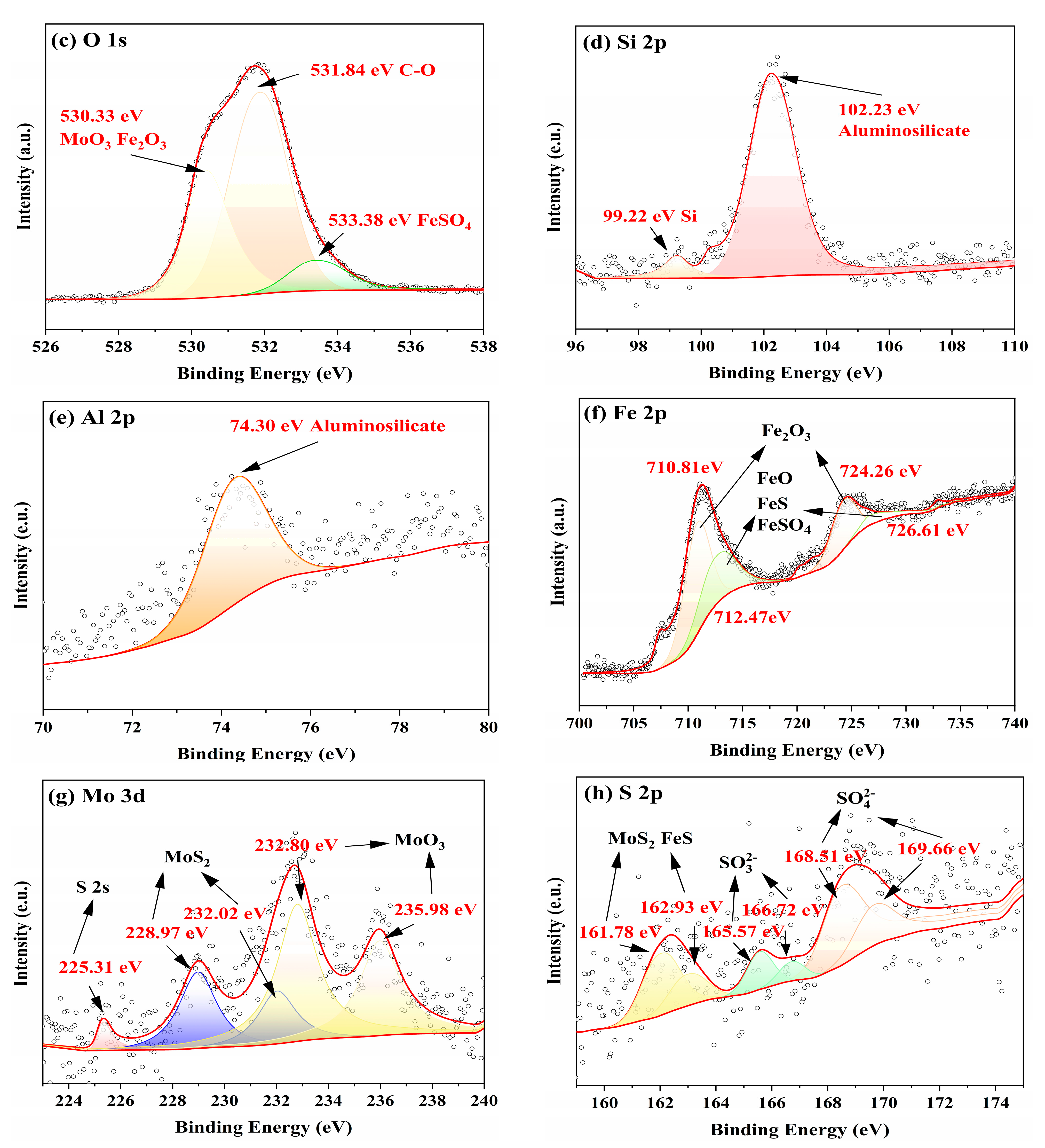
3.3. Worn Surface Analysis
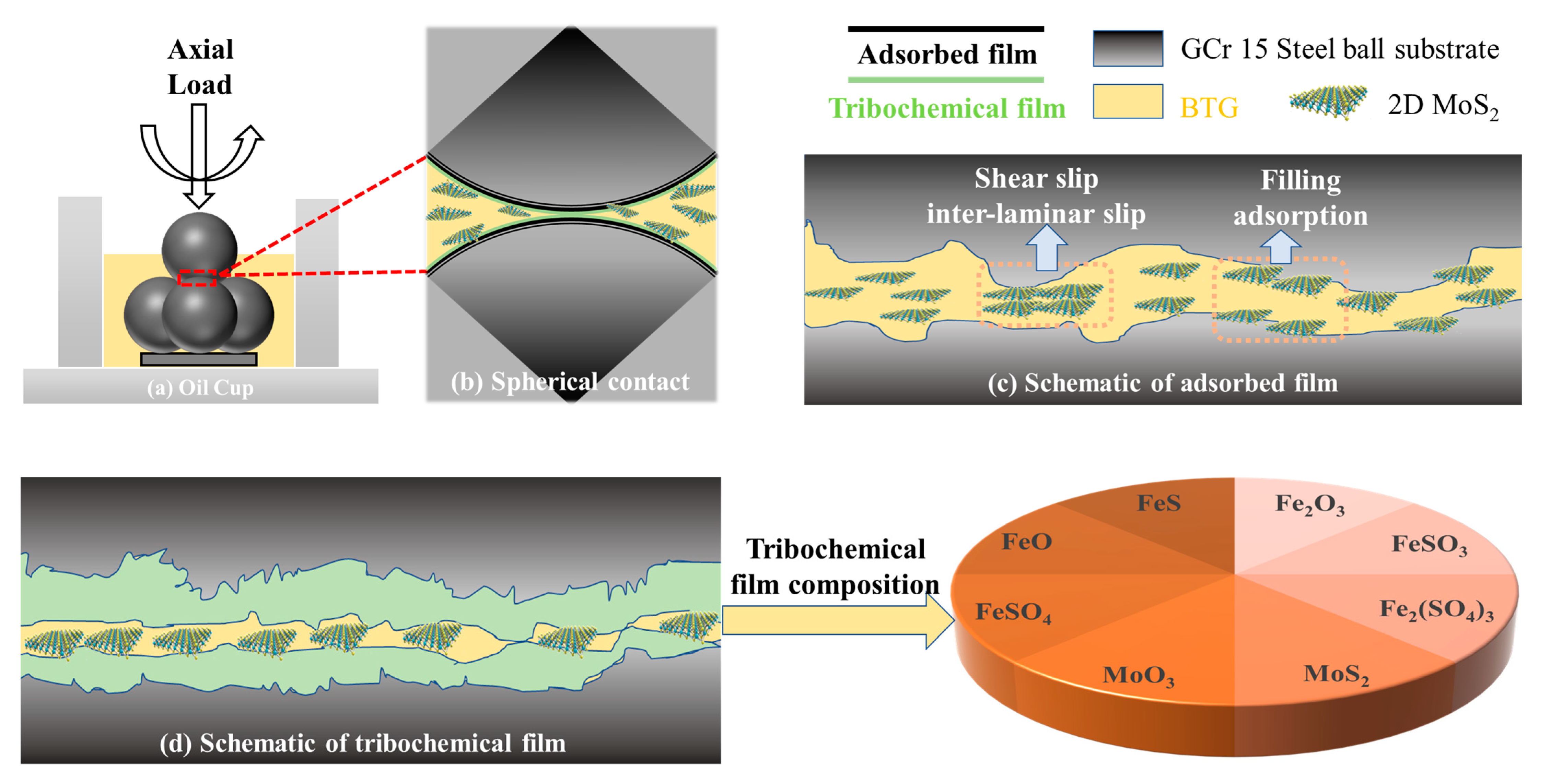
3.4. Lubrication Mechanism of 2D MoS2
4. Conclusions
- a
- The 2D MoS2 as lubricating additives utilized in the bentonite grease have significant effects on its penetration, dropping point, oil separation, evaporation, copper corrosion and friction-reducing and antiwear properties.
- b
- The COF, WSD, surface roughness and wear scar depth of BG + 1.2 wt.% 2D MoS2 were effectively reduced by approximately 22.15%, 23.14%, 55.15%, and 82.64%, respectilvely, in comparison with that of BG. In addition, the contact region was firmly in a state of boundary lubrication under the four-ball tribometer according to the calculation of the Dowson and Hamrock minimum film thickness formula.
- c
- Raman, EDS and XPS results collectively showed that a stable adsorption film and a robust tribochemical composed of Fe2O3, FeSO4, Fe2(SO4)3,FeSO3, FeS, FeO, and MoO3, which is typically more ductile than the GCr15 bearing steel substrate and protect the GCr15 bearing steel/GCr15 bearing steel substrate from severe wear by avoiding direct metal-to-metal contact.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Luo, J.; Min, L.; Ma, L. Origin of friction and the new frictionless technology—Superlubricity: Advancements and future outlook. Nano Energy 2021, 86, 106092. [Google Scholar] [CrossRef]
- Chen, C.; Liu, Y.; Tang, Q.; Xu, H.; Tang, M.; Li, X.; Liu, L.; Dong, J. Tribological and rheological performance of lithium grease with poly-α-olefin and alkyl-tetralin as base oils. Chin. J. Chem. Eng. 2023, 56, 180–192. [Google Scholar] [CrossRef]
- Zhou, C.; Ren, G.; Fan, X.; Lv, Y. Probing the effect of thickener microstructure on rheological and tribological properties of grease. J. Ind. Eng. Chem. 2022, 111, 51–63. [Google Scholar] [CrossRef]
- Calderon Salmeron, G.; Leckner, J.; Schwack, F.; Westbroek, R.; Glavatskih, S. Greases for electric vehicle motors: Thickener effect and energy saving potential. Tribol. Int. 2022, 167, 107400. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Y.; Li, C.; Zhou, Z.; Nie, X.; Chen, Y.; Cao, H.; Liu, B.; Zhang, N.; Said, Z.; et al. Extreme pressure and antiwear additives for lubricant: Academic insights and perspectives. Int. J. Adv. Manuf. Technol. 2022, 120, 1–27. [Google Scholar] [CrossRef]
- Vetter, W.; Sprengel, J.; Krätschmer, K. Chlorinated paraffins—A historical consideration including remarks on their complexity. Chemosphere 2022, 287, 132032. [Google Scholar] [CrossRef]
- Niu, W.; Yuan, M.; Wang, P.; Shi, Q.; Xu, H.; Dong, J. One-pot synthesis of SIB@ZIF-8 with enhanced anti-corrosion properties and excellent lubrication properties. Tribol. Int. 2020, 151, 106491. [Google Scholar] [CrossRef]
- Acharya, B.; Pardue, T.N.; Avva, K.S.; Krim, J. In situ, real time studies of thermal reaction film formation temperatures for iron and 304SS surfaces immersed in 5% tricresyl phosphate in base oil. Tribol. Int. 2018, 126, 106–115. [Google Scholar] [CrossRef]
- Mistry, K.K.; Morina, A.; Erdemir, A.; Neville, A. Tribological Performance of EP Lubricants with Phosphorus-Based Additives. Tribol. Trans. 2013, 56, 645–651. [Google Scholar] [CrossRef]
- Sato, K.; Watanabe, S.; Sasaki, S. High Friction Mechanism of ZDDP Tribofilm Based on in situ AFM Observation of Nano-Friction and Adhesion Properties. Tribol. Lett. 2022, 70, 94. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Li, C.; Zhao, G.; Wang, X. A study of 2,5-dimercapto-1,3,4-thiadiazole derivatives as multifunctional additives in water-based hydraulic fluid. Ind. Lubr. Tribol. 2014, 66, 402–410. [Google Scholar] [CrossRef]
- Guegan, J.; Southby, M.; Spikes, H. Friction Modifier Additives, Synergies and Antagonisms. Tribol. Lett. 2019, 67, 83. [Google Scholar] [CrossRef]
- Liu, S.; Yu, T.; Lich, L.V.; Yin, S.; Bui, T.Q. Size and surface effects on mechanical behavior of thin nanoplates incorporating microstructures using isogeometric analysis. Comput. Struct. 2019, 212, 173–187. [Google Scholar] [CrossRef]
- Baig, N. Two-dimensional nanomaterials: A critical review of recent progress, properties, applications, and future directions. Compos. Part A Appl. Sci. Manuf. 2023, 165, 107362. [Google Scholar] [CrossRef]
- Zhao, J.; Gao, T.; Li, Y.; He, Y.; Shi, Y. Two-dimensional (2D) graphene nanosheets as advanced lubricant additives: A critical review and prospect. Mater. Today Commun. 2021, 29, 102755. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Y.; Li, J.; Xiao, Y.; Mu, W.; Wang, Z.; Song, L.; Yu, J. Flame Retardancy of Epoxy Resins Modified with Few-Layer Black Phosphorus. Polymers 2023, 15, 1655. [Google Scholar] [CrossRef]
- Zang, C.; Yang, M.; Liu, E.; Qian, Q.; Zhao, J.; Zhen, J.; Zhang, R.; Jia, Z.; Han, W. Synthesis, characterization and tribological behaviors of hexagonal boron nitride/copper nanocomposites as lubricant additives. Tribol. Int. 2022, 165, 107312. [Google Scholar] [CrossRef]
- Yang, J.; Xu, X.; Liu, L. Plasma-assisted friction control of 2D MoS2 made by atomic layer deposition. Nanotechnology 2020, 31, 395711. [Google Scholar] [CrossRef]
- Yi, S.; Guo, Y.; Li, J.; Zhang, Y.; Zhou, A.; Luo, J. Two-dimensional molybdenum carbide (MXene) as an efficient nanoadditive for achieving superlubricity under ultrahigh pressure. Friction 2023, 11, 369–382. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y.; Liu, Y.; Zhao, J.; Li, J.; Wang, Q.; Luo, J. Tribological behavior of layered double hydroxides with various chemical compositions and morphologies as grease additives. Friction 2021, 9, 952–962. [Google Scholar] [CrossRef]
- Kang, J.; Sangwan, V.K.; Wood, J.D.; Hersam, M.C. Solution-based processing of monodisperse two-dimensional nanomaterials. Acc. Chem. Res. 2017, 50, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Ling, Z.; Wang, Z.; Zhang, M.; Yu, C.; Wang, G.; Dong, Y.; Liu, S.; Wang, Y.; Qiu, J. Sustainable synthesis and assembly of biomass-derived B/N co-doped carbon nanosheets with ultrahigh aspect ratio for high-performance supercapacitors. Adv. Funct. Mater. 2016, 26, 111–119. [Google Scholar] [CrossRef]
- He, Q.; Zeng, Z.; Yin, Z.; Li, H.; Wu, S.; Huang, X.; Zhang, H. Fabrication of flexible MoS2 thin-film transistor arrays for practical gas-sensing applications. Small 2012, 8, 2994–2999. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xie, Y.; Liu, Z.; Wang, X.; Chai, Y.; Yan, F. Two-dimensional material membranes: An emerging platform for controllable mass transport applications. Small 2014, 10, 4521–4542. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Tang, L.; Khan, U.; Yu, Q.; Cheng, H.-M.; Zou, X.; Liu, B. Morphology and surface chemistry engineering toward pH-universal catalysts for hydrogen evolution at high current density. Nat. Commun. 2019, 10, 269. [Google Scholar] [CrossRef]
- Lu, P.; Xiang, S.; Xu, S.; Chen, H.; Wang, H.; Wang, J.; Zhang, G. Tribological study on preparation of two-dimensional MoS2 as grease additive by ultrasonic liquid phase stripping. J. Mater. Eng. 2023, 51, 160–168. [Google Scholar]
- Ren, S.; Yu, Q.; Yu, X.; Rong, P.; Jiang, L.; Jiang, J. Graphene-supported metal single-atom catalysts: A concise review. Sci. China Mater. 2020, 63, 903–920. [Google Scholar] [CrossRef]
- Sun, X.; Liu, Z.; Welsher, K.; Robinson, J.T.; Goodwin, A.; Zaric, S.; Dai, H. Nano-graphene oxide for cellular imaging and drug delivery. Nano Res. 2008, 1, 203–212. [Google Scholar] [CrossRef]
- O’Neill, A.; Khan, U.; Coleman, J.N. Preparation of high concentration dispersions of exfoliated MoS2 with increased flake size. Chem. Mater. 2012, 24, 2414–2421. [Google Scholar] [CrossRef]
- Chaurasia, A.; Verma, A.; Parashar, A.; Mulik, R.S. Experimental and computational studies to analyze the effect of h-BN nanosheets on mechanical behavior of h-BN/polyethylene nanocomposites. J. Phys. Chem. C 2019, 123, 20059–20070. [Google Scholar] [CrossRef]
- Peng, L.; Zhu, Y.; Li, H.; Yu, G. Chemically integrated inorganicgraphene two-dimensional hybrid materials for flexible energy storage devices. Small 2016, 12, 6183–6199. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, J.; Li, J.; Yi, S.; Ge, X.; Zhang, X.; Luo, J. Shear-Induced Interfacial Structural Conversion Triggers Macroscale Su-perlubricity: From Black Phosphorus Nanoflakes to Phosphorus Oxide. ACS Appl. Mater. Interfaces 2021, 13, 31947–31956. [Google Scholar] [CrossRef] [PubMed]
- Hamrock, B.J.; Dowson, D.C. Isothermal Elastohydrodynamic Lubrication of Point Contacts: Part III—Fully Flooded Results. J. Lubr. Technol. 1976, 99, 264–275. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, P.; Ji, L.; Liu, X.; Wan, H.; Chen, L.; Li, H.; Jin, Z. Tribological properties of MoS2 coating for ultra-long wear-life and low coefficient of friction combined with additive g-C3N4 in air. Friction 2021, 9, 789–801. [Google Scholar] [CrossRef]
- Han, Y.; Qiao, D.; Guo, Y.; Feng, D.; Shi, L. Influence of Competitive Adsorption on Lubricating Property of Phosphonate Ionic Liquid Additives in PEG. Tribol. Lett. 2016, 64, 22. [Google Scholar] [CrossRef]
- Lu, Z.; Cao, Z.; Hu, E.; Hu, K.; Hu, X. Preparation and tribological properties of WS2 and WS2/TiO2 nanoparticles. Tribol. Int. 2019, 130, 308–316. [Google Scholar] [CrossRef]
- Rabaso, P.; Dassenoy, F.; Ville, F.; Diaby, M.; Vacher, B.; Le Mogne, T.; Belin, M.; Cavoret, J. An Investigation on the Reduced Ability of IF-MoS2 Nanoparticles to Reduce Friction and Wear in the Presence of Dispersants. Tribol. Lett. 2014, 55, 503–516. [Google Scholar] [CrossRef]
- Geng, S.; Yang, W.; Liu, Y.; Yu, Y. Engineering sulfur vacancies in basal plane of MoS2 for enhanced hydrogen evolution reaction. J. Catal. 2020, 391, 91–97. [Google Scholar] [CrossRef]
- Hu, H.; He, Y.; Wang, Q.; Tao, L. In-situ research on formation mechanisms of transfer films of a Polyimide-MoS2 composite in vacuum. Tribol. Int. 2023, 180, 108211. [Google Scholar] [CrossRef]
- Wei, X.; Li, W.; Fan, X.; Zhu, M. MoS2-functionalized attapulgite hybrid toward high-performance thickener of lubricating grease. Tribol. Int. 2023, 179, 108135. [Google Scholar] [CrossRef]

| Test Description | Result | Method |
|---|---|---|
| Kinematic viscosity (mm2/s) (40 °C) | 490.7 | ASTM D445 |
| Kinematic viscosity (mm2/s) (100 °C) | 31.75 | ASTM D445 |
| Viscosity Index | 95 | ASTM D2270 |
| Appearance | Clear to bright | Visual |
| Colour (ASTM) (Quantitative) | L2.0 | ASTM D1500 |
| Density (kg/m3) (15 °C) | 0.9012 | ASTM D4052 |
| Density (kg/m3) (30 °C) | 0.8917 | ASTM D4052 |
| Refractive index (20 °C) | 1.46 | ASTM D1218 |
| Pour point (°C) | −6 | ASTM D5950 |
| Flash point (°C) (PMcc) | 316 | ASTM D92 |
| Specific Gravity (60/60 °F) | 0.9017 | ASTM D4052 |
| Total acid number (mgKOH/g) | 0.01 | ASTM D664 |
| Cartxon residue (micro method) (wt.%) | 0.41 | ASTM D4530 |
| Sulphur content (wt.%) | 0.536 | ASTM D4294 |
| Water content (vol.%) | Nill | ASTM D95 |
| Parameter | BG-0 | BG-0.3 | BG-0.6 | BG-0.9 | BG-1.2 | BG-1.5 | BG-1.8 | BG-2.1 |
|---|---|---|---|---|---|---|---|---|
| Speed | 1200 rpm | |||||||
| Load | 392 N | |||||||
| Temerature | 75 °C | |||||||
| Test Duration | 60 min | |||||||
| Component | Elastic modulus (MPa) | Poisson ratio | Diameter | Rockwell harness (HR) | Surface roughness (Ra) | |||
| GCr15 | 2.085 × 105 | 0.3 | 12.7 mm | 60 ± 1 | 0.256 µm | |||
| Parameter | BG-0 | BG-0.3 | BG-0.6 | BG-0.9 | BG-1.2 | BG-1.5 | BG-1.8 | BG-2.1 | Method |
|---|---|---|---|---|---|---|---|---|---|
| Penetration/0.1 mm | 285 | 244 | 261 | 292 | 297 | 301 | 306 | 310 | GB/T 269 |
| Dropping point | 278 | 292 | 290 | 285 | 286 | 287 | 287 | 291 | GB/T 3498 |
| Oil separation | 0.93 | 0.54 | 1.46 | 1.22 | 0.92 | 0.91 | 1.42 | 1.64 | NB/SH/T 0324 |
| Evaporation loss | 0.20 | 0.41 | 0.47 | 0.40 | 0.39 | 0.43 | 0.45 | 0.52 | SH/T 0661 |
| Copper corrosion | 1a | 1a | 1a | 1a | 1a | 1a | 1a | 1a | GB 7326 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, S.; Xiang, S.; Yang, X.; Yang, X.; Bao, H.; He, H.; Du, X.; Zhang, Q.; Zhang, J.; Ma, K.; et al. Lubrication-Enhanced Mechanisms of Bentonite Grease Using 2D MoS2 with Narrow Lateral Size and Thickness Distributions. Lubricants 2024, 12, 447. https://doi.org/10.3390/lubricants12120447
Zhu S, Xiang S, Yang X, Yang X, Bao H, He H, Du X, Zhang Q, Zhang J, Ma K, et al. Lubrication-Enhanced Mechanisms of Bentonite Grease Using 2D MoS2 with Narrow Lateral Size and Thickness Distributions. Lubricants. 2024; 12(12):447. https://doi.org/10.3390/lubricants12120447
Chicago/Turabian StyleZhu, Shaoyicheng, Shuo Xiang, Xue Yang, Xin Yang, Hebin Bao, Hao He, Xin Du, Qinhui Zhang, Junjie Zhang, Kai Ma, and et al. 2024. "Lubrication-Enhanced Mechanisms of Bentonite Grease Using 2D MoS2 with Narrow Lateral Size and Thickness Distributions" Lubricants 12, no. 12: 447. https://doi.org/10.3390/lubricants12120447
APA StyleZhu, S., Xiang, S., Yang, X., Yang, X., Bao, H., He, H., Du, X., Zhang, Q., Zhang, J., Ma, K., Cao, Y., Liu, Y., Peng, L., Li, Z., & Fan, Y. (2024). Lubrication-Enhanced Mechanisms of Bentonite Grease Using 2D MoS2 with Narrow Lateral Size and Thickness Distributions. Lubricants, 12(12), 447. https://doi.org/10.3390/lubricants12120447





