Abstract
Vegetable oil (VO)-based lubricants are environmentally friendly replacements for mineral oils. This work critically reviews the literature and identifies the molecular structures in VO-based lubricants which have been used to improve performance. The specific roles that size, type, number, position, spatial arrangement, and symmetry play in determining lubricating functionality were highlighted. Data were systematically collected to identify the contributions of major structural components and relate them to specific physical functionality measurables. The relationships were presented to reveal structure–function trends. Empirical predictive relationships between flow and thermal transition properties and structures were established. Molecular mass was revealed to be a fundamental determinant of viscosity and transition temperatures, but these properties were shown to also be influenced by other structural factors such as polar functional groups, branching, and symmetry. Almost all the examined viscosity data plotted versus molecular mass are enclosed within the 95% prediction band of an exponential rise to a maximum function (R2 = 0.7897). Generally, for both flow and thermal transition, a given structure versus function follows simple linear or exponential functions with unbranched VO-based lubricants, lending themselves more easily to strong correlations. This review is a first step towards comprehensively relating structure to lubrication function. The revealed relationships of structural contributions to the lubricating functionality of VO-based lubricants provide insights that may be used to extend the ranges of chemical and physical properties of some molecular architectures examined.
1. Introduction
Vegetable oils (VOs) provide significant prospects for sustainable biomaterial production, including lubricants. VOs as a renewable feedstock for lubricants, however, also present barriers and challenges ranging from ethical concerns regarding the use of edible crops in the production of industrial materials, to environmental impacts of the synthetic routes used, economic viability, and consumer acceptance. These challenges are progressively addressed by a large and growing number of high-quality research communications and several successful VO-based products are already in use, albeit with limited market share. This review seeks to utilize this body of literature to extract structure–function relationships, with attention paid to differences under which the various derivatives have been evaluated in terms of lubricating properties.
1.1. Methodology and Layout of the Review
Although the effects of chemical structure on VO-based lubricant properties have been extensively studied [1] a systematic approach that would lead to predictive relationships is lacking. This work critically reviews the literature on VO-based lubricants from a perspective of structure–function relationships, focusing on flow and thermal transition behaviors. It identifies the contributions of the major structural components and relates them to function: it is, therefore, the first step towards relating structure to lubrication function, but by no means does it address the need for a fully comprehensive analysis of the relationships between structure and function of VO-based lubricants.
The data were organized to reveal structure–function trends; more specifically, roles of size, type, number, position, spatial arrangement, and symmetry on flow and phase transformation properties. Predictive empirical structure–property correlations were extracted from the literature data by interrogating separate studies under similar conditions. The change in Gibbs free energy at the solid–liquid transition (Equation (2)) was used as a theoretical basis to explain and predict the trends that structural elements impose on the enthalpy and entropy of lubricant molecules, which ultimately impact lubricating functionality. It was used as a guide to understanding the contribution of specific structural elements to the thermal transition parameters of lubricants given a particular molecular architecture.
This section (Section 1) introduces VO-based lubricants. Section 1.2 presents the categories and the physicochemical characteristics of lubricants generally and Section 1.3 those of biobased lubricants. The advantages and disadvantages of biobased lubricants are discussed in Section 1.4. The major constituent structures of biobased lubricants and their molecular arrangement which dictate their overall effect and contribution to the physicochemical properties are described in Section 1.5. Gibbs free energy is introduced in Section 1.6 as a guide to interrogate structural contributions to phase change.
Section 2 discusses VO and derivatives as feedstock to synthesize lubricants. Section 2 presents the VO chemical structure and Section 2.1 outlines the structural features of triacylglycerols (TAGs). Section 2.2 summarizes the chemical routes which have been used to prepare VO-based lubricants and lists the most common ester lubricant categories. The salient properties of existing commercial VO-based lubricants are tabulated in Section 3.
Section 4 discusses the role of structure in the rheological behavior of VO-based lubricants. The power index from the Herschel–Bulkley function is used in Section 4.1 to analyze the shear rate versus shear stress data of VO-based lubricants and to determine relationships between flow type and structure. The power index was expressed in terms of molecular mass, branching, saturation, and functional groups, discussed and modeled when clear and predictive trends are observed. Viscosity data of VO-based lubricants are analyzed and discussed in Section 4.2. When possible, the intricate effects of mass, double bonds, branching, and functional groups on viscosity were separated and modeled (Section 4.2.1, Section 4.2.2, Section 4.2.3 and Section 4.2.4).
The role of structure in the thermal transition behavior of VO-based lubricants is discussed in Section 5. The melting point/pour point was particularly examined to elucidate the roles of mass/chain length, number, configuration, and position of double bonds, branching, and functional groups (Section 5.1, Section 5.2, Section 5.3 and Section 5.4). The data were modeled with appropriate functions when they lent themselves to clear interpretation. Challenges faced by research on VO-based lubricants and perspectives for biobased lubricants are outlined in Section 6. A conclusion is provided in Section 7.
1.2. Lubricants Categories and Their Physicochemical Characteristics
1.2.1. Lubricants Categories
Lubricants are materials used in various industrial processes to essentially control friction, clean the points of contact and dissipate heat [2]. In these functions, lubricants increase operational efficiency and material service life [3]. The global lubricant market is considerable. The global volume demand for lubricants which was 37 million tons in 2020 [4] is expected to grow to 38.1 million tons by 2028 [5]. The global market value is projected to grow from USD 117.31 billion in 2022 to USD 131.33 billion by 2029 [6].
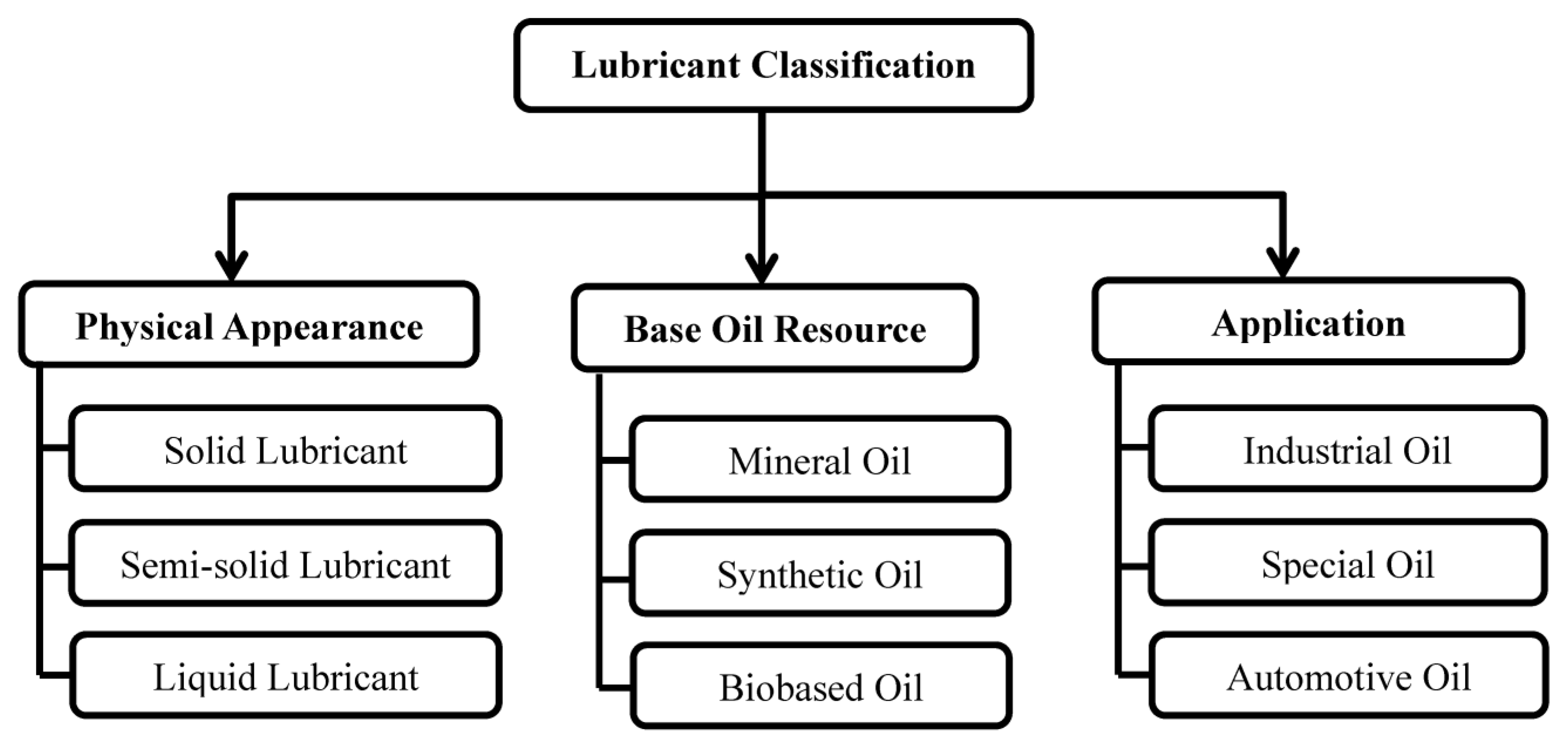
A typical lubricant consists of 70–90% base-stock components and various additives [7]. The selection of lubricants is based on their suitability to fulfill the lubrication specifications and requirements of particular applications [6]. The properties of a base oil determine the most important functions of lubricants. Additives are formulated to improve the properties of the base stocks [8]. Lubricants can be categorized by (i) physical appearance (ii) nature of the base oil and (iii) application. Figure 1 presents the classification of lubricants as established in the literature [9]. The source, manufacture, and main components of mineral, synthetic, and biobased base fluids [10,11] are summarized in Figure 1. Classification of lubricants by physical appearance, base oil resource, and application. The manufacture and main components of the mineral, synthetic and biobased base fluids [10,11] are summarized in Table 1.
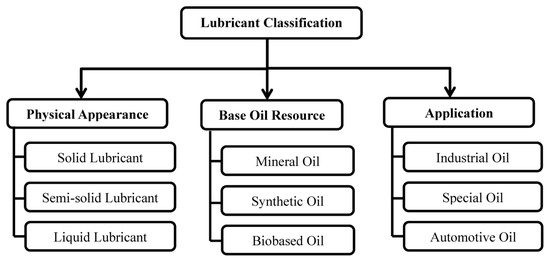
Figure 1.
Classification of lubricants by physical appearance, base oil resource, and application.

Table 1.
Base stocks for lubricant oils.
Mineral oil lubricants currently dominate the market, with approximately 74% of the market share. The automotive and transportation industry consumes 57% of the lubricant volume, the majority of which are mineral and synthetic oils [10]. Lubricant suppliers are well-established with Royal Dutch Shell, Exxon Mobil, Chevron Corporation, Total, and British Petroleum being the top five [18].
1.2.2. Physicochemical Characteristics of Lubricants
Standardized methods, e.g., ASTM International, (formerly known as the American Society for Testing and Materials), ISO: International Organization for Standardization, EN: Europäische Norm (European Standards) and DIN: Deutsches Institut für Normung (German Institute for Standardization) tests are typically used to determine the properties of lubricants [19]. ASTM International remains the most popular source for evaluation methods of lubricants [18]. The physicochemical characteristics of lubricants are generally divided into categories [20] as listed in Table 2. The sought-after physical and chemical properties of biobased lubricant relate to performance [5,21,22]. The key parameters used to characterize lubricants and their relevance to performance are shown in Table 3. These physicochemical properties, along with biodegradability and renewability are essential for lubricant development and selection.

Table 2.
Categories of physicochemical characteristics of lubricants.

Table 3.
Key parameters used to characterize lubricants and their relevance.
1.3. Biobased Lubricants
Mineral and synthetic oils are predominantly derived from petroleum and come with the known problems associated with fossil fuels such as lack of renewability and sustainability and contribution to climate change. They are also associated with added environmental harms due to total-loss applications and spillages which impact soil, water, and the atmosphere [23,24]. However, depending on the biodegradability of renewably sourced lubricants, these are not unique to fossil fuel-derived lubricants. This problem is particularly acute as it is estimated that about half of the total production of lubricants worldwide accumulates in the environment [25]. Consequently, the development of non-toxic, renewable, and biodegradable alternatives to petroleum-based lubricants, loosely termed “biobased lubricants” has become a high priority. The demand for biolubricants is encouraged by growing environmental awareness, the adoption of stringent government regulations governing petroleum-based lubricants, tougher requirements for renewability, and increasing consumer acceptance [6]. Current research in this area is aimed at extending the limits of renewable organic lubricants in terms of chemical and physical properties, particularly those directly relevant to lubrication [26].
To be considered environmentally friendly, lubricants require strict criteria, particularly regarding biodegradability, renewability, and toxicity. The term ‘biobased lubricant’ applies to all lubricants which are both rapidly biodegradable and non-toxic to humans and aquatic environments. Biobased lubricants are gaining commercial attention encouraged by continued advances in performance and economics. However, the use of biobased lubricants is still limited compared to mineral oil-based lubricants. The global biobased lubricants market is projected to grow from USD 3.1 billion in 2022 to USD 4.3 billion by 2029 [16]. Most commercial biobased lubricant base oils are esters—either mixtures of natural esters or synthetic esters derived from fatty acids or a mixture of both [5,27]. Biobased lubricants are developed and marketed by several major oil companies including British Petroleum, Chevron, Exxon Mobil, and Gulf, and are used in a wide variety of applications [28]. The transition to environmentally acceptable materials from renewable resources requires the involvement of several players in the value chain including milling, derivatization, and transformation facilities, the manufacturing industry, regulators, and end users.
A wide range of properties for biobased lubricants is essential for their potential substitution in many applications.
1.4. Advantages and Disadvantages of Biobased Lubricants
Mineral oils are sourced from fossil fuel and hence come with inherent problems including low biodegradation rates, a high potential for bioaccumulation, and significant toxicity. The disposal of mineral oils can pollute aquatic and terrestrial ecosystems. The trace metals emitted by internal combustion engines such as phosphorous, zinc, calcium, magnesium, and iron are derived mainly from the combustion of the lubrication oil [29]. In contrast, the lubricants derived from oleochemicals generally degrade faster, have a smaller residual footprint, do not bioaccumulate appreciably, and have lower toxicities. They also have lubrication advantages over mineral oil such as low volatility, better lubricity, winder viscosity ranges and higher viscosity index, flash point, and higher solubilizing capacity for additives [30,31]. On the contrary, biobased lubricant molecules also have inherent weaknesses, including susceptibility to chemical reactions, particularly in ester and alkene groups, crystallize at sub-zero temperatures, have susceptibility to thermo-oxidative degradation particularly at temperatures > 250 °C, and are more expensive compared to mineral oil [32]. A list of the environmental, performance, properties, and economic advantages and disadvantages of VOs and VO-based lubricants [14,31,33,34,35,36,37,38] is provided in Table 4.

Table 4.
Advantages and disadvantages of biobased lubricants. Viscosity Index (VI); Flash Point (FP); Pour Point (PP); Boiling Point (BP); Vegetable Oil (VO).
To achieve optimal performance and even perform better than conventional lubricants in various lubrication applications, chemical modification of the feedstock and base oils is required to overcome their inherent limitations such as poor or inadequate flow characteristics and poor thermal and oxidative stabilities. The performance is typically adjusted through formulation with additive packages.
1.5. Main Molecular Structures That Contribute to Lubricant Properties
The common structures of current VO-based lubricants are wax ester mimics, linear long aliphatic chains, polar functional groups, and double bonds [21]. The constituent structures and their arrangement in the molecule dictate the lubricant’s physicochemical properties. The most important structural elements affecting the properties of VO-based lubricants are [39]:
- Molecular mass and carbon chain length.
- Level of unsaturation refers to the number of unsaturation sites (cis C=C double bonds) in the molecule.
- Degree of branching refers to the number of pendent structures attached at the unsaturation sites of the molecule’s backbone.
- The presence of functional groups such as ester linkages, carboxyl groups, and hydroxyl groups.
The common structural design elements affecting the properties of VO-based lubricants include:
- Molecular symmetry.
- Isomerism.
- Odd–even effect.
The molecular symmetry can be expressed with the rotational symmetry number defined as the number of identical images that can be produced by rotation of the molecule within 360° in any direction.
Isomers are compounds that have the same molecular formula but different molecular structures. There are two general classes of isomers: (i) structural isomers and (ii) stereoisomers. The atoms of structural isomers are connected differently and differ in their bonding sequence. The number of structural isomers increases as a function of carbon numbers. Stereoisomers are isomers whose atoms are bonded together in the same sequence but differ from each other in their orientation in space [40].
The even and odd fatty chains present subtle differences in packing arrangement. The angle between the terminal carbon-carbon bonds is 180° for the even carbon chains and 109° for the odd carbon chains which results in optimal intermolecular contacts at both ends for the even fatty chain and at only one end for the odd fatty chains.
Table 5 provides a summary of the overall effect and contribution of the structural elements to key biobased lubricants properties. The contribution is classified as advantageous or disadvantageous depending on desired property outcome [6,21]. Table 5 shows that a given structure may provide conflicting advantageous and disadvantageous contributions. These are typically reconciled depending on the delivered desired property outcome.

Table 5.
Key structural elements and their influence on lubricant properties. Flash Point (FP); Cloud Point (CP); Pour Point (PP); Thermal Stability (TS); Oxidative Stability (OS); Viscosity Index (VI).
Information regarding the molecular structure and resulting physicochemical properties is key in lubricant formulation. The building blocks of potentially high-performance biobased lubricants and their arrangement in specific molecular architectures are designed through precise modification techniques. The fundamental knowledge of all these aspects is important to designing functional biobased oils and lubricants.
1.6. The Thermodynamics of Phase Change: Gibbs Free Energy as a Guide to Interrogate Structural Contributions to Phase Change
The expression for the change in Gibbs free energy at the solid–liquid transition (; Equation (1)) can be used as a theoretical construct to interrogate the influence of various structural elements on phase behavior. Phase behavior has a profound impact on lubricating functionality. Lubricants which undergo a glass transition or a phase transition in a temperature range over which the lubricant is expected to perform are not suitable. When the transition temperature (melting point ) is reached, the solid and liquid phases exist in thermal equilibrium, the Gibb free energy of the solid and liquid phases become equal and the following equation can be written for [47]:
and are the enthalpy and entropy of melting, respectively. is primarily related to the magnitude of the attractive intermolecular forces. is the change in molecular disorder and depends on molecular configuration [43].
The rearrangement of Equation (1) provides an expression for the entropy of transition in terms of the enthalpy and solid–liquid phase transition temperature:
Equation (2) provides a pathway to link the solid–liquid phase transition to the molecular structure via and [48]. is determined by the ratio of factors linked to the overall intermolecular interactions to factors linked to the size, geometry, and relative position of the structural elements in the molecule.
The change in a system’s enthalpy over a phase transition at constant temperature under constant pressure (P) is given by the increase in the internal energy and work of expansion which is relatively small. Enthalpy forces are equal to the sum of all the pairwise intermolecular attractions of the atoms and groups that comprise the molecule.
is largely dependent upon molecular geometry and can be evaluated from the molecular disorder introduced by molecular flexibility and spatial configuration. Entropy relates to the microstates which describe the number of different possible arrangements of molecular position and therefore measures the degree of randomness in a system. The Boltzmann equation (Equation (3)) gives the entropy as a function of the number of the different microstates available for a system in each configuration:
is the number of microstates compatible with the macroscopic parameters at a particular thermodynamic state and is the Boltzmann constant (1.38 × 10−23 J/K).
Enthalpy is an additive constitutive property and is determined by the intermolecular attractions that hold the molecules in their crystal lattices, whereas entropy favors the breakup of the crystal lattice. The contributions of the different structures of a lubricant molecule to the enthalpy and entropy of the solid–liquid transformation are interlinked in a complex manner. In the presence of polar groups or cyclic structures which can control the flexibility of nearby fatty chains, the molecules may achieve an optimal packing and high crystal order which contributes to high melting temperature despite geometry constraints [6,49]. In other ester molecules, including those with pendant groups, the structural factors that increase the entropy generally contribute to lower the enthalpy, which decreases . As a first approach to understanding the complex melting process, the enthalpy is normally taken as the primary determinant of the melting point as discussed in Yalkowsky et al. [50,51].
2. Vegetable Oil as an Alternative Feedstock for Biobased Lubricants
Like for fuel, chemicals, phase change material, and other biomaterials, VOs are sought-after feedstocks for the manufacture of lubricants [52]. VOs are typically biodegradable, non-toxic, and renewable. Most VOs are naturally composed of 88–96% triacylglycerols (TAGs) with some minor amounts of diacylglycerols (DAGs, usually <5%). monoacylglycerols (MAGs, <0.2%) and free fatty acids (FAs, <0.1%). Other minor components are tocopherols and tocotrienols and phytosterols (<0.1%) [50,53]. The oil from jojoba seeds is a molecularly unique vegetable oil that is comprised predominantly of long-chain unsaturated monoesters with total chain lengths between 40 and 42 carbons [54,55]. Compared to TAG oils, jojoba oil, and natural wax esters have better lubricant properties and possess an exceptionally higher thermal stability and viscosity index [56,57] but are not used in large-scale commercialization because of cost and availability [58].
VOs which are extracted from edible crops, such as coconut, corn, cottonseed, palm, canola, soybean, olive, peanut, safflower, sesame, and sunflower are currently the most researched feedstock to produce VO-based lubricants [16,59,60]. Wax esters analogs such as jojoba-like esters (JLEs) have been produced from commonly available and economical oleochemical feedstocks, such as oleic acid and erucic acid, and fatty alcohols using chemical or biotechnological means [42,58,61,62,63,64,65,66,67,68,69]. Environmentally acceptable strategies such as those based on lipases have shown potential at the laboratory scale for bulk production of wax esters [67,70,71]. These technologies are increasingly commercialized for the manufacture of pharmaceuticals and cosmetic ingredients [68]. Further work is needed in enzyme and reaction engineering to achieve efficient and cost-effective manufacturing of VO-based lubricants [66]. At present, because enzymatic technology is generally not economically feasible at the industrial scale, the mass production of wax esters relies on expensive and environmentally harmful processes such as those using chemically synthesized fatty alcohols [66,72].
Pure VOs comprise a relatively small fraction of the biobased lubricant market. A well-performing VO-based lubricant would ideally be produced by exploiting inherent attributes and potential synergies of the VO structures (fatty acids, unsaturation sites, ester functional groups) with the incorporation of select polar functional groups through chemical modification [71,73,74,75,76,77]. However, developing performing VO-based lubricants is not trivial, as the aforementioned structural elements often present conflicting contributions, and the known synergies are complex. This makes the design of biobased lubricant systems that use vegetable oil-derived esters more complex than hydrocarbon biobased lubricants.
2.1. TAG Structural Features
TAGs in their unmodified state have advantages and disadvantages for use in lubricant formulations. Their structures such as the ester group, fatty acids, and double bonds, however, allow for versatile chemical transformations which result in a very wide range of properties that meet lubricants requirements.
2.1.1. TAG Structural Features
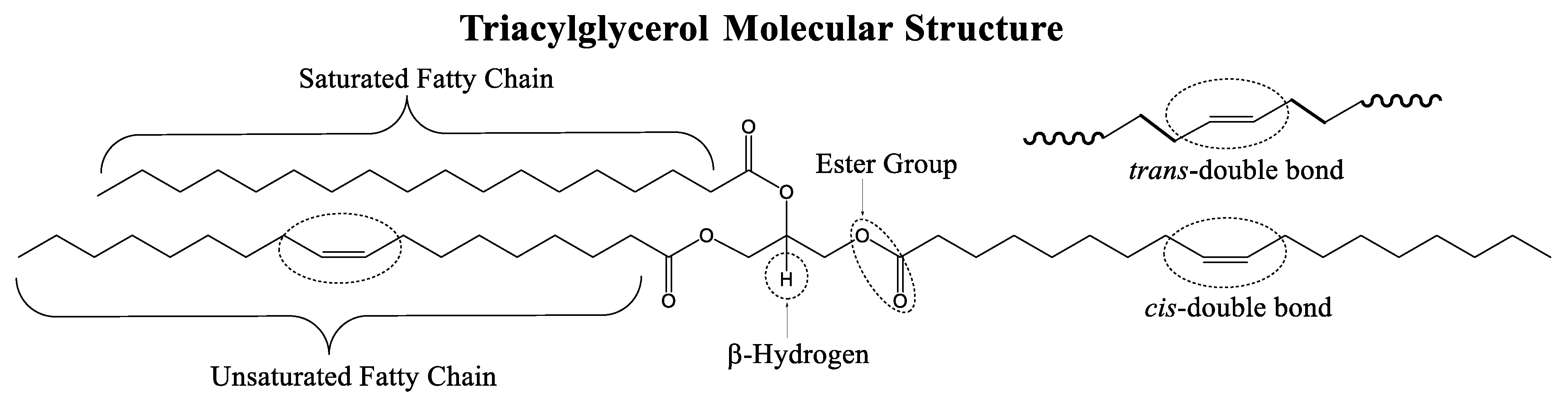
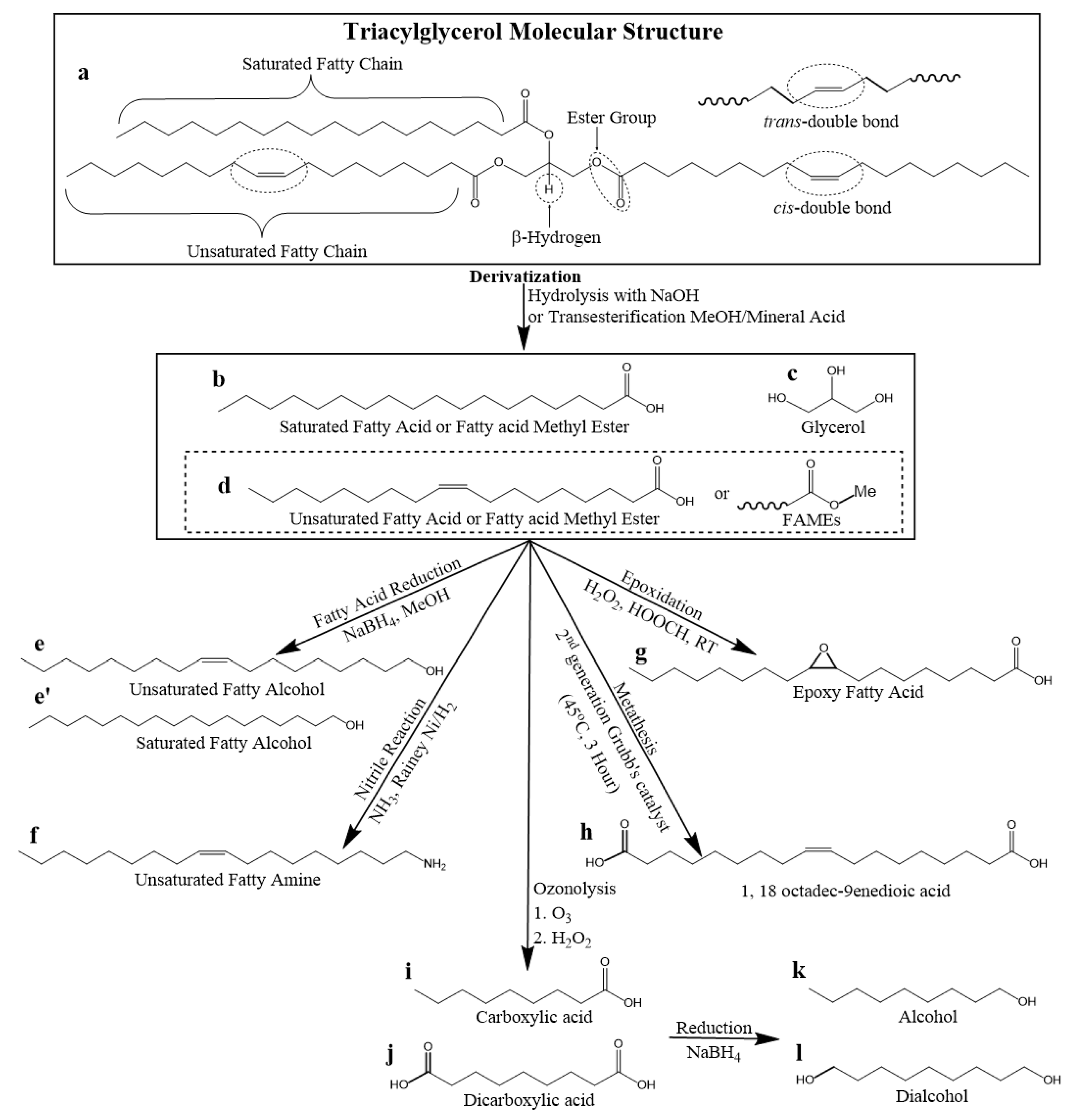
A TAG is a type of lipid molecule composed of three fatty acids esterified to a glycerol molecule. Each fatty acid can be the same or different and the length and degree of unsaturation of the fatty acid chain can vary. Scheme 1 presents the structural features in the TAG molecule, namely: (i) linear saturated and unsaturated fatty chains, (ii) cis/trans alkene functional group, (iii) ester functional group, and (iv) β-hydrogen.
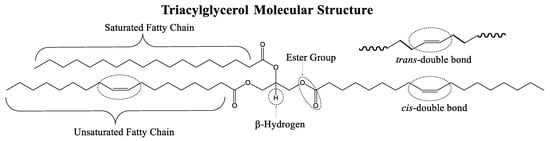
Scheme 1.
General triacylglycerol (TAG) molecular structure. Reaction sites are indicated surrounded by encircled dotted lines.
Fatty Chain
There are over 1000 known fatty acids with different chain lengths, configurations, and levels of unsaturation of which only about 20 widely occur in nature [21]. Fatty acids in TAG oils typically range from 8 to 24 carbon atoms and comprise 0 to 7 carbon-carbon double bonds, depending on the plant type and growth conditions [78]. The most common naturally occurring saturated fatty acids are capric, lauric, myristic, palmitic, and stearic acids, whereas the common unsaturated fatty acids are oleic, linoleic, linolenic acid, and arachidonic acids [79].
Linear saturated fatty chains (Scheme 1) are composed of sp3 hybridized C−C single bonds with tetrahedral molecular geometry and a bond angle of 109.5°. Linear saturated fatty chains in lubricant molecules can contribute to higher resistance to oxidation and thermal degradation which can improve the longevity and effectiveness of the lubricant. Additionally, linear saturated chains make lubricant molecules more rigid and less susceptible to shearing force which helps maintain the stability of the lubricating film.
Unsaturation Site
The C=C double bonds in unsaturated fatty chains are sp2 hybridized with trigonal planar and bond angle of 120°. The C=C double bond is connected by a strong σ-bond and a weaker π-bond. The π-bond is responsible for the characteristic chemical reactivity of the C=C bond such as the ability to undergo functionalized/derivatized and thermo-oxidative degradation [80]. Additionally, the C=C double bonds can exist in a cis or trans isomeric configuration in the fatty chain (Scheme 1). The cis configuration is kinked and allows the molecules to adopt a bent shape to give fluid structure whereas the trans configuration gives a more linear and rigid chain.
Ester Functional Group
Each fatty acid is attached to the TAG glycerol backbone via an ester linkage (Scheme 2). The ester group is composed of a carbonyl (C=O) group and an alkoxy (C–O–C) group attached to the carbonyl carbon. The ester group is flat trigonal planar with a bond angle of 120° because of the carbonyl carbon sp2 hybridized orbitals. The ester group alkoxy moiety has a low rotational energy barrier which makes the bond easily rotatable thus providing increased flexibility to the molecule [67,81]. Additionally, ester groups have low polarity and cannot form strong intermolecular hydrogen bonding interactions due to the absence of positively charged hydrogen dipoles which means that TAG-TAG interactions are mostly through van der Waals forces [43].
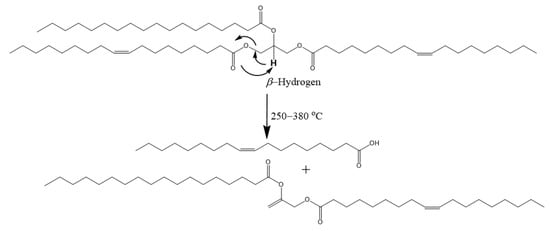
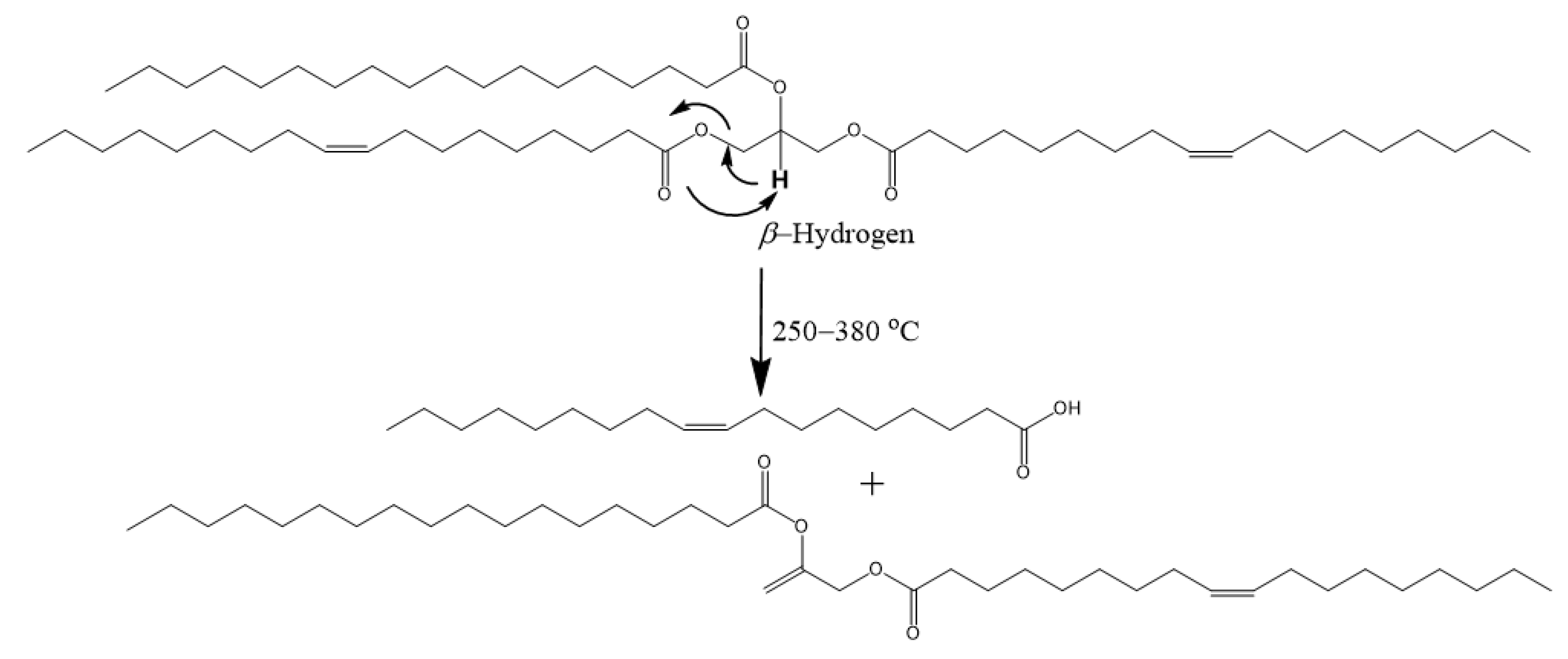
Scheme 2.
Thermal degradation mechanism of a TAG via the cis−elimination of β-hydrogen.
β-Hydrogen
C=C double bonds and β-hydrogens are undesirable in lubricants because of poor thermo-oxidative stability [82]. TAGs can be thermally degraded at elevated temperatures via the β-hydrogen elimination mechanism (Scheme 2) [83,84]. β-hydrogen elimination occurs at lower temperatures (~250–380 °C) and is detrimental to the TAG [82]. The β-hydrogen is acidic and cleaves as a hydrogen ion. The acidity of β-hydrogen (pKa~10) is relatively lower compared to hydrogens on the ester group (pKa~20) of linear esters and those attached to the fatty chains (pKa~50) [85]. The β-hydrogen is particularly undesirable because it degrades the TAG molecule when abstracted.
Among the oleochemical reactions of significance to manufacture lubricant base fluids from TAGs and their derivatives, the modifications of the carboxyl group of the fatty acids are preferred and account for about 90% of the oleochemical reactions [34]. The reactions across the C=C double bond, such as hydrogenation, epoxidation, metathesis, and ozonolysis, only account for less than 10% [86].
2.2. Synthetic Pathways to Modify TAG
The full potential of VO-based lubricants can be achieved by various chemical modification techniques. Chemical modification has the potential to enhance the desirable properties and mitigate the unwanted properties over a wide range of temperatures. The modification of a TAG molecule involves different processes, loosely classified as functionalization and derivatization.
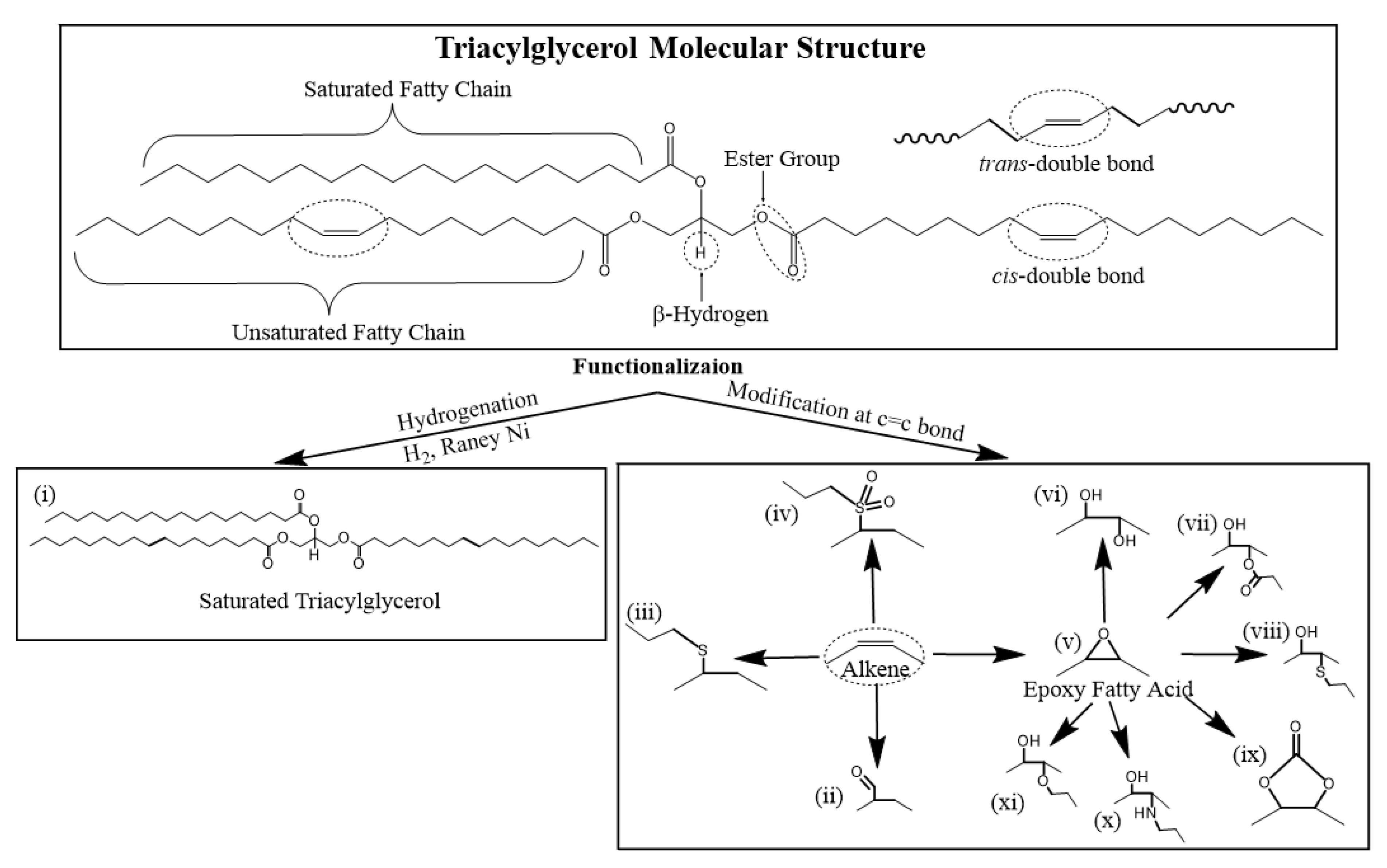
2.2.1. TAG Functionalization
Functionalization refers to the process of introducing new functional groups directly onto a TAG molecule without changing its original molecular structure. Functional groups are predominantly attached via the C=C double bond in the TAG molecule (Scheme 3). Synthetic pathways used to introduce new functional groups at the C=C double bond on a TAG include (i) hydrogenation, (ii) hydroformylation, (iii) thioetherification, (iv) sulfide oxidation, (v) epoxidation followed by ring opening with (vi) water, (vii) carboxylic acid, (viii) thiol, (ix) CO2, (x) amine and (xi) alcohol nucleophiles (Scheme 3).
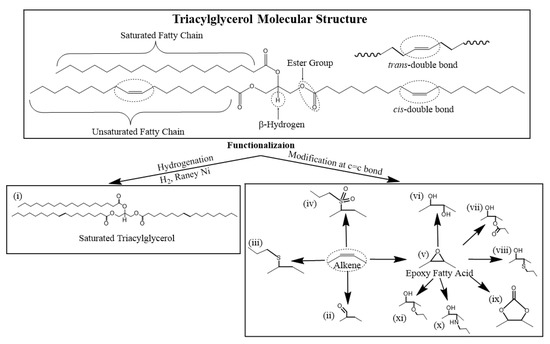
Scheme 3.
Common synthetic pathways to functionalize TAG molecules for use in lubricants.
Hydrogenation
Hydrogenation of TAGs is a chemical process that involves the addition of hydrogen to unsaturated sites of fatty chains to obtain fully saturated TAGs (See Scheme 3i). Hydrogenation is conducted at high-pressure and -temperature conditions using a metal catalyst. Typically, the reaction is carried out at temperatures between 250 and 300 °C and pressure between 25 and 35 MPa. Commonly used catalysts for hydrogenation are Raney nickel (Ni) or palladium (Pd) [87]. Partial hydrogenation can also be conducted to obtain partially saturated TAGs. The degree of hydrogenation can be controlled by restricting the amount of hydrogen, reaction temperature, time, and catalyst [88].
Hydroformylation
Hydroformylation consists of adding a formyl group over a C=C double bond in TAG and unsaturated fatty acids (Scheme 3ii). Hydroformylation is carried out by the reaction of C=C double bonds with carbon monoxide and hydrogen gas (CO/H2) using a cobalt carbonyl complex catalyst under high temperature (150–200 °C) and pressure (5–30 MPa) [89,90].
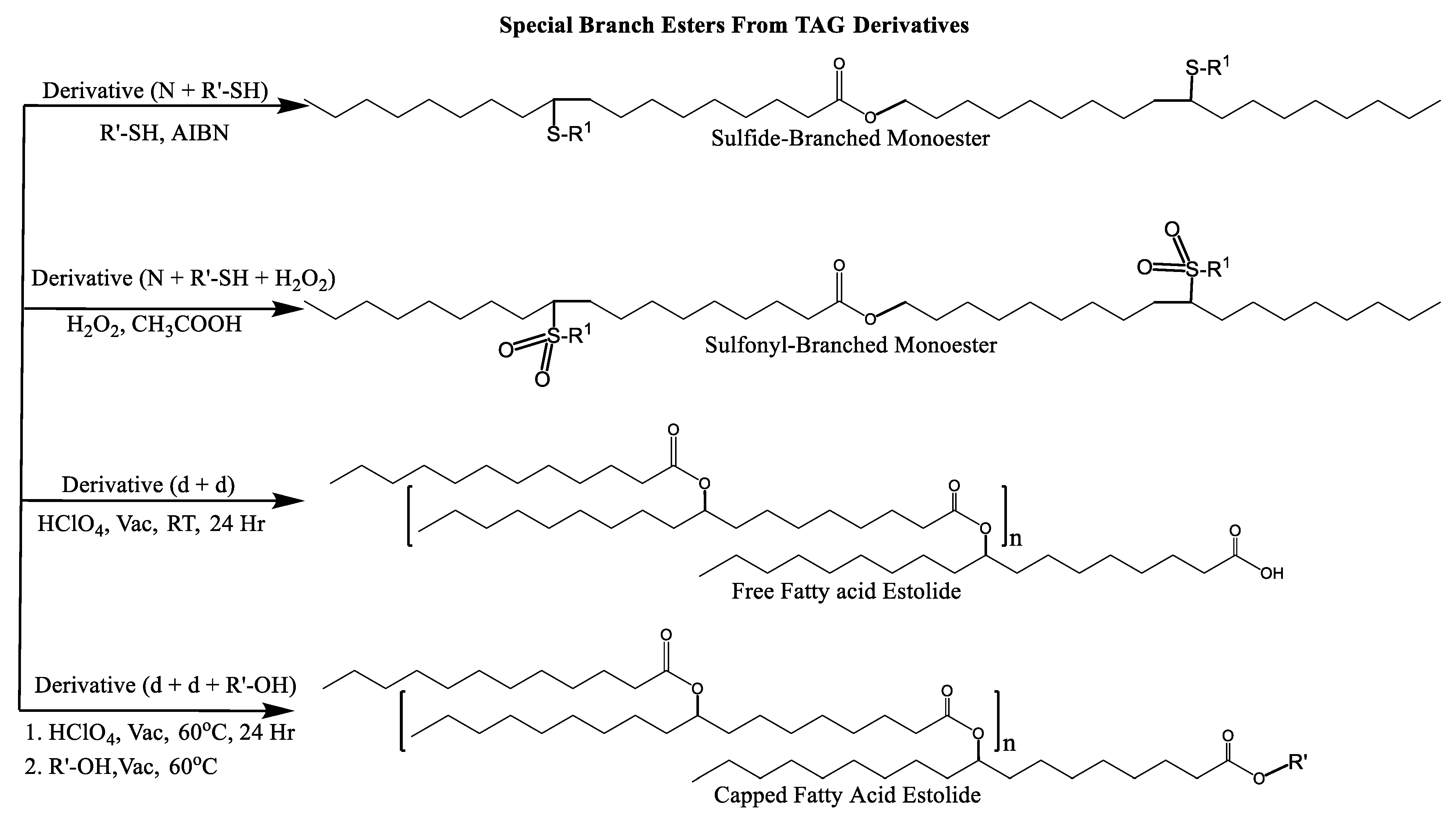
Thioetherification/Oxidation
Thioetherification can be used to introduce sulfide groups across a C=C double bond at unsaturated sites on TAGs and fatty acids (Scheme 3iii) [91,92,93,94,95,96,97]. It proceeds through free-radical additions and catalyzed Michael additions mechanisms. Sulfide functional group can be oxidized to sulfonyl functional through the oxidation with H2O2 and CH3COOH (Scheme 3iv).
Prilezhaev Epoxidation
Prilezhaev epoxidation is the common method used for selective functionalization of C=C double bond in TAG and unsaturated fatty acid to produce an epoxide functional group (Scheme 3v) via the addition of an electrophilic reagent [89]. Typically, Prilezhaev epoxidation is performed using organic peroxy acids which are formed in-situ by reaction with carboxylic acid (CH3COOH or HCOOH) and hydrogen peroxide (H2O2) in the presence of a strong mineral acid catalyst (HCl, H2SO4, HNO3) [86]. The optimization of epoxide formation can be achieved by tuning reactant mole time, ratio, temperature, solvent, catalyst (mineral acid and ion exchange resins), stirring speed, type of peroxy acid (peracetic, performic, m-chloroperbenzoic acid), rate of addition of H2O2/acetic acid and contacting patterns (batch/semi-batch mode/azeotropic distillation) [98,99].
Epoxide Ring Opening with Different Nucleophiles
The epoxide ring-opening reaction involves the cleavage of an epoxide ring by a nucleophilic attack at one of the ring carbons. The reaction is typically carried out under acid conditions in the presence of a nucleophile, such as carboxylic acid, alcohol, water, amine, and thiol. The ring-opening of epoxide with common nucleophiles, namely carboxylic acid, alcohol, thiol water, and amine will introduce an ester (Scheme 3vii), ether (Scheme 3xi), sulfide (Scheme 3viii), hydroxyl (Scheme 3vi), and amine (Scheme 3x) pendant groups across the C=C bond, respectively, along with a free hydroxyl group [86,93,100]. Additionally, a comprehensive review of many other epoxide ring-opening pathways, namely acylation, acetylation, hydrolysis, alkoxylation, aminolysis, hydrohalogenation, ketalization organophosphonation, and thioetherification has been conducted [94].
Carbonation
Carbonation of fatty epoxides with carbon dioxide (CO2), also referred to as carboxylation, produces ring-expanded cyclic carbonates (1,3-dioxolanes). Carbonation involves the coupling of CO2 to an epoxide ring to form a cyclic carbonate (Scheme 3ix). The coupling reaction of epoxide with CO2 is extensively studied and various homogeneous and heterogeneous catalysts have been reported for this reaction. Carbonation reactions are commonly carried out using CO2 at atmospheric pressure and tetra-n-butyl ammonium bromide ([Bu4N]Br]) as a catalyst [90]. Sodium iodide with 15-crown-5 and potassium iodide with 18-crown-6 are proven to be effective catalysts. Fatty cyclic carbonates can also be further ring open with different nucleophiles to produce lubricants [101].
2.2.2. TAG Derivatization
Derivatization refers to the process of chemically modifying the TAG molecule to create new molecules that can be used as building blocks for the synthesis of new lubricants. As with TAG functionalization (see Section 2.2.1), the alkene C=C double bond is reactive due to the presence of reactive C-C π-bonds and can undergo addition reactions and oxidative cleavage reactions. Moreover, ester groups are prone to nucleophilic substitution reactions with a variety of nucleophiles at the electrophilic carbon because its alkoxy group is a good leaving group. TAGs can be derivatized at the ester functional groups and C=C double bonds to make new molecular structures to expand the physical property ranges. Scheme 4 presents the common derivatization pathways.
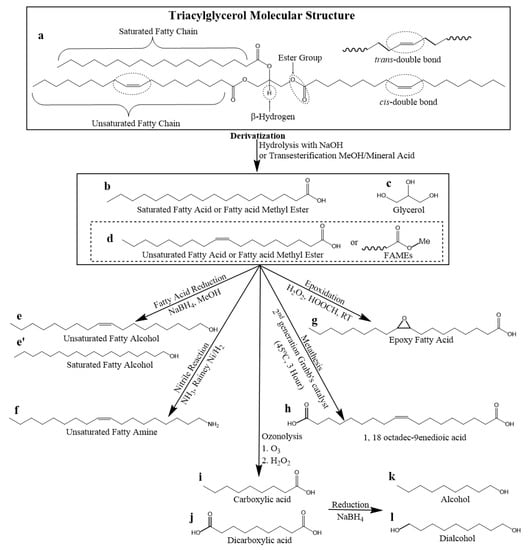
Scheme 4.
TAG derivatization pathways to obtain common derivatives to be used as building blocks for VO-based lubricants.
Hydrolysis and transesterification are synthetic pathways used to derivatize TAGs via the ester functional groups to obtain saturated (compound a in Scheme 4) and unsaturated (compound b in Scheme 4) fatty acids and fatty acid methyl esters (FAMEs), respectively. Fatty acid can be reduced to (compound e) fatty alcohols via fatty acid reduction and can also be converted to (compound f) fatty amine via nitrile reaction. Unsaturated fatty acids can be epoxidized to obtain fatty acid epoxide (compound g). The C=C double bonds are cleaved via ozonolysis to produce mono and dicarboxylic acids (compound i and j). Unsaturated dicarboxylic acids (compound h) can also be obtained via self-metathesis. Dicarboxylic acids and be reduced to dialcohols (compound l).
Hydrolysis
Fatty acids which are readily produced by hydrolysis of TAG are feedstocks of choice for a variety of synthetic biobased lubricant products as shown in Scheme 4b [86,97,98,99]. The three ester linkages in TAG molecules can be hydrolyzed either using an acid (e.g., HCl, H2SO4), base (e.g., NaOH), or enzymatic catalyst (e.g., lipases) to produce linear saturated and unsaturated fatty acids and glycerol byproduct [86,100].
Fatty Acid Reduction
The reduction of a fatty acid alkyl ester to a fatty alcohol with sodium borohydride (NaBH4) is a common chemical reaction used in the synthesis of fatty alcohols on a laboratory scale (Scheme 4e,e′,k) and dialcohols (Scheme 4l) [102]. Commercially, fatty acids and fatty acid methyl esters are reduced to fatty alcohols using supported metal catalysts such as Cu–Cr-based catalysts, ZnO, Al2O3, Cr2O3, and Fe2O under high temperature (250–350 °C) and pressure (10–35 MPa) reaction conditions [103,104]. This process is used at a commercial scale for the reduction of oleic acid (cis-9-octadecenoic acid) and methyl oleate (methyl-9-octadecenoate) to obtain oleyl alcohol (9-octadecen-1-ol) [103].
Nitrile Reaction
The conversion of fatty acid to fatty amine using a nitrile reaction involves the reaction of fatty acid with ammonia at a high temperature followed by dehydration and hydrogenation (Scheme 4f) [105]. The preparation of fatty amines occurs in two steps. First, fatty acid is treated with ammonia in the presence of a dehydration catalyst to form fatty nitriles. The nitriles are hydrogenated into fatty amines by a metal catalyst (Ni or Pd) in the second step [106].
Ozonolysis
Ozonolysis is a commonly used method to produce short-chain carboxylic acids (Scheme 4i) and dicarboxylic acids (Scheme 4j) from unsaturated fatty acids. Different chain lengths of linear dicarboxylic acids can be obtained depending on the unsaturated fatty acid starting material. For instance, 1,3 dicarboxylic acid, 1,4 dicarboxylic acid, 1,5 dicarboxylic acid, and 1,9 dicarboxylic acid be obtained through ozonolysis of linoleic/linolenic acid, docosahexaenoic acid, arachidonic acid, and oleic acid [90].
Metathesis
In metathesis, the double bonds between carbon atoms in unsaturated fatty acids are ruptured and new double bonds are formed with the formation of new products. There are different types of olefin metathesis such as self-metathesis, cross-metathesis, ring-closing metathesis, and ring-opening metathesis. In self-metathesis, the same olefins are involved, and produced oleo compounds have been used as precursors to synthesize lubricants (Scheme 4h). Different transition metal-based (e.g., Mo, W, Re, Pt, Pd, Rh, Ru, and Ir) homogeneous and heterogeneous catalysts have been employed in metathesis reactions. Grubbs first generation, second-generation, and third-generation Ru-based catalysts showed higher reactivity, higher selectivity, and greater production [90].
Esterification/Transesterification
Esterification is a condensation reaction between a fatty acid and alcohol to produce ester and water. The transesterification reaction of a TAG involves the glycerol moiety being replaced by another alcohol. Generally, esterification and transesterification reactions are catalyzed using mineral oils (HCl, H2SO4, or p-toluensulfonic acid), base (NaOH, KOH) [107] and heterogenous solid catalyst (Amberlyst-15, Nafion, sulfated inorganic oxides, alkaline earth oxides (CaO, MgO) and inorganic superacids (WO3/ZrO2, WO3/ZrO2-Al2O3) [102]. The physicochemical properties of synthetic esters are directly related to the type of acids or alcohols used in the esterification/transesterification reactions.
Estolide Formation
Estolides are branched esters that are formed when the carboxylic acid group of one fatty acid links to the site of unsaturation of another fatty acid (via carbocation) to form oligomeric esters using an acid catalyst (H2SO4) and an oxidant (HClO4). This carbocation can be subject to nucleophilic attacks by other fatty acids to form an estolide [108].
3. VO-Based Ester Lubricants from TAG Derivatives
Because of the structural weaknesses of TAGs (mentioned in Section 2.1.1), pure vegetable oils are only used in applications with low thermal stress such as total-loss applications [109]. Although VOs can be directly modified using synthetic pathways, they are mostly derivatized into their chemical components as shown in Scheme 4. TAG derivatization pathways to obtain common derivatives to be used as building blocks for VO-based lubricants. Scheme 4. The general approach is to alter the starting molecule to create new molecules with desired lubricant properties [25,90,103,105].
VO-based lubricants may include different functional groups on the fatty chain such as acids, alcohols, esters, amines, ethers, carbonates, sulfones, sulfides, and amides. C=C double bonds and fatty acid carboxylic groups are chemically modified to obtain different functional groups on the fatty chain such as alcohols, esters, amides, amines, and halides [21,108,109,110].
C=C double bonds present in linear unsaturated fatty chains can be chemically modified to introduce pendant groups of varying chain lengths and create branched fatty acid structures. Branch groups can be introduced at a C=C bond via synthetic routes such as epoxidation/ring-opening [4,25,34,78], etherification [109], acylation [111], and thioetherification. Thioetherification of a fatty halide with a fatty thiol produces a fatty sulfide which can then be further oxidized with hydrogen peroxide to fatty sulfones [110,112].
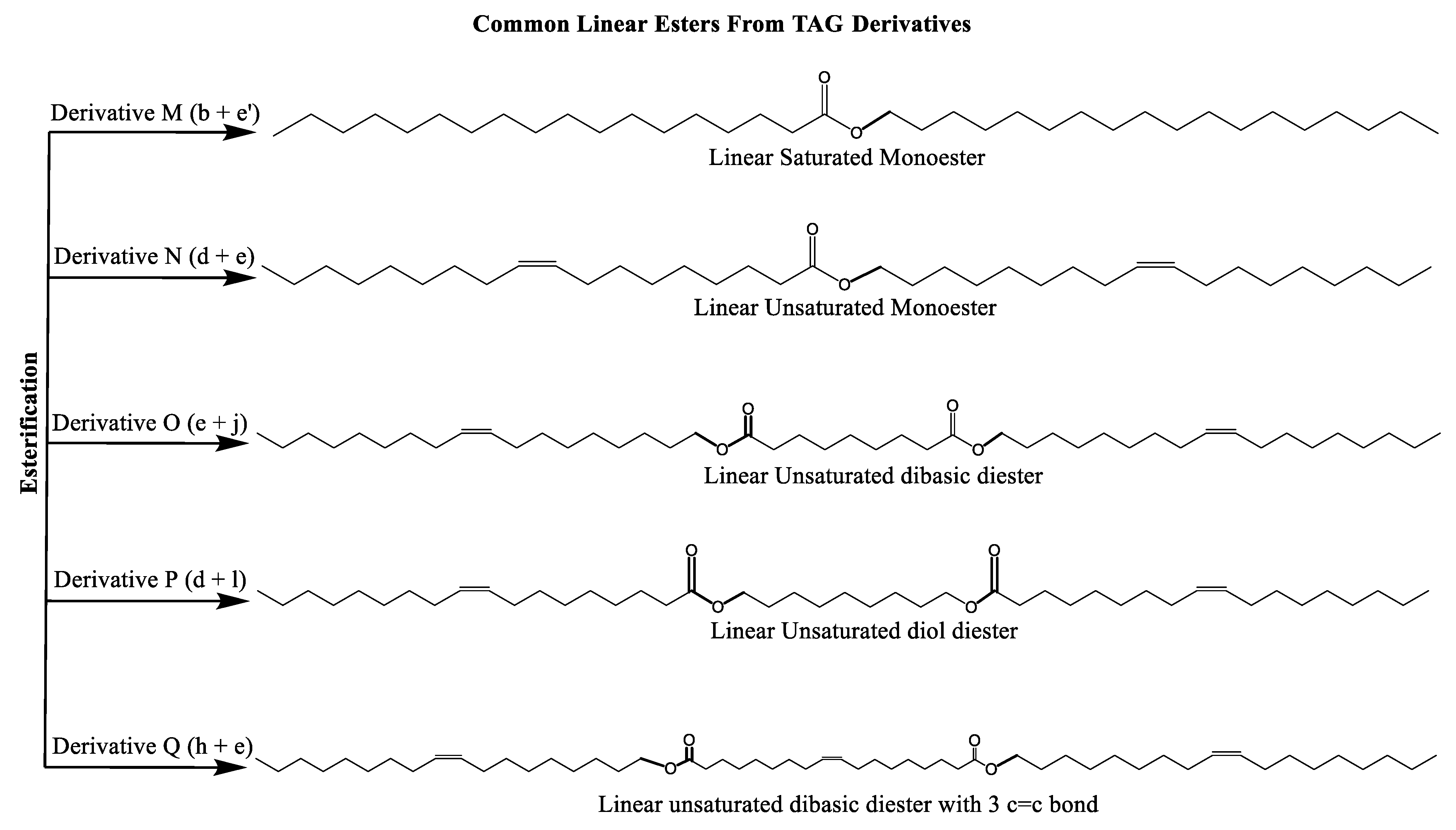
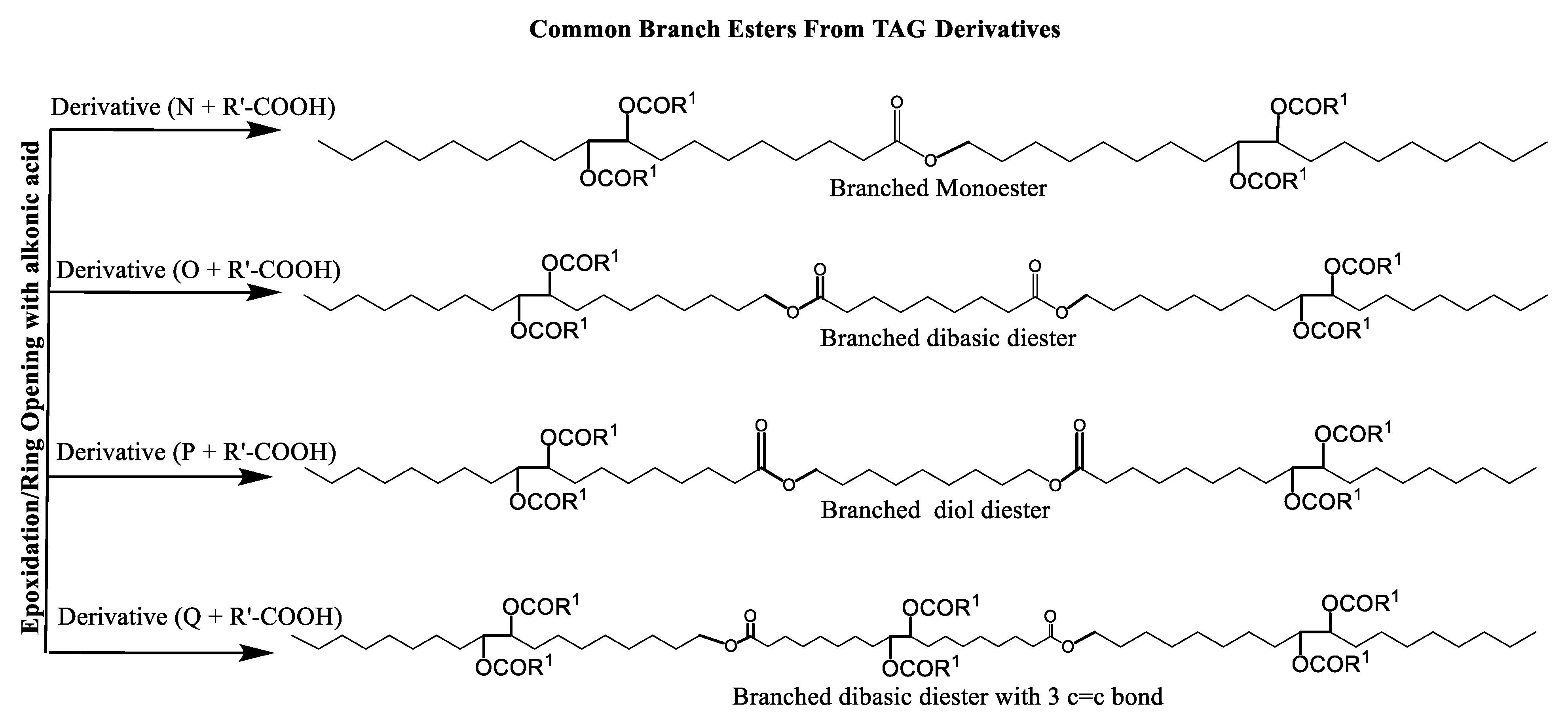
Multiple synthetic pathways are used to prepare functional lubricants from TAG derivatives. For instance, a variety of linear saturated and unsaturated monoesters and diesters with 0–3 C=C double bonds (Scheme 5) have been prepared by esterification of TAG derivatives shown in Scheme 4. In Scheme 6, unsaturated esters produced were branched via epoxidation/ring opening with different alkonic acids. In Scheme 7, sulfide, sulfonyl esters were obtained via thioetherification and oxidation, respectively. Moreover, estolides which have fatty ester branches were also possible through a condensation reaction.
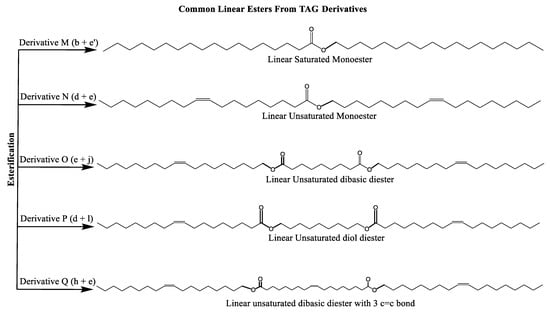
Scheme 5.
Linear saturated and unsaturated monoesters and diesters from TAG Derivatives.
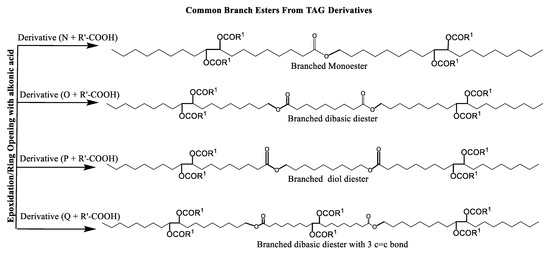
Scheme 6.
Ester-branched monoesters and diesters from TAG Derivatives.
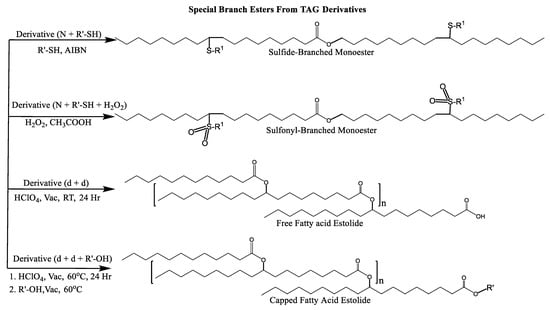
Scheme 7.
Sulfide, sulfonyl, and fatty acid branched VO-based lubricants.
Ester biobased lubricants are typically categorized into monoesters, dibasic diesters, diol diesters, and polyol esters. More complex esters such as oligoesters and functionalized TAGs have been extensively studied as lubricant base fluids [108,111,113,114,115,116,117,118,119].
Dibasic esters are prepared by the esterification reaction of linear dicarboxylic acids with linear fatty alcohols. Diol diesters are prepared by esterification of linear dialcohols with linear fatty acids. Dibasic and diol diesters were compared to demonstrate the effect of ester group directionality on physical properties.
Broadly, derivatized VO esters in Scheme 5 and Scheme 6 are classified into two categories: linear esters mimetics of synthetic oils and branched esters mimetics of polyalphaolefin (PAO), respectively [120]. Polyesters such as estolides have been extensively studied as lubricant base fluids. These lubricants have common structural elements that are associated with improved lubricating properties such as linear fatty chain segments, polar functional groups, low unsaturation, and branched chains [108].
Salient Properties of Current Commercial Ester-Based Lubricants
Commercial ester base fluids offer a wide range of physical properties suitable for different applications including automotive and marine engine oils, compressor oils, hydraulic fluids, and gear oils. They are used when the additional cost over mineral oils is justified [121]. The biodegradability of commercial ester-based lubricants varies from very high, such as for linear molecules particularly those derived from unmodified natural fatty acids, to very low, particularly for esters of aromatic and 2-alkyl substituted acids, or branched chains [122]. Select property ranges of different categories of commercial ester-based lubricant base oils as reported in the literature [2,6,47,123,124] and by a global supplier of synthetic ester oils in their website [42] are compiled in Table 6.

Table 6.
Property ranges of ester-based lubricant base oils.
4. Role of Structure in the Rheological Behavior of VO-Based Lubricants
Viscosity and flow characteristics are important factors in the design of lubricants. They strongly depend on temperature, pressure, and lubricant film thickness [125]. Because it affects heat generation during internal friction and governs the rate of oil consumption, viscosity is decided as a trade-off between lubrication performance and fuel economy [6]. Flow type is considered in the choice of lubricants to optimize fluid properties for specific applications. For example, Newtonian flow is required in applications where variations in viscosity cannot be tolerated, shear thinning is desirable in materials that are required to flow only during processing or use, and shear thickening is advantageous in applications in which absorption and dissipation of energy upon impact are necessary to enable oil performance under low shear [5]. This information is often overlooked for biobased lubricants.
4.1. Analysis of Structure-Flow Type Relationships in VO-Based Lubricants
The flow behavior of fluids is characterized by the shear stress ()—shear rate () relationship. It is usually expressed by Herschel–Bulkley model (Equation (4)).
where is the yield stress below which there is no flow, the consistency index, and the power index. depends on the constitutive properties of the material. For Newtonian fluids is equal to 1. The flow is shear thickening when and shear thinning when . In the absence of yield stress, the Herschel–Bulkley model transforms into the Power Law model [6,34].
4.1.1. Effect of Molecular Mass on Flow Type
The available data relating molecular mass to flow type of VO-based lubricants does not easily lend itself to modeling because of the complexity that molecular architecture introduces. The roles of structural features such as degree of oligomerization and branching are not easily separated from the effect of mass. Furthermore, even if distinctions were possible, they rapidly disappear with increasing temperature. The configuration of the different constituent structures in the molecular architecture appears to be the most important factor determining the rheological behavior of complex compounds as shown in dendritic polymers [126]. Flow type is ultimately dictated by complex short-range structuring in the melt involving intermolecular interactions as well as intramolecular forces and entropic considerations such as those from bulkiness, flexibility, and rotatability. For example, the formation of transient clusters by hydrodynamic interactions via hydrogen bonds, known as hydroclusters in colloid suspensions, is suggested to explain the promotion of shear thickening in biobased lubricants. The phenomenon is caused by complex short-range structuring in the melt involving contributions of mass, hydrogen bonding, chain flexibility, group rotations, and steric effects [124,127]. The effect of mass on the flow type of VO-based lubricants is best revealed in correlation with the effect of oligomerization and branching.
4.1.2. Effect of Branching on Flow Type
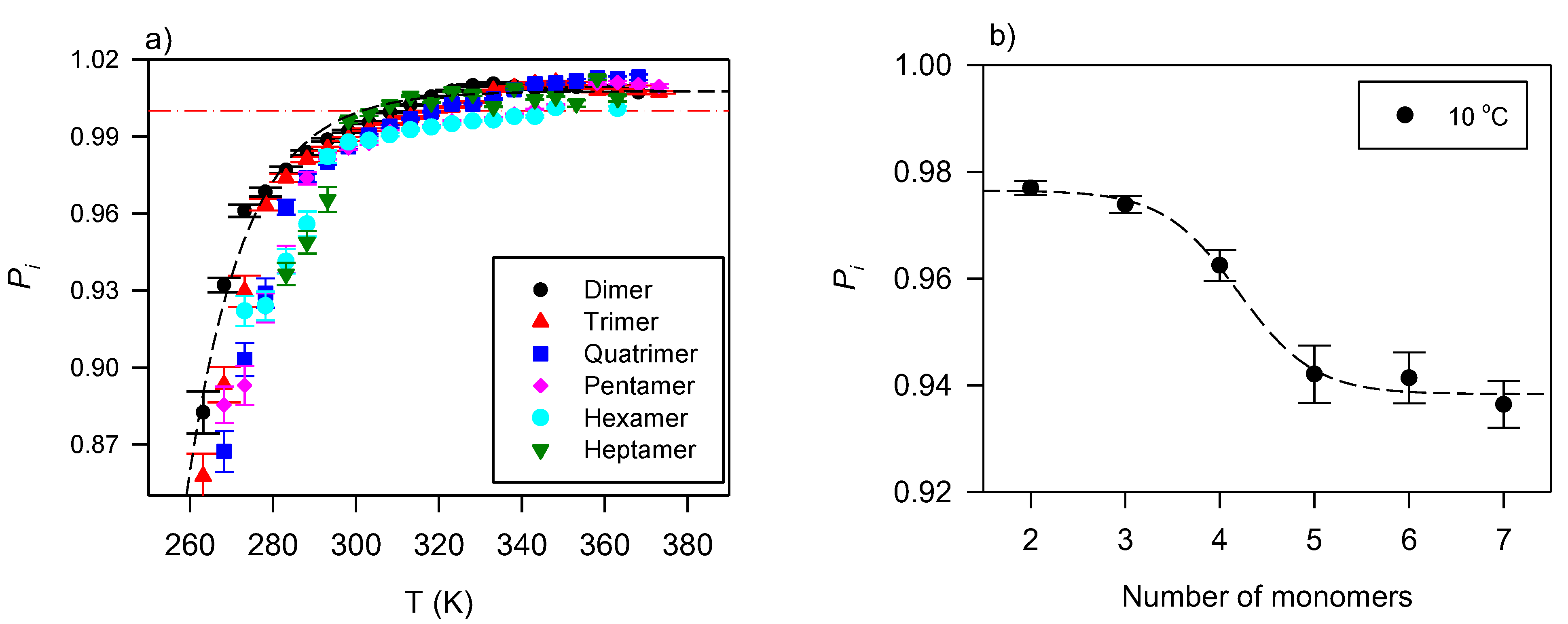
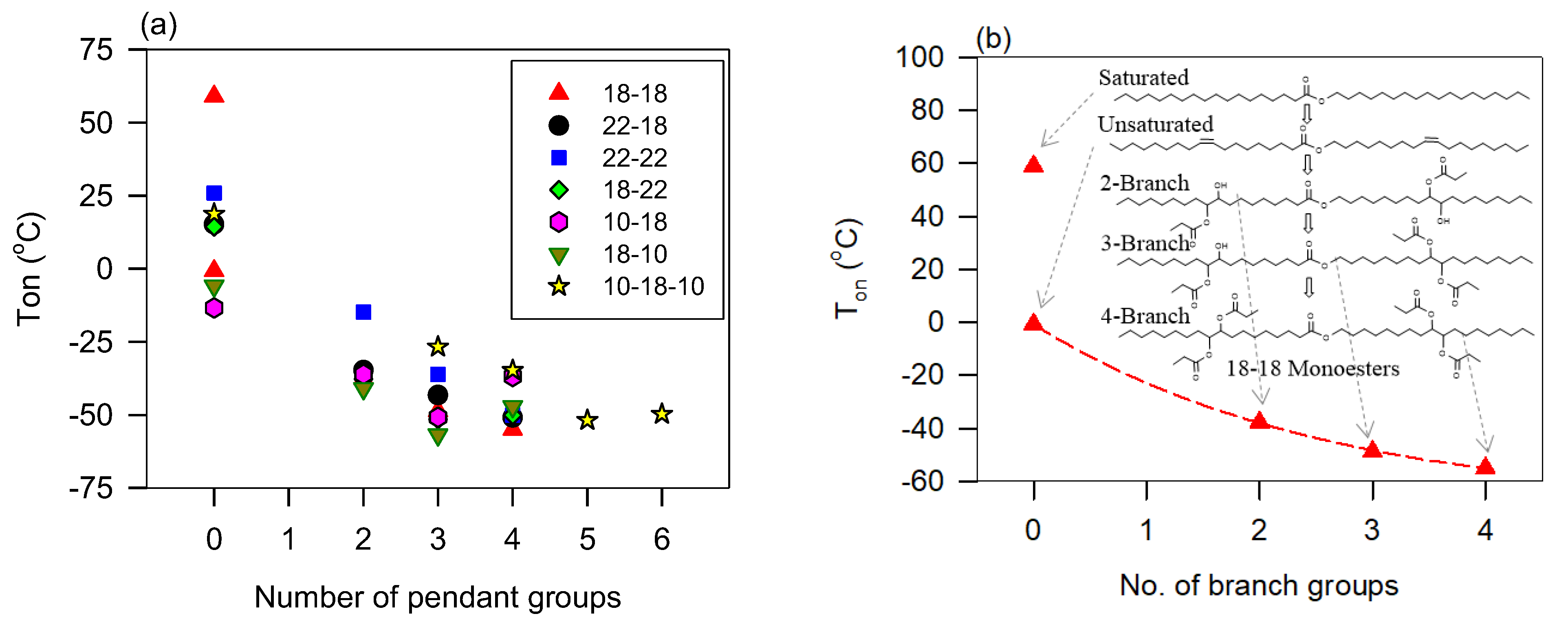
The effect of branching on the flow type of VO-based lubricants is associated with the bulky configuration of the pendant groups, their number, and the number of OH groups present. The information gathered from the shear rate–shear stress data of dimers to octamers of triolein provides an insight into the behavior [128]. Figure 2a shows the curves of these TAG oligomers and Figure 2b its variation with the number of monomers at 10 °C.
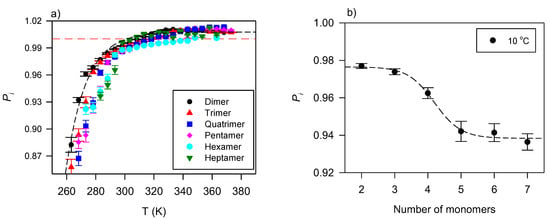
Figure 2.
(a) Power index versus temperature for TAG oligomers [128]. The dashed line is a fit to an exponential rise to a maximum function (Equation (5); R2 > 0.9899); (b) Power index versus the number of monomers in TAG oligomers. The line in (b) is a guide for the eye.
Figure 2a indicates a flow that progresses from shear thinning at low temperatures to slightly shear thickening for the smaller oligomers at high temperatures. Although variations are observed, one can fit in the provided temperature range to an exponential rise to maximum functions (Equation (5), R2 > 0.9899; 0.82 ≤ ≤ 0.95; 0.06 ≤ ≤ 0.19).
where is the plateau value, and the characteristic temperature or temperature constant of the function.
Overall, as illustrated in Figure 2b with data collected at 10 °C, the oligomers with five or more monomers (larger mass) present a measurably more pronounced shear-thinning character than the lower order oligomers (smaller mass). This difference is significant at low temperatures, diminishes then disappears as the temperature increases.
Monoesters are examples of rheologically complex fluids that are dramatically affected by branching. Monoesters that possess strong shear-thickening character such as those with terminal double bonds become Newtonian at all temperatures when branched, regardless of the number or position of the branches [129]. Shear thickening, a less widely observed behavior than shear thinning, is thought to be caused by changes in the particle arrangements under shear [5]. This suggests that branching limits the entropic effects that induce structuring which helps the establishment of shear-thickening flow.
Unbranched dibasic and diol-derived diesters are Newtonian at all temperatures with no distinct flow characteristics that could be directly attributed to any structural feature such as end group type, asymmetry, saturation, double bond position, or ester group position [130,131,132,133]. Compared to monoesters, the second ester of diesters introduces sufficient extra flexibility to establish a Newtonian flow. Diesters become shear thinning at low temperatures when branched. This may be explained by the rigidity introduced by pendant groups.
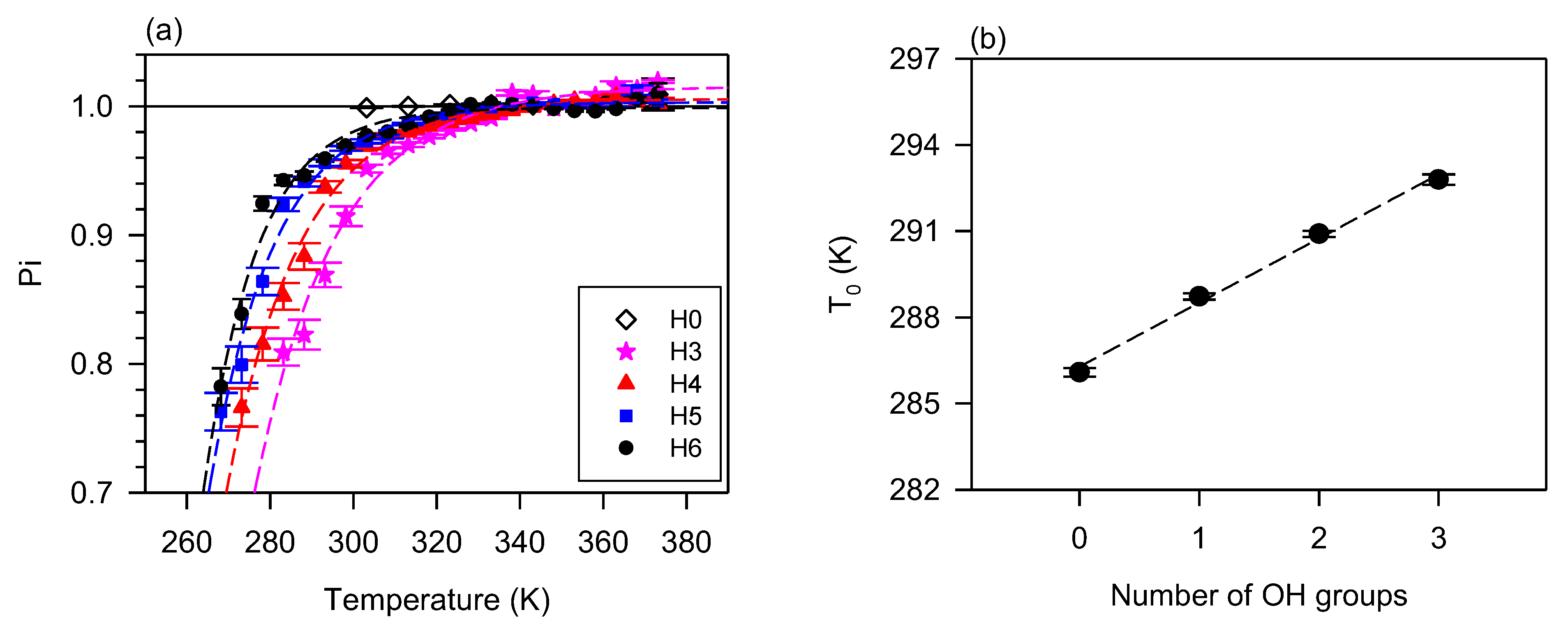
The effect of branching and oligomerization on the flow behavior is similar in many aspects. The study by Li et al. [128] and Raghunanan et al. [134] of branched esters shows the power index versus temperature follows the exponential rise to a maximum function (Equation (5)), R2 > 0.9879, Figure 3a shows of (E)-Didec-9-enyl Octadec-9-enedioate (H0) and its 3 to 6 branched derivatives. The derivatives with 3, 4, 5, and 6 pendant chains, labeled H3, H4, H5, and H6, comprise 3, 2, 1, and 0 OH groups, respectively [129]. The fit of the data to Equation (5) was very good (R2 > 0.9879). Newtonian flow was achieved at different temperatures (between 50 °C and 70 °C) depending on the number of branches/OH groups. At any given temperature, the shear-thinning character decreases with an increasing number of pendant groups (or decreasing number of OH groups). This relationship is captured in Figure 3b with the linear increase of the characteristic temperature () with the number of OH groups. This linear correlation indicates the compounding nature of the contribution of the hydrogen bonds to the structuring of liquid diesters.
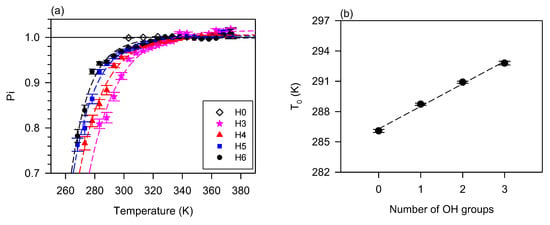
Figure 3.
(a) Power index () versus temperature for (E)-Didec-9-enyl Octadec-9-enedioate (H0) and its 3 to 6 branched derivatives (H3-H6) [72,73,129,134]. Lines are fitted to exponential rise to a maximum function (Equation (5); R2 > 0.9879; 0.65 ≤ ≤ 0.85; 0.15 ≤ ≤ 0.37); (b) critical temperature versus the number of OH groups in the branched derivatives (0 in H6, 1 in H5, 2 in H4 and 3 in H3). Dashed line: fit a straight line (; R2 = 0.9944; = 286.3, = 2.23).
The flow behavior of oligomers and branched diesters contrasts with that of branched monoesters which despite the added mass from bulky pendent chains remain Newtonian, suggesting that molecular mass must be large enough to counterbalance any entropic effect which might prevent the formation of transient clusters. As discussed above, although hydrogen bonding is prevalent, the presence of a second ester group is necessary for branching to induce shear thinning.
4.1.3. Role of the Ester Groups in Flow Type
As shown for linear aliphatic diesters studied by Bouzidi and Raghunanan [72,133], the presence of a second ester group promotes Newtonian flow with no yield stress regardless of other structural features such as double bond position (internal or terminal) or end group effects. The presence of the second ester group introduces an extra bulky moiety and two sites at which the fatty segments can rotate resulting in a large enough entropy to facilitate the rapid breakup of any formed transient clusters or completely prevent their formation.
4.1.4. Role of the Double Bonds in Flow Type
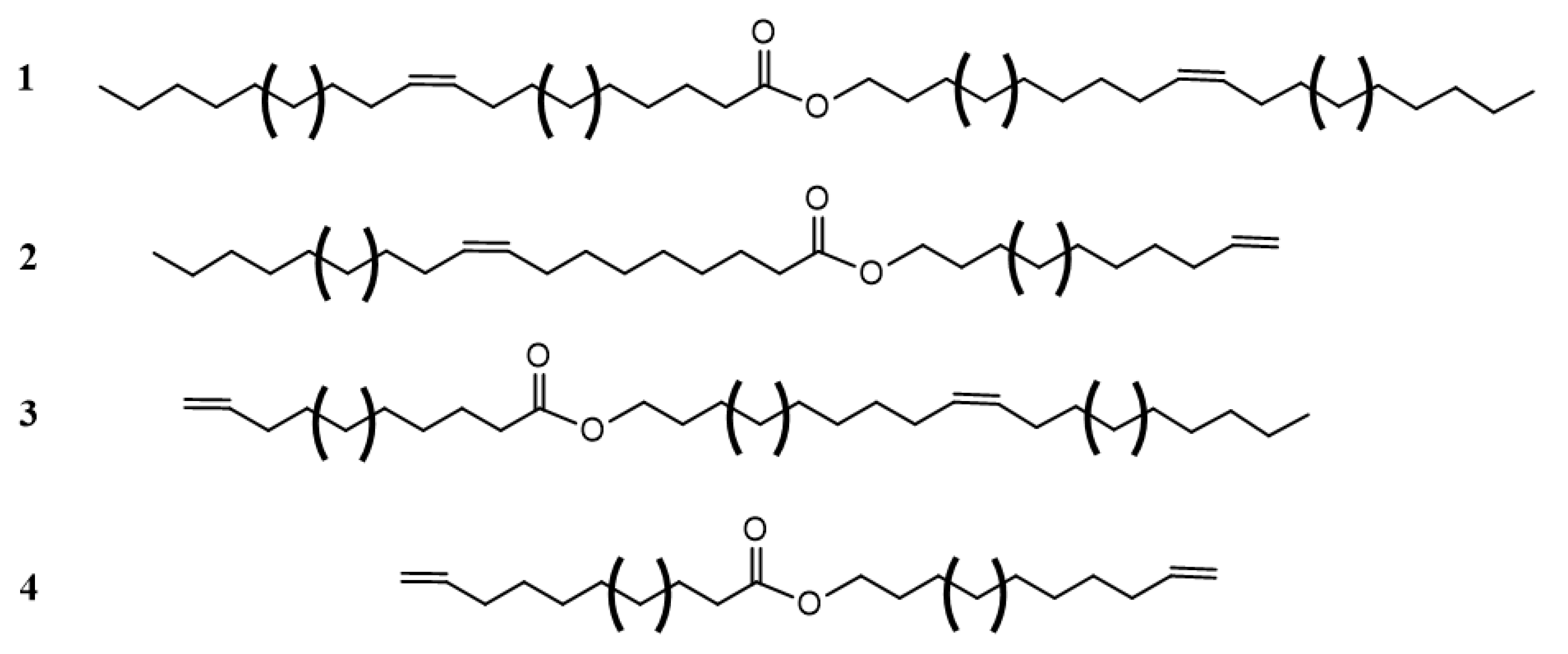
Double bonds in VO-based lubricants influence the flow type in a complex manner. The flow behavior of JLEs with internal double bonds differs from those with terminal double bonds [135]. The general structures of monoesters with internal and terminal double bonds are provided in Scheme 8.
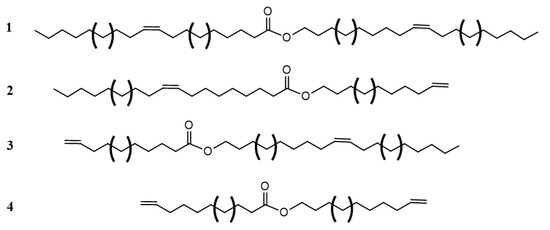
Scheme 8.
General structure of monoesters with internal and terminal double bonds. 1: two internal double bonds; 2: internal double bond on the fatty acid moiety and terminal double bond on the alcohol moiety, 3: terminal double bond on the fatty acid moiety and internal double bond on the alcohol moiety and 4: two terminal double bonds. Brackets indicate different chain lengths.
Linear aliphatic monoesters with internal double bonds demonstrated a Newtonian flow with no yield stress and those with at least one terminal double bond demonstrated a flow that is Newtonian at low temperature and increasingly shear thickening as the temperature increases (power index varied from 1.01 to 1.32) similar to what is observed in concentrated dispersions and so-called structured fluids [133]. The behavior can be explained by the formation of clusters of nearly touching particles that grow as the suspension thickens under shear [136]. The fact that the compounds with terminal double bonds present shear thickening and those with internal double bonds are Newtonian suggests that the internal double bonds prevent the formation of transient clusters whereas the linear geometry of the terminal double bonds may promote them under shear via end group interactions with other electron dense C=C end groups [73].
4.1.5. Effect of Isomerism on Flow Type
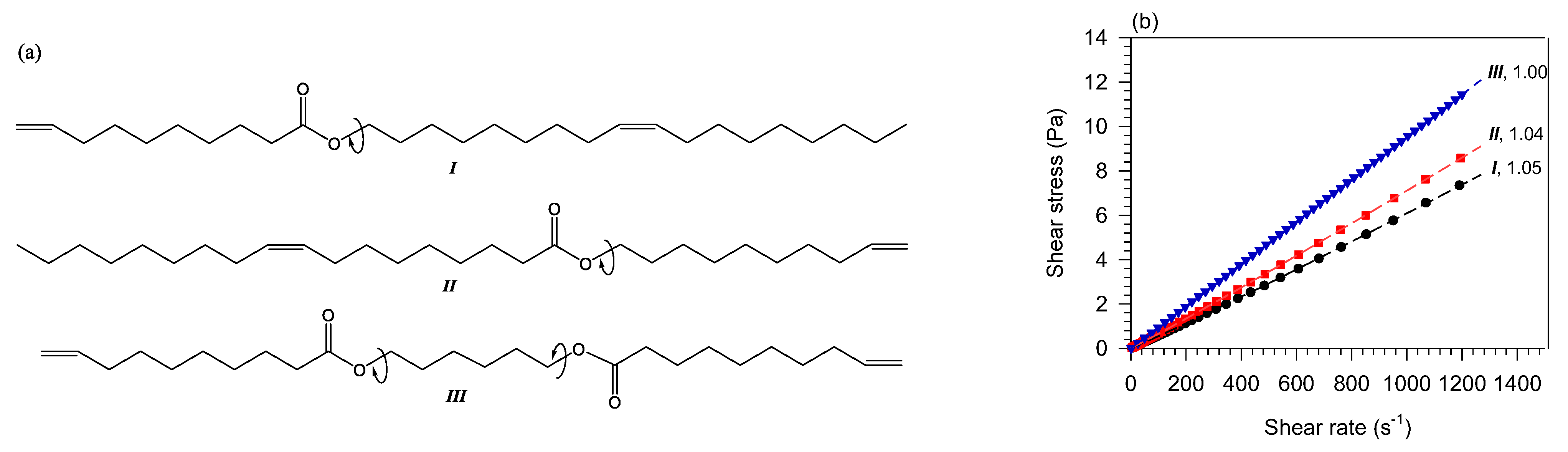
The study of monoester isomers reveals significant differences in the contribution of fatty acids and fatty alcohols to the flow type [73,137]. As illustrated with in Figure 4, showing the shear rate-share stress curves of dec-9-en-1-yl oleate (I in Figure 4), (Z)-octadec-9-en-1-yl dec-9-enoate, (II in Figure 4) and diesters hexane-1,6-diyl bis(dec-9-enoate) (III in Figure 4), for any given total length, the power index of the monoester with the shorter fatty alcohol is smaller than the monoester with the longer fatty alcohol [73,134]. This is explained by entropic effects due to the difference in the directional flexible rotation about the alkoxy C−O−C bond of the ester group (arrows on the schemes in compounds I and II in Figure 4). The isomer with shorter alcohol rotates more readily compared to isomer with longer alcohol and thus, presents a higher entropy which helps reduce the formation of transient clusters. The Newtonian flow of the symmetrical molecule of the same chain length (compound III in Figure 4) confirms that the effect is due to the imbalance in rotation about the center which prevents transient clusters from forming [134].
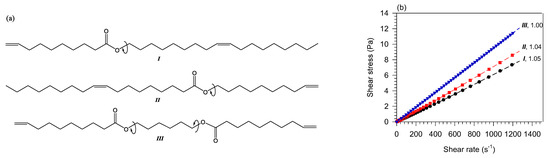
Figure 4.
Chemical structures (a,b) shear stress–shear rate at 40 °C of unsaturated monoesters (I: dec-9-en-1-yl oleate and II: (Z) -octadec-9-en-1-yl dec-9-enoate [73]) and diesters (III: hexane-1,6-diyl bis(dec-9-enoate) [134]).
4.2. Analysis of Structure-Viscosity Relationships in VO-Based Lubricants
Viscosity is an important parameter as it dictates the internal friction within a liquid lubricant. During liquid lubricating operations, the base oil viscosity influences the ability to form a lubricating film which is most critical for the control of friction and therefore protection of surfaces from wear and degradation. The viscosity of VO-based lubricants decreases exponentially with temperature and is modeled similarly to other organic fluids [138]. Models based on first principles and which provide physically meaningful insights into the flow behavior in relation to the configuration of the molecules are limited. Among these, the Andrade and the van Velzen models are simple but powerful with two (2) parameters, one describing the magnitude of the viscosity and the other the mass and complexity of the molecules [133,135,136,139,140]. Generalized forms of the Andrade and van Velzen models were successfully applied to VO-based lubricants such as in Li et al. [73,133]. The results of these works show that the parameter which describes the magnitude of the viscosity and the parameter which describes the complexity of the molecules can be predictively related to structural features such as the number of branches or OH groups. Further work is needed to unambiguously establish these relationships [133,138].
4.2.1. Effect of Molecular Mass
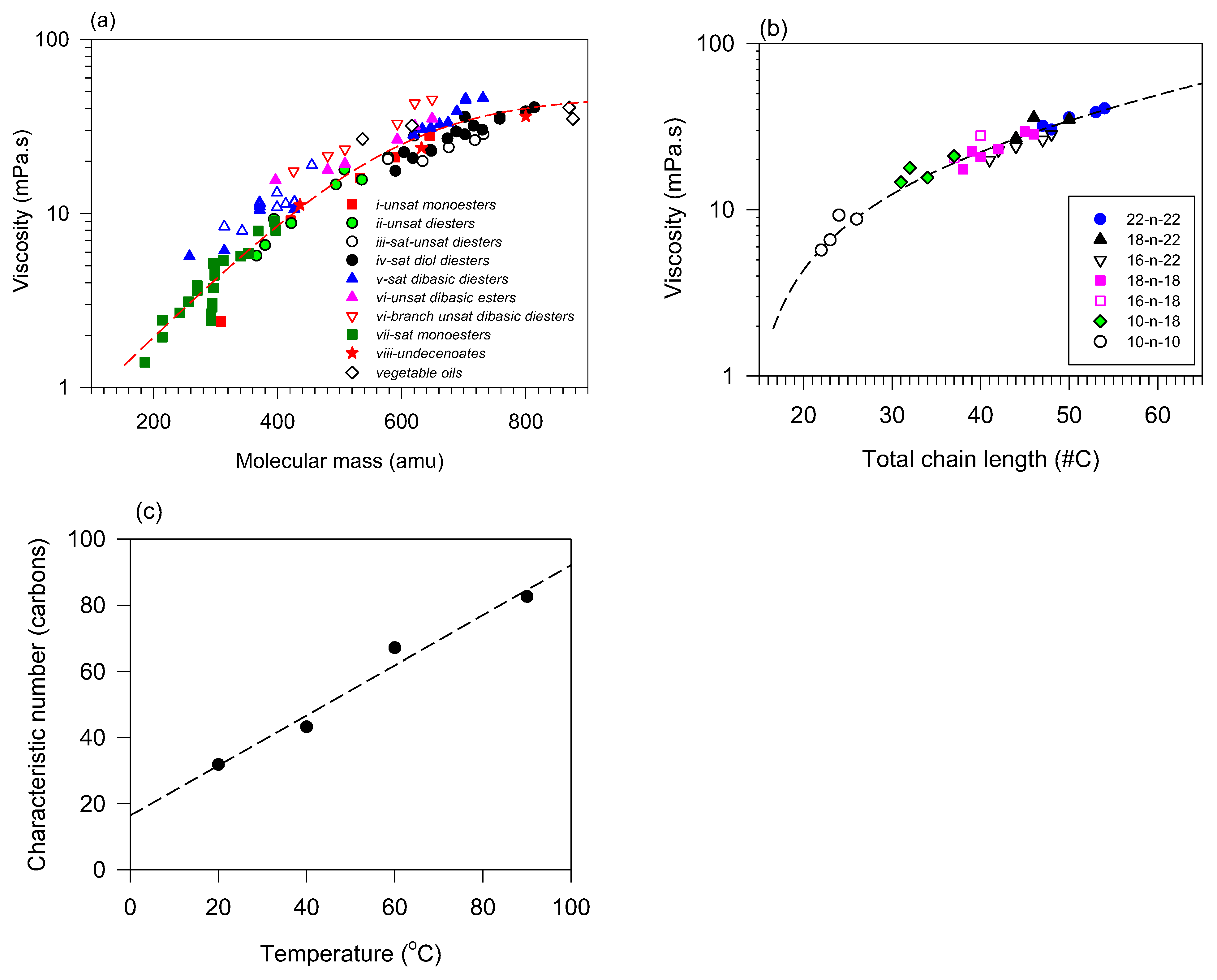
Viscosity generally tends to increase with increasing molecular mass, which may be desirable, or not, depending on the intended application [139,141]. Figure 5a summarizes the correlations of viscosity with molecular mass for vegetable oils and a series of different linear esters. These include saturated and unsaturated monoesters [73,140,142,143], saturated and unsaturated dibasic diesters [73,144], and unsaturated diol diesters [134,145]. The viscosities of select VOs are included in the figure for comparison.
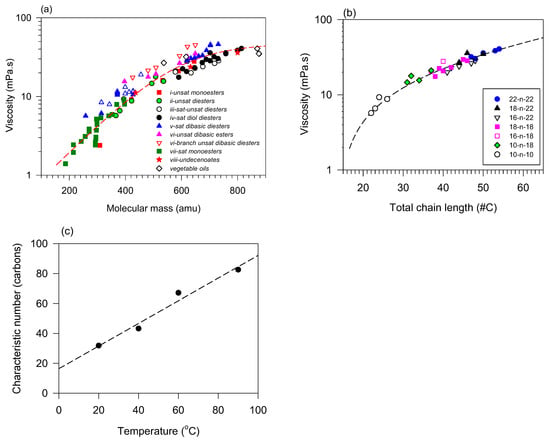
Figure 5.
Viscosity VO-based esters taken at 40 °C versus (a) molecular mass and (b) total chain length. Lines are fitted to exponential functions (Equation (6); R2 > 0.9297); (c) characteristic number () versus temperature for diesters [73,140,142,143]. The dashed line is a fit to a straight line (; R2 = 0.9736; = −15.2, = 0.74).
The viscosity versus molecular mass (MM) and total chain length (number of carbons n) for VO-based linear esters follow an exponential function (Equation (6)) such as typical hydrocarbons [146,147,148].
The fit of all the collected viscosity data versus molecular mass (x = MM, x0 = MM0) to an exponential function and reported in Figure 5a (Equation (6); R2 = 0.9297) yielded a 95% prediction band that encloses practically all the data points. The fact that the viscosity of all these compounds is enclosed in the prediction band is attributable to the predominance of entanglement interactions which form strong energy barriers to flow and mass transfer limitation over any other structural effect such as fatty chain type, symmetry, number, and position of double bonds and the number of functional groups [149,150]. This relatively simple relationship allows for a fair prediction of the viscosity in a large molecular mass range regardless of structural details. One can further refine the predictions for a series of datasets adequately sorted and predictive information can be extracted by structure type because the contribution of influential structures is generally not lost, particularly at low temperatures. The individuality of each series which manifest at low temperature due to structural differences is normally lost at a higher temperature where higher kinetic energy results in higher entropy and reduce internal friction and resistance to flow [151].
The viscosity of certain distinctive series such as simple unbranched monoesters and diesters can be modeled using this empirical function with a much better goodness of fit. In these cases, the total chain length in carbons (x = ) can be used as the variable with a characteristic number of carbons (), and the trend is explained by incremental increases in intermolecular attractions [152]. The approach has been used on saturated and monounsaturated fatty acids [129] as well as more complex esters such as JLEs [134,146]. An example of viscosity at 40 °C versus total chain length is presented in Figure 5b for different symmetrical and asymmetrical diesters (R2 > 0.9411). As shown in Figure 5c, the viscosity change with temperature is also captured in the fits with the characteristic number (). The linear trend observed for is a corroboration of the exponential behavior of the viscosity versus temperature.
Odd–Even Effect
The physical properties of odd-numbered carbon molecules (such as linear alkanes, fatty acids, and esters) are known to differ from those of even-numbered carbon molecules because of their different molecular conformations [153]. For example, the viscosity of even-numbered-derived diesters has a lower viscosity than their odd-numbered counterparts. This is attributed to the smaller particle size of the denser syn-conformation which facilitates particle–particle interactions and resistance to flow. Raghunanan et al. study [137] is an example of the influence of odd–even effects on linear aliphatic diol-derived diesters. The study shows the viscosity of the VO-based lubricant with odd-numbered carbon molecules follows the same trends as their even-number counterparts with slightly different parameter values.
4.2.2. Effect of Unsaturation: Type, Degree, and Position of Double Bonds
The presence of double bonds in the fatty chain increases viscosity because of the contribution of the interactions of the carbon-carbon double bonds [146,154]. Raghunanan et al. [133] showed that the unsaturated esters present consistently higher viscosities than the saturated esters. The VO-based lubricant with cis- double bonds are generally more viscous than the trans- counterparts. Example are given in Geist [155] and Zéberg-Mikkelsen [156] for cis- alkenes and their trans- alkene counterparts. Despite the expected increase in π-bond interactions, however, an increasing degree of unsaturation does not necessarily translate into higher viscosity. Rodrigues et al. [148,148] and Allen et al. [147] reported decreasing viscosity with increasing levels of unsaturation in the C18 fatty acid methyl esters of oleic, linoleic, and linolenic acids. Rodrigues [148] attributed this trend to the increasingly coiled geometries of the higher unsaturated molecules which restrict the π-bond interactions in the melt. This argument can be extended even further to include the restriction of chain entanglement and other particle–particle interactions in the melt due to the increasing rigidity of the molecules with increasing levels of cis- unsaturation.
The position of the double bonds (internal or terminal) also influences the molecule shape and hence its viscosity [2,134,157]. Bouzidi et al. [72,137] showed that unsaturated monoesters comprising internal double bonds present lower viscosity than those with terminal double bonds, and viscosity is higher when the internal double bond is on the acid moiety than in the alcohol moiety. This is likely caused by the difference introduced by the double bond to the fatty acid and fatty alcohol in intermolecular interactions via combined effects of size, conformation, and flexibility.
4.2.3. Influence of Functional Groups
Functional groups affect lubricant viscosity whether present in the backbone or pendant groups [148,155,156]. Those with permanent dipoles which can form strong dipole-dipole intermolecular attractions contribute to the rigidity and intermolecular attraction and so increase viscosity [147,148] whereas, flexible functional groups which form weak dipole-induced dipole attractions lower the viscosity [44,57,63,148,158,159,160,161,162]. Molecules with polar functional groups which are capable of forming hydrogen bonds form strong secondary intermolecular attractions which persist in the fluid state [159]. The presence of hydroxyl groups modifies the viscosity index and may contribute to improved lubricant oxidative stability by acting as scavengers for free radicals [160,163]. For instance, due mainly to polarity effects, lubricity can be enhanced with higher polarity functional groups [159]. Although polarity is beneficial for lubricity, it may cause seal-swelling and interfere with the action of polar additives by preferentially adsorbing onto metal surfaces [159,164,165,166].
4.2.4. Influence of Branch Groups and Hydroxyl Groups
The viscosity of esters is sensitive to branching because it reduces flexibility and alters the shape of VO-based lubricant molecules. The change in flexibility is shown to be the main driver in the reduction of viscosity as well as viscosity index (VI) [7,44,167]. This can be drastic as demonstrated for neopentyl diesters compared to analogous dibasic diesters where viscosity decreased by approximately 50% in the neopentyl diesters compared to dibasic diesters counterparts [5,44,144]. The preponderant effect of flexibility is further confirmed by the fact that viscosity is not significantly influenced by the branching of the rigid alcohol moiety [42].
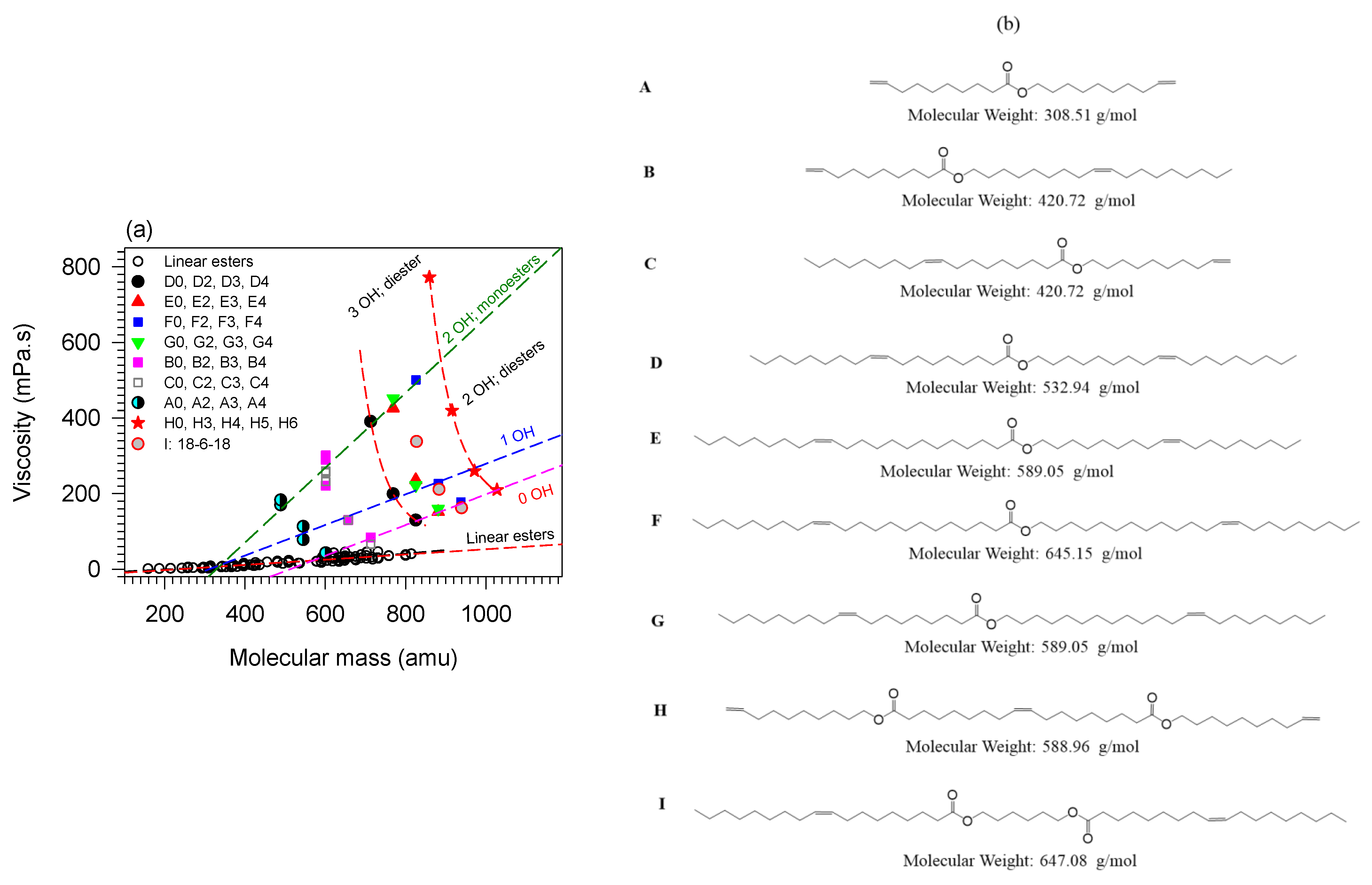
The effect of branching and the number of hydroxyl groups on viscosity is illustrated in Figure 6a with seven series of branched monoesters and two series of branched diesters [2,3,5,33,144,145,168]. The structures of the molecules are given in panel (b) of Figure 6. The pendant chain in all the molecules is propanoic acid. The viscosity of linear esters (empty circles, ○), fitted to a line, is included in Figure 6a as a baseline for comparison.
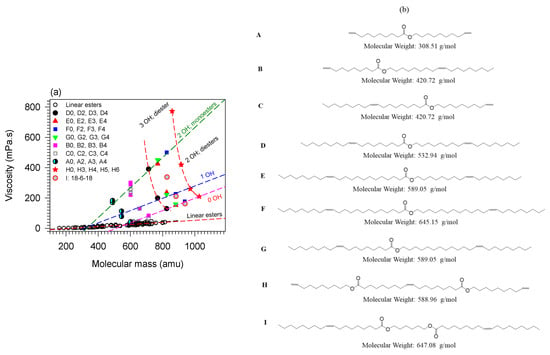
Figure 6.
(a) Viscosity of branched monoesters (A–G) and diesters (H,I) versus molecular mass. (b) molecular structures of the base compounds. The molecules are ordered from A to I in the molecular mass ascending order. The numeral value attached to the letter in panel (a) indicates the number of pendant chains of the branched derivative [73,148,152].
Figure 6 indicates that the effects of OH groups and mass combine to increase the viscosity whereas branches tend to decrease it. Disregarding less important contributions of other structures, and as a first approximation, the trends of the individual contributions of branching, OH groups and mass can be decoupled and predictive. The effect of branching alone can be evaluated by comparing the fully branched molecules (0-OH line in Figure 6) with the non-branched esters. One can for example appreciate the quantifiable departure of the viscosity of the branched compounds from the line observed for the unbranched esters. Additionally, the effect on the viscosity of branching esters is much greater than adding ester groups. For example, it is one order of magnitude compared to adding a second ester group to the backbone of a monoester molecule [73,148,152].
The contribution to the viscosity of hydrogen bonds combines with the effect of mass and branching flexibility in a predictive manner consistent with the strong intermolecular attractions incrementally provided by added OH groups. The effect is illustrated in Figure 6 with the trends shown by each same backbone series. Analysis of the data shows that the viscosity () decreases exponentially with increasing molecular mass (, R2 > 0.9881—See fit lines passing through the H3456 (★) and A234 (●) data in Figure 6). The value of the critical mass obtained for the fits is = 64 ± 5 g/mol for all the series shown in Figure 6. This value is close to the molecular mass of the propionic acid pendant chain which is 74 g/mol indicating that each branch contributes equally to the viscosity regardless of backbone size. This result also confirms the predominant effect of flexibility and the possibility to predict the effect of branching despite the overwhelming effect of the OH groups. The analysis shows that hydrogen bonds do not influence the change in flexibility introduced by the branches.
Overall, and for any given number of OH groups, the viscosity of esters increases linearly with mass. The rate of change is lowest for linear esters and increases with branching and increasing number of OH groups. The trend lines of the compounds with OH = 1 and OH = 0 (R2 > 0.9771; Figure 6) have a similar rate of change (slope = 0.4 mPa.s/gmol−1) and are 58 ± 1 mPa.s apart. The viscosity of the branched esters with 2OH groups increases 2.5 times more rapidly with increasing molecular mass than those with 1 or no OH groups.
The slope of the straight line fitting the viscosity versus the mass of the series of monoesters with 2 OH (= 1.0 mPa.s/gmol−1; R2 = 0.9491) is the same as the slope for the series of diesters with 2 OH groups. However, the viscosity of the monoesters is higher than that of the diesters (the 2OH diesters and 2OH monoesters lines of Figure 6 are 200 mPa.s apart). This indicates that the influence of OH groups is mitigated by added structural flexibility which in this case is the presence of the second ester group which introduces an extra center of rotation which lowers the viscosity. The 2OH line of the diesters is however above the 1OH line revealing the distinctive role of polarity in the viscosity of branched esters.
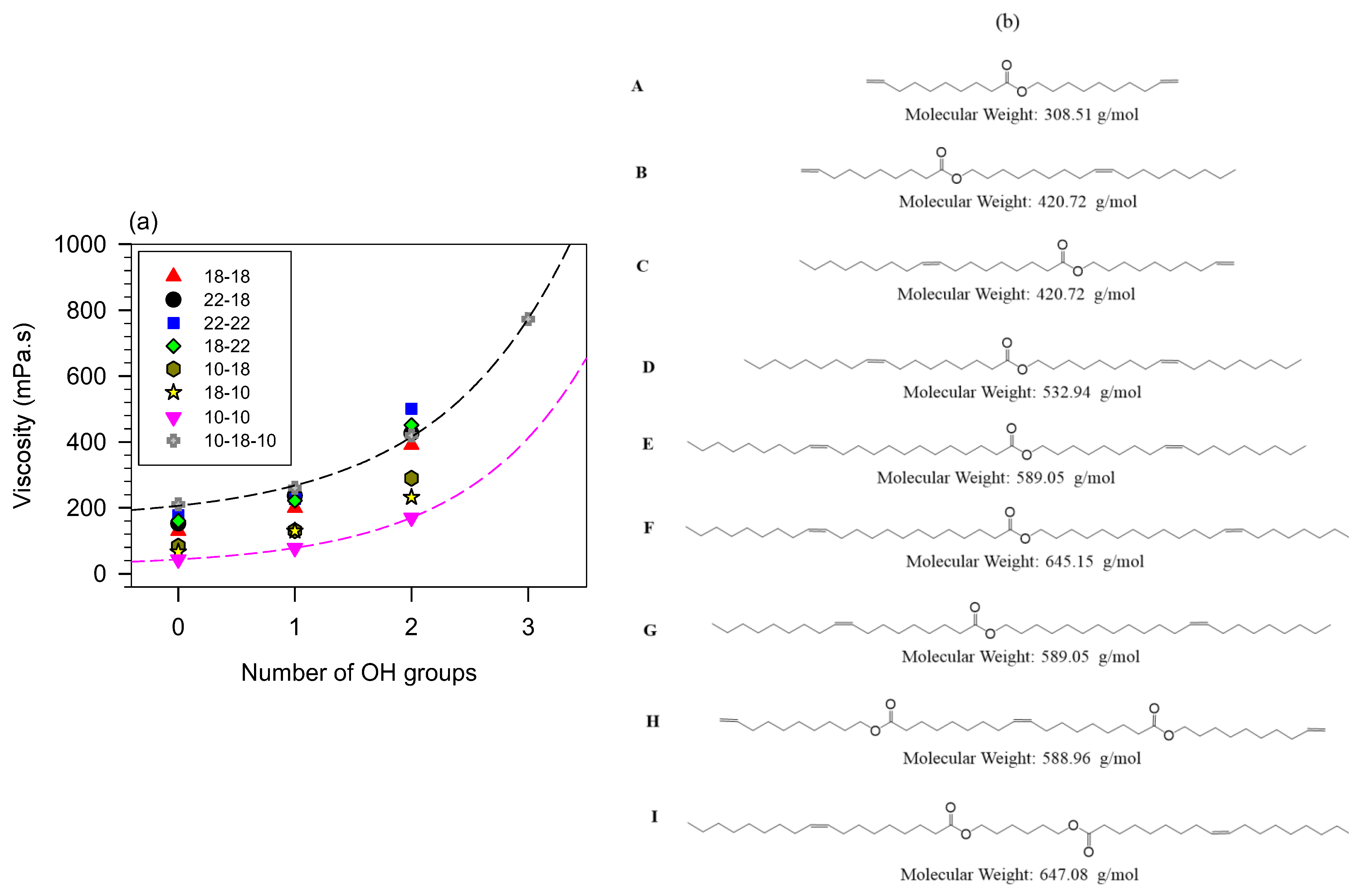
Viscosity versus the number of hydrogen bonds is illustrated in Figure 7 with branched oleyl oleate. The effect of mass is evident from Figure 7 and can be predictively related to viscosity for the same number of OH groups. It is, however, much less than the hydrogen bond contribution. For each series of compounds, viscosity versus the number of hydrogen bonds indicates an exponential increase () with a characteristic number of OH groups () of 1.1 ± 0.1 for all the series. This value indicates first that the OH groups contribute equally and additively to the viscosity and second that there is no significant hindrance of the hydrogen bonds from other structural features.
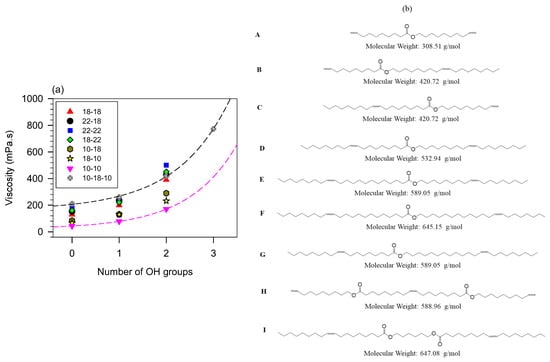
Figure 7.
Viscosity at 40 °C of branched monoesters and 10-18-10 diester versus the number of hydroxyl groups in their propyl branched derivatives. (b) molecular structures of the base esters of panel (a).
Although structural traits other than the number of OH groups are at play, the above data show that viscosity is predictively related to molecular mass, number of pendant chains, number of rotation centers, and number of hydrogen bonds. These are simple linear or exponential predictive relationships.
4.2.5. Effect of Isomerism, Directionality, and Number of Ester Groups
The effect of isomerism is particularly shown in branched esters. The data confirms the primary role of hydrogen bonds and the significance of the position of the hydroxyl groups on the backbone. Typically, branched fatty esters with longer fatty acid/shorter fatty alcohol present lower viscosities than their isomers [73]. The difference in viscosity decreases with the number of branch groups. This is because the decrease in the number of OH groups which provide the largest effect promotes a relatively much smaller effect of change in flexibility due to the increase of branches. This effect is absent for unbranched monoesters.
The influence of stereoisomerism on viscosity is well documented wherein the conformer with the greater flexibility presents a higher viscosity [73]. In the case of esters, the difference in viscosity is related to the influence of directionality of rotation about the flexible C−O−C alkoxy bonds of the ester groups. For example, the viscosity of the diol-derived diesters is higher than that of the dibasic-derived diesters [134]. The effect of ester group direction is noticeable even at temperatures as high as 100 °C as reported for alkyl-branched diester isomers [134]. The study showed that the difference in viscosity between isomers is consistent (~20 mPa·s) regardless of chain length. This indicates that it is primarily driven by the difference in the number of conformations formed by the outwards and inwards rotations in the dibasic and diol-derived diesters. The number of conformational structures in diol-derived diesters is limited by the steric bulk of the ester moieties, which, due to close proximity, restricts free rotation of the fatty acid moieties at the sides of the ester groups [133]. Simply, the larger number of molecular conformations in dibasic-derived diesters translates into greater resistance to flow and thus, higher viscosity.
Similar conformation arguments may be invoked to account for the similarity in viscosity of the unsaturated monoesters and diol-derived diesters, i.e., despite the presence of a second ester group, the viscosity of the unsaturated monoester (E)-octadec-9-en-1-yl (E)-octadec-9-enoate is close to that of the diol-derived diester (butane-1,4-diyl (9E,9’E)-bis(octadec-9-enoate). Compared to diesters, which possess large steric barriers to the rotation of the fatty acid tails about the flexible C−O−C alkoxy bond, the alcohol moieties of monoesters are free to rotate about their ester alkoxy bonds. The reduced flexibility of diesters compared to monoesters likely facilitates a decrease in the resistance to flow that was of similar magnitude to the increased resistance offered by the second ester group.
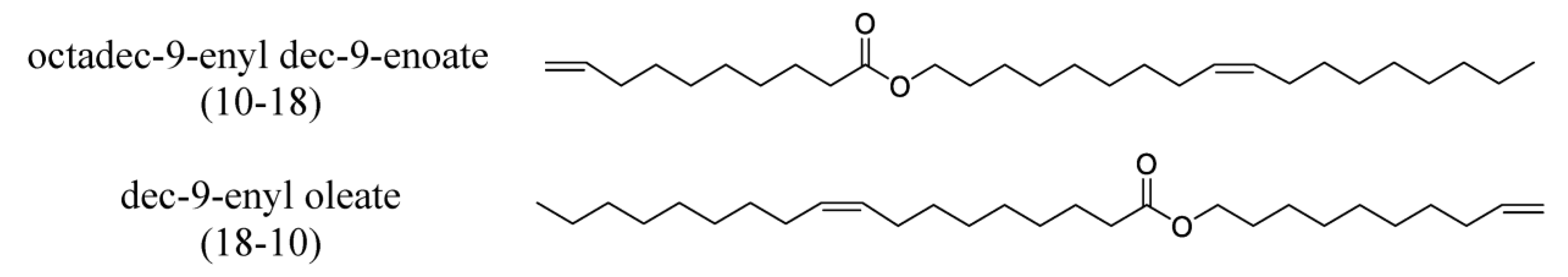
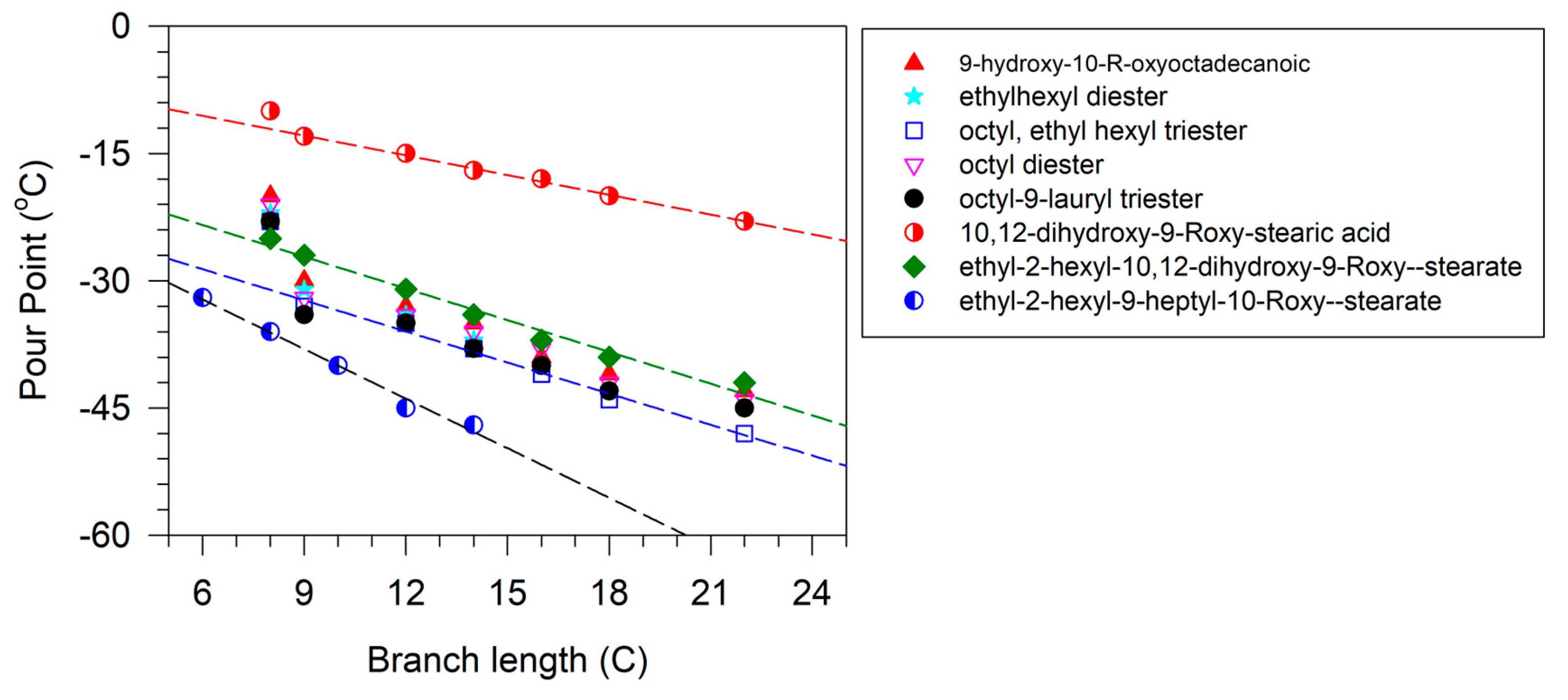
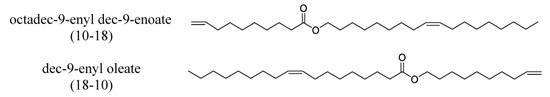
The importance of the position of the OH group/branches is illustrated with the branched derivatives of octadec-9-enyl dec-9-enoate (10-18) and dec-9-enyl oleate (18-10). Both have a terminal double bond (Scheme 9) which can be branched with the OH group in a terminal position or internal position. In both cases, the viscosity of the derivative with terminal OH is higher than that of the internal OH. The difference in viscosity between the isomers is 79 mPa.s when the fatty acid is functionalized and 29 mPa.s when the alcohol is functionalized [137].
Scheme 9.
Structure of octadec-9-enyl dec-9-enoate (10-18) and dec-9-enyl oleate (18-10).
5. Role of Structure in the Thermal Transition Behavior of VO-Based Lubricants
The low-temperature performance of lubricants is usually characterized by the cloud point (CP) and pour point (PP). CP gives the first temperature at which solid crystals can be observed under cooling (ASTM D2500), and PP gives the temperature at which a liquid stop flowing (ASTM D97). The DSC onset of crystallization () and melting offset () are related to the CP, and crystallization and melting peak temperatures () are related to the PP [73,129,134]. The melting parameters are more readily used because, unlike crystallization, melting does not usually depend on the rate at which the material is cooled or heated [129,133,156].
The main intermolecular forces provided by saturated and unsaturated fatty chains are dispersion forces [137,169]. Generally, saturated fatty chains help form closely packed crystals with high energy storage densities whereas the restricted rotation around double bonds and kinks associated with cis C=C double bonds cause those hydrocarbon chains to be more rigid and ‘bent’ at an angle, all detrimental to optimum crystal packing which leads to lower temperatures of crystallization/PP than straight saturated fatty chains [170,171,172]. The common flexible organic moieties in ester lubricants are the sp3 C−C bond in linear saturated fatty chains and C−O−C moieties from the ester, ether, and carbonyl groups [173,174]. The higher the degree of flexibility, the larger the entropy change upon melting. This is because a flexible molecule gains a higher degree of internal freedom when it melts as compared to a nonflexible molecule [175].
The thermal transition behavior of VO-based lubricants is discussed below in terms of the influence of the component structures on the melting enthalpy and melting entropy and hence on the observed melting points. The success with suppressing crystallization in oleochemical-derived esters is due to the combined effects of increasing the number of ester groups along the molecular backbone, total chain length, number of branched groups, and position of branched groups. Because the contributions of the different structures are generally complex and do not lend themselves to distinct trends, they are analyzed when independent relationships can be established and related to each other when possible.
5.1. Influence of Molecular Mass/Hydrocarbon Chain Length
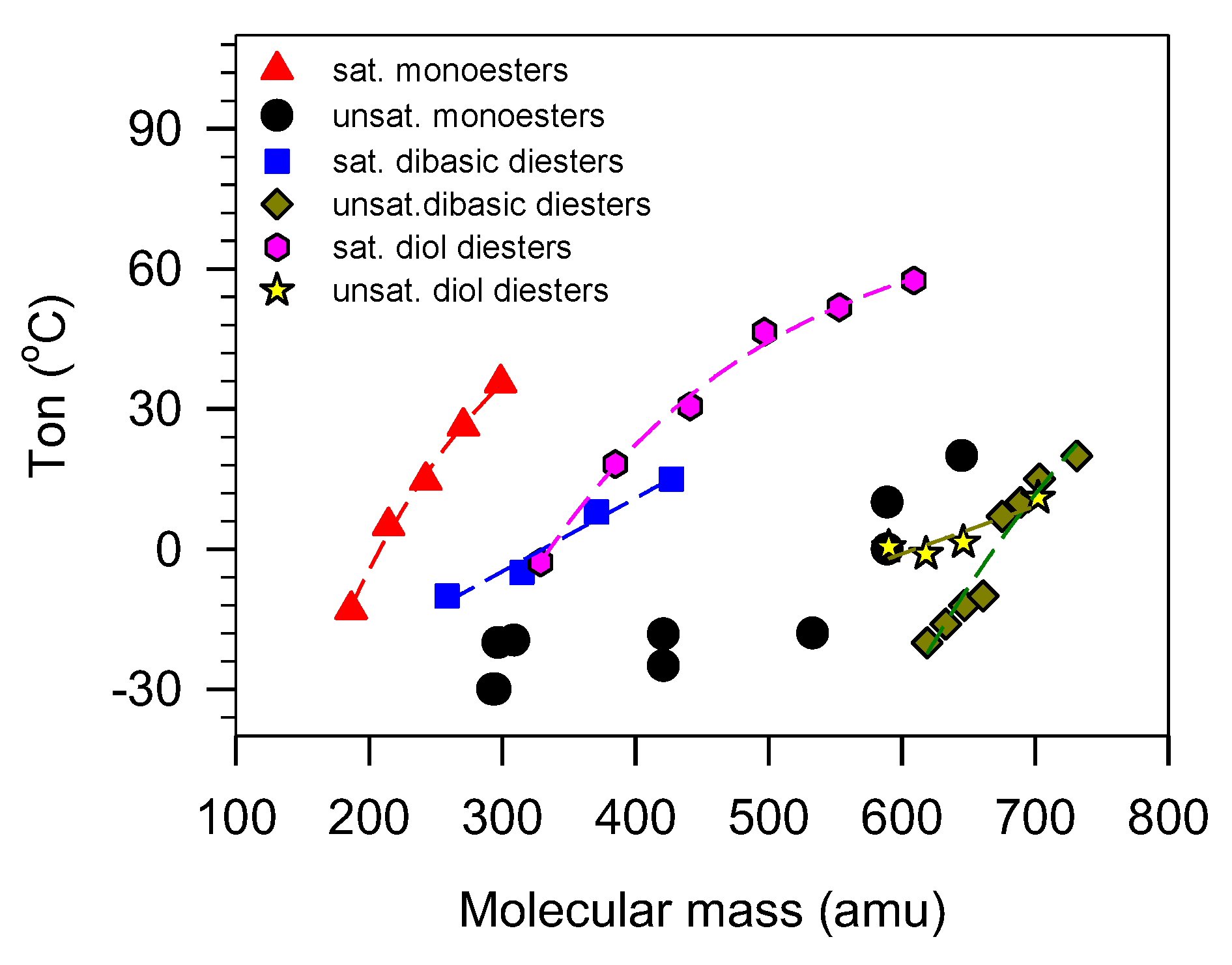
Although the thermal transition behavior of VO-based lubricants depends strongly on molecular mass, it is generally not a good indicator of PP because of the influence of other structural parameters which hinder access to unequivocal relationships [46,176]. Generally, the thermal parameters increase with increasing mass but the relationships are only predictive in simple linear esters where there is no other major structural driver [177,178]. As shown in Figure 8 for monoesters and diesters, simple correlation analysis is not normally possible. Even in usual saturated linear chains, model indicators other than molecular mass are needed to account for the differences that are observed between different molecular architectures.
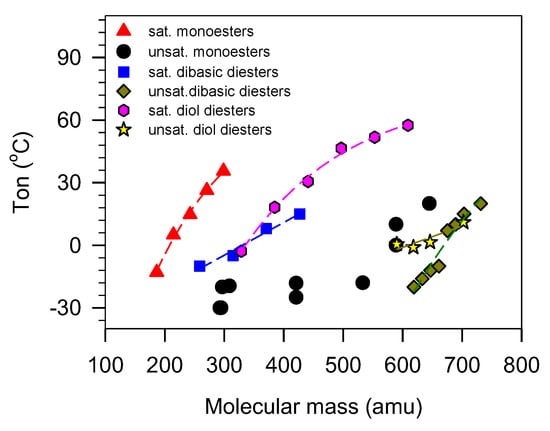
Figure 8.
Onset temperature of crystallization () of unbranched monoesters and diesters versus molecular mass [51,179,180,181,182].
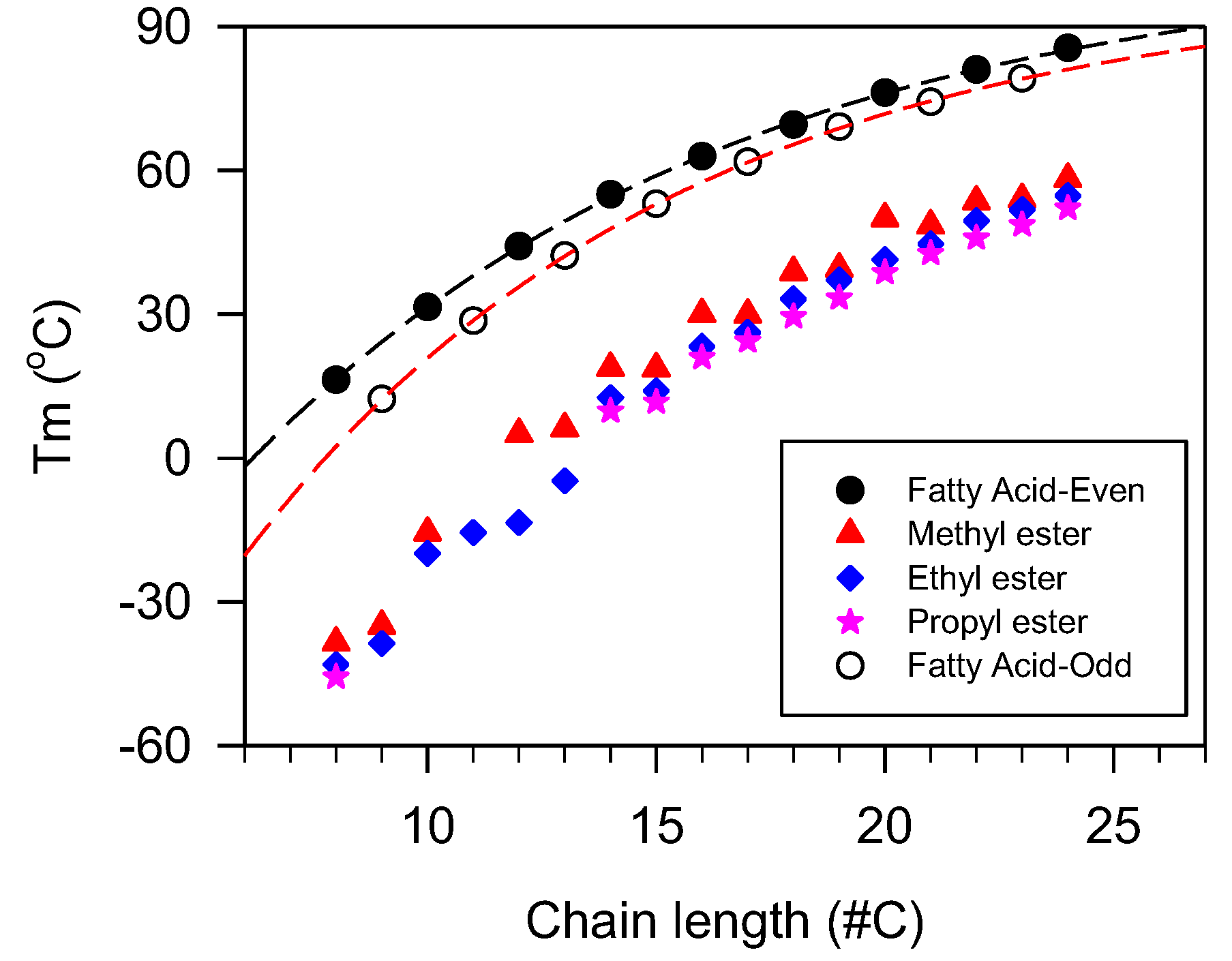
In linear esters in which the fatty chains are the main source of the intermolecular interaction, the number of carbons is an excellent indicator used to model the thermal transition properties. In this case, the increase in methylene units (CH2) adds predictable incremental van der Waals intermolecular attractions and hence provides the possibility to establish predictive relationships. The increment in temperature of melting estimated from saturated monoesters data, in the region where mass limitation effects are not overwhelming, is 1–2 °C per methylene unit added [179]. Generally, the crystallization and melting temperatures versus fatty chain length follow an exponential rise to maximum functions due to the combined effects of the incremental increase of the dispersion forces mitigated by mass and heat transfer limitations [180,181]. This trend is well documented for wax esters [51], methyl esters of jojoba oil [182], and saturated fatty acids [148,183]. The plots of melting temperature versus chain length of different esters are presented in Figure 9.
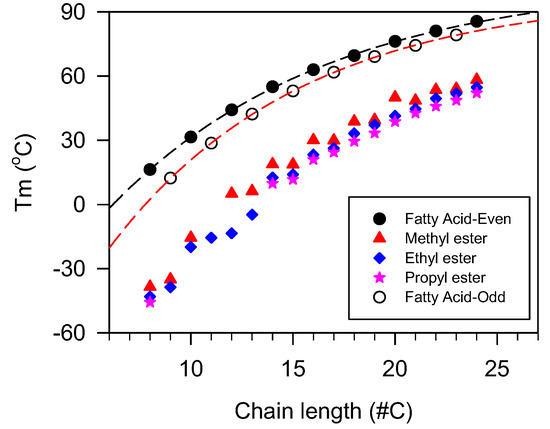
Figure 9.
Peak melting temperature versus chain length of esters.
Lubricants with even or odd fatty chains tend to have different thermal transition values [177,179]. However, as shown in Figure 9 for the case of , the properties follow the same trends and can be described with the same exponential function (. In the case of the fatty acids data shown in Figure 9, the fit yielded coefficients of determination R2 > 0.9999 and a characteristic number of carbons of 10.3 for the even fatty acids and 9.6 for odd fatty acids which account for the difference between their melting points, and their narrowing as the number of carbons increases.
The molecular mass and chain length are good predictors of the thermal transition parameters. They however compete directly with the degree of unsaturation which tends to lower the pour point.
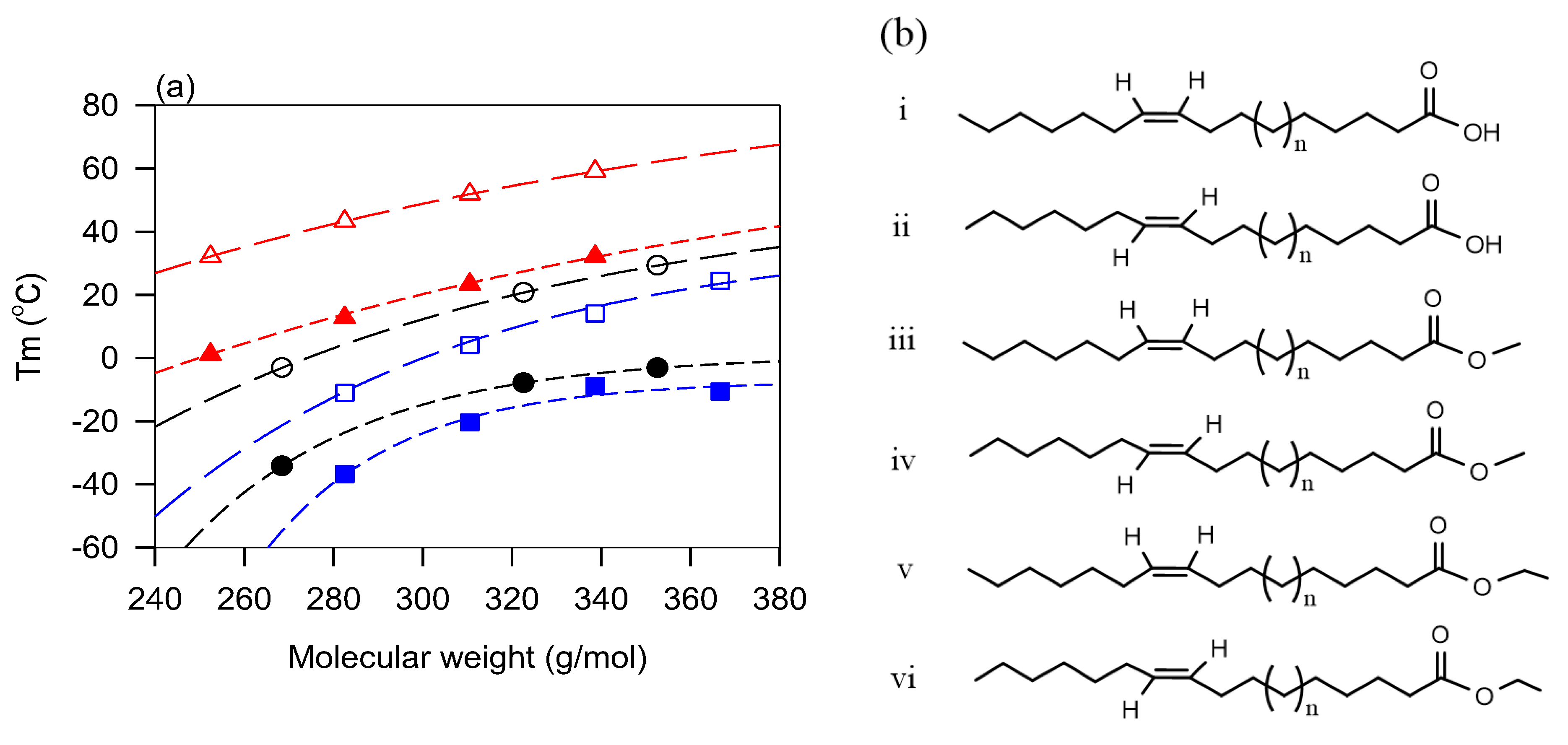
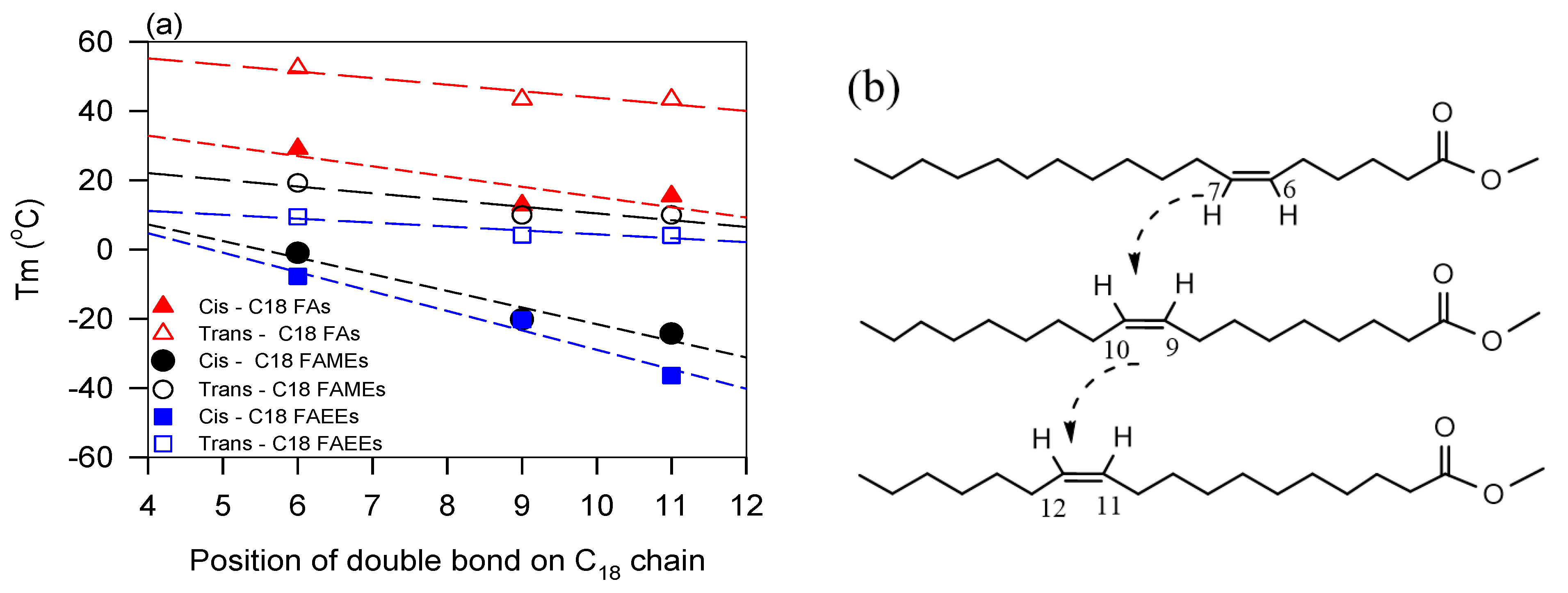
5.2. Role of Double Bonds—Effect of the Degree of Unsaturation
The degree of unsaturation significantly affects many properties including key thermal parameters such as PP. The number of double bonds, their nature (cis or trans), and their position contribute to the properties in a complex manner. The presence of double bonds in lubricant molecules is the primary cause of poor oxidation stability [177,183]. The monounsaturated VOs have better oxidative stability (OS) than polyunsaturated vegetable oils [184]. It is common to selectively hydrogenate VO-based feedstocks to convert polyunsaturated fatty acids into monounsaturated fatty acids to increase OS [109,185]. However, saturating too many double bonds results in poorer cold-temperature performance. A balance between pour point and OS requirements is usually achieved by a balance between saturated and unsaturated fatty moieties. The abundant monounsaturated oleic acid is typically preferred over polyunsaturated fatty acids because it better achieves this balance.
5.2.1. Number, Nature (Cis or Trans), and Position of the Double Bonds
The influence of the hydrocarbon chain length on the pour point of unsaturated esters is complex and depends on the number, nature (cis or trans), and position of the double bonds [45,186,187]. Generally, as shown in Figure 10 for C16, C18, C20, and C22 omega-7 fatty acids and their methyl (FAMEs) and ethyl esters (FAEEs), the melting points of the trans configuration are monotonously higher than those of the cis configuration. Figure 10 shows that a simple exponential function may be used to describe the variation with molecular mass for each series (R2 > 0.8978). Furthermore, the data indicate that the difference between of the cis- and trans- configurations is independent of molecular mass.
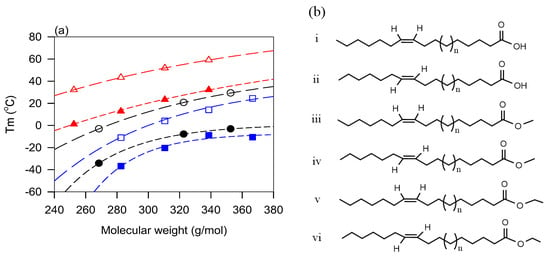
Figure 10.
(a) Effect of cis- and trans- configuration on the melting point of C16, 18, 20, and 22 monounsaturated fatty acid and its methyl and ethyl esters. (b) Molecular structures of (FAs; cis/trans: (i) ▲/(ii) △;), fatty acid methyl esters (FAMEs: (iii) ●/(iv) ○;) and fatty acid ethyl esters (FAEEs: (v) ■/(vi) □).
5.2.2. Position of the Double Bonds
The position of the double bonds is consequential. Notably, it was observed that double bonds on the alcohol moiety of fatty wax esters provide lower melting points compared to the fatty acid moiety [188]. Figure 11 shows that the effect of the position of the cis and trans double bond on of C18 monounsaturated fatty acids, fatty acid methyl esters (FAMEs), and fatty acid ethyl esters (FAEEs) is predictable. The melting point () of all these molecules linearly decreases as the double bond position shifts to higher numbers on the backbone. One can note again that of the trans configuration is higher than that of the cis configuration for all the molecules.
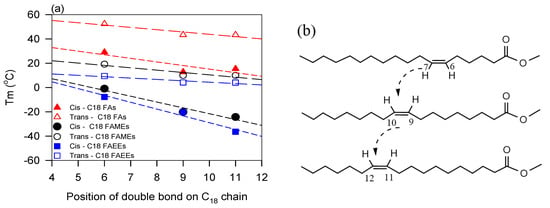
Figure 11.
(a) Melting temperature versus double bond position of C18 monounsaturated fatty acids (FAs), fatty acid methyl esters (FAMEs), and fatty acid ethyl esters (FAEEs). (b) Chemical structures of the C18 FAMEs with the double bond in the 6, 9, and 11C positions. cis- and trans-double bonds: full and open symbols, respectively.
5.3. Role of Functional Groups
The functional groups affect lubricant thermal properties in a variety of ways [4]. The contribution depends on the functional group’s type and position in the molecule. The polarity of common functional groups as quantified by the estimated dipole moment of the electronegative bonds is provided in Table 7. The polarity values of Table 7 can be used to evaluate the contribution of the functional group to the intermolecular forces [7,109,189].

Table 7.
Dipole moments of common functional groups [7,44,57,63,148,158,159,161,162,163,164,165,190].
The functional groups with permanent dipoles that can form strong dipole-dipole intermolecular attractions such as sulfonyl (–SO2–) amide (–CONH–), carboxylic (–COOH), hydroxyl (–OH), and amine (–NH2) groups increase the crystallization temperature and enthalpy [159,160] whereas flexible functional groups which form weak dipole-induced dipole attractions such as sulfide (–S–) ether (–O–), ester (–COO–) and carbonate (–CO3–) result in lower crystallization temperature and enthalpy because of increased bulkiness and flexibility which increase much more the entropy than the added dipole-induced dipole attractions [57,159,161,162,163,164,165].
5.4. Ester Group Directionality Isomerism
Chirality is beneficial in molecular structures designed for biobased lubricants. The presence of chiral centers in lubricant molecular structures introduces stereoisomers which increases [160]. The study of over 50 chiral compounds showed that of racemic mixtures tend to be lower than those of their pure D and L isomers [163].
5.5. Role of Pendant Groups—Degree of Branching
The presence of pendent chains reduces the ability of individual molecules to stack in an orderly fashion, which lowers the pour point and viscosity of lubricants [159]. Branching also has the associated benefit of reducing the levels of unsaturation which increases oxidative stability [159,164,165]. However, branched molecules which tend to have more tertiary carbons, have lower OS than corresponding linear biobased lubricant molecules because of oxidative degradation reactions at their C–H bonds [50,57].
5.5.1. Degree of Branching—Number and Type of Pendant Groups
The increase in the number of branch groups and branch chain length in a linear lubricant backbone decreases because pendant groups inhibit regular molecular stacking during crystallization [6]. Additionally, branch chains contribute to via increased molecular flexibility, particularly when they are long (>4 carbons) [181,191]. This can dramatically reduce the PP, therefore improving the low-temperature performance of the VO-based lubricant [6,7,50,114,115,116,192]. For instance, various biobased isoalkanes available commercially can achieve pour points as low as −81 °C despite being fully saturated [178]. Figure 12a shows the effect of the number of branch groups on for different series of branched ester-based lubricants. Figure 12b illustrates the structural changes on using 18-18 monoesters as a representative series.
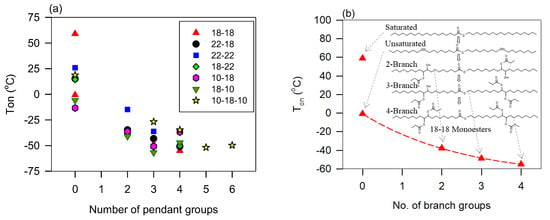
Figure 12.
(a) Onset temperature of crystallization () versus the number of branch groups on the pour point for the various series of esters and ester-based lubricants with constant backbone length. and (b) variation of pour point of 18-18 monoesters with the number of pendant groups.
5.5.2. Length of Branches
The chain length of the branches is shown to linearly decrease PP. The branching of tri-ester derivatives of oleic acid with chains of 6 to 14 carbons followed straight lines (R2 > 0.9799, Figure 13) with slopes varying from −1.95 to −0.78 °C/carbon atom [4,31,193].
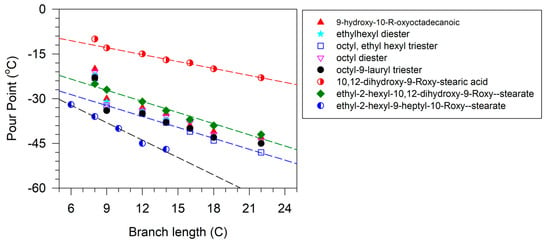
Figure 13.
Pour point versus branch length of branched tri-ester derivatives of oleic acid [4,31,194].
5.5.3. Position of the Branches
The position of the branch also has an effect: branching towards the center of the molecule provides a greater decrease in PP [7,195,196]. The location of the branching also affects cold-flow performance, with the alcohol moiety having the most influence on the thermal transition behavior of ester materials. clearly illustrates the effect of symmetry on ester compounds. For example, Yao et al. [197] reported that the PP of diesters with the 2-ethylhexyl alcohol moieties is lower (−60 °C) than those with the 2-ethylbutyl alcohol moiety (−35 °C). Generally, for the same chain length, the PP of hydrocarbon-branched diesters decreases with increasing the alcohol chain.
The gains achieved in PP, and from pendant chains depend on their nature, size, and location in the molecule backbone. The contributions of these structural features can be advantageous or disadvantageous.
5.6. Influence of Molecular Symmetry
The dependence of thermal transition behavior on molecular symmetry is reported for saturated wax monoesters as well as hydrocarbon-branched esters. Symmetrical molecules have higher rotational symmetry numbers than asymmetrical molecules. The symmetry number can be used to estimate the probability of the molecule being properly oriented for incorporation into the crystal lattice [152]. Lubricants with high molecular symmetry reduce because the molecules have a higher probability of presenting in the correct orientation for incorporation into the crystal lattice [50]. For lubricants, designing molecules with low symmetry numbers is desirable as it increases . Symmetrical molecules are more easily crystallized and have higher due to lower than asymmetrical molecules of the same size [127].
Patel et al. [179] reported that saturated asymmetric esters present melting points 1 °C to 5 °C lower than saturated symmetric esters. The structural changes in the alcohol moiety of a given ester are shown to influence thermal transition behavior more significantly compared to similar changes in the fatty acid moieties. For the same total chain length, the thermal transition temperatures of jojoba such as monoesters (JLE) with longer alcohol chains than the fatty acid chain are consistently higher [7,45,198].
All the structures of a biobased lubricant are constrained by molecular symmetry. The analysis of contributions from chain type, unsaturation, functional groups, and branching, discussed above obey the rules dictated by symmetry considerations.
6. Challenges and Perspectives
6.1. Challenges
The first issue facing VO-based lubricants is the source itself which poses major ethical problems in terms of competition with food and land requirements [199]. Even if new sources of renewable oils that do not compete with food are found, their transformation into usable materials is still challenging. The large-scale production of biobased lubricants requires increased crop cultivation which may lead to deforestation, habitat destruction, and increased water and pesticide usage unless they are produced in a bioreactor [200].
Current biobased lubricants have substandard oxidative stability because of the high susceptibility of their double bonds and β-CH group of their ester groups to radical attack. This leads to oxidative degradation and produces insoluble deposits that increase the biobased lubricant acidity and viscosity [201]. Biobased lubricants also have poor corrosion protection especially when moisture is present. The low-temperature characteristics of biobased lubricants include cloudiness, precipitation, poor flowability, and crystallization at relatively high temperatures [42]. Furthermore, biobased lubricants may not be compatible with certain machinery and equipment materials, such as seals and gaskets, which can result in swelling, leaking, or premature degradation.
Before using VOs as feedstock, physical and chemical refining is necessary. Multiple synthetic routes are needed to convert the TAG, fatty acids, and fatty alcohol molecules into crude lubricants. The resulting crude lubricants may need to be purified to ensure optimal performance. The refining, multiple synthesis steps, characterization, and analysis increase the cost of production of the VO-based lubricants. Because of the multiple-step production processes, biobased lubricant costs between 30 and 40% more compared to conventional lubricants [202].
6.2. Perspectives
There are opportunities to improve on the disadvantages of biobased lubricants. The first is using wasteland, growing non-edible, cheaper, and reliable oil-bearing feedstocks, and developing more efficient production methods [200]. Genetic engineering of oilseed crops can be tailored to increase yields and produce desired lipid profiles for lubricant production. The recycling and reusing of waste VOs, such as cooking oils, can provide a cost-effective source for VO-based lubricants. In that regard, innovative technologies are being developed to convert waste oils into high-quality lubricants through processes such as refining, purification, and modification. The blending of different feedstocks may be a means to improve properties and harness synergies that may emerge. Feedstock produced in bioreactors by algae and bacteria may also benefit from such approaches.
A critical part of the process of making biobased lubricants is the chemical transformations involved. New synthetic pathways to make novel molecular structures and cheaper biobased lubricants need to be further explored. As the industry develops, along with improved production efficiency, greener chemical routes need to be developed. A part of this endeavor is the development of efficient catalysts. Besides improving on the well-known modifications such as transesterification, epoxidation, hydrogenation, and esterification, emerging techniques such as enzymatic modification and microbial fermentation can be further developed to make specialized material with improved stability, reduced viscosity and enhanced thermal and oxidative stabilities [78]. Advanced computational modeling techniques, such as molecular dynamics simulations, can help optimize the structures and properties of biobased lubricants and guide the development of new formulations with improved performance.
Additives are an important component of lubricants that cannot be ignored in biobased formulations. The drive to use performance and environmentally friendly additives such as antioxidants, anti-wear agents, friction modifiers, and viscosity modifiers are a hot topic of research that would benefit from further research and development [203]. Nano-additives may be used to enhance wear resistance, reduce friction and provide better load-carrying capacity, therefore increasing the overall performance of the lubricant.
Another step to take these materials from the laboratory to the market is needed. It is imperative to confirm the tribological behavior of the new biobased lubricants and test them within existing machinery and systems to determine performance properties such as friction coefficient and wear. Additionally, their hydrolytic and oxidative stabilities which influence the shelf and use life of the materials need to be part of this research effort.
Consumer acceptance of VO-based lubricants is a major concern. Additionally, the environmental benefits, particularly the biodegradability and carbon footprint of VO-based lubricants need to be popularized for a larger informed acceptance. Life cycle and environmental assessment and management are therefore a priority for research and industry.
Meaningful engagements between manufacturers, environmental organizations, and regulatory agencies should be encouraged to seek the establishment of protocols for the development and implementation of technologies in ways that are socially beneficial, ecologically sustainable, and economically viable.
7. Conclusions
Biobased lubricants are an important category of products in the transition from fossil sources towards renewable and biobased products. They present several advantages over conventional lubricants derived from mineral oils, but their application on a commercial scale is still limited because of limitations in the requirements for production, performance, and cost.
This critical review of the literature on VO-based lubricants identified the molecular mass, level of unsaturation, degree of branching, and functional groups such as ester linkages and hydroxyl groups as the key molecular structures that determine lubricant performance. The contributions of these structures to lubricating functionality depend on common structural design elements including molecular symmetry, isomerism, and the odd–even effect. The specific roles that size, type, number, position, spatial arrangement, and symmetry play in determining lubricating functionality were elucidated.
The collation of flow and thermal transition data with attention paid to differences in conditions under which the various derivatives have been evaluated in terms of lubricating properties allowed the establishment of empirical predictive relationships between these properties and structures such as fatty chain length, number and type of functional groups. The role of symmetry and isomerism on these properties was quantified. A key finding is that function is generally linked to a structure by a simple linear or exponential function. Molecular mass, for example, is a fundamental determinant of viscosity and transition temperatures, but it is not a definitive predictor because of the distinct influence of other factors such as polar groups, branching, and symmetry. For example, within the 95% prediction band that an exponential rise to a maximum function encloses practically all the viscosity data versus molecular mass (R2 = 0.7897), particular and more refined predictive relationships can be extracted for particular molecular architectures. Not surprisingly, the unbranched VO-based lubricants lend themselves more easily to predictive structure–function relationships.
The VO-based ester lubricants demonstrate excellent low temperature and flow behavior, as well as high thermal and oxidative stabilities. These excellent properties are non-trivial achievements, and these esters represent the next generation of superior VO-based materials with increased inherent lubricity and biodegradability for use in commercial lubricants.
The knowledge gained in this work is unique, never reported before, and may be a milestone in the understanding of a class of compounds that is just starting to be researched. As with any other work, however, many questions have been raised that need to be answered. The use of functional groups in lipid-based lubricants remains an area for future investigation and optimization. Additionally, instead of using the oleyl oleate monoester backbone, other unsaturated backbones such as diesters and triesters can be used to create new VO-based ester lubricants with superior viscosity and cold-flow performance. Other new chemical and physical properties may emerge from these materials.
The new insights gained can be used to extend the ranges of chemical and physical properties of some of the molecules examined. The structure–property relationships presented in this work have been semi-empirically derived. These may be used to feed existing databases, or as a basis to develop first-principle models. Either way, this is the first step in the development of robust predictive systems with the goal of informing smart molecular designs and decreasing dependence upon expensive semi-empirical approaches for materials development.
Author Contributions
N.S.: Formal analysis, Writing Original draft preparation, Investigation, Data curation, Visualization, Validation. L.B.: Conceptualization, Formal analysis, Writing—Reviewing and Editing, Methodology, Software, Validation, Term, Validation, Visualization. S.S.N.: Conceptualization, Formal analysis, Writing—Reviewing and Editing, Supervision, Visualization, Funding acquisition, Project Administration, Validation, Methodology, Resources. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The Data used for this work was mined from the literature. It is duly referenced and can be easily found. No data that is not available from published works is used.
Conflicts of Interest
The authors declare no conflict of interest.
Nomenclature/Abbreviations and Names and Molecular Structures of Compounds Used
| ASTM International | Formerly American Society for Testing and Materials | Units |
| BP | Boiling Point | °C |
| CFP | Cold-Flow Properties | -- |
| CP | Cloud Point | °C |
| DAG | Diacylglycerol | -- |
| DIN | Deutsches Institut für Normung (German Institute for Standardization) | -- |
| EN | Europäische Norm (European Standards) | -- |
| FAEE | Fatty Acid Ethyl Esters | -- |
| FAME | Fatty Acid Methyl Ester | -- |
| FP | Flash Point | °C |
| FWHM | Full Width at Half Maximum | °C |
| ISO | International Organization for Standardization | -- |
| JLE | Jojoba-Like Ester | -- |
| Boltzmann constant | J/K | |
| MM | Molecular Mass | amu |
| Characteristic molecular mass | amu | |
| Chain length in number of carbons | -- | |
| Characteristic Number of Carbons | -- | |
| OS | Oxidative Stability | -- |
| PAO | Polyalphaolefin | -- |
| PP | Pour Point | °C |
| Power Index | ||
| TAG | Triacylglycerol | -- |
| Onset temperature | °C | |
| Peak temperature | °C | |
| Melting Point | °C | |
| Offset temperature | °C | |
| Characteristic Temperature | °C | |
| TS | Thermal Stability | -- |
| VI | Viscosity Index | -- |
| VO | Vegetable Oil | -- |
| Gibbs Free Energy at Solid–Liquid Transition | J | |
| Change in Enthalpy of Melting | J/g | |
| Change in Entropy of Melting | J/(g·°C) | |
| Viscosity | Pa·s | |
| Number of Hydroxyl Groups (OH) | ||
| Characteristic Number of Hydroxyl Groups (OH) |
| Names and Molecular Structures | |||
| Class of Compounds | Scheme # | Figure # | Generalized Molecular Structure |
| Vegetable Oil TAG | Scheme 3 | Figure 5a | 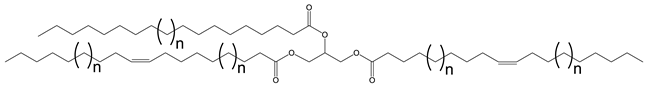 |
| Linear Saturated Monoesters | Scheme 5 | Figure 5a and Figure 8 | 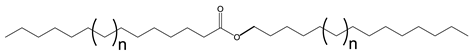 |
| Linear unsaturated Monoesters | Scheme 5 | Figure 5a, Figure 6a, Figure 7 and Figure 8 | 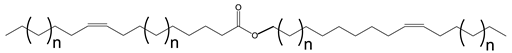 |
| Linear saturated diol diesters | -- | Figure 5a and Figure 8 | 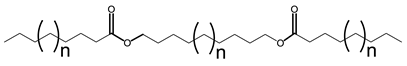 |
| Linear saturated dibasic diesters | -- | Figure 5a and Figure 8 | 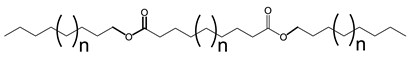 |
| Linear unsaturated diol diesters | Scheme 5 | Figure 5a,b and Figure 8 | 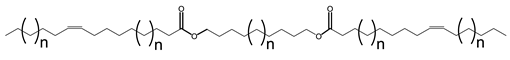 |
| Linear unsaturated dibasic diesters | Scheme 5 | Figure 5a and Figure 8 |  |
| Undecenoates | -- | Figure 5a |  |
| Branched-unsaturated dibasic diesters | -- | Figure 5a and Figure 13 | 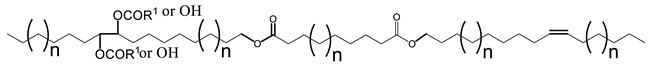 |
| Branched Monoesters | -- | Figure 6, Figure 12a,b and Figure 13 |  |
| Branched diol diesters | Scheme 6 | Figure 5a and Figure 6a |  |
| Branched dibasic diesters | Scheme 6 | Figure 5a, Figure 6a and Figure 7 |  |
| Sulfide-branched monoesters | Scheme 7 | -- |  |
| Sulfonyl-branched monoesters | Scheme 7 | -- | 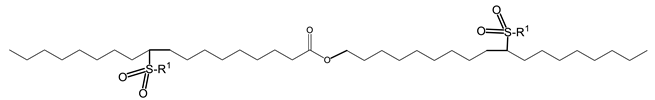 |
| Diester (H0-H6) | -- | Figure 3 and Figure 6 | 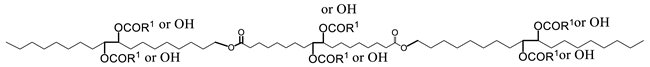 |
| Saturated fatty acids | -- | Figure 9 |  |
| Unsaturated fatty acid | -- | Figure 10 and Figure 11 |  |
| Saturated fatty acid methyl esters (FAMEs) | -- | Figure 9 | 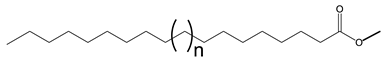 |
| Unsaturated fatty acid methyl esters (FAMEs) | -- | Figure 10 and Figure 11 | 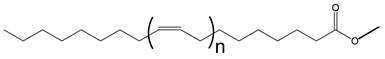 |
| Saturated fatty acid ethyl esters (FAEEs) | -- | Figure 9 | 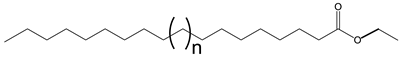 |
| Unsaturated fatty acid ethyl esters (FAEEs) | -- | Figure 10 | 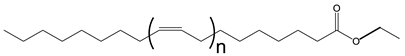 |
| Saturated fatty acid propyl esters (FAPEs) | -- | Figure 9 |  |
References
- Lee, C.T.; Lee, M.B.; Mong, G.R.; Chong, W.W.F. A bibliometric analysis on the tribological and physicochemical properties of vegetable oil–based bio-lubricants (2010–2021). Environ. Sci. Pollut. Res. 2022, 29, 56215–56248. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.P. Plant-oil-based lubricants and hydraulic fluids. J. Sci. Food Agr. 2006, 86, 1769–1780. [Google Scholar] [CrossRef]
- Reeves, C.J.; Siddaiah, A.; Menezes, P.L. A Review on the Science and Technology of Natural and Synthetic Biolubricants. J. Bio. Tribo. Corros. 2017, 3, 11. [Google Scholar] [CrossRef]
- Bart, J.C.J.; Gucciardi, E.; Cavallaro, S. 6—Chemical transformations of renewable lubricant feedstocks. In Biolubricants; Bart, J.C.J., Gucciardi, E., Cavallaro, S., Eds.; Woodhead Publishing: Philadelphia, PA, USA, 2013; pp. 249–350. [Google Scholar]
- Rudnick, L.R. Synthetics, Mineral Oils, and Bio-Based Lubricants: Chemistry and Technology; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Chan, C.-H.; Tang, S.W.; Mohd, N.K.; Lim, W.H.; Yeong, S.K.; Idris, Z. Tribological behavior of biolubricant base stocks and additives. Renew. Sust. Energ. Rev. 2018, 93, 145–157. [Google Scholar] [CrossRef]
- Mortier, R.M.; Orszulik, S.T.; Fox, M.F. Chemistry and Technology of Lubricants; Springer: Berlin/Heidelberg, Germany, 2010; Volume 107115. [Google Scholar]
- Giakoumis, E.G. Analysis of 22 vegetable oils’ physico-chemical properties and fatty acid composition on a statistical basis, and correlation with the degree of unsaturation. Renew. Energy 2018, 126, 403–419. [Google Scholar] [CrossRef]
- Shafi, W.K.; Charoo, M.; Hanief, M. Effect of fatty acid composition on the lubricating properties of bio-based green lubricants. In Tribology and Sustainability; CRC Press: Boca Raton, FL, USA, 2021; pp. 231–250. [Google Scholar]
- Syahir, A.Z.; Zulkifli, N.W.M.; Masjuki, H.H.; Kalam, M.A.; Alabdulkarem, A.; Gulzar, M.; Khuong, L.S.; Harith, M.H. A review on bio-based lubricants and their applications. J. Clean. Prod. 2017, 168, 997–1016. [Google Scholar] [CrossRef]
- Minami, I. Molecular Science of Lubricant Additives. Appl. Sci. 2017, 7, 445. [Google Scholar] [CrossRef]
- MaximizeMarketResearch. Global Lubricant Market: Industry Analysis and Forecast (2021–2027) by Type, Fluid Type, and Region. Available online: https://www.maximizemarketresearch.com/market-report/global-lubricant-market/29070/ (accessed on 28 June 2023).
- Statista. Global Lubricant Demand 2000–2028. Available online: https://www.statista.com/statistics/411616/lubricants-demand-worldwide (accessed on 28 June 2023).
- fortunebusinessinsights. Lubricants Market Sise, Shares & COVID-19 Impact Analysis. Available online: https://www.fortunebusinessinsights.com/industry-reports/lubricants-market-101771 (accessed on 28 June 2023).
- Pirro, D.M.; Webster, M.; Daschner, E. Lubrication Fundamentals: Revised and Expanded, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2016; p. 578. [Google Scholar]
- Narayana Sarma, R.; Vinu, R. Current Status and Future Prospects of Biolubricants: Properties and Applications. Lubricants 2022, 10, 70. [Google Scholar] [CrossRef]
- Rawat, S.S.; Harsha, A.P. Current and Future Trends in Grease Lubrication. In Automotive Tribology; Katiyar, J.K., Bhattacharya, S., Patel, V.K., Kumar, V., Eds.; Springer: Singapore, 2019; pp. 147–182. [Google Scholar]
- Negi, P.; Singh, Y.; Tiwari, K. A review on the production and characterization methods of bio-based lubricants. Mater. Today Proc. 2021, 46, 10503–10506. [Google Scholar] [CrossRef]
- Sancheti, S.V.; Yadav, G.D. Synthesis of environment-friendly, sustainable, and nontoxic bio-lubricants: A critical review of advances and a path forward. Biofuel Bioprod. Bior. 2022, 16, 1172–1195. [Google Scholar] [CrossRef]
- Whitby, R.D. Lubricant Blending and Quality Assurance; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Cecilia, J.A.; Ballesteros Plata, D.; Alves Saboya, R.M.; Tavares de Luna, F.M.; Cavalcante, C.L.; Rodríguez-Castellón, E. An Overview of the Biolubricant Production Process: Challenges and Future Perspectives. Processes 2020, 8, 257. [Google Scholar] [CrossRef]
- Singh, Y.; Farooq, A.; Raza, A.; Mahmood, M.A.; Jain, S. Sustainability of a non-edible vegetable oil based bio-lubricant for automotive applications: A review. Process. Saf. Environ. Prot. 2017, 111, 701–713. [Google Scholar] [CrossRef]
- Farfan-Cabrera, L.I.; Franco-Morgado, M.; González-Sánchez, A.; Pérez-González, J.; Marín-Santibáñez, B.M. Microalgae Biomass as a New Potential Source of Sustainable Green Lubricants. Molecules 2022, 27, 1205. [Google Scholar] [CrossRef]
- Pawar, R.V.; Hulwan, D.B.; Mandale, M.B. Recent advancements in synthesis, rheological characterization, and tribological performance of vegetable oil-based lubricants enhanced with nanoparticles for sustainable lubrication. J. Clean. Prod. 2022, 378, 134454. [Google Scholar] [CrossRef]
- McNutt, J. Development of biolubricants from vegetable oils via chemical modification. J. Ind. Eng. Chem. 2016, 36, 1–12. [Google Scholar] [CrossRef]
- Mobarak, H.M.; Niza Mohamad, E.; Masjuki, H.H.; Kalam, M.A.; Al Mahmud, K.A.H.; Habibullah, M.; Ashraful, A.M. The prospects of biolubricants as alternatives in automotive applications. Renew. Sust. Energ. Rev. 2014, 33, 34–43. [Google Scholar] [CrossRef]
- Bartz, W.J. Lubricants and the environment. Tribol. Int. 1998, 31, 35–47. [Google Scholar] [CrossRef]
- Polaris-Market-Research. Bio-Lubricants Market Share, Size, Trends, Industry Analysis Report, By Base Oil Type (Vegetable Oils, Animal Fats, Others); By Application; By End-Use; By Region; Segment Forecast, 2022–2030. Available online: https://www.polarismarketresearch.com/industry-analysis/bio-lubricants-market (accessed on 26 June 2023).
- Betton, C.I. Lubricants and Their Environmental Impact. In Chemistry and Technology of Lubricants; Mortier, R.M., Fox, M.F., Orszulik, S.T., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 435–457. [Google Scholar]
- Sosa, Y. Acceleration to lubricants sustainability. Tribol. Lubr. Technol. 2022, 78, 44–50. [Google Scholar]
- Erhan, S.Z.; Sharma, B.K.; Liu, Z.; Adhvaryu, A. Lubricant base stock potential of chemically modified vegetable oils. J. Agr. Food Chem. 2008, 56, 8919–8925. [Google Scholar] [CrossRef]
- Freschi, M.; Paniz, A.; Cerqueni, E.; Colella, G.; Dotelli, G. The Twelve Principles of Green Tribology: Studies, Research, and Case Studies—A Brief Anthology. Lubricants 2022, 10, 129. [Google Scholar] [CrossRef]
- Salimon, J.; Salih, N.; Yousif, E. Biolubricants: Raw materials, chemical modifications and environmental benefits. Eur. J. Lipid Sci. Technol. 2010, 112, 519–530. [Google Scholar] [CrossRef]
- Ho, C.K.; McAuley, K.B.; Peppley, B.A. Biolubricants through renewable hydrocarbons: A perspective for new opportunities. Renew. Sust. Energ. Rev. 2019, 113, 109261. [Google Scholar] [CrossRef]
- Miller, A.L.; Stipe, C.B.; Habjan, M.C.; Ahlstrand, G.G. Role of Lubrication Oil in Particulate Emissions from a Hydrogen-Powered Internal Combustion Engine. Environ. Sci. Technol. 2007, 41, 6828–6835. [Google Scholar] [CrossRef] [PubMed]
- Salih, N.; Salimon, J. A review on new trends, challenges and prospects of ecofriendly friendly green food-grade biolubricants. Biointerface Res. Appl. Chem. 2021, 12, 1185–1207. [Google Scholar] [CrossRef]
- De Wilde, B.; Mortier, N.; Siotto, M.; Briassoulis, D.; Mistriotis, A.; van der Zee, M. Biodegradability Standards Assessment Report; European Union: Ghent, Belgium, 2015; p. 40. [Google Scholar]
- Arnsek, A.; Vizintin, J. Scuffing load capacity of rapeseed-based oils (c). Lubric. Eng. 1999, 55, 11–18. [Google Scholar]
- Singh, R. Progress of environment friendly cutting fluids/solid lubricants in turning-A review. Mater. Today: Proc. 2021, 37, 3577–3580. [Google Scholar] [CrossRef]
- Uppar, R.; Dinesha, P.; Kumar, S. A critical review on vegetable oil-based bio-lubricants: Preparation, characterization, and challenges. Environ. Dev. Sustain. 2022, 24, 1471–11513. [Google Scholar] [CrossRef]
- EPA. Environmentally Acceptable Lubricants; EPA 800-R-11-002; United States Environmental Protection Agency: Washington, DC, USA, 2011; p. 27. [Google Scholar]
- Zainal, N.; Zulkifli, N.; Gulzar, M.; Masjuki, H. A review on the chemistry, production, and technological potential of bio-based lubricants. Renew. Sust. Energ. Rev. 2018, 82, 80–102. [Google Scholar] [CrossRef]
- Ouellette, R.J.; Rawn, J.D. 1—Structure and Bonding in Organic Compounds. In Organic Chemistry, 2nd ed.; Ouellette, R.J., Rawn, J.D., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 1–30. [Google Scholar]
- Knothe, G.; Steidley, K.R. Lubricity of Components of Biodiesel and Petrodiesel. The Origin of Biodiesel Lubricity. Energ. Fuel 2005, 19, 1192–1200. [Google Scholar] [CrossRef]
- Knothe, G.; Dunn, R.O. A comprehensive evaluation of the melting points of fatty acids and esters determined by differential scanning calorimetry. J. Am. Oil Chem. Soc. 2009, 86, 843–856. [Google Scholar] [CrossRef]
- Wagner, J.P.; Schreiner, P.R. London Dispersion in Molecular Chemistry—Reconsidering Steric Effects. Angew. Chem. Int. Ed. 2015, 54, 12274–12296. [Google Scholar] [CrossRef]
- Reeves, C.J.; Menezes, P.L.; Jen, T.-C.; Lovell, M.R. The influence of fatty acids on tribological and thermal properties of natural oils as sustainable biolubricants. Tribol. Int. 2015, 90, 123–134. [Google Scholar] [CrossRef]
- Bunn, C.W. The melting points of chain polymers. J. Polym. Sci. B: Polym. Phys. 1996, 34, 799–819. [Google Scholar] [CrossRef]
- Wiberg, K.B.; Laidig, K.E. Barriers to rotation adjacent to double bonds. 3. The carbon-oxygen barrier in formic acid, methyl formate, acetic acid, and methyl acetate. The origin of ester and amide resonance. J. Am. Chem. Soc. 1987, 109, 5935–5943. [Google Scholar] [CrossRef]
- Yalkowsky, S.H.; Alantary, D. Estimation of Melting Points of Organics. J. Pharm. Sci. 2018, 107, 1211–1227. [Google Scholar] [CrossRef]
- Lian, B.; Yalkowsky, S.H. Molecular geometry and boiling related thermodynamic properties. J. Chem. Thermodyn. 2012, 54, 250. [Google Scholar] [CrossRef]
- Jabbarzadeh, A.; Atkinson, J.D.; Tanner, R.I. The effect of branching on slip and rheological properties of lubricants in molecular dynamics simulation of Couette shear flow. Tribol. Int. 2002, 35, 35–46. [Google Scholar] [CrossRef]
- Boyde, S. Esters—Synthetics, Mineral Oils, and Bio-Based Lubricants. In Synthetics, Mineral Oils, and Bio-Based Lubricants; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Mackay, D.; Boethling, R.S. Handbook of Property Estimation Methods for Chemicals: Environmental Health Sciences; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Yalkowsky, S.H. Carnelley’s Rule and the Prediction of Melting Point. J. Pharm. Sci. 2014, 103, 2629–2634. [Google Scholar] [CrossRef]
- Soodoo, N.; Poopalam, K.D.; Bouzidi, L.; Narine, S.S. Exploiting aromaticity in fatty terephthalate diesters to enhance melting point and prevent polymorphism. Sol. Energy Mater. Sol. 2022, 238, 111650. [Google Scholar] [CrossRef]
- Soodoo, N.; Bouzidi, L.; Narine, S.S. Effect of Pendant Sulfide and Sulfonyl groups on the Thermal, Flow and Antioxidative Properties of Lipid Based Aliphatic Monoesters. Ind. Eng. Chem. Res. 2023, 18601–18612. [Google Scholar] [CrossRef]
- Sanchez, N.; Martinez, M.; Aracil, J.; Corma, A. Synthesis of oleyl oleate as a jojoba oil analog. J. Am. Oil Chem. Soc. 1992, 69, 1150–1153. [Google Scholar] [CrossRef]
- Hammond, E. Vegetable oils Types and Properties. 2003. Available online: https://www.sciencedirect.com/topics/neuroscience/vegetable-oil (accessed on 28 June 2023).
- Miwa, T.K. Jojoba oil wax esters and derived fatty acids and alcohols: Gas chromatographic analyses. J. Am. Oil Chem. Soc. 1971, 48, 259–264. [Google Scholar] [CrossRef]
- Sánchez, M.; Marchetti, J.M.; Boulifi, N.E.; Martínez, M.; Aracil, J. Jojoba oil biorefinery using a green catalyst. Part I: Simulation of the process. Biofuel Bioprod. Bior. 2015, 9, 129–138. [Google Scholar] [CrossRef]
- Canoira, L.; Alcantara, R.; García-Martínez, M.J.; Carrasco, J. Biodiesel from Jojoba oil-wax: Transesterification with methanol and properties as a fuel. Biomass Bioenerg. 2006, 30, 76–81. [Google Scholar] [CrossRef]
- El Kinawy, O. Comparison Between Jojoba Oil and Other Vegetable Oils as a Substitute to Petroleum. Energy Sources 2004, 26, 639–645. [Google Scholar] [CrossRef]
- No, S.-Y. Inedible vegetable oils and their derivatives for alternative diesel fuels in CI engines: A review. Renew. Sust. Energ. Rev. 2011, 15, 131–149. [Google Scholar] [CrossRef]
- Demirbas, A. Progress and recent trends in biodiesel fuels. Energy Convers. Manag. 2009, 50, 14–34. [Google Scholar] [CrossRef]
- Bouzidi, L.; Li, S.; Di Biase, S.; Rizvi, S.Q.; Dawson, P.; Narine, S.S. Lubricating and Waxy Esters II: Synthesis, Crystallization, and Melt Behavior of Branched Monoesters. Ind. Eng. Chem. Res. 2012, 51, 14892–14902. [Google Scholar] [CrossRef]
- Martinez, M.; Torrano, E.; Aracil, J. Synthesis of esters of high molecular weight. An analog of jojoba oil. A statistical approach. Ind. Eng. Chem. Res. 1988, 27, 2179–2182. [Google Scholar] [CrossRef]
- Coteron, A.; Sánchez, N.; Martinez, M.; Aracil, J. Optimisation of the synthesis of an analogue of jojoba oil using a fully central composite design. Canadian J. Chem. Eng. 1993, 71, 485–488. [Google Scholar] [CrossRef]
- Yadav, G.D.; Lathi, P.S. Kinetics and mechanism of synthesis of butyl isobutyrate over immobilised lipases. Biochem. Eng. J. 2003, 16, 245–252. [Google Scholar] [CrossRef]
- Aracil, J.; Martinez, M.; Sanchez, N.; Corma, A. Formation of a Jojoba Oil Analog by Esterification of Oleic-Acid Using Zeolites as Catalyst. Zeolites 1992, 12, 233–236. [Google Scholar] [CrossRef]
- Vilas Bôas, R.N.; de Castro, H.F. A review of synthesis of esters with aromatic, emulsifying, and lubricant properties by biotransformation using lipases. Biotechnol. Bioeng. 2022, 119, 725–742. [Google Scholar] [CrossRef]
- Bouzidi, L.; Li, S.; Di Biase, S.; Rizvi, S.Q.; Narine, S.S. Lubricating and waxy esters. 4. Synthesis, crystallization behavior, melt behavior, and flow behavior of linear monoesters incorporating 9-decenol and 9-decenoic Acid. Ind. Eng. Chem. Res. 2013, 52, 2740–2749. [Google Scholar] [CrossRef]
- Li, S.; Bouzidi, L.; Narine, S.S. Lubricating and Waxy Esters, V: Synthesis, Crystallization, and Melt and Flow Behaviors of Branched Monoesters Incorporating 9-Decenol and 9-Decenoic Acid. Ind. Eng. Chem. Res. 2014, 53, 12339–12354. [Google Scholar] [CrossRef]
- Bolina, I.C.A.; Gomes, R.A.B.; Mendes, A.A. Biolubricant Production from Several Oleaginous Feedstocks Using Lipases as Catalysts: Current Scenario and Future Perspectives. Bioenerg. Res. 2021, 14, 1039–1057. [Google Scholar] [CrossRef]
- Mendes, A.A.; Soares, C.M.F.; Tardioli, P.W. Recent advances and future prospects for biolubricant base stocks production using lipases as environmentally friendly catalysts: A mini-review. World J. Microbiol. Biotechnol. 2022, 39, 25. [Google Scholar] [CrossRef]
- Wu, S.; Snajdrova, R.; Moore, J.C.; Baldenius, K.; Bornscheuer, U.T. Biocatalysis: Enzymatic Synthesis for Industrial Applications. Angew. Chem. Int. Ed. 2021, 60, 88–119. [Google Scholar] [CrossRef]
- Erdem, E.; Woodley, J.M. Industrially useful enzymology: Translating biocatalysis from laboratory to process. Chem. Catalysis 2022, 2, 2499–2505. [Google Scholar] [CrossRef]
- Appiah, G.; Tulashie, S.K.; Akpari, E.E.A.; Rene, E.R.; Dodoo, D. Biolubricant production via esterification and transesterification processes: Current updates and perspectives. Int. J. Energy Res. 2021, 46, 3860–3890. [Google Scholar] [CrossRef]
- Sammaiah, A.; Padmaja, K.V.; Prasad, R.B.N. Synthesis and Evaluation of Novel Acyl Derivatives from Jatropha Oil as Potential Lubricant Basestocks. J. Agr. Food Chem. 2014, 62, 4652–4660. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Hammond, E.; Wang, T.; Bhuyan, S.; Sundararajan, S. Synthesis and Physical Properties of Potential Biolubricants based on Ricinoleic Acid. J. Am. Oil Chem. Soc. 2010, 87, 937–945. [Google Scholar] [CrossRef]
- Ieda, N.; Mantri, K.; Miyata, Y.; Ozaki, A.; Komura, K.; Sugi, Y. Esterification of Long-Chain Acids and Alcohols Catalyzed by Ferric Chloride Hexahydrate. Ind. Eng. Chem. Res. 2008, 47, 8631–8638. [Google Scholar] [CrossRef]
- Gunstone, F.D.; Harwood, J.L.; Dijkstra, A.J. The Lipid Handbook with CD-ROM, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Garcia, T.; Martinez, M.; Aracil, J. Enzymatic synthesis of an analogue of jojoba oil: Optimization by statistical analysis. Enzyme Microb. Technol. 1993, 15, 607–611. [Google Scholar] [CrossRef]
- Vonghia, E.; Boocock, D.G.; Konar, S.K.; Leung, A. Pathways for the deoxygenation of triglycerides to aliphatic hydrocarbons over activated alumina. Energ. Fuel. 1995, 9, 1090–1096. [Google Scholar] [CrossRef]
- Zhang, C.; Garrison, T.F.; Madbouly, S.A.; Kessler, M.R. Recent advances in vegetable oil-based polymers and their composites. Prog. Polym. Sci. 2017, 71, 91–143. [Google Scholar] [CrossRef]
- Moser, B.R.; Cermak, S.C.; Doll, K.M.; Kenar, J.A.; Sharma, B.K. A review of fatty epoxide ring opening reactions: Chemistry, recent advances, and applications. J. Am. Oil Chem. Soc. 2022, 99, 801–842. [Google Scholar] [CrossRef]
- Gupta, M.K. Chapter 7—Hydrogenation_Practical Guide to Vegetable Oil Processing (Second Edition). In Practical Guide to Vegetable Oil Processing, 2nd ed.; Gupta, M.K., Ed.; AOCS Press: Urbana, IK, USA, 2017; pp. 171–215. [Google Scholar]
- Freeman, I.P. Margarines and Shortenings_Ullmann’s Encyclopedia of Industrial Chemistry. In Ullmann’s Encyclopedia of Industrial Chemistry; Wileys: Hobokan, NJ, USA, 2000. [Google Scholar]
- Vanbesien, T.; Hapiot, F.; Monflier, E. Hydroformylation of vegetable oils and the potential use of hydroformylated fatty acids. Lipid Technol. 2013, 25, 175–178. [Google Scholar] [CrossRef]
- Karmakar, G.; Ghosh, P.; Kohli, K.; Sharma, B.K.; Erhan, S.Z. Chemicals from Vegetable Oils, Fatty Derivatives, and Plant Biomass. In Innovative Uses of Agricultural Products and Byproducts; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2020; Volume 1347, pp. 1–31. [Google Scholar]
- Sammaiah, A.; Padmaja, K.V.; Prasad, R.B. Tribology and oxidation studies of fatty acid sulfide derivatives synthesized via thiol-ene “Click” additions. Eur. J. Lipid Sci. Technol. 2016, 118, 495–502. [Google Scholar] [CrossRef]
- Wu, J.; Shashi, F.; Dimuthu, W.; Zhigang, C.; Webster, D. Synthesis of Soybean Oil-Based Thiol Oligomers. ChemSusChem 2011, 4, 1135–1142. [Google Scholar] [CrossRef]
- Ionescu, M.; Radojčić, D.; Wan, X.; Petrović, Z.S.; Upshaw, T.A. Functionalized vegetable oils as precursors for polymers by thiol-ene reaction. Eur. Polym. J. 2015, 67, 439–448. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Hupin, S.; Lecamp, L.; Vuluga, D.; Afonso, C.; Burel, F.; Loutelier-Bourhis, C. Thiol–ene chemistry of vegetable oils and their derivatives under UV and air: A model study by using infrared spectroscopy and mass spectrometry. RSC Adv. 2017, 7, 3343–3352. [Google Scholar] [CrossRef]
- Montero de Espinosa, L.; Meier, M.A.R. Plant oils: The perfect renewable resource for polymer science?! Eur. Polym. J. 2011, 47, 837–852. [Google Scholar] [CrossRef]
- Bantchev, G.B.; Kenar, J.A.; Biresaw, G.; Han, M.G. Free Radical Addition of Butanethiol to Vegetable Oil Double Bonds. J. Agr. Food Chem. 2009, 57, 1282–1290. [Google Scholar] [CrossRef]
- Desroches, M.; Caillol, S.; Lapinte, V.; Auvergne, R.; Boutevin, B. Synthesis of Biobased Polyols by Thiol−Ene Coupling from Vegetable Oils. Macromolecules 2011, 44, 2489–2500. [Google Scholar] [CrossRef]
- Wai, P.T.; Jiang, P.; Shen, Y.; Zhang, P.; Gu, Q.; Leng, Y. Catalytic developments in the epoxidation of vegetable oils and the analysis methods of epoxidized products. RSC Adv. 2019, 9, 38119–38136. [Google Scholar] [CrossRef]
- Saurabh, T.; Patnaik, M.; Bhagt, S.; Renge, V. Epoxidation of vegetable oils: A review. Int. J. Adv. Eng. Technol. 2011, 2, 491–501. [Google Scholar]
- Karmakar, G.; Ghosh, P.; Sharma, B.K. Chemically Modifying Vegetable Oils to Prepare Green Lubricants. Lubricants 2017, 5, 44. [Google Scholar] [CrossRef]
- Kenar, J.A.; Knothe, G.; Copes, A.L. Synthesis and characterization of dialkyl carbonates prepared from mid-, long-chain, and guerbet alcohols. J. Am. Oil Chem. Soc. 2004, 81, 285–291. [Google Scholar] [CrossRef]
- Orrego Vallejo, A.; Ferretti, C.A.; Díez, V.K. Reduction of Vegetable Oil-Derived Fatty Acid Methyl Esters toward Fatty Alcohols without the Supply of Gaseous H2. J. Am. Oil Chem. Soc. 2020, 97, 1029–1042. [Google Scholar] [CrossRef]
- Sánchez, M.A.; Torres, G.C.; Mazzieri, V.A.; Pieck, C.L. Selective hydrogenation of fatty acids and methyl esters of fatty acids to obtain fatty alcohols–a review. J. Chem. Technol. Biotechnol. 2017, 92, 27–42. [Google Scholar] [CrossRef]
- Knothe, G.; Derksen, J.T.P. Recent Developments in the Synthesis of Fatty Acid Derivatives; Taylor & Francis: Milton Park, UK, 1999. [Google Scholar]
- Biswas, A.; Sharma, B.K.; Willett, J.L.; Erhan, S.Z.; Cheng, H.N. Soybean oil as a renewable feedstock for nitrogen-containing derivatives. Energy Environ. Sci. 2008, 1, 639–644. [Google Scholar] [CrossRef]
- Biermann, U.; Bornscheuer, U.T.; Feussner, I.; Meier, M.A.R.; Metzger, J.O. Fatty Acids and their Derivatives as Renewable Platform Molecules for the Chemical Industry. Angew. Chem. Int. Ed. 2021, 60, 20144–20165. [Google Scholar] [CrossRef] [PubMed]
- Salimon, J.; Abdullah, B.M.; Salih, N. Hydrolysis optimization and characterization study of preparing fatty acids from Jatropha curcas seed oil. Chem. Cent. J. 2011, 5, 67. [Google Scholar] [CrossRef]
- Chen, Y.; Biresaw, G.; Cermak, S.C.; Isbell, T.A.; Ngo, H.L.; Chen, L.; Durham, A.L. Fatty Acid Estolides: A Review. J. Am. Oil Chem. Soc. 2020, 97, 231–241. [Google Scholar] [CrossRef]
- Wagner, H.; Luther, R.; Mang, T. Lubricant base fluids based on renewable raw materials: Their catalytic manufacture and modification. Appl. Catal. Gen. 2001, 221, 429–442. [Google Scholar] [CrossRef]
- Raghunanan, L.; Narine, S.S. Influence of structure on chemical and thermal stability of aliphatic diesters. J. Phys. Chem. B 2013, 117, 14754–14762. [Google Scholar] [CrossRef]
- Schmidt, M.A.; Dietrich, C.R.; Cahoon, E.B. Biotechnological enhancement of soybean oil for lubricant applications. In Synthetics, Mineral Oils, and Bio-Based Lubricants, 1st ed.; Rudnick, L.R., Ed.; CRC Press: Boca Raton, FL, 2005; pp. 407–418. [Google Scholar]
- Kreutzer, U.R. Manufacture of fatty alcohols based on natural fats and oils. J. Am. Oil Chem. Soc. 1984, 61, 343–348. [Google Scholar] [CrossRef]
- Voeste, T.; Buchold, H. Production of fatty alcohols from fatty acids. J. Am. Oil Chem. Soc. 1984, 61, 350–352. [Google Scholar] [CrossRef]
- Castro, W.; Perez, J.M.; Erhan, S.Z.; Caputo, F. A study of the oxidation and wear properties of vegetable oils: Soybean oil without additives. J. Am. Oil Chem. Soc. 2006, 83, 47–52. [Google Scholar] [CrossRef]
- Fox, N.J.; Stachowiak, G.W. Vegetable oil-based lubricants—A review of oxidation. Tribol. Int. 2007, 40, 1035–1046. [Google Scholar] [CrossRef]
- Lathi, P.S.; Mattiasson, B. Green approach for the preparation of biodegradable lubricant base stock from epoxidized vegetable oil. Appl. Catal. B Environ. 2007, 69, 207–212. [Google Scholar] [CrossRef]
- Kulkarni, R.D.; Deshpande, P.S.; Mahajan, S.U.; Mahulikar, P.P. Epoxidation of mustard oil and ring opening with 2-ethylhexanol for biolubricants with enhanced thermo-oxidative and cold flow characteristics. Ind. Crop. Prod. 2013, 49, 586–592. [Google Scholar] [CrossRef]
- Kazemi, M.; Kohzadi, H.; Abdi, O. Alkylation of Thiols in Green Mediums. J. Mater. Environ. Sci. 2015, 6, 1451–1456. [Google Scholar]
- Soodoo, N.; Raghunanan, L.; Bouzidi, L.; Narine, S. Phase behavior of monosulfones: Use of high polarity sulfonyl groups to improve the thermal properties of lipid-based materials for PCM applications. Sol. Energy Mater. Sol. 2019, 201, 110115. [Google Scholar] [CrossRef]
- Padmaja, K.V.; Rao, B.V.S.K.; Reddy, R.K.; Bhaskar, P.S.; Singh, A.K.; Prasad, R.B.N. 10-Undecenoic acid-based polyol esters as potential lubricant base stocks. Ind. Crop. Prod. 2012, 35, 237–240. [Google Scholar] [CrossRef]
- Cermak, S.C.; Isbell, T.A. Synthesis and physical properties of estolide-based functional fluids. Ind. Crop. Prod. 2003, 18, 183–196. [Google Scholar] [CrossRef]
- Paredes, X.; Pensado, A.S.; Comuñas, M.J.P.; Fernández, J. Experimental Dynamic Viscosities of Dipentaerythritol Ester Lubricants at High Pressure. J. Chem. Eng. Data 2010, 55, 3216–3223. [Google Scholar] [CrossRef]
- Rani, S.; Joy, M.L.; Nair, K.P. Evaluation of physiochemical and tribological properties of rice bran oil—Biodegradable and potential base stoke for industrial lubricants. Ind. Crop. Prod. 2015, 65, 328–333. [Google Scholar] [CrossRef]
- Zhang, J.; Tan, A.; Spikes, H. Effect of Base Oil Structure on Elastohydrodynamic Friction. Tribol. Lett. 2016, 65, 13. [Google Scholar] [CrossRef]
- Jackson, A. Synthetic versus mineral fluids in lubrication. In Proceedings of the The International Tribology Conference, Melbourne, Australia, 2–4 December 1987. [Google Scholar]
- Kuhlman, R.E. Environmentally Friendly Lubrication Issues. In Encyclopedia of Tribology; Wang, Q.J., Chung, Y.-W., Eds.; Springer US: Boston, MA, USA, 2013; pp. 985–991. [Google Scholar]
- Salih, N.; Salimon, J.; Yousif, E. The physicochemical and tribological properties of oleic acid based triester biolubricants. Ind. Crop. Prod. 2011, 34, 1089–1096. [Google Scholar] [CrossRef]
- Li, S.; Bouzidi, L.; Narine, S.S. Synthesis and physical properties of triacylglycerol oligomers: Examining the physical functionality potential of self-metathesized highly unsaturated vegetable oils. Ind. Eng. Chem. Res. 2013, 52, 2209–2219. [Google Scholar] [CrossRef]
- Narine, S.; Li, S.; Mahdevari, A.; Bouzidi, L.; DiBiase, S.A.; Rizvi, S.Q. Esters for Use as a Base Stock and in Lubricant Applications. U.S. Patent 8,741,822, 3 June 2014. [Google Scholar]
- Bird, R.B. Transport phenomena. Appl. Mec. Rev. 2002, 55, R1–R4. [Google Scholar] [CrossRef]
- Brown, E.; Jaeger, H.M. Through Thick and Thin. Science 2011, 333, 1230–1231. [Google Scholar] [CrossRef] [PubMed]
- Sendijarevic, I.; McHugh, A.J. Effects of Molecular Variables and Architecture on the Rheological Behavior of Dendritic Polymers. Macromolecules 2000, 33, 590–596. [Google Scholar] [CrossRef]
- Raghunanan, L.; Narine, S.S. Engineering Green Lubricants IV: Influence of Structure on the Thermal Behavior of Linear and Branched Aliphatic Fatty Acid-Derived Diesters. ACS Sustain. Chem. Eng. 2016, 4, 4868–4874. [Google Scholar] [CrossRef]
- Raghunanan, L.; Narine, S.S. Branched Biobased Diesters with Exceptional Low Temperature and Flow Properties for Use in Lubricant Formulations. ACS Sustain. Chem. Eng. 2016, 4, 2542–2549. [Google Scholar] [CrossRef]
- Brown, E.; Jaeger, H.M. Shear thickening in concentrated suspensions: Phenomenology, mechanisms and relations to jamming. Rep. Prog. Phys. 2014, 77, 046602. [Google Scholar] [CrossRef]
- Cheng, X.; McCoy, J.H.; Israelachvili, J.N.; Cohen, I. Imaging the microscopic structure of shear thinning and thickening colloidal suspensions. Science 2011, 333, 1276–1279. [Google Scholar] [CrossRef]
- Raghunanan, L.; Floros, M.C.; Narine, S.S. Thermal stability of renewable diesters as phase change materials. Thermochim. Acta 2016, 644, 61–68. [Google Scholar] [CrossRef]
- Monnery, W.D.; Svrcek, W.Y.; Mehrotra, A.K. Viscosity: A critical review of practical predictive and correlative methods. Canadian J. Chem. Eng. 1995, 73, 3–40. [Google Scholar] [CrossRef]
- van Velzen, D.; Cardozo, R.L.; Langenkamp, H. A liquid viscosity-temperature-chemical constitution relation for organic compounds. Ind. Eng. Chem. Fundam. 1972, 11, 20–25. [Google Scholar] [CrossRef]
- Viswanath, D.S.; Ghosh, T.K.; Prasad, D.H.L.; Dutt, N.V.K.; Rani, K.Y. Correlation and Estimation of Pure Liquid Viscosity. In Viscosity of Liquids Theory, Estimation, Experiment, and Data; Springer: Dordrecht, The Netherlands, 2007; pp. 135–405. [Google Scholar]
- Andrade, E.N.D.C. The Viscosity of Liquids. Nature 1930, 125, 309–310. [Google Scholar] [CrossRef]
- Reid, R.C.; Prausnitz, J.M.; Poling, B.E. The Properties of Gases and Liquids; McGraw-Hill: New York, NY, USA, 1987; p. 742. [Google Scholar]
- Harris, K.R. Temperature and pressure dependence of the viscosities of 2-ethylhexyl benzoate, bis (2-ethylhexyl) phthalate, 2, 6, 10, 15, 19, 23-hexamethyltetracosane (squalane), and diisodecyl phthalate. J. Chem. Eng. Data 2009, 54, 2729–2738. [Google Scholar] [CrossRef]
- Totten, G.E. Fuels and Lubricants Handbook: Technology, Properties, Performance, and Testing, 2nd ed.; ASTM International: Conshoboken, PA, USA, 2019. [Google Scholar]
- Eychenne, V.; Mouloungui, Z. Relationships between structure and lubricating properties of neopentylpolyol esters. Ind. Eng. Chem. Res. 1998, 37, 4835–4843. [Google Scholar] [CrossRef]
- Moore, R.J.; Gibbs, P.; Eyring, H. Structure of the liquid state and viscosity of-hydrocarbons. J. Phys. Chem. 1953, 57, 172–178. [Google Scholar] [CrossRef]
- Allen, C.A.W.; Watts, K.C.; Ackman, R.G.; Pegg, M.J. Predicting the viscosity of biodiesel fuels from their fatty acid ester composition. Fuel 1999, 78, 1319–1326. [Google Scholar] [CrossRef]
- Rodrigues, J.d.A., Jr.; Cardoso, F.d.P.; Lachter, E.R.; Estevão, L.R.M.; Lima, E.; Nascimento, R.S.V. Correlating chemical structure and physical properties of vegetable oil esters. J. Am. Oil Chem. Soc. 2006, 83, 353–357. [Google Scholar] [CrossRef]
- Salimon, J.; Abdo, W.; Salih, N.; Yarmo, A.; Derawi, D. Lubricity and Tribological Properties of Dicarboxylic Acid and Oleyl Alcohol Based Esters. Sains Malays. 2015, 44, 405–412. [Google Scholar] [CrossRef]
- Yasa, S.R.; Cheguru, S.; Krishnasamy, S.; Korlipara, P.V.; Rajak, A.K.; Penumarthy, V. Synthesis of 10-undecenoic acid based C22-dimer acid esters and their evaluation as potential lubricant basestocks. Ind. Crop. Prod. 2017, 103, 141–151. [Google Scholar] [CrossRef]
- Ahmed, W.A.; Salimon, J.; Yarmo, M.A. Lubricity Characterizations of Sebacic Acid Based Ester. Int. J. Adv. Sci. Eng. Inf. Technol. 2014, 4, 1–6. [Google Scholar] [CrossRef]
- Ahmed, W.A.; Ambar, Y.; Nadia, S.; Mohd Darfizzi, D.; Muhammad Rahimi, Y.; Jumat, S. Synthesis and Lubricity Properties Analysis of Branched Dicarboxylate Esters Based Lubricant. Malays. J. Anal. Sci. 2015, 19, 106–117. [Google Scholar]
- Knapstad, B.; Skjoelsvik, P.A.; Oeye, H.A. Viscosity of pure hydrocarbons. J. Chem. Eng. Data 1989, 34, 37–43. [Google Scholar] [CrossRef]
- Ceriani, R.; Gonçalves, C.B.; Rabelo, J.; Caruso, M.; Cunha, A.C.; Cavaleri, F.W.; Batista, E.A.; Meirelles, A.J. Group contribution model for predicting viscosity of fatty compounds. J. Chem. Eng. Data 2007, 52, 965–972. [Google Scholar] [CrossRef]
- Geist, J.; Cannon, M. Viscosities of pure hydrocarbons. Ind. Eng. Chem. 1946, 18, 611–613. [Google Scholar] [CrossRef]
- Zéberg-Mikkelsen, C.K.; Baylaucq, A.; Barrouhou, M.; Boned, C. The effect of stereoisomerism on dynamic viscosity: A study of cis-decalin and trans-decalin versus pressure and temperature. Phys. Chem. Chem. Phys. 2003, 5, 1547–1551. [Google Scholar] [CrossRef]
- Lowitz, D.A.; Spencer, J.W.; Webb, W.; Schiessler, R.W. Temperature-Pressure-Structure Effects on the Viscosity of Several Higher Hydrocarbons. Chem. Phys. 1959, 30, 73–83. [Google Scholar] [CrossRef]
- Noureddini, H.; Teoh, B.; Clements, L.D. Viscosities of vegetable oils and fatty acids. J. Am. Oil Chem. Soc. 1992, 69, 1189–1191. [Google Scholar] [CrossRef]
- Nelson Jr, R.D.; Lide, D.R., Jr.; Maryott, A.A. Selected Values of Electric Dipole Moments for Molecules in The Gas Phase; National Standard Reference Data System: Gaitherburg, MD, USA, 1967. [Google Scholar]
- Wang, F.; Zhao, N.; Xiao, F.; Wei, W.; Sun, Y. The Design and Simulation of the Synthesis of Dimethyl Carbonate and the Product Separation Process Plant. In Distillation Advances from Modelling to Applications; IntechOpen: London, UK, 2012. [Google Scholar]
- Minkin, V.I.; Osip, A.O.; Zhdanov, Y.A. Dipole Moments in Organic Chemistry; Vaughan, W.E., Ed.; Springer Science & Business Media: Boston, MA, USA, 2012. [Google Scholar]
- Marks, M.; Thompson, J.; Krishnan, G. An experimental study of die attach polymer bleedout in ceramic packages. Thin Solid. Films 1994, 252, 54–60. [Google Scholar] [CrossRef]
- Durig, J.R.; Jin, Y.; Phan, H.V.; Liu, J.; Durig, D.T. Far-Infrared Spectra, Conformational Stability, Barriers to Internal Rotation, Ab Initio Calculations, r0 Structural Parameters, and Vibrational Assignment of Ethyl Methyl Ether. Struct. Chem. 2002, 13, 1–26. [Google Scholar] [CrossRef]
- Krisher, L.C.; Saegebarth, E. Microwave Spectrum of Acetic Acid, CH3COOH and CD3COOH. Chem. Phys. 1971, 54, 4553–4558. [Google Scholar] [CrossRef]
- Clark, T.; Murray, J.S.; Lane, P.; Politzer, P. Why are dimethyl sulfoxide and dimethyl sulfone such good solvents? J. Mol. Model. 2008, 14, 689–697. [Google Scholar] [CrossRef]
- Deming, T.J. Functional modification of thioether groups in peptides, polypeptides, and proteins. Bioconjug. Chem. 2017, 28, 691–700. [Google Scholar] [CrossRef]
- Pafford, B.J.; Godici, P.E.; Schlosberg, R.H.; Aldrich, H.S.; Krevalis, M.A.; Kim, J.T. Polyol ester compositions with unconverted hydroxyl groups for use as lubricant base stocks. EP0938536A1, 1 September 1999. [Google Scholar]
- Gryglewicz, S.; Piechocki, W.; Gryglewicz, G. Preparation of polyol esters based on vegetable and animal fats. Bioresour. Technol. 2003, 87, 35–39. [Google Scholar] [CrossRef]
- Brekan, J.; Quinn, J.; Mandla, K.; Littich, R. Branched Diesters for Use as a Base Stock and in Lubricant Applications. U.S. Patent 8,683,196, 20 June 2017. [Google Scholar]
- Dunn, R.O. Thermal analysis of alternative diesel fuels from vegetable oils. J. Am. Oil Chem. Soc. 1999, 76, 109–115. [Google Scholar] [CrossRef]
- Mohanan, A.; Bouzidi, L.; Narine, S.S. Harnessing the synergies between lipid-based crystallization modifiers and a polymer pour point depressant to improve pour point of biodiesel. Energy 2017, 120, 895–906. [Google Scholar] [CrossRef]
- Quinchia, L.; Delgado, M.; Franco, J.; Spikes, H.; Gallegos, C. Low-temperature flow behaviour of vegetable oil-based lubricants. Ind. Crop. Prod. 2012, 37, 383–388. [Google Scholar] [CrossRef]
- Sato, K.; Ueno, S.; Yano, J. Molecular interactions and kinetic properties of fats. Prog. Lipid Res. 1999, 38, 91–116. [Google Scholar] [CrossRef]
- Himawan, C.; Starov, V.M.; Stapley, A.G.F. Thermodynamic and kinetic aspects of fat crystallization. Adv. Colloid. Interface Sci. 2006, 122, 3–33. [Google Scholar] [CrossRef]
- Sato, K. Crystallization behaviour of fats and lipids—A review. Chem. Eng. Sci. 2001, 56, 2255–2265. [Google Scholar] [CrossRef]
- Clayden, J.; Greeves, N.; Warren, S. Organic Chemistry; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- Perez, J.M.; Rudnick, L.R.; Erhan, S.Z. Natural Oils as Lubricants. In Synthetics, Mineral Oils, and Bio-Based Lubricants: Chemistry and Technology, 2nd ed.; Rudnick, L.R., Ed.; CRC Press: Boca Raton, FL, USA, 2013; p. 375. [Google Scholar]
- De Torres, M.; Jiménez-Osés, G.; Mayoral, J.A.; Pires, E. Fatty acid derivatives and their use as CFPP additives in biodiesel. Bioresource Technol. 2011, 102, 2590–2594. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Nelson, D.R.; Gibbs, A.G. Chemical and physical analyses of wax ester properties. J. Insect Sci. 2001, 1. [Google Scholar] [CrossRef] [PubMed]
- Jayadas, N.H.; Nair, K.P. Coconut oil as base oil for industrial lubricants—Evaluation and modification of thermal, oxidative and low temperature properties. Tribol. Int. 2006, 39, 873–878. [Google Scholar] [CrossRef]
- Yalkowsky, S.H. Estimation of Entropies of Fusion of Organic Compounds. Ind. Eng. Chem. Fundam. 1979, 18, 108–111. [Google Scholar] [CrossRef]
- Li, S.; Bouzidi, L.; Narine, S.S. Synthesis, crystallization, and melting behavior of metathesis-like triacylglycerol oligomers: Effects of saturation, isomerism, and size. Ind. Eng. Chem. Res. 2014, 53, 14579–14591. [Google Scholar] [CrossRef]
- Misra, R.; Murthy, M. Blending of additives with biodiesels to improve the cold flow properties, combustion and emission performance in a compression ignition engine—A review. Renew. Sust. Energ. Rev. 2011, 15, 2413–2422. [Google Scholar] [CrossRef]
- Kenar, J.A. The use of lipids as phase change materials for thermal energy storage. Lipid Technol. 2014, 26, 154–156. [Google Scholar] [CrossRef]
- Shah, S.N.; Sharma, B.K.; Moser, B.R.; Erhan, S.Z. Preparation and evaluation of jojoba oil methyl esters as biodiesel and as a blend component in ultra-low sulfur diesel fuel. Bioenerg. Res. 2010, 3, 214–223. [Google Scholar] [CrossRef]
- Sharma, B.K.; Doll, K.M.; Erhan, S.Z. Ester hydroxy derivatives of methyl oleate: Tribological, oxidation and low temperature properties. Bioresource Technol. 2008, 99, 7333–7340. [Google Scholar] [CrossRef]
- Costa, J.C.S.; Mendes, A.; Santos, L.M.N.B.F. Chain length dependence of the thermodynamic properties of n-alkanes and their monosubstituted derivatives. J. Chem. Eng. Data 2018, 63, 1–20. [Google Scholar] [CrossRef]
- Boese, R.; Weiss, H.-C.; Bläser, D. The melting point alternation in the short-chain n-alkanes: Single-crystal X-Ray analyses of propane at 30 K and of n-butane to n-nonane at 90 K. Angew. Chem. Int. Ed. 1999, 38, 988–992. [Google Scholar] [CrossRef]
- Marinova, E.; Seizova, K.; Totseva, I.; Panayotova, S.S.; Marekov, I.N.; Momchilova, S.M. Oxidative changes in some vegetable oils during heating at frying temperature. Bulg. Chem. Commun. 2012, 44, 57–63. [Google Scholar]
- Pierce, L.; Hayashi, M. Microwave Spectrum, Dipole Moment, Structure, and Internal Rotation of Dimethyl Sulfide. Chem. Phys. 1961, 35, 479–485. [Google Scholar] [CrossRef]
- Adhvaryu, A.; Erhan, S.Z.; Perez, J.M. Tribological studies of thermally and chemically modified vegetable oils for use as environmentally friendly lubricants. Wear 2004, 257, 359–367. [Google Scholar] [CrossRef]
- Boyde, S. Hydrolytic stability of synthetic ester lubricants. J. Synth. Lubr. 2000, 16, 297–312. [Google Scholar] [CrossRef]
- Reaume, S.J.; Ellis, N. Use of isomerization and hydroisomerization reactions to improve the cold flow properties of vegetable oil based biodiesel. Energies 2013, 6, 619–633. [Google Scholar] [CrossRef]
- Sharma, B.K.; Adhvaryu, A.; Liu, Z.; Erhan, S.Z. Chemical modification of vegetable oils for lubricant applications. J. Am. Oil Chem. Soc. 2006, 83, 129–136. [Google Scholar] [CrossRef]
- Miller, S.; O’Rear, D.; Rosenbaum, J. Lubricant Base Oils with Optimized Branching. U.S. Patent AU2004281378A1, 28 April 2005. [Google Scholar]
- Pettersson, A. High-performance base fluids for environmentally adapted lubricants. Tribol. Int. 2007, 40, 638–645. [Google Scholar] [CrossRef]
- Yao, L.; Hammond, E.G. Isolation and melting properties of branched-chain esters from lanolin. J. Am. Oil Chem. Soc. 2006, 83, 547–552. [Google Scholar] [CrossRef]
- Salih, N.; Salimon, J.; Yousif, E. The effect of chemical structure on pour point, oxidative stability and tribological properties of oleic acid triester derivatives. Malays. J. Anal. Sci. 2013, 17, 119–128. [Google Scholar]
- Miller, S.A.; Landis, A.E.; Theis, T.L.; Reich, R.A. A comparative life cycle assessment of petroleum and soybean-based lubricants. Environ. Sci. Technol. 2007, 41, 4143–4149. [Google Scholar] [CrossRef] [PubMed]
- Almasi, S.; Ghobadian, B.; Najafi, G.; Soufi, M.D. A review on bio-lubricant production from non-edible oil-bearing biomass resources in Iran: Recent progress and perspectives. J. Clean. Prod. 2021, 290, 125830. [Google Scholar] [CrossRef]
- Murru, C.; Badía-Laíño, R.; Díaz-García, M.E. Oxidative Stability of Vegetal Oil-Based Lubricants. ACS Sustain. Chem. Eng. 2021, 9, 1459–1476. [Google Scholar] [CrossRef] [PubMed]
- Woma, T.; Lawal, S.; Abdulrahman, A.S.; Olutoye, M.; Ojapah, M. Vegetable Oil Based Lubricants: Challenges and Prospects. Tribol. Online 2019, 14, 60–70. [Google Scholar] [CrossRef]
- Kurre, S.K.; Yadav, J. A review on bio-based feedstock, synthesis, and chemical modification to enhance tribological properties of biolubricants. Ind. Crop. Prod. 2023, 193, 116122. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).