Rheological Behavior of Different Calf Sera before, during and after Biomechanical Testing
Abstract
:1. Introduction
- Do NBCS and BCS show different rheological properties?
- Do the rheological properties of the two fluids change after different testing intervals?
2. Materials and Methods
2.1. Test Fluid
2.2. Test Setup
2.3. Density Measurements
2.4. Rheologic Behavior
2.5. Protein Measurements
2.6. Statistical Analysis
3. Results
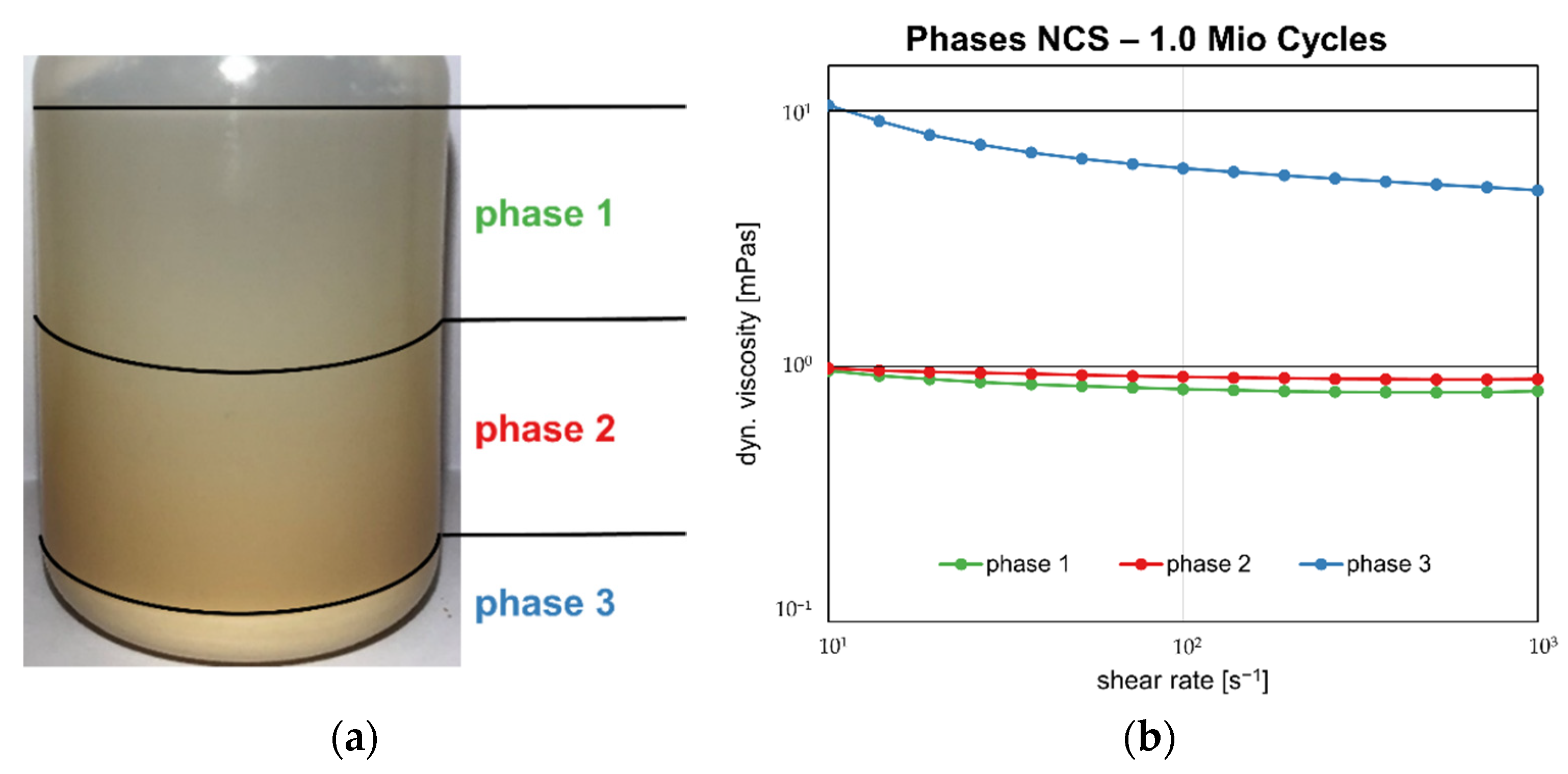
3.1. Optical Observation
3.2. Density Measurement
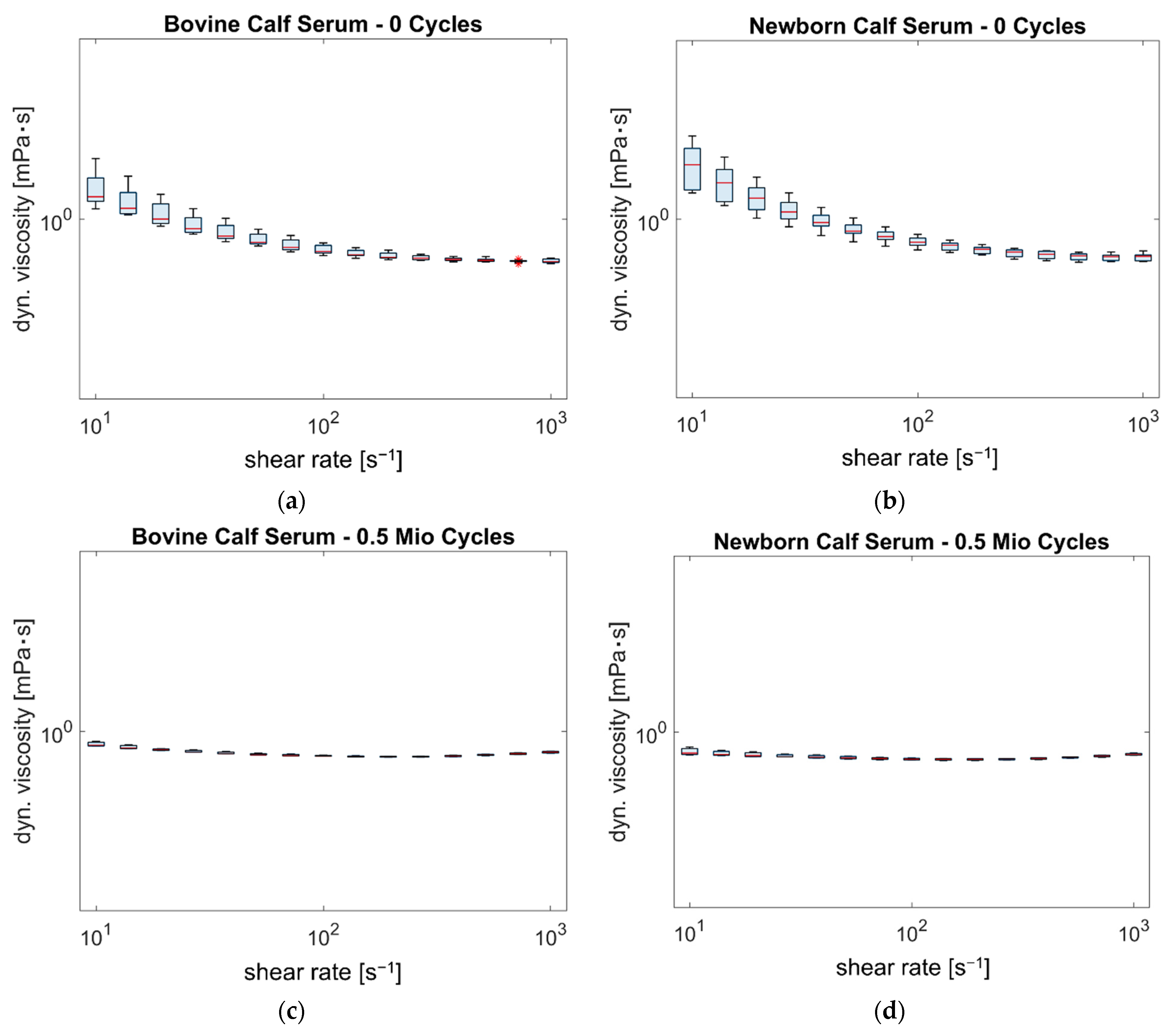
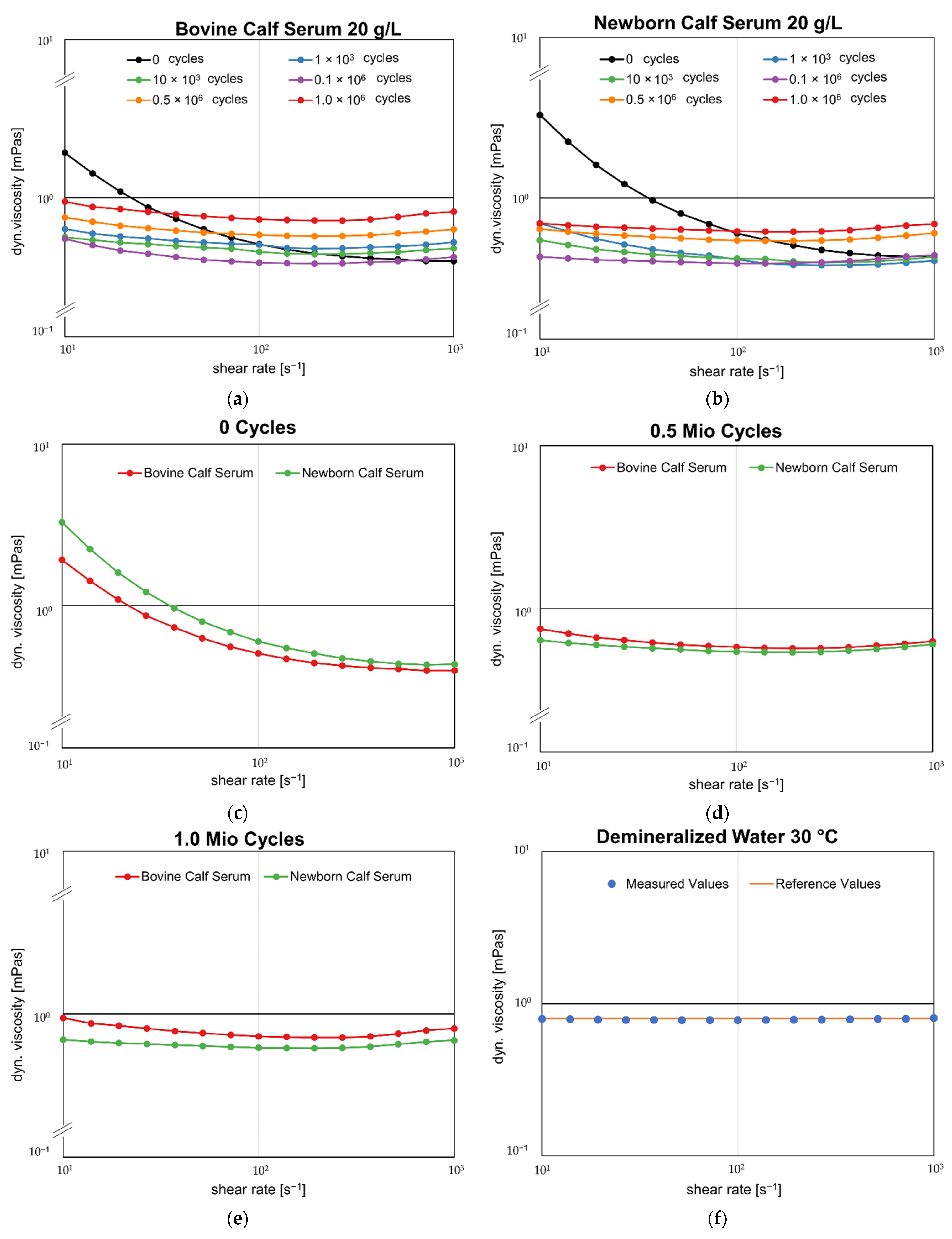
3.3. Rheologic Behavior
3.4. Protein Concentration
4. Discussion
4.1. Do NBCS and BCS Show Different Rheological Properties?
4.2. Do the Rheological Properties of the Two Fluids Change after Different Testing Intervals?
4.3. Limitations
5. Conclusions
- BSC and NBCS do not differ from each other in terms of their rheological behavior neither before (0 cycles) nor after biomechanical examinations (0.5 × 106 cycles).
- The rheological behavior of test sera changes as soon as they are mechanically loaded. In addition, the replacement interval of the serum plays a decisive role in biomechanical testing, as the fluid exhibits a more viscous behavior with increasing test duration.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Australian Orthopaedic Associatopn National Joint Replacement Registry [AOANJRR]. Hip, Knee & Shoulder Athroplasty: 2021 Annual Report; AOA: Adelaide, Australia, 2021; pp. 1–432. [Google Scholar]
- Lombardi, A.V., Jr.; Berend, K.R.; Adams, J.B. Why knee replacements fail in 2013: Patient, surgeon, or implant? Bone Jt. J. 2014, 96, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Grimberger, A.; Jansson, V.; Lützner, J.; Melsheimer, O.; Morlock, M.; Steinbrück, A. German Arthroplasty Registry (Endoprothesenregister Deutschland—EPRD); EPRD: Berlin, Germany, 2020. [Google Scholar] [CrossRef]
- MacDonald, D.W.; Higgs, G.; Parvizi, J.; Klein, G.; Hartzband, M.; Levine, H.; Kraay, M.; Rimnac, C.M.; Kurtz, S.M. Oxidative properties and surface damage mechanisms of remelted highly crosslinked polyethylenes in total knee arthroplasty. Int. Orthop. 2013, 37, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Andriacchi, T.P.; Mundermann, A.; Smith, R.L.; Alexander, E.J.; Dyrby, C.O.; Koo, S. A framework for the in vivo pathomechanics of osteoarthritis at the knee. Ann. Biomed. Eng. 2004, 32, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Sundfeldt, M.; Carlsson, L.V.; Johansson, C.B.; Thomsen, P.; Gretzer, C. Aseptic loosening, not only a question of wear: A review of different theories. Acta Orthop. 2006, 77, 177–197. [Google Scholar] [CrossRef]
- Kretzer, J.P.; Reinders, J.; Sonntag, R.; Hagmann, S.; Streit, M.; Jeager, S.; Moradi, B. Wear in total knee arthroplasty—Just a question of polyethylene?: Metal ion release in total knee arthroplasty. Int. Orthop. 2014, 38, 335–340. [Google Scholar] [CrossRef]
- Fisher, J.; McEwen, H.M.; Tipper, J.L.; Galvin, A.L.; Ingram, J.; Kamali, A.; Stone, M.H.; Ingham, E. Wear, debris, and biologic activity of cross-linked polyethylene in the knee: Benefits and potential concerns. Clin. Orthop. Relat. Res. 2004, 428, 114–119. [Google Scholar] [CrossRef]
- Gallo, J.; Slouf, M.; Goodman, S.B. The relationship of polyethylene wear to particle size, distribution, and number: A possible factor explaining the risk of osteolysis after hip arthroplasty. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 94, 171–177. [Google Scholar] [CrossRef]
- Glyn-Jones, S.; Pandit, H.; Kwon, Y.M.; Doll, H.; Gill, H.S.; Murray, D.W. Risk factors for inflammatory pseudotumour formation following hip resurfacing. J. Bone Jt. Surg. Br. 2009, 91, 1566–1574. [Google Scholar] [CrossRef]
- ISO 14242-1:2012; Implants for Surgery—Wear of Total Hip-Joint Prostheses—Part 1: Loading and Displacement Parameters for Wear-Testing Machines and Corresponding Environmental Conditions for Test. International Organization for Standardization: Geneva, Switzerland, 2012.
- ISO 14243-1:2009; Implants for Surgery—Wear of Total Knee-Joint Prostheses—Part 1: Loading and Displacement Parameters for Wear Testing Machines with Load Control and Corresponding Environmental Conditions for Test. International Organization for Standardization: Geneva, Switzerland, 2019.
- Reinders, J.; Sonntag, R.; Vot, L.; Gibney, C.; Nowack, M.; Kretzer, J.P. Wear testing of moderate activities of daily living using in vivo measured knee joint loading. PLoS ONE 2015, 10, e0123155. [Google Scholar] [CrossRef] [Green Version]
- Kretzer, J.P.; Zietz, C.; Schroder, C.; Reinders, J.; Middelborg, L.; Paulus, A.; Sonntag, R.; Bader, R.; Utzschneider, S. Principles of tribological analysis of endoprostheses. Orthopade 2012, 41, 844–852. [Google Scholar] [CrossRef]
- Schwiesau, J.; Schilling, C.; Utzschneider, S.; Jansson, V.; Fritz, B.; Blomer, W.; Grupp, T.M. Knee wear simulation under conditions of highly demanding daily activities—Influence on an unicompartmental fixed bearing knee design. Med. Eng. Phys. 2013, 35, 1204–1211. [Google Scholar] [CrossRef]
- Wang, A.; Essner, A.; Stark, C.; Dumbleton, J.H. Comparison of the size and morphology of UHMWPE wear debris produced by a hip joint simulator under serum and water lubricated conditions. Biomaterials 1996, 17, 865–871. [Google Scholar] [CrossRef]
- Beaule, P.E.; Campbell, P.A.; Walker, P.S.; Schmalzried, T.P.; Dorey, F.J.; Blunn, G.W.; Bell, C.J.; Yahia, L.; Amstutz, H.C. Polyethylene wear characteristics in vivo and in a knee stimulator. J. Biomed. Mater. Res. 2002, 60, 411–419. [Google Scholar] [CrossRef]
- Liao, Y.S.; Benya, P.D.; McKellop, H.A. Effect of protein lubrication on the wear properties of materials for prosthetic joints. J. Biomed. Mater. Res. 1999, 48, 465–473. [Google Scholar] [CrossRef]
- Brandt, J.M.; Charron, K.; Zhao, L.; MacDonald, S.J.; Medley, J.B. Calf serum constituent fractions influence polyethylene wear and microbial growth in knee simulator testing. Proc. Inst. Mech. Eng. H 2012, 226, 427–440. [Google Scholar] [CrossRef]
- Dowson, D. Paper 10: Elastohydrodynamics. Proc. Inst. Mech. Eng. 1967, 182, 151–167. [Google Scholar] [CrossRef]
- Ateshian, G.A. The role of interstitial fluid pressurization in articular cartilage lubrication. J. Biomech. 2009, 42, 1163–1176. [Google Scholar] [CrossRef]
- More, S.; Kotiya, A.; Kotia, A.; Ghosh, S.K.; Spyrou, L.A.; Sarris, I.E. Rheological properties of synovial fluid due to viscosupplements: A review for osteoarthritis remedy. Comput. Methods Programs Biomed. 2020, 196, 105644. [Google Scholar] [CrossRef]
- Balazs, E.A.; Watson, D.; Duff, I.F.; Roseman, S. Hyaluronic acid in synovial fluid. I. Molecular parameters of hyaluronic acid in normal and arthritis human fluids. Arthritis Rheum 1967, 10, 357–376. [Google Scholar] [CrossRef]
- Balazs, E.A. Viscosupplementation for treatment of osteoarthritis: From initial discovery to current status and results. Surg. Technol. Int. 2004, 12, 278–289. [Google Scholar]
- Anadere, I.; Chmiel, H.; Laschner, W. Viscoelasticity of “normal” and pathological synovial fluid. Biorheology 1979, 16, 179–184. [Google Scholar] [CrossRef]
- Wonerow, T.; Uhler, M.; Nuppnau, J.; Kretzer, J.P.; Mantwill, F. Rheologic Behavior of Bovine Calf Serum. Materials 2021, 14, 2538. [Google Scholar] [CrossRef]
- Rothammer, B.; Marian, M.; Rummel, F.; Schroeder, S.; Uhler, M.; Kretzer, J.P.; Tremmel, S.; Wartzack, S. Rheological behavior of an artificial synovial fluid—Influence of temperature, shear rate and pressure. J. Mech. Behav. Biomed. Mater. 2021, 115, 104278. [Google Scholar] [CrossRef] [PubMed]
- Reinders, J.; Sonntag, R.; Kretzer, J.P. How do gait frequency and serum-replacement interval affect polyethylene wear in knee-wear simulator tests? J. Mater. Sci. Mater. Med. 2014, 25, 2463–2469. [Google Scholar] [CrossRef] [PubMed]
- Shahemi, N.H.; Kamis, S.L.; Sawae, Y.; Morita, T. Effect of Protein Interactions on the UHMWPE Composites After the Mul-tidirectional Wear Test. Res. Sq. 2021, 1, 1–34. [Google Scholar] [CrossRef]
- Reinders, J.; Sonntag, R.; Kretzer, J.P. Synovial fluid replication in knee wear testing: An investigation of the fluid volume. J Orthop. Res. 2015, 33, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, S.; Braun, S.; Mueller, U.; Sonntag, R.; Jaeger, S.; Kretzer, J.P. Particle analysis of shape factors according to American Society for Testing and Materials. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 225–233. [Google Scholar] [CrossRef]
- Mezger, T.G. Das Rheologie Handbuch—Für Anwender von Rotations- und Ozillations-Rheometern; Vincentz Network: Hannover, Germany, 2016; p. 5. [Google Scholar]
- Bortel, E.L.; Charbonnier, B.; Heuberger, R. Development of a Synthetic Synovial Fluid for Tribological Testing. Lubricants 2015, 3, 664–686. [Google Scholar] [CrossRef]
- Brandt, J.M.; Charron, K.D.; Zhao, L.; MacDonald, S.J.; Medley, J.B. Lubricant Biochemistry Affects Polyethylene Wear in Knee Simulator Testing. Biotribology 2021, 27, 100185. [Google Scholar] [CrossRef]
- Nečas, D.; Sadecká, K.; Vrbka, M.; Gallo, J.; Galandáková, A.; Křupka, I.; Hartl, M. Observation of lubrication mechanisms in knee replacement: A pilot study. Biotribology 2019, 17, 1–7. [Google Scholar] [CrossRef]
- Harsha, A.P.; Joyce, T.J. Challenges associated with using bovine serum in wear testing orthopaedic biopolymers. Proc. Inst. Mech. Eng. H 2011, 225, 948–958. [Google Scholar] [CrossRef]
- Lu, Z.; McKellop, H. Frictional heating of bearing materials tested in a hip joint wear simulator. Proc. Inst. Mech. Eng. H 1997, 211, 101–108. [Google Scholar] [CrossRef]
- Wiedersich, J.; Kohler, S.; Skerra, A.; Friedrich, J. Temperature and pressure dependence of protein stability: The engineered fluorescein-binding lipocalin FluA shows an elliptic phase diagram. Proc. Natl. Acad. Sci. USA 2008, 105, 5756–5761. [Google Scholar] [CrossRef]
- Takeda, K.; Wada, A.; Yamamoto, K.; Moriyama, Y.; Aoki, K. Conformational change of bovine serum albumin by heat treatment. J. Protein Chem. 1989, 8, 653–659. [Google Scholar] [CrossRef]
- Tateiwa, T.; Clarke, I.C.; Shirasu, H.; Masaoka, T.; Shishido, T.; Yamamoto, K. Effect of low protein concentration lubricants in hip simulators. J. Orthop. Sci. 2006, 11, 204–211. [Google Scholar] [CrossRef]
- Stevenson, H.; Cann, P.M. Protein Content of Model Synovial Fluid and CoCrMo Wear. Biotribology 2021, 26, 100172. [Google Scholar] [CrossRef]
- Shiramizu, K.; Vizesi, F.; Bruce, W.; Herrmann, S.; Walsh, W.R. Tibiofemoral contact areas and pressures in six high flexion knees. Int. Orthop. 2009, 33, 403–406. [Google Scholar] [CrossRef]
- Cheng, C.-K.; Huang, C.-H.; Liau, J.-J.; Huang, C.-H. The influence of surgical malalignment on the contact pressures of fixed and mobile bearing knee prostheses––A biomechanical study. Clin. Biomech. 2003, 18, 231–236. [Google Scholar] [CrossRef]
- Guenther, L.E.; Turgeon, T.R.; Bohm, E.R.; Brandt, J.M. The biochemical characteristics of wear testing lubricants affect polyethylene wear in orthopaedic pin-on-disc testing. Proc. Inst. Mech. Eng. H 2015, 229, 77–90. [Google Scholar] [CrossRef]
- Fullam, S.; He, J.; Scholl, C.S.; Schmid, T.M.; Wimmer, M.A. Competitive Binding of Bilirubin and Fatty Acid on Serum Albumin Affects Wear of UHMWPE. Lubricants 2020, 8, 53. [Google Scholar] [CrossRef]
- Sakoda, H.; Niimi, S. Impact of lipid-induced degradation on the mechanical properties of ultra-high molecular weight polyethylene for joint replacements. J. Mech. Behav. Biomed. Mater. 2016, 53, 218–225. [Google Scholar] [CrossRef]
- Sakoda, H.; Okamoto, Y.; Haishima, Y. In vitro estimation of reduction in strength and wear resistance of UHMWPE for joint prostheses due to lipid-induced degradation. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 3155–3161. [Google Scholar] [CrossRef]


| Unit | BCS (Raw) | NBCS (Raw) | |
|---|---|---|---|
| Parameter/Biochemical Assay | |||
| Cholesterol | [mg/100 mL] | 138.1 | 167.5 |
| Triglycerides | [mg/100 mL] | 8.9 | 14.6 |
| Glucose | [mg/100 mL] | 110.1 | 81.3 |
| Total protein | [mg/mL] | 72.12 | 78.95 |
| Capillary Electrophoresis | |||
| Albumine absolute | [mg/mL] | 32.90 | 36.63 |
| α-Globuline absolute | [mg/mL] | 15.80 | 17.68 |
| β-Globuline absolute | [mg/mL] | 10.20 | 8.13 |
| γ-Globuline absolute | [mg/mL] | 13.20 | 16.50 |
| Albumin/Globulin-quotient | n.a. | 0.84 | 0.86 |
| Other | |||
| pH value | n.a. | 7.81 | 8.03 |
| Osmolality | [mOsm/kg] | 300 | 299 |
| Hemoglobin | [mg/100 mL] | 14.7 | 22.7 |
| Endotoxin | [EU/mL] | 2.6 | 9.256 |
| Test Serum | Absolute Density [g/cm3] |
|---|---|
| BCS | 1.00441 ± 0.00001 |
| NBCS | 1.00420 ± 0.00001 |
| Shear Rate [s−1] | Test Fluids and Intervals | |||||
|---|---|---|---|---|---|---|
| dyn. Viscosity BCS [mPa∙s] | ||||||
| 0 Cycles | 1 × 103 Cycles | 10 × 103 Cycles | ||||
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | |
| 10 | 1.122 | [1.068; 1.176] | 0.942 | [0.789; 1.094] | 0.919 | [0.792; 1.045] |
| 100 | 0.888 | [0.739; 0.897] | 0.891 | [0.858; 0.923] | 0.874 | [0.823; 0.925] |
| 1000 | 0.850 | [0.835; 0.857] | 0.891 | [0.882; 0.899] | 0.876 | [0.869; 0.884] |
| 0.1 × 106 Cycles | 0.5 × 106 Cycles | 1.0 × 106 Cycles | ||||
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | |
| 10 | 0.899 | [0.783; 1.017] | 0.951 | [0.927; 0.974] | 0.990 | [0.966; 1.014] |
| 100 | 0.846 | [0.821; 0.871] | 0.909 | [0.904; 0.914] | 0.946 | [0.922; 0.969] |
| 1000 | 0.859 | [0.837; 0.880] | 0.922 | [0.912; 0.931] | 0.965 | [0.943; 0.986] |
| dyn. Viscosity NBCS [mPa∙s] | ||||||
| 0 Cycles | 1 × 103 Cycles | 10 × 103 Cycles | ||||
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | |
| 10 | 1.220 | [1.146; 1.295] | 0.875 | [0.797; 0.953] | 0.899 | [0.826; 0.972] |
| 100 | 0.912 | [0.899; 0.925] | 0.837 | [0.824; 0.871] | 0.848 | [0.836; 0.880] |
| 1000 | 0.863 | [0.854; 0.872] | 0.851 | [0.834; 0.867] | 0.862 | [0.841; 0.883] |
| 0.1 × 106 Cycles | 0.5 × 106 Cycles | 1.0 × 106 Cycles | ||||
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | |
| 10 | 0.862 | [0.831; 0.893] | 0.925 | [0.887; 0.963] | 0.931 | [0.932; 0.943] |
| 100 | 0.848 | [0.838; 0.857] | 0.898 | [0.889; 0.908] | 0.919 | [0.913; 0.925] |
| 1000 | 0.865 | [0.852; 0.879] | 0.915 | [0.905; 0.926] | 0.936 | [0.931; 0.942] |
| BCS | ||||||
| 0 | 1 × 103 | 10 × 103 | 0.1 × 106 | 0.5 × 106 | 1.0 × 106 | |
| 0 | ||||||
| 1 × 103 | <0.001 * | |||||
| 10 × 103 | 0.001 * | 1.000 | ||||
| 0.1 × 106 | 0.144 | 0.014 * | 0.390 | |||
| 0.5 × 106 | <0.001 * | 0.045 * | 0.003 * | <0.001 * | ||
| 1.0 × 106 | <0.001 * | <0.001 * | <0.001 * | <0.001 * | 0.005 * | |
| NBCS | ||||||
| 0 | 1 × 1023 | 10 × 1023 | 0.1 × 106 | 0.5 × 106 | 1.0 × 106 | |
| 0 | ||||||
| 1 × 103 | <0.001 * | |||||
| 10 × 103 | 0.025 * | 1.000 | ||||
| 0.1 × 106 | 0.020 * | 1.000 | 1.000 | |||
| 0.5 × 106 | 0.019 * | <0.001 * | <0.001 * | <0.001 * | ||
| 1.0 × 106 | <0.001 * | <0.001 * | <0.001 * | <0.001 * | 0.211 | |
| Protein Concentration NBCS [g/L] | ||||
|---|---|---|---|---|
| 0 Cycles | 1.0 × 106 Cycles | |||
| Phase 1 | Phase 2 | Phase 3 | ||
| MV ± SD | 19.98 ± 0.07 | 2.63 ± 0.12 | 16.30 ± 0.72 | 55.27 ± 10.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uhler, M.; Schonhoff, M.; Nees, T.A.; Wonerow, T.; Nuppnau, J.; Mantwill, F.; Kretzer, J.P.; Schroeder, S. Rheological Behavior of Different Calf Sera before, during and after Biomechanical Testing. Lubricants 2022, 10, 224. https://doi.org/10.3390/lubricants10090224
Uhler M, Schonhoff M, Nees TA, Wonerow T, Nuppnau J, Mantwill F, Kretzer JP, Schroeder S. Rheological Behavior of Different Calf Sera before, during and after Biomechanical Testing. Lubricants. 2022; 10(9):224. https://doi.org/10.3390/lubricants10090224
Chicago/Turabian StyleUhler, Maximilian, Mareike Schonhoff, Timo A. Nees, Tanja Wonerow, Jens Nuppnau, Frank Mantwill, Jan Philippe Kretzer, and Stefan Schroeder. 2022. "Rheological Behavior of Different Calf Sera before, during and after Biomechanical Testing" Lubricants 10, no. 9: 224. https://doi.org/10.3390/lubricants10090224
APA StyleUhler, M., Schonhoff, M., Nees, T. A., Wonerow, T., Nuppnau, J., Mantwill, F., Kretzer, J. P., & Schroeder, S. (2022). Rheological Behavior of Different Calf Sera before, during and after Biomechanical Testing. Lubricants, 10(9), 224. https://doi.org/10.3390/lubricants10090224






