Synthesis, Wear and Corrosion of Novel Electrospark and Electrospark–Electrochemical Hybrid Coatings Based on Carbon Steels
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussions
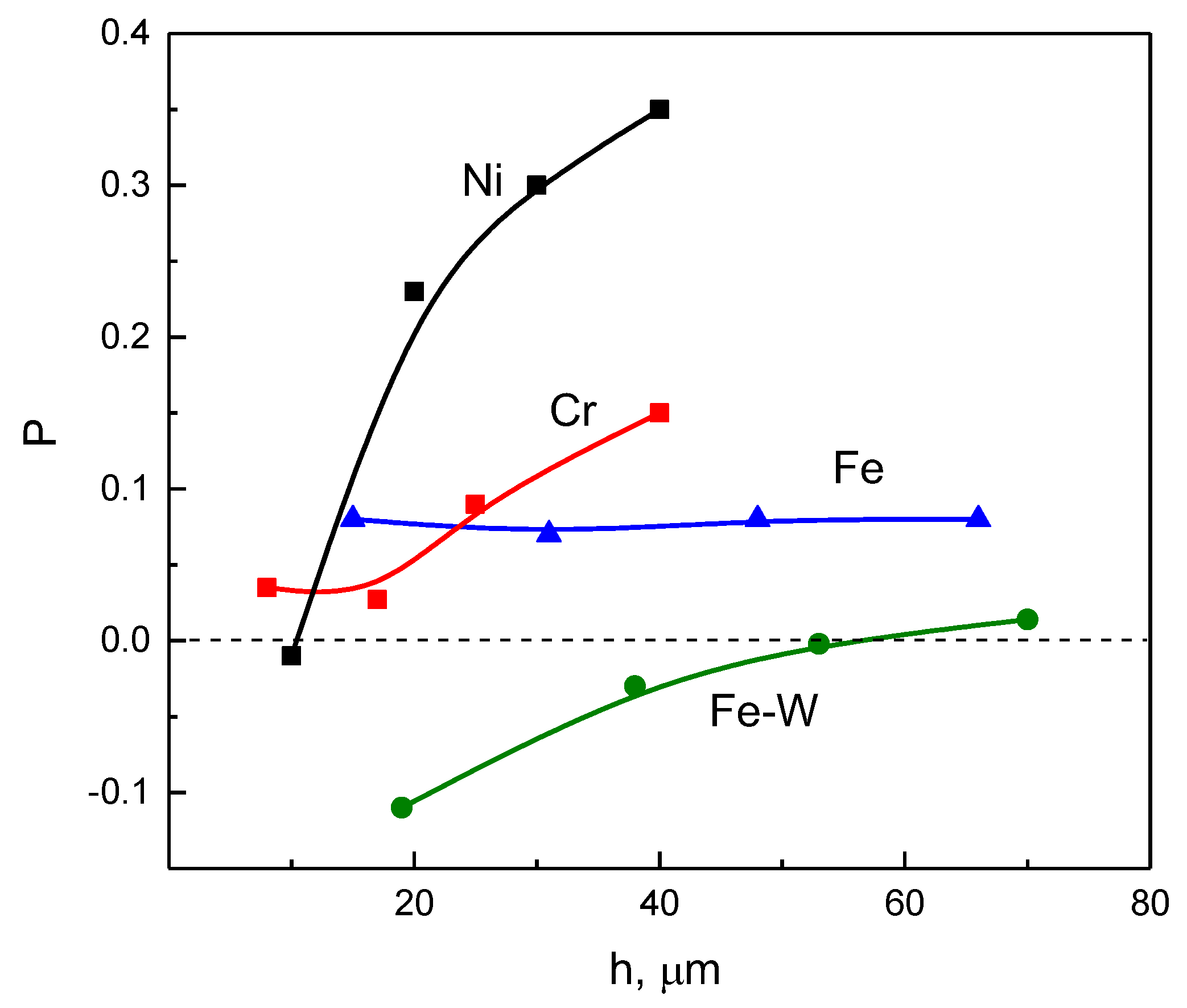
3.1. Leveling Power of Investigated Electrolytes
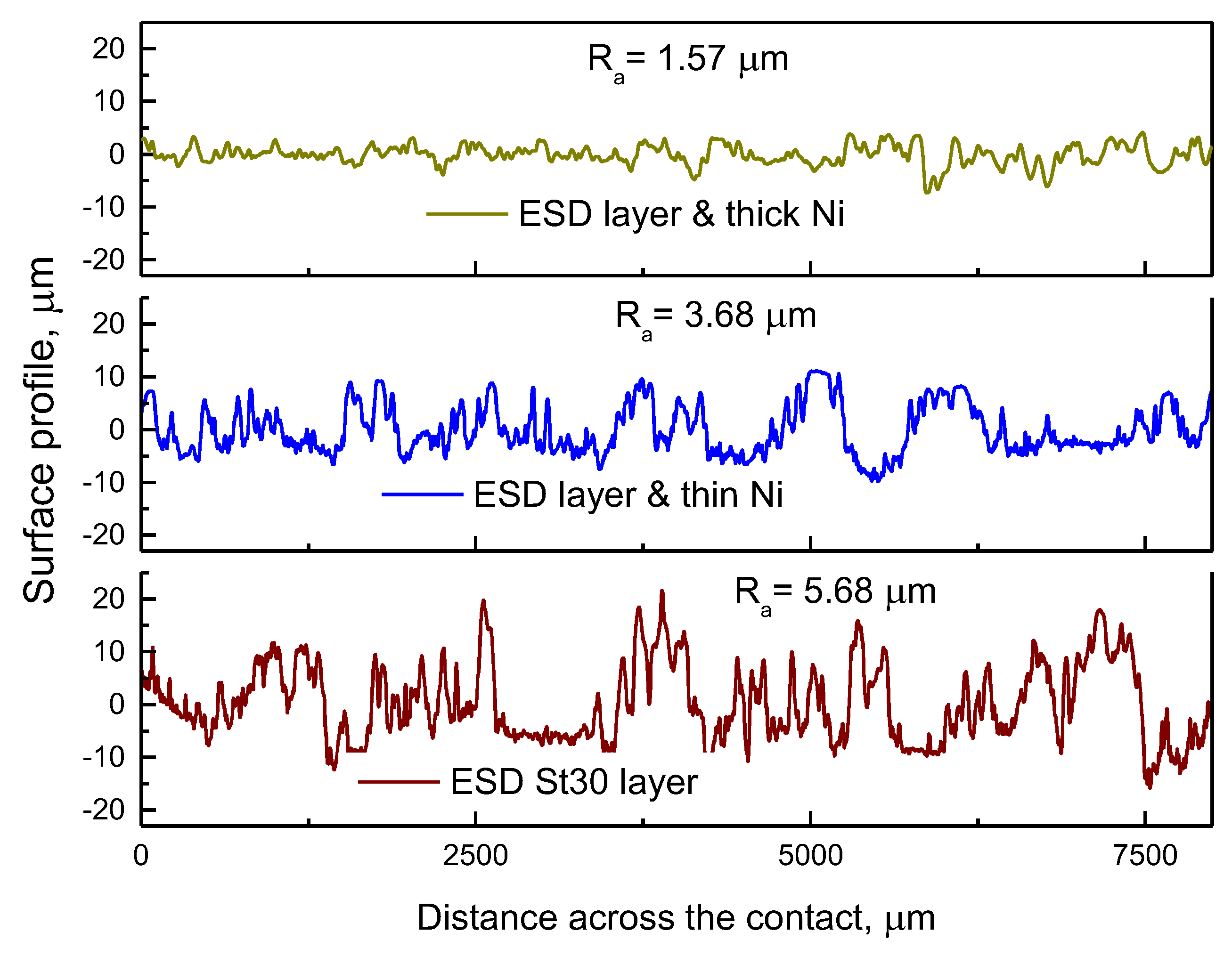
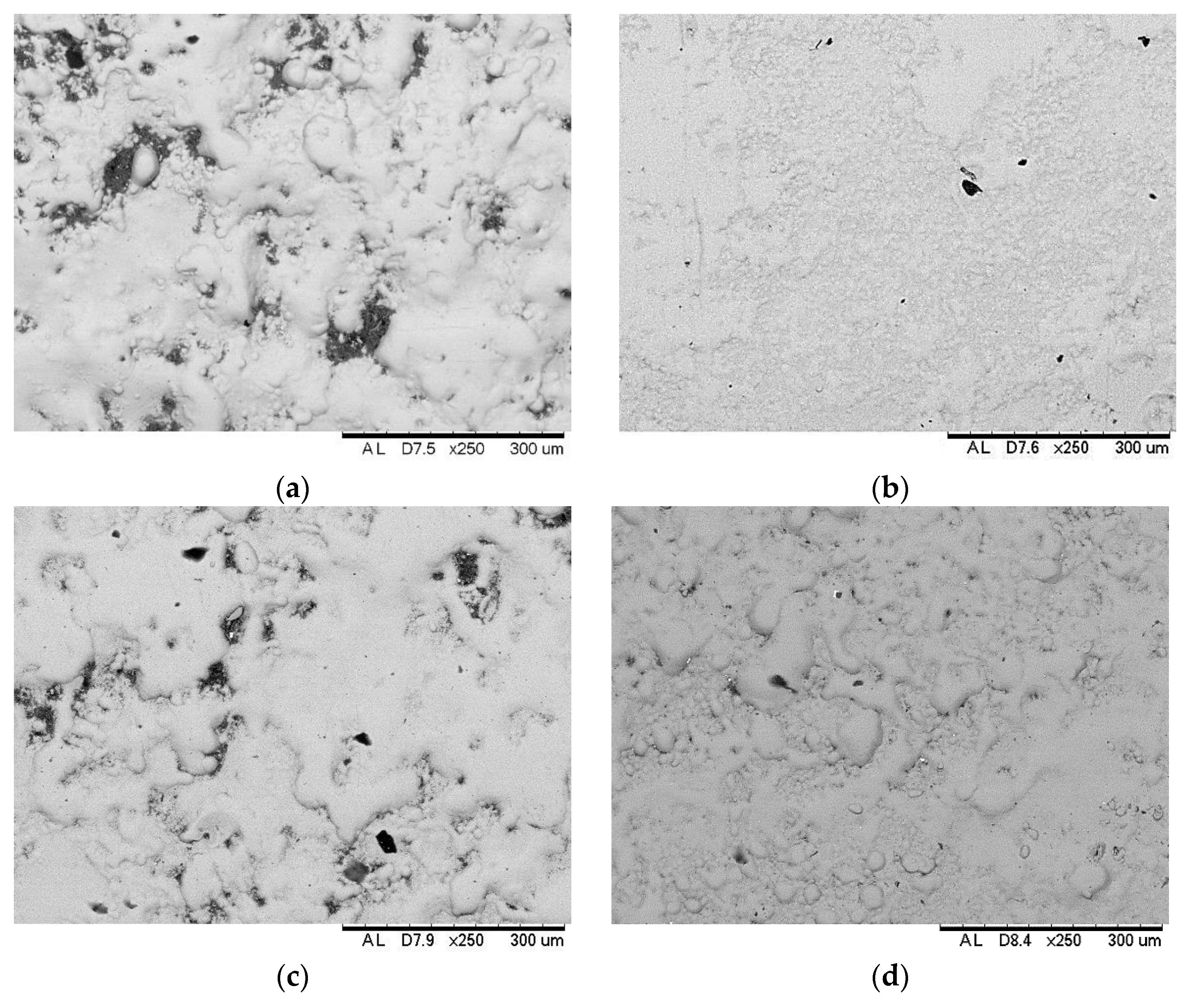
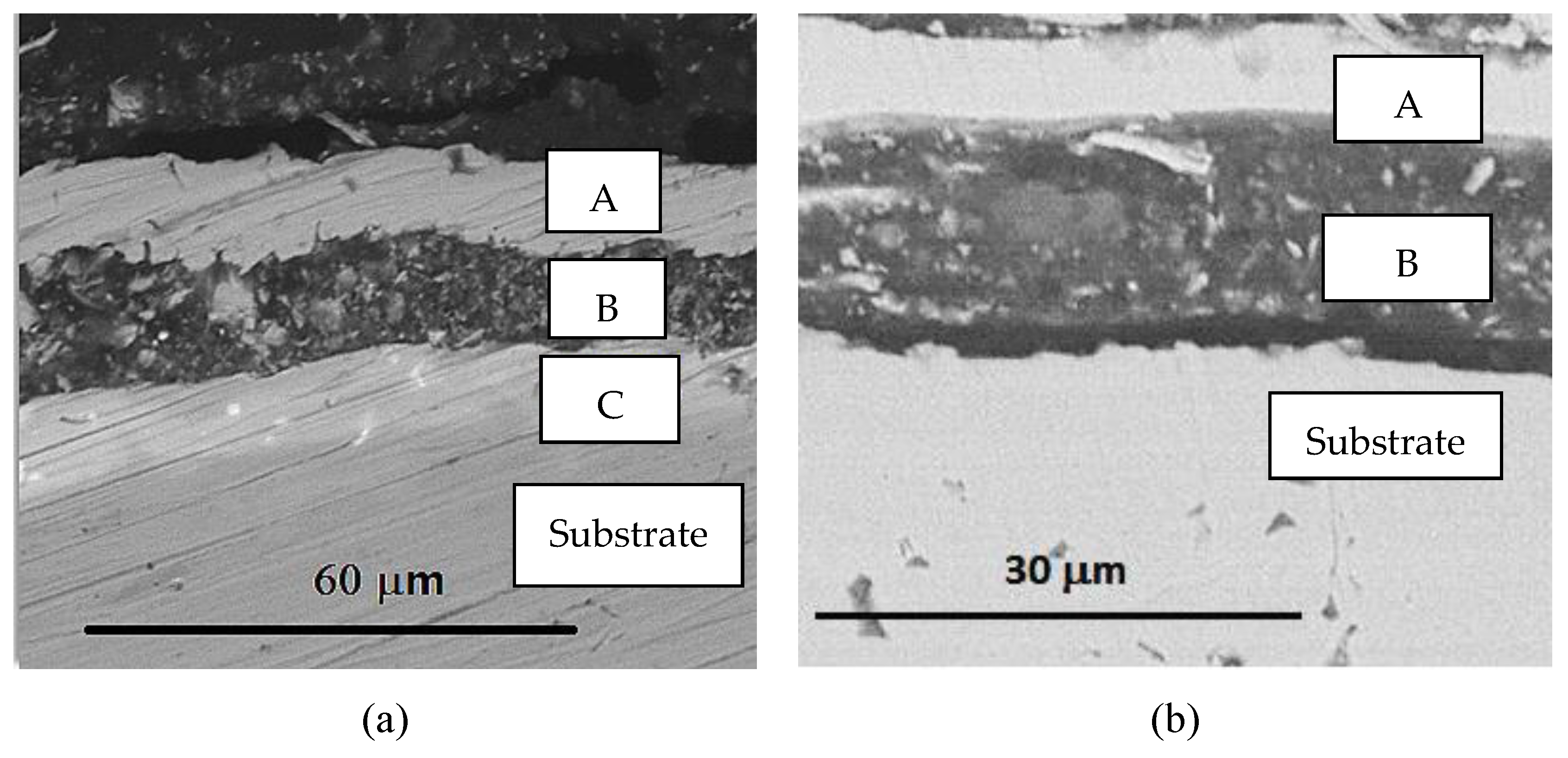
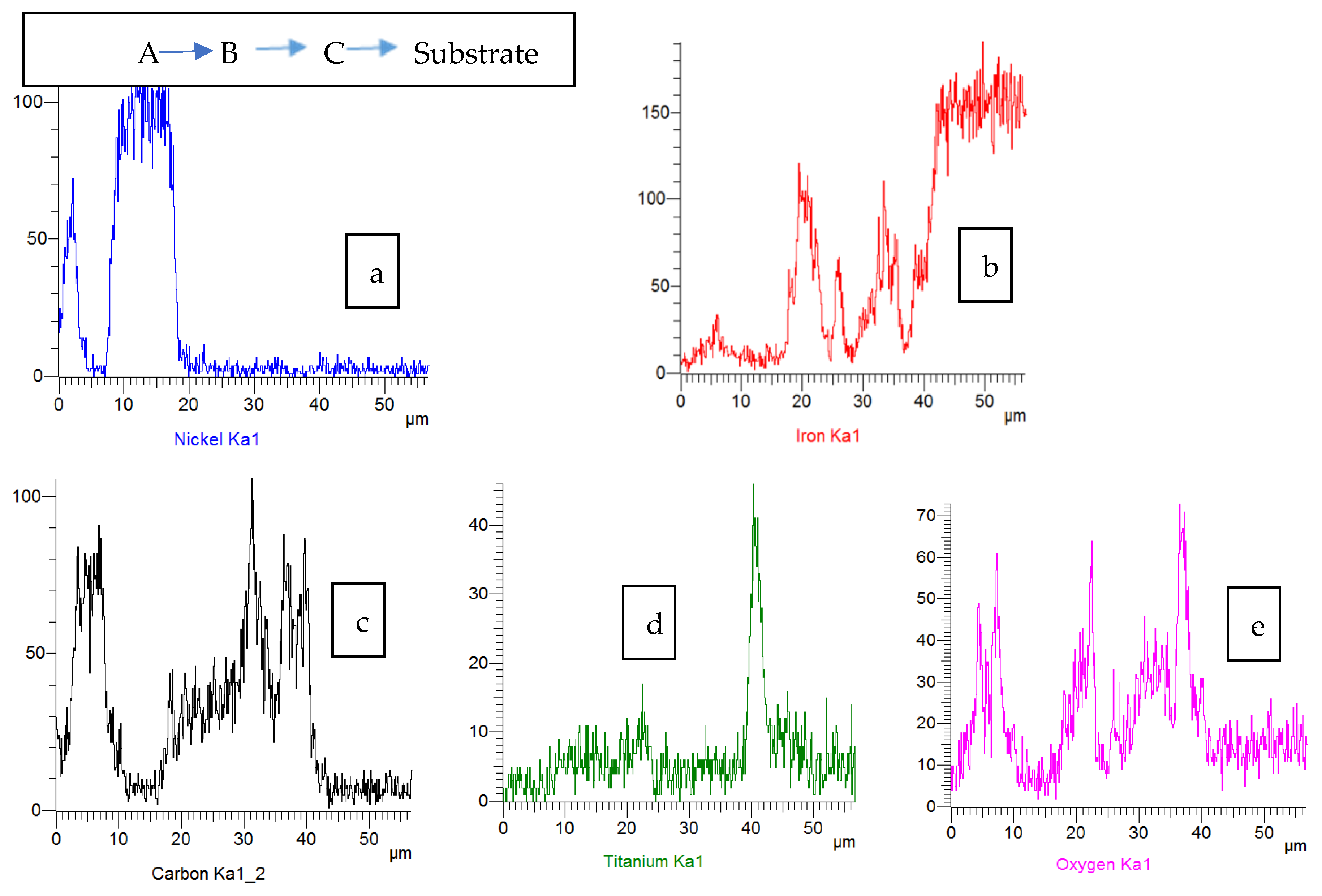
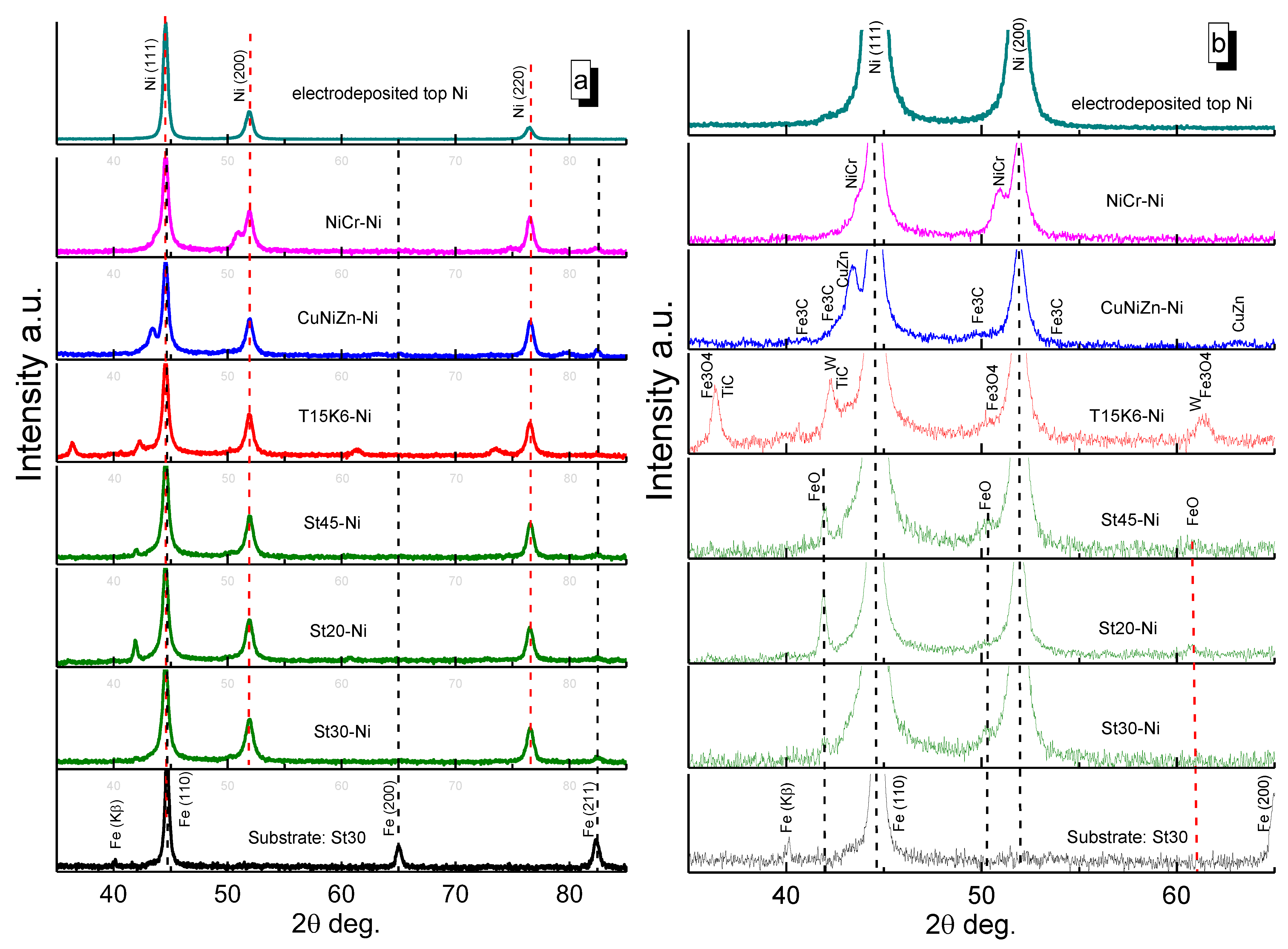
3.2. Surface Morphology, Composition and Structure of ESD-Ni Hybrid Coatings
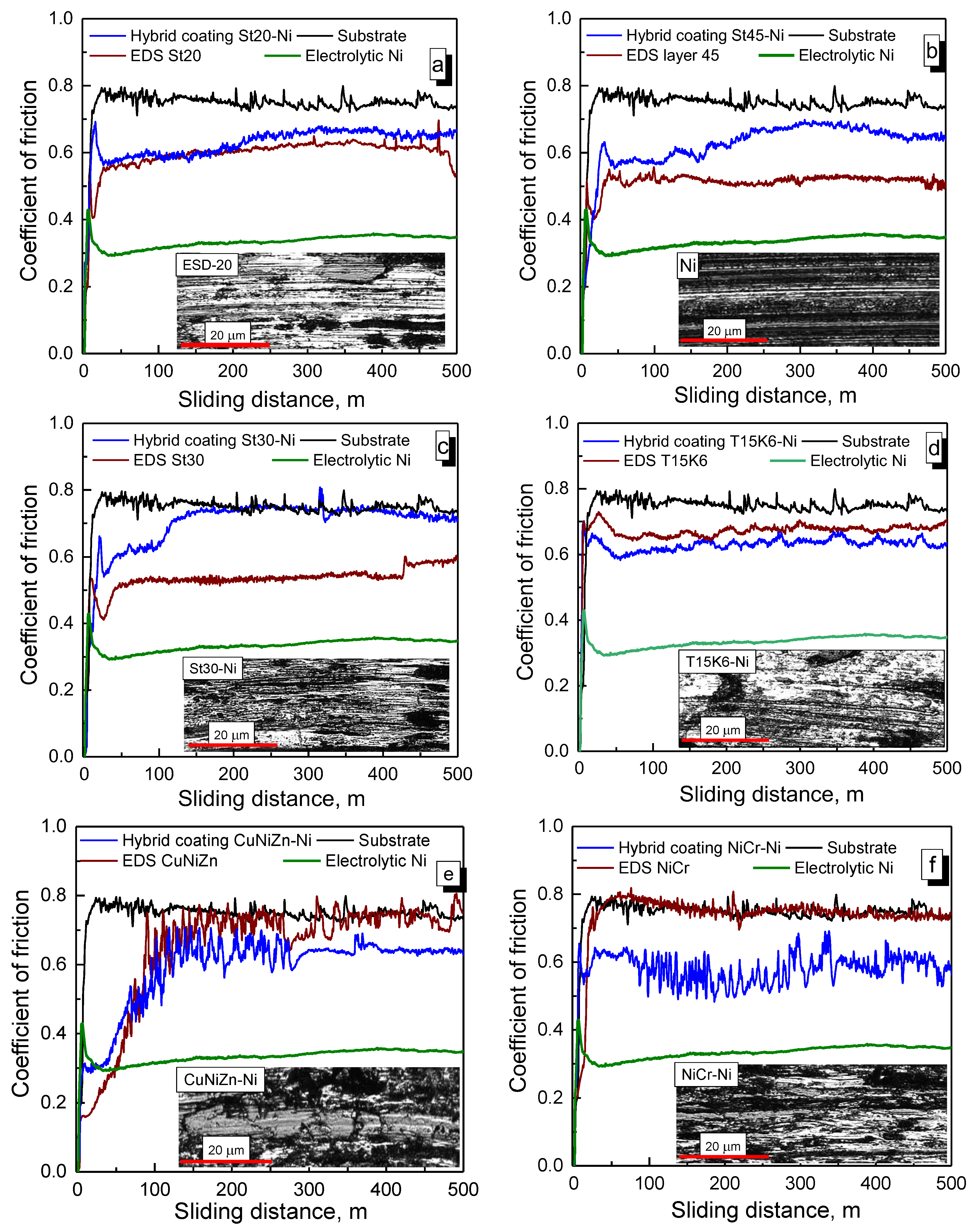
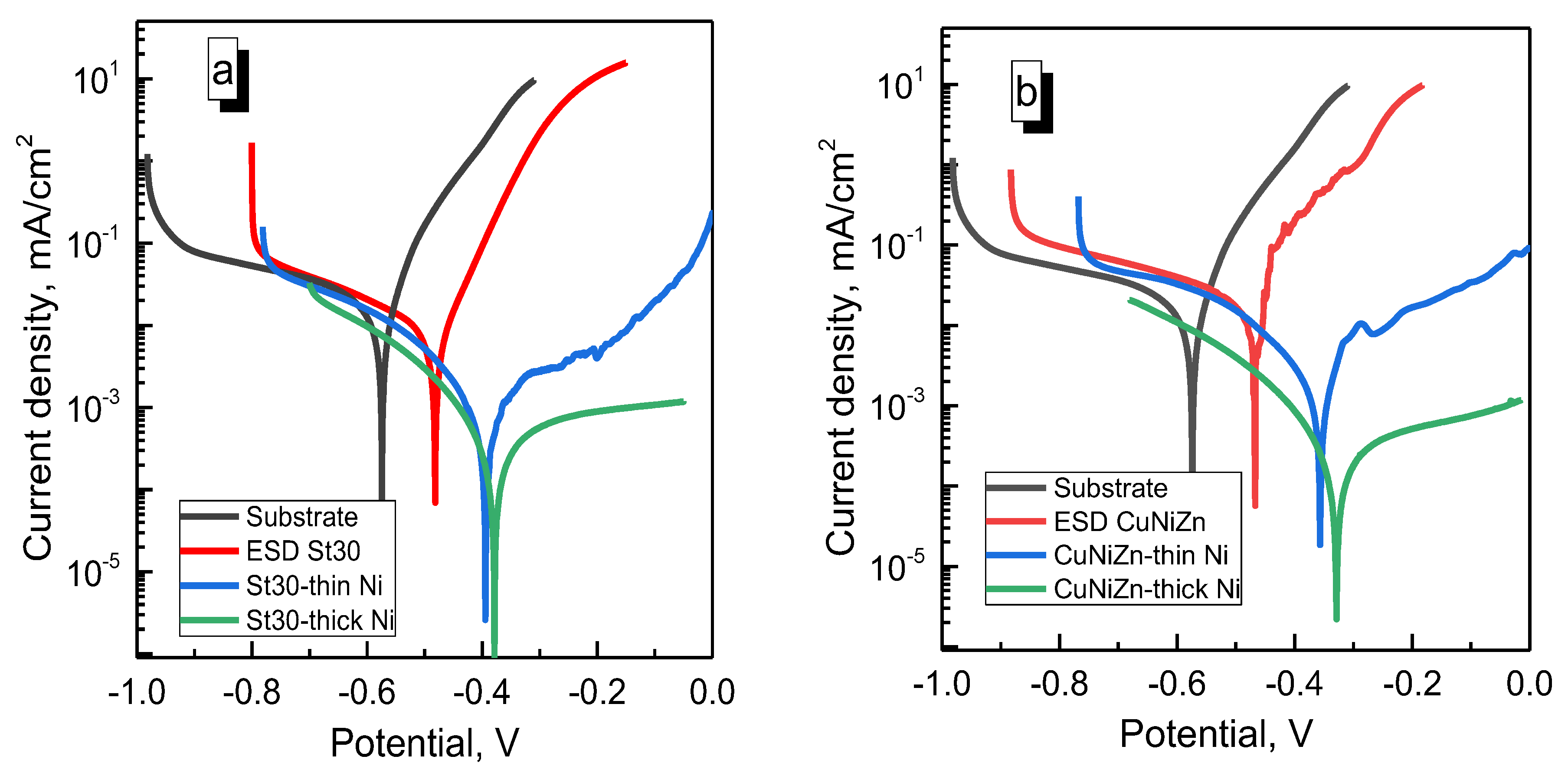
3.3. Wear and Corrosion Resistance of Hybrid Coatings
3.3.1. Wear Tests
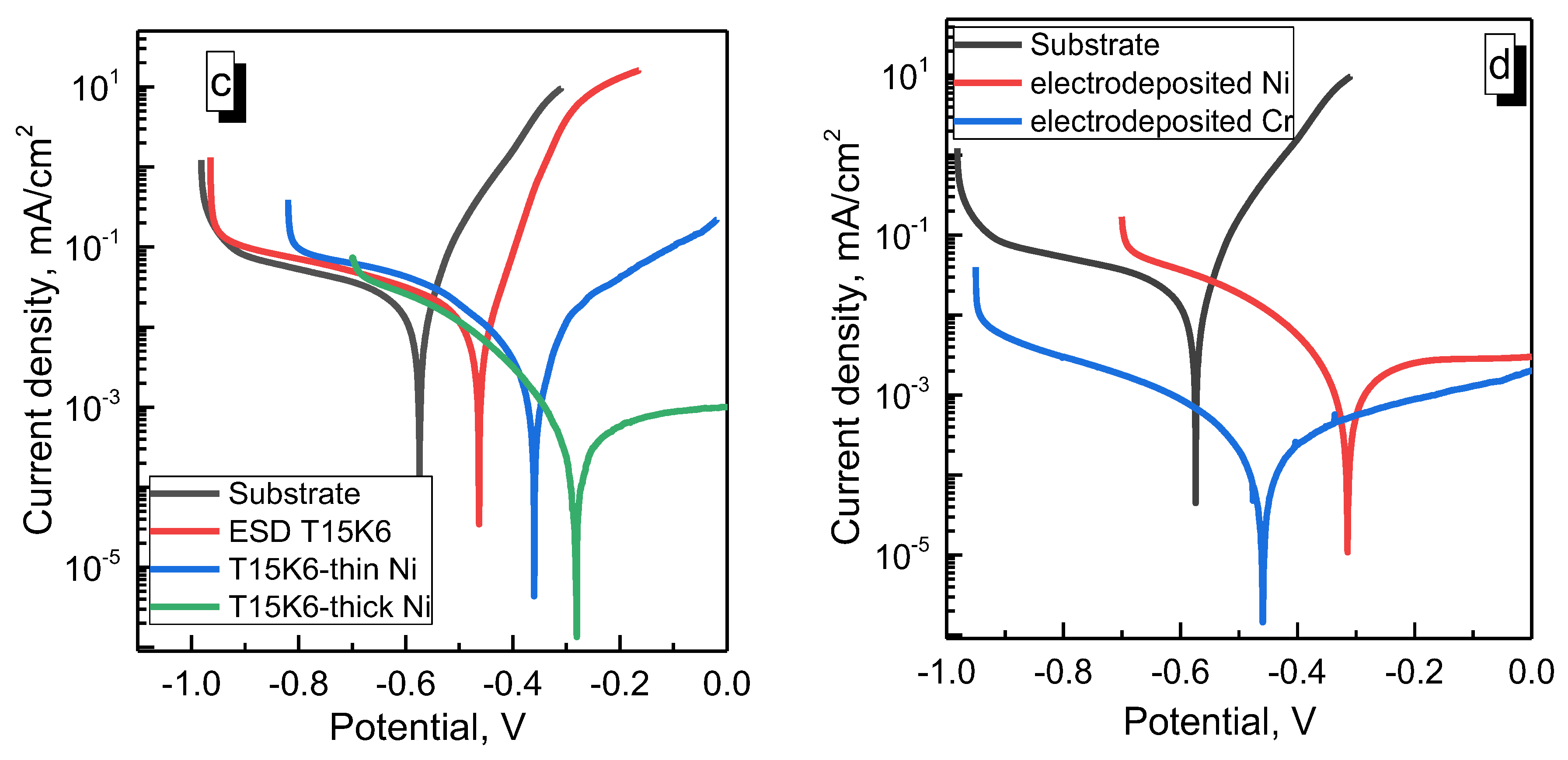
3.3.2. Corrosion Tests
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fotovvati, B.; Namdari, N.; Dehghanghadikolaei, A. On Coating Techniques for Surface Protection: A Review. J. Manuf. Mater. Process. 2019, 3, 28. [Google Scholar] [CrossRef]
- Zielinski, A.; Bartmanski, M. Electrodeposited Biocoatings, Their Properties and Fabrication Technologies: A Review. Coatings 2020, 10, 782. [Google Scholar] [CrossRef]
- Felix, L.M.; Kwan, C.C.; Zhou, N.Y. The Effect of Pulse Energy on the Defects and Microstructure of Electro-Spark-Deposited Inconel 718. Metall. Mater. Trans. A 2019, 50, 4223–4231. [Google Scholar] [CrossRef]
- Lazarenko, B.R.; Lazarenko, N.I. Electrospark Machining of Metals; US Cons Bureau: New York, NY, USA, 1964. [Google Scholar]
- Renna, G.; Leo, P.; Casalino, G.; Cerri, E. Repairing 2024 Aluminum Alloy via Electrospark Deposition Process: A Feasibility Study. Adv. Mater. Sci. Eng. 2018, 2018, 8563054. [Google Scholar] [CrossRef]
- Barile, C.; Casavola, C.; Pappalettera, G.; Renna, G. Advancements in Electrospark Deposition (ESD) Technique: A Short Review. Coatings 2022, 12, 1536. [Google Scholar] [CrossRef]
- Cao, G.; Zhng, X.; Tang, G.; Ma, X. Microstructure and corrosion behavior of Cr coating on M50 steel fabricated electrospark deposition. J. Mater. Eng. Perfom. 2019, 28, 4086–4094. [Google Scholar]
- Rukanskis, H. Control of metal surfaces mechanical and tribological characteristics using cast effective electrospark deposition. Surf. Eng. Appl. Electrochem. 2019, 55, 607–619. [Google Scholar] [CrossRef]
- Topala, P.; Ojegov, E.; Ursaki, V. Nanostructures of obtaining using electric discharges at atmospheric pressure. In Nanostructures and Thin Films of Multifunctional Applications; Tigineanu, I., Topala, P., Ursaki, V., Eds.; Springer International Publishing AG: Berlin/Heidelberg, Germany, 2016; pp. 43–83. [Google Scholar]
- Padgurskas, J.; Kreivaitis, R.; Rukuiža, R.; Mihailov, V.; Agafii, V.; Kriūkienė, R.; Baltušnikas, A. Tribological properties of coatings obtained by electro-spark alloying C45 steel surfaces. Surf. Coat. Technol. 2017, 311, 90–97. [Google Scholar] [CrossRef]
- Myslyvchenko, O.; Gaponova, O.; Tarelnyk, V.; Krapivka, M.O. The Structure Formation and Hardness of High-Entropy Alloy Coatings Obtained by Electrospark Deposition. Powder Metall. Met. Ceram. 2020, 59, 201–208. [Google Scholar] [CrossRef]
- Sheveyko, A.N.; Kuptsov, K.A.; Kiryukhantsev-Korneev, P.V.; Kaplansky, Y.Y.; Orekhov, A.S.; Levashov, E.A. Protective coatings for LPBF Ni-based superalloys using a combination of electrospark deposition and pulsed arc evaporation methods. Appl. Surf. Sci. 2022, 581, 152357. [Google Scholar] [CrossRef]
- Vizureanu, P.; Perju, M.; Achitei, D.; Nejneru, C. Advanced Electro-Spark Deposition Process on Metallic Alloys. In Advanced Surface Engineering Research; Chowdhury, M.A., Ed.; IntechOpen: London, UK, 2018; pp. 45–68. [Google Scholar]
- Paustovskii, A.V.; Tkachenko, Y.G.; Alfintseva, R.A.; Kirilenko, S.N.; Yurchenko, D.Z. Optimization of the composition, structure, and properties of electrode materials and electrospark coatings for strengthening and reconditioning of metal surfaces. Surf. Engin. Appl. Electrochem. 2013, 49, 4–12. [Google Scholar] [CrossRef]
- Levashov, E.A.; Malochkin, O.V.; Kudryashov, A.E.; Gammel, F.; Suchentrunk, R. Effects of nanocrystalline powders additions on the characteristics of combustion process, phase and structure-formation, and properties of SHS alloys on titanium carbide base. J. Mater. Synth. Process. 2002, 10, 231–236. [Google Scholar] [CrossRef]
- Celis, J.P.; Drees, D.; Huq, M.Z.; Wu, P.Q.; De Bonte, M. Hybrid processes—A versatile technique to match process requirements and coating needs. Surf. Coat. Technol. 1999, 113, 165–181. [Google Scholar] [CrossRef]
- Gleiter, H. Nanostructural materials: Basic concepts. Acta Mater. 2000, 48, 1–29. [Google Scholar] [CrossRef]
- Onishchenko, D.V.; Reva, V.P.; Kuryavyi, V.G.; Petrov, V.V.; Kim, V.A. Innovative Technology for Forming Hard Alloy T15K6 Using Amorphous Carbon from Renewable Plant Raw Material. Metallurgist 2013, 57, 548–552. [Google Scholar] [CrossRef]
- Dobruchowska, E.; Koprowska, J. Corrosion Behaviour of CuSn- and CuZnNi-coated Polypropylene Nonwoven. Fibres Text. East. Eur. 2006, 24, 72–78. [Google Scholar] [CrossRef]
- Yu, H.; Shi, H.X.; Wang, Y.L.; Zhang, K.K.; Wang, W.Y.; Han, L.J.; Pang, Q.H. NiCr alloy coating deposited on the surface of 35CrMo steel by the electrospark process. Mater. Sci. Forum 2008, 575–578, 827–832. [Google Scholar] [CrossRef]
- Kozyr, A.; Konevtsov, L.; Konovalov, S.; Kovalenko, S.; Ivashenko, V. Research on heat resistance properties of coatings deposited by electrospark alloying on steel C45 by nickel-chromium alloys. Lett. Mater. 2018, 8, 140–145. [Google Scholar] [CrossRef]
- Bobanova, Z.; Petrenko, V.; Tsyntsaru, N.; Dikusar, A. Leveling power of Co-W and Fe-W electrodeposited coatings. Key Eng. Mater. 2019, 813, 248–253. [Google Scholar] [CrossRef]
- Cesiulis, H.; Tsyntsaru, N.; Podlaha, E.; Li, D.; Sort, J. Electrodeposition of iron-group alloys into nanostructured oxide membranes: Synthetic challenges and properties. Curr. Nanosci. 2018, 14, 1–16. [Google Scholar] [CrossRef]
- Revathy, S.; Nair, A.S.; Sreejakumari, S.S. Recent trends and developments in two-dimensional materials based electrodeposited nickel nanocomposite coatings. FlatChem 2022, 36, 100434. [Google Scholar]
- Zhao, H.; Liu, L.; Zhu, J.; Tang, Y.; Hu, W. Microstructure and corrosion behavior of electrodeposited nickel prepared from a sulphamate bath. Mater. Lett. 2007, 61, 1605–1608. [Google Scholar] [CrossRef]
- Hasanpour, P.; Salehikahrizsangi, P.; Raeissi, K.; Santamaria, M.; Calabrese, L.; Proverbio, E. Dual Ni/Ni-Co Electrodeposited Coatings for Improved Erosion-Corrosion Behaviour. Surf. Coat. Technol. 2019, 368, 147–161. [Google Scholar] [CrossRef]
- Yang, W.; Liang, Z.; Yang, D.; Chen, D.; Luo, X.; Xilin Luo, X.; Gong, C. Corrosion of Electrodeposited Ni Coatings of Agricultural Machinery by Farming Fertilizers. J. Mater. Eng. Perform. 2021, 30, 7275–7282. [Google Scholar] [CrossRef]
- Zhu, W.; Xu, Y.; Kong, D. Microstructure and Tribological Performance of Electrodeposited Cr Coating with Trivalent–Chromium Electrolyte. JOM 2022, 74, 4575–4582. [Google Scholar] [CrossRef]
- Wei, T.; Zhang, R.; Yang, H.; Liu, H.; Qiu, S.; Wang, Y.; Du, P.; He, K.; Hu, X.; Dong, C. Microstructure, corrosion resistance and oxidation behavior of Cr-coatings on Zircaloy-4 prepared by vacuum arc plasma deposition. Corros. Sci. 2019, 158, 108077. [Google Scholar] [CrossRef]
- Scinti.net. Available online: https://www.scinti.net/electrospark-treatment-edm/alier-31 (accessed on 29 March 2023).
- Kruglikov, S.S. Leveling Power of Electrolytes and Microgeometric Characteristics of Surfaces of Electrodeposited Metals. Russ. J. Electrochem. 2001, 37, 657–660. [Google Scholar] [CrossRef]
- Huang, C.A.; Hsu, F.Y.; Yao, S.J. Microstructure analysis of the martensitic stainless steel surface fine-cut by the wire electrode discharge machining (WEDM). Mater. Sci. Eng. 2004, A371, 119–126. [Google Scholar] [CrossRef]
- Zhou, G.; Zhu, Y.; Wang, X.; Xia, M.; Zhang, Y.; Ding, H. Sliding tribological properties of 0.45% carbon steel lubricated with Fe3O4 magnetic nano-particle additives in baseoil. Wear 2013, 301, 753–757. [Google Scholar] [CrossRef]
- Hatipoglu, G.; Kartal, M.; Uysal, M.; Cetinkaya, T.; Akbulut, H. The effect of sliding speed on the wear behavior of pulse electro Co-deposited Ni/MWCNT nanocomposite coatings. Tribol. Int. 2016, 98, 59–73. [Google Scholar] [CrossRef]
- Goel, S.; Joshi, S.S.; Abdelal, G.; Agrawal, A. Molecular dynamics simulation of nanoindentation of Fe3C and Fe4C. Mater. Sci. Eng. A 2014, 597, 331–341. [Google Scholar] [CrossRef]
- Medabalimi, S.R.; Ramesh, M.R.; Kadoli, R. Developing partially oxidized NiCr coatings using the combined flame spray and plasma spray process for improved wear behaviour at high temperature. Wear 2021, 478–479, 203885. [Google Scholar] [CrossRef]
- Bersirova, O.; Cesiulis, H.; Donten, M.; Krolikowski, A.; Stojek, Z.; Baltrunas, G. Corrosion and anodic behavior of electrodeposited Ni-Mo alloys. Physchem. Mech. Mat. 2004, 4, 620–625. [Google Scholar]
- Safonov, V.A.; Vykhodtseva, L.N.; Edigaryan, A.A.; Aliev, A.D.; Molodkina, E.B.; Danilov, A.I.; Lubnin, E.N.; Polukarov, Y.M. Corrosion–electrochemical behavior of chromium deposits obtained from sulfuric acid solutions containing oxalates. Russ. J. Electrochem. 2001, 37, 127–134. [Google Scholar] [CrossRef]
- Makarava, I.; Esmaeili, M.; Kharytonau, D.S.; Pelcastre, L.; Ryl, J.; Bilesan, M.R.; Vuorinen, E.; Repo, E. Influence of CeO2 and TiO2 Particles on Physicochemical Properties of Composite Nickel Coatings Electrodeposited at Ambient Temperature. Materials 2022, 15, 5550. [Google Scholar] [CrossRef]
- Shozib, I.; Ahmad, A.; Abdul-Rani, A.; Beheshti, M.; Aliyu, A. A review on the corrosion resistance of electroless Ni-P based composite coatings and electrochemical corrosion testing methods. Corros. Rev. 2022, 40, 1–37. [Google Scholar] [CrossRef]


| Sample No. | Electrode Material Used for ESD Layer | ESD Parameters |
|---|---|---|
| 1 | T15K6 (WC 79 %; TiC 15 %, Co 6%) | Manual mode Pulse energy: 0.07 J; Application speed 2.5 cm2/min; Deposition time: 4 min. |
| 2 | CuNiZn (Cu 52%, Ni 11%, Zn 33%, Mn 4%) | |
| 3 | Ni-Cr (Ni 79%, Cr 19%, Fe 2%) | |
| 4 | AISI 1045 (St45) | |
| 5 | AISI 1020 (St20) | |
| 6 | AISI 1030 (St30) |
| No | St30 Substrate Ra, µm | ESD Layer | Hybrid Coating | |||
|---|---|---|---|---|---|---|
| “thin” Ni | “thick” Ni | Abbreviation | ||||
| Electrode tool | Ra, µm | Ra, µm | ||||
| 1 | 0.3 ± 0.1 | T15K6 | 4.9 ± 0.4 | 3.8 ± 0.4 | 2.3 ± 0.2 | T15K6-Ni |
| 2 | CuNiZn | 2.3 ± 0.4 | 2.5 ± 0.7 | 1.2 ± 0.2 | CuNiZn-Ni | |
| 3 | NiCr | 3.8 ± 0.5 | 3.1 ± 0.4 | 2.5 ± 0.1 | NiCr-Ni | |
| 4 | St45 | 3.8 ± 0.3 | 4.4 ± 0.4 | 2.3 ± 0.1 | St45-Ni | |
| 5 | St20 | 3.7 ± 0.2 | 4.7 ± 0.9 | 3.7 ± 0.3 | St20-Ni | |
| 6 | St30 | 5.7 ± 0.5 | 3.6 ± 0.6 | 1.6 ± 0.2 | St30-Ni | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benkovsky, I.; Tsyntsaru, N.; Silkin, S.; Petrenko, V.; Pakstas, V.; Cesiulis, H.; Dikusar, A. Synthesis, Wear and Corrosion of Novel Electrospark and Electrospark–Electrochemical Hybrid Coatings Based on Carbon Steels. Lubricants 2023, 11, 205. https://doi.org/10.3390/lubricants11050205
Benkovsky I, Tsyntsaru N, Silkin S, Petrenko V, Pakstas V, Cesiulis H, Dikusar A. Synthesis, Wear and Corrosion of Novel Electrospark and Electrospark–Electrochemical Hybrid Coatings Based on Carbon Steels. Lubricants. 2023; 11(5):205. https://doi.org/10.3390/lubricants11050205
Chicago/Turabian StyleBenkovsky, Iurii, Natalia Tsyntsaru, Serhii Silkin, Vladimir Petrenko, Vidas Pakstas, Henrikas Cesiulis, and Alexandr Dikusar. 2023. "Synthesis, Wear and Corrosion of Novel Electrospark and Electrospark–Electrochemical Hybrid Coatings Based on Carbon Steels" Lubricants 11, no. 5: 205. https://doi.org/10.3390/lubricants11050205
APA StyleBenkovsky, I., Tsyntsaru, N., Silkin, S., Petrenko, V., Pakstas, V., Cesiulis, H., & Dikusar, A. (2023). Synthesis, Wear and Corrosion of Novel Electrospark and Electrospark–Electrochemical Hybrid Coatings Based on Carbon Steels. Lubricants, 11(5), 205. https://doi.org/10.3390/lubricants11050205







