Tribological Response of δ-Bi2O3 Coatings Deposited by RF Magnetron Sputtering
Abstract
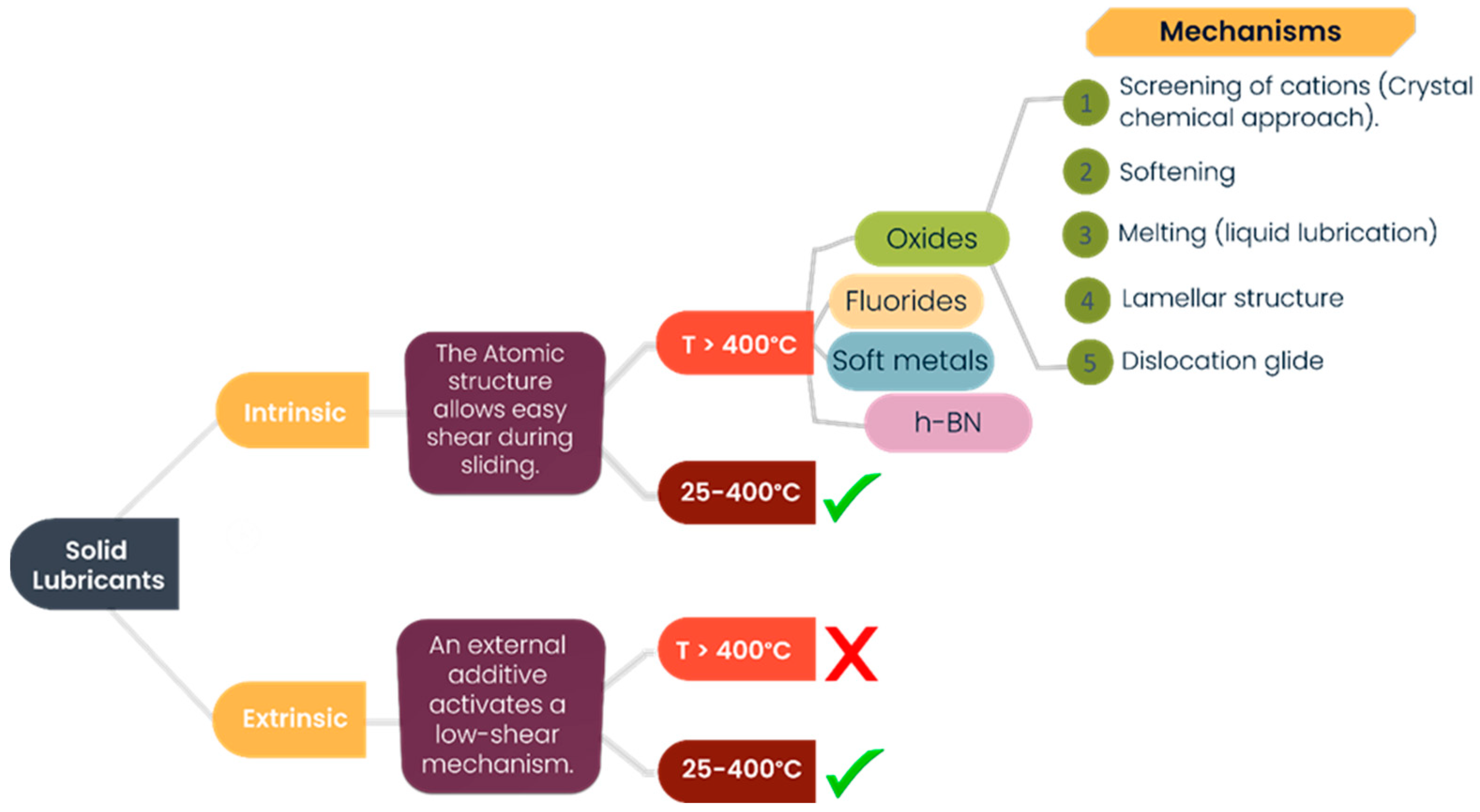
1. Introduction
2. Materials and Methods
3. Results
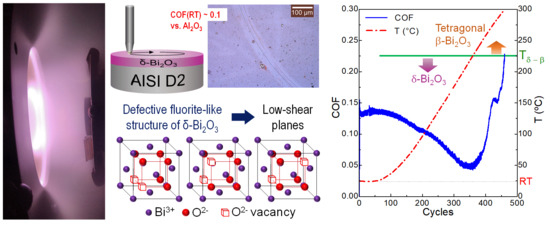
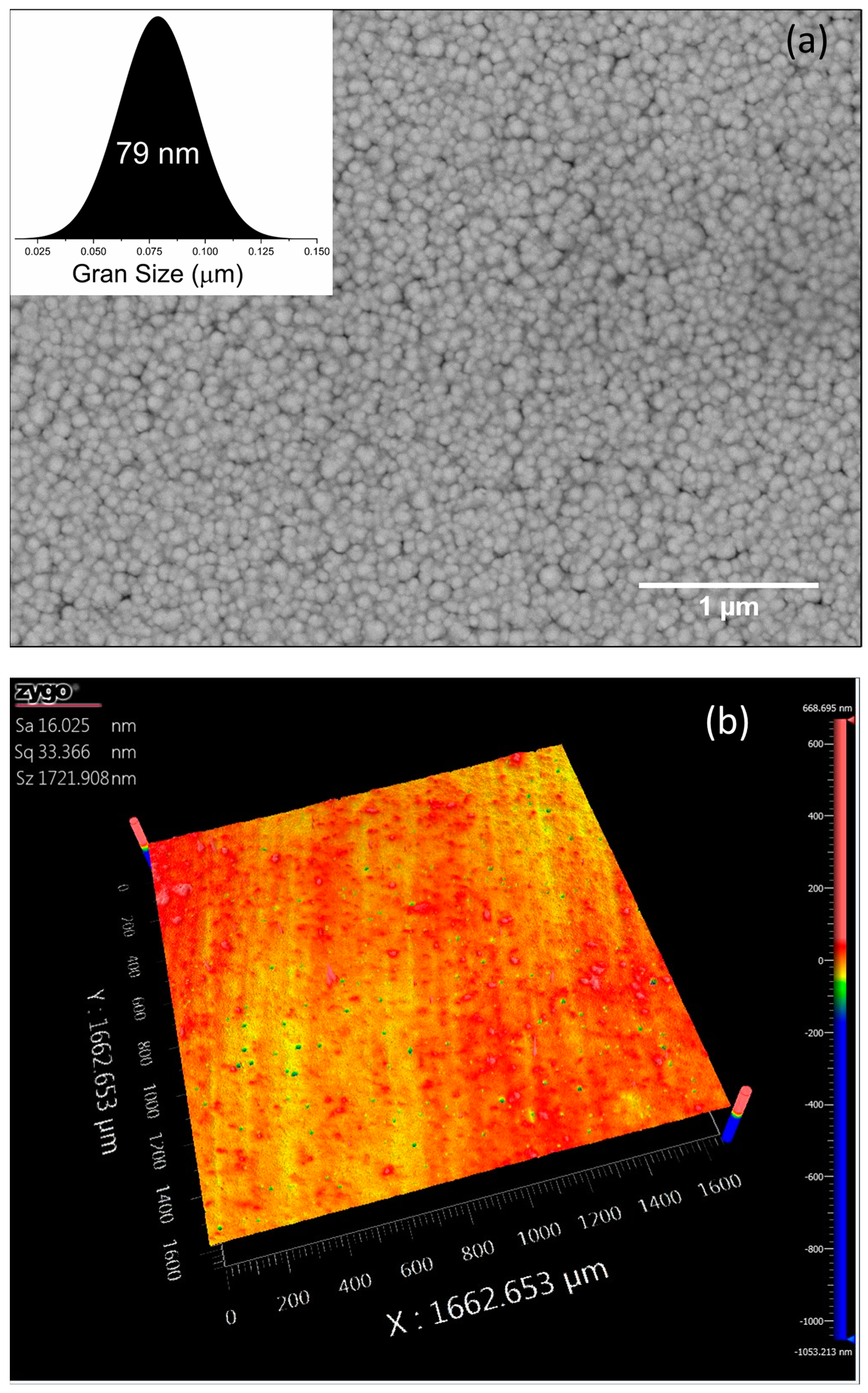
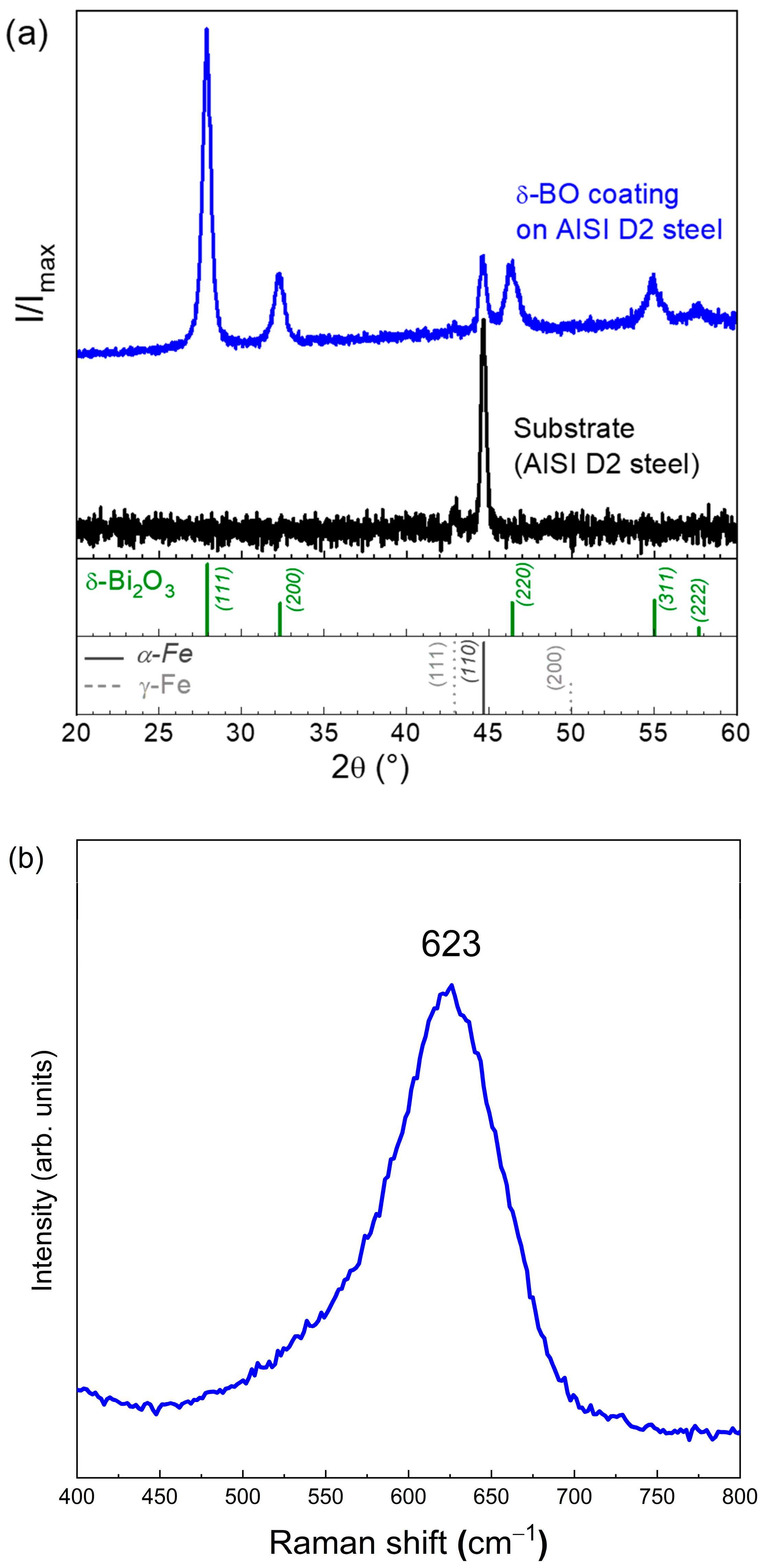
3.1. Structural Characterization
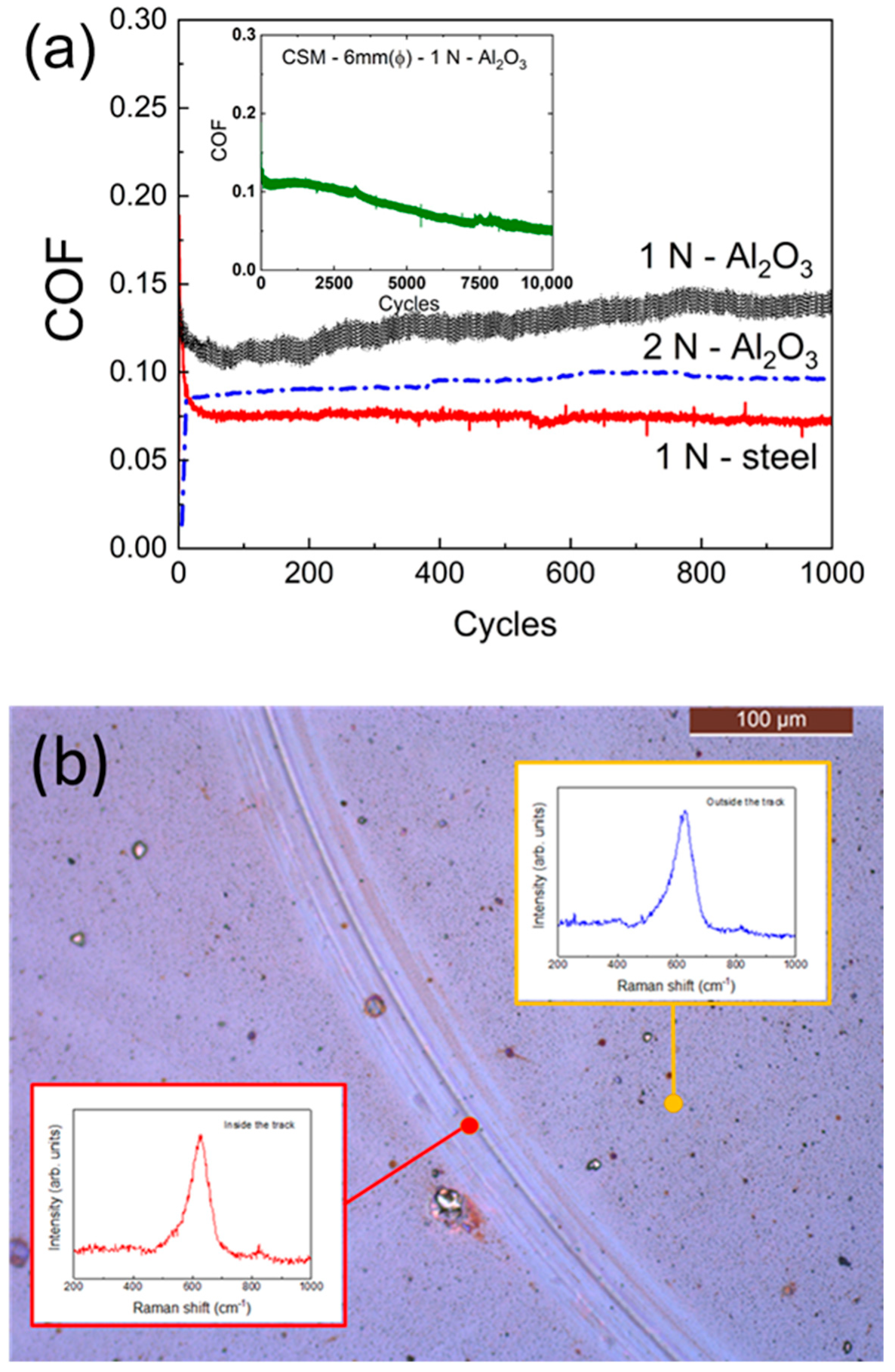
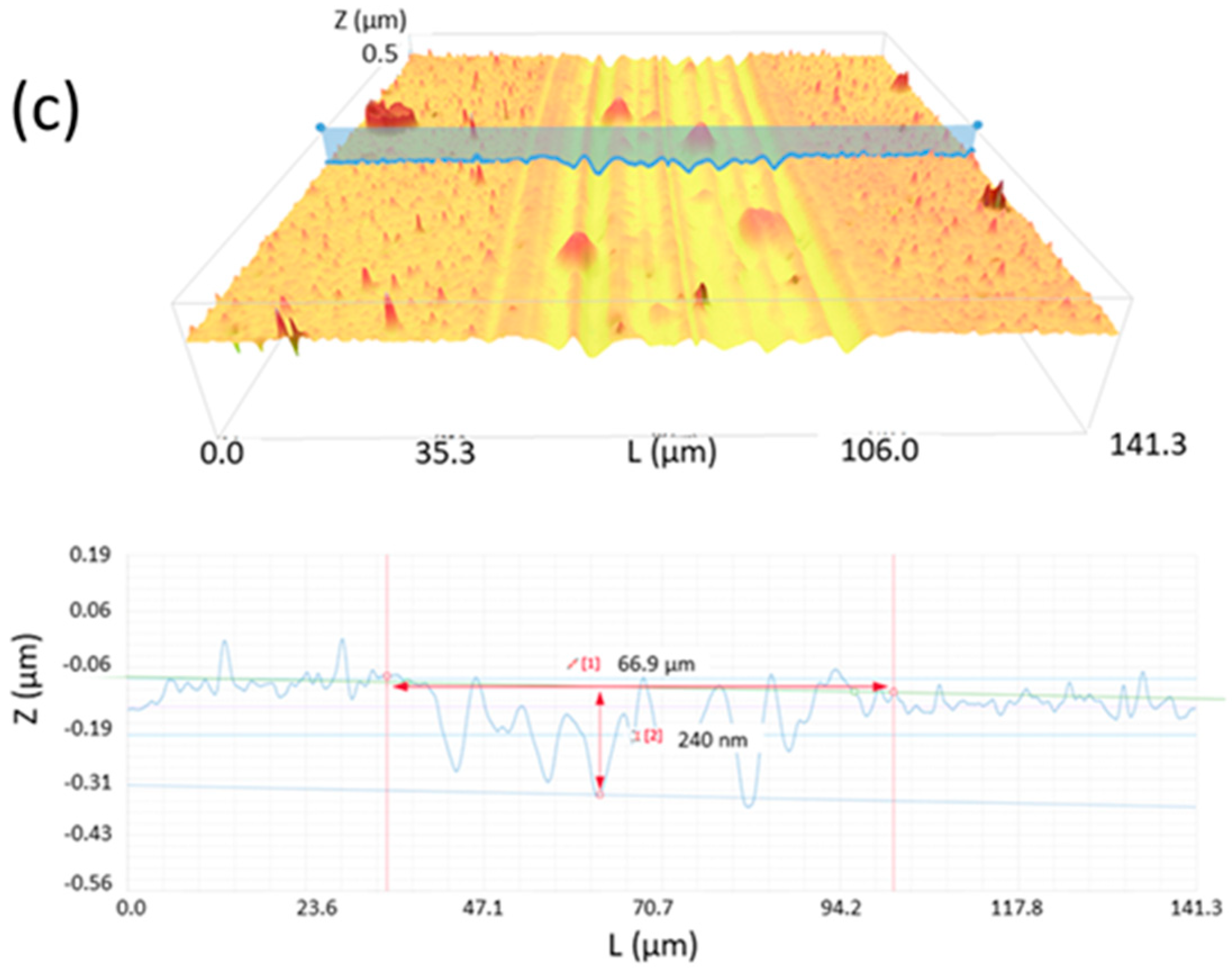
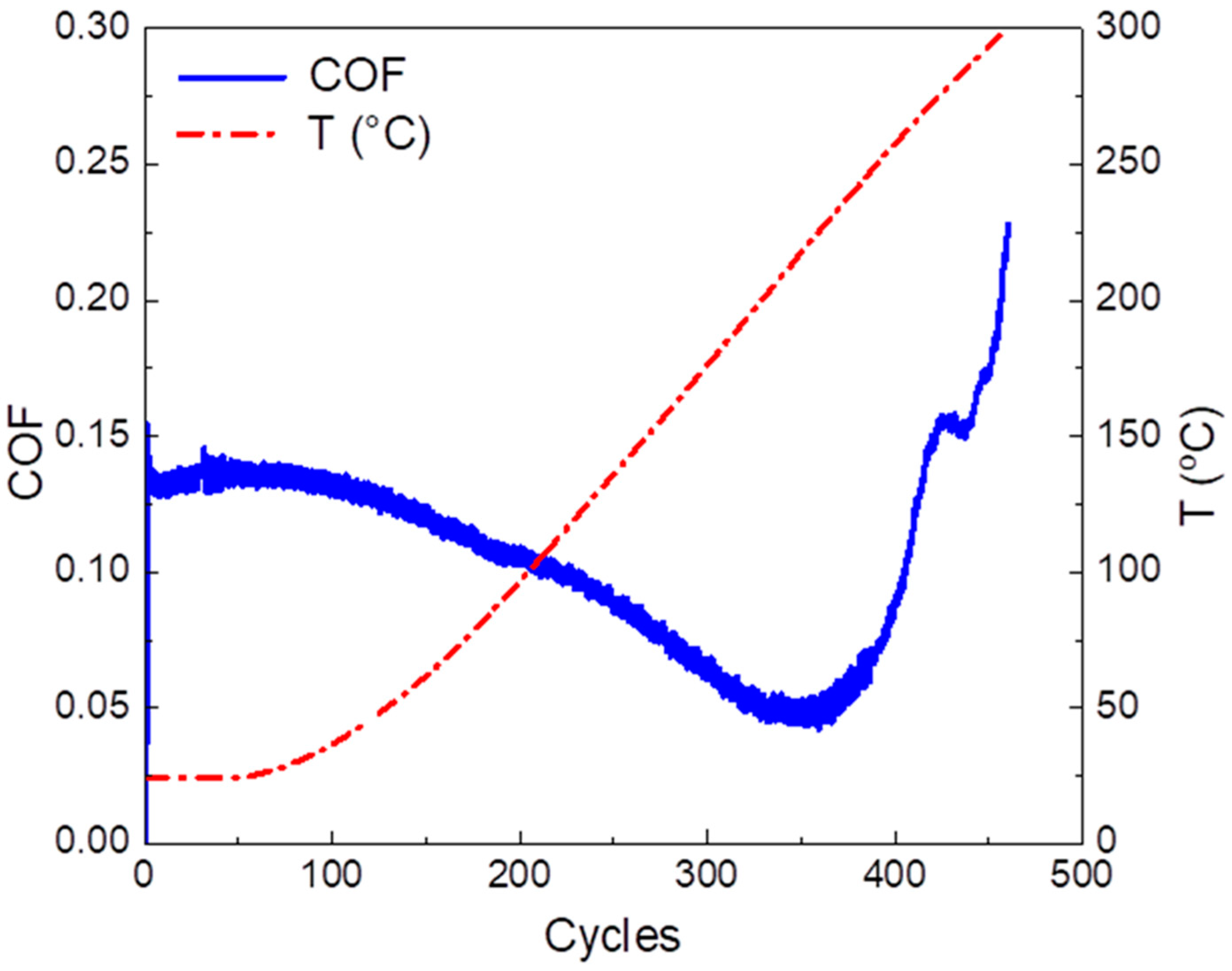
3.2. Tribological Characterization
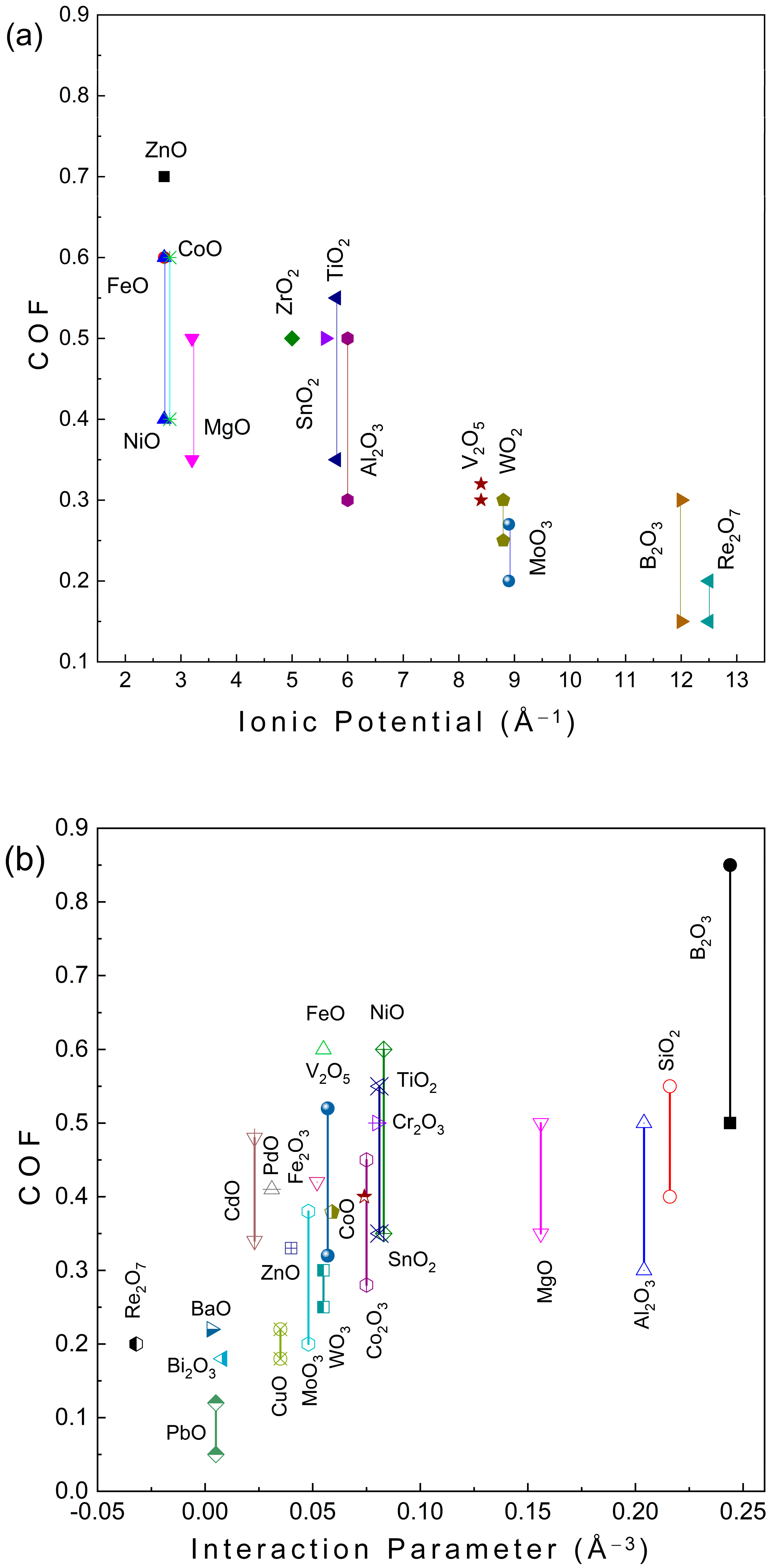
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ouyang, J.-H.; Li, Y.-F.; Zhang, Y.-Z.; Wang, Y.-M.; Wang, Y.-J. High-Temperature Solid Lubricants and Self-Lubricating Composites: A Critical Review. Lubricants 2022, 10, 177. [Google Scholar] [CrossRef]
- Quinn, T.F.J.; Sullivan, J.L.; Rowson, D.M. Origins and development of oxidational wear at low ambient temperatures. Wear 1984, 94, 175–191. [Google Scholar] [CrossRef]
- Tong, Y.; Zhang, T.; Zhang, S. Influence of oxides on the formation of self-lubricating layer and anti-wear performance during sliding. Tribol. Int. 2023, 179, 108188. [Google Scholar] [CrossRef]
- Aouadi, S.M.; Gao, H.; Martini, A.; Scharf, T.W.; Muratore, C. Lubricious oxide coatings for extreme temperature applications: A review. Surf. Coat. Technol. 2014, 257, 266–277. [Google Scholar] [CrossRef]
- He, N.; Li, H.; Ji, L.; Liu, X.; Zhou, H.; Chen, J. Reusable chromium oxide coating with lubricating behavior from 25 to 1000 °C due to a self-assembled mesh-like surface structure. Surf. Coat. Technol. 2017, 321, 300–308. [Google Scholar] [CrossRef]
- Muratore, C.; Voevodin, A.A. Chameleon Coatings: Adaptive Surfaces to Reduce Friction and Wear in Extreme Environments. Annu. Rev. Mater. Res. 2009, 39, 297–324. [Google Scholar] [CrossRef]
- Skopp, A.; Woydt, M. Ceramic and Ceramic Composite Materials with Improved Friction and Wear Properties. Tribol. Trans. 1995, 38, 233–242. [Google Scholar] [CrossRef]
- Erdemir, A. A crystal-chemical approach to lubrication by solid oxides. Tribol. Lett. 2000, 8, 97–102. [Google Scholar] [CrossRef]
- Prakash, B.; Celis, J.P. The Lubricity of Oxides Revised Based on a Polarisability Approach. Tribol. Lett. 2007, 27, 105–112. [Google Scholar] [CrossRef]
- Gardos, M.N. Magneli phases of anion-deficient rutile as lubricious oxides. Part I. Tribological behavior of single-crystal and polycrystalline rutile (TinO2n−1). Tribol. Lett. 2000, 8, 65–78. [Google Scholar] [CrossRef]
- Magnéli, A. Structures of the ReO3-type with Recurrent Dislocations of atoms: ‘Homologous Series’ of Molybdenum and Tungsten Oxides. Acta Crystallogr. 1953, 6, 495–500. [Google Scholar] [CrossRef]
- Erdemir, A.; Li, S.; Jin, Y. Relation of Certain Quantum Chemical Parameters to Lubrication Behavior of Solid Oxides. Int. J. Mol. Sci. 2005, 6, 203–218. [Google Scholar] [CrossRef]
- Martini, A.; Eder, S.J.; Dörr, N. Tribochemistry: A Review of Reactive Molecular Dynamics Simulations. Lubricants 2020, 8, 44. [Google Scholar] [CrossRef]
- Erdemir, A.; Ramirez, G.; Eryilmaz, O.L.; Narayanan, B.; Liao, Y.; Kamath, G.; Sankaranarayanan, S.K.R.S. Carbon-based tribofilms from lubricating oils. Nature 2016, 536, 67–71. [Google Scholar] [CrossRef]
- Yamashita, J.; Kurosawa, T. The Theory of the Dielectric Constant of Ionic Crystals III. J. Phys. Soc. Jpn. 1955, 10, 610–633. [Google Scholar] [CrossRef]
- Dimitrov, V.; Komatsu, T. Classification of Simple Oxides: A Polarizability Approach. J. Solid State Chem. 2002, 163, 100–112. [Google Scholar] [CrossRef]
- Woydt, M.; Skopp, A.; Dorfel, I.; Witke, K. Wear engineering oxides/anti-wear oxides. Wear 1998, 218, 84–95. [Google Scholar] [CrossRef]
- Fateh, N.; Fontalvo, G.A.; Gassner, G.; Mitterer, C. The Beneficial Effect of High-Temperature Oxidation on the Tribological Behaviour of V and VN Coatings. Tribol. Lett. 2007, 28, 1–7. [Google Scholar] [CrossRef]
- Gassner, G.; Mayrhofer, P.H.; Kutschej, K.; Mitterer, C.; Kathrein, M. A new low friction concept for high temperatures: Lubricious oxide formation on sputtered VN coatings. Tribol. Lett. 2004, 14, 751–756. [Google Scholar] [CrossRef]
- Kato, H.; Komai, K. Tribofilm formation and mild wear by tribo-sintering of nanometer-sized oxide particles on rubbing steel surfaces. Wear 2007, 262, 36–41. [Google Scholar] [CrossRef]
- Gonzalez-Rodriguez, P.; van den Nieuwenhuijzen, K.J.; Lette, W.; Schipper, D.J.; Ten Elshof, J.E. Tribochemistry of Bismuth and Bismuth Salts for Solid Lubrication. ACS Appl. Mater. Interfaces 2016, 8, 7601–7606. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Yi, G.; Wan, S.; Shi, P.; Yang, J.; Pham, S.T.; Tieu, A.K.; Ta, T.D. Effect of adding soft Bi2O3 on structural modification and tribological regulation of Ni-5 wt% Al composite coating in wide temperatures range. Surf. Coat. Technol. 2021, 405, 126517. [Google Scholar] [CrossRef]
- Levin, E.M.; Roth, R.S. Polymorphism of Bismuth Sesquioxide. II. Effect of Oxide Additions on Polymorphism of Bi2O3. J. Res. Natl. Bur. Stand. Sect. A Phys. Chem. 1964, 68A, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Li, W. Preparation and structure characteristics of nano-Bi2O3 powders with mixed crystal structure. J. Cent. South Univ. Technol. 2005, 12, 243–245. [Google Scholar] [CrossRef]
- Switzer, J.A.; Shumsky, M.G.; Bohannan, E.W. Electrodeposited ceramic single crystals. Science 1999, 284, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Bohannan, E.W.; Jaynes, C.C.; Shumsky, M.G.; Barton, J.K.; Switzer, J.A. Low-temperature electrodeposition of the high-temperature cubic polymorph of bismuth(III) oxide. Solid State Ion. 2000, 131, 97–107. [Google Scholar] [CrossRef]
- Fan, H.T.; Pan, S.S.; Teng, X.M.; Ye, C.; Li, G.H. Structure and thermal stability of delta-Bi2O3 thin films deposited by reactive sputtering. J. Phys. D Appl. Phys. 2006, 39, 1939–1943. [Google Scholar] [CrossRef]
- Gomez, C.L.; Depablos-Rivera, O.; Silva-Bermudez, P.; Muhl, S.; Zeinert, A.; Lejeune, M.; Charvet, S.; Barroy, P.; Camps, E.; Rodil, S.E. Opto-electronic properties of bismuth oxide films presenting different crystallographic phases. Thin Solid Film. 2015, 578, 103–112. [Google Scholar] [CrossRef]
- Gomez, C.L.; Rodil, S.E. High stability and ac-conductivity of cubic fluorite-Bi2O3 films synthesized by magnetron sputtering. Solid State Ion. 2017, 309, 100–109. [Google Scholar] [CrossRef]
- Depablos-Rivera, O.; Martínez, A.; Rodil, S.E. Interpretation of the Raman spectra of bismuth oxide thin films presenting different crystallographic phases. J. Alloy. Compd. 2021, 853, 157245. [Google Scholar] [CrossRef]
- Gomez, C.L.; Depablos-Rivera, O.; Medina, J.C.; Silva-Bermudez, P.; Muhl, S.; Zeinert, A.; Rodil, S.E. Stabilization of the delta-phase in Bi2O3 thin films. Solid State Ion. 2014, 255, 147–152. [Google Scholar] [CrossRef]
- Leontie, L.; Caraman, M.; Evtodiev, I.; Cuculescu, E.; Mija, A. Optical properties of bismuth oxide thin films prepared by reactive d.c. magnetron sputtering onto p-GaSe (Cu). Phys. Status Solidi (A) 2008, 205, 2052–2056. [Google Scholar] [CrossRef]
- Klinkova, L.A.; Nikolaichik, V.I.; Barkovskii, N.V.; Fedotov, V.K. Thermal stability of Bi2O3. Russ. J. Inorg. Chem. 2007, 52, 1822–1829. [Google Scholar] [CrossRef]
- Matsumoto, A.; Koyama, Y.; Tanaka, I. Structures and energetics of Bi2O3 polymorphs in a defective fluorite family derived by systematic first-principles lattice dynamics calculations. Phys. Rev. B 2010, 81, 094117. [Google Scholar] [CrossRef]
- Sillen, L.G. Die Kristallstruktur des monoklinen α-Bi2O3. Die Nat. 1940, 28, 206–207. [Google Scholar] [CrossRef]
- Gattow, G.; Schroder, H. Uber Wismutoxide. III. Die Kristallstruktur Der Hochtemperaturmodifikation Von Wismut(Iii)-Oxid (Delta-Bi2O3). Z. Anorg. Allg. Chem. 1962, 318, 176–189. [Google Scholar] [CrossRef]
- Storz, O.; Gasthuber, H.; Woydt, M. Tribological properties of thermal-sprayed Magnéli-type coatings with different stoichiometries (TinO2n−1). Surf. Coat. Technol. 2001, 140, 76–81. [Google Scholar] [CrossRef]
- Lugscheider, E.; Bärwulf, S.; Barimani, C. Properties of tungsten and vanadium oxides deposited by MSIP–PVD process for self-lubricating applications. Surf. Coat. Technol. 1999, 120–121, 458–464. [Google Scholar] [CrossRef]
- Ciancio, R.; Carlino, E.; Rossi, G.; Aruta, C.; Di Uccio, U.S.; Vittadini, A.; Selloni, A. Magnéli-like phases in epitaxial anatase TiO2 thin films. Phys. Rev. B 2012, 86, 104110. [Google Scholar] [CrossRef]
- Franz, R.; Mitterer, C. Vanadium containing self-adaptive low-friction hard coatings for high-temperature applications: A review. Surf. Coat. Technol. 2013, 228, 1–13. [Google Scholar] [CrossRef]
- Schwingenschlögl, U.; Eyert, V. The vanadium Magnéli phases VnO2n−1. Ann. Der Phys. 2004, 13, 475–510. [Google Scholar] [CrossRef]
- Kutschej, K.; Mayrhofer, P.H.; Kathrein, M.; Polcik, P.; Mitterer, C. Influence of oxide phase formation on the tribological behaviour of Ti–Al–V–N coatings. Surf. Coat. Technol. 2005, 200, 1731–1737. [Google Scholar] [CrossRef]
- Restrepo, J.; Mondragon-Rodriguez, G.; Gonzalez-Carmona, J.M.; Alvarado-Orozco, J.M.; Garcia-Zarco, O.; Rodil, S.E. Cathodic Arc Evaporation of Self-Lubricating TiSiVN Coatings. J. Mater. Eng. Perform. 2022, 31, 1857–1869. [Google Scholar] [CrossRef]
- Franz, R.; Neidhardt, J.; Kaindl, R.; Sartory, B.; Tessadri, R.; Lechthaler, M.; Polcik, P.; Mitterer, C. Influence of phase transition on the tribological performance of arc-evaporated AlCrVN hard coatings. Surf. Coat. Technol. 2009, 203, 1101–1105. [Google Scholar] [CrossRef]
- Mirabal-Rojas, R.; Depablos-Rivera, O.; Gómez, C.L.; Fonseca-Garcia, A.; Medina, J.C.; Barrera-Ortega, C.C.; Pérez-Alvarez, J.; Muhl, S.; Camps, E.; Rodil, S.E. Reduction of the coefficient of friction of niobium nitride coatings by the addition of bismuth. Vacuum 2016, 125, 146–153. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodil, S.E.; Depablos-Rivera, O.; Sánchez-López, J.C. Tribological Response of δ-Bi2O3 Coatings Deposited by RF Magnetron Sputtering. Lubricants 2023, 11, 207. https://doi.org/10.3390/lubricants11050207
Rodil SE, Depablos-Rivera O, Sánchez-López JC. Tribological Response of δ-Bi2O3 Coatings Deposited by RF Magnetron Sputtering. Lubricants. 2023; 11(5):207. https://doi.org/10.3390/lubricants11050207
Chicago/Turabian StyleRodil, Sandra E., Osmary Depablos-Rivera, and Juan Carlos Sánchez-López. 2023. "Tribological Response of δ-Bi2O3 Coatings Deposited by RF Magnetron Sputtering" Lubricants 11, no. 5: 207. https://doi.org/10.3390/lubricants11050207
APA StyleRodil, S. E., Depablos-Rivera, O., & Sánchez-López, J. C. (2023). Tribological Response of δ-Bi2O3 Coatings Deposited by RF Magnetron Sputtering. Lubricants, 11(5), 207. https://doi.org/10.3390/lubricants11050207