Abstract
The depletion of oil reserves and concerns about the environmental impact of the use and incorrect disposal of mineral lubricants have been promoting the development of bio-based lubricants. In this study, biolubricants obtained from fatty acids of castor oil were synthesized by esterification (>wt.%93), epoxidation (>wt.%92), and oxirane ring opening reactions using water (>wt.%92) or 2-ethylhexanol (>wt.%94) as nucleophilic agents. The frictional characteristics of the synthesized samples were obtained through tribological tests performed in a four-ball tester and compared with a commercial mineral oil. The sample obtained through oxirane ring opening with water showed the best frictional performance (FC = 0.0699 ± 0.0007) among the prepared samples, with equivalent wear rate (WSD = 281.2 ± 5.54 μm) and ca. 20% lower friction coefficient when compared to the commercial mineral oil, indicating its great potential for replacing mineral fossil oils.
1. Introduction
Lubrication, which is the application of a fluid film between the contacting metal surfaces in static and dynamic operating conditions, is used to minimize the effects of friction and wear on machinery and equipment. Currently, most lubricants are produced from mineral oil; although these lubricants meet technical requirements, their production is limited by the availability of oil reserves. Increased worldwide concerns about the environmental impact of the incorrect disposal of mineral oils and emissions of metals generated during the operation of internal combustion engines led, for example, to the introduction of non-technical criteria (ecotoxicological and sustainability properties) to reduce the environmental impact of lubricants [1,2,3,4].
Thus, efforts are devoted to the development of bio-based lubricants produced from renewable and biodegradable sources. Vegetable oils, particularly non-edible vegetable oils, are used as the main basis for the production of biolubricants. Although edible vegetable oils may serve as a basis for the production of biolubricants, such usage is associated with sustainability concerns because increased demand for edible oils would result in the expansion of plantations which could lead to deforestation and eutrophication [1,5]. At the same time, non-edible oilseeds can be cultivated in low-fertility dry and semi-arid environments [6].
Castor oil stands out among non-edible vegetable oils because of its main composition of hydroxylated fatty acids [4]. Ricinoleic acid comprises wt.% 82–90 of fatty acids in castor oil (Ricinus communis L.) [7,8,9]. Ricinoleic acid contains a hydroxyl group (–OH) in its chain, which provides good lubricity, especially for applications as hydraulic fluid, in which the lubricant operates in the mixed lubrication/EHL and hydrodynamic regimes since the hydroxyl group (-OH) forms a thick and viscous lubricant due to intermolecular hydrogen bonding. [10,11,12,13]. Although ricinoleic acid has good tribological properties, the presence of a double bond in its structure increases its susceptibility to oxidation, which is disadvantageous for direct applications in mechanical systems [14,15].
To improve the oxidative stability and other physicochemical properties of unsaturated vegetable oils, studies have been carried out to obtain esters through chemical processes [16,17]. The most common chemical modification routes are transesterification/esterification, hydrogenation, estolide formation, and epoxidation reactions [18,19,20]. The transesterification route consists of replacing the glycerol portions of the triglyceride molecule of vegetable oils with a long- or branched-chain alcohol and performing esterification with free fatty acids extracted from vegetable oils [19,21]. Hydrogenation consists of the isomerization of cis- and trans-acids. Estolide esters are obtained by bonding a carboxylic acid group to the double bond of another fatty acid [22]. Epoxidation is the removal of double carbon bonds through the introduction of an oxygen atom, resulting in the formation of an epoxide functional group [20]. Although epoxidation of vegetable oils removes unsaturations, it yields biolubricants with poor low-temperature properties, which need to be further submitted to an oxirane ring opening reaction and subsequent esterification [16,17,18,19,20,21,22]. The possibility of using a variety of vegetable oils, alcohols, and other materials results in a wide variety of products with diverse physicochemical and tribological characteristics.
Recent studies have used castor oil and/or ricinoleic acid to obtain biolubricants via an estolide formation route [23]. The obtained products showed a similar pour point and higher oxidative stability than castor oil-based lubricants reported in the literature. Encinar et al., (2020) used castor oil transesterification to obtain biolubricants with a higher flash point than mineral oils [8]. Saboya et al., (2017) obtained castor oil-based biolubricants by esterification and demonstrated that 2-ethylhexyl ricinoleate exhibits excellent low-flow properties, viscosity index (VI) comparable to that of commercial synthetic oils, biodegradability higher than mineral oil, and better oxidative stability than castor oil [24]. Rios et al., (2020) prepared biolubricants through epoxidation followed by an oxirane ring opening, and the obtained products exhibited excellent low-temperature properties with high oxidative stability [7].
Although the mentioned studies report significant improvements in physicochemical properties, neither of them evaluated the frictional characteristics of the obtained products. Tribological tests simulate lubricant operation, and proper performance (high energy efficiency, low friction coefficients (FC), and wear scar diameter (WSD)) in the test is paramount to validate the use of the considered lubricant in mechanical systems [13,25,26,27]. The present study aimed at producing biolubricants from fatty acids of castor oil through esterification, epoxidation, and oxirane ring opening reactions with 2-ethylhexanol and water used as nucleophilic agents to produce the ether branches, since these consecutive reactions improve their properties at low temperatures, viscosity index, and oxidative stability [13]. The study of their tribological characteristics becomes of fundamental importance to meet all the technical criteria for their application in machinery lubrication. Therefore, the main physicochemical properties of the products were determined, and the tribological characteristics were evaluated using a four-ball tester.
2. Materials and Methods
2.1. Materials
A sample of fatty acids from castor oil was purchased from Azevedo Ind. e Com. de Óleos LTDA (São Paulo, Brazil). The sample presented in its composition a mixture of ricinoleic acid (82–90 wt.%), linoleic acid (wt.% 2–8), oleic acid (wt.% 2–7), stearic acid (2 wt.%), and palmitic acid (wt.% 2). Acetone (>wt.% 99.5), p-toluenesulfonic acid (>wt.% 98), toluene (>wt.% 99), and sodium bicarbonate (>wt.% 99) were supplied by Neon (Suzano, Brazil). Hydrogen peroxide (35.5% v/v), anhydrous sodium sulfate (>wt.% 99), and formic acid (>wt.% 85) were purchased from Dinâmica (São Paulo, Brazil). Amberlyst 15 resin, 2-ethylhexanol (>wt.% 99), potassium bromide, and deuterated chloroform (CDCl3, 99.8%) were supplied by Sigma-Aldrich (San Luis, Missouri, USA). A commercial mineral oil sample (SAE 20W50) was purchased from Maxon Oil (São José dos Pinhas, Brazil), with physicochemical properties listed in Table 1.

Table 1.
Physicochemical properties of the commercial mineral oil (SAE 20W50).
2.2. Synthesis Procedure
Biolubricants were obtained using the synthesis route adapted from the previous studies [7,28]. A scheme of the reactions used to synthesize the bio-based lubricants is shown in Figure 1. The sample of fatty acids from castor oil (FACO) was submitted to esterification (Figure 1b), epoxidation (Figure 1c), and oxirane ring opening reactions (Figure 1d,e).
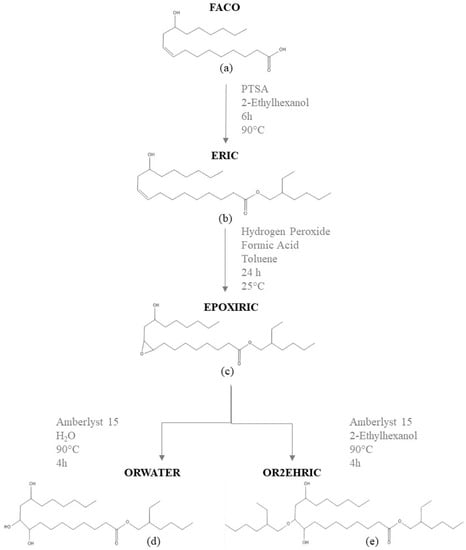
Figure 1.
Scheme of the synthesis routes used to obtain bio-based lubricants. (a) FACO (fatty acids from castor oil), (b) ERIC (esterification reaction product), (c) EPOXIRIC (epoxidation reaction product), (d) ORWATER (product of the oxirane ring opening reaction with water as a nucleophilic agent), and (e) OR2EHRIC (product of the oxirane ring opening reaction with 2-ethylhexanol as a nucleophilic agent).
Esterification was performed in a 3-neck flask, where 100 g (0.336 mol) of fatty acids from castor oil (FACO) were mixed with 131 g (1.01 mol) of 2-ethylhexanol (molar ratio FACO:2-ethylhexanol = 1:3) and 10 g of p-toluenesulfonic acid (10% of fatty acids by weight). The reaction was conducted for 6 h at 90 °C, under reflux and constant stirring at 900 rpm. Then, the mixture was transferred to a separatory funnel, and the organic phase was washed with a solution of NaHCO3 (5% w/v) and distilled water until pH 7 was attained. The product was dried with anhydrous Na2SO4 and distilled in a Kugelrohr system under vacuum (3∙10−2 mbar) at 110 °C to remove the excess of 2-ethylhexanol. The final product of this step was named ERIC.
Next, the ERIC sample was submitted to epoxidation: in a flat-bottom flask, a solution of 70 g (0.171 mol) of ERIC, 7.4 mL (0.171 mol) of formic acid, and 50 mL of toluene were prepared. Subsequently, 57 mL (0.684 mol) of the hydrogen peroxide solution (molar ratio ERIC:CH2O2:H2O2 1:1:4) was added dropwise to the reaction solution. The reaction was conducted for 24 h at room temperature and under constant stirring at 900 rpm. Then, the liquid was transferred from the flask to a separatory funnel. The upper phase was neutralized with a solution of NaHCO3 (5% w/v), washed with distilled water, and dried with anhydrous sodium sulfate. The toluene was removed using a rotary evaporator, under reduced pressure at 90 °C for 40 min. The product of this step was named EPOXIRIC.
Via the oxirane ring opening reaction, two products were obtained from the EPOXIRIC sample with 2-ethylhexanol (named OR2EHRIC) and with water (named ORWATER) used for the nucleophilic attack with molar ratios of 10:1 water/EPOXIRIC and 3:1 alcohol/EPOXIRIC. Dry Amberlyst 15 (A15) was used as a catalyst at a mass ratio (EPOXIRIC/A15) of 10:1. The reaction was conducted in a three-neck flask at 90 °C for 4 h under reflux and constant magnetic stirring (900 rpm). Subsequently, the product was filtered to separate the catalyst and washed in a separatory funnel with a solution of sodium bicarbonate (5% w/v) and distilled water, until reaching pH 7. The samples were dried with anhydrous Na2SO4. Then, the ORWATER (ring opening with water) sample was stored, and the OR2EHRIC (ring opening with 2-ethylhexanol) product was distilled in a Kugelrohr system under vacuum (3∙10−2 mbar) at 110 °C to remove excess 2-ethylhexanol.
2.3. Physicochemical Characterization
All samples were analyzed by one-dimensional proton nuclear magnetic resonance spectroscopy (1H NMR; Bruker Avance DRX-500, Billerica, Massachusetts, USA) operating at 500 MHz with deuterated chloroform as a solvent.
Fourier-transform infrared spectroscopy (FTIR; Shimadzu IRTracer-100, Quioto, Japan) was performed for all samples to confirm the esterification, epoxidation, and oxirane ring opening reactions. The analysis was carried out using a potassium bromide (KBr) tablet in the scanning range of 400–4000 cm−1 [29]. The tablet was prepared by pressing at a force of 8 kN. Thirty-two scans were performed at a resolution of 4 cm−1.
Density at 20 °C and kinematic viscosity at 40 °C and 100 °C of all samples were determined following ASTM D7042 and ASTM D445 methods, respectively, in an Anton Paar SVM 3000 equipment (Graz, Austria) [30,31]. The ASTM D2270 method was used to obtain the viscosity index value (VI) [32].
2.4. Tribological Evaluation
All samples were taken to a tribological test, using a four-ball tester coupled to a DHR-3 rheometer (TA Instruments, New Castle, Delaware, USA). More information about the tribological tester may be found in reference [33]. During the test, one ball rotated at a constant sliding speed (0.228 m/s), temperature of 40 °C, and under loading force (55 N) against three fixed balls submerged in the evaluated lubricant, as shown in Figure 2. The test was conducted for 1 h. The balls used in this test were made of chrome steel alloy (AISI52100) with a hardness of 64 HRC, diameter of 12.7 mm, and an initial surface roughness of 0.015 μm. Prior to the test, the balls were cleaned with acetone and dried under ambient conditions. Optical microscopy (Zeiss, Oberkochen, Germany) was used to evaluate the wear morphology and determine the WSD for each test. New balls were used for each new test. The mineral oil sample (SAE 20W50) was used as a reference for tribological comparison, using friction coefficients (FC) and wear scar diameters (WSD) of the biolubricants.
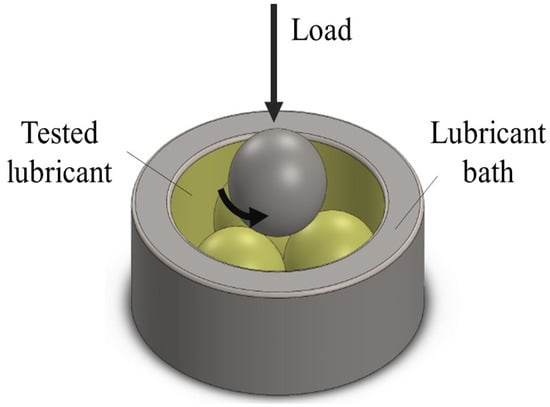
Figure 2.
Four-ball tribological test setup.
3. Results and Discussion
3.1. Physicochemical Characterization
Densities at 20 °C, kinematic viscosities at 40 °C and 100 °C, and viscosity indices obtained for all samples are listed in Table 2. In lubricants, density is used for evaluation of used lubricants: an increase in density compared to the fresh lubricant may indicate the presence of insolubles, water, higher-density products, and oxidized compounds, while a decrease in density may indicate the presence of lower-density contaminants and/or fuel [34]. The ERIC, EPOXIRIC, and OR2EHRIC samples showed lower densities than the FACO sample, whereas the ORWATER sample presented a higher density than the FACO sample. These results may be related to the lower viscosities of the ERIC, EPOXIRIC, and OR2EHRIC samples and the higher viscosity of the ORWATER sample, if compared to the FACO sample, since density values depend on viscosity, quality, and additives content of the sample [35,36,37].

Table 2.
Physicochemical properties of the FACO, ERIC, EPOXIRIC, OR2EHRIC, and ORWATER samples.
Viscosity is a measurement of the shear strength of a lubricant and depends primarily on the molecular interactions within the lubricant [34]. The FACO sample is mainly composed of ricinoleic acid, which contains a hydroxyl group that enhances attractive intermolecular interactions due to hydrogen bonding. For this reason, its viscosity is higher than those of other fatty acids [7,38,39]. The lower viscosity of the ERIC sample if compared to the FACO sample may be ascribed to the introduction of a branched ethyl group that makes intermolecular interactions more difficult [7,40].
The insertion of the epoxy group into the EPOXIRIC sample led to an increase in viscosity because of the intermolecular interactions induced by the presence of an oxygen atom in the ring [41]. Through oxirane ring opening reactions, the OR2EHRIC and ORWATER samples were obtained using 2-ethylhexanol and water, respectively, as nucleophilic agents. The ORWATER sample showed higher viscosities due to the introduction of two new hydroxyl groups that strengthened the molecular interactions through hydrogen bonds [13]. At the same time, in the OR2EHRIC sample, an epoxy group was transformed into an alcohol group and an ether group. Due to the lower number of hydrogen bonds, the OR2EHRIC sample showed lower viscosities than ORWATER sample [7]. The viscosity index (VI) describes the effect of temperature on the viscosity of the lubricant. The higher the VI, the lower the influence of temperature on viscosity [42,43,44]. The studied samples presented VI values (71.8–105) within the limits of mineral lubricants (groups I and II), according to the API classification [45].
The FTIR spectra of all samples are shown in Figure 3. In the FACO sample, the band at 1710 cm−1 was attributed to the carbonyl group (C=O) in the long-chain fatty acids. For the ERIC sample, this peak shifts from 1710 to 1740 cm−1, indicating the presence of an ethyl ester C=O group. This result indicates that the functional groups of carboxylic acids present in the spectrum of the FACO sample were indeed transformed into esters after the esterification reaction [46]. Furthermore, for the ERIC sample, a peak may be observed at around 1170 cm−1; this peak is absent in the FACO sample and is characteristic of the presence of the C=O functional group, from the ester formation [47,48]. In addition, the CH3 group corresponding to the ethyl ester appears in the ERIC sample spectrum at 1461 cm−1 [49].
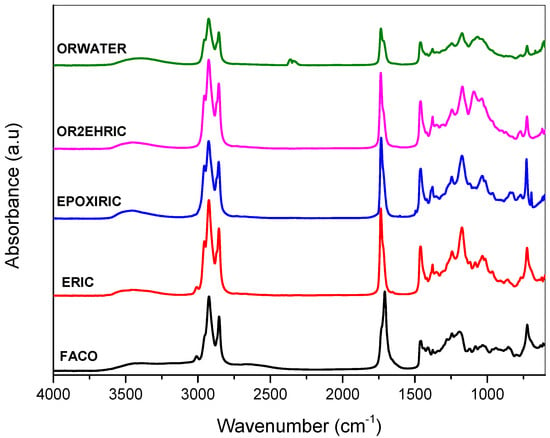
Figure 3.
FTIR spectra of the FACO, ERIC, EPOXIRIC, OR2EHRIC, and ORWATER samples.
The differences between the spectra of the FACO and ERIC samples demonstrate the effective esterification of the fatty acids from castor oil. In the EPOXIRIC sample, bands at 825 and 841 cm−1, ascribed to the epoxide groups, are observed. These bands are absent in the spectra of the OR2EHRIC and ORWATER samples, demonstrating the successful oxirane ring opening. In addition, in the OR2EHRIC and ORWATER samples, the bands at 1737 cm−1 represent carbonyl elongation (C=O). Bands at 1090, 1172, and 1244 cm−1 represent the C–O of the ether and ester, whereas the band at 3470 cm−1 represents the elongation of the –OH group [21,41,50,51].
The esterification, epoxidation, and oxirane ring opening reactions were also confirmed through the 1H NMR spectra (Figure 4). In the FACO sample (Figure 4), peak (a) is ascribed to the bonding of the hydrogen of the alkene to carbons 9 and 10 [7,52], whereas peak (b) is associated with the hydrogen of the terminal chain (–CH3). Finally, peaks (c) and (d) correspond to the hydrogen bonded to the sp3 carbon (–CH2–) [7,53]. For the ERIC sample (Figure 3), the peak (e) corresponds to the hydrogen bonded to the carbon near the sp3 oxygen of the ester functional group, confirming successful esterification. In the spectrum of the EPOXIRIC sample (Figure 3), peak (f), representing unsaturated bonds (still seen in the ERIC sample), has disappeared whereas peak (g) appears, indicating the formation of the epoxide ring [28,54]. Lastly, the spectra of OR2EHRIC and ORWATER samples (Figure 3) show the absence of peak (g) and the presence of peak (h), corresponding to the hydrogen bonded to the hydroxyl carbon (–CH(OH)–) [28,54].
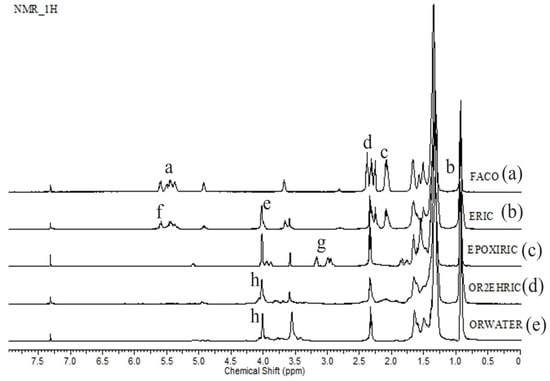
Figure 4.
1H NMR spectra of the (a) FACO, (b) ERIC, (c) EPOXIRIC, (d) OR2EHRIC and (e) ORWATER samples.
3.2. Tribological Results
The FC curves and WSD values of the FACO, ERIC, OR2EHRIC, and ORWATER samples are shown in Figure 5, along with the results obtained for the mineral oil (SAE 20W50). The FC curves of the ORWATER and the mineral oil samples show essentially constant friction values. On the other hand, the FC curves of the FACO and OR2EHRIC samples gradually decrease with time. Interestingly, the FC curve of the ERIC sample fluctuates throughout the test. According to the concept of friction traces, steady state friction coefficients are classified into four types: type A—the FC is constant during the steady state; type B—the FC gradually increases during the test; type C—the FC gradually decreases during the test; and type D—the FC fluctuates throughout the test [55,56,57]. Type A is usually associated with low wear; for this reason, the ORWATER and the mineral oil samples show the lowest WSD values observed in this study, 281.2 ± 5.54 and 225.2 ± 2.16 μm, respectively [55,57]. Types B and C may be acceptable for lubricants, whereas type D is unacceptable because it is normally associated with high wear. As seen, the FACO and OR2EHRIC samples show friction curves with type C characteristics and thus exhibit intermediate WSD values. Finally, the ERIC sample presents a type D friction curve and thus exhibits the highest WSD observed in this study.
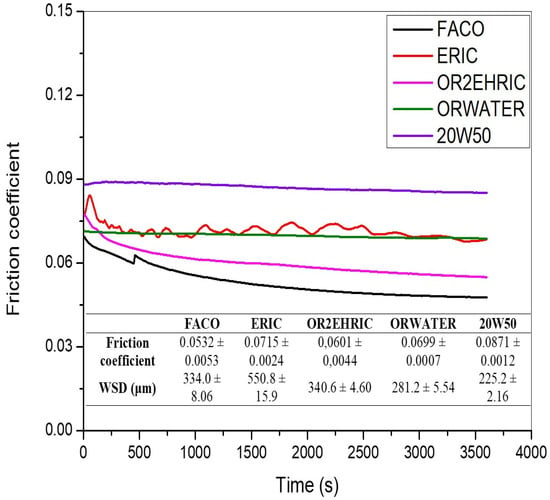
Figure 5.
Friction coefficient curves in tribological testes of the bio-based samples, and of the commercial mineral oil sample (SAE 20W50).
All bio-based samples evaluated in this study present FC values lower than that of the 20W50 mineral oil sample. This may be attributed to the molecular structure of the biolubricants samples, which have polar and non-polar regions, whereas the 20W50 mineral oil contains predominantly non-polar molecules. The polar region of biolubricants is responsible for the absorption or adhesion to the metallic surfaces, and the non-polar region is responsible for the wear resistance of the lubricant film [13,58]. Previous studies suggest that lubricity and wear protection are affected by criteria related to molecular structure: degree of molecular branching, chain length, and number of ester functional groups [13].
In this study, the branching of the ERIC structure with 2-ethylhexanol (forming the OR2EHRIC structure) reduced the FC and WSD, corroborating those previously reported results. Additionally, the use of water as the nucleophilic agent to open the oxirane ring produced the most efficient biolubricant sample for wear reduction. The presence of three hydroxyls (–OH) in the ORWATER molecule improved the wear resistance of the biolubricant due to the strong molecular interactions induced by hydrogen bonding [10,13,59]. The WSD of the ORWATER sample (281.2 ± 5.54 μm) was close to that of the mineral oil (225.2 ± 2.16 μm), demonstrating its promising potential for use as a lubricant, especially considering that the commercial mineral oil sample (20W50) contains anti-wear additives that significantly improve the wear reduction capacity of mineral oil [60,61,62].
The morphologies of the wear surfaces of the studied samples are presented in Table 3. The grooves in the direction of application are caused by wear through abrasion and adhesion [26,63,64]. Among the bio-based samples, ORWATER showed the highest wear resistance of the lubricant film, demonstrating its stronger absorption on the metallic surfaces of the balls. Thus, the wear surfaces are smoother when using ORWATER. Although the 20W50 sample yielded the lowest WSD values, its wear morphology is visually rougher than those of the bio-based lubricants samples, which appear to be more efficient in forming a monomolecular (or multimolecular) layered structure oriented toward the polar end, forming a film that inhibits the metal-to-metal contact. This would yield lower roughness of the worn surfaces [26,65,66].

Table 3.
Wear morphology of the balls lubricated with the bio-based samples, and of the balls lubricated with the commercial mineral oil sample (SAE 20W50), after tribological tests.
4. Conclusions
In the present study, biolubricants samples were prepared through esterification (ERIC), epoxidation (EPOXIRIC), and oxirane ring opening reactions, using 2-ethylhexanol (OR2EHRIC) and water (ORWATER) as nucleophilic agents to create ether branches, and compared to a commercial mineral oil sample (SAE 20W50). The viscosities of the samples varied from 25.2 to 420.46 cSt at 40 °C and from 4.55 to 24.39 cSt at 100 °C. Successful syntheses were confirmed using FTIR and NMR analyses. The ORWATER and 20W50 samples showed constant FC curves throughout the tribological test, suggesting that they produce films that effectively reduce wear. The FC curve of the OR2EHRIC sample showed type C friction behavior, whereas that of the ERIC sample showed type D behavior. The ORWATER sample obtained from fatty acids of castor oil showed effective lubricant film formation capacity, with similar wear resistance and FC lower than that of the commercial mineral oil.
Author Contributions
Conceptualization, P.R.C.F.R.F. and M.R.d.N.; methodology, P.R.C.F.R.F., M.R.d.N. and S.S.O.d.S.; validation, P.R.C.F.R.F., M.R.d.N. and S.S.O.d.S.; formal analysis, F.M.T.d.L., E.R.-C. and C.L.C.J.; investigation, P.R.C.F.R.F.; resources, F.M.T.d.L., E.R.-C., and C.L.C.J.; data curation, P.R.C.F.R.F.; writing—original draft preparation, P.R.C.F.R.F., F.M.T.d.L., E.R.-C. and C.L.C.J.; writing—review and editing, P.R.C.F.R.F., F.M.T.d.L., E.R.-C. and C.L.C.J.; supervision, F.M.T.d.L., E.R.-C. and C.L.C.J.; project administration, F.M.T.d.L., E.R.-C. and C.L.C.J.; funding acquisition, F.M.T.d.L., E.R.-C. and C.L.C.J. All authors have read and agreed to the published version of the manuscript.
Funding
We thank CNPq (Conselho Nacional de Pesquisa e Desenvolvimento Científico) Projects: 304950/2019-0 (Tavares de Luna, F.M.), 309046/2018-1 (Loureiro Cavalcante, C., Jr.) and FUNCAP (Fundação Cearense de Apoio ao Desenvolvimento Científico e Tecnológico) Project E1-0079-0004301 for financial support for this study. Ribeiro-Filho thanks the FAPEMA (Fundação de Amparo à Pesquisa e ao Desenvolvimento Científico e Tecnológico do Maranhão) Project 01131/18 (Campos Flexa Ribeiro Filho, P.R.) and Universidade Estadual do Maranhão (UEMA) for financial support. E.R.-C. thanks Junta de Andalucía, project P20-00375 and FEDER funds.
Data Availability Statement
Not applicable.
Acknowledgments
We thank CNPq (Conselho Nacional de Pesquisa e Desenvolvimento Científico) and FUNCAP (Fundação Cearense de Apoio ao Desenvolvimento Científico e Tecnológico) for financial support for this study. Ribeiro-Filho thanks the FAPEMA (Fundação de Amparo à Pesquisa e ao Desenvolvimento Científico e Tecnológico do Maranhão) and Universidade Estadual do Maranhão (UEMA) for financial support. E.R.-C. thanks Junta de Andalucía (Spain) project P20_00375 and FEDER funds.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shah, R.; Woydt, M.; Zhang, S. The Economic and Environmental Significance of Sustainable Lubricants. Lubricants 2021, 9, 21. [Google Scholar] [CrossRef]
- Singh, Y.; Sharma, A.; Singla, A. Non-edible vegetable oil–based feedstocks capable of bio-lubricant production for automotive sector applications—A review. Environ. Sci. Pollut. Res. 2019, 26, 14867–14882. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.L.; Stipe, C.B.; Habjan, M.C.; Ahlstrand, G.G. Role of lubrication oil in particulate emissions from a hydrogen-powered internal combustion engine. Environ. Sci. Technol. 2007, 41, 6828–6835. [Google Scholar] [CrossRef] [PubMed]
- Perera, M.; Yan, J.; Xu, L.; Yang, M.; Yan, Y. Bioprocess development for biolubricant production using non-edible oils, agro-industrial byproducts and wastes. J. Clean. Prod. 2022, 357, 131956. [Google Scholar] [CrossRef]
- Singh, Y.; Farooq, A.; Raza, A.; Mahmood, M.A.; Jain, S. Sustainability of a non-edible vegetable oil based bio-lubricant for automotive applications: A review. Process Saf. Environ. Prot. 2017, 111, 701–713. [Google Scholar] [CrossRef]
- Neupane, D.; Bhattarai, D.; Ahmed, Z.; Das, B.; Pandey, S.; Solomon, J.K.Q.; Qin, R.; Adhikari, P. Growing Jatropha (Jatropha curcas L.) as a Potential Second-Generation Biodiesel Feedstock. Inventions 2021, 6, 60. [Google Scholar] [CrossRef]
- Rios, I.C.; Cordeiro, J.P.; Arruda, T.B.M.G.; Rodrigues, F.E.A.; Uchoa, A.F.J.; Luna, F.M.T.; Cavalcante, C.L.; Ricardo, N.M.P.S. Chemical modification of castor oil fatty acids (Ricinus communis) for biolubricant applications: An alternative for Brazil’s green market. Ind. Crops Prod. 2020, 145, 112000. [Google Scholar] [CrossRef]
- Encinar, J.M.; Nogales-Delgado, S.; Sánchez, N.; González, J.F. Biolubricants from Rapeseed and Castor Oil Transesterification by Using Titanium Isopropoxide as a Catalyst: Production and Characterization. Catalysts 2020, 10, 366. [Google Scholar] [CrossRef]
- Singh, A.K. Castor oil-based lubricant reduces smoke emission in two-stroke engines. Ind. Crops Prod. 2011, 33, 287–295. [Google Scholar] [CrossRef]
- Quinchia, L.A.; Delgado, M.A.; Reddyhoff, T.; Gallegos, C.; Spikes, H.A. Tribological studies of potential vegetable oil-based lubricants containing environmentally friendly viscosity modifiers. Tribol. Int. 2014, 69, 110–117. [Google Scholar] [CrossRef]
- Quinchia, L.A.; Delgado, M.A.; Valencia, C.; Franco, J.M.; Gallegos, C. Viscosity modification of different vegetable oils with EVA copolymer for lubricant applications. Ind. Crops Prod. 2010, 32, 607–612. [Google Scholar] [CrossRef]
- Isbell, T.A.; Lowery, B.A.; DeKeyser, S.S.; Winchell, M.L.; Cermak, S.C. Physical properties of triglyceride estolides from lesquerella and castor oils. Ind. Crops Prod. 2006, 23, 256–263. [Google Scholar] [CrossRef]
- Chan, C.H.; Tang, S.W.; Mohd, N.K.; Lim, W.H.; Yeong, S.K.; Idris, Z. Tribological behavior of biolubricant base stocks and additives. Renew. Sustain. Energy Rev. 2018, 93, 145–157. [Google Scholar] [CrossRef]
- Araújo, S.V.; Luna, F.M.T.; Rola, E.M.; Azevedo, D.C.S.; Cavalcante, C.L. A rapid method for evaluation of the oxidation stability of castor oil FAME: Influence of antioxidant type and concentration. Fuel Process. Technol. 2009, 90, 1272–1277. [Google Scholar] [CrossRef]
- Choi, U.S.; Ahn, B.G.; Kwon, O.K.; Chun, Y.J. Tribological behavior of some antiwear additives in vegetable oils. Tribol. Int. 1997, 30, 677–683. [Google Scholar] [CrossRef]
- Cecilia, J.A.; Ballesteros Plata, D.; Alves Saboya, R.M.; Tavares de Luna, F.M.; Cavalcante, C.L., Jr.; Rodríguez-Castellón, E. An Overview of the Biolubricant Production Process: Challenges and Future Perspectives. Processes 2020, 8, 257. [Google Scholar] [CrossRef]
- Owuna, F.J.; Dabai, M.U.; Sokoto, M.A.; Dangoggo, S.M.; Bagudo, B.U.; Birnin-Yauri, U.A.; Hassan, L.G.; Sada, I.; Abubakar, A.L.; Jibrin, M.S. Chemical modification of vegetable oils for the production of biolubricants using trimethylolpropane: A review. Egypt. J. Pet. 2020, 2, 75–82. [Google Scholar] [CrossRef]
- Inkerd, R.; Dussadee, N.; Thararux, C.; Chanathaworn, J.; Ramaraj, R. An experimental investigation of palm oil as an environment friendly biolubricant. In Proceedings of the 22nd Tri-U International Joint Seminar and Symposium, Jiangsu University, Zhen Jiang, China, 23 October 2015. [Google Scholar]
- Annisa, A.N.; Widayat, W. A review of bio-lubricant production from vegetable oils using esterification transesterification process. MATEC Web Conf. 2018, 156, 06007. [Google Scholar] [CrossRef]
- McNutt, J.; He, Q.S. Development of biolubricants from vegetable oils via chemical modification. J. Ind. Eng. Chem. 2016, 36, 1–12. [Google Scholar] [CrossRef]
- Salih, N.; Salimon, J. Yousif, E. The physicochemical and tribological properties of oleic acid based triester biolubricants. Ind. Crops Prod. 2011, 34, 1089–1096. [Google Scholar] [CrossRef]
- Syahir, A.Z.; Zulkifli, N.W.M.; Masjuki, H.H.; Kalam, M.A.; Alabdulkarem, A.; Gulzar, M.; Khuong, L.S.; Harith, M.H. A review on bio-based lubricants and their applications. J. Clean. Prod. 2017, 168, 997–1016. [Google Scholar] [CrossRef]
- Greco-Duarte, J.; Cavalcanti-Oliveira, E.D.; Da Silva, J.A.C.; Fernandez-Lafuente, R.; Freire, D.M.G. Two-step enzymatic production of environmentally friendly biolubricants using castor oil: Enzyme selection and product characterization. Fuel 2017, 202, 196–205. [Google Scholar] [CrossRef]
- Saboya, R.M.A.; Cecilia, J.A.; García-Sancho, C.; Sales, A.V.; Luna, F.M.T.; Rodríguez-Castellón, E.; Cavalcante, C.L. Synthesis of biolubricants by the esterification of free fatty acids from castor oil with branched alcohols using cationic exchange resins as catalysts. Ind. Crops Prod. 2017, 104, 52–61. [Google Scholar] [CrossRef]
- Suresha, B.; Hemanth, G.; Rakesh, A.; Adarsh, K.M. Tribological behaviour of pongamia oil as lubricant with and without halloysite nanotubes using four-ball tester. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2019; Volume 2128, p. 030011. [Google Scholar] [CrossRef]
- Ribeiro Filho, P.R.C.F.; da Silva, S.S.O.; do Nascimento, M.R.; de Aguiar Soares, S.; de Luna, F.M.T.; Cavalcante, C.L., Jr. Tribological properties of bio-based lubricant basestock obtained from pequi oil (Caryocar brasiliensis). J. Braz. Soc. Mech. Sci. Eng. 2022, 44, 1–9. [Google Scholar] [CrossRef]
- Sethuramiah, A.; Kumar, R. Chapter 2—Lubricants and their formulation. In Modeling of Chemical Wear; Elsevier: Oxford, UK, 2016; pp. 25–39. [Google Scholar] [CrossRef]
- Marques, J.P.C.; Rios, Í.C.; Parente, E.J.S.; Quintella, S.A.; Luna, F.M.T.; Cavalcante, C.L. Synthesis and Characterization of Potential Bio-Based Lubricant Basestocks via Epoxidation Process. J. Am. Oil Chem. Soc. 2020, 97, 437–446. [Google Scholar] [CrossRef]
- dos Santos, R.C.M.; Gurgel, P.C.; Pereira, N.S.; Breves, R.A.; de Matos, P.R.R.; Silva, L.P.; Sales, M.J.A.; Lopes, R.V.V. Ethyl esters obtained from pequi and macaúba oils by transesterification with homogeneous acid catalysis. Fuel 2020, 259, 116206. [Google Scholar] [CrossRef]
- ASTM International. Standard Test Method for Dynamic Viscosity and Density of Liquids by Stabinger Viscometer (and the Calculation of Kinematic Viscosity); ASTM International: West Conshohocken, PA, USA, 2014. [Google Scholar]
- ASTM International. Standard Test Method for Kinematic Viscosity of Transparent and Opaque Liquids: (and Calculation of Dynamic Viscosity); ASTM International: West Conshohocken, PA, USA, 2006. [Google Scholar]
- ASTM International. Standard Practice for Calculating Viscosity Index from Kinematic Viscosity at 40 and 100 °C; ASTM International: West Conshohocken, PA, USA, 2016. [Google Scholar]
- TA Instruments. Available online: https://www.tainstruments.com/tribo-rheometry-accessory/ (accessed on 27 September 2022).
- Carreteiro, R.P.; Belmiro, P.N.A. Lubrificantes e Lubrificação Industrial; Interciência: Rio de Janeiro, Brazil, 2006. [Google Scholar]
- API 1509, “Appendix E—API Base Oil Interchangeability Guidelines for Passenger Car Motor Oils and Diesel Engine Oils”. Available online: https://www.api.org/~/media/files/certification/engine-oil-diesel/publications/annerev043019%20rev043019.pdf (accessed on 13 November 2022).
- Lin, L.; Kedzierski, M.A. Density and viscosity of a polyol ester lubricant: Measurement and molecular dynamics simulation. Int. J. Refrig. 2020, 118, 188–201. [Google Scholar] [CrossRef]
- Ewen, J.P.; Gattinoni, C.; Thakkar, F.M.; Morgan, N.; Spikes, H.A.; Dini, D. A Comparison of Classical Force-Fields for Molecular Dynamics Simulations of Lubricants. Materials 2016, 9, 651. [Google Scholar] [CrossRef]
- Keera, S.T.; El Sabagh, S.M.; Taman, A.R. Castor oil biodiesel production and optimization. Egypt. J. Pet. 2018, 27, 979–984. [Google Scholar] [CrossRef]
- Hernandez, N.L.P.; Bonon, A.J.; Bahú, J.O.; Barbosa, M.I.R.; Maciel, M.R.W.; Filho, R.M. Epoxy monomers obtained from castor oil using a toxicity-free catalytic system. J. Mol. Catal. A Chem. 2017, 426 Pt B, 550–556. [Google Scholar] [CrossRef]
- Mukherjee, S.; Ghosh, M. Studies on performance evaluation of a green plasticizer made by enzymatic esterification of furfuryl alcohol and castor oil fatty acid. Carbohydr. Polym. 2017, 157, 1076–1084. [Google Scholar] [CrossRef]
- do Valle, C.P.; Rodrigues, J.S.; Fechine, L.M.U.D.; Cunha, A.P.; Malveira, J.Q.; Luna, F.M.T.; Ricardo, N.M.P.S. Chemical modification of Tilapia oil for biolubricant applications. J. Clean. Prod. 2018, 191, 158–166. [Google Scholar] [CrossRef]
- Narayana Sarma, R.; Vinu, R. Current Status and Future Prospects of Biolubricants: Properties and Applications. Lubricants 2022, 10, 70. [Google Scholar] [CrossRef]
- Verdier, S.; Coutinho, J.A.P.; Silva, A.M.S.; Alkilde, O.F.; Hansen, J.A. A critical approach to viscosity index. Fuel 2009, 88, 2199–2206. [Google Scholar] [CrossRef]
- Braga, J.W.B.; Junior, A.A.S.; Martins, I.S. Determination of viscosity index in lubricant oils by infrared spectroscopy and PLSR. Fuel 2014, 120, 171–178. [Google Scholar] [CrossRef]
- Pirro, D.M.; Daschner, E. Lubrication Fundamentals; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar] [CrossRef]
- Zhou, J.; Xiong, Y.; Gong, Y.; Liu, X. Analysis of the oxidative degradation of biodiesel blends using FTIR, UV–Vis, TGA and TD-DES methods. Fuel 2017, 202, 23–28. [Google Scholar] [CrossRef]
- Arbain, N.H.; Salimon, J. Synthesis and characterization of ester trimethylolpropane based jatropha curcas oil as biolubricant base stocks. J. Sci. Technol. 2010, 2. Available online: https://publisher.uthm.edu.my/ojs/index.php/JST/article/view/245 (accessed on 13 November 2022).
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S.; Vyvyan, J.A. Introduction to Spectroscopy; Cengage Learning: North Ryde, NSW, Australia, 2014. [Google Scholar]
- Bhardwaj, R.M. Control and Prediction of Solid-State of Pharmaceuticals: Experimental and Computational Approaches; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar] [CrossRef]
- Sharma, B.K.; Adhvaryu, A.; Liu, Z.; Erhan, S.Z. Chemical modification of vegetable oils for lubricant applications. J. Am. Oil Chem. Soc. 2006, 83, 129–136. [Google Scholar] [CrossRef]
- Ob-eye, J.; Chaiendoo, K.; Itthibenchapong, V. Catalytic Conversion of Epoxidized Palm Fatty Acids through Oxirane Ring Opening Combined with Esterification and the Properties of Palm Oil-Based Biolubricants. Ind. Eng. Chem. Res. 2021, 60, 15989–15998. [Google Scholar] [CrossRef]
- Torrentes-Espinoza, G.; Miranda, B.C.; Vega-Baudrit, J.; Mata-Segreda, J.F. Castor oil (Ricinus communis) supercritical methanolysis. Energy 2017, 140, 426–435. [Google Scholar] [CrossRef]
- Slivniak, R.; Domb, A.J. Macrolactones and polyesters from ricinoleic acid. Biomacromolecules 2005, 6, 1679–1688. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, R.D.; Deshpande, P.S.; Mahajan, S.U.; Mahulikar, P.P. Epoxidation of mustard oil and ring opening with 2-ethylhexanol for biolubricants with enhanced thermo-oxidative and cold flow characteristics. Ind. Crops Prod. 2013, 49, 586–592. [Google Scholar] [CrossRef]
- Minami, I.; Hong, H.S.; Mathur, N.C. Lubrication performance of model organic compounds in high oleic sunflower oil. J. Synth. Lubr. 1999, 16, 1–12. [Google Scholar] [CrossRef]
- Gates, R.S.; Hsu, S.M. Effect of selected chemical compounds on the lubrication of silicon nitride. Tribol. Trans. 1991, 34, 417–425. [Google Scholar] [CrossRef]
- Fox, N.J.; Tyrer, B.; Stachowiak, G.W. Boundary lubrication performance of free fatty acids in sunflower oil. Tribol. Lett. 2004, 16, 275–281. [Google Scholar] [CrossRef]
- Rudnick, L.R. (Ed.) Synthetics, Mineral Oils, and Bio-Based Lubricants: Chemistry and Technology; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Qi, C.; Renhui, Z.; Zhongyi, H.; Liping, X. Effect of carbon-chain length and hydroxyl number on lubrication performance of alcohols. J. Hebei Univ. Sci. Technol. 2021, 42, 1–7. [Google Scholar] [CrossRef]
- Watkins, R.C. The antiwear mechanism of zddp’s: Part II. Tribol. Int. 1982, 15, 13–15. [Google Scholar] [CrossRef]
- Kim, B.; Jiang, J.C.; Aswath, P.B. Mechanism of wear at extreme load and boundary conditions with ashless anti-wear additives: Analysis of wear surfaces and wear debris. Wear 2011, 270, 181–194. [Google Scholar] [CrossRef]
- Papay, A.G. Antiwear and extreme-pressure additives in lubricants. Lubr. Sci. 1998, 10, 209–224. [Google Scholar] [CrossRef]
- Noorawzi, N.; Samion, S. Tribological effects of vegetable oil as alternative lubricant: A pin-on-disk tribometer and wear study. Tribol. Trans. 2016, 59, 831–837. [Google Scholar] [CrossRef]
- Afifah, A.N.; Syahrullail, S.; Azlee, N.I.W.; Sidik, N.A.C.; Yahya, W.J.; Abd Rahim, E. Biolubricant production from palm stearin through enzymatic transesterification method. Biochem. Eng. J. 2019, 148, 178–184. [Google Scholar] [CrossRef]
- Bahari, A. Investigation into Tribological Performance of Vegetable Oils as Biolubricants at Severe Contact Conditions. Ph.D. Thesis, University of Sheffield, Sheffield, UK, 2017. [Google Scholar]
- de Andrade Souza, L.; Moreira, D.R.; Ricardo, N.M.P.S.; Maier, M.E.; Ribeiro Filho, P.R.C.F.; Luna, F.M.T.; Petzhold, C.L. Esters from castor oil functionalized with aromatic amines as a potential lubricant. J. Am. Oil Chem. Soc. 2022, 1–10. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).














