Evaluation of Wear Resistance of AISI L6 and 5140 Steels after Surface Hardening with Boron and Copper
Abstract
1. Introduction
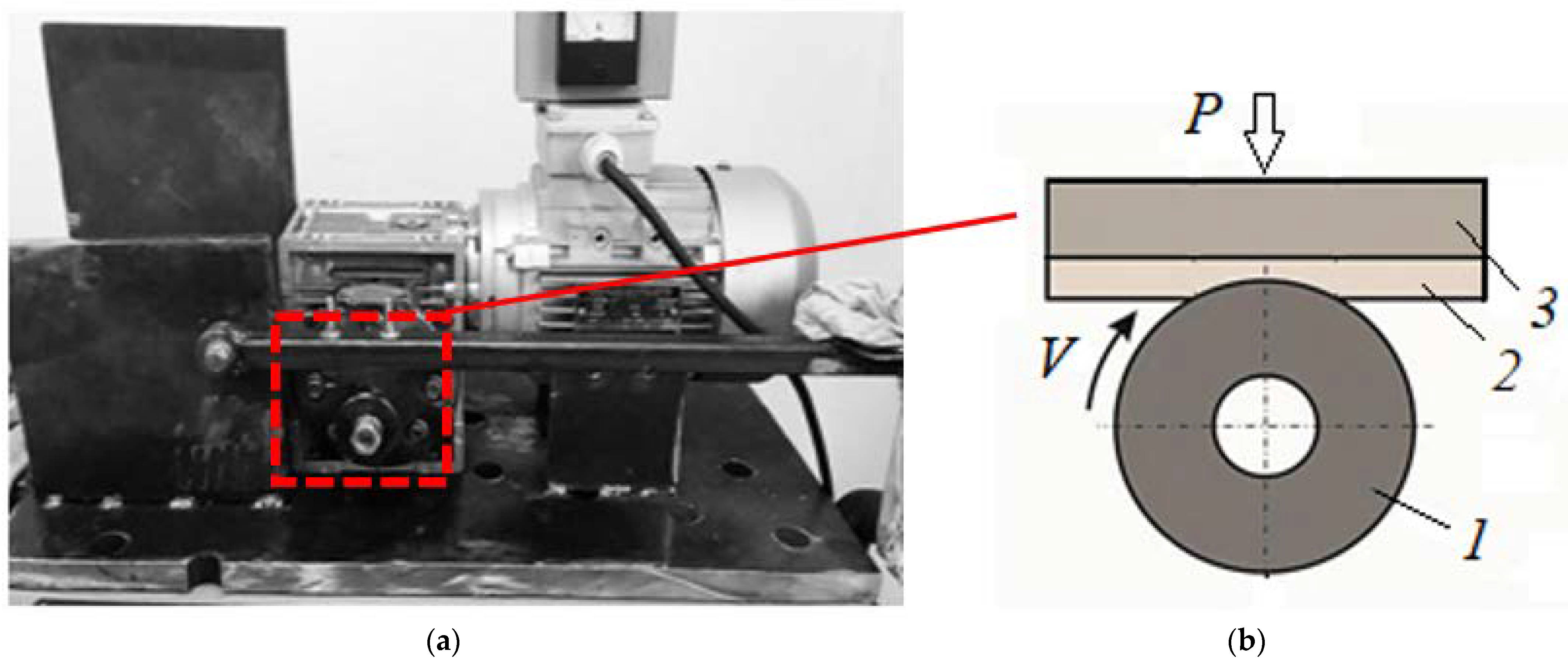
2. Materials and Methods
3. Results
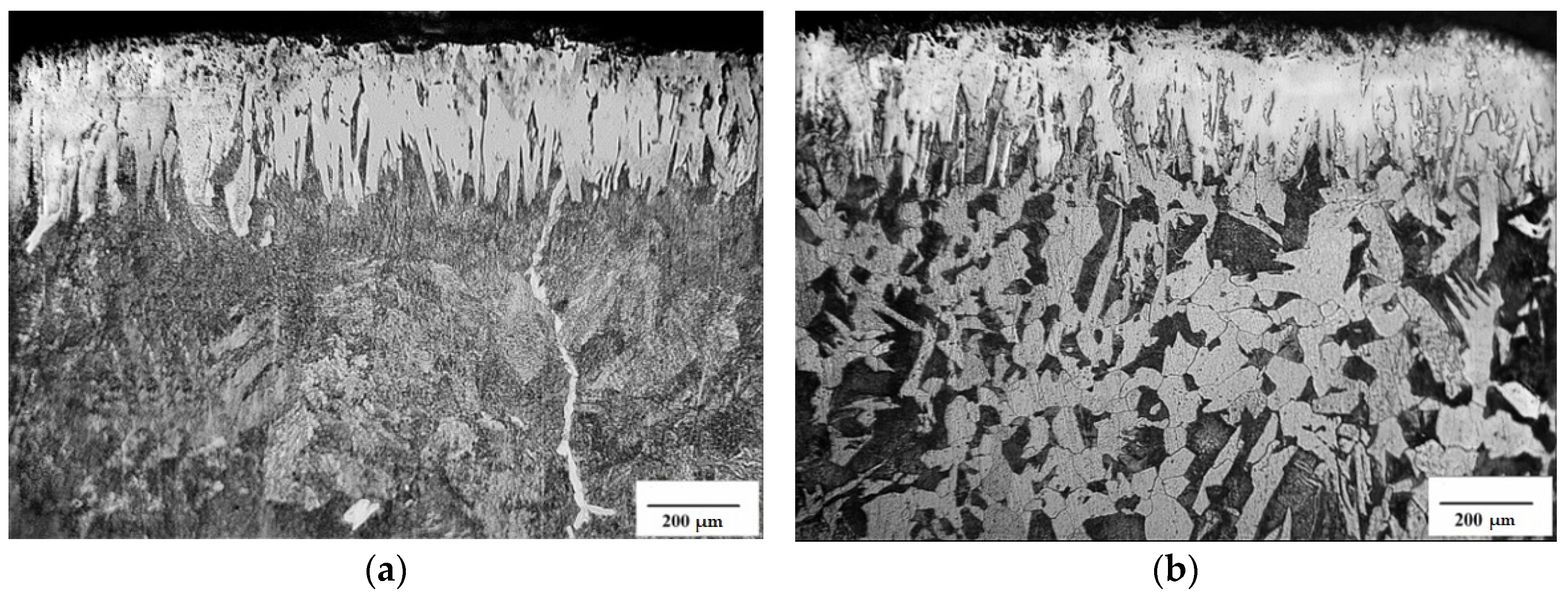
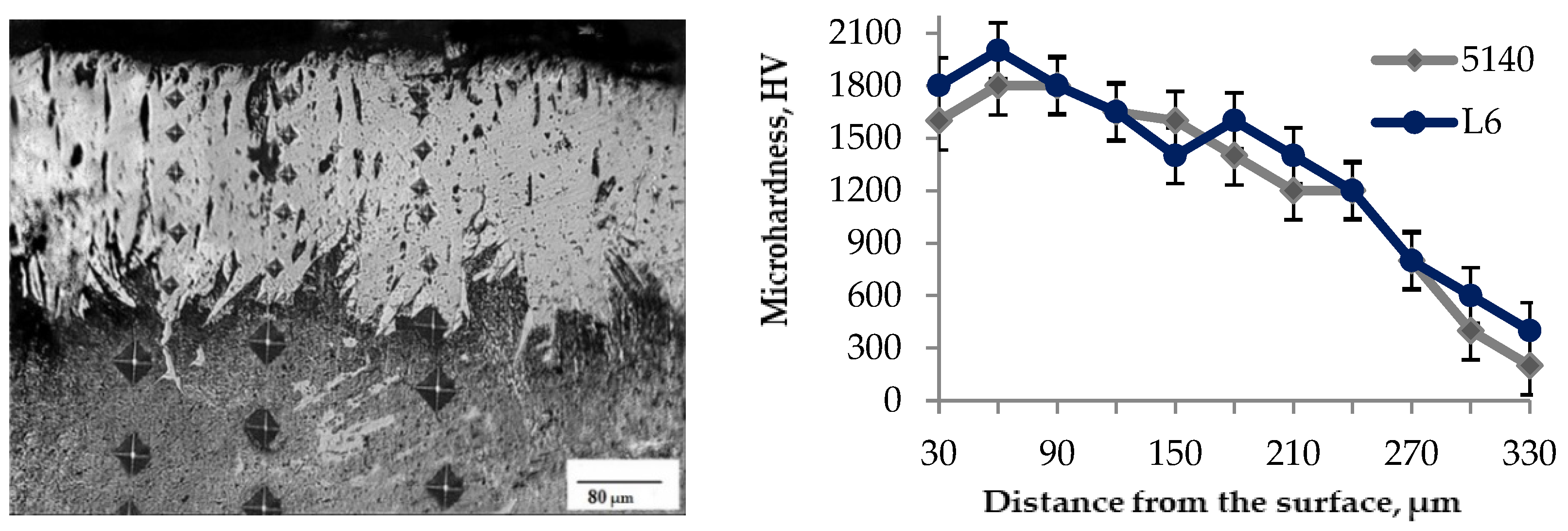
3.1. Microstructure, Microhardness and Phase Composition
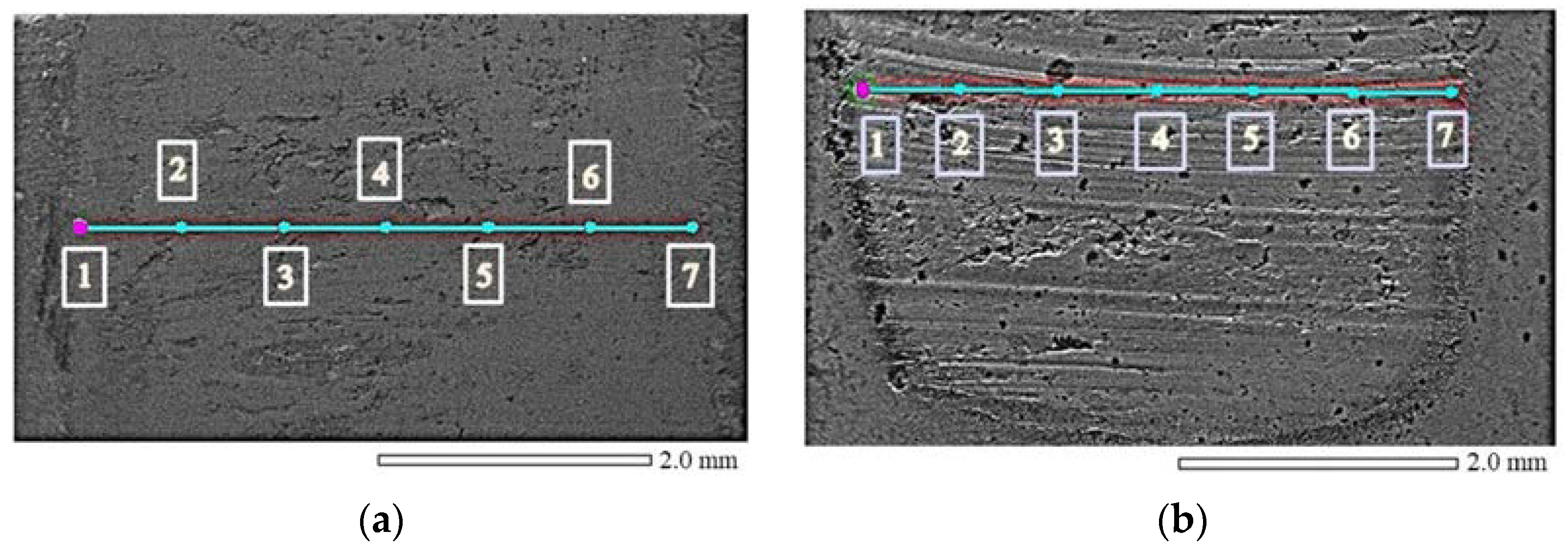
3.2. Wear Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schneider, M.J.; Chatterjee, M.S. Introduction to Surface Hardening of Steels. In ASM Handbook. In Steel Heat Treating Fundamentals and Processes; Dossett, J.L., Totten, G.E., Eds.; ASM International: Novelty, OH, USA, 2013; Volume 4A, pp. 389–398. [Google Scholar] [CrossRef]
- Korotkov, V.A.; Ananyev, S.P.; Zlokazov, M.V. Iznosostoikost Stalei s Plazmennoi Zakalkoi i Karbonitraciei [Wear Resistance of Plasma-Hardened and Carbonitrated Steels]; NTI (branch) UrFU: Nizhny Tagil, Russia, 2014; pp. 4–6. Available online: https://elar.urfu.ru/bitstream/10995/27013/1/978-5-9544-0065-6.pdf (accessed on 20 November 2022). (In Russian)
- Mertgenç, E.; Kayali, Y. Diffusion kinetics and boronizing of high entropy alloy produced by TIG melting reverse suction method. Can. Metall. Q. 2022, 1–10. [Google Scholar] [CrossRef]
- Campos-Silva, I.; Palomar-Pardavé, M.; Pérez Pastén-Borja, R.; Kahvecioglu Feridun, O.; Bravo-Bárcenas, D.; López-García, C.; Reyes-Helguera, R. Tribocorrosion and cytotoxicity of FeB-Fe2B layers on AISI 316 L steel. Surf. Coat. Technol. 2018, 349, 986–997. [Google Scholar] [CrossRef]
- Kulka, M. Trends in thermochemical techniques of boriding. In Current Trends in Boriding; Engineering Materials; Springer: Cham, Switzerland, 2019; pp. 17–98. [Google Scholar] [CrossRef]
- Bricín, D.; Kříž, A.; Novotný, J.; Špirit, Z. The effect of boriding and heat treatment on the structure and properties of 100Cr6 steel. Manuf. Technol. 2022, 22, 2–9. [Google Scholar] [CrossRef]
- Mishigdorzhiyn, U.; Chen, Y.; Ulakhanov, N.; Liang, H. Microstructure and Wear Behavior of Tungsten Hot-Work Steel afterBoriding and Boroaluminizing. Lubricants 2020, 8, 26. [Google Scholar] [CrossRef]
- Ouladsaad, S.; Allaoui, O.; Daas, A. Boro-Aluminizing of XC38 steel. Indian J. Chem. Technol. 2019, 26, 239–243. Available online: https://www.researchgate.net/publication/334225476_Boro-aluminizing_of_XC38_steel (accessed on 21 November 2022).
- Bartkowska, A.; Bartkowski, D.; Przestacki, D.; Hajkowski, J.; Miklaszewski, A. Microstructural and Mechanical Properties of B-Cr Coatings Formed on 145Cr6 Tool Steel by Laser Remelting of Diffusion Borochromized Layer Using Diode Laser. Coatings 2021, 11, 608. [Google Scholar] [CrossRef]
- Bartkowska, A.; Pertek, A.; Popławski, M.; Bartkowski, D.; Przestacki, D.; Miklaszewski, A. Effect of laser modification of B-Ni complex layer on wear resistance and microhardness. Opt. Laser Technol. 2015, 72, 116–124. [Google Scholar] [CrossRef]
- Krukovich, M.G.; Prusakov, B.A.; Sizov, I.G. Methods of Reducing the Brittleness of Boronized Layers: The Parameters of Boriding Technology Aimed at Determining the Plasticity of Boronized Layers. In Plasticity of Boronized Layers; Springer Series in Materials Science; Springer: Cham, Switerland, 2016; Volume 237, pp. 111–196. [Google Scholar] [CrossRef]
- Lysykh, S.A.; Kharaev, Y.P.; Kornopoltsev, V.N.; Zhong, H.S.; Lygdenov, B.D.; Guryev, A.M. Formirovanie diffusionnuh sloev i isslodovanie sherohovatosti pri kompleksnom nasushenii poverhnosti stali 5KhNM borom i mediu [Formation of diffusion layers and study of roughness under complex saturation of the surface of 5KhNM steel with boron and copper]. Polzunovsky Bull. 2020, 3, 77–82. (In Russian) [Google Scholar] [CrossRef]
- Russian Federation. Sposob Boromednenia Stalnuh Izdelii v Vibrokipushem Sloe [The Method of Borocoppering Steel Products in a Vibrating Boiling Layer]. Patent No. 2005811, 15 January 1994. Available online: https://findpatent.ru/patent/200/2004619.html (accessed on 21 November 2022). (In Russian).
- Belskyi, E.I.; Sitkevich, M.V.; Ponkratin, E.I.; Stefanovich, V.A. Khimiko-Termicheskaya Obraborka Instrumentalnykh Materialov [Thermal-Chemical Treatment of Tool Materials]; Nauka i tekhnika; USSR: Minsk, Belarus, 1986; pp. 61–121. Available online: https://elibrary.ru/item.asp?id=45613821 (accessed on 23 November 2022). (In Russian)
- Electrochemical Copper Plating Applicable to Hole Metallization of Printed Board. Patent No. CN102127781A, 20 July 2011. Available online: https://patents.google.com/patent/CN102127781A/en (accessed on 23 November 2022).
- Lysykh, S.A.; Kornopoltsev, V.N.; Mishigdorzhiyn, U.L.; Kharaev, Y.P.; Dasheev, D.E. Issledovaniye var’irovaniya razmerov posle termodiffuzionnogo nasyshcheniya stali 5KhNM v poroshkovykh smesyakh soderzhashchikh bor i med’ [Investigation of the variation in the size of samples made of 5CNM steel under thermal diffusion saturation with powder mixtures containing boron and copper]. Strength. Technol. Coat. 2021, 17, 498–502. (In Russian) [Google Scholar] [CrossRef]
- Kharaev, Y.P.; Kornopoltsev, V.N.; Lysyh, S.A. Opredeleniye Sostava Smesi Pri Poverkhnostnom Uprochnenii Stali Borom i med’yu [Identification of a Mixture Composition for Surface Hardening of Steel with Boron and Copper]. Polzunovskiy Al’manakh 2016, 4, 142–144. Available online: https://elibrary.ru/item.asp?id=27711859 (accessed on 24 November 2022). (In Russian).
- Roberts, G.; Krauss, G.; Kennedy, R. Low-Alloy Special-Purpose Tool Steels. In Tool Steels, 5th ed.; ASM International: Novelty, OH, USA, 1998; pp. 141–163. [Google Scholar] [CrossRef]
- ASTM-A29A29M-2004; Standard Specification for Steel Bars Carbon and Alloy Hot Wrought. Available online: http://www.astmsteel.com/wp-content/uploads/2015/10/ASTM-A29A29M-2004_Standard-Specification-for-Steel-Bars-Carbon-and-Alloy-Hot-Wrought.pdf (accessed on 24 November 2022).
- Krelling, A.P.; Teixeira, F.F.; Costa, C.E.; Almeida, E.A.; Zappelino, B.F.; Milan, J.C. Microabrasive wear behavior of borided steel abraded by SiO2 particles. J. Mater. Res. Technol. 2019, 8, 766–776. [Google Scholar] [CrossRef]
- Milinovi´c, A.; Stojši´c, J.; Kladari´c, I.; Matijevi´c, B. Evaluation of boride layers on C70W2 steel using a new approach to characterization of boride layers. Materials 2022, 15, 3891. [Google Scholar] [CrossRef] [PubMed]
- Prince, M.; Raj, G.S.; Kumar, D.Y.; Gopalakrishnan, P. Boriding of Steels: Improvement of Mechanical Properties—A Review. High Temp. Mater. Processes Int. Q. High-Technol. Plasma Processes 2022, 26, 43–89. [Google Scholar] [CrossRef]
- Zimmerman, C. Boriding (boronizing) of Metals. In ASM Handbook, Steel Heat Treating Fundamentals and Processes; Dossett, J.L., Totten, G.E., Eds.; ASM International: Novelty, OH, USA, 2013; Volume 4A, pp. 709–724. [Google Scholar] [CrossRef]
- Sun, X.; Hao, W.; Geng, G.; Ma, T.; Li, Y. Solidification microstructure evolution of undercooled Cu-15 wt.% Fe alloy melt. Adv. Mater. Sci. Eng. 2018, 2018, 6304518. [Google Scholar] [CrossRef]
- Jacob, K.T.; Priya, S.; Waseda, Y. Measurement of the activity of boron in liquid copper using a four-phase equilibrium technique. Metall. Mater. Trans. A 2000, 31, 2674–2678. [Google Scholar] [CrossRef]
- Chino-Ulloa, A.; Ruiz-Trabolsi, P.A.; Torres-Avila, I.P.; Orozco-Álvarez, C.; Tadeo-Rosas, R.; Velázquez, J.C.; Hernández-Sánchez, E. Kinetics and mechanical characterization of hard layers obtained by boron diffusion in 80/20 nickel–chromium alloy. Coatings 2022, 12, 1387. [Google Scholar] [CrossRef]
- Lysykh, S.A.; Mishigdorzhiyn, U.L.; Kornopoltsev, V.N.; Kharaev, Y.P.; Milonov, A.S.; He, X.Z. Snizheniye khrupkosti boridnykh sloyov na poverkhnosti stali 45 kompleksnym nasyshcheniyem borom i med’yu [The fragility reduction of boride layers on the surface of 45 steel by complex saturation with boron and copper]. In Proceedings of the VIII International Conference on Problems in the Mechanics of Modern Machines, Ulan-Ude, Russia, 4–9 July 2022. [Google Scholar] [CrossRef]
- Dearnley, P.A.; Bell, T. Engineering the surface with boron based materials. Surf. Eng. 1985, 1, 203–217. [Google Scholar] [CrossRef]
- Sánchez Huerta, D.; López Perrusquia, N.; Hilerio Cruz, I.; Doñu Ruiz, M.A.; García Bustos, E.D.; Flores Martínez, M. Growth kinetics and mechanical characterization of a hard boron coating on a tool steel. Defect Diffus. Forum 2017, 380, 29–34. [Google Scholar] [CrossRef]
- Bayca, S.U.; Bican, O.; Yamanel, B.; Hekimoğlu, A.P.; Calis, M. The Effect of solid boriding time on the structure, hardness and corrosion properties of AISI 5140 Steel. Prot. Metals Phys. Chem. Surf. 2020, 56, 591–597. [Google Scholar] [CrossRef]
- Kisuka, F.; Wu, C.-Y.; Hare, C. Friction-induced heat generation between two particles. EPJ Web Conf. 2021, 249, 05007. [Google Scholar] [CrossRef]
- Luo, Q. Tribofilms in Solid Lubricants. In Encyclopedia of Tribology; Wang, Q.J., Chung, Y.W., Eds.; Springer: Boston, MA, USA, 2013; pp. 3760–3767. [Google Scholar] [CrossRef]
- Balandin, Y.A. Povysheniye Iznosostoykosti Stal’nykh Izdeliy Diffuzionnym Boromedneniyem, Khromirovaniyem i Borokhromirovaniyem v Psevdoozhizhennom Sloye [Increasing the Wear Resistance of Steel Products by Diffusion Borocoppering, Chromizing and Borochromizing in a Fluidized Bed]. News Chelyabinsk Sci. Cent. Ural Branch Russian Acad. Sci. 2003, 1, 76–77. Available online: https://elibrary.ru/item.asp?id=8386064 (accessed on 25 November 2022).
- Bruce, D.; Hancock, P. Note on the temperature stability of wüstite in surface oxide films on iron. Br. Corros. J. 1969, 4, 221–222. [Google Scholar] [CrossRef]
- Lee, S.; Hsu, H.C.; Tuan, W. Oxidation Behavior of Copper at a Temperature below 300 °C and the Methodology for Passivation. Mater. Res.-Ibero-Am. J. Mater. 2016, 19, 51–56. [Google Scholar] [CrossRef]
- Totik, Y.; Alsaran, A.; Celik, A.; Efeoglu, I. The wear behaviour of duplex treated AISI 5140 steel. Ind. Lubr. Tribol. 2011, 63, 344–349. [Google Scholar] [CrossRef]
- Piasecki, A.; Kotkowiak, M.; Makuch, N.; Kulka, M. Wear behavior of self-lubricating boride layers produced on Inconel 600-alloy by laser alloying. Wear 2019, 426–427, 919–933. [Google Scholar] [CrossRef]
- Scherge, M.; Böttcher, R.; Kürten, D.; Linsler, D. Multi-phase friction and wear reduction by copper nanopartices. Lubricants 2016, 4, 36. [Google Scholar] [CrossRef]




| Steel Grade | C | Mn | Si | Cr | Ni | V | Mo | Fe |
|---|---|---|---|---|---|---|---|---|
| L6 | 0.65–0.75 | 0.25–0.8 | 0.25 | 0.6–1.2 | 1.25–2 | 0.2–0.3 1 | up to 0.5 | 94.2–97.0 |
| 5140 | 0.38–0.43 | 0.7–0.9 | 0.15–0.35 | 0.7–0.9 | - | - | - | 97.42–98.07 |
| No Spectrum | Elemental Composition, wt % | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| B | C | O | Al | Cr | Ni | Cu | Mo | Fe | |
| 1 | - | 23.18 | 8.07 | 5.86 | 2.22 | 1.48 | 5.68 | - | 53.51 |
| 2 | 0.7 | 38.73 | 3.50 | 0.08 | - | 0.02 | 3.00 | - | 53.97 |
| 3 | 0.51 | 79.44 | 4.47 | - | - | - | - | - | 15.58 |
| 4 | - | 14.92 | 17.98 | 0.28 | 0.62 | 0.03 | 0.53 | 4.26 | 61.38 |
| 5 | 0.85 | - | 21.19 | - | - | 64.84 | 13.12 | - | - |
| 6 | - | 99.85 | 0.05 | - | - | - | - | 0.10 | - |
| 7 | - | - | 20.86 | - | - | 45.03 | 34.11 | - | - |
| No Spectrum | Elemental Composition, wt % | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| B | C | O | Al | Cr | Ni | Cu | Mn | Fe | |
| 1 | 2.48 | 47.76 | 12.86 | 1.68 | 0.33 | 0.29 | 0.67 | - | 33.93 |
| 2 | 0.4 | 15.68 | 19.62 | 1.72 | 0.29 | 0.10 | - | - | 62.19 |
| 3 | 0.4 | 24.84 | 17.57 | 1.55 | 0.24 | 0.03 | 0.32 | 0.46 | 54.59 |
| 4 | 0.03 | 22.46 | 19.00 | 3.53 | 0.78 | 0.06 | 0.98 | - | 53.16 |
| 5 | - | 25.34 | 18.10 | 3.06 | 0.35 | 0.78 | 0.10 | - | 52.27 |
| 6 | - | 23.58 | 18.61 | 3.25 | 0.43 | 0.46 | 0.81 | 0.19 | 52.67 |
| 7 | - | 20.22 | 19.68 | 4.10 | 0.51 | 1.25 | 2.22 | - | 52.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lysykh, S.; Kornopoltsev, V.; Mishigdorzhiyn, U.; Kharaev, Y.; Xie, Z. Evaluation of Wear Resistance of AISI L6 and 5140 Steels after Surface Hardening with Boron and Copper. Lubricants 2023, 11, 48. https://doi.org/10.3390/lubricants11020048
Lysykh S, Kornopoltsev V, Mishigdorzhiyn U, Kharaev Y, Xie Z. Evaluation of Wear Resistance of AISI L6 and 5140 Steels after Surface Hardening with Boron and Copper. Lubricants. 2023; 11(2):48. https://doi.org/10.3390/lubricants11020048
Chicago/Turabian StyleLysykh, Stepan, Vasily Kornopoltsev, Undrakh Mishigdorzhiyn, Yuri Kharaev, and Zhongliang Xie. 2023. "Evaluation of Wear Resistance of AISI L6 and 5140 Steels after Surface Hardening with Boron and Copper" Lubricants 11, no. 2: 48. https://doi.org/10.3390/lubricants11020048
APA StyleLysykh, S., Kornopoltsev, V., Mishigdorzhiyn, U., Kharaev, Y., & Xie, Z. (2023). Evaluation of Wear Resistance of AISI L6 and 5140 Steels after Surface Hardening with Boron and Copper. Lubricants, 11(2), 48. https://doi.org/10.3390/lubricants11020048







