MoS2 Nanomaterials as Lubricant Additives: A Review
Abstract
:1. Introduction
2. Structure and Synthesis
2.1. Structure of MoS2
2.2. Synthesis Method of Nano-MoS2 Nanomaterials
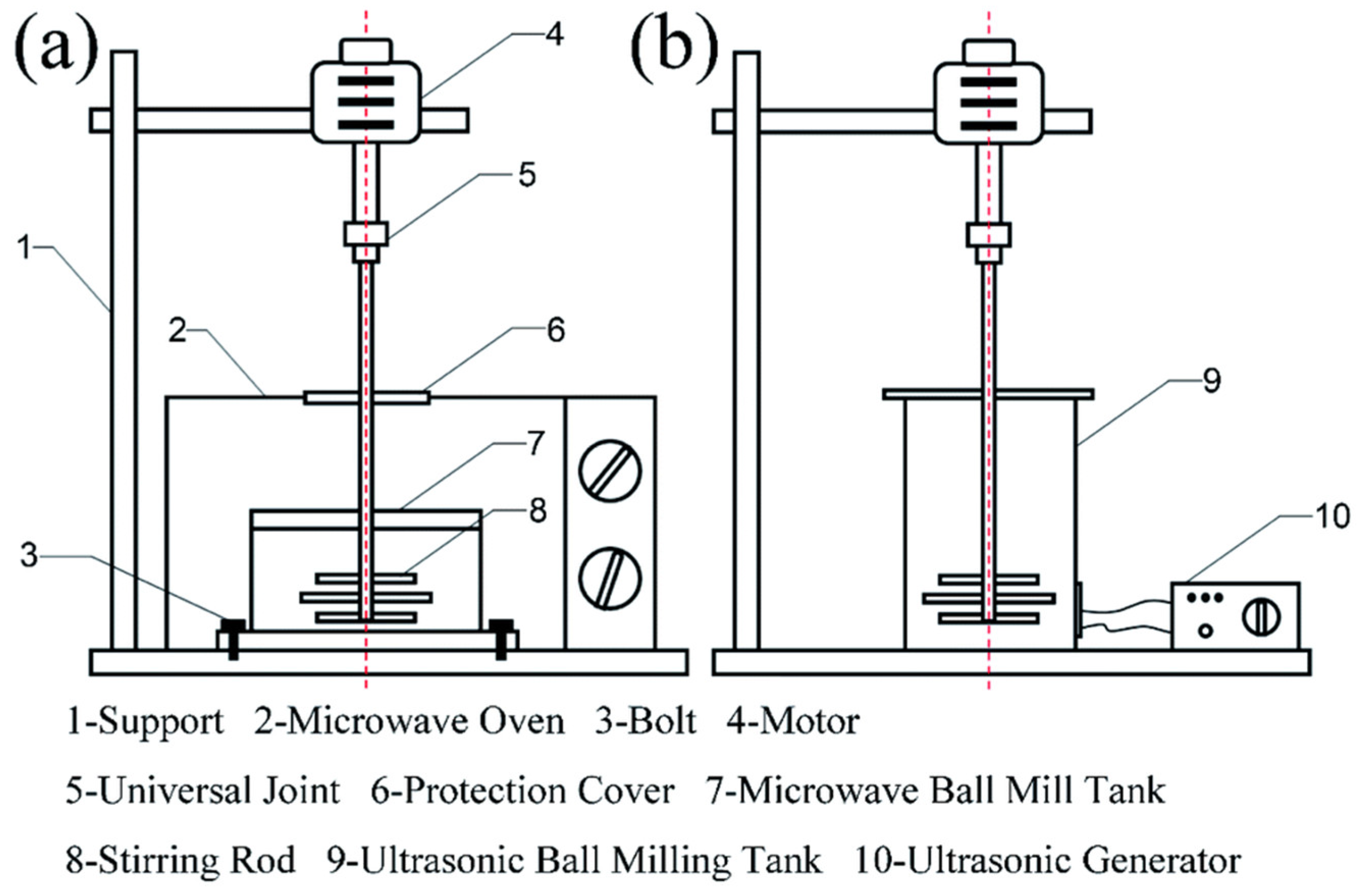
2.2.1. Mechanical Exfoliation
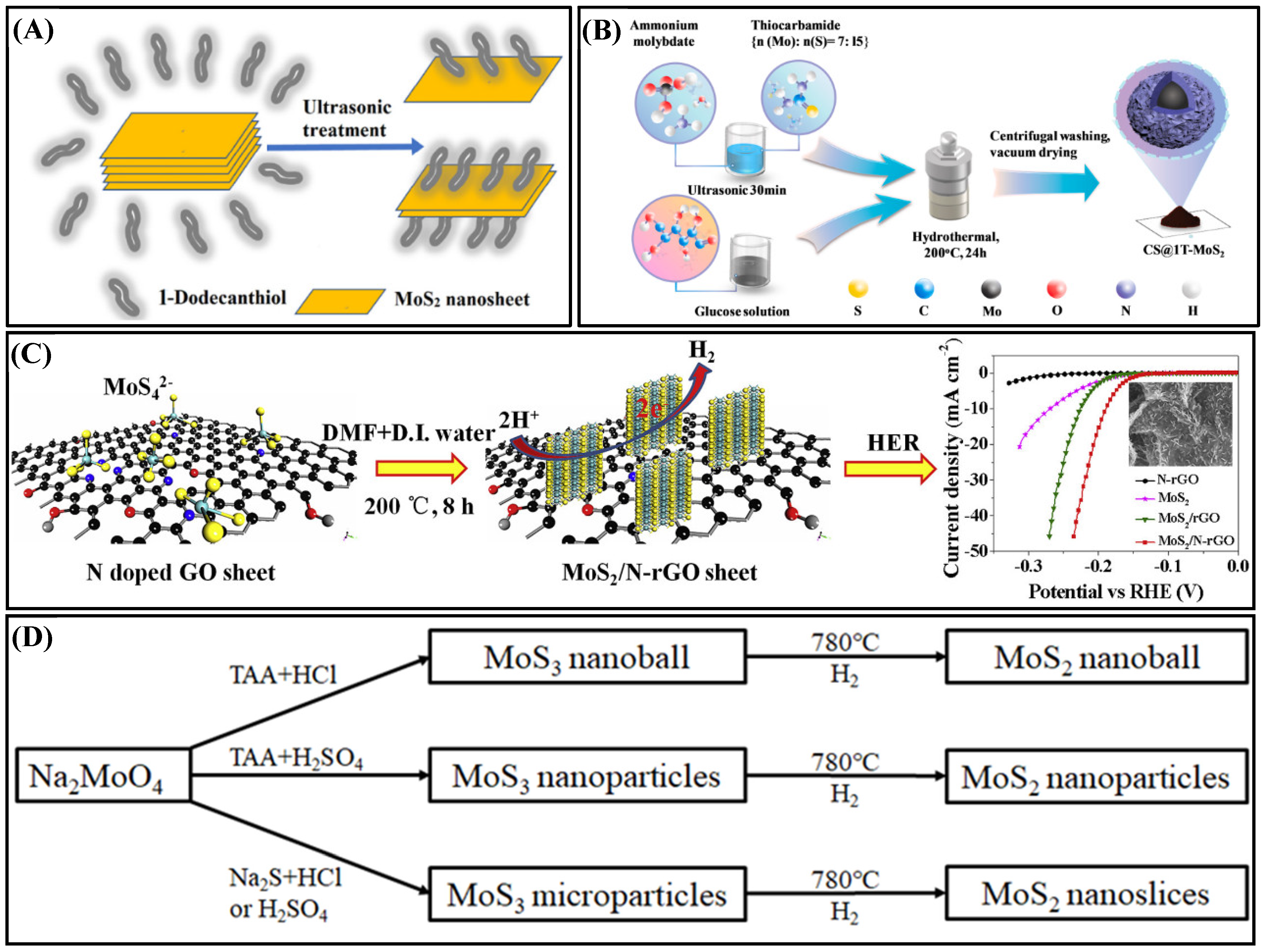
2.2.2. Hydrothermal
2.2.3. Solvothermal
2.2.4. Liquid-Phase Precipitation
3. Stability of MoS2 Nanomaterial Dispersion
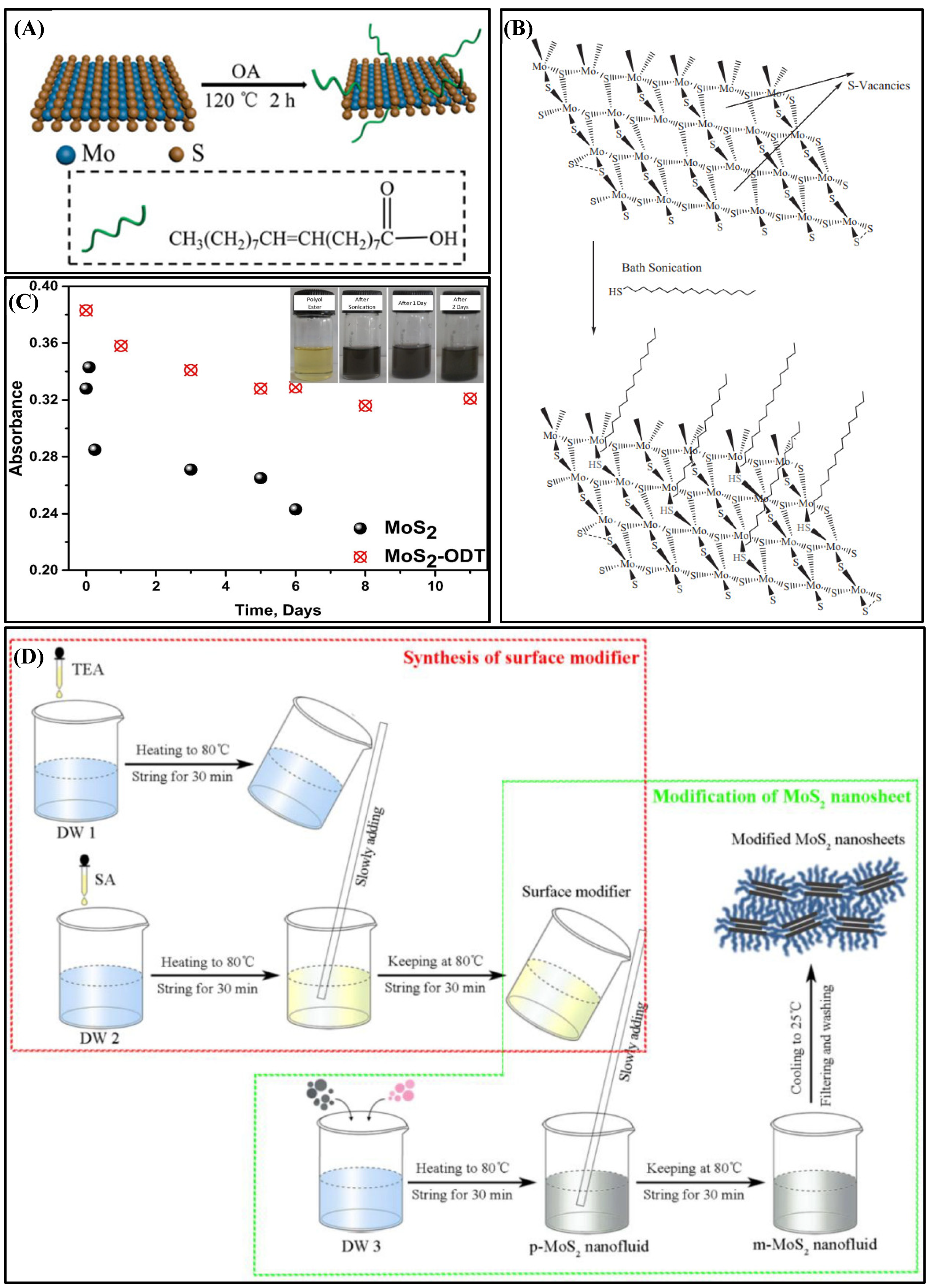
3.1. Physical Methods
3.2. Chemical Methods
4. Tribological Behavior and Lubrication Mechanism of MoS2 Nanomaterials
4.1. Tribological Properties of Nano-MoS2
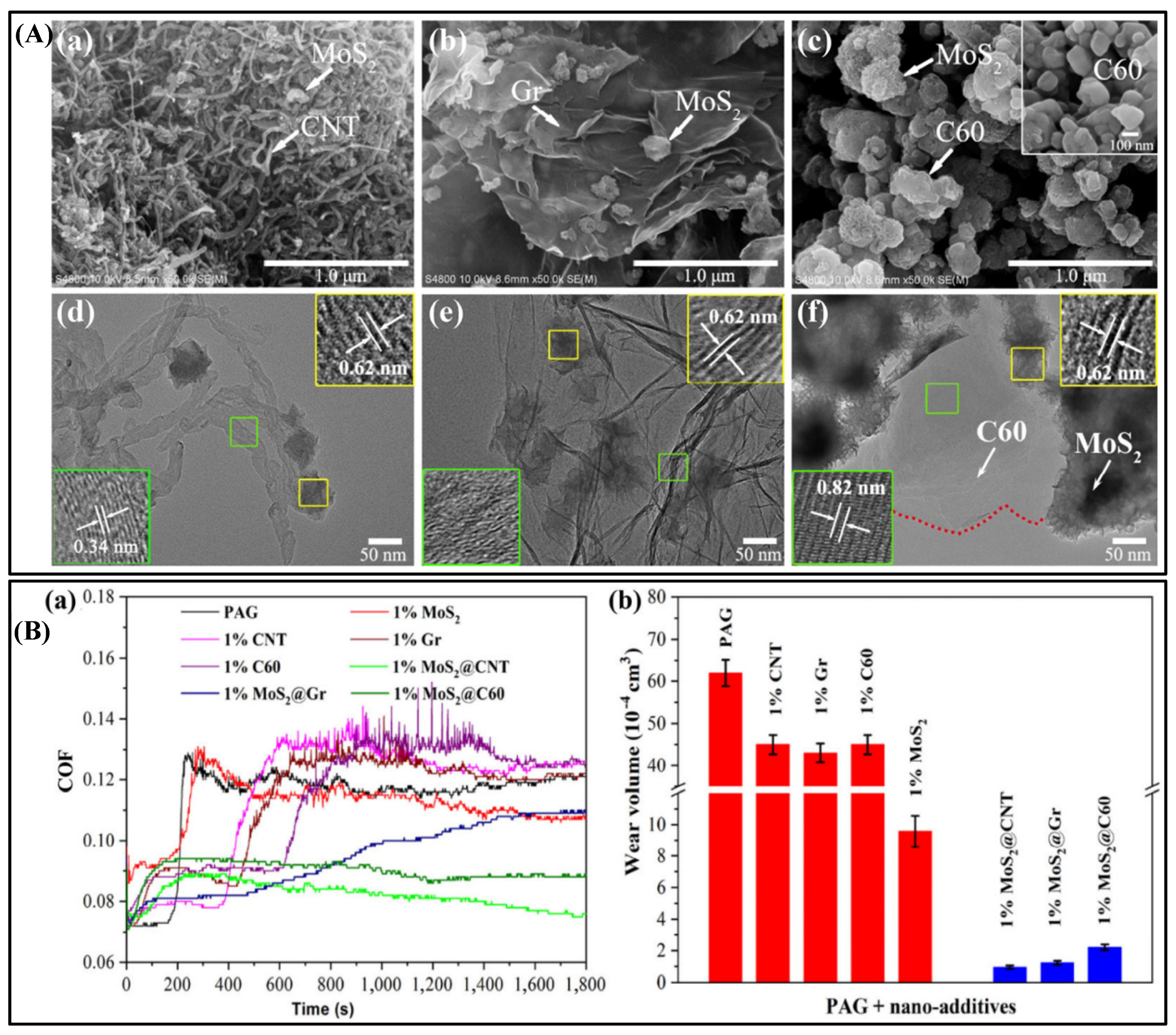
4.2. Tribological Properties of MoS2 Composites
4.3. Lubrication Mechanism
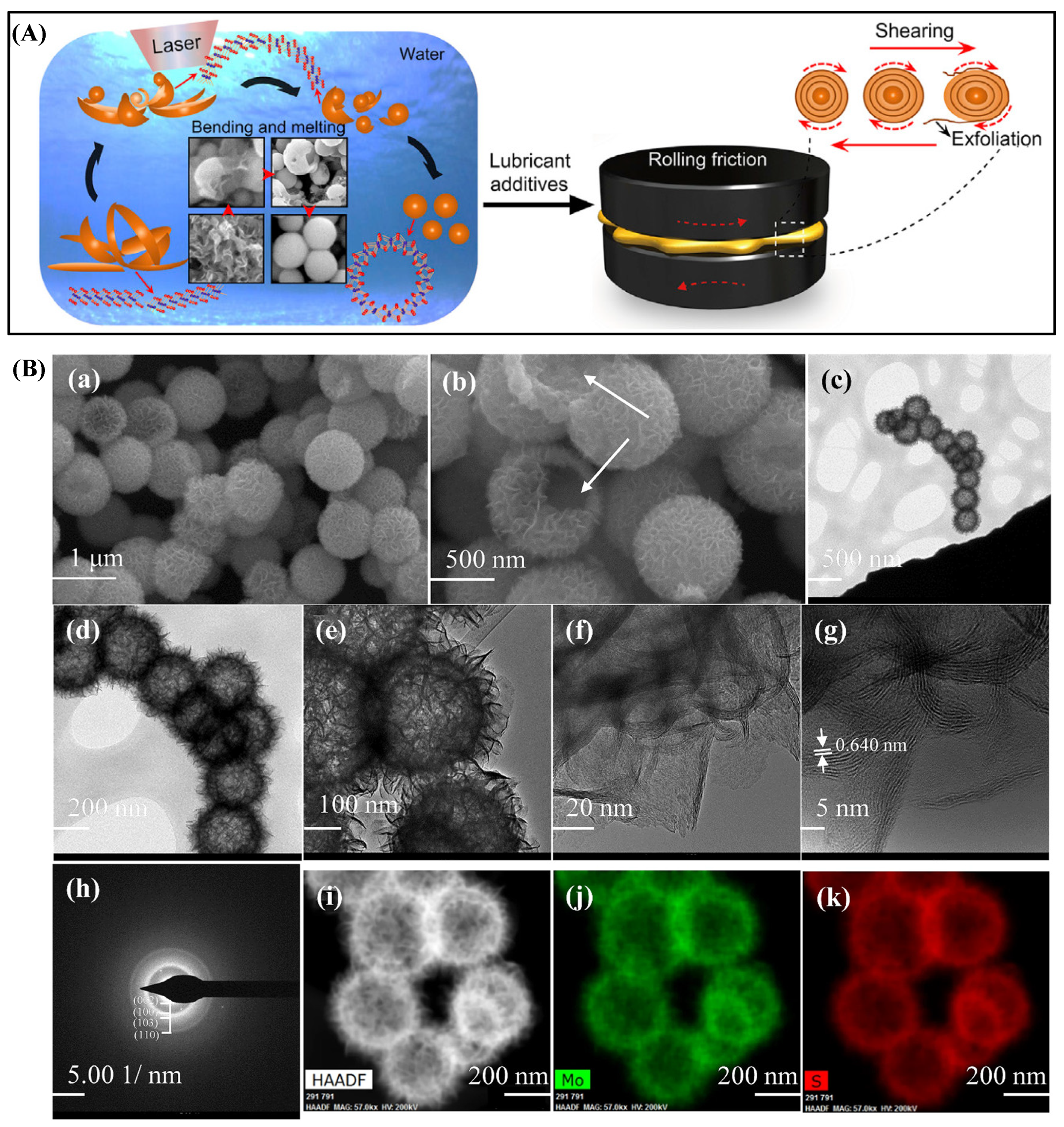
4.3.1. Rolling Mechanism
4.3.2. Shear Slip
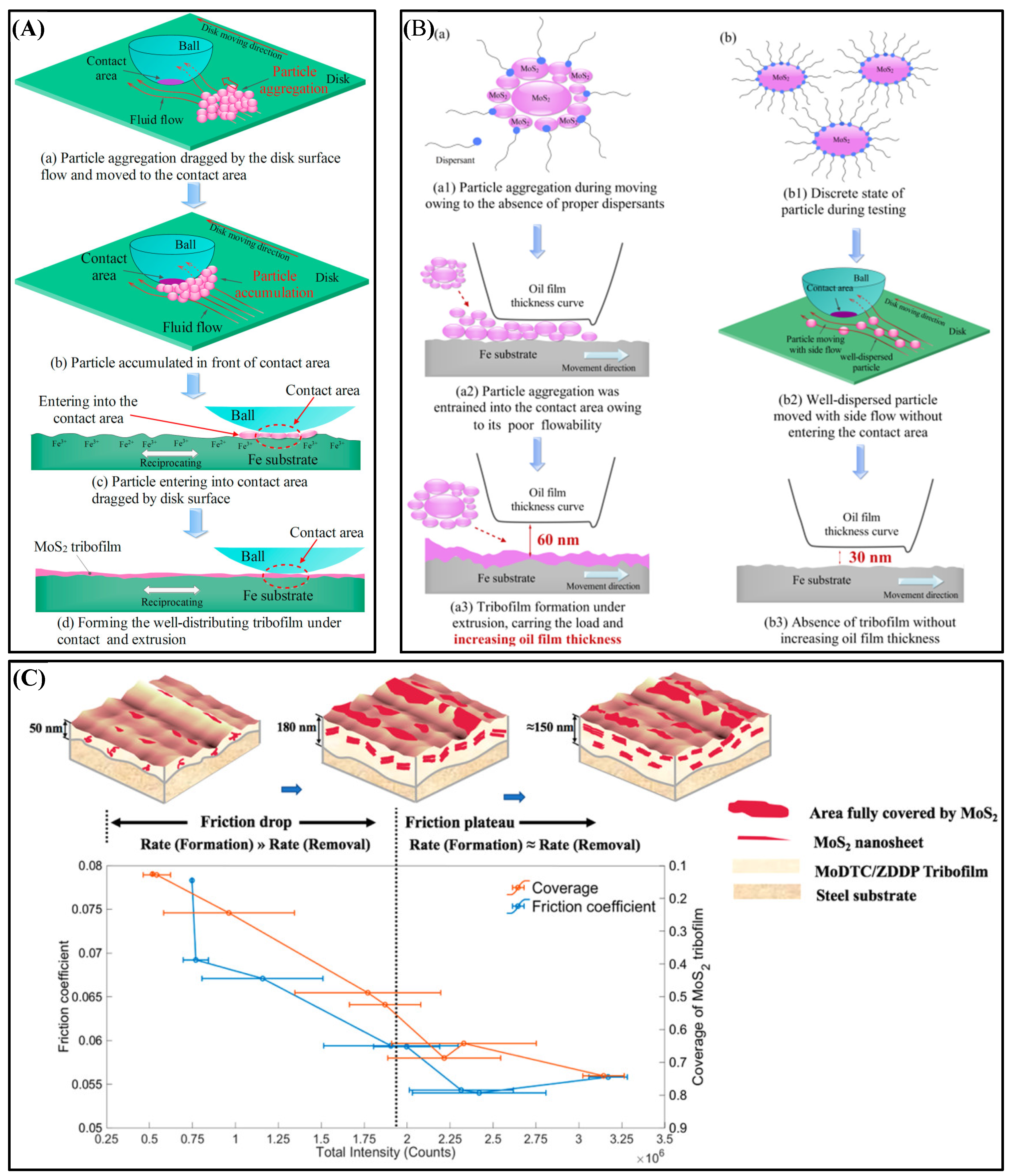
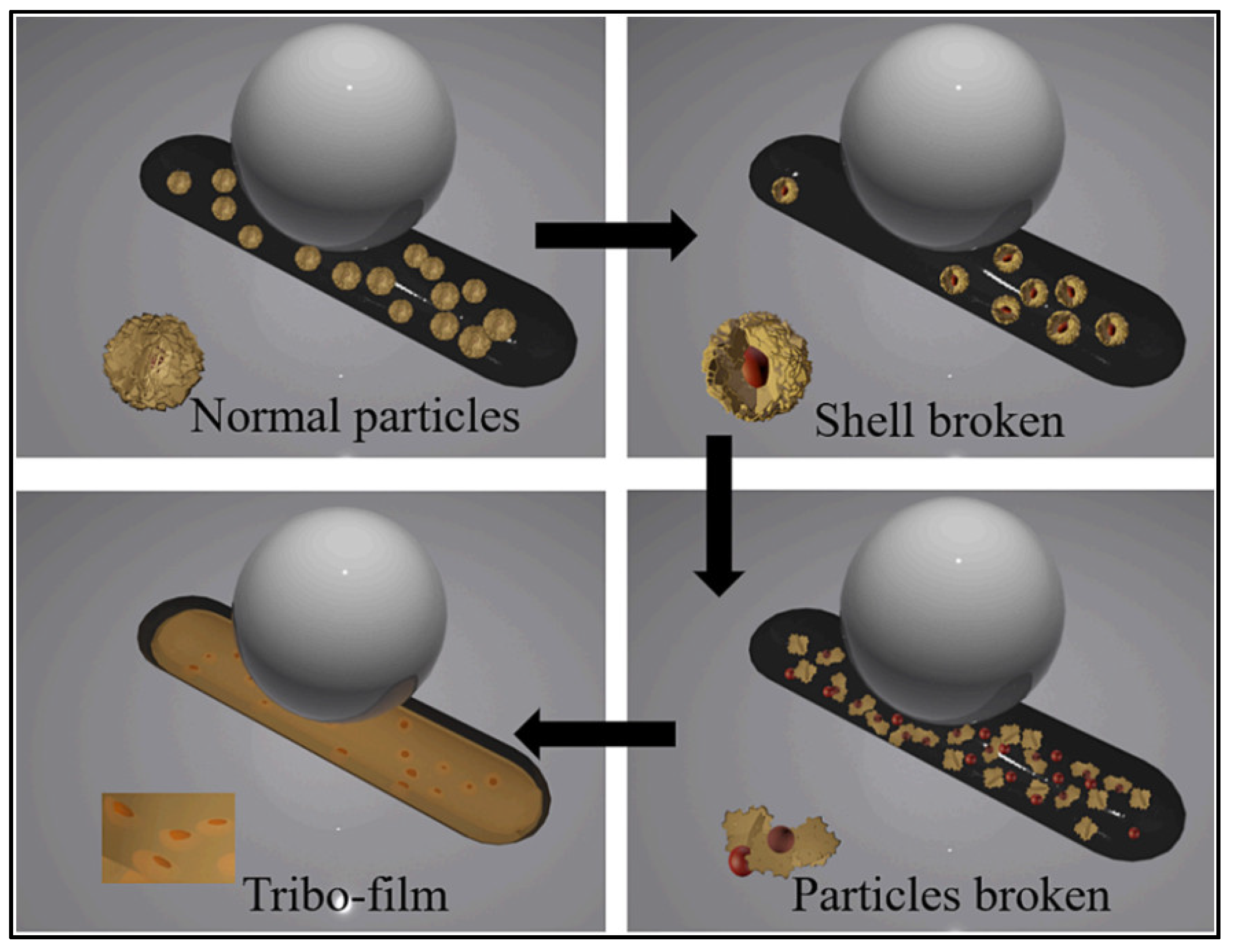
4.3.3. Tribofilm Formation
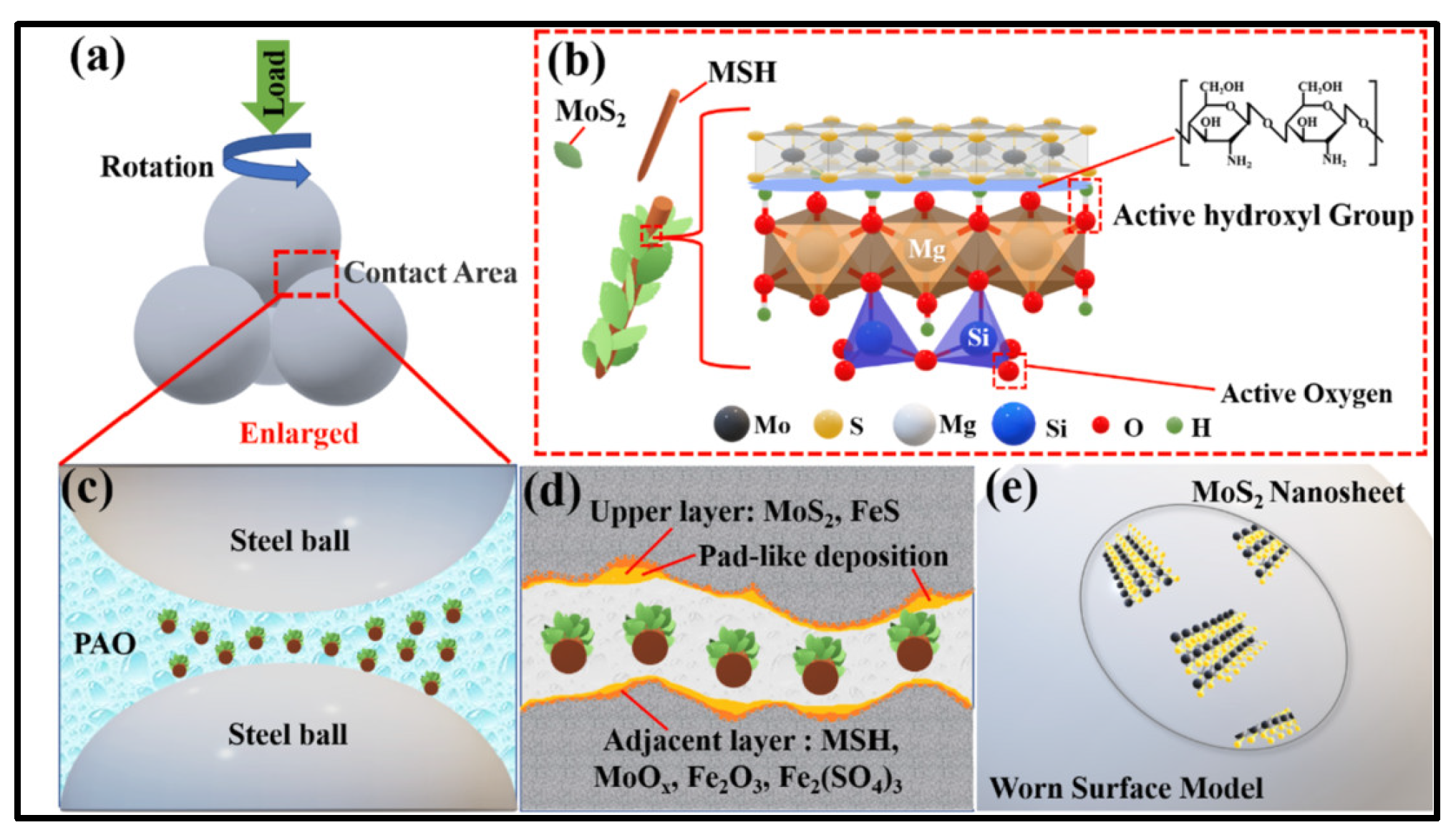
4.3.4. Cooperative Lubrication
4.3.5. Other Mechanisms
4.3.6. Diversification of Lubrication Mechanism
5. Summary and Outlook
- (1)
- The dispersion stability of MoS2 nanomaterials in lubricating oils has not been adequately addressed. During the friction process, the organic modifier is prone to degradation due to the heat generated by friction, which leads to the re-colonization of MoS2 nanoparticles in the lubricating oil. Therefore, research on the long-term dispersion stability of MoS2 nanomaterials is still necessary.
- (2)
- In terms of lubrication mechanism, there are fewer systematic descriptions of the lubrication mechanism of MoS2 nanomaterials with different morphologies in lubricants. The influence of each component on the tribological performance is still unclear, such as the interaction between additive materials and lubricants, the synergistic lubrication of each component in composite nanomaterials, and the interaction between nanomaterials and modifiers. It is necessary to further elucidate the relevant mechanisms by combining advanced characterization techniques, molecular dynamics simulations, and theoretical calculations.
- (3)
- Most reported additives for MoS2-based nanomaterials have been studied under laboratory conditions. In the future, there is a need to develop new additives with good tribological properties under extreme conditions or multiple environments. In addition, low-cost, large-scale preparation routes and evaluation of tribological properties in practical applications are essential for the practical application of these additives.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Abbreviations
| MoDTC | Molybdenum dialkyl dithiocarbamate |
| ZDDP | Zinc dialkyl dithiophosphate |
| TMDs | Transition metal dichalcogenides |
| CS/HAc | chitosan/acetic acid |
| DMF | N, N-dimethylformamide |
| NMP | N-methylpyrrolidone |
| TAA | Thioacetamide |
| OA | Oleic acid |
| ODT | Octadecanethiol |
| TEA | Triethanolamine |
| SA | Stearic acid |
| h-BN | hexagonal boron nitride |
| CTAB | Cetyltrimethylammonium bromide |
| FrGO | Functionalized reduced graphene oxide |
| MSH | Magnesium silicate hydroxide |
| Gr-MS-Zn | ZnO-modified reduced graphene oxide/MoS2 |
| PAO | Polyalphaolefin |
| LP | Liquid paraffin |
| SEM | Scanning electron microscope |
| TEM | Transmission electron microscopy |
| RHC/MoS2 | Rice husk charcoal/MoS2 |
| PEG | Polyethylene glycol |
| CNT | Carbon nanotubes |
| PAG | Polyalkylene glycol |
| CS @ 1 T-MoS2 | carbon sphere @ metallographic MoS2 |
| g-C3N4 | graphitic phase carbon nitride |
| MoS2-O-OLA | Alkylamine-grafted MoS2 oxide |
| PIBS | Polyisobutylene amine succinimide |
| COF | Coefficient of friction |
| Raman | Raman microscopy |
| AFM | Atomic force microscopy |
| K10 | Montmorillonite K10 |
| FA | Fly ash |
References
- Holmberg, K.; Andersson, P.; Erdemir, A. Global energy consumption due to friction in passenger cars. Tribol. Int. 2012, 47, 221–234. [Google Scholar] [CrossRef]
- Holmberg, K.; Andersson, P.; Nylund, N.-O.; Mäkelä, K.; Erdemir, A. Global energy consumption due to friction in trucks and buses. Tribol. Int. 2014, 78, 94–114. [Google Scholar] [CrossRef]
- Zhao, J.; Huang, Y.; He, Y.; Shi, Y. Nanolubricant additives: A review. Friction 2020, 9, 891–917. [Google Scholar] [CrossRef]
- Mang, T.; Bobzin, K.; Bartels, T. Industrial Tribology: Tribosystems, Friction, Wear and Surface Engineering, Lubrication; John Wiley and Sons, Ltd.: New York, NY, USA, 2011. [Google Scholar]
- Mang, T.; Dresel, W. Lubricants and Lubrication; John Wiley and Sons, Ltd.: New York, NY, USA, 2007. [Google Scholar]
- Neville, A.; Morina, A.; Haque, T.; Voong, M. Compatibility between tribological surfaces and lubricant additives—How friction and wear reduction can be controlled by surface/lube synergies. Tribol. Int. 2007, 40, 1680–1695. [Google Scholar] [CrossRef]
- Umer, J.; Morris, N.; Rahmani, R.; Balakrishnan, S.; Rahnejat, H. Nanoscale frictional characterisation of base and fully formulated lubricants based on activation energy components. Tribol. Int. 2020, 144, 106115. [Google Scholar] [CrossRef]
- Rudnick, L.R. Lubricant Additives: Chemistry and Applications, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Guo, D.; Xie, G.; Luo, J. Mechanical properties of nanoparticles: Basics and applications. J. Phys. D Appl. Phys. 2014, 47, 013001. [Google Scholar] [CrossRef]
- Han, Y.; Pan, L.; Zhang, H.; Zeng, Y.; Yin, Z. Effect of lubricant additives of Cu, Fe and bimetallic CuFe nanoparticles on tribological properties. Wear 2022, 508–509, 204485. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, H.; Li, N.; Jiang, Z. Friction and Wear Characteristics of Fe3O4 Nano-Additive Lubricant in Micro-Rolling. Lubricants 2023, 11, 434. [Google Scholar] [CrossRef]
- Liu, C.; Friedman, O.; Meng, Y.; Tian, Y.; Golan, Y. CuS Nanoparticle Additives for Enhanced Ester Lubricant Performance. ACS Appl. Nano Mater. 2018, 1, 7060–7065. [Google Scholar] [CrossRef]
- Liu, Y.; Ge, X.; Li, J. Graphene lubrication. Appl. Mater. 2020, 20, 100662. [Google Scholar] [CrossRef]
- Liu, W.; Li, W.; Li, R.; Lu, Z.; Li, D.; Zhang, G.; Wu, Z. Green oil additive g-C3N4: A feasible strategy to enhance the tribological properties of DLC film. Mater. Res. Express 2019, 6, 115036. [Google Scholar] [CrossRef]
- Zhang, S.; Hu, L.; Feng, D.; Wang, H. Anti-wear and friction-reduction mechanism of Sn and Fe nanoparticles as additives of multialkylated cyclopentanes under vacuum condition. Vacuum 2013, 87, 75–80. [Google Scholar] [CrossRef]
- Xiao, H.; Liu, S. 2D nanomaterials as lubricant additive: A review. Mater. Des. 2017, 135, 319–332. [Google Scholar] [CrossRef]
- Uflyand, I.E.; Zhinzhilo, V.A.; Burlakova, V.E. Metal-containing nanomaterials as lubricant additives: State-of-the-art and future development. Friction 2019, 7, 93–116. [Google Scholar] [CrossRef]
- Bhushan, B. Introduction to Tribology; John Wiley and Sons, Ltd.: New York, NY, USA, 2013. [Google Scholar]
- Zhao, X.; Perry, S.S. The role of water in modifying friction within MoS2 sliding interfaces. ACS Appl. Mater. Interfaces 2010, 2, 1444–1448. [Google Scholar] [CrossRef] [PubMed]
- Chhowalla, M.; Amaratunga, G. Thin films of fullerene-like MoS2 nanoparticles with ultra-low friction and wear. Nature 2000, 407, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Donnet, C.; Martin, J.M.; Le Mogne, T.; Belin, M. Super-low friction of MoS2 coatings in various environments. Tribol. Int. 1996, 29, 123–128. [Google Scholar] [CrossRef]
- Khare, H.; Burris, D. Surface and subsurface contributions of oxidation and moisture to room temperature friction of molybdenum disulfide. Tribol. Lett. 2014, 53, 329–336. [Google Scholar] [CrossRef]
- Winer, W.O. Molybdenum disulfide as a lubricant: A review of the fundamental knowledge. Wear 1967, 10, 422–452. [Google Scholar] [CrossRef]
- Lansdown, A.R. Molybdenum Disulphide Lubrication; Elsevier: Amsterdam, The Netherlands, 1999. [Google Scholar]
- Acikgoz, O.; Guerrero, E.; Yanilmaz, A.; Dagdeviren, O.; Çelebi, C.; Strubbe, D.A.; Baykara, M.Z. Intercalation leads to inverse layer dependence of friction on chemically doped MoS2. Nanotechnology 2023, 34, 015706. [Google Scholar] [CrossRef]
- Bojarska, Z.; Kopytowski, J.; Mazurkiewicz-Pawlicka, M.; Bazarnik, P.; Gierlotka, S.; Rożeń, A.; Makowski, Ł. Molybdenum disulfide-based hybrid materials as new types of oil additives with enhanced tribological and rheological properties. Tribol. Int. 2021, 160, 106999. [Google Scholar] [CrossRef]
- Singh, K.K.; Prabhu, B.R.; Choudhary, S.; Pramanik, C.; John, N.S. Effect of graphene and MoS2 flakes in industrial oils to enhance lubrication. ACS Omega 2019, 4, 14569–14578. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Fan, X.; Yue, Z.; Zhu, M. Mechanism on heterogeneous transfer film formed by diamond-like carbon film under molybdenum disulfide hybrid polyethylene glycol lubrication. Carbon 2023, 210, 118030. [Google Scholar] [CrossRef]
- Toh, R.J.; Sofer, Z.; Luxa, J.; Sedmidubský, D.; Pumera, M. 3R phase of MoS2 and WS2 outperforms the corresponding 2H phase for hydrogen evolution. Chem. Commun. 2017, 53, 3054–3057. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, U.; Kaur, M.; Singh, K.; Kumar, M.; Kumar, A. A synoptic review of MoS2: Synthesis to applications. Superlattices Microstruct. 2019, 128, 274–297. [Google Scholar] [CrossRef]
- Wang, Q.H.; Kourosh-Zadeh, K.; Kis, A.; Coleman, J.N.; Strano, M.S. Electronics and optoelectronics of two-dimensional transition metal dichalcogenides. Nat. Nanotechnol. 2012, 7, 699–712. [Google Scholar] [CrossRef]
- Eda, G.; Yamaguchi, H.; Voiry, D.; Fujita, T.; Chen, M.W.; Chhowalla, M. Photoluminescence from chemically exfoliated MoS2. Nano Lett. 2011, 11, 5111–5116. [Google Scholar] [CrossRef]
- Frindt, R.F. Single crystals of MoS2 several molecular layers thick. J. Appl. Phys. 1966, 37, 1928–1929. [Google Scholar] [CrossRef]
- Liu, Y.; Li, R. Study on ultrasound-assisted liquid-phase exfoliation for preparing graphene-like molybdenum disulfide nanosheets. Ultrason. Sonochem. 2020, 63, 104923. [Google Scholar] [CrossRef]
- Yao, Q.; Guo, J.; Guan, F.; Zhao, M.; Zhang, S.; Tuo, X.; Yang, Q. Ultrasonic-Assisted Exfoliation Bulk-Phase of MoS2 with Chitosan/Acetic Acid Solution. JOM 2023, 75, 701–707. [Google Scholar] [CrossRef]
- Xie, M.; Pan, B.; Liu, H.; Li, N.; Chen, Z.; Yan, J.; Fu, Z.; Guo, S.; Wang, H. One-step synthesis of carbon sphere@1T-MoS2 towards superior antiwear and lubricity. Tribol. Int. 2022, 176, 107927. [Google Scholar] [CrossRef]
- Zhao, L.; Hong, C.; Lin, L.; Wu, H.; Su, Y.; Zhang, X.; Liu, A. Controllable nanoscale engineering of vertically aligned MoS2 ultrathin nanosheets by nitrogen doping of 3D graphene hydrogel for improved electrocatalytic hydrogen evolution. Carbon 2017, 116, 223–231. [Google Scholar] [CrossRef]
- Hu, K.; Hu, X.; Jiang, P. Large-scale and morphology-controlled synthesis of nano-sized molybdenum disulphide particles by different sulphur sources. Int. J. Mater. Prod. Technol. 2010, 39, 378–387. [Google Scholar] [CrossRef]
- Pallikkarathodi Mani, N.; Cyriac, J. Green approach to synthesize various MoS2 nanoparticles via hydrothermal process. Bull. Mater. Sci. 2022, 45, 184. [Google Scholar] [CrossRef]
- Park, S.Y.; Lee, J.E.; Kim, Y.H.; Kim, J.J.; Shim, Y.-S.; Kim, S.Y.; Lee, M.H.; Jang, H.W. Room temperature humidity sensors based on rGO/MoS2 hybrid composites synthesized by hydrothermal method. Sens. Actuators B Chem. 2018, 258, 775–782. [Google Scholar] [CrossRef]
- Tang, G.; Zhang, J.; Liu, C.; Zhang, D.; Wang, Y.; Tang, H.; Li, C. Synthesis and tribological properties of flower-like MoS2 microspheres. Ceram. Int. 2014, 40, 11575–11580. [Google Scholar] [CrossRef]
- Li, J.; Wang, D.; Ma, H.; Pan, Z.; Jiang, Y.; Li, M.; Tian, Z. Ionic liquid assisted hydrothermal synthesis of hollow core/shell MoS2 microspheres. Mater. Lett. 2015, 160, 550–554. [Google Scholar] [CrossRef]
- Vijaya, S.; Landi, G.; Wu, J.J.; Anandan, S. MoS2 nanosheets based counter electrodes: An alternative for Pt-free dye-sensitized solar cells. Electrochim. Acta 2019, 294, 134–141. [Google Scholar] [CrossRef]
- Gan, Y.X.; Jayatissa, A.H.; Yu, Z.; Chen, X.; Li, M. Hydrothermal synthesis of nanomaterials. J. Nanomater. 2020, 2020, 8917013. [Google Scholar] [CrossRef]
- Li, L.; Qin, Z.; Ries, L.; Hong, S.; Michel, T.; Yang, J.; Salameh, C.; Bechelany, M.; Miele, P.; Kaplan, D.; et al. Role of sulfur vacancies and undercoordinated Mo regions in MoS2 nanosheets toward the evolution of hydrogen. ACS Nano 2019, 13, 6824–6834. [Google Scholar] [CrossRef]
- Li, X.; Feng, Z.; Zai, J.; Ma, Z.-F.; Qian, X. Incorporation of Co into MoS2/graphene nanocomposites: One effective way to enhance the cycling stability of Li/Na storage. J. Power Sources 2018, 373, 103–109. [Google Scholar] [CrossRef]
- He, D.; Yang, Y.; Liu, Z.; Shao, J.; Wu, J.; Wang, S.; Shen, L.; Bao, N. Solvothermal-assisted assembly of MoS2 nanocages on graphene sheets to enhance the electrochemical performance of lithium-ion battery. Nano Res. 2020, 13, 1029–1034. [Google Scholar] [CrossRef]
- Wang, C.; Jiang, J.; Ruan, Y.; Ao, X.; Ostrikov, K.; Zhang, W.; Lu, J.; Li, Y.Y. Construction of MoO2 quantum dot–graphene and MoS2 nanoparticle–graphene nanoarchitectures toward ultrahigh lithium storage capability. ACS Appl. Mater. Interfaces 2017, 9, 28441–28450. [Google Scholar] [CrossRef]
- Cao, H.; Bai, Z.; Li, Y.; Xiao, Z.; Zhang, X.; Li, G. Solvothermal Synthesis of Defect-Rich Mixed 1T-2MoS2 Nanoflowers for Enhanced Hydrodesulfurization. ACS Sustain. Chem. Eng. 2020, 8, 7343–7352. [Google Scholar] [CrossRef]
- Chen, J.; Xu, Z.; Hu, Y.; Yi, M. PEG-assisted solvothermal synthesis of MoS2 nanosheets with enhanced tribological property. Lubr. Sci. 2020, 32, 273–282. [Google Scholar] [CrossRef]
- Najafi, L.; Bellani, S.; Martín-García, B.; Oropesa-Nuñez, R.; Del Rio Castillo, A.E.; Prato, M.; Moreels, I.; Bonaccorso, F. Solution-processed hybrid graphene flake/2H-MoS2 quantum dot heterostructures for efficient electrochemical hydrogen evolution. Chem. Mater. 2017, 29, 5782–5786. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, Y.; Fan, X.; Wang, S.; Li, Y.; Zhang, F.; Zhang, G.; Peng, W. (0D/3D) MoS2 on porous graphene as catalysts for enhanced electrochemical hydrogen evolution. Carbon 2017, 121, 163–169. [Google Scholar] [CrossRef]
- Huang, H.; Huang, J.; Liu, W.; Fang, Y.; Liu, Y. Ultradispersed and single-layered MoS2 nanoflakes strongly coupled with graphene: An optimized structure with high kinetics for the hydrogen evolution reaction. ACS Appl. Mater. Interfaces 2017, 9, 39380–39390. [Google Scholar] [CrossRef]
- Tan, L.; Li, X.; Wang, Z.; Guo, H.; Wang, J. Lightweight Reduced Graphene Oxide@MoS2 Interlayer as Polysulfide Barrier for High-Performance Lithium–Sulfur Batteries. ACS Appl. Mater. Interfaces 2018, 10, 3707–3713. [Google Scholar] [CrossRef]
- Hou, S.; Li, Y.; Huo, Y.; Wu, C.; Zhang, D.; Zhang, H. Preparation of nano-scale molybdenum disulfide by liquid phase precipitation method and its lubricating properties. Ferroelectrics 2018, 524, 79–85. [Google Scholar] [CrossRef]
- Hu, K.H.; Wang, Y.R.; Hu, X.G.; Wo, H.Z. Preparation and characterisation of ball-like MoS2 nanoparticles. Mater. Sci. Technol. 2007, 23, 242–246. [Google Scholar] [CrossRef]
- Hu, K.H.; Hu, X.G.; Sun, X.J.; Jing, H.F.; Zhan, S. Synthesis and characterization of nanosize molybdenum disulfide particles by quick homogeneous precipitation method. Key Eng. Mater. 2007, 353–358, 2107–2110. [Google Scholar] [CrossRef]
- Hu, X.; Sun, X.; Hu, K. Preparation Parameters Optimization of Noncrystalline MoS3–The Precursor of Nano-MoS2. Solid State Phenom. 2007, 121–123, 1309–1312. [Google Scholar] [CrossRef]
- Hu, K.; Hu, X. Formation, exfoliation and restacking of MoS2 nanostructures. Mater. Sci. Technol. 2009, 25, 407–414. [Google Scholar] [CrossRef]
- Xu, Y.; Hu, E.; Hu, K.; Xu, Y.; Hu, X. Formation of an adsorption film of MoS2 nanoparticles and dioctyl sebacate on a steel surface for alleviating friction and wear. Tribol. Int. 2015, 92, 172–183. [Google Scholar] [CrossRef]
- Kim, D.; Archer, L.A. Nanoscale organic-inorganic hybrid lubricants. Langmuir 2011, 27, 3083–3094. [Google Scholar] [CrossRef]
- Bakunin, V.N.; Suslov, A.Y.; Kuzmina, G.N.; Parenago, O.P. Recent achievements in the synthesis and application of inorganic nanoparticles as lubricant components. Lubr. Sci. 2005, 17, 127–145. [Google Scholar] [CrossRef]
- Devendiran, D.K.; Amirtham, V.A. A review on preparation, characterization, properties and applications of nanofluids. Renew. Sustain. Energy Rev. 2016, 60, 21–40. [Google Scholar] [CrossRef]
- Huang, W.; Liu, W.; Wu, D.H. Investigations into lubrication in grinding processes using MWCNTs nanofluids with ultrasonic-assisted dispersion. J. Clean. Prod. 2016, 137, 1553–1559. [Google Scholar] [CrossRef]
- Maheswaran, R.; Sunil, J. Experimental analysis of tribological properties of ultrasonically dispersed garnet nanoparticles in SN500 grade lubricating oil. Ind. Lubr. Tribol. 2018, 70, 250–255. [Google Scholar] [CrossRef]
- Ashour, M.; Mohamed, A.; Elshalakany, A.B.; Osman, T.; Khatab, A. Rheological behavior of lithium grease with CNTs/GNPs hybrid nanocomposite as an additive. Ind. Lubr. Tribol. 2018, 70, 331–338. [Google Scholar] [CrossRef]
- Mosleh, M.; Atnafu, N.D.; Belk, J.H.; Nobles, O.M. Modification of sheet metal forming fluids with dispersed nanoparticles for improved lubrication. Wear 2009, 267, 1220–1225. [Google Scholar] [CrossRef]
- Li, W.; Cheng, Z.-L.; Liu, Z. Novel Preparation of Calcium Borate/Graphene Oxide Nanocomposites and Their Tribological Properties in Oil. J. Mater. Eng. Perform. 2017, 26, 285–291. [Google Scholar] [CrossRef]
- Shi, D.; Yang, M.; Chang, B.; Ai, Z.; Zhang, K.; Shao, Y.; Wang, S.; Wu, Y.; Hao, X. Ultrasonic-ball milling: A novel strategy to prepare large-size ultrathin 2D materials. Small 2020, 16, 1906734. [Google Scholar] [CrossRef] [PubMed]
- Guerra, V.; Wan, C.; Degirmenci, V.; Sloan, J.; Presvytis, D.; Watson, M.; McNally, T. Characterisation of graphite nanoplatelets (GNP) prepared at scale by highpressure homogenization. J. Mater. Chem. C 2019, 7, 6383–6390. [Google Scholar] [CrossRef]
- Kumar, D.P.; Hong, S.; Reddy, D.A.; Kim, T.K. Ultrathin MoS2 layers anchored exfoliated reduced graphene oxide nanosheet hybrid as a highly efficient cocatalyst for CdS nanorods towards enhanced photocatalytic hydrogen production. Appl. Catal. B 2017, 212, 7–14. [Google Scholar] [CrossRef]
- Upadhyay, R.K.; Kumar, A. Effect of humidity on the synergy of friction and wear properties in ternary epoxy-graphene-MoS2 composites. Carbon 2019, 146, 717–727. [Google Scholar] [CrossRef]
- Alazemi, A.A.; Dysart, A.D.; Phuah, X.L.; Pol, V.G.; Sadeghi, F. MoS2 nanolayer coated carbon spheres as an oil additive for enhanced tribological performance. Carbon 2016, 110, 367–377. [Google Scholar] [CrossRef]
- Xu, Y.; Peng, Y.; Dearn, K.D.; Zheng, X.; Yao, L.; Hu, X. Synergistic lubricating behaviors of graphene and MoS2 dispersed in esterified bio-oil for steel/steel contact. Wear 2015, 342–343, 297–309. [Google Scholar] [CrossRef]
- Wang, S.; Chen, D.; Chen, Y.; Zhu, K. Dispersion stability and tribological properties of additives introduced by ultrasonic and microwave assisted ball milling in oil. RSC Adv. 2020, 10, 25177–25185. [Google Scholar] [CrossRef]
- Sahoo, R.R.; Biswas, S.K. Deformation and friction of MoS2 particles in liquid suspensions used to lubricate sliding contact. Thin Solid Films 2010, 518, 5995–6005. [Google Scholar] [CrossRef]
- Hou, X.; Jiang, H.; Ali, M.K.A.; Liu, H.; Su, D.; Tian, Z. Dispersion behavior assessment of the molybdenum disulfide nanomaterials dispersed into poly alpha olefin. J. Mol. Liq. 2020, 311, 113303. [Google Scholar] [CrossRef]
- Gulzar, M.; Mahmood, K.; Zahid, R.; Alabdulkarem, A.; Masjuki, H.H.; Kalam, M.A.; Varman, M.; Zulkifli, N.W.M.; Ahmad, P.; Malik, M.S.S. The effect of particle size on the dispersion and wear protection ability of MoS2 particles in polyalphaolefin and trimethylolpropane ester. Proc. Inst. Mech. Eng. Part J J. Eng. Tribol. 2018, 232, 987–998. [Google Scholar] [CrossRef]
- Paramashivaiah, B.M.; Rajashekhar, C.R. Studies on effect of various surfactants on stable dispersion of graphene nano particles in simarouba biodiesel. IOP Conf. Ser. Mater. Sci. Eng. 2016, 149, 012083. [Google Scholar] [CrossRef]
- Large, M.J.; Ogilvie, S.P.; Graf, A.A.; Lynch, P.J.; O’Mara, M.A.; Waters, T.; Jurewicz, I.; Salvage, J.P.; Dalton, A.B. Large-scale surfactant exfoliation of graphene and conductivity-optimized graphite enabling wireless connectivity. Adv. Mater. Technol. 2020, 5, 2000284. [Google Scholar] [CrossRef]
- Zhao, J.; Gao, T.; Li, Y.; He, Y.; Shi, Y. Two-dimensional (2D) graphene nanosheets as advanced lubricant additives: A critical review and prospect. Mater. Today Commun. 2021, 29, 102755. [Google Scholar] [CrossRef]
- Fu, Z.; Gu, X.; Hu, L.; Li, Y.; Li, J. Radiation Induced Surface Modification of Nanoparticles and Their Dispersion in the Polymer Matrix. Nanomaterials 2020, 10, 2237. [Google Scholar] [CrossRef] [PubMed]
- Khazaei, M.A.; Bastani, D.; Mohammadi, A.; Kordzadeh, A. Adsorption Dynamics of Surface-Modified Silica Nanoparticles at Solid–Liquid Interfaces. Langmuir 2022, 38, 12421–12431. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zheng, S.; Chen, Q.; Cao, B. A new method for surface modification of TiO2/Al2O3 nanocomposites with enhanced anti-friction properties. Mater. Chem. Phys. 2012, 134, 38–42. [Google Scholar] [CrossRef]
- Wu, P.; Li, W.; Liu, Z.; Cheng, Z. Preparation and tribological properties of oleic acid-decorated MoS2 nanosheets with good oil dispersion. J. Dispers. Sci. Technol. 2018, 39, 1742–1751. [Google Scholar] [CrossRef]
- Jiang, H.; Hou, X.; Qian, Y.; Liu, H.; Ali, M.K.A.; Dearn, K.D. A tribological behavior assessment of steel contacting interface lubricated by engine oil introducing layered structural nanomaterials functionalized by oleic acid. Wear 2023, 524–525, 204675. [Google Scholar] [CrossRef]
- Kumari, S.; Mungse, H.P.; Gusain, R.; Kumar, N.; Sugimura, H.; Khatri, O.P. Octadecanethiol-grafted molybdenum disulfide nanosheets as oil-dispersible additive for reduction of friction and wear. FlatChem 2017, 3, 16–25. [Google Scholar] [CrossRef]
- Meng, Y.; Sun, J.; He, J.; Yang, F.; Wu, P. Surface modification induced improvement of dispersion stability and tribological properties of MoS2 nanosheets. J. Dispers. Sci. Technol. 2023, 44, 1010–1020. [Google Scholar] [CrossRef]
- Aralihalli, S.; Biswas, S.K. Crafting of dispersants on MoS2 nanoparticles in base oil lubrication of steel. Tribol. Lett. 2013, 49, 61–76. [Google Scholar] [CrossRef]
- Lee, J.D. Concise of Ingoranic Chemistry, 5th ed.; Oxford University Press: Oxford, UK, 2013. [Google Scholar]
- Sahoo, R.R.; Math, S.; Biswas, S.K. Mechanics of deformation under traction and friction of a micrometric monolithic MoS2 particle in comparison with those of an agglomerate of nanometric MoS2 particles. Tribol. Lett. 2010, 37, 239–249. [Google Scholar] [CrossRef]
- Kumari, S.; Chouhan, A.; Kumar Konathala, L.N.S.; Sharma, O.P.; Ray, S.S.; Ray, A.; Khatri, O.P. Chemically functionalized 2D/2D hexagonal boron Nitride/Molybdenum disulfide heterostructure for enhancement of lubrication properties. Appl. Surf. Sci. 2022, 579, 152157. [Google Scholar] [CrossRef]
- Farsadi, M.; Bagheri, S.; Ismail, N.A. Nanocomposite of functionalized graphene and molybdenum disulfide as friction modifier additive for lubricant. J. Mol. Liq. 2017, 244, 304–308. [Google Scholar] [CrossRef]
- Guan, Z.; Wu, Z.; Liu, J.; Tu, X.; Li, S. Controllable fabrication of magnesium silicate hydroxide reinforced MoS2 hybrid nanomaterials as effective lubricant additives in PAO. Appl. Surf. Sci. 2022, 597, 153777. [Google Scholar] [CrossRef]
- Chouhan, A.; Sarkar, T.K.; Kumari, S.; Vemuluri, S.; Khatri, O.P. Synergistic lubrication performance by incommensurately stacked ZnO-decorated reduced graphene oxide/MoS2 heterostructure. J. Colloid Interface Sci. 2020, 580, 730–739. [Google Scholar] [CrossRef]
- Rabaso, P.; Ville, F.; Dassenoy, F.; Diaby, M.; Afanasiev, P.; Cavoret, J.; Vacher, B.; Le Mogne, T. Boundary lubrication: Influence of the size and structure of inorganic fullerene-like MoS2 nanoparticles on friction and wear reduction. Wear 2014, 360, 161–178. [Google Scholar] [CrossRef]
- Jia, X.; Huang, J.; Li, Y.; Yang, J.; Song, H. Monodisperse Cu nanoparticles @ MoS2 nanosheets as a lubricant additive for improved tribological properties. Appl. Surf. Sci. 2019, 494, 430–439. [Google Scholar] [CrossRef]
- Song, W.; Yan, J.; Ji, H. Fabrication of GNS/MoS2 composite with different morphology and its tribological performance as a lubricant additive. Appl. Surf. Sci. 2019, 469, 226–235. [Google Scholar] [CrossRef]
- Hu, K.H.; Hu, X.G.; Xu, Y.F.; Huang, F.; Liu, J.S. The effect of morphology on the tribological properties of MoS2 in liquid paraffin. Tribol. Lett. 2010, 40, 155–165. [Google Scholar] [CrossRef]
- Uzoma, P.C.; Hu, H.; Khadem, M.; Penkov, O.V. Tribology of 2D Nanomaterials: A Review. Coatings 2020, 10, 897. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, F.; Yang, K.; Xiong, Y.; Tang, J.; Chen, H.; Duan, M.; Li, Z.; Zhang, H.; Xiong, B. Review of two-dimensional nanomaterials in tribology: Recent developments, challenges and prospects. Adv. Colloid Interface Sci. 2023, 321, 103004. [Google Scholar] [CrossRef] [PubMed]
- Cho, D.H.; Wang, L.; Kim, J.S.; Lee, G.H.; Kim, E.S.; Lee, S.; Lee, S.Y.; Hone, J.; Lee, C. Effect of surface morphology on friction of graphene on various substrates. Nanoscale 2013, 5, 3063–3069. [Google Scholar] [CrossRef] [PubMed]
- Tontini, G.; Semione, G.D.L.; Bernardi, C.; Binder, R.; de Mello, J.D.B.; Drago, V. Synthesis of nanostructured flower-like MoS2 and its friction properties as additive in lubricating oils. Ind. Lubr. Tribol. 2016, 68, 658–664. [Google Scholar] [CrossRef]
- Luo, T.; Chen, X.; Wang, L.; Wang, P.; Li, C.; Zeng, H.; Cao, B. Green laser irradiation-stimulated fullerene-like MoS2 nanospheres for tribological applications. Tribol. Int. 2018, 122, 119–124. [Google Scholar] [CrossRef]
- Xu, W.; Fu, C.; Hu, Y.; Chen, J.; Yang, Y.; Yi, M. Synthesis of hollow core-shell MoS2 nanoparticles with enhanced lubrication performance as oil additives. Bull. Mater. Sci. 2021, 44, 88. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, H.B.; Guo, Z.; Lou, X.W.D. Facile synthesis of carbon-coated MoS2 nanorods with enhanced lithium storage properties. Electrochem. Commun. 2012, 20, 7–10. [Google Scholar] [CrossRef]
- Li, W.J.; Shi, E.W.; Ko, J.M.; Chen, Z.Z.; Ogino, H.; Fukuda, T. Hydrothermal synthesis of MoS2 nanowires. J. Cryst. Growth 2003, 250, 418–422. [Google Scholar] [CrossRef]
- Xu, Y.; Fu, K.; Liu, K.; Sun, K.; Dong, Y.; Yao, L. A State of the Art Review of the Tribology of Graphene/MoS2 Nanocomposites. Mater. Today Commun. 2022, 34, 105108. [Google Scholar] [CrossRef]
- Hu, E.; Su, E.; Chen, Y.; Subedi, A.; Wang, J.; Hu, K.; Tang, L. Preparation and Tribological Behaviors of Modified Rice Husk Carbon/MoS2 Composite Particles as a Functional Additive in Polyethylene Glycol. Tribol. Trans. 2022, 65, 564–577. [Google Scholar] [CrossRef]
- Gong, K.; Lou, W.; Zhao, G.; Wu, X.; Wang, X. MoS2 nanoparticles grown on carbon nanomaterials for lubricating oil additives. Friction 2021, 9, 747–757. [Google Scholar] [CrossRef]
- Jiang, H.; Hou, X.; Ma, Y.; Su, D.; Qian, Y.; Ahmed Ali, M.K.; Dearn, K.D. The tribological performance evaluation of steel-steel contact surface lubricated by polyalphaolefins containing surfactant-modified hybrid MoS2/h-BN nano-additives. Wear 2022, 504–505, 204426. [Google Scholar] [CrossRef]
- Min, C.; Yang, Y.; Liang, H.; He, Z.; Zhu, J.; Li, Q. Covalently Bonded 2D/0D g-C3N4/MoS2 Nanocomposites for Enhanced Tribological Properties in Oil. ChemistrySelect 2021, 6, 1661–1668. [Google Scholar] [CrossRef]
- Guan, Z.; Zhang, P.; Florian, V.; Wu, Z.; Zeng, D.; Liu, J.; Wang, B.; Tu, X.; Li, S.; Li, W. Preparation and tribological behaviors of magnesium silicate hydroxide-MoS2 nanoparticles as lubricant additive. Wear 2022, 492–493, 204237. [Google Scholar] [CrossRef]
- Luo, T.; Wei, X.; Huang, X.; Huang, L.; Yang, F. Tribological properties of Al2O3 nanoparticles as lubricating oil additives. Ceram. Int. 2014, 40, 7143–7149. [Google Scholar] [CrossRef]
- Aldana, P.U.; Dassenoy, F.; Vacher, B.; Le Mogne, T.; Thiebaut, B. WS2 nanoparticles anti-wear and friction reducing properties on rough surfaces in the presence of ZDDP additive. Tribol. Int. 2016, 102, 213–221. [Google Scholar] [CrossRef]
- Ku, B.C.; Han, Y.C.; Lee, J.E.; Lee, J.K.; Park, S.H.; Hwang, Y.J. Tribological effects of fullerene (C60) nanoparticles added in mineral lubricants according to its viscosity. Int. J. Precis. Eng. Manuf. 2010, 11, 607–611. [Google Scholar] [CrossRef]
- Bao, Y.Y.; Sun, J.L.; Kong, L.H. Tribological properties and lubricating mechanism of SiO2 nanoparticles in waterbased fluid. IOP Conf. Ser. Mater. Sci. Eng. 2017, 182, 12025. [Google Scholar] [CrossRef]
- Azman, N.F.; Syahrullail, S.; Sot, M.N.H.M. Investigation of tribological properties of CuO/palm oil nanolubricant using pin-on-disc tribotester. Green Mater. 2018, 6, 30–37. [Google Scholar] [CrossRef]
- Xie, H.; Wang, Y.; Wang, P.; Liu, S.; Ye, Q.; Liu, W. Poly (tannic acid) functionalized onion-like carbon nanoparticles derived from candle soot serving as potent lubricant additives. J. Mol. Liq. 2023, 379, 121697. [Google Scholar] [CrossRef]
- Kotia, A.; Ghosh, G.K.; Srivastava, I.; Deval, P.; Ghosh, S.K. Mechanism for improvement of friction wear by using Al2O3 and SiO2/Gear oil nanolubricants. J. Alloys Compd. 2019, 782, 592–599. [Google Scholar] [CrossRef]
- Dai, W.; Kheireddin, B.; Gao, H.; Liang, H. Roles of nanoparticles in oil lubrication. Tribol. Int. 2016, 102, 88–98. [Google Scholar] [CrossRef]
- Guo, J.; Peng, R.; Du, H.; Shen, Y.; Li, Y.; Li, J.; Dong, G. The Application of Nano-MoS2 Quantum Dots as Liquid Lubricant Additive for Tribological Behavior Improvement. Nanomaterials 2020, 10, 200. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Chen, X.; Li, P.; Wang, P.; Li, C.; Cao, B.; Luo, J.; Yang, S. Laser irradiation-induced laminated graphene/MoS2 composites with synergistically improved tribological properties. Nanotechnology 2018, 29, 265704. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Jiang, B.; He, J.; Xia, X.; Pan, F. Lubrication performance of MoS2 and SiO2 nanoparticles as lubricant additives in magnesium alloy-steel contacts. Tribol. Int. 2016, 93, 63–70. [Google Scholar] [CrossRef]
- Meng, Y.; Sun, J.; He, J.; Yang, F.; Wu, F. Interfacial interaction induced synergistic lubricating performance of MoS2 and SiO2 composite nanofluid. Colloids Surf. A Physicochem. Eng. Asp. 2021, 626, 126999. [Google Scholar] [CrossRef]
- Wang, G.; Yang, C.; Bai, Q.; Guan, K.; Shang, K.; Li, D.; Guo, S. Alkylamine-Grafted Molybdenum Disulfide Nanosheets for Enhanced Dispersion Stability and Lubricity Properties. Langmuir 2023, 39, 12476–12487. [Google Scholar] [CrossRef]
- Liu, T.; Qin, J.; Wang, J.; Li, J. On the tribological properties of RGO–MoS2 composites surface modified by oleic acid. Tribol. Lett. 2022, 70, 14. [Google Scholar] [CrossRef]
- Kong, L.; Sun, J.; Bao, Y. Preparation, characterization and tribological mechanism of nanofluids. RSC Adv. 2017, 7, 12599. [Google Scholar] [CrossRef]
- Hu, K.H.; Liu, M.; Wang, Q.J.; Xu, Y.F.; Schraube, S.; Hu, X.G. Tribological properties of molybdenum disulfide nanosheets by monolayer restacking process as additive in liquid paraffin. Tribol. Int. 2009, 42, 33–39. [Google Scholar] [CrossRef]
- Gong, K.; Wu, X.; Zhao, G.; Wang, X. Nanosized MoS2 deposited on graphene as lubricant additive in polyalkylene glycol for steel/steel contact at elevated temperature. Tribol. Int. 2017, 110, 1–7. [Google Scholar] [CrossRef]
- Wu, H.; Qin, L.; Dong, G.; Hua, M.; Yang, S.; Zhang, J. An investigation on the lubrication mechanism of MoS2 nano sheet in point contact: The manner of particle entering the contact area. Tribol. Int. 2017, 107, 48–55. [Google Scholar] [CrossRef]
- Wu, H.; Wang, L.; Dong, G. Origin of the tribofilm from MoS2 nanoparticle oil additives: Dependence of oil film thickness on particle aggregation in rolling point contact. Friction 2021, 9, 1436–1449. [Google Scholar] [CrossRef]
- Xu, D.; Wang, C.; Espejo, C.; Wang, J.; Neville, A.; Morina, A. Understanding the friction reduction mechanism based on molybdenum disulfide tribofilm formation and removal. Langmuir 2018, 34, 13523–13533. [Google Scholar] [CrossRef]
- Song, W.; Yan, J.; Ji, H. Tribological Study of the SOCNTs@MoS2 Composite as a Lubricant Additive: Synergistic Effect. Ind. Eng. Chem. Res. 2018, 57, 6878–6887. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, X.; Duan, F.; Meng, Y. An efficient lubrication approach to mitigate soot-induced wear: Synergistic repair effect of magnetic MoS2 composites and magnetic field. Wear 2022, 488–489, 204182. [Google Scholar] [CrossRef]
- Hu, K.; Cai, Y.; Hu, X.; Xu, Y. Synergistic lubrication of MoS2 particles with different morphologies in liquid paraffin. Ind. Lubr. Tribol. 2013, 65, 143–149. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, K.; Hu, E.; Guo, J.; Han, C.; Hu, X. Double hollow MoS2 nano-spheres: Synthesis, tribological properties, and functional conversion from lubrication to photocatalysis. Appl. Surf. Sci. 2017, 392, 1144–1152. [Google Scholar] [CrossRef]
- Cheng, L.; Hu, E.; Chao, X.; Zhu, R.; Hu, K.; Hu, X. MoS2/montmorillonite nanocomposite: Preparation, tribological properties and inner synergistic lubrication. Nano 2018, 13, 1850144. [Google Scholar] [CrossRef]
- Hu, K.; Miao, Y.; Lu, Z. Preparation and tribological performance of MoS2 nanoparticles supported on fly ash microparticles. Ind. Lubr. Tribol. 2023, 75, 51–59. [Google Scholar] [CrossRef]
- Yi, C.; Hu, C.; Shi, L.; Bai, M.; Li, Y.; Tang, D. Frictional properties of MoS2 on a multi-level rough wall under starved lubrication. Phys. Chem. Chem. Phys. 2023, 25, 14348–14358. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; Zhang, C. The synthesis of MoS2 particles with different morphologies for tribological application. Tribol. Int. 2017, 116, 285–294. [Google Scholar] [CrossRef]
- Zhang, Z.; Fan, X.; Ma, X.; Zhu, M. Synergistic lubrication mechanism of core/shell C@MoS2 particles as lubricant additives. Appl. Surf. Sci. 2023, 639, 158234. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Z.; Lin, Q.; Cao, Z.; Li, W.; Gong, J.; Wang, Y.; Hu, K.; Hu, X. MoS2 Nanomaterials as Lubricant Additives: A Review. Lubricants 2023, 11, 527. https://doi.org/10.3390/lubricants11120527
Lu Z, Lin Q, Cao Z, Li W, Gong J, Wang Y, Hu K, Hu X. MoS2 Nanomaterials as Lubricant Additives: A Review. Lubricants. 2023; 11(12):527. https://doi.org/10.3390/lubricants11120527
Chicago/Turabian StyleLu, Ziyan, Qingqing Lin, Zhaotao Cao, Wanyuan Li, Junjie Gong, Yan Wang, Kunhong Hu, and Xianguo Hu. 2023. "MoS2 Nanomaterials as Lubricant Additives: A Review" Lubricants 11, no. 12: 527. https://doi.org/10.3390/lubricants11120527
APA StyleLu, Z., Lin, Q., Cao, Z., Li, W., Gong, J., Wang, Y., Hu, K., & Hu, X. (2023). MoS2 Nanomaterials as Lubricant Additives: A Review. Lubricants, 11(12), 527. https://doi.org/10.3390/lubricants11120527





