Abstract
Graphene is a single atomic plane of sp2-bound carbon that has attracted considerable interest in various technologies. On the basis of its unique physical, mechanical, and chemical properties, graphene is a potentially strong candidate as a lubricant additive in its liquid-based form to reduce friction and protect surfaces from degrading. Furthermore, graphene on wear performance acts as a heat dissipation source for liquid lubricants. This review explores and addresses the fundamental mechanisms illuminating the exceptional tribological behaviours of graphene family materials and their limitations. Although graphene additives were reported to improve friction coefficients and wear properties, several challenges remain a hindrance, such as production costs, dispersion stability, and lack of information regarding graphene optimisation. Thus, this review can provide a standard methodological framework for graphene additives in improving tribological performance. Moreover, this review provides an up-to-date review of current tribological experiments based on ultrafine particles incorporated with graphene as an additive for lubricating liquids.
1. Introduction
Tribology is an interdisciplinary field focusing on friction, wear, and lubrication. Hence, the field investigates the interaction between moving surfaces and every facet of wear, lubrication, adhesion, abrasion, tribochemistry, and other related topics. In numerous industries, including those in the automobile sector, bearings, space, sports, cuisine, biomedicine, and renewable energy, tribology is also utilised as a solution. As frictional and wear losses consume energy that could be conserved, tribology becomes increasingly important to tackle this concern effectively. A small investment in research and development of better tribological practices could prevent between 1 and 1.4% of the gross domestic product from being spent [1]. In addition to its financial advantages, tribology can aid in protecting the environment by boosting energy efficiency and lowering CO2 emissions. By implementing cutting-edge tribology technologies, CO2 emissions from the transportation sector can also be significantly reduced, which is a significant portion produced by energy consumers. Therefore, significant societal issues can be resolved using the tribological study findings [2]. In terms of lubricated surfaces, tribology is found between journal and thrust bearings, cam mechanisms, gear teeth, and hydraulic systems. Figure 1 presents a selection of these tribological interfaces, which include examples of tribological interfaces spanning from the contact between mechanical devices to human joints. The study of lubricants contributes a significant portion of the field of tribology.
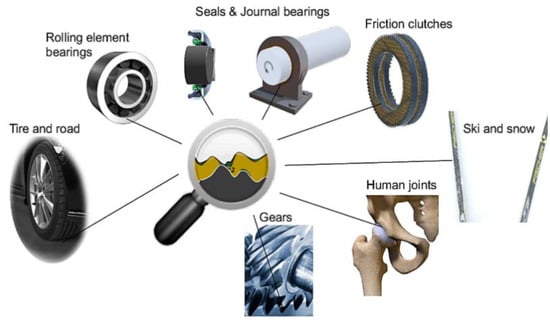
Figure 1.
Standard classical tribological interfaces. Reproduced with permission from [3].
A lubricant is a substance that effectively improves solid objects to move relative to one another by reducing wear and friction on interacting surfaces (see Figure 2). Additionally, automated systems often generate friction from sliding, rolling, or rotating contact interfaces. Hence, friction results in significant energy losses, part failures, and potentially fatal mechanical mishaps [4]. In addition, friction wastes at least 5% of the total wealth in the world by converting productive energy into worthless heat. To effectively reduce the negative impact, reducing friction between two surfaces can produce moving parts in machines and electromechanical devices with significant energy, resource, and maintenance savings [5,6,7]. Various equipment and devices are utilised daily in which these machines and devices have several moving parts that require proper lubricants. Hence, lubricants should be chemically and thermally stable [8], involatile, non-corrosive, and time durable. In order to comply with the criteria for environmental protection, they must also be environmentally friendly and biodegradable [9]. The primary purposes and benefits of lubricants can be summarised as follows:

Figure 2.
Schematic diagram representing the role of lubricants in reducing friction. The image on the left demonstrates two rough surfaces with high friction, creating resistance. The image on the right demonstrates how adding lubricant creates a thin film that produces more accessible sliding materials.
- Lowering the amount of loading and extending its service life by producing a lubricating layer at the contact between two components.
- Enhancing beneficial driving qualities such as reduced noise or friction.
- Radiating the generated heat outside to avoid overheating the bearings and degradation of lubrication. If the circulation lubrication method is used, the performance improves incredibly well.
- Lessening corrosion by limiting rust and the penetration of foreign materials.
- Reducing the amount of wear and tear on the surfaces by putting lubricants between the surfaces that rub against each other and avoiding metal/metal contacts.
- Lessening material deterioration and metal expansion brought on by frictional heat.
- Functioning as a coolant for metal due to its heat transfer medium.
- Decreasing wear and avoiding rough relative motion.
- Lowering the expense of maintenance.
- Lessening internal combustion engine power loss.
Despite the advantages of lubricant systems, the physical and chemical properties can highly influence the tribological lubricant’s performance. The three main categories of lubricants are grease, solid lubricants, and lubricating oils. A liquid lubricant, such as water or natural or synthetic oils, may reduce friction by preventing sliding between contact interfaces (metal-to-metal or metal-to-non-metal contacts). The lubricant’s performance is determined by the load on the contact, the sliding velocity, and the lubricant’s viscosity (higher viscosity indicates stronger lubricating film). Nonetheless, the film will break down in extreme load cases, and the surfaces will come into direct contact (boundary lubrication), which can cause increased friction and wear [10]. While oil and grease-based lubricants are easy to handle, they offer only moderate performance and can be contaminated by external dust [11]. Excessive oil-based lubricant may also cause combustion when operated under extreme temperatures. Conversely, solid lubricants, such as molybdenum disulphide, require careful application as a thin film but can wear out quickly and are sensitive to the environment [12]. Clearly, additives are necessary to augment lubricants’ physical and chemical properties to overcome the shortfalls presented here.
An excellent option to resolve this challenge involves the use of nanoparticle additives. These additives can avoid direct contact, lowering the friction coefficient and speeding up wear due to the rolling impact of nanoparticles on lubricated contact surfaces. Therefore, the use of nanoparticles produces higher effortless sliding and prevents metal-to-metal contact, which improves the tribological characteristics of the lubricating system [13]. Recently, nanoparticles were efficiently demonstrated in various fields [14,15,16,17] for their advanced features, including lubricant additives. The tribological performance of nanoparticles currently utilised as lubricant additives produces impressive results [18,19,20]. Due to their small size and unique microstructure, nanoparticles can easily create lubricated tribofilms on substrates by contacting the contact surfaces [4]. The most common particle-based lubricant additives are pure metals, metal oxides, metal sulphides, metal hydroxides, and metal salts. For the first time in the 1980s–1990s, Hisakado et al. discovered that Cu nanoparticles as lubricant additives demonstrated good tribological qualities in base oils [21]. Nevertheless, the use of lubricant as additive is fraught with difficulties. One of the most crucial disadvantages of particle additives is their dispersion stability. Good dispersion performance allows nanoparticles to enter the frictional contact zone more easily and reduces clogging and lubrication degradation caused by agglomeration [22]. Thus, several modification strategies should be examined to increase the dispersion stability of lubricant additives.
Using large quantities of additives increases the viscosity of the primary lubricant. As a result, when incorporating nano additives into essential lubricants, their physicochemical qualities must be kept within acceptable ranges. Furthermore, the high cost of nanomaterial synthesis is a considerable barrier. Hence, the nanomaterials manufacturing process must be adjusted to increase nano additives’ economic application [22]. Meanwhile, MoS2 is also hampered by its high density and nanoparticle aggregation. Although having improved dispersion, MoS2 has a limited load-bearing capability when used alone. Therefore, a significant quantity of MoS2 is generally required to minimise the coefficient of friction (CoF). This amount might offer a considerable obstacle to the matrix’s overall mechanical behaviour [23,24], whereby the difficulty lies in achieving very low friction while maintaining a low particle concentration.
Conventional lubricant additives, such as organic phosphates, organic sulphides, and organic metallic compounds, have good dispersing stabilities and tribological properties. In contrast, these additives have varying degrees of toxicity, releasing sulphated ash, phosphorous, and sulphur (SAPS), which can result in air pollution, such as acid rain and hazy weather, and chemical corrosion [25,26]. While some additives, including ionic liquids, offer exceptional tribological performance and are environmentally benign, their high cost prevents them from being widely used in industry [27,28]. As a result, it is critical to creating environmentally friendly and long-lasting lubricating nanomaterials. Nanocarbon-based materials, graphene, and graphene-like substances are considered future possibilities. The foundation of practical applications in lubrication systems is the stability of the additives, which is of great importance. Nonetheless, there are still challenges in improving the dispersion stability of nano lubricant additives, as physically treated nanoparticles are susceptible to reaggregation [29].
Since the most durable material is crucial as a lubricant-based material, it should be environmentally insensitive, easy to handle, and able to reach contact interfaces to reduce friction. Thus, this review investigated the exceptional graphene properties that are highly resistant to wear, regardless of test environments, with insensitivity towards environmental factors. As graphene was discovered in 2004, it has already amazed researchers globally with its unique properties [30,31]. Therefore, this review intends to highlight graphene’s unique properties as a superior lubricant additive in liquid-based settings to reduce wear and friction coefficient. Moreover, this review presents a collection of work from various research groups globally, highlighting the various applications of graphene as a lubricant additive to augment its chemical and physical properties. On the basis of this review, the development of graphene additives in lubricant technology can be accelerated.
2. Graphene and Carbon-Based Additives
Graphene exhibits remarkable and unparalleled qualities, making it highly desirable for tribological applications due to its exceptionally high mechanical strength, outstanding conductivity, low shear strength, and high surface area. The tribological properties of graphene are controlled by various techniques used for its synthesis and the presence of functional groups, such as residual oxygen functionalities, thickness and lateral dimensions of each sheet, number of atomic lamellae in a sheet, and structural flaws. Furthermore, the ease of surface functionalisation enhances the potential of graphene-based materials for aqueous lubrication. Such improvements are due to oxygen functionalities, excellent conductivity for dissipating heat, an ultra-low thickness that allows entrance into tribo-interfaces, a good affinity for forming protective tribo-thin films at contact interfaces, and low shear strength [32]. In addition, graphene provides excellent potential for use as an ultra-thin protective layer for many precision components owing to its greater strength [33].
Before the discovery of graphene, graphite was the most common form of carbon used for lubrication [34,35]. Graphite comprises multiple layers of graphene sheets and can easily take on a layered structure [35]. Nevertheless, the use of graphite as a lubricant has many shortcomings when used in a liquid-phase solution. Graphite cannot be dispersed evenly in a liquid, which causes a decrease in fluidity and lubrication performance [36]. Graphite also has difficulty entering the contact area and forming a continuous protective film, resulting in a lack of lubrication. Thus, graphite is not an ideal lubricant for liquid-phase solutions [37]. Graphene, on the other hand, is a single layer of carbon atoms arranged in a two-dimensional (2D) hexagonal lattice. Unlike graphite, the single-layer structure of graphene can be stabilised easily within the liquid phase by using surfactants [36].
The effects of few-layered graphene (FLG) could also contribute to a graphite-like friction reduction due to the sliding of graphene layers [38]. Saurin et al. [39] studied the effect of graphene structure by comparing two forms of commercial graphene nanomaterials, such as 1–2 layer and 1–10-layer graphene. In future works, they were used as additives in 1-octyl-3-methylimidazolim tetrafluoroborate in steel-epoxy resin and sapphire-steel contacts. The 1–2 layered graphene formed large agglomerates, resulting in abrasive wear. In contrast, the 1–10 layered graphene prevented wear by avoiding direct contact between asperities. Furthermore, compared to graphite, graphene-based additives are more resistant to oxidation and corrosion and possess superior thermal [40] and tribo-logical [38,41,42] properties. This section maps the advantages of graphene’s inherent chemical and physical properties to the desirable properties for a lubricant additive. Then, the synthesis techniques for graphene-based lubricant additives are discussed. Several researchers [43,44,45,46,47] demonstrated the superior chemical resistance of graphene. A study by Topsakal et al. [43] described a first principles analysis of the oxidation protection effect of graphene. The study revealed that the graphene coating demonstrated efficient protection from oxidation. A high energy barrier to the path of oxygen atoms’ path was observed, which could have penetrated from the top of graphene to the higher reactive regions located below. Meanwhile, Su et al. [44] reported that graphene films synthesised via graphene oxide (GO) laminate reduction produced a barrier capable of blocking aggressive chemicals, such as hydrofluoric acid. The high level of graphitisation of the laminates and minimal structural destruction during the reduction process are the reasons for their impressive barrier properties. Therefore, this research revealed the potential of thin protective coatings with high stability and inertness like that of graphene and graphite, with the potential for a wide range of applications. In a more practical approach, Chen et al. [45] demonstrated the corrosion resistance of graphene toward hydrogen peroxide. The graphene was produced via chemical vapour deposition (CVD) to protect metallic growth substrates, such as Ni and Cu. The experimental work agreed with the properties predicted by Topsakal et al.’s simulations [43]. In addition to graphene’s chemical inertness and barrier-like properties, multiple researchers [48,49,50] indicated that graphene has strong antibacterial properties. This makes graphene very appealing as an anti-fouling additive in various bio-lubricant forms. In particular, Li et al. [50] believed that the charge transfer properties of graphene are responsible for the antibacterial actions of graphene.
Besides the chemical properties, graphene was discovered to have superior tribological performance when compared to other materials [36,37,38,41,42,51]. The tribological performance is often attributed to the ability of graphene layers to slide smoothly against each other [52,53,54,55]. These results were confirmed by Xu et al. [56] in their molecular dynamics simulation work, which demonstrates the relationship between the number of layers in graphene and friction. The model provides a comprehensive explanation of the results, which showed remarkable stability across a wide range of temperatures, shear velocities, and pressures, predicting that the friction force approached zero as the graphene approaches two or three layers.
In terms of the application of graphene in lubricants, Pape et al. [38] reported experiments conducted with a rolling bearing test setup that evaluated the implementation of graphene platelets as a dry lubricant and grease additive in machinery components. Different thicknesses of the graphene platelets, ranging from 2 to 11–15 nm, were tested in angular contact ball-bearing surfaces. The results indicated that the graphene platelets formed a thin film, and the presence of the material in the grease improved the lubrication of the bearings. Meanwhile, Berman et al. [54] reported the effect of adding few-layer graphene (FLG) on a steel surface for wear reduction. The added FLG layers act as a two-dimensional nanomaterial that conforms to the contact interface, allowing for shearing and slowing down tribo-corrosion. Thus, it reduced wear between the sliding contacts by almost four orders of magnitude and friction coefficients by a factor of 6. The thermal conductivity of graphene also plays an essential role as a lubricant additive [40]. Graphene has excellent thermal properties, as shown through theoretical approaches [24,57,58] and experimental approaches [59,60]. Hence, adding graphene to lubricating media will improve its thermal conductivity [61,62,63,64]. Al-Janabi et al. [62,65] conducted a series of experiments to determine the stability, thermal conductivity, and rheological properties of graphene and multi-walled carbon nanotubes (MWCNT) in lubricant with different surfactants [66]. The study described that the highest overall value for thermal conductivity obtained was for the graphene sample, with a reported thermal conductivity of 0.145 W/mK. The researchers also discovered that the samples with added nanoparticles all recorded higher values for thermal conductivity when compared to samples without nanoparticles. Alternatively, Naddaf et al. [63] studied the thermal and electrical conductivity of nanofluids containing graphene nano-platelets (GNP) and MWCNT at different weight concentrations. The results exhibited increased conductivity for both thermal and electrical conductivity with increased weight concentrations of the carbon-based additives. In this case, the dispersion of the carbon-based nanoparticle plays a crucial role in improving the thermal conductivity of the fluid.
On the basis of the extensive research on graphene, it has been found to be a superior material for use as a lubricant additive due to its superior chemical and physical properties. Graphene-based lubricants resist oxidation and corrosion with superior thermal, tribological, and rheological properties. Additionally, graphene’s excellent thermal properties also make it an attractive option for improving the thermal conductivity of lubricants. Although graphene is a popular option, synthesising graphene-based lubricants is challenging, and further research is needed to understand the best synthesising methods. Nevertheless, graphene has the potential to revolutionise the lubricant industry, and its use as a lubricant additive can produce significant improvements in our current lubrication systems.
2.1. Synthesis of Graphene
Graphene was first isolated and characterised in 2004 by Geim et al. [30] at the University of Manchester. The authors used scotch tape to mechanically exfoliate graphene films from a small mesa of highly oriented pyrolytic graphite. This approach allowed for the preparation of FLG films up to 10 μm in size, with even thicker films that were up to 100 μm across and visible to the naked eye (d ≈ 3 nm). Since then, much research has been devoted to understanding the properties of graphene and developing methods to synthesise it [67,68,69]. The summary of the synthesis technique for graphene is displayed in Figure 3, in which the most common method was chemical vapour deposition (CVD) [70,71,72,73]. CVD is a process where a material is deposited on a substrate by decomposing a gas in a controlled environment. The resulting CVD graphene/graphitic films are often used as a protection layer for micro-electromechanical systems (MEMS)/ nano-electromechanical systems (NEMS) devices [74], gas barriers [75,76], and sensors [77]. In contrast, CVD graphene is usually deposited in situ and may not be suitable for manufacturing graphene for liquid-phase lubricants. The synthesis of liquid-phase graphene additives may be generalised into a few broad steps mechanical exfoliation of graphene oxide or other chemically modified graphene compounds [51,78], followed by chemical reduction or modification [79,80,81] or thermal reduction [82,83,84,85].
One of the most common routes for graphene production involves the mechanical exfoliation of graphite oxide into graphene oxide via a method known as Hummer’s method [80,81,83,84,85]. The Hummers’ method and most of its modified synthesis route involve the oxidation of graphite via a chemical reaction that introduces oxygen molecules to pure graphene layers which make up graphite powder. Usually, sulfuric acid is used as an oxidation agent. Then, potassium permanganate and sodium nitrate are added to act as catalysts for the reaction between the graphene and the concentrated sulfuric acid. The resultant graphene oxide (GO) layers have a weaker inter-layer attraction and thus are easily separated by mechanical agitation in the liquid. Liang et al. [78] introduced an in situ graphene exfoliation method for water-based lubricants. Their method relies on using a non-ionic surfactant (Triton-X) in the exfoliation process. The graphene was mechanically exfoliated via ultrasonic sonification after mixing with the non-ionic surfactant. Liang et al. reported an 80% enhancement in friction properties in water-based lubricants with graphene additives. Alternatively, Patel et al. [79] used off-the-shelf reduced graphene oxide (rGO) nano-platelets as additives to lubricants and reduction of wear and friction by up to 51.86%.
The chemical reduction of GO involves using a chemical agent to remove the oxygen atoms bonded to the GO after some form of mechanical exfoliation is applied to separate the GO layers. Reduced graphene oxide (rGO) is the product of this reduction process, which typically involves using hydrazine hydrate. The rGO has a higher degree of crystallinity than the GO and thus exhibits higher mechanical properties. The rGO has been used as an additive in lubricants that can reduce friction and wear [86]. Due to the toxicity of hydrazine hydrate, various alternatives were proposed [80,81]. Satheesh et al. [81] proposed an alternative reduction route utilising thiourea as a reducing agent. The resulting graphene flakes showed stable thermal performances. Silva et al. [80] summarised using green alternatives to hydrazine hydrate. Amongst the available alternatives, ascorbic acid was known as the most promising reducing agent due to its low toxicity, low cost, and non-carcinogenic properties. Therefore, the resulting graphene demonstrated good electrical and thermal properties.
An alternative method for the reduction of GO involves the use of thermal reduction. Thermal reduction is usually made at an elevated temperature to reduce the GO to graphene. On the contrary, in most cases, the rGO may still have some hydroxyl, carbonyl, and carboxylic acid groups attached to the surface [83,84,85]. Alam et al. reported a modified Hummers method for synthesising rGO with a thermal reduction [85]. The thermal stability of the rGO was the main factor to consider for thermal reduction, as the higher temperature needed to purge the hydroxyl, carbonyl, and carboxylic acid groups may also cause a mass loss in the carbon skeleton due to combustion. The rGO experiences less mass loss at lower temperatures, but the final product may have a higher density of impurities [83,85]. Oliveira et al. [83] suggested that a rapid rate of heating to high temperatures, as opposed to slow annealing, may be the key to creating high-quality rGO. The higher heating rate causes the oxygen-containing group on the graphene surface to detach rapidly, forming high-pressure vapour and pushing the layers apart.
Although the liquid phase exfoliation of graphene may be the best way to produce graphene for lubricant additives, researchers need to be aware of the fundamental limitations and impurities present in the final products [80,81,87]. Ambrosi et al. [87] presented evidence that natural and synthetic graphite contains substantial metallic impurities in the graphite oxide samples after oxidation and in chemically reduced graphene after reduction. Despite some of the impurities being removed during the oxidation process of the graphite, a substantial amount was still present, causing a major impact on the electrochemical properties of the rGO produced [87].
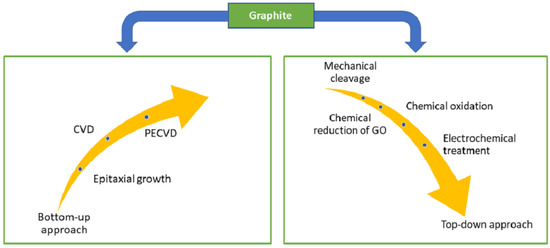
Figure 3.
Synthesis approaches of graphene from graphite. Reproduced with permission from [88].
2.2. Lubrication Mechanisms of Graphene
Graphene-based nanocomposites are used as lubricant additives to reduce friction and wear. The sliding pair wear resistance and friction reduction were greatly enhanced by investigating the tribological behaviour of base oil with graphene lubrication. In invading the rubbing interface, graphene successfully stopped tribo-pairs from making mechanical contact with one another. Thus, with the addition of graphene, base oil viscosity, and film thickness increased. The creation of protective films, interlayer shearing, and surface mending was lubrication mechanisms that helped to lower the friction coefficient and worn scar diameter [4,89,90,91].
Wu et al. [92] investigated graphene’s interlayer shearing and surface mending behaviour. Since the interlayer shearing stress of graphene was smaller than the shear force between sliding pairs, the friction coefficient of a tribo-pair decreased. The graphene interlayer shearing lubrication mechanism that occurred during the running-in stage is shown in Figure 4a, in which the surfaces of the tribo-pairs were covered with many dimples and peaks. Graphene was interlayer sheared during sliding because of the shear stress between tribo-pairs, which reduced the friction coefficient. For the heavy load, the larger graphene nanoplates were broken into smaller ones, which allowed the smaller graphene to reach the rubbing surface much more quickly. Graphene is also used to stop the rubbing surface from oxidising while sliding. After interlayer shearing and tearing during friction, graphene was absorbed into the worn surface to fix the deep dimples and severe scratches. The oil film thickness investigation proved that graphene accessed the contact area and significantly lowered the friction coefficient and wear. Hence, a protective tribofilm can be developed in a constant state of friction.
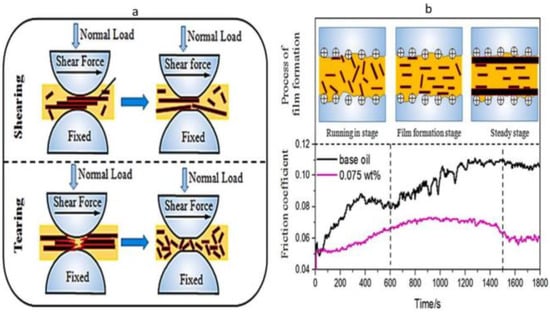
Figure 4.
(a) Graphene nanoparticle lubrication mechanism in interlayer shearing. (b) Protective film formation process for graphene lubrication. Reproduced with permission from [92].
Another experiment was conducted to observe the effect of film formation of graphene by Wu et al. [92]. Figure 4b describes the outcome of combining the friction coefficient curves and the protective film formation process. When tribo-pairs were lubricated with aviation lubricant containing 0.075 wt.% of graphene, the friction coefficient significantly decreased compared to the base oil. Three stages, namely, the running-in stage, the protective film formation stage, and the steady stage, were found concerning the increasing sliding time. The interlayer shearing operated when sliding pairs were lubricated by oil containing graphene in the running-in stage, as opposed to lubrication with base oil. Therefore, the graphene nanoplates penetrated the contact area and promiscuously dispersed into the lubricating fluid as the large graphene pieces were broken into little pieces. As the electric charge is combined with shear stress, graphene tends to be parallel to the rubbing surface during the formation of the protective coating. This stage enhanced friction reduction and wear resistance by using graphene to heal severe wear caused by jogging. After adhering graphene to the rubbing surface, the friction coefficient curves remain stable during the steady stage. Finally, protective coatings are created under the impact of average load and shear stress. As a result, sliding pairs’ tribological characteristics were greatly enhanced [92].
3. Graphene as Lubricant Additive in Liquid Form
3.1. Overview of Graphene Tribology at Ultrafine Particles to Reduce Friction and Protect Surfaces from Wear in Liquid Form
This section demonstrates the tribological properties of liquid-based graphene in reducing friction and wear. It also is noted that the tribological properties of the targeted materials highly depend on the type of lubricant and should be discretely studied. Table 1 summarises the various targeted applications lubricated using liquid-based graphene. On the basis of the literature, several studies involve the use of graphene-related compounds, such as multi-layer, few-layer, single-layer, reduced, and fluorinated graphene-based materials.

Table 1.
Summary of liquid-based graphene tribological properties on various applications.
A study reported by Zin et al. demonstrated the use of graphene nanostructures in poly-alkylene glycol oil (PAG) for compressors operating with CO2 refrigerant [94]. The 0.2 wt.% of graphene-added lubricant revealed the most efficient amount. Thus, the decreased contact area produced by the unusual form of nanohorns and rolling or sliding processes was linked to friction reduction. Several studies on improving polyalphaolefin (PAO)-based oils with graphene were also observed. Kong et al. reported utilising multi-layer graphene in polyalphaolefin-4 (PAO4) oil with a ball-on-plate tribotester [111]. By varying the thickness of the graphene layer, multi-layer graphene outperformed few-layer graphene, owing to the interlaminar structure. Furthermore, when smaller-size graphene was used instead of large graphene films, the friction and wear rate improved by 37% and 47%, respectively. Similarly, Guo et al. also studied multi-layer graphene in polyalphaolefin-2 (PAO2) oil [42]. In this study, 0.05 wt.% multi-layer graphene in PAO2 demonstrated a 78% friction coefficient reduction and wear scar diameter under a load of 120 N. In another PAO study, polyalphaolefin-9 (PAO9) oil was investigated by adding micro-graphene materials (see Figure 5). The study effectively showed that low concentrations of graphene improved friction coefficient and wear scare diameter by 17% and 14%, respectively [107].
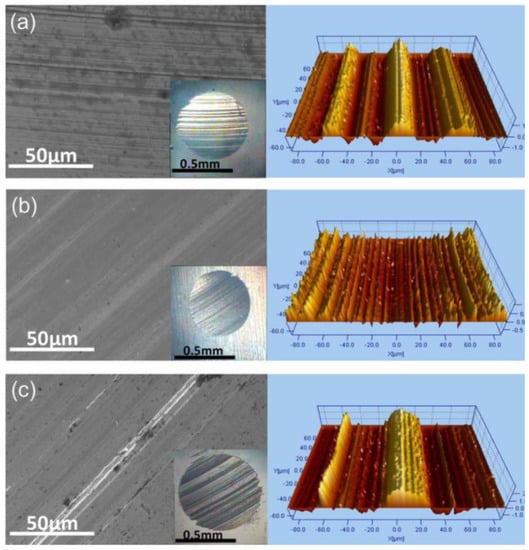
Figure 5.
SEM images of wear scars of (a) base oil, (b) 0.06 wt.% graphene, and (c) 5 wt.% graphene with their corresponding topographic images. Reproduced with permission from [107].
Several studies have reported using graphene in grease-based samples, such as titanium complex, lithium, and calcium-based greases [102,104,106]. Wang et al. explored the use of graphene in lithium-based oil, and the tribological performance was performed under point, line, and surface contacts [104]. All three contacts reported reduced average friction and wear loss, owing to the mechanical strength of graphene. Interestingly, the study demonstrates that graphene can act as a catalyst to stimulate Fe2O3 and Li2O tribofilm formation. Graphene nanosheets were also examined in calcium grease, in which the amount of graphene was varied [106]. An initial observation revealed that increasing the graphene content until 3 wt.% was sufficient to improve tribological properties, owing to the thin laminated structure. In addition, the authors successfully increased the non-seizure load from 150 to 240 N of the graphene-based calcium grease.
Less explored materials, such as fluorinated graphene, were performed on liquid paraffin [113]. This study reduced the friction coefficient and wear rate by 51.4% and 90.9%, respectively. The authors confirmed that multi-layer use was more efficient than single-layer fluorinated graphene. Furthermore, a stable and concrete tribofilm was successfully developed with self-lubricating behaviour. On the other hand, reduced graphene oxide, which is essentially known as graphene but with higher functional oxide groups, was also explored as a lubricant in silicon substrates [114]. Through a covalently assembled multiple-step route, the reduced graphene oxide was demonstrated to reduce the friction coefficient of silicon substrates down to 0.23.
Although the study of liquid-based graphene was initiated long ago, the number of reported works seems to be dwindling. Such observation was noticed as researchers moved from pure graphene to doped- or composite-based graphene materials (which are mentioned in the following section). These newer materials offer a limitless combination of graphene-based lubricants with other compounds, which reported higher significant improvements in reducing coefficient frictions and wear rates. Additionally, a few reports have mentioned graphene oxides, which may play a more efficient role than liquid-based graphene. A study by Xie et al. deduced that graphene oxide performs better than graphene in magnesium alloy and steel contacts [102]. On the basis of the improved friction coefficient (21.9 to 77.5%) and wear rate (13.5 to 90%) values, they tremendously improved when graphene was replaced with graphene oxide, owing to the excellent affinity between graphene oxide sheets and magnesium alloy surface, high water dispersibility, and superior wetting characteristic. Recently, Goralka et al. [115] focused on the use of graphene as a nanofluid additive for titanium alloy (Ti-6Al-4V) and cemented tungsten carbide (WC–Co) contacts as they were commonly used in machining operations [116]. They studied the effect of graphene concentration on the surface roughness of pin-on-disk tests made by Ti-6Al-4V and WC–Co. They found that the 0.10 wt.% of graphene concentration in distilled water demonstrated the lowest friction coefficient (0.29) and wear rate (4.8 × 10−4 mm3. Nm−1) than pure water. Thus, studies on pure graphene materials should be further studied and optimised to compete with other graphene-based materials, as the field still has so much to offer. Through this, the advancement of the tribological applications of pure graphene in various applications can be successfully achieved.
3.2. Overview on Modification of Graphene as a Lubricant Additive in Liquid Form
The tribological performance of graphene as a function lubricant depended on modifications made on the surface of the graphene. The graphene’s tribological properties can be made via several methods, including chemically modified graphene surfaces with fluorine, hydrogen, or oxygen. These chemical modifications on the surface of graphene have been widely investigated via experimental and theoretical works.
3.2.1. Functionalised Graphene as Lubricant Additive in Liquid Form
According to Wang et al. [117], the oxidised graphene increased the friction between two sliding graphene layers due to the presents of epoxied and hydroxyl groups. These domination groups created an electrostatic and produce non-stable hydrogen bond interactions. The formation of hydrogen bonds within oxidised graphene may also require an enormous energy barrier, resulting in high energy dissipation. For hydrogenated graphene sheets, the friction decreased significantly compared to pure graphene [118]. The lower friction value was explained by electrons accumulated between the carbon atom and the attached hydrogen atom, resulting in a decreased repulsive interaction of two contact sheets and, thus, reduced energy generation. Functionalised graphene with fluorine, hydrogen, or oxygen can increase friction. Furthermore, the adhesive properties for fluorinated graphene were decreased, while modification of hydrogenated and oxidised graphene remains almost unchanged. This tribological effect is due to the reduction of the Van der Waals on the contact area. Thus, these findings show that other factors might also appear, which could lead to the tribological behaviour of the graphene’s interfaces.
3.2.2. Polymer Graphene and Carbon-Based Additive for Liquid Lubricant
Friction and wear decrease the efficiency and lifetimes of mechanical devices. Nonetheless, only a few researchers have reported the fabrication of graphene and polymer to enhance the lubricating performance of a liquid-based substance. Wu et al. [119] reported the fabrication of polysaccharide-based hydrogels such as alginate-incorporated graphene to form a nanocomposite with outstanding strength and high tribological performance. They fabricated graphene-enhanced and in situ-formed alginate hybrid hydrogels with the addition of gluconic acid-δ-lactone (GDL) and calcium carbonate (CaCO3) as a substitute to control uniform gelation of alginates. The tribological performance for an ideal lubricant is performed via polymer–metal friction pairs using a ball-on-disc test mode. The introduction of graphene enhanced the mechanical performances of alginate hydrogels and endowed them with better bearing capacity and wear resistance. From the results, the friction coefficient and wear volume were decreased by 37% (0.106, represented by G1) and 50% (represented by G3), respectively, as compared to deionised water. The study also claimed that water molecules released within the hydrogels network formed a self-lubricating of graphene composite to reduce the friction coefficient. Additionally, the mechanism of graphene-reinforced alginate hydrogel lubricating and its superior tribological performance are depicted in Figure 6. On the contrary, most polymer-based incorporated graphene is used as a solid coating lubricant for reduced friction and wear at dry sliding conditions [120,121].
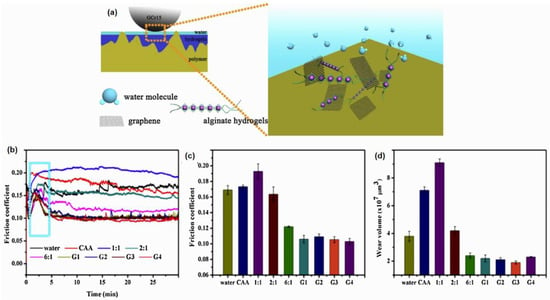
Figure 6.
(a) Schematic diagram of the lubrication mechanism, (b) friction coefficient curves, (c) average friction coefficient, and (d) wear volume of graphene-enhanced and in situ-formed alginate hydrogels. Reproduced with permission from [119].
3.2.3. Oleic-Modified Graphene-Composite-Based Additive for Liquid Lubricant
The decoration of graphene with functional groups such as hydroxyl, carboxyl, and epoxy has received attention among researchers due to the possibility of grafting with other molecules to enhance lubricating performance by synergistic effect. Although the significant modification improved functionalised graphene’s tribological properties, they still need to fulfil the demand for lifetime anti-wear. Thus, composite-based graphene material was constructed to obtain highly effective lubricating performance with an ideal anti-wear lifetime. For instance, oleic-modified graphene has been found to improve lube oil’s friction-reduction property, wear resistance, and load-bearing capacity due to its small diameter and extremely thin laminated structure [122]. Cheng et al. [122] synthesised oleic-diethanolamide-capped zinc-borate-coated graphene oxide composites by a liquid-phase-based ultrasonic-assisted stripping technique which allowed the composites to enter and deposited on the contact area to prevent direct contact with the rough surface of a four-ball wear machine.
3.2.4. TMD-Modified Graphene-Composite-Based Additive for Liquid Lubricant
Recently, several researchers reported the development of a hybrid graphene-based composite consisting of transition metals such as MoS2. The weak Van der Waals bonding between successive layers of transition metal dichalcogenides (TMDs) enables the growth of these heterostructures with minimal concern for lattice mismatch and the subsequent strain common in epitaxial growth [123]. In particular, the dispersion of a graphene/MoS2 composite improved the tribological properties of esterified bio-oil (EBO), thus reducing friction and wear behaviours of steel/steel pairs compared to the pristine graphene and MoS2 dispersion, respectively [124]. From the investigation, Xu et al. reported a synergistic lubricating effect of EBO consisting of 0.3 wt.% graphene and 0.2 wt.% MoS2. The ratios significantly reduced the friction coefficient and wear of the steel specimens. Furthermore, the existence of MoS2 in composites restricted the grinding of graphene into smaller sizes and prevented defective graphene, thus protecting the contact area during the rubbing process. A mutual influence of graphene and MoS2 during the frictional process that contributed to a significant decrease in the average friction coefficient and wear of the EBO with composite, up to 300 N, is illustrated in Figure 7.
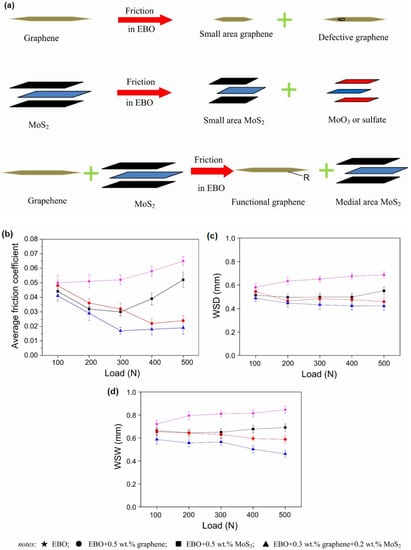
Figure 7.
(a) Illustration of the frictional process of (a) graphene, (b) MoS2, and (c) 0.3 wt.% graphene + 0.2 wt.% MoS2 and the effects of loads on (a) average friction coefficient and (b) wear scar diameter (WSD) and (d) wear scar width (WSW) of steel specimens lubricated by EBO with and without additives (rotational speed: 1000 rpm; testing time: 30 min). Reproduced with permission from [124].
Farsadi et al. [125] reported that the combination of functionalised reduced graphene oxide (FrGO) and MoS2 demonstrated better dispersibility over one month in oil than GO and the monocomponent of MoS2. Nonetheless, the MoS2-based FrGO composite exhibited a slight reduction in coefficient of friction (CoF) compared to the MoS2 graphene-based composite developed by Xu et al. [124]. The effects of various loading amounts of MoS2 and GO on lubricating performance were further investigated by Hou et al. [126]. Hou et al. prepared two feed ratios of GO and MoS2 precursors to design composites containing high and low amounts of MoS2 represented by RGO/MoS2-1 and RGO/MoS2-2, respectively. Later, the tribological behaviours were evaluated as an additive in paraffin oil operated by ball-on-disk mode. From the results, the unique combination structure of cocked MoS2 nanosheets of the RGO/MoS2-1 sample demonstrated enhanced dispersibility and stability. In addition, both composites demonstrated the lowest CoF of 0.09, with RGO/MoS2-1 composite exhibiting a higher wear resistance capability than other additives (see Figure 8).
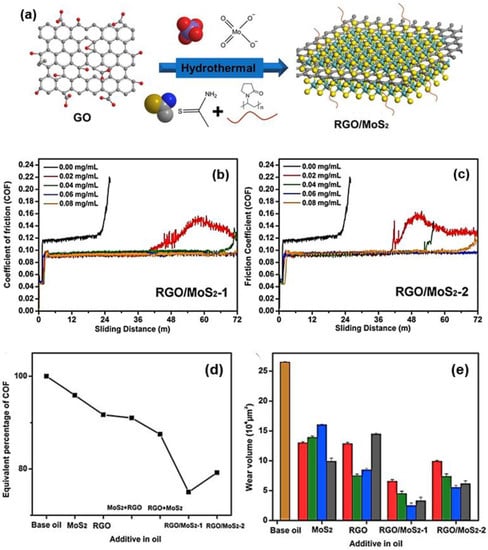
Figure 8.
(a) Schematic preparation of RGO/MoS2 heterostructure with a graphene layer sandwiched between two MoS2 layers, (b) CoF variation against sliding distance in paraffin oil formulated with RGO/MoS2-1 and (c) RGO/MoS2-2 heterostructures at various concentrations, (d) the percentage of CoF by setting the CoF of paraffin oil as a maximum value of 100%, and (e) wear volumes of wear tracks in base oil and oil formulated with different additives at various concentrations. Reproduced with permission from [126].
Despite the excellent tribological behaviour of graphene-based MoS2 composite materials, other concerns corresponding to the nanosized MoS2 have also been focused upon by several researchers. Wu et al. reported for the first time the preparation of MoS2 on graphene (MoS2/Gr), then later utilised it as an additive in perfluoropolyether (PFPE) base oil [127]. As a result, the carried-out investigation discovered that the MoS2/Gr nanocomposites demonstrated a highly stable dispersion in PFPE-based oil over two weeks. Subsequently, nanocomposite friction reduction and anti-wear properties are improved in comparison with pure PFPE oil or monocomponent-based oil. The tribological test used a steel/steel contact under a high vacuum, as they believed the improvement of lubricating performance is indicated by the effect of nanosized MoS2 on graphene dispersed in PFPE. The fact that nanosized MoS2 on graphene could improve tribological performance was agreed upon by Gong et al. [128], who developed nanosized MoS2/Gr. The materials were used as a lubricating oil additive in PAG dispersion for steel/steel contacts of an optimal SRV-IV oscillating friction and wear tester. The authors observed that the tribological performance of 0.5 wt.% MoS2/Gr in PAG oil performed the largest friction reduction and had better anti-wear properties compared to other additives (see Figure 9). Moreover, they summarised that nanosized MoS2 on graphene could enter the contact area between the sliding surfaces and then form thin, durable, and stable surface boundary layers. Thus, it can maintain low friction and wear.
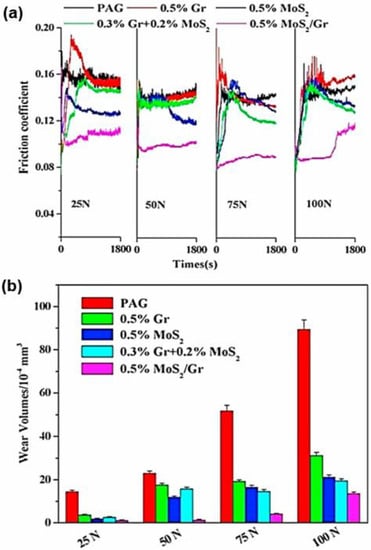
Figure 9.
(a) Friction coefficient and (b) wear volumes of the discs lubricated by pure PAG oil and the different additives in base oil formulated with 0.5 wt.% Gr, 0.5 wt.% MoS2, 0.3 wt.% Gr + 0.2 wt.% MoS2, and 0.5 wt.% MoS2/Gr at different loads. Reproduced with permission from [128].
3.2.5. Metal-Modified Graphene-Composite-Based Additive for Liquid Lubricant
Because of their nanoscale size and high specific surface area, several metallic nanoparticles such as TiO2, Fe2O3, CuO, and Ni have been widely used as anti-wear lubricant additives in lubricant systems [129,130]. Furthermore, these graphene-based nanomaterials are driven by the development of energy-efficient lubricant additives concerned with energy savings and environmental protection. For example, Jaswal et al. [130] described titanium-dioxide-reinforced boron and nitrogen co-doped reduced graphene oxide (TiO2-B-N-MRG) hybrid materials via a simple solution mixing technique. The tribological properties of the hybrid material were tested in neutral paraffin oil using a four-ball machine at an optimised additive concentration of 0.15% w/v. The addition of elements B and N doped into hybrid material resulted in the formation of a protective tribofilm in situ within the contact surfaces, reducing asperity–asperity adhesion.
The TiO2 nanoparticles deposited on the reduced graphene layers prevent those layers from agglomerating. Remarkably, the nanoparticle shape of TiO2, which is spherical, acts as a dual additive effect by providing it as a nano bearing between reduced graphene layers and tribopairs to smooth the sliding phenomenon during the rubbing process, thus reducing the CoF and wear rates under high-stress conditions. Recently, the dual additive effects of TiO2 graphene-based nanoparticles were reported by Garmroudi et al. [131] and Zhao et al. [132] without boron and nitrogen elements. On top of that, these composites revealed high stability in base oil and reduced average friction coefficient and wear rate at more significant percentages than pure oil.
Song et al. [133] fabricated α-Fe2O3 nanorods/GO composites for the first time via a simple hydrolysis method. The tribological performance was tested using a universal ball-plate microtribotester in paraffin oil. The α-Fe2O3 nanorods with 3–5 nm diameter and 15–30 nm length anchored on the GO nanosheets act as a spacer during the rubbing process, which can prevent wear on the contact surfaces. The sample also formed a continuous film to reduce shear stress, thus lowering the friction coefficient and WDS rate. Majeed et al. [134] reported on the suspension of Fe2O3 nanoparticles within exfoliated graphene, demonstrating similar outcomes. From other perspectives, a multilayer of graphene amazingly contributed greatly to a lower friction coefficient and wear rate, as reported by Zhou et al. [135]. They tested the tribological performance of multilayer graphene/Fe2O3 nanocomposite on a sliding titanium (TC11) alloy’s interface. A stable double-layer tribo-layer formed a protective layer on TC11 alloy, preventing it from wear. Furthermore, a weak Van der Waals interaction among the graphene layers facilitated smooth sliding motion and reduced its shear force [136].
As discussed above, the nanoparticle size of the additive plays a significant factor that can improve the lubricating performance due to its synergistic effect between graphene nanosheets and nanoparticles. Nevertheless, Meng et al. [137] reported that the presence of surfactant elements is also essential and can contribute to reducing the friction coefficient and wear rate. Therefore, they prepared copper nanoparticles deposited on graphene oxide nanosheets with the assistance of a supercritical carbon dioxide (Sc-Cu/GO) composite. The investigation found that the Sc-Cu/GO composite demonstrated better lubricating performance than Cu/GO composite without ScCO2. This improved performance is because the ScSO2 acts as an active platform to uniformly distribute the Cu nanoparticles on GO nanosheets. Thus, the composite could maximise its role during the sliding process in liquid paraffin oil. Besides preparing Cu nanoparticles, Meng and her colleagues also reported synthesising graphene oxide dotted with nickel nanoparticles with supercritical CO2 to reduce friction and wear on the contact steel balls [138]. The main contributing factor was also agreed by Song et al., where the authors prepared Cu nanoparticles decorated on the polydopamine-functionalised GO nanosheets (Cu/PDA/GO) via a simple wet chemical reduction route [139]. As a result, they concluded that the PDA serves as an active platform to uniformly immobilise the Cu nanoparticles anchored on the GO nanosheet’s surfaces, as depicted in Figure 10b,c. Moreover, introducing various functional groups within the composite increased the dispersibility of soybean oil. During the tribological test within soybean oil, the Cu/PDA/GO composites exhibited a reduction in friction and acted as a protective coating against wear and deformation of the sliding steel surfaces (see Figure 10d,e).
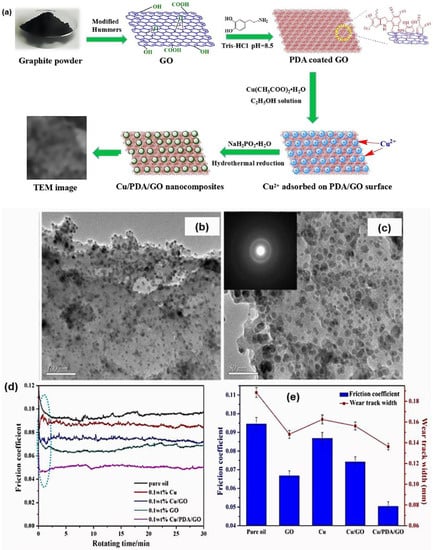
Figure 10.
(a) Schematic for forming Cu/PDA/GO composites, (b,c) TEM image of Cu/PDA/GO composites, (d) average friction coefficient, and (e) wear rate of steel disk in soybean oil formulated with different additives. Reproduced with permission from [139].
Verma et al. reported that the dual-metal-doped composite preparation consists of zinc oxide (ZnO) and magnesium-doped zinc oxide nanoparticles Zn0.88Mg0.12O (ZMO), decorated on the rGO nanosheets [140]. To study the tribological effect of doping metals on the lubricating performance, they investigated it by using a four-ball lubricant tester in paraffin oil. The solid synergistic interactions between rGO and nanoparticles contributed to a remarkable improvement in the triboactivity of nanocomposites compared to other additives, as demonstrated in Figure 11. Furthermore, the friction/wear-reducing efficiency and load-carrying capacity of different additives in paraffin oil were lies, as they followed the order: ZMO−rGO > ZnO−rGO > ZMO > ZnO > rGO > paraffin oil.
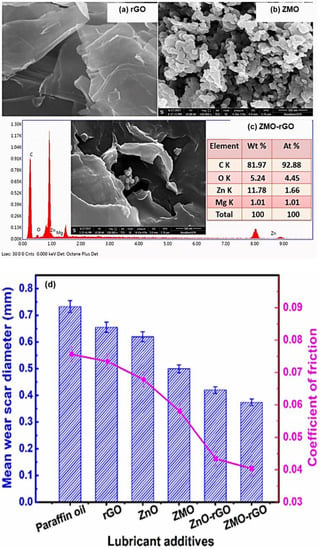
Figure 11.
HR-SEM images of (a) rGO, (b) ZMO, and (c) ZMO−rGO with the EDX spectrum, and (d) mean wear scar diameter and coefficient of friction in paraffin oil formulated with various nano additives (concentration of 0.125% w/v) at a given load of 392 N; sliding speed of 1200 rpm; a temperature of 75 °C; test period 60 min. Reproduced with permission from [140].
In another study, Zhang et al. [141] prepared a boehmite/graphene oxide nano-hybrid via a simple covalent bond method used as a lubricant-oil-based additive. The investigation as the lubricating oil additive was tested in a ball-on-disc testing machine and a four-ball machine. They discovered that the graphene composite that contained modified boehmite reduced friction efficiency and improved anti-wear ability compared to pure oil (VHVI8). Specifically, the friction coefficient (CoF), wear scar diameter (WSD), and wear rate were reduced by 14%, 28%, and 73%, respectively.
3.2.6. Noble-Metal-Modified Graphene-Composite-Based Additive for Liquid Lubricant
After subsequent investigation on the effect of metal-doping graphene-based composites, Meng et al. also fabricated noble metals such as silver (Ag) decorated on graphene nanocomposites to be employed as an additive in engine oil (10W40) at concentrations of 0.06–0.10 wt.% [142]. As a result, they found that the effect of adding silver has the ability to reduce the friction coefficient and wear by 30.4% and 27.4%, respectively. Later on, Wang et al. [4] prepared a unique structure of silver/graphene nanocomposite via a simple and effective one-step laser irradiation method. The fabricated L-Ag@rGO nanocomposite showed excellent lubricating performance on contact surfaces of a four-ball tribometer in a paraffin oil base. The L-Ag@rGO as a lubricant additive demonstrated superior friction reduction of about 40% and a wear rate of about 36% in comparison with pure Ag nanoparticles, GO sheets, Ag@GO, and several commercial additives. The size of Ag nanospheres also contributed to the main factor for the dispersibility of the composite that can remain for long-time stability in base oil (more than 60 days). Thus, the L-Ag@rGO composite offers a tremendous potential prospect as an additive material for the lubricating industry.
Graphene has emerged as a promising reinforcement material to improve wear resistance in composite materials. The tribological performance of hybrid graphene-based composites is summarised in Table 2. The significant previous research on composite-based graphene materials displayed a good dispersion in liquid-based form that allowed for excellent anti-friction and anti-wear ability compared with conventional graphene materials. Additionally, using composite as an additive only requires a small amount; thus, the fabrication of graphene-composite-based additives could still be attractive for industrial applications.

Table 2.
Overview of graphene-composite material tribological properties of widely used liquid lubricants.
4. Conclusion, Challenges, and Future Prospects
4.1. Conclusion
The improved mechanical, electrical, optical, and structural features of graphene have sparked a great deal of interest in basic research and a wide variety of practical applications. Graphene is a potential candidate for tribological applications, which aim to lessen friction and wear on engineering surfaces. These applications could be made possible by graphene’s weak Van der Waals interaction between its atomic-thick lamellae, excellent mechanical strength, remarkable thermal conductivity, and high surface area. In recent years, the utilisation of graphene, graphene that has been chemically modified, and graphene-based composites as additions to various lubricating media have grown significantly. Hence, this review outlined the most recent developments in graphene-based nanomaterials for lubricant additive applications, concentrating primarily on synthesis techniques, structure, lubrication mechanism, tribological performance assessment, and applications of graphene-based nanomaterial additives. Surprisingly, the dispersibility, stability, and tribological properties of other materials could be easily controlled and enhanced by mixing graphene. Graphene-based nanomaterials were dispersed in various lubricants to address various environmental conditions, which could broaden their scope of tribology application. The development of an additive lubricant based on the graphene family was investigated vigorously, and it promotes a suitable additive in liquid-based form. Graphene performed excellent tribologically for many lubricant systems as an additive lubricant due to its extraordinary physical and chemical properties.
4.2. Challenges
Although the family of graphene as an additive can play a vital role in lubrication and wear resistance performances, there are still some limitations that need to be addressed as follows:
- (1)
- For instance, dispersion stability is a significant concern for graphene family additives since it is related to instability in liquid-based lubrication systems.
- (2)
- The high-temperature-induced wear debris and material degradation can significantly impact the long-term stability of graphene family additives, which still needs to be investigated in further research.
- (3)
- The high cost of additives belonging to the graphene family for industrial applications is still a large challenge to resolve. It is imperative to develop an industrial-scale, cost-effective preparation procedure for synthesising additives belonging to the graphene family for practical applications [31].
- (4)
- Currently, there are no widely acknowledged standards for creating graphene family materials as additives. For example, the optimal parameters for a given application situation, such as particle size, layer number, type of particle, and concentration of functional groups, are yet unknown.
- (5)
- An in-depth assessment of the factors involved and advanced approaches will be necessary to discover low-cost graphene preparation and noble materials as additives. This assessment will drive the future design and deployment of graphene family compounds as additives.
- (6)
- Some of the regularly utilised organic and inorganic components (such as sodium dodecyl sulphate (SDS) [143] and MoS2 [144]) in graphene-based nanomaterials contain sulphur elements that can readily induce pollutant release. Consequently, developing “green” graphene-based nanoparticles as efficient lubricant additives without compromising friction and wear qualities is highly desired.
- (7)
- The dispersion stability of graphene-based nanostructures in different liquid lubricants remains a mystery. Organic modifiers are prone to degradation, owing to friction-induced heat during the rubbing process, which results in the re-aggregation of graphene nanosheets in the lubricants. Therefore, long-term dispersibility has been a significant concern for industrial lubricant uses.
By overcoming the challenges mentioned above, graphene can be potentially used as additives in the commercialisation of lubrication and wear resistance technology.
4.3. Future Prospects
Once these challenges are resolved, graphene family materials might serve as outstanding lubrication options in various and vast areas, saving enormous amounts of energy and reducing environmental pollution caused by friction. By resolving the problems in the upcoming few years, it is anticipated that considerable breakthroughs in graphene-based nanomaterials with desired characteristics will be possible. Before preparation, the type and structure of the functionalised molecules and the physical structure of graphene-based nanocomposites determine the performance of graphene nanocomposites due to the various tribological states in various application scenarios. Therefore, it is necessary to model and calculate their chemical characteristics. Alternatively, creating graphene-based nanocomposites with tailored characteristics, controlled morphologies, and optimised structures is simpler. Moreover, it encourages using industrial lubricating additives and graphene in the micro-nano area. Developing a novel method for creating graphene nanocomposites with precisely regulated architectures is crucial. Graphene-based nanocomposites will shine in micro-nano friction systems, bio-tribology, and industrial lubrication by continually refining preparation processes and techniques, optimising their anti-friction with anti-wear and lubrication mechanisms.
Author Contributions
Conceptualisation, A.R.M.; resources, A.R.M. and G.S.H.T.; data curation, A.R.M., G.S.H.T., M.S., T.Y.L., and A.H.; writing—original draft preparation, A.R.M., G.S.H.T., M.S., T.Y.L., and A.H.; writing—review and editing, A.R.M., G.S.H.T., M.S., T.Y.L., and A.H.; visualisation, A.R.M. and G.S.H.T.; supervision, A.R.M., K.-Y.C., and M.R.J.; project administration, A.R.M.; funding acquisition, A.R.M., K.-Y.C., and M.R.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Ministry of Higher Education (MoHE) Malaysia (PR003-2022) and University of Malaya for providing financial support through the Research University grant (ST045-2021).
Acknowledgments
We acknowledge the Ministry of Higher Education (MoHE) Malaysia (PR003-2022) and University of Malaya for providing financial support through the Research University grant (ST045-2021).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jost, H.P. Tribology Micro & Macro Economics: A Road to Economic Savings. Tribol. Lubr. Technol. 2005, 61, 18. [Google Scholar]
- Pranay Kumar Parsi Introduction to Tribology. Available online: https://www.tribonet.org/wiki/introduction-to-tribology/ (accessed on 25 November 2022).
- Almqvist, A.; Ràfols, F.P. Scientific Computing with Applications in Tribology: A Course Compendium. 2022. Available online: https://www.diva-portal.org/smash/record.jsf?pid=diva2%3A1289574&dswid=9285 (accessed on 22 November 2022).
- Wang, L.; Gong, P.; Li, W.; Luo, T.; Cao, B. Mono-Dispersed Ag/Graphene Nanocomposite as Lubricant Additive to Reduce Friction and Wear. Tribol. Int. 2020, 146, 106228. [Google Scholar] [CrossRef]
- Urbakh, M. Towards Macroscale Superlubricity. Nat. Nanotechnol. 2013, 8, 893–894. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.I. Tribology and Energy Efficiency: From Molecules to Lubricated Contacts to Complete Machines. Faraday Discuss. 2012, 156, 361–382. [Google Scholar] [CrossRef]
- Boyde, S. Green Lubricants. Environmental Benefits and Impacts of Lubrication. Green Chem. 2002, 4, 293–307. [Google Scholar] [CrossRef]
- Christensen, G.; Younes, H.; Hong, G.; Lou, D.; Hong, H.; Widener, C.; Bailey, C.; Hrabe, R. Hydrogen Bonding Enhanced Thermally Conductive Carbon Nano Grease. Synth. Met. 2020, 259, 116213. [Google Scholar] [CrossRef]
- Fan, M.; Yang, D.; Wang, X.; Liu, W.; Fu, H. Doss–Based QAILs: As Both Neat Lubricants and Lubricant Additives with Excellent Tribological Properties and Good Detergency. Ind. Eng. Chem. Res. 2014, 53, 17952–17960. [Google Scholar] [CrossRef]
- Minami, I. Ionic Liquids in Tribology. Molecules 2009, 14, 2286–2305. [Google Scholar] [CrossRef] [PubMed]
- Jarfors, A.E.W.; Castagne, S.J.; Danno, A.; Zhang, X. Tool Wear and Life Span Variations in Cold Forming Operations and Their Implications in Microforming. Technologies 2016, 5, 3. [Google Scholar] [CrossRef]
- Erdemir, A. Solid Lubricants and Self-Lubricating Films. Mod. Tribol. Handb. 2001, 2, 787–818. [Google Scholar]
- Ahmed, E.; Nabhan, A.; Ghazaly, N.M.; Abd El Jaber, G.T. Tribological Behavior of Adding Nano Oxides Materials to Lithium Grease: A Review. Am. J. Nanomater. 2020, 8, 1–9. [Google Scholar]
- Hashem, A.; Marlinda, A.R.; Hossain, M.A.; Al Mamun, M.; Shalauddin, M.; Simarani, K.; Johan, M.R. A Unique Oligonucleotide Probe Hybrid on Graphene Decorated Gold Nanoparticles Modified Screen-Printed Carbon Electrode for Pork Meat Adulteration. Electrocatalysis 2022, 14, 1–16. [Google Scholar] [CrossRef]
- Hashem, A.; Hossain, M.A.M.; Al Mamun, M.; Simarani, K.; Johan, M.R. Nanomaterials Based Electrochemical Nucleic Acid Biosensors for Environmental Monitoring: A Review. Appl. Surf. Sci. Adv. 2021, 4, 100064. [Google Scholar] [CrossRef]
- Sagadevan, S.; Marlinda, A.R.; Johan, M.R.; Umar, A.; Fouad, H.; Alothman, O.Y.; Khaled, U.; Akhtar, M.S.; Shahid, M.M. Reduced Graphene/Nanostructured Cobalt Oxide Nanocomposite for Enhanced Electrochemical Performance of Supercapacitor Applications. J. Colloid Interface Sci. 2020, 558, 68–77. [Google Scholar] [CrossRef]
- Marlinda, A.R.; Sagadevan, S.; Yusoff, N.; Pandikumar, A.; Huang, N.M.; Akbarzadeh, O.; Johan, M.R. Gold Nanorods-Coated Reduced Graphene Oxide as a Modified Electrode for the Electrochemical Sensory Detection of NADH. J. Alloys Compd. 2020, 847, 156552. [Google Scholar] [CrossRef]
- Song, X.; Qiu, Z.; Yang, X.; Gong, H.; Zheng, S.; Cao, B.; Wang, H.; Möhwald, H.; Shchukin, D. Submicron-Lubricant Based on Crystallized Fe3O4 Spheres for Enhanced Tribology Performance. Chem. Mater. 2014, 26, 5113–5119. [Google Scholar] [CrossRef]
- Tao, C.; Wang, B.; Barber, G.C.; Schall, J.D.; Lan, H. Tribological Behaviour of SnO2 Nanoparticles as an Oil Additive on Brass. Lubr. Sci. 2018, 30, 247–255. [Google Scholar] [CrossRef]
- Younes, H.; Hong, H.; Peterson, G.P. A Novel Approach to Fabricate Carbon Nanomaterials–Nanoparticle Solids through Aqueous Solutions and Their Applications. Nanomanufacturing Metrol. 2021, 4, 226–236. [Google Scholar] [CrossRef]
- Hisakado, T.; Tsukizoe, T.; Yoshikawa, H. Lubrication Mechanism of Solid Lubricants in Oils. J. Lubr. Technol. 1983, 105, 245–252. [Google Scholar] [CrossRef]
- Liu, W.; Qiao, X.; Liu, S.; Chen, P. A Review of Nanomaterials with Different Dimensions as Lubricant Additives. Nanomaterials 2022, 12, 3780. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Liu, S. 2D Nanomaterials as Lubricant Additive: A Review. Mater. Des. 2017, 135, 319–332. [Google Scholar] [CrossRef]
- Senatore, A.; Hong, H.; D’Urso, V.; Younes, H. Tribological Behavior of Novel CNTs-Based Lubricant Grease in Steady-State and Fretting Sliding Conditions. Lubricants 2021, 9, 107. [Google Scholar] [CrossRef]
- Spikes, H. Low-and Zero-sulphated Ash, Phosphorus and Sulphur Anti-wear Additives for Engine Oils. Lubr. Sci. 2008, 20, 103–136. [Google Scholar] [CrossRef]
- Hasan, M.S.; Kordijazi, A.; Rohatgi, P.K.; Nosonovsky, M. Machine Learning Models of the Transition from Solid to Liquid Lubricated Friction and Wear in Aluminum-Graphite Composites. Tribol. Int. 2022, 165, 107326. [Google Scholar] [CrossRef]
- Huang, G.; Yu, Q.; Ma, Z.; Cai, M.; Zhou, F.; Liu, W. Oil-Soluble Ionic Liquids as Antiwear and Extreme Pressure Additives in Poly-α-Olefin for Steel/Steel Contacts. Friction 2019, 7, 18–31. [Google Scholar] [CrossRef]
- Jiang, C.; Li, W.; Nian, J.; Lou, W.; Wang, X. Tribological Evaluation of Environmentally Friendly Ionic Liquids Derived from Renewable Biomaterials. Friction 2018, 6, 208–218. [Google Scholar] [CrossRef]
- Zhao, J.; Huang, Y.; He, Y.; Shi, Y. Nanolubricant Additives: A Review. Friction 2021, 9, 891–917. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, S.; Shi, Q.; Ge, X.; Wang, W. Graphene-Family Lubricant Additives: Recent Developments and Future Perspectives. Lubricants 2022, 10, 215. [Google Scholar] [CrossRef]
- Chouhan, A.; Kumari, S.; Sarkar, T.K.; Rawat, S.S.; Khatri, O.P. Graphene-Based Aqueous Lubricants: Dispersion Stability to the Enhancement of Tribological Properties. ACS Appl. Mater. Interfaces 2020, 12, 51785–51796. [Google Scholar] [CrossRef]
- Penkov, O.; Kim, H.-J.; Kim, H.-J.; Kim, D.-E. Tribology of Graphene: A Review. Int. J. Precis. Eng. Manuf. 2014, 15, 577–585. [Google Scholar] [CrossRef]
- Al Faruque, M.A.; Syduzzaman, M.; Sarkar, J.; Bilisik, K.; Naebe, M. A Review on the Production Methods and Applications of Graphene-Based Materials. Nanomaterials 2021, 11, 2414. [Google Scholar] [CrossRef] [PubMed]
- Hansora, D.P.; Shimpi, N.G.; Mishra, S. Graphite to Graphene via Graphene Oxide: An Overview on Synthesis, Properties, and Applications. Jom 2015, 67, 2855–2868. [Google Scholar] [CrossRef]
- Avilés, M.-D.; Saurín, N.; Sanes, J.; Carrión, F.-J.; Bermúdez, M.-D. Ionanocarbon Lubricants. The Combination of Ionic Liquids and Carbon Nanophases in Tribology. Lubricants 2017, 5, 14. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, M.; Li, X.; Jin, L.; Su, G.; Mo, Y.; Li, L.; Zhu, H.; Tian, Y. Research Progress in Application of 2D Materials in Liquid-Phase Lubrication System. Materials 2018, 11, 1314. [Google Scholar] [CrossRef]
- Pape, F.; Poll, G. Investigations on Graphene Platelets as Dry Lubricant and as Grease Additive for Sliding Contacts and Rolling Bearing Application. Lubricants 2019, 8, 3. [Google Scholar] [CrossRef]
- Saurín, N.; Sanes, J.; Bermúdez, M.-D. New Graphene/Ionic Liquid Nanolubricants. Mater. Today Proc. 2016, 3, S227–S232. [Google Scholar] [CrossRef]
- Renteria, J.D.; Nika, D.L.; Balandin, A.A. Graphene Thermal Properties: Applications in Thermal Management and Energy Storage. Appl. Sci. 2014, 4, 525–547. [Google Scholar] [CrossRef]
- Garcia, I.; Guerra, S.; de Damborenea, J.; Conde, A. Reduction of the Coefficient of Friction of Steel-Steel Tribological Contacts by Novel Graphene-Deep Eutectic Solvents (DESs) Lubricants. Lubricants 2019, 7, 37. [Google Scholar] [CrossRef]
- Guo, Y.-B.; Zhang, S.-W. The Tribological Properties of Multi-Layered Graphene as Additives of PAO2 Oil in Steel–Steel Contacts. Lubricants 2016, 4, 30. [Google Scholar] [CrossRef]
- Topsakal, M.; Şahin, H.; Ciraci, S. Graphene Coatings: An Efficient Protection from Oxidation. Phys. Rev. B 2012, 85, 155445. [Google Scholar] [CrossRef]
- Su, Y.; Kravets, V.G.; Wong, S.L.; Waters, J.; Geim, A.K.; Nair, R.R. Impermeable Barrier Films and Protective Coatings Based on Reduced Graphene Oxide. Nat. Commun. 2014, 5, 4843. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Brown, L.; Levendorf, M.; Cai, W.; Ju, S.-Y.; Edgeworth, J.; Li, X.; Magnuson, C.W.; Velamakanni, A.; Piner, R.D. Oxidation Resistance of Graphene-Coated Cu and Cu/Ni Alloy. ACS Nano 2011, 5, 1321–1327. [Google Scholar] [CrossRef] [PubMed]
- Nine, M.J.; Cole, M.A.; Tran, D.N.H.; Losic, D. Graphene: A Multipurpose Material for Protective Coatings. J. Mater. Chem. A 2015, 3, 12580–12602. [Google Scholar] [CrossRef]
- An’amt, M.N.; Yusoff, N.; Sagadevan, S.; Wahab, Y.A.; Johan, M.R. Recent Progress in Nitrates and Nitrites Sensor with Graphene-Based Nanocomposites as Electrocatalysts. Trends Environ. Anal. Chem. 2022, 34, e00162. [Google Scholar]
- Chen, J.; Peng, H.; Wang, X.; Shao, F.; Yuan, Z.; Han, H. Graphene Oxide Exhibits Broad-Spectrum Antimicrobial Activity against Bacterial Phytopathogens and Fungal Conidia by Intertwining and Membrane Perturbation. Nanoscale 2014, 6, 1879–1889. [Google Scholar] [CrossRef]
- Lu, B.; Li, T.; Zhao, H.; Li, X.; Gao, C.; Zhang, S.; Xie, E. Graphene-Based Composite Materials Beneficial to Wound Healing. Nanoscale 2012, 4, 2978–2982. [Google Scholar] [CrossRef]
- Li, J.; Wang, G.; Zhu, H.; Zhang, M.; Zheng, X.; Di, Z.; Liu, X.; Wang, X. Antibacterial Activity of Large-Area Monolayer Graphene Film Manipulated by Charge Transfer. Sci. Rep. 2014, 4, 4359. [Google Scholar] [CrossRef]
- Xu, Y.; Cao, H.; Xue, Y.; Li, B.; Cai, W. Liquid-Phase Exfoliation of Graphene: An Overview on Exfoliation Media, Techniques, and Challenges. Nanomaterials 2018, 8, 942. [Google Scholar] [CrossRef]
- Geim, A.K.; Grigorieva, I. V Van Der Waals Heterostructures. Nature 2013, 499, 419–425. [Google Scholar] [CrossRef]
- Chen, X.; Li, J. Superlubricity of Carbon Nanostructures. Carbon 2020, 158, 1–23. [Google Scholar] [CrossRef]
- Berman, D.; Erdemir, A.; Sumant, A. V Graphene: A New Emerging Lubricant. Mater. Today 2014, 17, 31–42. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Y.; Wang, Y.; Mao, J.; He, Y.; Luo, J. Mild Thermal Reduction of Graphene Oxide as a Lubrication Additive for Friction and Wear Reduction. RSC Adv. 2017, 7, 1766–1770. [Google Scholar] [CrossRef]
- Xu, L.; Ma, T.-B.; Hu, Y.-Z.; Wang, H. Vanishing Stick–Slip Friction in Few-Layer Graphenes: The Thickness Effect. Nanotechnology 2011, 22, 285708. [Google Scholar] [CrossRef]
- Goyal, V.; Balandin, A.A. Thermal Properties of the Hybrid Graphene-Metal Nano-Micro-Composites: Applications in Thermal Interface Materials. Appl. Phys. Lett. 2012, 100, 73113. [Google Scholar] [CrossRef]
- Zhong, W.-R.; Zhang, M.-P.; Ai, B.-Q.; Zheng, D.-Q. Chirality and Thickness-Dependent Thermal Conductivity of Few-Layer Graphene: A Molecular Dynamics Study. Appl. Phys. Lett. 2011, 98, 113107. [Google Scholar] [CrossRef]
- Ghosh, S.; Bao, W.; Nika, D.L.; Subrina, S.; Pokatilov, E.P.; Lau, C.N.; Balandin, A.A. Dimensional Crossover of Thermal Transport in Few-Layer Graphene. Nat. Mater. 2010, 9, 555–558. [Google Scholar] [CrossRef]
- Nika, D.L.; Balandin, A.A. Thermal Transport in Graphene, Few-Layer Graphene and Graphene Nanoribbons. Therm. Transp. low Dimens. 2016, 339–363. [Google Scholar] [CrossRef]
- Sarafraz, M.M.; Safaei, M.R.; Tian, Z.; Goodarzi, M.; Bandarra Filho, E.P.; Arjomandi, M. Thermal Assessment of Nano-Particulate Graphene-Water/Ethylene Glycol (WEG 60: 40) Nano-Suspension in a Compact Heat Exchanger. Energies 2019, 12, 1929. [Google Scholar] [CrossRef]
- Al-Janabi, A.S.; Hussin, M.; Abdullah, M.Z. Stability, Thermal Conductivity and Rheological Properties of Graphene and MWCNT in Nanolubricant Using Additive Surfactants. Case Stud. Therm. Eng. 2021, 28, 101607. [Google Scholar] [CrossRef]
- Naddaf, A.; Heris, S.Z. Experimental Study on Thermal Conductivity and Electrical Conductivity of Diesel Oil-Based Nanofluids of Graphene Nanoplatelets and Carbon Nanotubes. Int. Commun. Heat Mass Transf. 2018, 95, 116–122. [Google Scholar] [CrossRef]
- Zhao, J.; Gao, T.; Li, Y.; He, Y.; Shi, Y. Two-Dimensional (2D) Graphene Nanosheets as Advanced Lubricant Additives: A Critical Review and Prospect. Mater. Today Commun. 2021, 29, 102755. [Google Scholar] [CrossRef]
- Al-Janabi, A.S.; Hussin, M. Stability and thermal conductivity of graphene in polyester nanolubricant. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2020; Volume 2267, p. 20066. [Google Scholar]
- Cao, H.-Y.; Guo, Z.-X.; Xiang, H.; Gong, X.-G. Layer and Size Dependence of Thermal Conductivity in Multilayer Graphene Nanoribbons. Phys. Lett. A 2012, 376, 525–528. [Google Scholar] [CrossRef]
- Bhuyan, M.; Alam, S.; Uddin, M.; Islam, M.; Bipasha, F.A.; Hossain, S.S. Synthesis of Graphene. Int. Nano Lett. 2016, 6, 65–83. [Google Scholar] [CrossRef]
- Lin, L.; Peng, H.; Liu, Z. Synthesis Challenges for Graphene Industry. Nat. Mater. 2019, 18, 520–524. [Google Scholar] [CrossRef] [PubMed]
- Whitener, K.E., Jr.; Sheehan, P.E. Graphene Synthesis. Diam. Relat. Mater. 2014, 46, 25–34. [Google Scholar] [CrossRef]
- Saeed, M.; Alshammari, Y.; Majeed, S.A.; Al-Nasrallah, E. Chemical Vapour Deposition of Graphene—Synthesis, Characterisation, and Applications: A Review. Molecules 2020, 25, 3856. [Google Scholar] [CrossRef] [PubMed]
- Deng, B.; Liu, Z.; Peng, H. Toward Mass Production of CVD Graphene Films. Adv. Mater. 2019, 31, 1800996. [Google Scholar] [CrossRef]
- Deokar, G.; Avila, J.; Razado-Colambo, I.; Codron, J.-L.; Boyaval, C.; Galopin, E.; Asensio, M.-C.; Vignaud, D. Towards High Quality CVD Graphene Growth and Transfer. Carbon 2015, 89, 82–92. [Google Scholar] [CrossRef]
- Kataria, S.; Wagner, S.; Ruhkopf, J.; Gahoi, A.; Pandey, H.; Bornemann, R.; Vaziri, S.; Smith, A.D.; Ostling, M.; Lemme, M.C. Chemical Vapor Deposited Graphene: From Synthesis to Applications. Phys. Status Solidi 2014, 211, 2439–2449. [Google Scholar] [CrossRef]
- Rana, S.; Reynolds, J.D.; Ling, T.Y.; Shamsudin, M.S.; Pu, S.H.; Chong, H.M.H.; Pamunuwa, D. Nano-Crystalline Graphite for Reliability Improvement in MEM Relay Contacts. Carbon 2018, 133, 193–199. [Google Scholar] [CrossRef]
- Fishlock, S.J.; Pu, S.H.; Bhattacharya, G.; Han, Y.; McLaughlin, J.; McBride, J.W.; Chong, H.M.H.; O’Shea, S.J. Micromachined Nanocrystalline Graphite Membranes for Gas Separation. Carbon 2018, 138, 125–133. [Google Scholar] [CrossRef]
- Seo, T.H.; Lee, S.; Cho, H.; Chandramohan, S.; Suh, E.-K.; Lee, H.S.; Bae, S.K.; Kim, S.M.; Park, M.; Lee, J.K. Tailored CVD Graphene Coating as a Transparent and Flexible Gas Barrier. Sci. Rep. 2016, 6, 24143. [Google Scholar] [CrossRef]
- Ling, T.Y.; Pu, S.H.; Fishlock, S.J.; Han, Y.; Reynolds, J.D.; McBride, J.W.; Chong, H.M.H. Sensing Performance of Nanocrystalline Graphite-Based Humidity Sensors. IEEE Sens. J. 2019, 19, 5421–5428. [Google Scholar] [CrossRef]
- Liang, S.; Shen, Z.; Yi, M.; Liu, L.; Zhang, X.; Ma, S. In-Situ Exfoliated Graphene for High-Performance Water-Based Lubricants. Carbon 2016, 96, 1181–1190. [Google Scholar] [CrossRef]
- Patel, J.; Kiani, A. Effects of Reduced Graphene Oxide (RGO) at Different Concentrations on Tribological Properties of Liquid Base Lubricants. Lubricants 2019, 7, 11. [Google Scholar] [CrossRef]
- De Silva, K.K.H.; Huang, H.-H.; Joshi, R.K.; Yoshimura, M. Chemical Reduction of Graphene Oxide Using Green Reductants. Carbon 2017, 119, 190–199. [Google Scholar] [CrossRef]
- Satheesh, K.; Jayavel, R. Synthesis and Electrochemical Properties of Reduced Graphene Oxide via Chemical Reduction Using Thiourea as a Reducing Agent. Mater. Lett. 2013, 113, 5–8. [Google Scholar] [CrossRef]
- Nassef, M.G.A.; Soliman, M.; Nassef, B.G.; Daha, M.A.; Nassef, G.A. Impact of Graphene Nano-Additives to Lithium Grease on the Dynamic and Tribological Behavior of Rolling Bearings. Lubricants 2022, 10, 29. [Google Scholar] [CrossRef]
- Oliveira, A.E.F.; Braga, G.B.; Tarley, C.R.T.; Pereira, A.C. Thermally Reduced Graphene Oxide: Synthesis, Studies and Characterization. J. Mater. Sci. 2018, 53, 12005–12015. [Google Scholar] [CrossRef]
- Saleem, H.; Haneef, M.; Abbasi, H.Y. Synthesis Route of Reduced Graphene Oxide via Thermal Reduction of Chemically Exfoliated Graphene Oxide. Mater. Chem. Phys. 2018, 204, 1–7. [Google Scholar] [CrossRef]
- Alam, S.N.; Sharma, N.; Kumar, L. Synthesis of Graphene Oxide (GO) by Modified Hummers Method and Its Thermal Reduction to Obtain Reduced Graphene Oxide (RGO). Graphene 2017, 6, 1–18. [Google Scholar] [CrossRef]
- Rani, A.; Nam, S.; Oh, K.A.; Park, M. Electrical Conductivity of Chemically Reduced Graphene Powders under Compression. Carbon Lett. 2010, 11, 90–95. [Google Scholar] [CrossRef]
- Ambrosi, A.; Chua, C.K.; Khezri, B.; Sofer, Z.; Webster, R.D.; Pumera, M. Chemically Reduced Graphene Contains Inherent Metallic Impurities Present in Parent Natural and Synthetic Graphite. Proc. Natl. Acad. Sci. USA 2012, 109, 12899–12904. [Google Scholar] [CrossRef]
- Rahman, M.A.; Sagadevan, S.; Johan, M.R. Graphene and Its Composites BT—Contemporary Nanomaterials in Material Engineering Applications; Mubarak, N.M., Khalid, M., Walvekar, R., Numan, A., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 21–35. ISBN 978-3-030-62761-4. [Google Scholar]
- Zhai, W.; Shi, X.; Wang, M.; Xu, Z.; Yao, J.; Song, S.; Wang, Y. Grain Refinement: A Mechanism for Graphene Nanoplatelets to Reduce Friction and Wear of Ni3Al Matrix Self-Lubricating Composites. Wear 2014, 310, 33–40. [Google Scholar] [CrossRef]
- Liu, Y.; Shin, D.-G.; Xu, S.; Kim, C.-L.; Kim, D.-E. Understanding of the Lubrication Mechanism of Reduced Graphene Oxide Coating via Dual In-Situ Monitoring of the Chemical and Topographic Structural Evolution. Carbon 2021, 173, 941–952. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, Q.; Gao, L.; Ma, T.; Qiu, M.; Hu, Y.; Wang, H.; Luo, J. A Molecular Dynamics Study of Lubricating Mechanism of Graphene Nanoflakes Embedded in Cu-Based Nanocomposite. Appl. Surf. Sci. 2020, 511, 145620. [Google Scholar] [CrossRef]
- Wu, L.; Gu, L.; Jian, R. Lubrication Mechanism of Graphene Nanoplates as Oil Additives for Ceramics/Steel Sliding Components. Ceram. Int. 2021, 47, 16935–16942. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, E.; Zhong, H.; Wang, J.; Subedi, A.; Hu, K.; Hu, X. Characterization and Tribological Performances of Graphene and Fluorinated Graphene Particles in PAO. Nanomaterials 2021, 11, 2126. [Google Scholar] [CrossRef]
- Zin, V.; Barison, S.; Agresti, F.; Colla, L.; Pagura, C.; Fabrizio, M. Improved Tribological and Thermal Properties of Lubricants by Graphene Based Nano-Additives. RSC Adv. 2016, 6, 59477–59486. [Google Scholar] [CrossRef]
- Zheng, D.; Cai, Z.; Shen, M.; Li, Z.; Zhu, M. Investigation of the Tribology Behaviour of the Graphene Nanosheets as Oil Additives on Textured Alloy Cast Iron Surface. Appl. Surf. Sci. 2016, 387, 66–75. [Google Scholar] [CrossRef]
- Şenel, M.C.; Gürbüz, M.; Koç, E. Mechanical and Tribological Behaviours of Aluminium Matrix Composites Reinforced by Graphene Nanoplatelets. Mater. Sci. Technol. 2018, 34, 1980–1989. [Google Scholar] [CrossRef]
- Lin, J.; Wang, L.; Chen, G. Modification of Graphene Platelets and Their Tribological Properties as a Lubricant Additive. Tribol. Lett. 2011, 41, 209–215. [Google Scholar] [CrossRef]
- Azman, S.S.N.; Zulkifli, N.W.M.; Masjuki, H.; Gulzar, M.; Zahid, R. Study of Tribological Properties of Lubricating Oil Blend Added with Graphene Nanoplatelets. J. Mater. Res. 2016, 31, 1932–1938. [Google Scholar] [CrossRef]
- Yang, J.; Xia, Y.; Song, H.; Chen, B.; Zhang, Z. Synthesis of the Liquid-like Graphene with Excellent Tribological Properties. Tribol. Int. 2017, 105, 118–124. [Google Scholar] [CrossRef]
- Niu, M.; Qu, J.; Gu, L. Synthesis of Titanium Complex Grease and Effects of Graphene on Its Tribological Properties. Tribol. Int. 2019, 140, 105815. [Google Scholar] [CrossRef]
- Huang, J.; Tan, J.; Fang, H.; Gong, F.; Wang, J. Tribological and Wear Performances of Graphene-Oil Nanofluid under Industrial High-Speed Rotation. Tribol. Int. 2019, 135, 112–120. [Google Scholar] [CrossRef]
- Xie, H.; Jiang, B.; Dai, J.; Peng, C.; Li, C.; Li, Q.; Pan, F. Tribological Behaviors of Graphene and Graphene Oxide as Water-Based Lubricant Additives for Magnesium Alloy/Steel Contacts. Materials 2018, 11, 206. [Google Scholar] [CrossRef]
- Suresha, B.; Hemanth, G.; Rakesh, A.; Adarsh, K.M. Tribological Behaviour of Neem Oil with and without Graphene Nanoplatelets Using Four-Ball Tester. Adv. Tribol. 2020, 2020, 1984931. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X.; He, Y.; Jiang, M.; Gu, K. Tribological Characteristics of Graphene as Grease Additive under Different Contact Forms. Tribol. Int. 2018, 127, 457–469. [Google Scholar] [CrossRef]
- Kiu, S.S.K.; Yusup, S.; Soon, C.V.; Arpin, T.; Samion, S.; Kamil, R.N.M. Tribological Investigation of Graphene as Lubricant Additive in Vegetable Oil. J. Phys. Sci. 2017, 28, 257–267. [Google Scholar]
- Kamel, B.M.; Mohamed, A.; El Sherbiny, M.; Abed, K.A.; Abd-Rabou, M. Tribological Properties of Graphene Nanosheets as an Additive in Calcium Grease. J. Dispers. Sci. Technol. 2017, 38, 1495–1500. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, M.; Zhu, H.; Tian, Y.; Wang, K.; Wei, J.; Ji, F.; Li, X.; Li, Z.; Zhang, P. Tribological Properties of Oleic Acid-Modified Graphene as Lubricant Oil Additives. J. Phys. D. Appl. Phys. 2011, 44, 205303. [Google Scholar] [CrossRef]
- Rasheed, A.K.; Khalid, M.; Javeed, A.; Rashmi, W.; Gupta, T.; Chan, A. Heat Transfer and Tribological Performance of Graphene Nanolubricant in an Internal Combustion Engine. Tribol. Int. 2016, 103, 504–515. [Google Scholar] [CrossRef]
- Shi, Z.; Shum, P.; Wasy, A.; Zhou, Z.; Li, L.K.-Y. Tribological Performance of Few Layer Graphene on Textured M2 Steel Surfaces. Surf. Coat. Technol. 2016, 296, 164–170. [Google Scholar] [CrossRef]
- Wu, L.; Gu, L.; Xie, Z.; Zhang, C.; Song, B. Improved Tribological Properties of Si3N4/GCr15 Sliding Pairs with Few Layer Graphene as Oil Additives. Ceram. Int. 2017, 43, 14218–14224. [Google Scholar] [CrossRef]
- Kong, S.; Wang, J.; Hu, W.; Li, J. Effects of Thickness and Particle Size on Tribological Properties of Graphene as Lubricant Additive. Tribol. Lett. 2020, 68, 1–10. [Google Scholar] [CrossRef]
- Xu, Z.; Shi, X.; Zhai, W.; Yao, J.; Song, S.; Zhang, Q. Preparation and Tribological Properties of TiAl Matrix Composites Reinforced by Multilayer Graphene. Carbon 2014, 67, 168–177. [Google Scholar] [CrossRef]
- Fan, K.; Chen, X.; Wang, X.; Liu, X.; Liu, Y.; Lai, W.; Liu, X. Toward Excellent Tribological Performance as Oil-Based Lubricant Additive: Particular Tribological Behavior of Fluorinated Graphene. ACS Appl. Mater. Interfaces 2018, 10, 28828–28838. [Google Scholar] [CrossRef]
- Ou, J.; Wang, J.; Liu, S.; Mu, B.; Ren, J.; Wang, H.; Yang, S. Tribology Study of Reduced Graphene Oxide Sheets on Silicon Substrate Synthesized via Covalent Assembly. Langmuir 2010, 26, 15830–15836. [Google Scholar] [CrossRef]
- Goralka, C.; Bridges, J.; Jahan, M.; Sidebottom, M.; Cameron, T.; Lu, Y.; Ye, Z. Friction and Wear Reduction of Tungsten Carbide and Titanium Alloy Contacts via Graphene Nanolubricant. Lubricants 2022, 10, 272. [Google Scholar] [CrossRef]
- Ezugwu, E.O.; Wang, Z.M. Titanium Alloys and Their Machinability—A Review. J. Mater. Process. Technol. 1997, 68, 262–274. [Google Scholar] [CrossRef]
- Wang, L.-F.; Ma, T.-B.; Hu, Y.-Z.; Wang, H. Atomic-Scale Friction in Graphene Oxide: An Interfacial Interaction Perspective from First-Principles Calculations. Phys. Rev. B 2012, 86, 125436. [Google Scholar] [CrossRef]
- Wang, J.; Wang, F.; Li, J.; Wang, S.; Song, Y.; Sun, Q.; Jia, Y. Theoretical Study of Superlow Friction between Two Single-Side Hydrogenated Graphene Sheets. Tribol. Lett. 2012, 48, 255–261. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, Z.; Yang, M.; Yuan, J.; Li, P.; Men, X. Graphene Enhanced and in Situ-Formed Alginate Hydrogels for Reducing Friction and Wear of Polymers. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 589, 124434. [Google Scholar] [CrossRef]
- Min, C.; Liu, D.; Qian, J.; He, Z.; Jia, W.; Song, H.; Guo, L. High Mechanical and Tribological Performance Polyimide Nanocomposites Using Amine-Functionalized Graphene Nanosheets. Tribol. Int. 2019, 131, 1–10. [Google Scholar] [CrossRef]
- Mura, A.; Adamo, F.; Wang, H.; Leong, W.S.; Ji, X.; Kong, J. Investigation about Tribological Behavior of ABS and PC-ABS Polymers Coated with Graphene. Tribol. Int. 2019, 134, 335–340. [Google Scholar] [CrossRef]
- Cheng, Z.-L.; Li, W.; Liu, Z. Preparation, Characterization, and Tribological Properties of Oleic Diethanolamide-Capped Zinc Borate-Coated Graphene Oxide Composites. J. Alloys Compd. 2017, 705, 384–391. [Google Scholar] [CrossRef]
- Walsh, L.A.; Addou, R.; Wallace, R.M.; Hinkle, C.L. Chapter 22—Molecular Beam Epitaxy of Transition Metal Dichalcogenides. In Molecular Beam Epitaxy, 2nd ed.; Henini, M.B.T.-M.B.E., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 515–531. ISBN 978-0-12-812136-8. [Google Scholar]
- Xu, Y.; Peng, Y.; Dearn, K.D.; Zheng, X.; Yao, L.; Hu, X. Synergistic Lubricating Behaviors of Graphene and MoS2 Dispersed in Esterified Bio-Oil for Steel/Steel Contact. Wear 2015, 342–343, 297–309. [Google Scholar] [CrossRef]
- Farsadi, M.; Bagheri, S.; Ismail, N.A. Nanocomposite of Functionalized Graphene and Molybdenum Disulfide as Friction Modifier Additive for Lubricant. J. Mol. Liq. 2017, 244, 304–308. [Google Scholar] [CrossRef]
- Hou, K.; Wang, J.; Yang, Z.; Ma, L.; Wang, Z.; Yang, S. One-Pot Synthesis of Reduced Graphene Oxide/Molybdenum Disulfide Heterostructures with Intrinsic Incommensurateness for Enhanced Lubricating Properties. Carbon 2017, 115, 83–94. [Google Scholar] [CrossRef]
- Wu, X.; Zhao, G.; Zhao, Q.; Gong, K.; Wang, X.; Liu, W.; Liu, W. Investigating the Tribological Performance of Nanosized MoS 2 on Graphene Dispersion in Perfluoropolyether under High Vacuum. RSC Adv. 2016, 6, 98606–98610. [Google Scholar] [CrossRef]
- Gong, K.; Wu, X.; Zhao, G.; Wang, X. Nanosized MoS2 Deposited on Graphene as Lubricant Additive in Polyalkylene Glycol for Steel/Steel Contact at Elevated Temperature. Tribol. Int. 2017, 110, 1–7. [Google Scholar] [CrossRef]
- Liang, H.; Bu, Y.; Zhang, J.; Cao, Z.; Liang, A. Graphene Oxide Film as Solid Lubricant. ACS Appl. Mater. Interfaces 2013, 5, 6369–6375. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, V.; Umrao, S.; Rastogi, R.B.; Kumar, R.; Srivastava, A. Synthesis, Characterization, and Tribological Evaluation of TiO2-Reinforced Boron and Nitrogen Co-Doped Reduced Graphene Oxide Based Hybrid Nanomaterials as Efficient Antiwear Lubricant Additives. ACS Appl. Mater. Interfaces 2016, 8, 11698–11710. [Google Scholar] [CrossRef]
- Garmroudi, A.; Kheirollahi, M.; Mousavi, S.A.; Fattahi, M.; Mahvelati, E.H. Effects of Graphene Oxide/TiO2 Nanocomposite, Graphene Oxide Nanosheets and Cedr Extraction Solution on IFT Reduction and Ultimate Oil Recovery from a Carbonate Rock. Petroleum 2020, 8, 476–482. [Google Scholar] [CrossRef]
- Zhao, Z.; Fan, X.; Li, W.; He, Y.; Sun, Q.; Zhu, M. Multi-Layer Interface Lubrication of in-Situ Synthesized Titanium Dioxide/Reduced Graphene Oxide Nanocomposites. Appl. Surf. Sci. 2022, 604, 154571. [Google Scholar] [CrossRef]
- Song, H.-J.; Jia, X.-H.; Li, N.; Yang, X.-F.; Tang, H. Synthesis of α-Fe 2 O 3 Nanorod/Graphene Oxide Composites and Their Tribological Properties. J. Mater. Chem. 2012, 22, 895–902. [Google Scholar] [CrossRef]
- Majeed, F.S.A.; Yusof, N.B.M.; Suhaimi, M.A.; Elsiti, N.M. Effect of Paraffin Oil with XGnP and Fe2O3 Nanoparticles on Tribological Properties. Mater. Today Proc. 2020, 27, 1685–1688. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, S.Q.; Huang, K.Z.; Zhang, B.; Wen, G.H.; Cui, X.H. Improvement of Tribological Performance of TC11 Alloy via Formation of a Double-Layer Tribo-Layer Containing Graphene/Fe2O3 Nanocomposite. Tribol. Int. 2017, 109, 485–495. [Google Scholar] [CrossRef]
- Feng, X.; Kwon, S.; Park, J.Y.; Salmeron, M. Superlubric Sliding of Graphene Nanoflakes on Graphene. ACS Nano 2013, 7, 1718–1724. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Su, F.; Chen, Y. Synthesis of Nano-Cu/Graphene Oxide Composites by Supercritical CO2-Assisted Deposition as a Novel Material for Reducing Friction and Wear. Chem. Eng. J. 2015, 281, 11–19. [Google Scholar] [CrossRef]
- Meng, Y.; Su, F.; Chen, Y. A Novel Nanomaterial of Graphene Oxide Dotted with Ni Nanoparticles Produced by Supercritical CO2-Assisted Deposition for Reducing Friction and Wear. ACS Appl. Mater. Interfaces 2015, 7, 11604–11612. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Wang, Z.; Yang, J.; Jia, X.; Zhang, Z. Facile Synthesis of Copper/Polydopamine Functionalized Graphene Oxide Nanocomposites with Enhanced Tribological Performance. Chem. Eng. J. 2017, 324, 51–62. [Google Scholar] [CrossRef]
- Verma, D.K.; Kumar, B.; Kavita; Rastogi, R. B. Zinc Oxide-and Magnesium-Doped Zinc Oxide-Decorated Nanocomposites of Reduced Graphene Oxide as Friction and Wear Modifiers. ACS Appl. Mater. Interfaces 2018, 11, 2418–2430. [Google Scholar] [CrossRef]
- Zhang, L.; He, Y.; Feng, S.; Zhang, L.; Zhang, L.; Jiao, Z.; Zhan, Y.; Wang, Y. Preparation and Tribological Properties of Novel Boehmite/Graphene Oxide Nano-Hybrid. Ceram. Int. 2016, 42, 6178–6186. [Google Scholar] [CrossRef]
- Meng, Y.; Su, F.; Chen, Y. Supercritical Fluid Synthesis and Tribological Applications of Silver Nanoparticle-Decorated Graphene in Engine Oil Nanofluid. Sci. Rep. 2016, 6, 37841. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, Z. Tribological Properties of Sodium Dodecyl Sulfate Aqueous Dispersion of Graphite-Derived Carbon Materials. RSC Adv. 2014, 4, 9980–9985. [Google Scholar] [CrossRef]
- Shi, X.; Song, S.; Zhai, W.; Wang, M.; Xu, Z.; Yao, J.; ud Din, A.Q.; Zhang, Q. Tribological Behavior of Ni3Al Matrix Self-Lubricating Composites Containing WS2, Ag and HBN Tested from Room Temperature to 800 °C. Mater. Des. 2014, 55, 75–84. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).