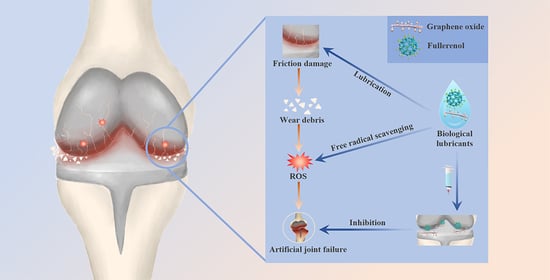
Synergistic Lubrication and Antioxidation Efficacies of Graphene Oxide and Fullerenol as Biological Lubricant Additives for Artificial Joints
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
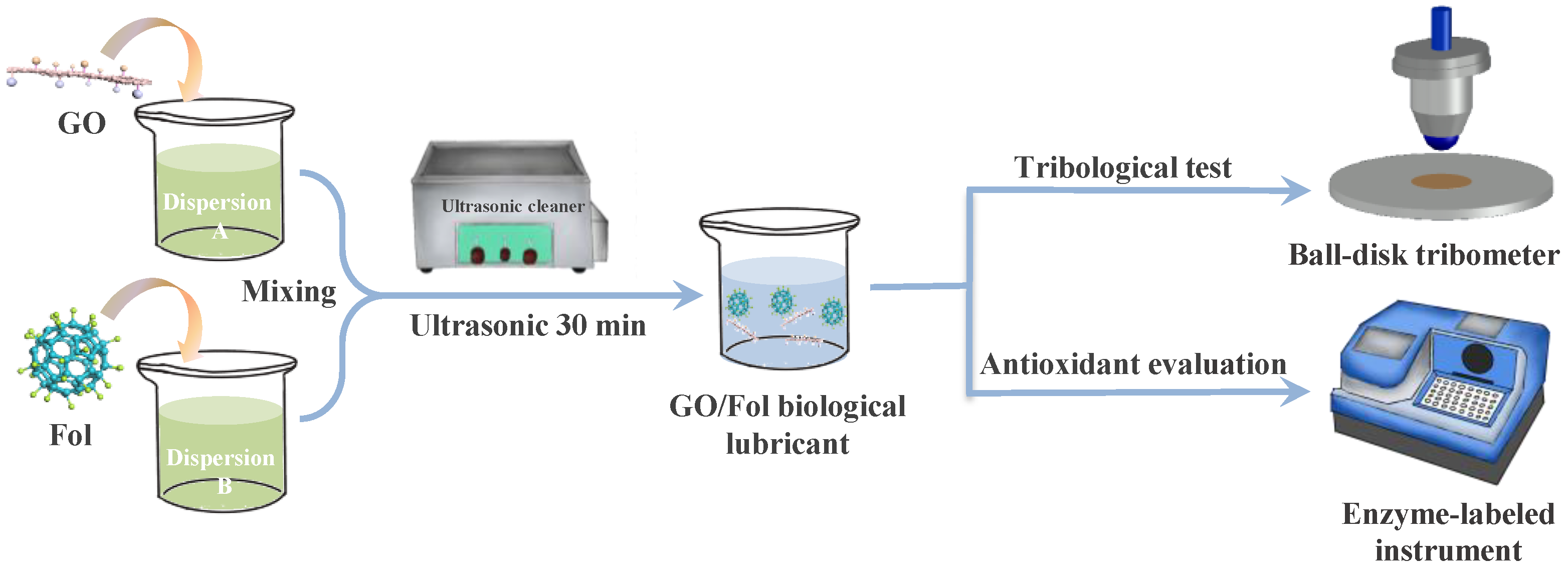
2.2. Preparation and Characterization of Biological Lubrication Additives
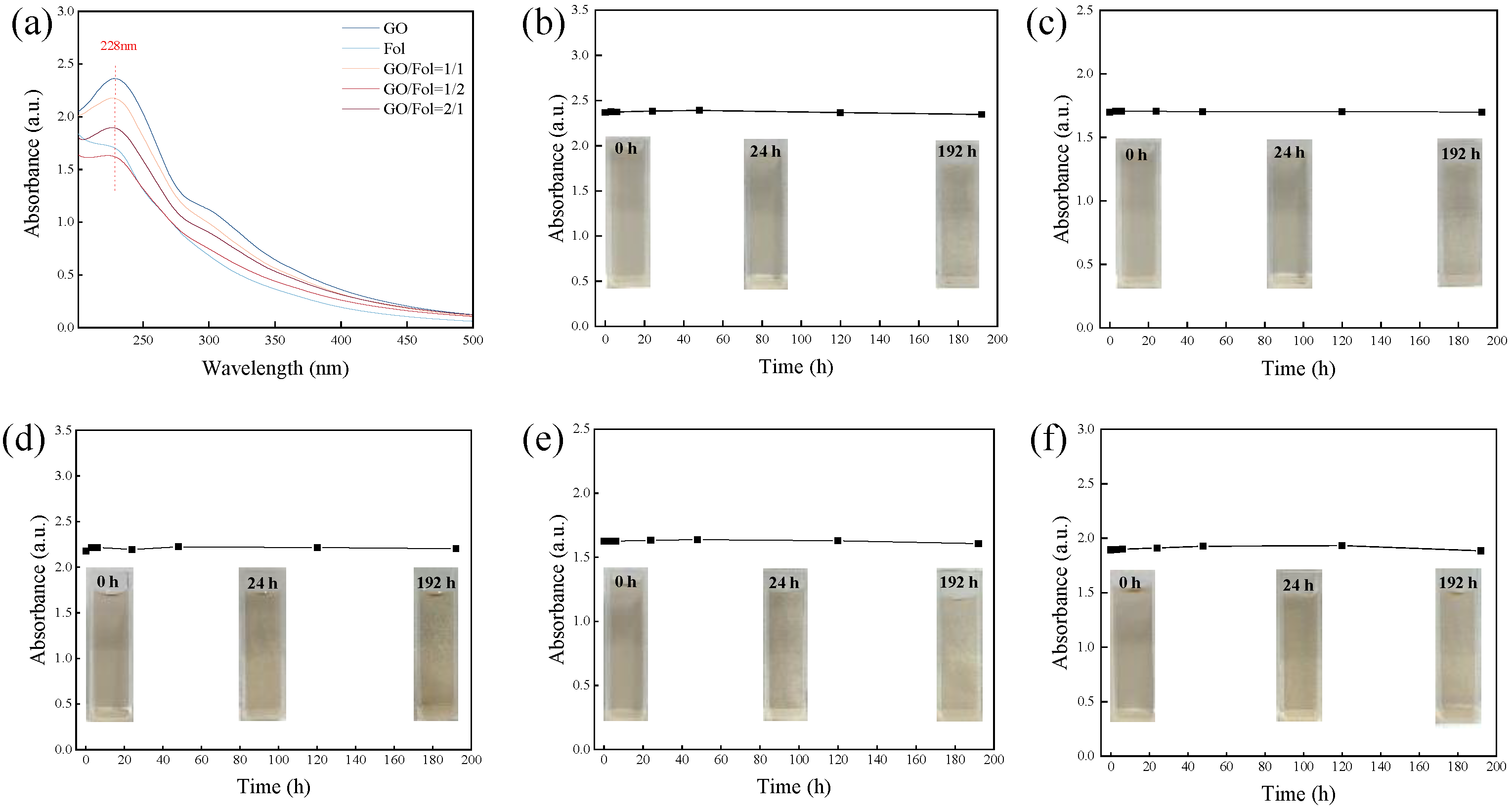
2.3. Dispersion Stability of Biological Lubrication Additives
2.4. Tribological Tests
2.5. Antioxidant Activity
2.5.1. DPPH Free Radical Scavenging Activity
2.5.2. Hydroxyl Free Radical Scavenging Activity
3. Results and Discussion
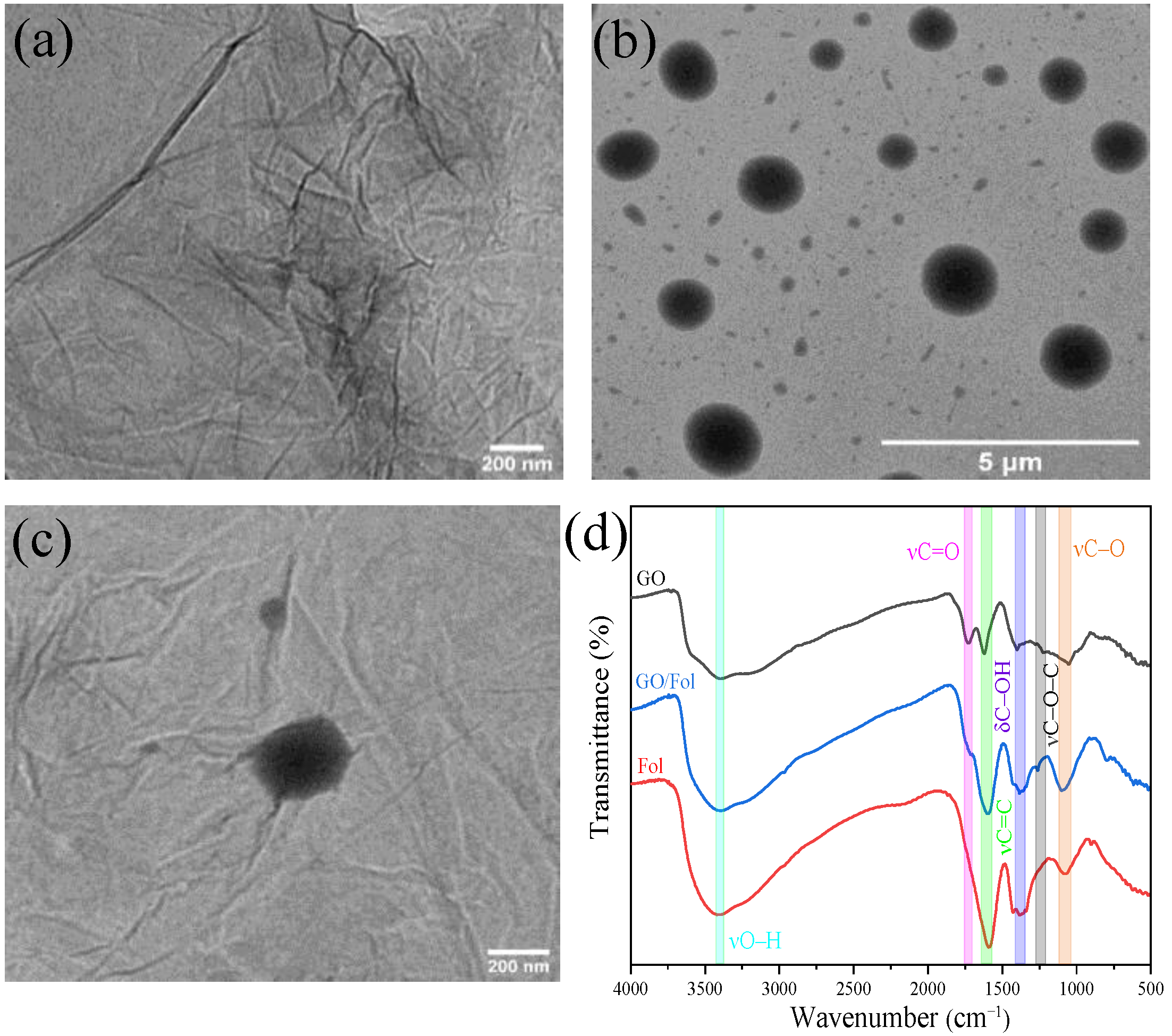
3.1. Characterization
3.2. Evaluation of Lubrication Performance
3.2.1. Friction Reduction Properties of Biological Lubrication Additives
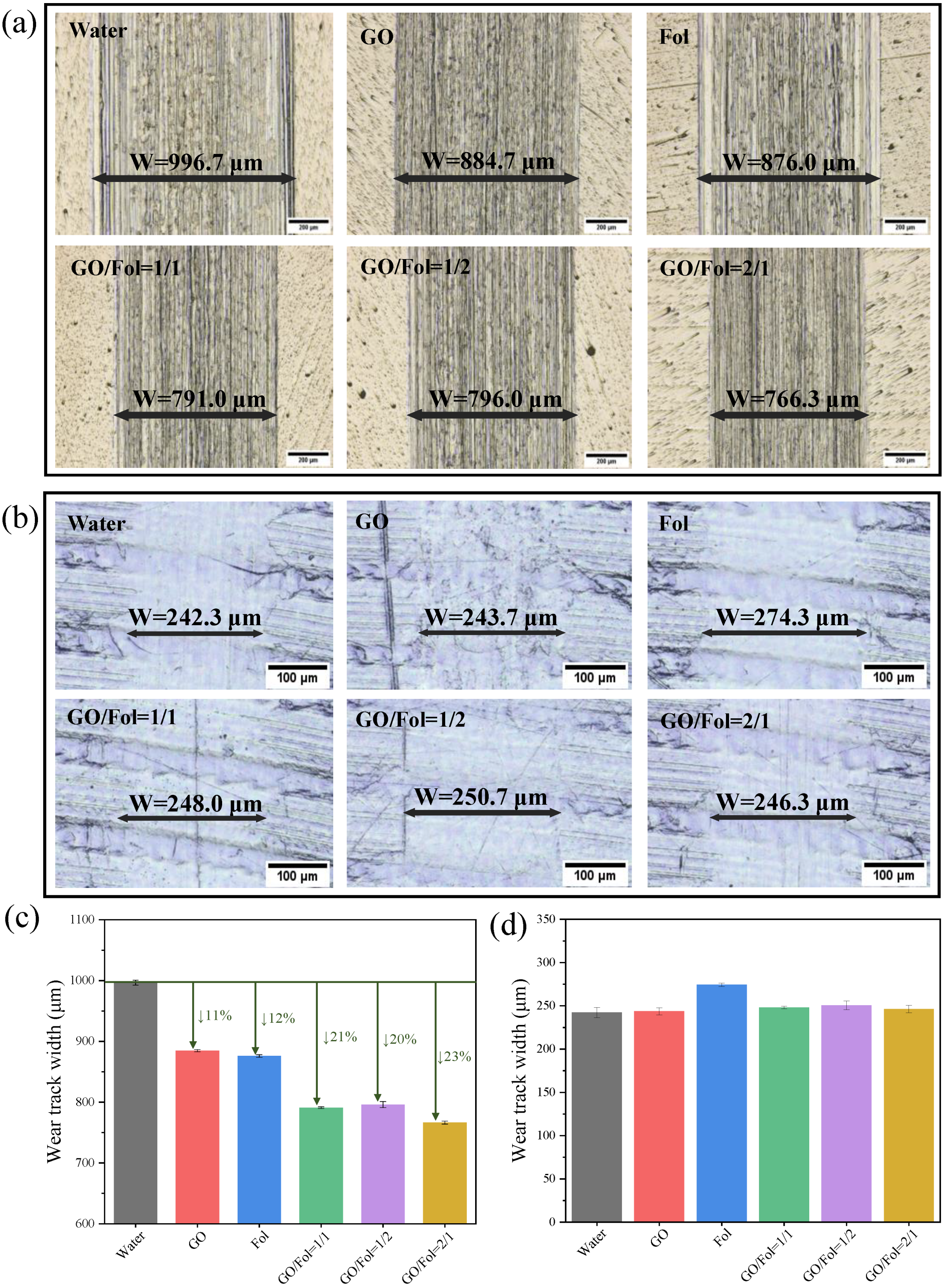
3.2.2. Antiwear Properties of Biological Lubrication Additives
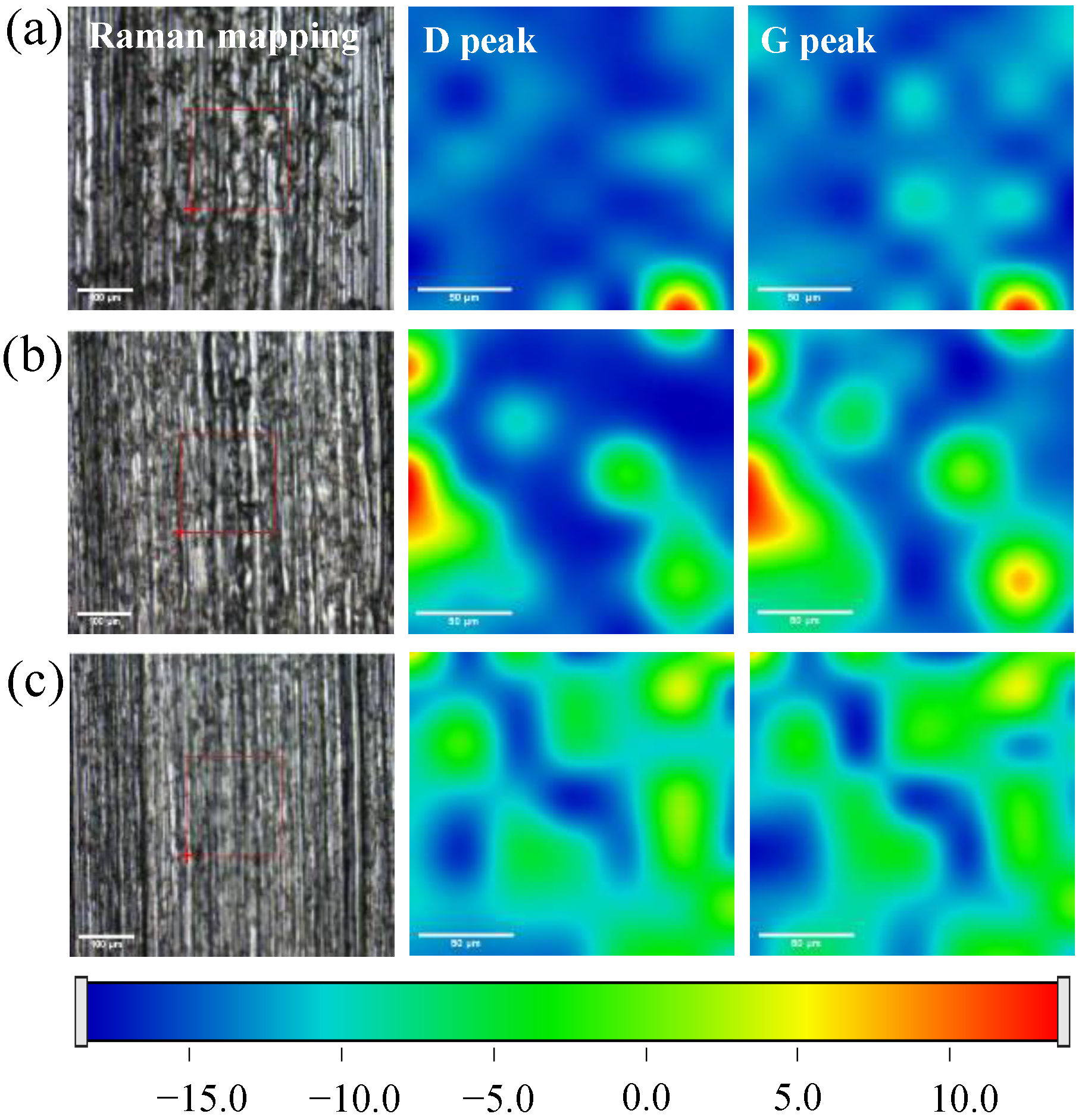
3.2.3. Composition Analysis of Wear Scars
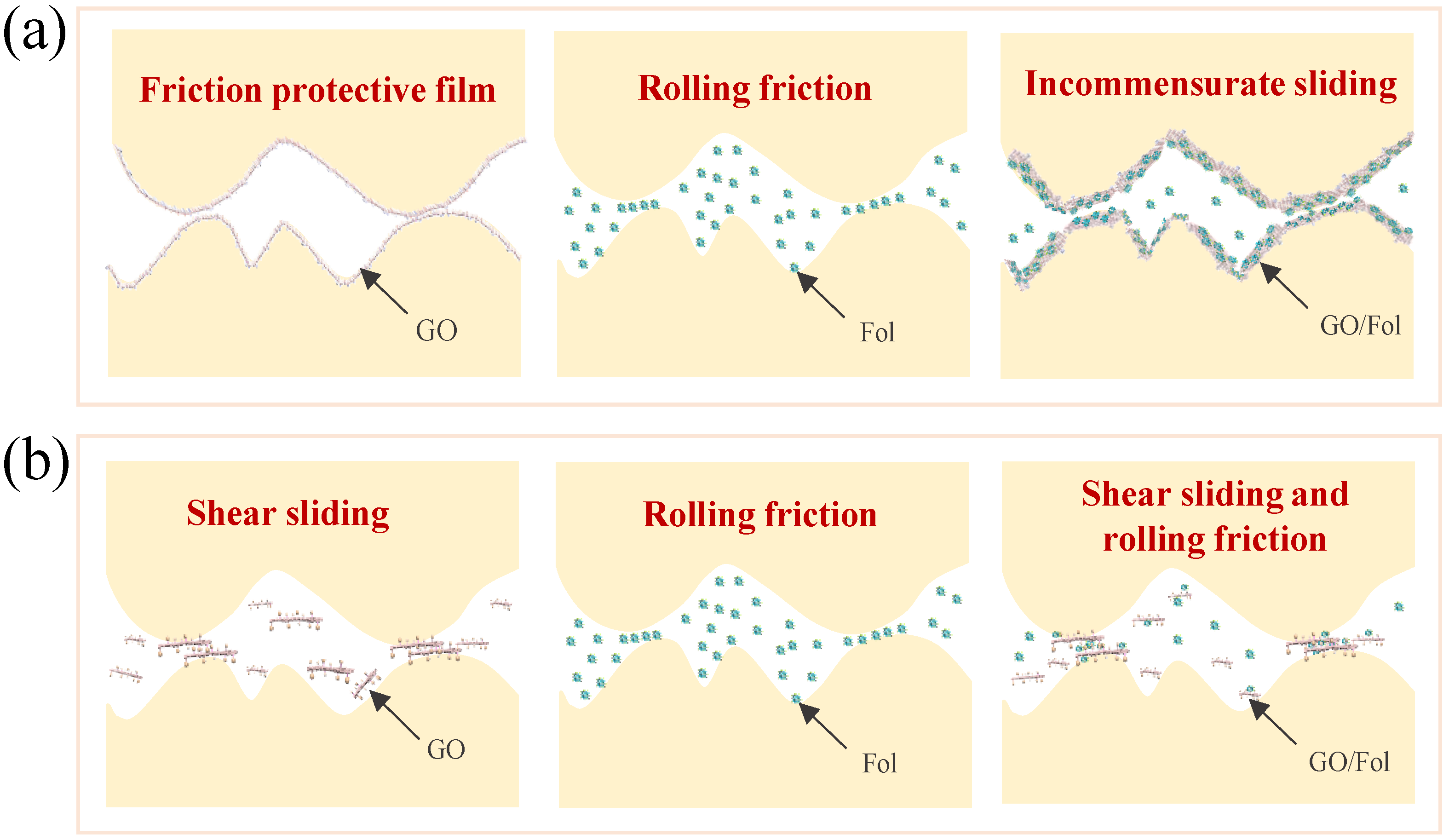
3.2.4. Lubrication Mechanism
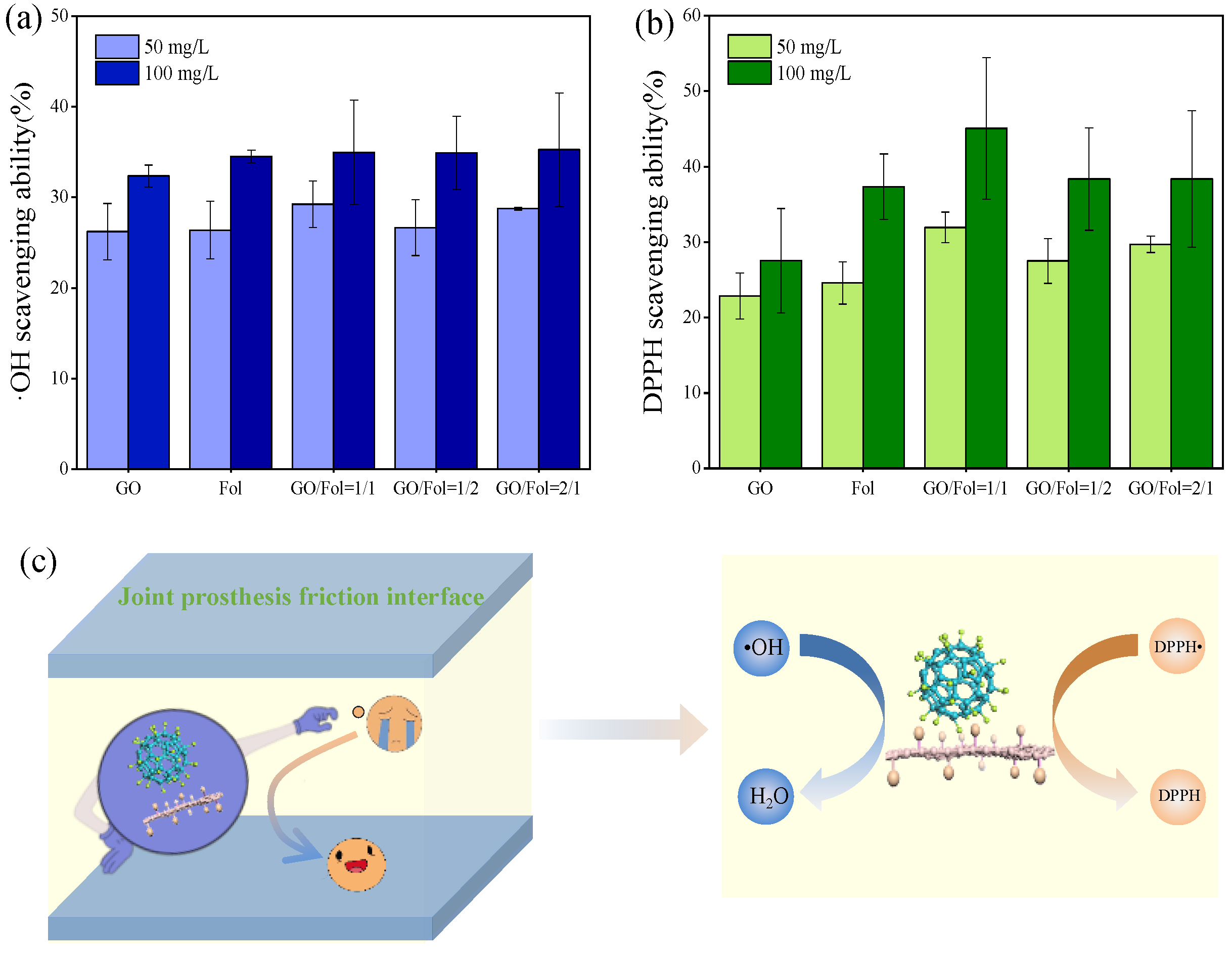
3.3. Antioxidant Activity of Biological Lubrication Additives
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Berenbaum, F.; Wallace, I.J.; Lieberman, D.E.; Felson, D.T. Modern-day environmental factors in the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2018, 14, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Merola, M.; Affatato, S. Materials for hip prostheses: A review of wear and loading considerations. Materials 2019, 12, 495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rim, Y.A.; Ju, J.H. The role of fibrosis in osteoarthritis progression. Life 2020, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Nine, M.J.; Choudhury, D.; Hee, A.C.; Mootanah, R.; Osman, N.A.A. Wear debris characterization and corresponding biological response: Artificial hip and knee joints. Materials 2014, 7, 980–1016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, F.; Tang, J.; Dai, K.; Huang, Y. Metallic wear debris collected from patients induces apoptosis in rat primary osteoblasts via reactive oxygen species-mediated mitochondrial dysfunction and endoplasmic reticulum stress. Mol. Med. Rep. 2019, 19, 1629–1637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hameister, R.; Kaur, C.; Dheen, S.T.; Lohmann, C.H.; Singh, G. Reactive oxygen/nitrogen species (ROS/RNS) and oxidative stress in arthroplasty. J. Biomed. Mater. Res. Part B 2020, 108, 2073–2087. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.G.; Zhang, Y.L.; Jin, Z.M. A review of the bio-tribology of medical devices. Friction 2022, 10, 4–30. [Google Scholar] [CrossRef]
- Méndez, P.A.; Ortiz, B.L.; Vásquez, G.M.; López, B.L. Mucoadhesive chitosan/OA nanoparticles charged with celecoxib inhibit prostaglandin E 2 LPS-induced in U 937 cell line. J. Appl. Polym. Sci. 2017, 134, 45288. [Google Scholar] [CrossRef]
- Everhart, J.S.; DiBartola, A.C.; Swank, K.; Pettit, R.; Hughes, L.; Lewis, C.; Flanigan, D.C. Cartilage damage at the time of anterior cruciate ligament reconstruction is associated with weaker quadriceps function and lower risk of future ACL injury. Knee Surg. Sport. Traumatol. Arthrosc. 2020, 28, 576–583. [Google Scholar] [CrossRef]
- Gleghorn, J.P.; Bonassar, L.J. Lubrication mode analysis of articular cartilage using Stribeck surfaces. J. Biomech. 2008, 41, 1910–1918. [Google Scholar] [CrossRef]
- Huang, H.; Lou, Z.; Zheng, S.; Wu, J.; Yao, Q.; Chen, R.; Kou, L.; Chen, D. Intra-articular drug delivery systems for osteoarthritis therapy: Shifting from sustained release to enhancing penetration into cartilage. Drug Deliv. 2022, 29, 767–791. [Google Scholar] [CrossRef]
- Šimek, M.; Nešporová, K.; Kocurková, A.; Foglová, T.; Ambrožová, G.; Velebný, V.; Kubala, L.; Hermannová, M. How the molecular weight affects the in vivo fate of exogenous hyaluronan delivered intravenously: A stable-isotope labelling strategy. Carbohydr. Polym. 2021, 263, 117927. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wu, B.; Chen, X.; Li, L.; Wang, X.; Gao, X.; Wang, X.; Hu, K.; Hu, X. Synergistic Lubricating Performance of Graphene Oxide and Modified Biodiesel Soot as Water Additives. Lubricants 2022, 10, 175. [Google Scholar] [CrossRef]
- Song, H.-J.; Li, N. Frictional behavior of oxide graphene nanosheets as water-base lubricant additive. Appl. Phys. A 2011, 105, 827–832. [Google Scholar] [CrossRef]
- Sarno, M.; Senatore, A.; Cirillo, C.; Petrone, V.; Ciambelli, P. Oil lubricant tribological behaviour improvement through dispersion of few layer graphene oxide. J. Nanosci. Nanotechno. 2014, 14, 4960–4968. [Google Scholar] [CrossRef]
- Zhao, J.; Mao, J.; Li, Y.; He, Y.; Luo, J. Friction-induced nano-structural evolution of graphene as a lubrication additive. Appl. Surf. Sci. 2018, 434, 21–27. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, Y.; Luo, J. Tribological properties of few-layer graphene oxide sheets as oil-based lubricant additives. Chin. J. Mech. Eng. 2016, 29, 439–444. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Liu, P.; Zheng, J.; Shu, C.; Pan, G.; Luo, J. Modification on the tribological properties of ceramics lubricated by water using fullerenol as a lubricating additive. Sci. China Technol. Sci. 2012, 55, 2656–2661. [Google Scholar] [CrossRef]
- Chen, S.; Ding, Q.; Gu, Y.; Quan, X.; Ma, Y.; Jia, Y.; Xie, H.; Tang, J. Study of tribological properties of fullerenol and nanodiamonds as additives in water-based lubricants for amorphous carbon (aC) coatings. Nanomaterials 2021, 12, 139. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, J.; Ma, H.; Zhen, M.; Guo, J.; Wang, L.; Jiang, L.; Shu, C.; Wang, C. Biocompatible [60]/[70] Fullerenols: Potent defense against oxidative injury induced by reduplicative chemotherapy. ACS Appl. Mater. Interfaces 2017, 9, 35539–35547. [Google Scholar] [CrossRef]
- Voitko, K.V.; Goshovska, Y.V.; Demianenko, E.M.; Sementsov, Y.I.; Zhuravskyi, S.V.; Mys, L.A.; Korkach, Y.P.; Kolev, H.; Sagach, V.F. Graphene oxide nanoflakes prevent reperfusion injury of Langendorff isolated rat heart providing antioxidative activity in situ. Free Radical Res. 2022, 56, 328–341. [Google Scholar] [CrossRef] [PubMed]
- Halenova, T.; Raksha, N.; Vovk, T.; Savchuk, O.; Ostapchenko, L.; Prylutskyy, Y.; Kyzyma, O.; Ritter, U.; Scharff, P. Effect of C60 fullerene nanoparticles on the diet-induced obesity in rats. Int. J. Obes. 2018, 42, 1987–1998. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef] [PubMed]
- Goodarzi, S.; Da Ros, T.; Conde, J.; Sefat, F.; Mozafari, M. Fullerene: Biomedical engineers get to revisit an old friend. Mater. Today 2017, 20, 460–480. [Google Scholar] [CrossRef] [Green Version]
- Wu, B.; Song, H.; Zhang, Q.; Hu, X. Controllable synthesis and friction reduction of ZnFe2O4@ C microspheres with diverse core-shell architectures. Tribol. Int. 2021, 153, 106614. [Google Scholar] [CrossRef]
- Jaiswal, R.; Saha, U.; Goswami, T.H.; Srivastava, A.; Prasad, N.E. ‘Pillar effect’of chemically bonded fullerene in enhancing supercapacitance performances of partially reduced fullerenol graphene oxide hybrid electrode material. Electrochim. Acta 2018, 283, 269–290. [Google Scholar] [CrossRef]
- Xie, H.; Jiang, B.; Dai, J.; Peng, C.; Li, C.; Li, Q.; Pan, F. Tribological behaviors of graphene and graphene oxide as water-based lubricant additives for magnesium alloy/steel contacts. Materials 2018, 11, 206. [Google Scholar] [CrossRef] [Green Version]
- Su, F.; Chen, G.; Huang, P. Lubricating performances of graphene oxide and onion-like carbon as water-based lubricant additives for smooth and sand-blasted steel discs. Friction 2020, 8, 47–57. [Google Scholar] [CrossRef] [Green Version]
- Song, H.; Wang, Z.; Yang, J. Tribological properties of graphene oxide and carbon spheres as lubricating additives. Appl. Phys. A. 2016, 122, 933. [Google Scholar] [CrossRef]
- Hao, T.; Li, J.; Yao, F.; Dong, D.; Wang, Y.; Yang, B.; Wang, C. Injectable fullerenol/alginate hydrogel for suppression of oxidative stress damage in brown adipose-derived stem cells and cardiac repair. ACS Nano 2017, 11, 5474–5488. [Google Scholar] [CrossRef]
- Abdelhalim, A.O.; Meshcheriakov, A.A.; Maistrenko, D.N.; Molchanov, O.E.; Ageev, S.V.; Ivanova, D.A.; Iamalova, N.R.; Luttsev, M.D.; Vasina, L.V.; Sharoyko, V.V. Graphene oxide enriched with oxygen-containing groups: On the way to an increase of antioxidant activity and biocompatibility. Colloids Surf. B 2022, 210, 112232. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hu, Z.; Xu, J.; Zhao, Y. Therapeutic applications of low-toxicity spherical nanocarbon materials. NPG Asia Mater. 2014, 6, e84. [Google Scholar] [CrossRef]
- Wang, Z.; Gao, X.; Zhao, Y. Mechanisms of antioxidant activities of fullerenols from first-principles calculation. J. Phys. Chem. A 2018, 122, 8183–8190. [Google Scholar] [CrossRef] [PubMed]
- Podolsky, N.E.; Marcos, M.A.; Cabaleiro, D.; Semenov, K.N.; Lugo, L.; Petrov, A.V.; Charykov, N.A.; Sharoyko, V.V.; Vlasov, T.D.; Murin, I.V. Physico-chemical properties of C60 (OH) 22–24 water solutions: Density, viscosity, refraction index, isobaric heat capacity and antioxidant activity. J. Mol. Liq. 2019, 278, 342–355. [Google Scholar] [CrossRef]
- Awan, F.; Bulger, E.; Berry, R.M.; Tam, K.C. Enhanced radical scavenging activity of polyhydroxylated C60 functionalized cellulose nanocrystals. Cellulose 2016, 23, 3589–3599. [Google Scholar] [CrossRef]


| Frictional Pair | Ball | Disk |
|---|---|---|
| Frictional pair 1 | Al2O3 (Ra, 3 µm) | Ti6Al4V (Ra, 1 µm) |
| Frictional pair 2 | Ti6Al4V (Ra, 3 µm) | Ultra-high-molecular-weight polyethylene (UHMWPE) (Ra, 1 µm) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Q.; Li, H.; Wu, L.; Bo, Z.; Wang, C.; Cheng, L.; Wang, C.; Peng, C.; Li, C.; Hu, X.; et al. Synergistic Lubrication and Antioxidation Efficacies of Graphene Oxide and Fullerenol as Biological Lubricant Additives for Artificial Joints. Lubricants 2023, 11, 11. https://doi.org/10.3390/lubricants11010011
Wu Q, Li H, Wu L, Bo Z, Wang C, Cheng L, Wang C, Peng C, Li C, Hu X, et al. Synergistic Lubrication and Antioxidation Efficacies of Graphene Oxide and Fullerenol as Biological Lubricant Additives for Artificial Joints. Lubricants. 2023; 11(1):11. https://doi.org/10.3390/lubricants11010011
Chicago/Turabian StyleWu, Qian, Honglin Li, Liangbin Wu, Zihan Bo, Changge Wang, Lei Cheng, Chao Wang, Chengjun Peng, Chuanrun Li, Xianguo Hu, and et al. 2023. "Synergistic Lubrication and Antioxidation Efficacies of Graphene Oxide and Fullerenol as Biological Lubricant Additives for Artificial Joints" Lubricants 11, no. 1: 11. https://doi.org/10.3390/lubricants11010011
APA StyleWu, Q., Li, H., Wu, L., Bo, Z., Wang, C., Cheng, L., Wang, C., Peng, C., Li, C., Hu, X., Li, C., & Wu, B. (2023). Synergistic Lubrication and Antioxidation Efficacies of Graphene Oxide and Fullerenol as Biological Lubricant Additives for Artificial Joints. Lubricants, 11(1), 11. https://doi.org/10.3390/lubricants11010011