Methyltrioctylammonium Octadecanoate as Lubricant Additive to Different Base Oils
Abstract
:1. Introduction
2. Materials and Methods
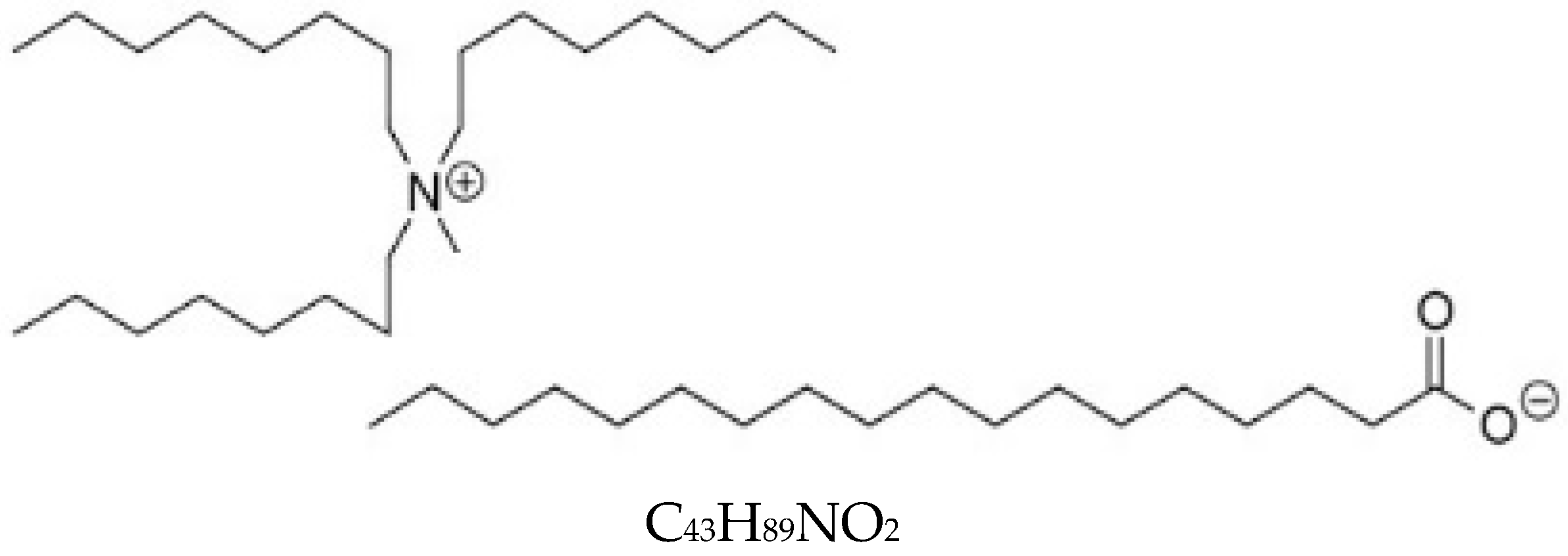
2.1. Ionic Liquid and Base Oils
2.2. Blend Characterization: Miscibility, Viscosity and Density
2.3. Tribological Tests
2.4. Surface Analysis
3. Results and Discussion
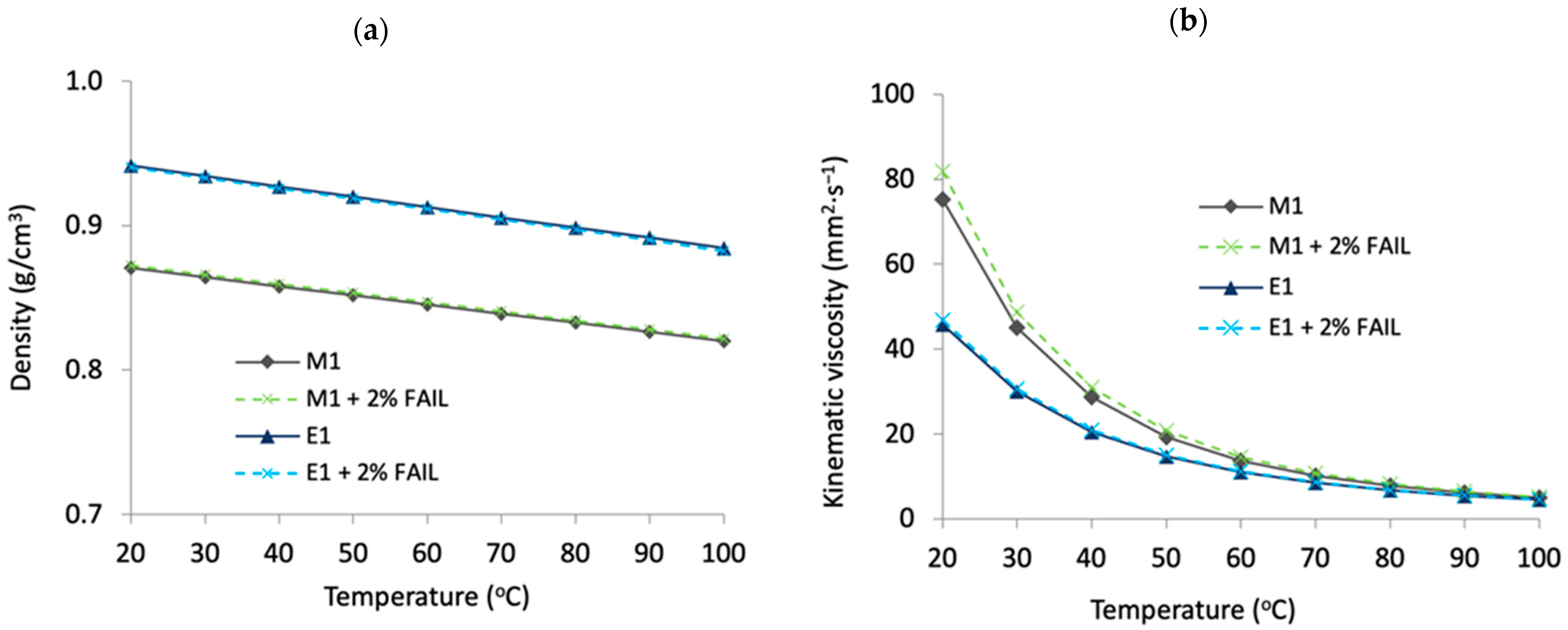
3.1. Miscibility, Viscosity and Density Values
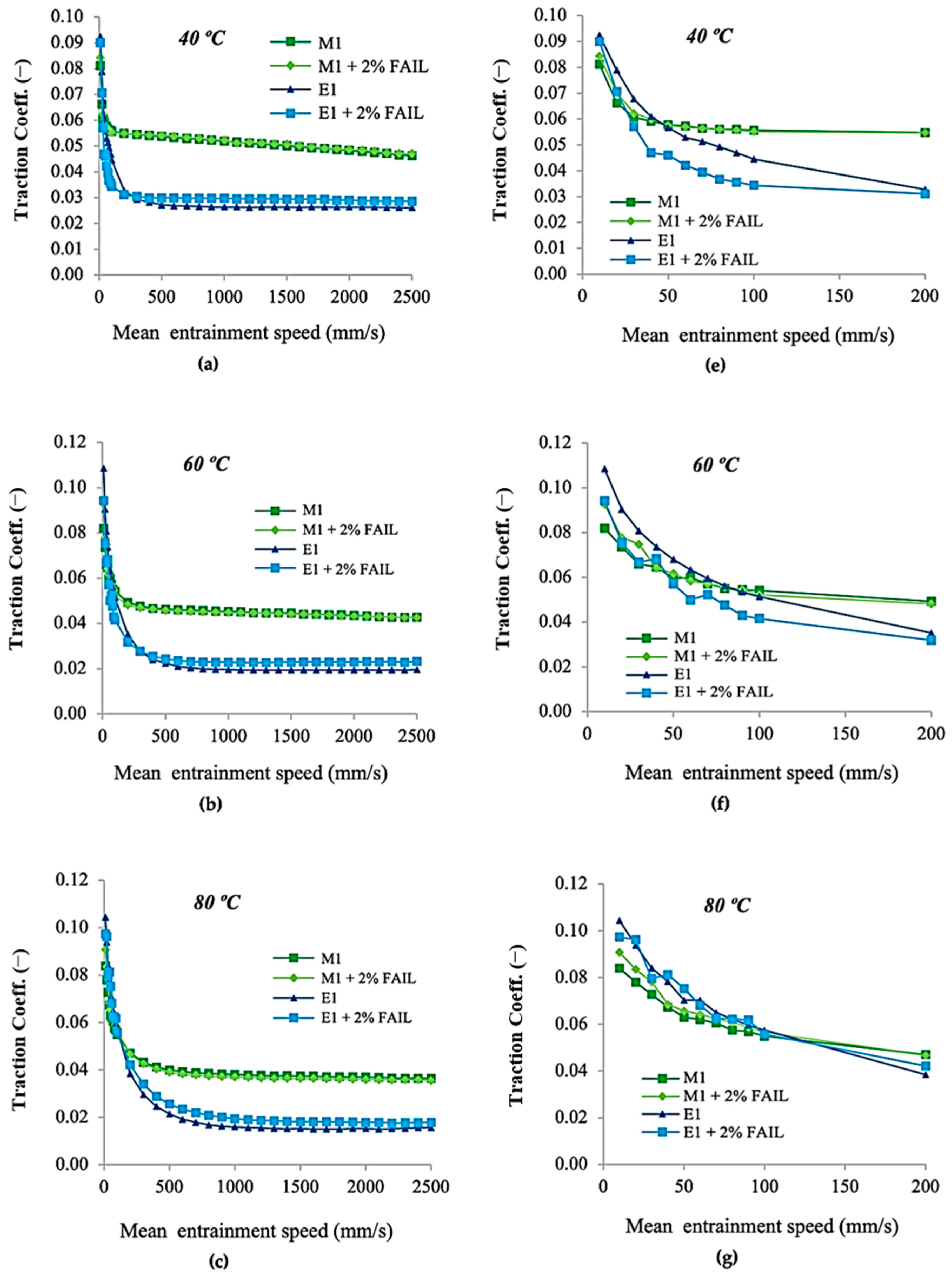
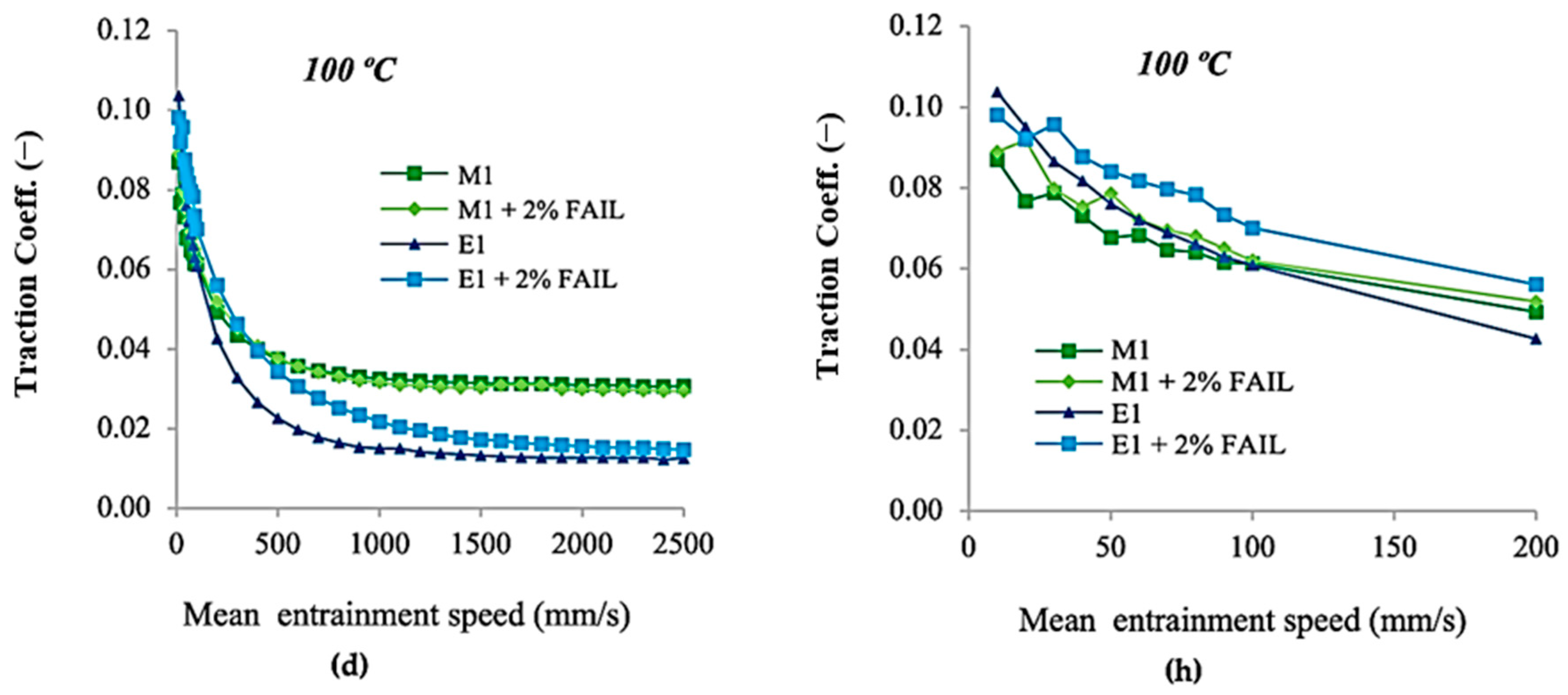
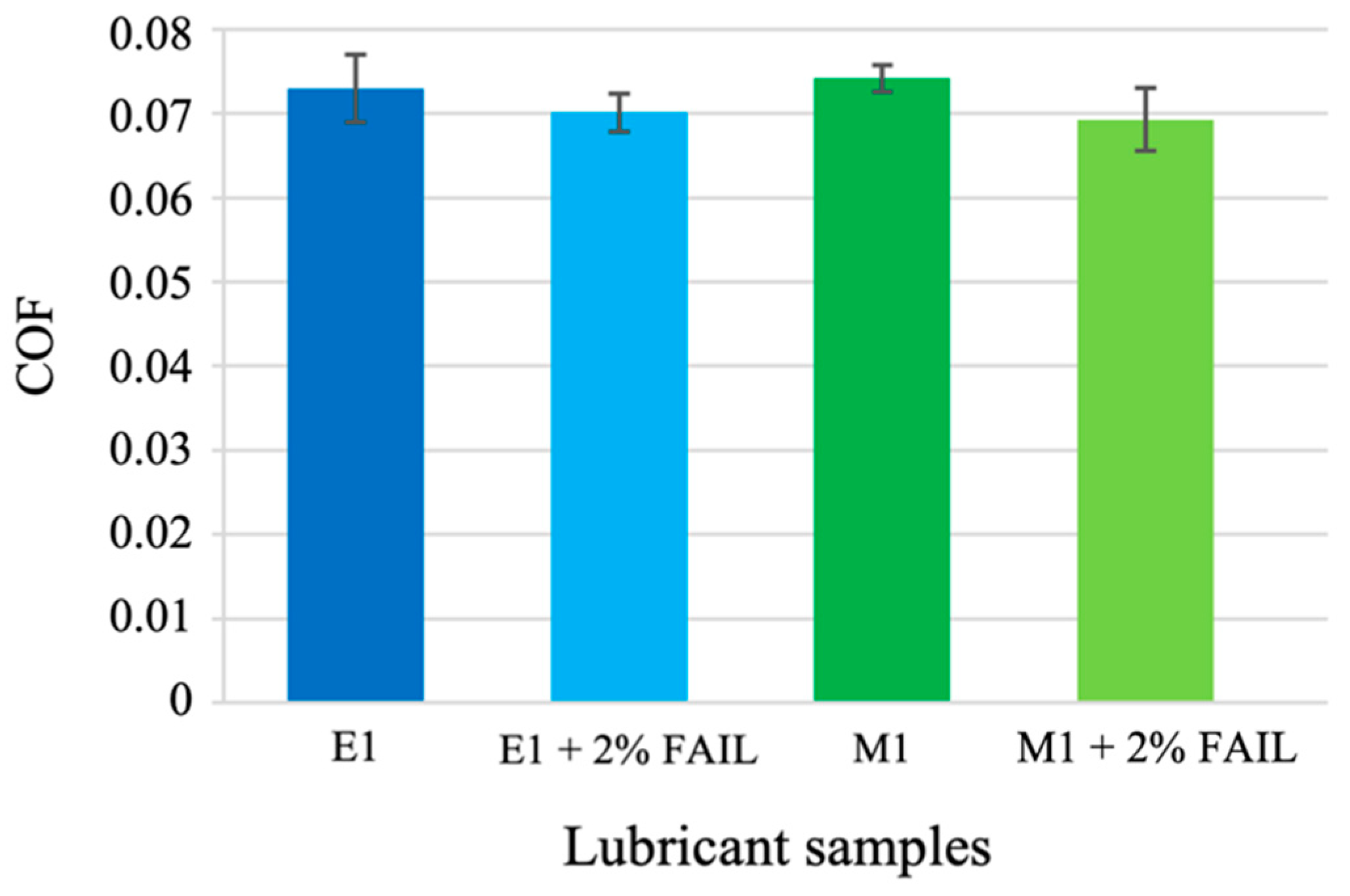
3.2. Tribological Tests
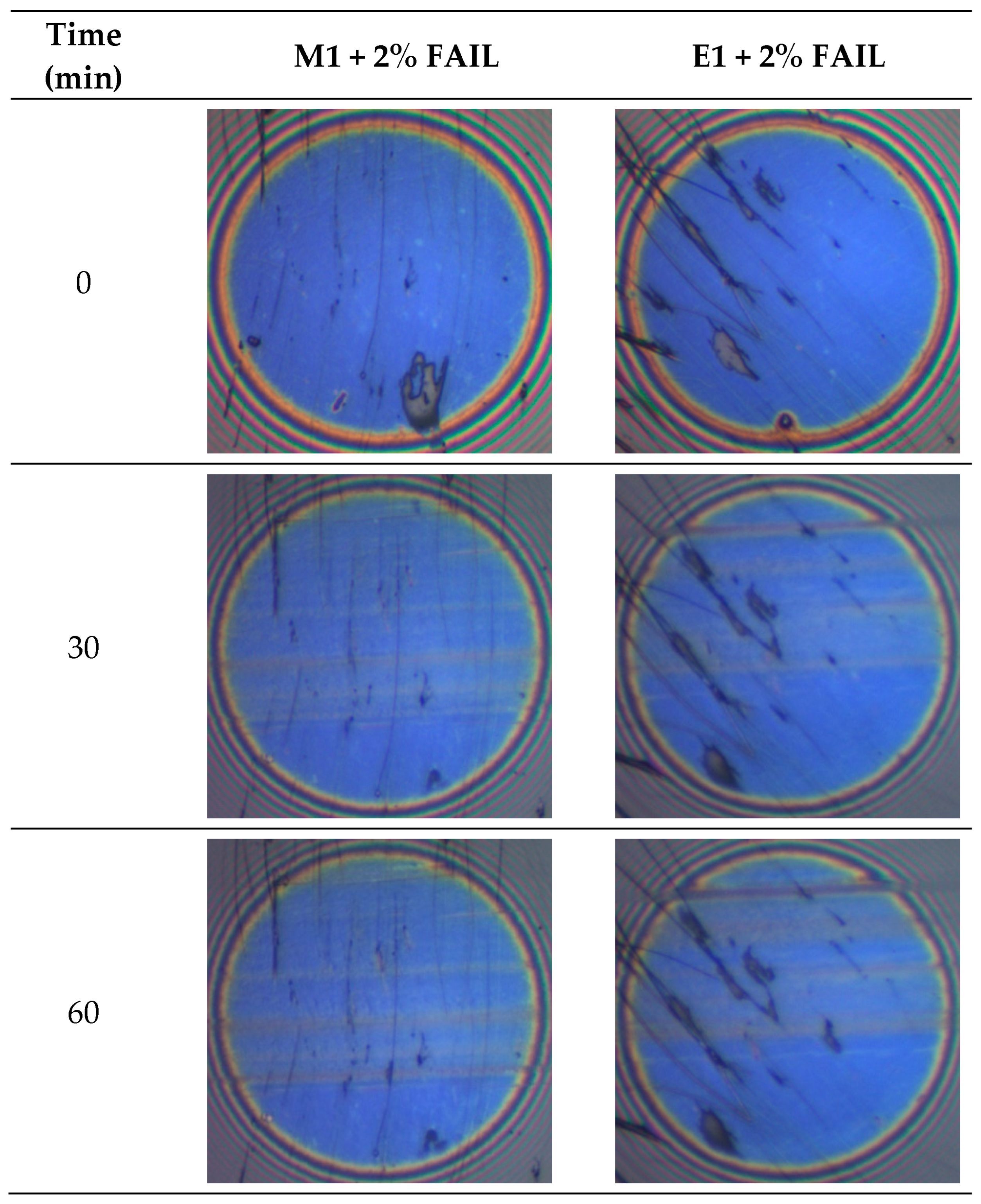
3.3. Surface Analysis
4. Conclusions
- The FAIL was miscible in the two base oils and no important changes in density and viscosity were found by using it as an additive at 2 wt.% concentration.
- The M1 + FAIL blend showed the highest friction values under the EHL regime and the lowest ones under the ML regime, corresponding to its higher viscosity, which facilitates the formation of a thicker lubricant film at lower speeds. In contrast, the E1 + FAIL showed the lowest friction values under EHL, probably due to its higher polarity and affinity for the metallic surface.
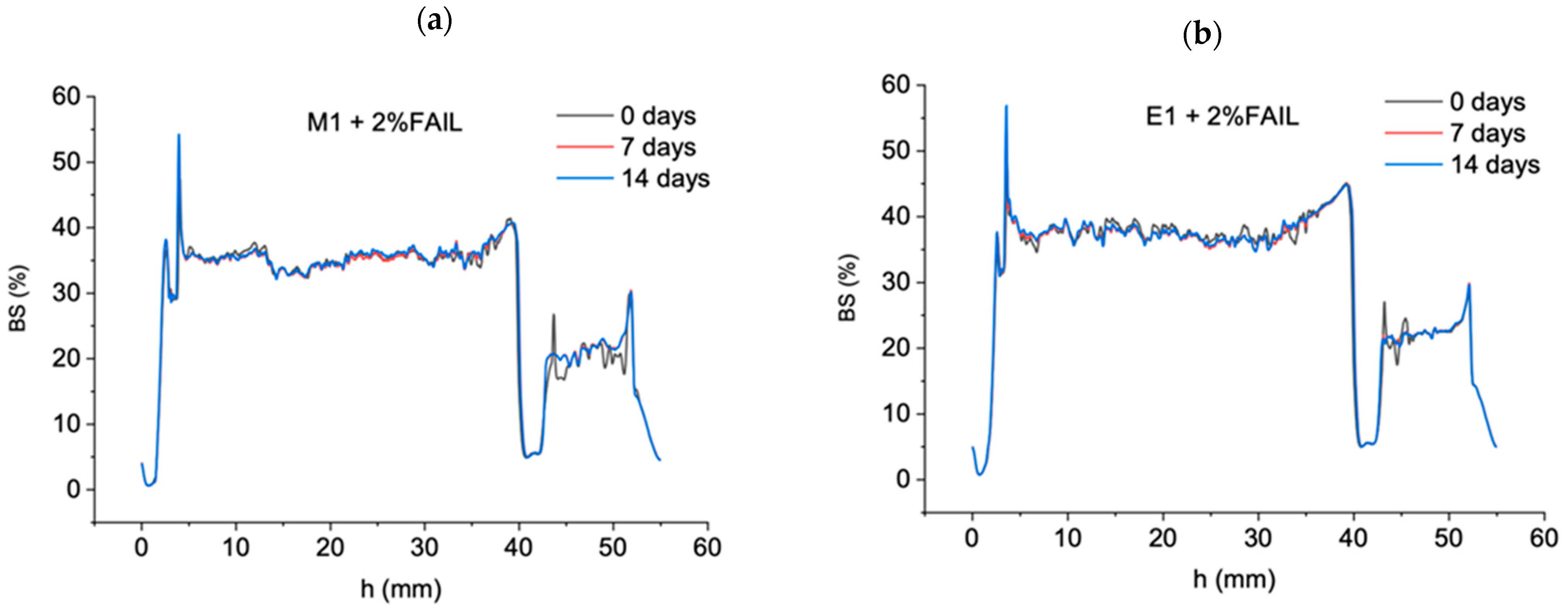
- E1 + FAIL showed a constant increase in the tribofilm thickness during the test, achieving the highest values recorded for the two lubricant samples studied. On the other hand, the M1 + FAIL only registered a very slight increase in tribofilm thickness.
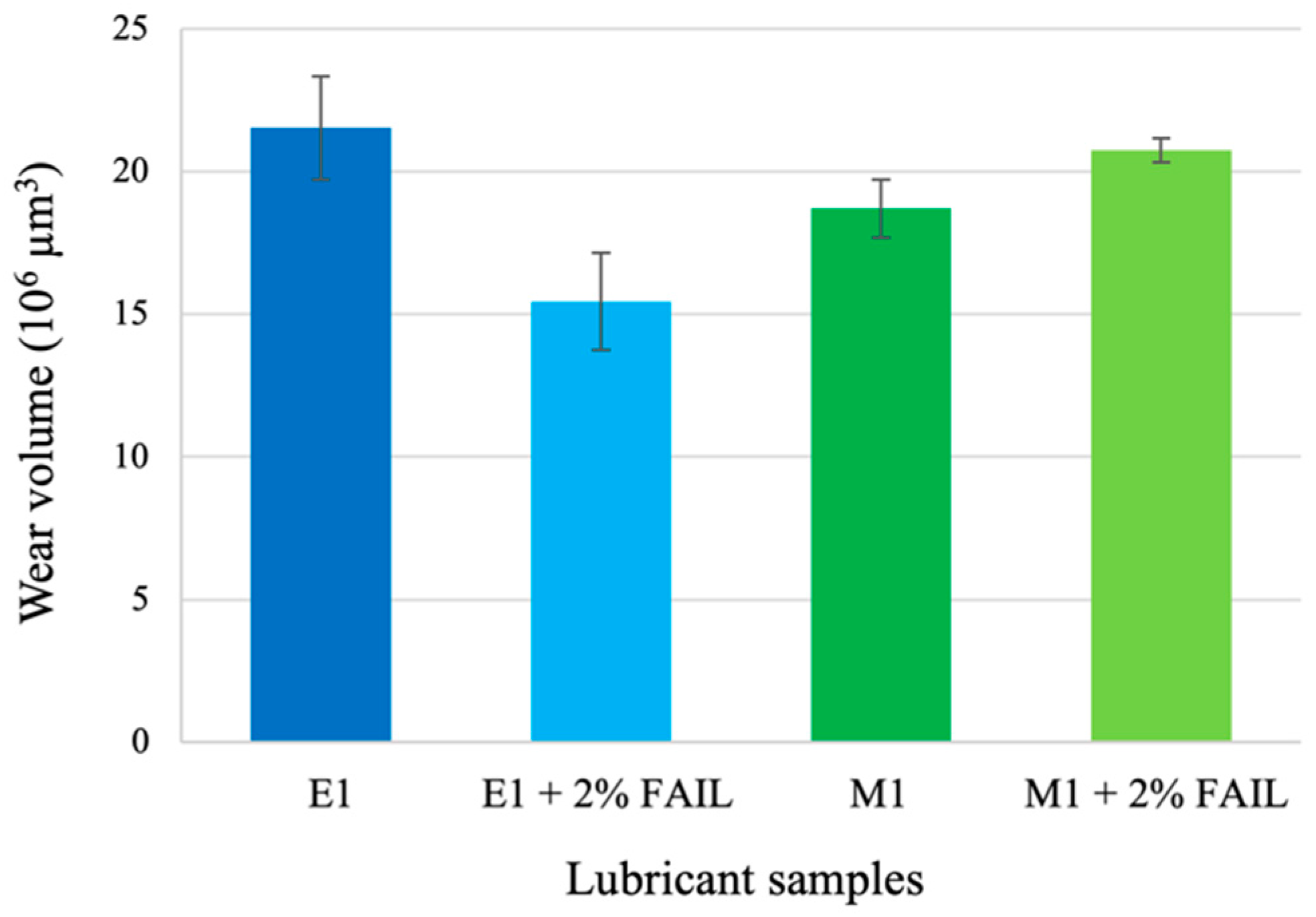
- The E1 + FAIL blend outperformed the antiwear behavior of the base oil, probably due to the better chemical affinity (higher polarity) of this blend for the metallic surface.
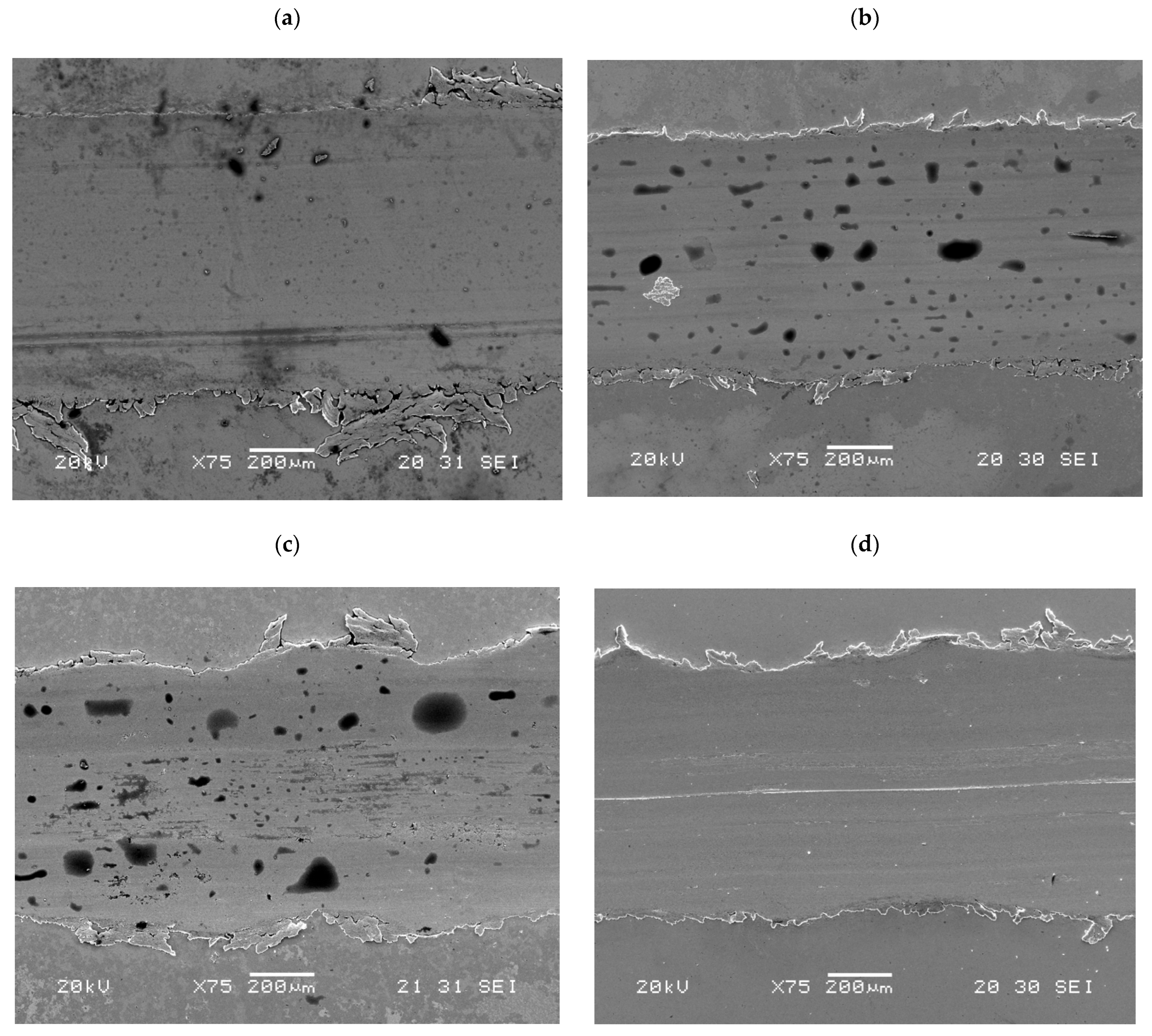
- The predominant wear mechanism found after wear tests was of the adhesive type with plastic deformation, and the presence of increasing amounts of organic oxygen (C–O plus C=O) on the wear scar led to better antiwear performance when the E1 + FAIL blend was used.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walden, P. Molecular weights and electrical conductivity of several fused salts. Bull. Imp. Acad. Sci. 1914, 8, 405–422. [Google Scholar]
- Chum, H.L.; Koch, V.R.; Miller, L.L.; Osteryoung, R.A. Electrochemical scrutiny of organometallic iron complexes and hexamethylbenzene in a room temperature molten salt. J. Am. Chem. Soc. 1975, 97, 3264–3265. [Google Scholar] [CrossRef]
- Wilkes, J.S.; Levisky, J.A.; Wilson, R.A.; Hussey, C.L. Dialkylimidazolium chloroaluminate melts: A new class of room-temperature ionic liquids for electrochemistry, spectroscopy and synthesis. Inorg. Chem. 1982, 21, 1263–1264. [Google Scholar] [CrossRef]
- Wilkes, J.S.; Zaworotko, M.J. Air and water stable 1-ethyl-3-methylimidazolium based ionic liquids. J. Chem. Soc. Chem. Commun. 1992, 13, 965–967. [Google Scholar] [CrossRef]
- Olivier, H. Recent developments in the use of non-aqueous ionic liquids for two-phase catalysis. J. Mol. Catal. A Chem. 1999, 146, 285–289. [Google Scholar] [CrossRef]
- Wassercheid, P.; Welton, T. Ionic Liquid in Synthesis, 2nd ed.; Wiley: Berlin, Germany, 2008. [Google Scholar]
- Welton, T. Room-Temperature Ionic Liquids. Solvents for Synthesis and Catalysis. Chem. Rev. 1999, 99, 2071–2084. [Google Scholar] [CrossRef]
- Hagiwara, R.; Ito, Y. Room temperature ionic liquids of alkylimidazolium cations and fluoroanions. J. Fluor. Chem. 2000, 105, 221–227. [Google Scholar] [CrossRef]
- Ye, C.F.; Liu, W.M.; Chen, Y.X.; Yu, L.G. Room-temperature ionic liquids: A novel versatile lubricant. Chem. Commun. 2001, 21, 2244–2245. [Google Scholar] [CrossRef]
- Jiménez, A.E.; Bermúdez, M.D.; Iglesias, P.; Carrión, F.J.; Martínez-Nicolás, G. 1-N-alkyl-3-methylimidazolium ionic liquids as neat lubricants and lubricant additives in steel–aluminium contacts. Wear 2006, 260, 766–782. [Google Scholar] [CrossRef]
- Iglesias, P.; Bermúdez, M.D.; Carrión, F.J.; Martıínez-Nicolás, G. Friction and wear of aluminium–steel contacts lubricated with ordered fluids-neutral and ionic liquid crystals as oil additives. Wear 2004, 256, 386–392. [Google Scholar] [CrossRef]
- Wang, H.; Lu, Q.; Ye, C.; Liu, W.; Cui, Z. Friction and wear behaviors of ionic liquid of alkylimidazolium hexafluorophosphates as lubricants for steel/steel contact. Wear 2004, 256, 44–48. [Google Scholar] [CrossRef]
- Imran; Amanullah. Phosphorus and Boron Application Optimizing Biofortification of P and Productivity of French Bean (Phaseolus vulgaris L.). Commun. Soil Sci. Plant Anal. 2021, 52, 2876–2883. [Google Scholar] [CrossRef]
- Cigno, E.; Magagnoli, C.; Pierce, M.; Iglesias, P. Lubricating ability of two phosphonium-based ionic liquids as additives of a bio-oil for use in wind turbines gearboxes. Wear 2017, 376–377, 756–765. [Google Scholar] [CrossRef]
- Qu, J.; Truhan, J.J.; Dai, S.; Luo, H.; Blau, P.J. Ionic liquids with ammonium cations as lubricants or additives. Tribol. Lett. 2006, 22, 207–214. [Google Scholar] [CrossRef]
- Kamimura, H.; Kubo, T.; Minami, I.; Mori, S. Effect andmechanism ofadditives for ionic liquids as new lubricants. Tribol. Int. 2007, 40, 620–625. [Google Scholar] [CrossRef]
- Fox, M.F.; Priest, M. Tribological properties of ionic liquids as lubricants and additives. Part 1: Synergistic tribofilm formation between ionic liquids and tricresyl phosphate. Proc. Inst. Mech. Eng. J J. Eng. Tribol. 2008, 222, 291–303. [Google Scholar] [CrossRef]
- Minami, I. Ionic Liquids in Tribology. Molecules 2009, 14, 2286–2305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bermúdez, M.D.; Jiménez, A.-E.; Sanes, J.; Carrión, F.-J. Ionic Liquids as Advanced Lubricant Fluids. Molecules 2009, 14, 2888–2908. [Google Scholar] [CrossRef] [PubMed]
- Matczak, L.; Johanning, C.; Gil, E.; Guo, H.; Smith, T.W.; Schertzer, M.; Iglesias, P. Effect of cation nature on the lubricating and physicochemical properties of three ionic liquids. Tribol. Int. 2018, 124, 23–33. [Google Scholar] [CrossRef]
- Zhou, F.; Liang, Y.; Liu, W. Ionic liquid lubricants: Designed chemistry for engineering applications. Chem. Soc. Rev. 2009, 38, 2590–2599. [Google Scholar] [CrossRef]
- Fraser, K.J.; Macfarlane, D.R. Phosphonium-Based Ionic Liquids: An Overview. Aust. J. Chem. 2009, 62, 309–321. [Google Scholar] [CrossRef]
- Palacio, M.; Bhushan, B. A Review of Ionic Liquids for Green Molecular Lubrication in Nanotechnology. Tribol. Lett. 2010, 40, 247–268. [Google Scholar] [CrossRef]
- Somers, A.E.; Howlett, P.C.; Macfarlane, D.R.; Forsyth, M. A Review of Ionic Liquid Lubricants. Lubricants 2013, 1, 3–21. [Google Scholar] [CrossRef] [Green Version]
- Otero, I.; López, E.R.; Reichelt, M.; Villanueva, M.; Salgado, J.; Fernández, J. Ionic Liquids Based on Phosphonium Cations As Neat Lubricants or Lubricant Additives for a Steel/Steel Contact. ACS Appl. Mater. Interfaces 2014, 6, 13115–13128. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Gabler, C.; Doerr, N.; Aswath, P.B. Mechanism of tribofilm formation with P and S containing ionic liquids. Tribol. Int. 2015, 92, 353–364. [Google Scholar] [CrossRef]
- Battez, A.E.H.; Bartolome, M.; Blanco, D.; Viesca, J.L.; Fernández-González, A.; González, R. Phosphonium cation-based ionic liquids as neat lubricants: Physicochemical and tribological performance. Tribol. Int. 2016, 95, 118–131. [Google Scholar] [CrossRef]
- Xiao, H. Ionic Liquid Lubricants: Basics and Applications. Tribol. Trans. 2016, 60, 20–30. [Google Scholar] [CrossRef]
- Battez, A.H.; Fernandes, C.M.; Martins, R.C.; Graça, B.M.; Anand, M.; Blanco, D.; Seabra, J.H. Two phosphonium cation-based ionic liquids used as lubricant additive. Part II: Tribofilm analysis and friction torque loss in cylindrical roller thrust bearings at constant temperature. Tribol. Int. 2017, 109, 496–504. [Google Scholar] [CrossRef]
- Battez, A.H.; Fernandes, C.M.; Martins, R.C.; Bartolomé, M.; González, R.; Seabra, J.H. Two phosphonium cation-based ionic liquids used as lubricant additive. Tribol. Int. 2016, 107, 233–239. [Google Scholar] [CrossRef]
- Zhao, D.; Liao, Y.; Zhang, Z. Toxicity of Ionic Liquids. CLEAN—Soil Air Water 2007, 35, 42–48. [Google Scholar] [CrossRef]
- Pretti, C.; Chiappe, C.; Baldetti, I.; Brunini, S.; Monni, G.; Intorre, L. Acute toxicity of ionic liquids for three freshwater organisms: Pseudokirchneriella subcapitata, Daphnia magna and Danio rerio. Ecotoxicol. Environ. Saf. 2009, 72, 1170–1176. [Google Scholar] [CrossRef] [PubMed]
- Ventura, S.P.; Marques, C.S.; Rosatella, A.A.; Afonso, C.A.; Gonçalves, F.; Coutinho, J.A. Toxicity assessment of various ionic liquid families towards Vibrio fischeri marine bacteria. Ecotoxicol. Environ. Saf. 2012, 76, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Ben Ghanem, O.; Papaiconomou, N.; Mutalib, M.A.; Viboud, S.; El-Harbawi, M.; Uemura, Y.; Gonfa, G.; Bustam, M.A.; Lévêque, J.-M. Thermophysical properties and acute toxicity towards green algae and Vibrio fischeri of amino acid-based ionic liquids. J. Mol. Liq. 2015, 212, 352–359. [Google Scholar] [CrossRef]
- Salgado, J.; Parajó, J.; Teijeira, T.; Cruz, O.; Proupín, J.; Villanueva, M.; Rodríguez-Añón, J.; Verdes, P.; Reyes, O. New insight into the environmental impact of two imidazolium ionic liquids. Effects on seed germination and soil microbial activity. Chemosphere 2017, 185, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Predel, T.; Schlücker, E.; Wasserscheid, P.; Gerhard, D.; Arlt, W. Ionic Liquids as Operating Fluids in High Pressure Applications. Chem. Eng. Technol. 2007, 30, 1475–1480. [Google Scholar] [CrossRef]
- Nainaparampil, J.J.; Eapen, K.C.; Sanders, J.H.; Voevodin, A. Ionic-liquid lubrication of sliding MEMS contacts: Comparison of AFM liquid cell and device-level tests. J. Microelectromechan. Syst. 2007, 16, 836–843. [Google Scholar] [CrossRef]
- Street, K.W.; Morales, W.; Koch, V.R.; Valco, D.J.; Richard, R.M.; Hanks, N. Evaluation of Vapor Pressure and Ultra-High Vacuum Tribological Properties of Ionic Liquids. Tribol. Trans. 2011, 54, 911–919. [Google Scholar] [CrossRef]
- Mo, Y.; Huang, F.; Zhao, F. Functionalized imidazolium wear-resistant ionic liquid ultrathin films for MEMS/NEMS applications. Surf. Interface Anal. 2010, 43, 1006–1014. [Google Scholar] [CrossRef]
- Zhang, S.; Hu, L.; Qiao, D.; Feng, D.; Wang, H. Vacuum tribological performance of phosphonium-based ionic liquids as lubricants and lubricant additives of multialkylatedcyclopentanes. Tribol. Int. 2013, 66, 289–295. [Google Scholar] [CrossRef]
- Jiang, D.; Hu, L.; Feng, D. Tribological properties of crown-type phosphate ionic liquids as lubricating additives in rapeseed oils. Lubr. Sci. 2012, 25, 195–207. [Google Scholar] [CrossRef]
- García, A.; González, R.; Battez, A.E.H.; Viesca, J.L.; Monge, R.; Fernández-González, A.; Hadfield, M. Ionic liquids as a neat lubricant applied to steel–steel contacts. Tribol. Int. 2014, 72, 42–50. [Google Scholar] [CrossRef] [Green Version]
- Jimenez, A.E.; Bermúdez, M.D.; Carrión, F.J.; Martínez-Nicolás, G. Room temperature ionic liquids as lubricant additives in steel–aluminium contacts: Influence of sliding velocity, normal load and temperature. Wear 2006, 261, 347–359. [Google Scholar] [CrossRef]
- Yu, B.; Bansal, D.G.; Qu, J.; Sun, X.; Luo, H.; Dai, S.; Blau, P.J.; Bunting, B.G.; Mordukhovich, G.; Smolenski, D.J. Oil-miscible and non-corrosive phosphonium-based ionic liquids as candidate lubricant additives. Wear 2012, 289, 58–64. [Google Scholar] [CrossRef]
- Totolin, V.; Minami, I.; Gabler, C.; Brenner, J.; Dörr, N. Lubrication Mechanism of Phosphonium Phosphate Ionic Liquid Additive in Alkylborane–Imidazole Complexes. Tribol. Lett. 2014, 53, 421–432. [Google Scholar] [CrossRef]
- González, R.; Bartolomé, M.; Blanco, D.; Viesca, J.L.; Fernández-González, A.; Battez, A.E.H. Effectiveness of phosphonium cation-based ionic liquids as lubricant additive. Tribol. Int. 2016, 98, 82–93. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Qu, J. Ionic Liquids as Lubricant Additives: A Review. ACS Appl. Mater. Interfaces 2017, 9, 3209–3222. [Google Scholar] [CrossRef] [PubMed]
- Sanes, J.; Avilés, M.-D.; Saurín, N.; Espinosa, T.; Carrión, F.-J.; Bermúdez, M.-D. Synergy between graphene and ionic liquid lubricant additives. Tribol. Int. 2017, 116, 371–382. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, Q.; Cai, M.; Shi, L.; Zhou, F.; Liu, W. Ibuprofen-Based Ionic Liquids as Additives for Enhancing the Lubricity and Antiwear of Water–Ethylene Glycol Liquid. Tribol. Lett. 2017, 65, 55. [Google Scholar] [CrossRef]
- Viesca, J.L.; Mallada, M.; Blanco, D.; Fernández-González, A.; Casado, J.E.; González, R.; Battez, A.H. Lubrication performance of an ammonium cation-based ionic liquid used as an additive in a polar oil. Tribol. Int. 2017, 116, 422–430. [Google Scholar] [CrossRef]
- Huang, G.; Yu, Q.; Ma, Z.; Cai, M.; Zhou, F.; Liu, W. Oil-soluble ionic liquids as antiwear and extreme pressure additives in poly-α-olefin for steel/steel contacts. Friction 2017, 7, 18–31. [Google Scholar] [CrossRef] [Green Version]
- Guangteng, G.; Spikes, H. Fractionation of liquid lubricants at solid surfaces. Wear 1996, 200, 336–345. [Google Scholar] [CrossRef]
- Rico, J.F.; Battez, A.H.; Cuervo, D.G. Wear prevention characteristics of binary oil mixtures. Wear 2002, 253, 827–831. [Google Scholar] [CrossRef]
- Cambiella, A.; Benito, J.M.; Pazos, C.; Coca, J.; Hernández, A.; Fernández, J.E. Formulation of emulsifiable cutting fluids and extreme pressure behaviour. J. Mater. Process. Technol. 2007, 184, 139–145. [Google Scholar] [CrossRef]
- Battez, A.E.H.; González, R.; Viesca, J.L.; Blanco, D.; Asedegbega, E.; Osorio, A. Tribological behaviour of two imidazolium ionic liquids as lubricant additives for steel/steel contacts. Wear 2009, 266, 1224–1228. [Google Scholar] [CrossRef]
- Mistry, K.; Fox, M.F.; Priest, M. Lubrication of an electroplated nickel matrix silicon carbide coated eutectic aluminium—Silicon alloy automotive cylinder bore with an ionic liquid as a lubricant additive. Proc. Inst. Mech. Eng. J J. Eng. Tribol. 2009, 223, 563–569. [Google Scholar] [CrossRef]
- Lu, R.; Nanao, H.; Kobayashi, K.; Kubo, T.; Mori, S. Effect of Lubricant Additives on Tribochemical Decomposition of Hydrocarbon Oil on Nascent Steel Surfaces. J. Jpn. Pet. Inst. 2010, 53, 55–60. [Google Scholar] [CrossRef] [Green Version]
- Blanco, D.; Battez, A.E.H.; Viesca, J.L.; González, R.; Fernández-González, A. Lubrication of CrN Coating With Ethyl-Dimethyl-2-Methoxyethylammonium Tris(pentafluoroethyl)Trifluorophosphate Ionic Liquid as Additive to PAO 6. Tribol. Lett. 2011, 41, 295–302. [Google Scholar] [CrossRef]
- Blanco, D.; González, R.; Battez, A.H.; Viesca, J.L.; Fernández-González, A. Use of ethyl-dimethyl-2-methoxyethylammonium tris(pentafluoroethyl)trifluorophosphate as base oil additive in the lubrication of TiN PVD coating. Tribol. Int. 2011, 44, 645–650. [Google Scholar] [CrossRef]
- Cai, M.; Liang, Y.; Yao, M.; Xia, Y.; Zhou, F.; Liu, W. Imidazolium Ionic Liquids as Antiwear and Antioxidant Additive in Poly(ethylene glycol) for Steel/Steel Contacts. ACS Appl. Mater. Interfaces 2010, 2, 870–876. [Google Scholar] [CrossRef]
- Jiménez, A.-E.; Bermúdez, M.-D. Short alkyl chain imidazolium ionic liquid additives in lubrication of three aluminium alloys with synthetic ester oil. Tribol.—Mater. Surf. Interfaces 2012, 6, 109–115. [Google Scholar] [CrossRef]
- Somers, A.E.; Khemchandani, B.; Howlett, P.C.; Sun, J.; MacFarlane, D.R.; Forsyth, M. Ionic Liquids as Antiwear Additives in Base Oils: Influence of Structure on Miscibility and Antiwear Performance for Steel on Aluminum. ACS Appl. Mater. Interfaces 2013, 5, 11544–11553. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Liang, Y.; Zhou, F.; Liu, W. A novel imidazolium salt with antioxidation and anticorrosion dual functionalities as the additive in poly(ethylene glycol) for steel/steel contacts. Wear 2013, 306, 197–208. [Google Scholar] [CrossRef]
- Qiao, D.; Wang, H.; Feng, D. Tribological Performance and Mechanism of Phosphate Ionic Liquids as Additives in Three Base Oils for Steel-on-aluminum Contact. Tribol. Lett. 2014, 55, 517–531. [Google Scholar] [CrossRef]
- Kronberger, M.; Pagano, F.; Pejaković, V.; Igartua, A.; Urbistondo, E.; Kalin, M. Miscibility and tribological investigations of ionic liquids in biodegradable esters. Lubr. Sci. 2014, 26, 463–487. [Google Scholar] [CrossRef]
- Gusain, R.; Gupta, P.; Saran, S.; Khatri, O.P. Halogen-Free Bis(imidazolium)/Bis(ammonium)-Di[bis(salicylato)borate] Ionic Liquids as Energy-Efficient and Environmentally Friendly Lubricant Additives. ACS Appl. Mater. Interfaces 2014, 6, 15318–15328. [Google Scholar] [CrossRef]
- Zhu, L.; Zhao, Q.; Wu, X.; Zhao, G.; Wang, X. A novel phosphate ionic liquid plays dual role in synthetic ester oil: From synthetic catalyst to anti-wear additive. Tribol. Int. 2016, 97, 192–199. [Google Scholar] [CrossRef]
- Qiu, X.; Lu, L.; Qu, Z.; Liao, J.; Fan, Q.; Shah, F.U.; Zhang, W.; An, R. Probing the nanofriction of non-halogenated phosphonium-based ionic liquid additives in glycol ether oil on titanium surface. Friction 2022, 10, 268–281. [Google Scholar] [CrossRef]
- Blanco, D.; González, R.; Viesca, J.L.; Fernández-González, A.; Bartolomé, M.; Battez, A.H. Antifriction and Antiwear Properties of an Ionic Liquid with Fluorine-Containing Anion Used as Lubricant Additive. Tribol. Lett. 2017, 65, 66. [Google Scholar] [CrossRef]
- Battez, A.H.; Ramos, D.; Blanco, D.; González, R.; Fernández-González, A.; Viesca, J.L. Lubrication Properties of the Ionic Liquid Dodecyl-3 Methylimidazolium bis(trifluoromethylsulfonyl)imide. Tribol. Lett. 2017, 66, 19. [Google Scholar] [CrossRef]
- González, R.; Ramos, D.; Blanco, D.; Viesca, J.L.; Hadfield, M.; Battez, A.H. Tribological performance of tributylmethylammonium bis (trifluoromethylsulfonyl) amide as neat lubricant and as an additive in a polar oil. Friction 2019, 7, 282–288. [Google Scholar] [CrossRef] [Green Version]
- Willing, A. Lubricants based on renewable resources—An environmentally compatible alternative to mineral oil products. Chemosphere 2001, 43, 89–98. [Google Scholar] [CrossRef]
- Mannekote, J.K.; Menezes, P.L.; Kailas, S.V.; Sathwik, R.K.C. Tribology of Green Lubricants. In Tribology Scientists and Engineers; Springer: New York, NY, USA, 2013; pp. 495–521. [Google Scholar]
- Holmberg, K.; Erdemir, A. Influence of tribology on global energy consumption, costs and emissions. Friction 2017, 5, 263–284. [Google Scholar] [CrossRef]
- Guo, H.; Iglesias, P. Tribological behavior of ammonium-based protic ionic liquid as lubricant additive. Friction 2021, 9, 169–178. [Google Scholar] [CrossRef]
- Yu, Q.; Zhang, C.; Dong, R.; Shi, Y.; Wang, Y.; Bai, Y.; Zhang, J.; Cai, M.; Zhou, F.; Liu, W. Physicochemical and tribological properties of gemini-type halogen-free dicationic ionic liquids. Friction 2021, 9, 344–355. [Google Scholar] [CrossRef]
- Costa, S.P.F.; Azevedo, A.M.O.; Pinto, P.C.A.G.; Saraiva, M.L.M.F.S. Environmental Impact of Ionic Liquids: Recent Advances in (Eco)toxicology and (Bio)degradability. ChemSusChem 2017, 10, 2321–2347. [Google Scholar] [CrossRef]
- Adawiyah, N.; Hawatulaila, S.; Aini, A.; Vijaya, A.; Ibrahim, M.; Moniruzzaman, M. Synthesis, characterization, ecotoxicity and biodegradability evaluations of novel biocompatible surface active lauroyl sarcosinate ionic liquids. Chemosphere 2019, 229, 349–357. [Google Scholar] [CrossRef]
- Parmentier, D.; Metz, S.J.; Kroon, M.C. Tetraalkylammonium oleate and linoleate based ionic liquids: Promising extractants for metal salts. Green Chem. 2012, 15, 205–209. [Google Scholar] [CrossRef]
- Rocha, M.A.A.; Bruinhorst, A.V.D.; Schröer, W.; Rathke, B.; Kroon, M.C. Physicochemical properties of fatty acid based ionic liquids. J. Chem. Thermodyn. 2016, 100, 156–164. [Google Scholar] [CrossRef]
- Gusain, R.; Dhingra, S.; Khatri, O.P. Fatty-Acid-Constituted Halogen-Free Ionic Liquids as Renewable, Environmentally Friendly, and High-Performance Lubricant Additives. Ind. Eng. Chem. Res. 2016, 55, 856–865. [Google Scholar] [CrossRef]
- Gusain, R.; Khatri, O.P. Fatty acid ionic liquids as environmentally friendly lubricants for low friction and wear. RSC Adv. 2016, 6, 3462–3469. [Google Scholar] [CrossRef]
- Mezzetta, A.; Guazzelli, L.; Seggiani, M.; Pomelli, C.S.; Puccini, M.; Chiappe, C. A general environmentally friendly access to long chain fatty acid ionic liquids (LCFA-ILs). Green Chem. 2017, 19, 3103–3111. [Google Scholar] [CrossRef] [Green Version]
- Fan, M.; Ma, L.; Zhang, C.; Wang, Z.; Ruan, J.; Han, M.; Ren, Y.; Zhang, C.; Yang, D.; Zhou, F.; et al. Biobased Green Lubricants: Physicochemical, Tribological and Toxicological Properties of Fatty Acid Ionic Liquids. Tribol. Trans. 2017, 61, 195–206. [Google Scholar] [CrossRef]
- Khatri, P.K.; Aathira, M.S.; Thakre, G.D.; Jain, S.L. Synthesis and tribological behavior of fatty acid constituted tetramethylguanidinium (TMG) ionic liquids for a steel/steel contact. Mater. Sci. Eng. C 2018, 91, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Ding, T.; Huang, Y.; Zheng, L.; Ren, T. Fatty acid based phosphite ionic liquids as multifunctional lubricant additives in mineral oil and refined vegetable oil. Tribol. Int. 2018, 123, 316–324. [Google Scholar] [CrossRef]
- Blanco, D.; Rivera, N.; Oulego, P.; Díaz, M.; González, R.; Battez, A.H. Novel fatty acid anion-based ionic liquids: Contact angle, surface tension, polarity fraction and spreading parameter. J. Mol. Liq. 2019, 288, 110995. [Google Scholar] [CrossRef]
- Rivera, N.; Blanco, D.; Viesca, J.L.; Fernández-González, A.; González, R.; Battez, A.H. Tribological performance of three fatty acid anion-based ionic liquids (FAILs) used as lubricant additive. J. Mol. Liq. 2019, 296, 111881. [Google Scholar] [CrossRef]
- Khan, A.; Gusain, R.; Sahai, M.; Khatri, O.P. Fatty acids-derived protic ionic liquids as lubricant additive to synthetic lube base oil for enhancement of tribological properties. J. Mol. Liq. 2019, 293, 111444. [Google Scholar] [CrossRef]
- Rivera, N.; García, A.; Fernández-González, A.; Blanco, D.; González, R.; Battez, A.H. Tribological behavior of three fatty acid ionic liquids in the lubrication of different material pairs. J. Mol. Liq. 2019, 296, 111858. [Google Scholar] [CrossRef]
- Ali, K.; Moshikur, R.; Wakabayashi, R.; Tahara, Y.; Moniruzzaman, M.; Kamiya, N.; Goto, M. Synthesis and characterization of choline–fatty-acid-based ionic liquids: A new biocompatible surfactant. J. Colloid Interface Sci. 2019, 551, 72–80. [Google Scholar] [CrossRef]
- Oulego, P.; Faes, J.; Gonzalez, R.; Viesca, J.L.; Blanco, D.; Battez, A.H. Relationships between the physical properties and biodegradability and bacteria toxicity of fatty acid-based ionic liquids. J. Mol. Liq. 2019, 292, 111451. [Google Scholar] [CrossRef]
- Gundolf, T.; Weyhing-Zerrer, N.; Sommer, J.; Kalb, R.; Schoder, D.; Rossmanith, P.; Mester, P. Biological Impact of Ionic Liquids Based on Sustainable Fatty Acid Anions Examined with a Tripartite Test System. ACS Sustain. Chem. Eng. 2019, 7, 15865–15873. [Google Scholar] [CrossRef]
- Gusain, R.; Khan, A.; Khatri, O.P. Fatty acid-derived ionic liquids as renewable lubricant additives: Effect of chain length and unsaturation. J. Mol. Liq. 2019, 301, 112322. [Google Scholar] [CrossRef]
- Sernaglia, M.; Blanco, D.; Battez, A.H.; Viesca, J.L.; González, R.; Bartolomé, M. Two fatty acid anion-based ionic liquids—part I: Physicochemical properties and tribological behavior as neat lubricants. J. Mol. Liq. 2020, 305, 112827. [Google Scholar] [CrossRef]
- Sernaglia, M.; Blanco, D.; Battez, A.H.; González, R.; Fernández-González, A.; Bartolomé, M. Two fatty acid anion-based ionic liquids—Part II: Effectiveness as an additive to a polyol ester. J. Mol. Liq. 2020, 310, 113158. [Google Scholar] [CrossRef]
- Faes, J.; González, R.; Battez, A.H.; Blanco, D.; Fernández-González, A.; Viesca, J.L. Friction, Wear and Corrosion Behavior of Environmentally-Friendly Fatty Acid Ionic Liquids. Coatings 2021, 11, 21. [Google Scholar] [CrossRef]
- Viesca, J.; Faes, J.; Rivera, N.; Rodríguez, E.; Cadenas, M.; González, R. Thermal stability, traction and tribofilm formation of three fatty acid-derived ionic liquids. Tribol. Int. 2021, 154, 106712. [Google Scholar] [CrossRef]
- Battez, A.E.H.; Rivera, N.; Blanco, D.; Oulego, P.; Viesca, J.L.; González, R. Physicochemical, traction and tribofilm formation properties of three octanoate-, laurate- and palmitate-anion based ionic liquids. J. Mol. Liq. 2019, 284, 639–646. [Google Scholar] [CrossRef]
- Kapadia, R.; Glyde, R.; Wu, Y. In situ observation of phosphorous and non-phosphorous antiwear films using a mini traction machine with spacer layer image mapping. Tribol. Int. 2007, 40, 1667–1679. [Google Scholar] [CrossRef]
- National Institute of Standards and Technology (NIST). Available online: https://srdata.nist.gov/xps/main_search_menu.aspx (accessed on 26 February 2022).
- Thermo Scientific XPS: Knowledge Base. Available online: https://xpssimplified.com/ (accessed on 26 February 2022).
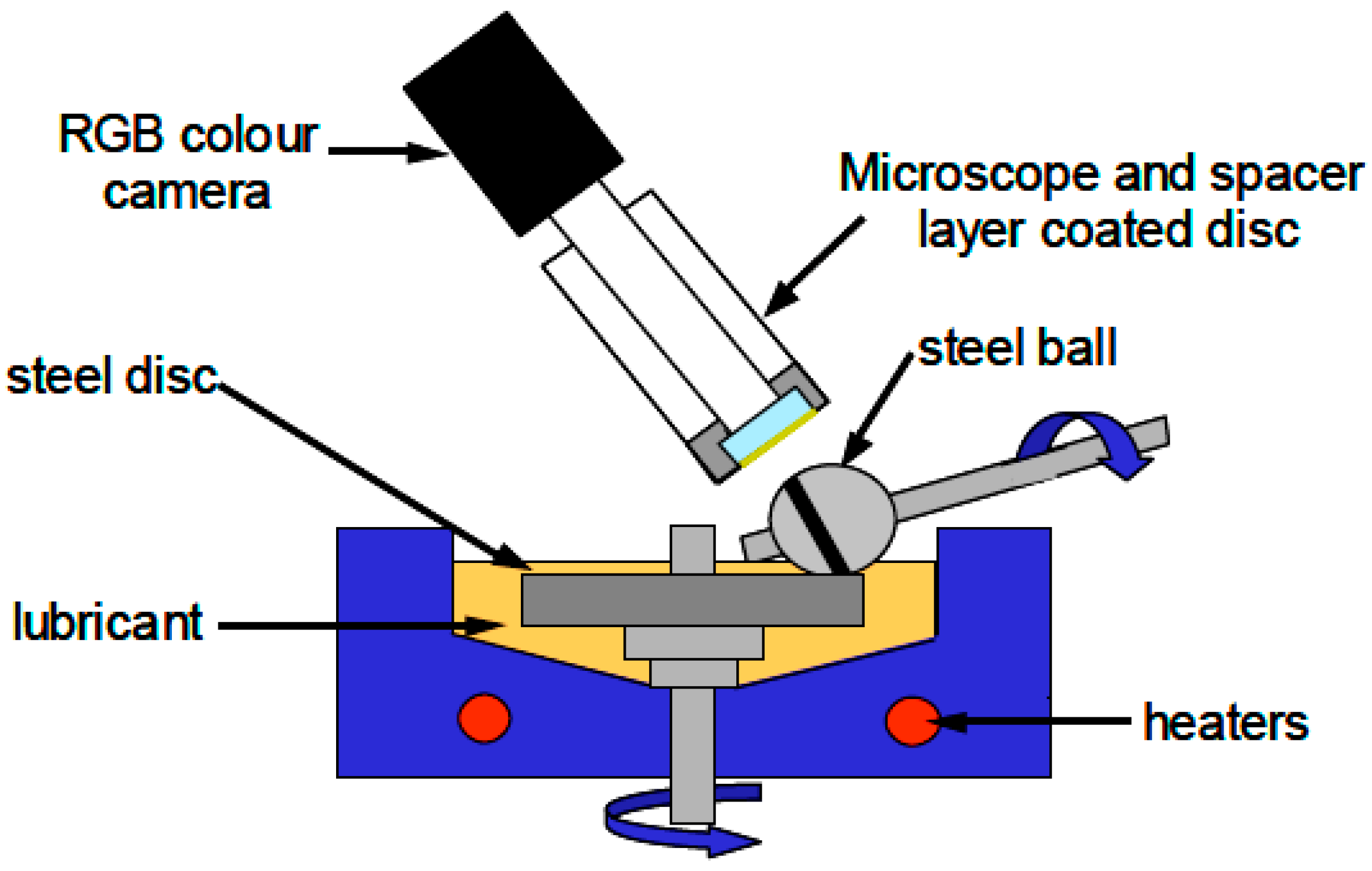


| Load (N)/Max. Contact Pressure (GPa) | Main Entrainment Speed a (mm‧s−1) | Slide-to-Roll Ratio b (%) | Temperature (°C) | |
|---|---|---|---|---|
| Stribeck measurement | 30/0.95 | 2500 to 10 c | 50 | 40, 60, 80, 100 |
| Film formation measurement | 50/1.13 | 150 | 50 | 100 |
| From | To | ||
|---|---|---|---|
| O1s | C=O | 532.0 | 532.2 |
| C–O | 533.3 | 533.5 | |
| Oxihydroxides | 530.9 | 531.1 | |
| Oxides | 529.4 | 529.7 | |
| Fe2p | Fe2O3 | 707.6 | 710.3 |
| FeO | 710.0 | 711.2 | |
| FeOOH | 712.5 | 713.4 |
| Oxides | C–O | FeOOH | C=O | |
|---|---|---|---|---|
| E1 | 54.66 | 8.44 | 20.85 | 16.05 |
| E1 + 2%FAIL | 45.65 | 13.58 | 18.75 | 22.03 |
| M | 54.01 | 7.04 | 22.6 | 16.34 |
| M1 + 2%FAIL | 57.74 | 6.99 | 20.41 | 14.86 |
| Fe2O3 | FeO | FeOOH | |
|---|---|---|---|
| E1 | 62.89 | 21.77 | 15.34 |
| E1 + 2%FAIL | 10.43 | 63.55 | 26.01 |
| M1 | 25.92 | 57.22 | 16.86 |
| M1 + 2%FAIL | 19.63 | 60.47 | 19.89 |
| C | O | N | Fe | |
|---|---|---|---|---|
| E1 | 72 | 23 | 0.2 | 4.3 |
| E1 + 2%FAIL | 76 | 20 | 0.2 | 3.4 |
| M1 | 70 | 24 | 0.5 | 5.3 |
| M1 + 2%FAIL | 60 | 30 | 1.0 | 9.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faes, J.; González, R.; Blanco, D.; Fernández-González, A.; Hernández-Battez, A.; Iglesias, P.; Viesca, J.L. Methyltrioctylammonium Octadecanoate as Lubricant Additive to Different Base Oils. Lubricants 2022, 10, 128. https://doi.org/10.3390/lubricants10060128
Faes J, González R, Blanco D, Fernández-González A, Hernández-Battez A, Iglesias P, Viesca JL. Methyltrioctylammonium Octadecanoate as Lubricant Additive to Different Base Oils. Lubricants. 2022; 10(6):128. https://doi.org/10.3390/lubricants10060128
Chicago/Turabian StyleFaes, Javier, Rubén González, David Blanco, Alfonso Fernández-González, Antolin Hernández-Battez, Patricia Iglesias, and José Luis Viesca. 2022. "Methyltrioctylammonium Octadecanoate as Lubricant Additive to Different Base Oils" Lubricants 10, no. 6: 128. https://doi.org/10.3390/lubricants10060128
APA StyleFaes, J., González, R., Blanco, D., Fernández-González, A., Hernández-Battez, A., Iglesias, P., & Viesca, J. L. (2022). Methyltrioctylammonium Octadecanoate as Lubricant Additive to Different Base Oils. Lubricants, 10(6), 128. https://doi.org/10.3390/lubricants10060128








