New Water-Ethylene Glycol Lubricants with Stearate Ionic Liquid Crystal Additive
Abstract
1. Introduction
2. Materials and Methods
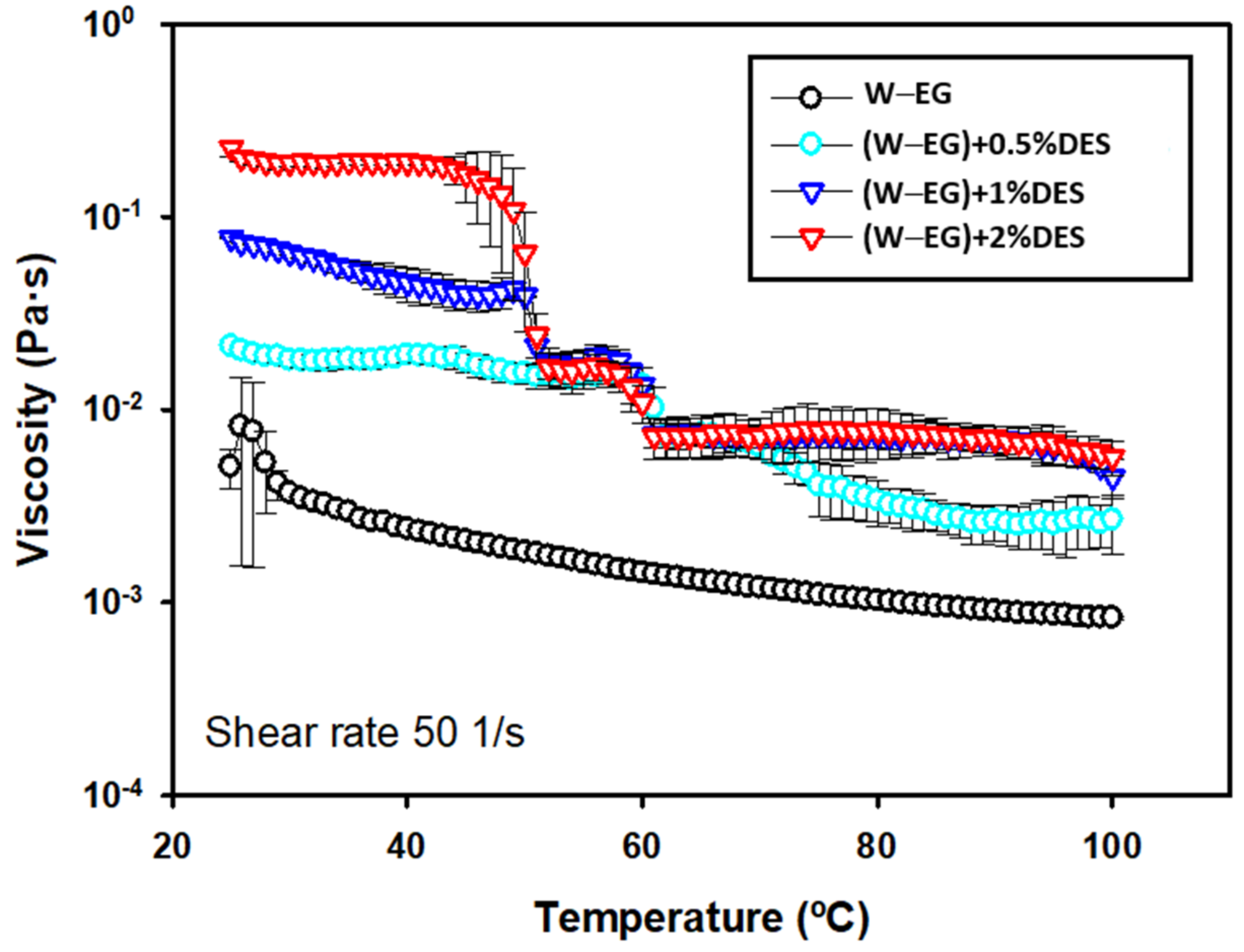
3. Results and Discussion
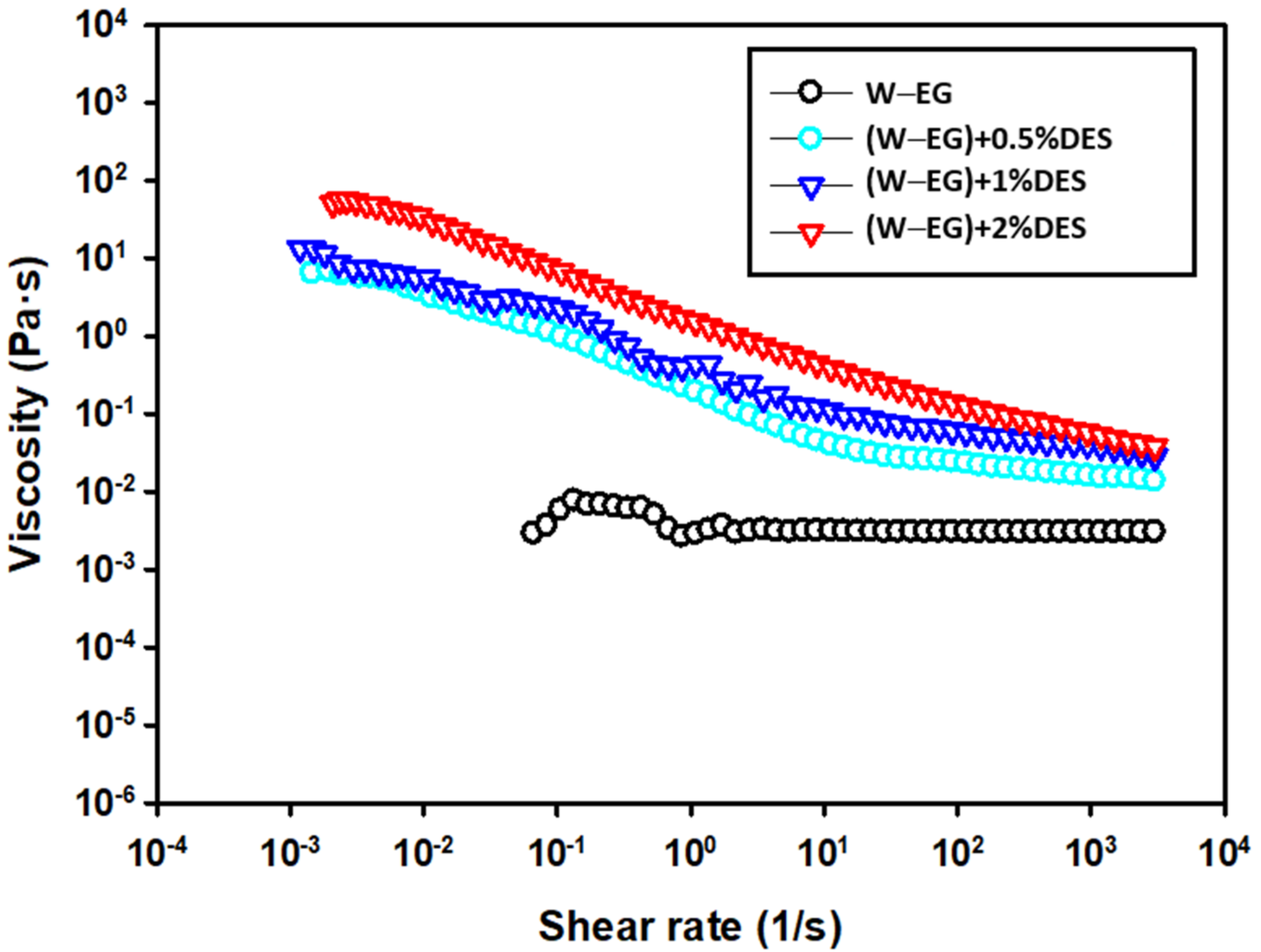
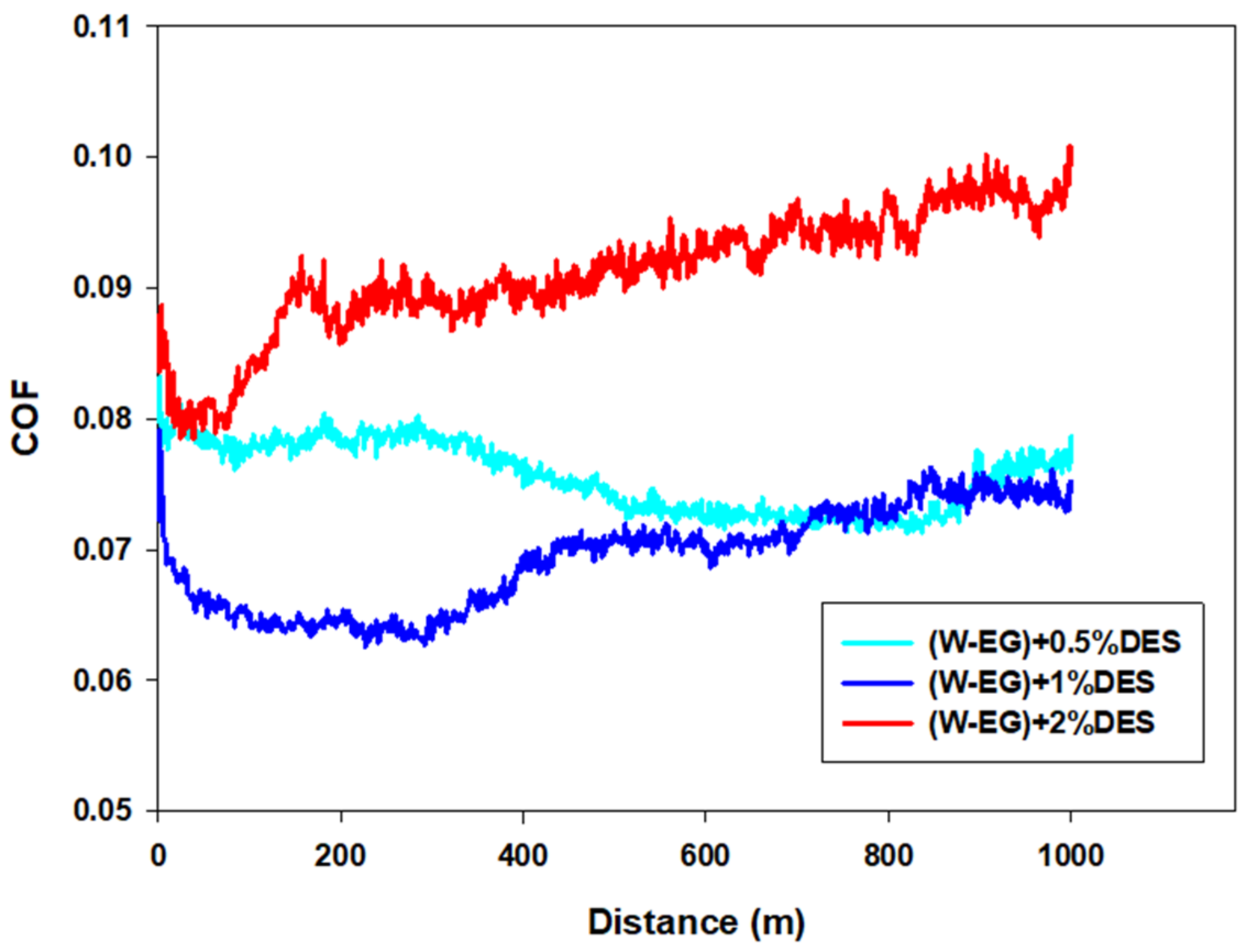
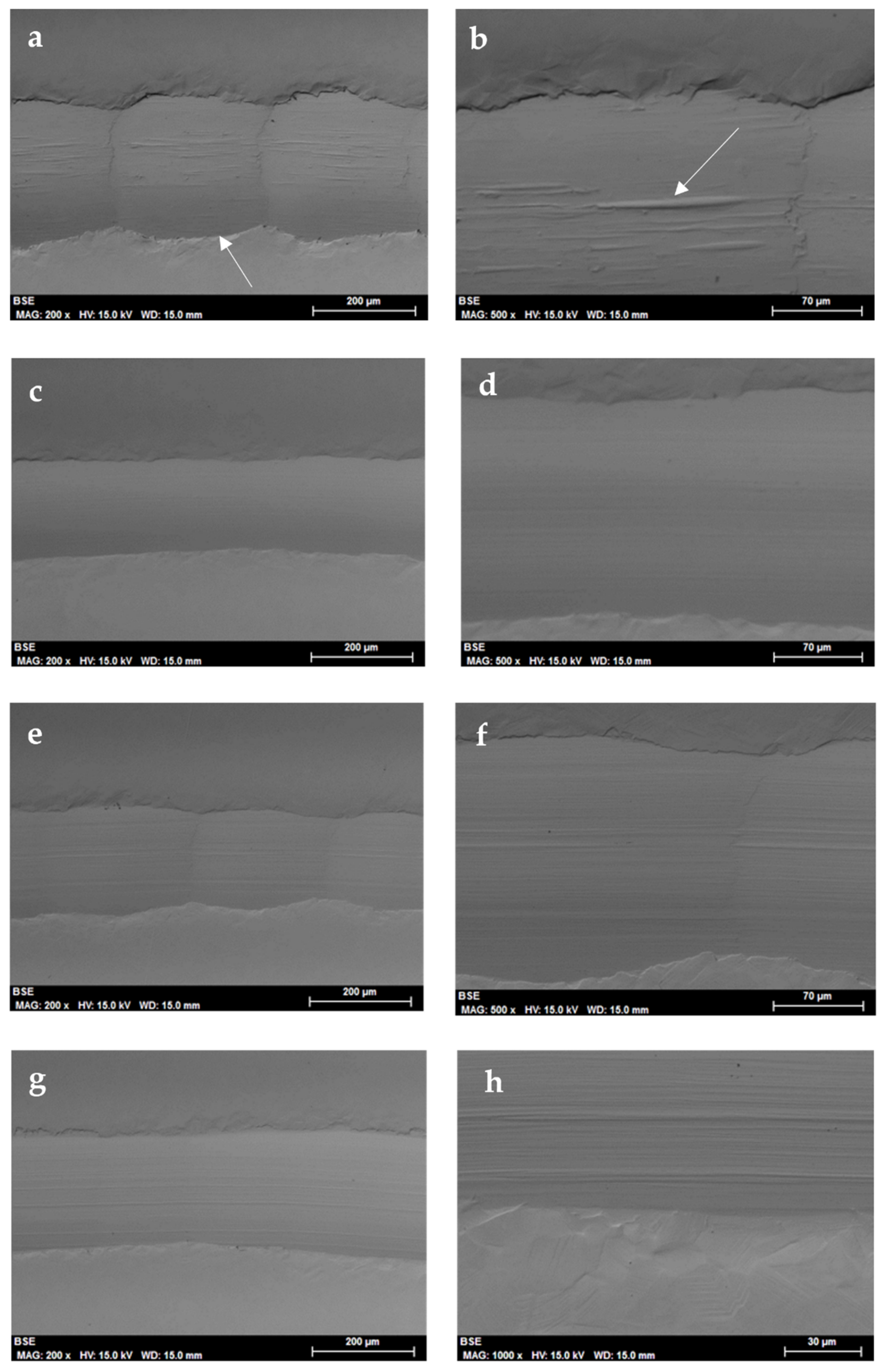
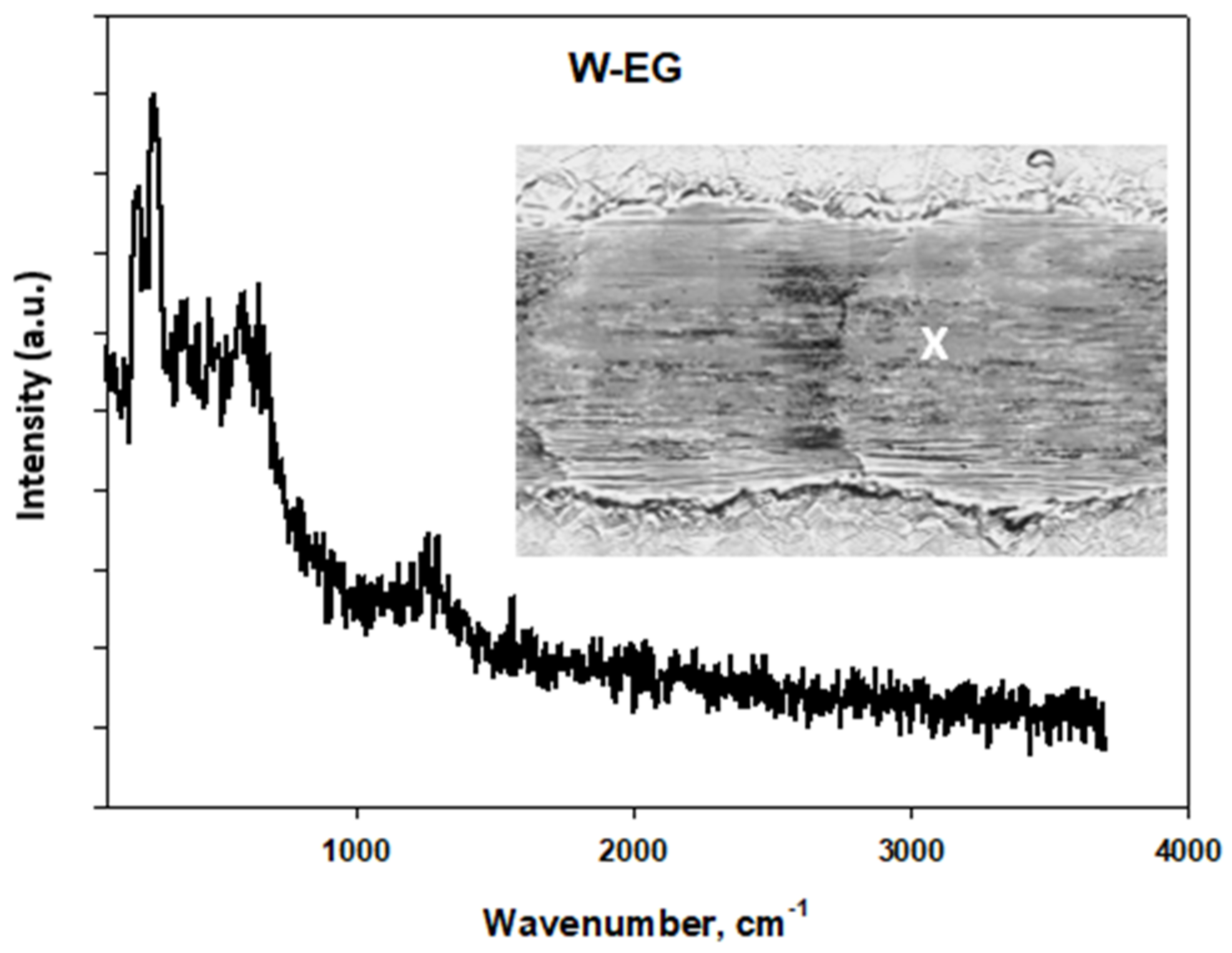
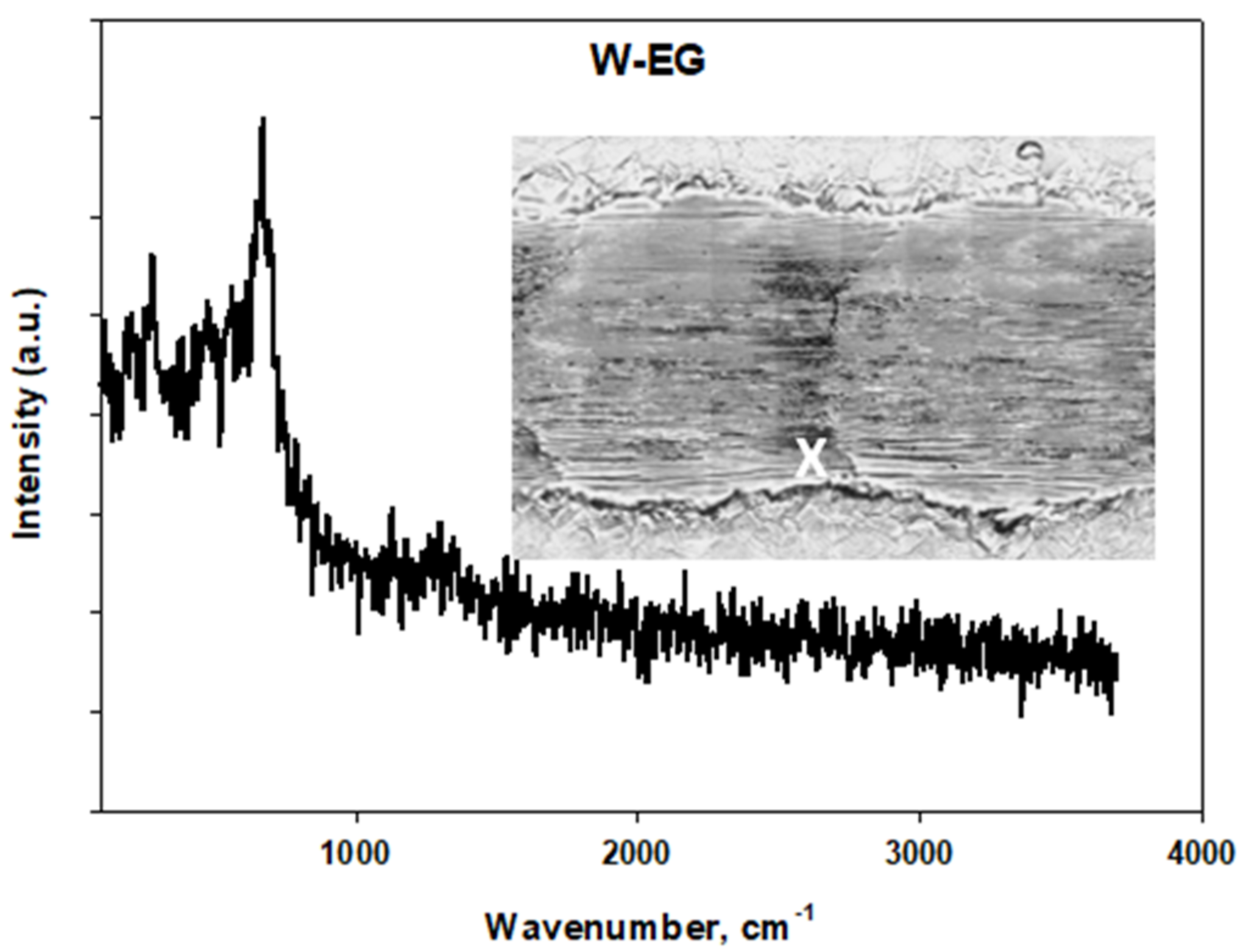
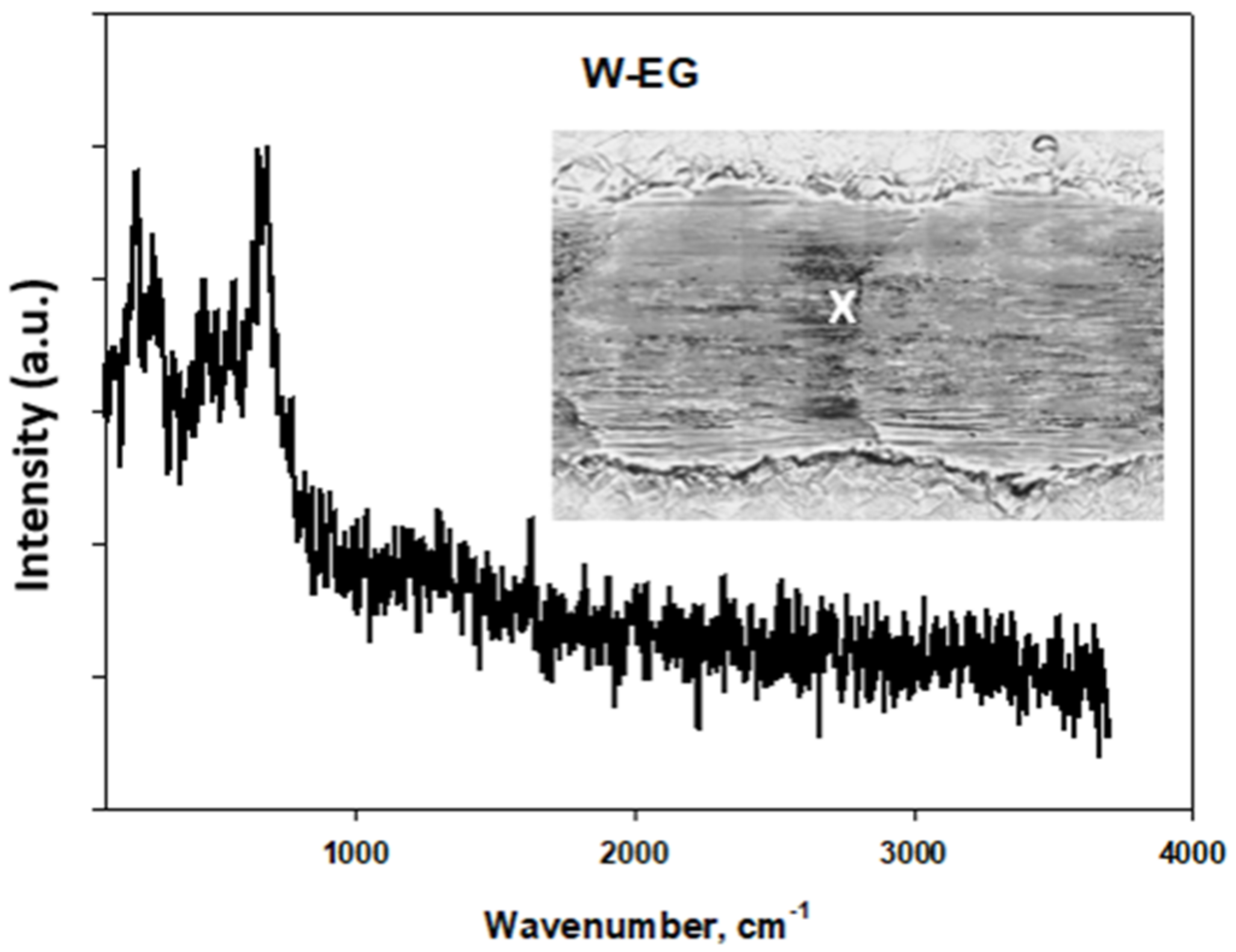
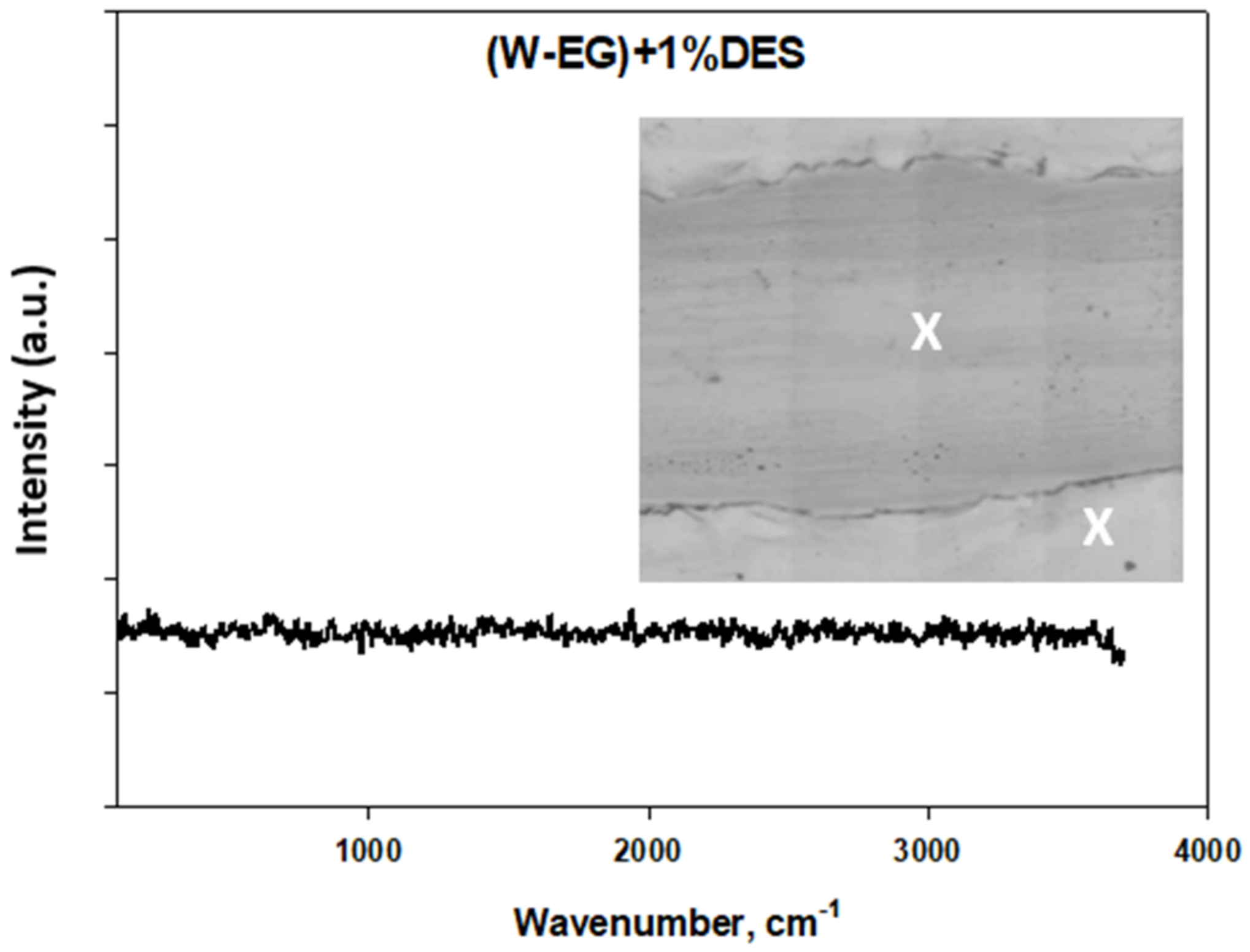
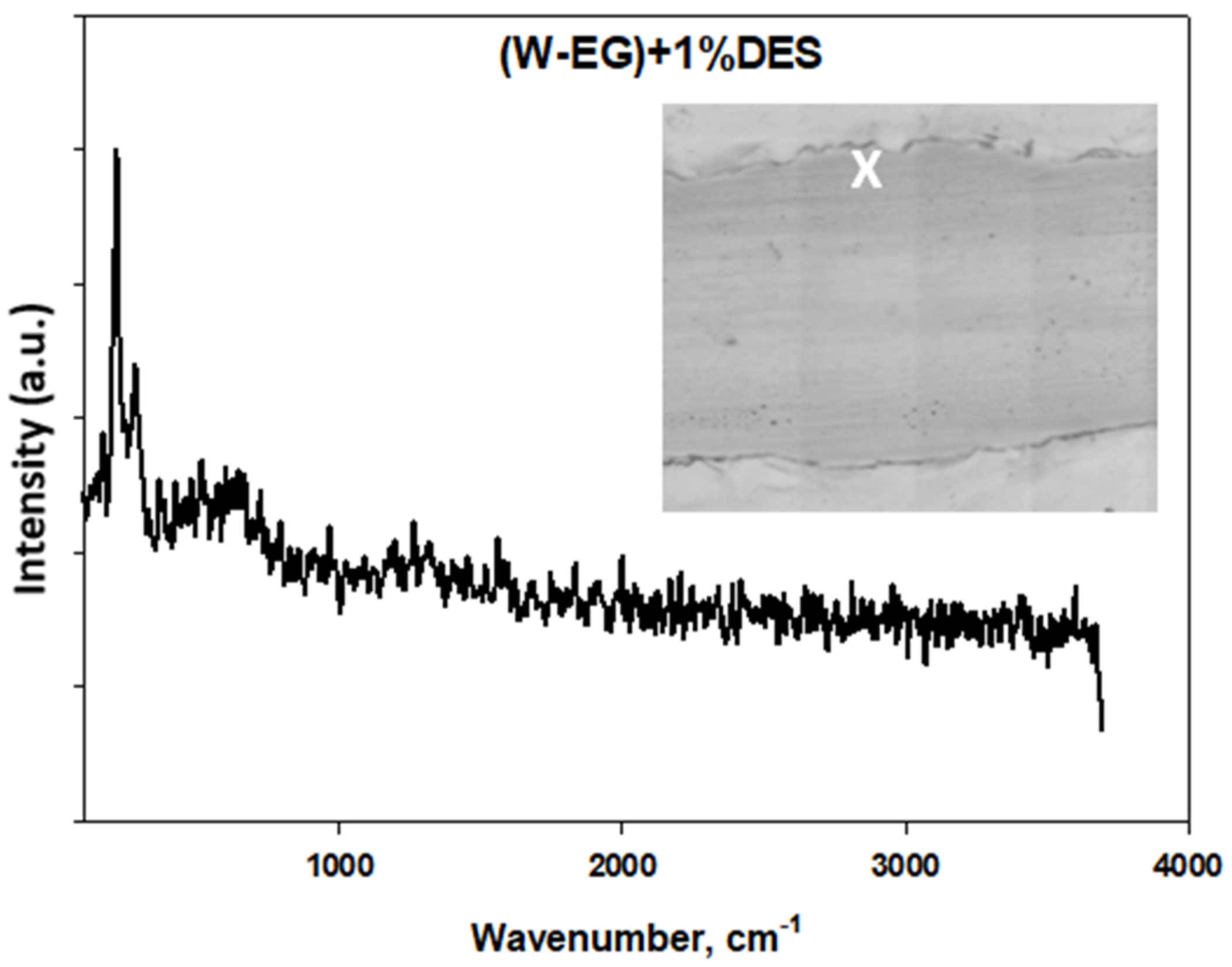
Tribological Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tomala, A.; Karpinska, A.; Werner, W.S.M.; Olver, A.; Störi, H. Tribological properties of additives for water-based lubricants. Wear 2010, 269, 804–810. [Google Scholar] [CrossRef]
- Igari, S.; Mori, S.; Takikawa, Y. Effects of molecular structure of aliphatic diols and polyalkylene glycol as lubricants on the wear of aluminum. Wear 2000, 244, 180–184. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Liu, W.; Liu, Y.; Wang, K.; Li, J.; Ma, T.; Eryilmaz, O.L.; Shi, Y.; Erdemir, A. Superlubricity of polyalkylene glycol aqueous solutions enabled by ultrathin layered double hydroxide nanosheets. ACS Appl. Mater. Interfaces 2019, 11, 20249–20256. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, C.; Deng, M.; Luo, J. Reduction of friction stress of ethylene glycol by attached hydrogen ions. Sci. Rep. 2014, 4, 7226. [Google Scholar] [CrossRef]
- Lu, X.H.; Yao, K.W.; Ouyang, J.; Tian, Y. Tribological characteristics and tribo-chemical mechanisms of Al-Mg-Ti-B coatings under water-glycol lubrication. Wear 2015, 326, 68–73. [Google Scholar] [CrossRef]
- Singh, T.; Jain, M.; Ganguli, D.; Ravi, K. Evaluation of water glycol hydraulic fluids: A tribological approach. J. Synthetic Lubrication 2006, 23, 177–184. [Google Scholar] [CrossRef]
- Kumar, B.; Singh, T.; Rao, K.S.; Pal, A.; Kumar, A. Thermodynamic and spectroscopic studies on binary mixtures of imidazolium ionic liquids in ethylene glycol. J. Chem. Thermodyn. 2008, 40, 32–39. [Google Scholar] [CrossRef]
- Singh, T.; Kumar, A. Volumetric behaviour of 1-butyl-3-methyl imidazolium hexafluorophosphate with ethylene glycol derivatives: Application of Prigogine-Flory-Patterson theory. J. Mol. Liq. 2010, 153, 117–123. [Google Scholar] [CrossRef]
- Pal, A.; Gaba, R.; Singh, T.; Kumar, A. Excess thermodynamic properties of binary mixtures of ionic liquid (1-butyl-3-methylimidazolium hexafluorophosphate) with alkoxyalkanols at several temperatures. J. Mol. Liq. 2010, 154, 41–46. [Google Scholar] [CrossRef]
- Somers, A.E.; Howlett, P.C.; MacFarlane, D.R.; Forsyth, M. A Review of Ionic Liquid Lubricants. Lubricants 2013, 1, 3–21. [Google Scholar] [CrossRef]
- Zhou, Y.; Qu, J. Ionic liquids as lubricant additives: A review. ACS Appl. Mater. Interfaces 2017, 9, 3209–3222. [Google Scholar] [CrossRef]
- Zhao, S.; Yan, L.; Cao, M.; Huang, L.; Yang, K.; Wu, S.; Lan, M.; Niu, G.; Zhang, W. Near-infrared light-triggered lysosome-targetable carbon dots for photothermal therapy of cancer. ACS Appl. Mater. Interfaces 2021, 13, 53610–53617. [Google Scholar] [CrossRef]
- Greaves, T.L.; Drummond, C.J. Protic ionic liquids: Evolving structure–property relationships and expanding applications. Chem. Rev. 2015, 115, 11379–11448. [Google Scholar] [CrossRef]
- Jordan, A.; Gathergood, N. Biodegradation of ionic liquids—A critical review. Chem. Soc. Rev. 2015, 44, 8200–8237. [Google Scholar] [CrossRef]
- Santos, D.; Costa, F.; Franceschi, E.; Santos, A.; Dariva, C.; Mattedi, S. Synthesis and physico-chemical properties of two protic ionic liquids based on stearate anion. Fluid Phase Equilibria 2014, 376, 132–140. [Google Scholar] [CrossRef]
- Rahman, M.H.; Warneke, H.; Webbert, H.; Rodriguez, J.; Austin, E.; Tokunaga, K.; Rajak, D.K.; Menezes, P.L. Water-Based Lubricants: Development, Properties, and Performances. Lubricants 2021, 9, 73. [Google Scholar] [CrossRef]
- Del Sol, I.; Gamez, A.J.; Rivero, A.; Iglesias, P. Tribological performance of ionic liquids as additives of water-based cutting fluids. Wear 2019, 426, 845–852. [Google Scholar] [CrossRef]
- Sagraloff, N.; Dobler, A.; Tobie, T.; Stahl, K.; Ostrowski, J. Development of an oil-free water-based lubricant for gear applications. Lubricants 2019, 7, 33. [Google Scholar] [CrossRef]
- Khanmohammadi, H.; Wijanarko, W.; Espallargas, N. Ionic liquids as additives in water-based lubricants: From surface adsorption to tribofilm formation. Tribol. Lett. 2020, 68, 130. [Google Scholar] [CrossRef]
- Dong, R.; Yu, Q.; Bai, Y.; Ma, Z.; Zhang, J.; Zhang, C.; Yu, B.; Zhou, F.; Liu, W.; Cai, M. Towards superior lubricity and anticorrosion performances of proton-type ionic liquids additives for water-based lubricating fluids. Chem. Eng. J. 2020, 383, 123201. [Google Scholar] [CrossRef]
- Espinosa, T.; Jiménez, M.; Sanes, J.; Jimenez, A.E.; Iglesias, M.; Bermudez, M.D. Ultralow friction with a protic ionic liquid boundary film at the water-lubricated sapphire-stainless steel interface. Tribol. Lett. 2014, 53, 1–9. [Google Scholar] [CrossRef]
- Avilés, M.D.; Carrión, F.J.; Sanes, J.; Bermúdez, M.D. Bio-based ionic liquid crystal for stainless steel-sapphire high temperature ultralow friction. Wear 2021, 485, 204020. [Google Scholar] [CrossRef]
- Avilés, M.D.; Pamies, R.; Sanes, J.; Arias-Pardilla, J.; Carrión, F.J.; Bermúdez, M.D. Protic ammonium bio-based ionic liquid crystal lubricants. Tribol. Int. 2021, 158, 106917. [Google Scholar] [CrossRef]
- Avilés, M.D.; Cao, V.D.; Sánchez, C.; Arias-Pardilla, J.; Carrión-Vilches, F.J.; Sanes, J.; Kjøniksen, A.L.; Bermúdez, M.D.; Pamies, R. Effect of temperature on the rheological behavior of a new aqueous liquid crystal bio-lubricant. J. Mol. Liquids 2020, 301, 112406. [Google Scholar] [CrossRef]
- Su, T.; Song, G.; Zheng, D.; Ju, C.; Zhao, Q. Facile synthesis of protic ionic liquids hybrid for improving antiwear and anticorrosion properties of water-glycol. Tribol. Int. 2021, 153, 106660. [Google Scholar] [CrossRef]
- Zheng, D.; Su, T.; Ju, C. Influence of ecofriendly protic ionic liquids on the corrosion and lubricating properties of water-glycol. Tribol. Int. 2022, 165, 107283. [Google Scholar] [CrossRef]
- Avilés, M.D.; Carrión, F.J.; Sanes, J.; Bermúdez, M.D. Effects of protic ionic liquid crystal additives on the water-lubricated sliding wear and friction of sapphire against stainless steel. Wear 2018, 408–409, 56–64. [Google Scholar] [CrossRef]
- Avilés, M.D.; Pamies, R.; Sanes, J.; Carrión, F.J.; Bermúdez, M.D. Fatty acid-derived ionic liquid lubricant. Protic ionic liquid crystal as additive of protic ionic liquid. Coatings 2019, 9, 710. [Google Scholar] [CrossRef]
- Wang, Y.R.; Yu, Q.L.; Cai, M.R.; Shi, L.; Zhou, F.; Liu, W.M. Ibuprofen-based ionic liquids as additives for enhancing the lubricity and antiwear of water-ethylene glycol liquid. Tribol. Lett. 2017, 65, 13. [Google Scholar] [CrossRef]
- Zheng, G.; Zhang, T.; Ding, X.; Xiang, F.; Li, T.; Lui, S.; Zheng, L. Tribological properties and surface interaction of novel water-soluble ionic liquid in water-glycol. Tribol. Int. 2017, 116, 440–448. [Google Scholar] [CrossRef]
- Krishan, K.; Krishan, R.S. Raman and infrared spectra of ethylene glycol. Proc. Indian Acad. Sci. A 1988, 64, 111. [Google Scholar] [CrossRef]
- Binnemans, K. Ionic Liquid Crystals. Chem. Rev. 2006, 105, 4148–4204. [Google Scholar] [CrossRef]
- Maximo, G.J.; Santos, R.J.B.N.; Lopes-da-Silva, J.A.; Costa, M.C.; Meirelles, A.J.A.; Coutinho, J.A.P. Lipidic Protic Ionic Liquid Crystals. ACS Sustain. Chem. Eng. 2014, 2, 672–682. [Google Scholar] [CrossRef]
- Maleki, A.; Zhu, K.; Pamies, R.; Rodríguez-Schmidt, R.; Kjøniksen, A.L.; Karlsson, G.; Hernández-Cifre, J.G.; García de la Torre, J.; Nyström, B. Effect of polyethylene glycol (PEG) length on the association properties of temperature-sensitive amphiphilic triblock copolymers (PNIPAAMm-b-PEGn-b-PNIPAAMm) in aqueous solution. Soft Matter 2011, 7, 8111–8119. [Google Scholar] [CrossRef]
- Ramya, S.; Anita, T.; Shaikh, H.; Dayal, R.K. Laser Raman microscopic studies of passive films formed on type 316LN stainless steels during pitting in chloride solution. Corrosion Sci. 2010, 52, 2114–2121. [Google Scholar] [CrossRef]
- Cui, Y.; Shuming, L.; Smith, K.; Kanghua, Y.; Hongying, H.; Jiang, W.; Li, Y. Characterization of corrosion scale formed on stainless steel delivery pipe for reclaimed water treatment. Water Res. 2016, 88, 816–825. [Google Scholar] [CrossRef]

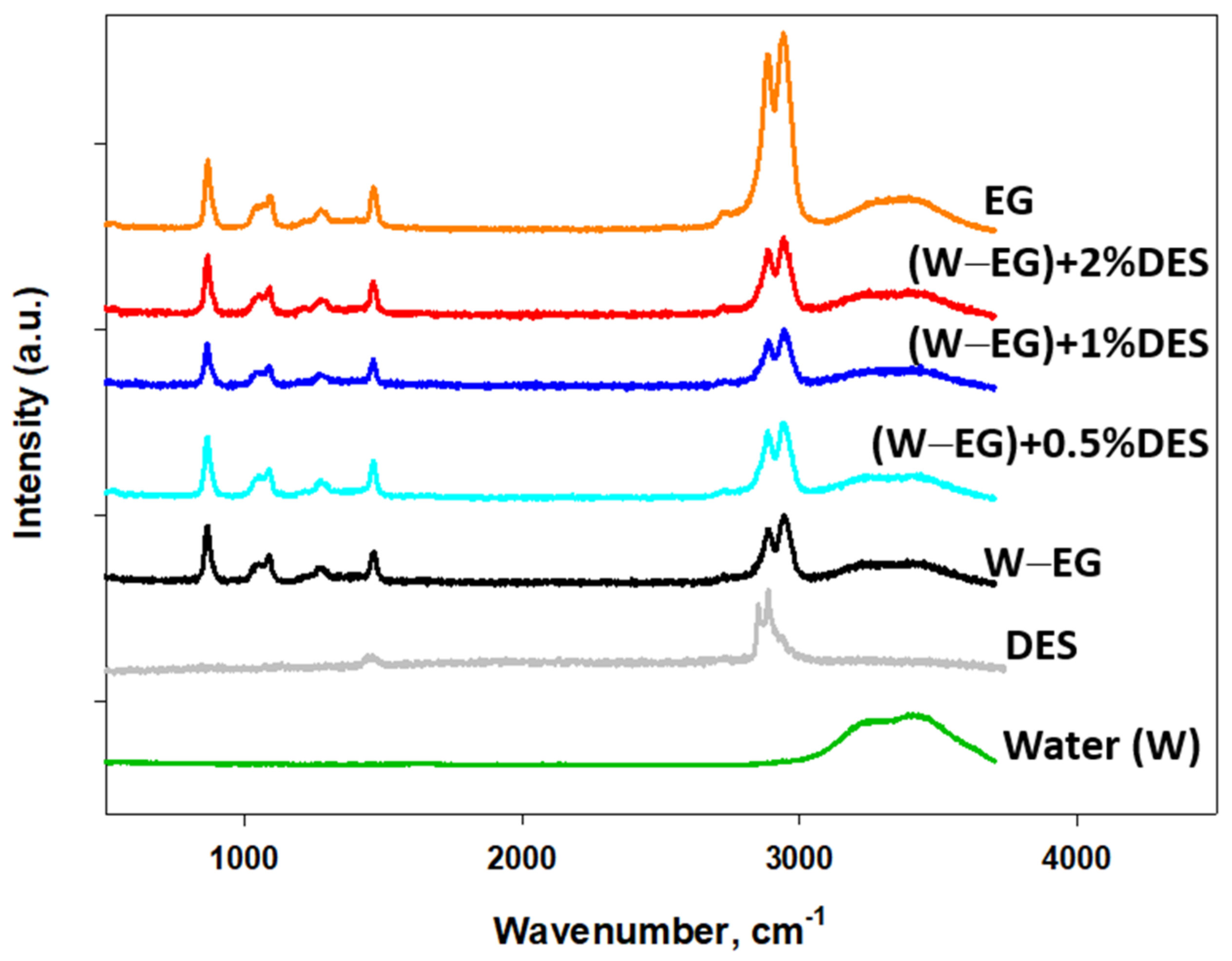
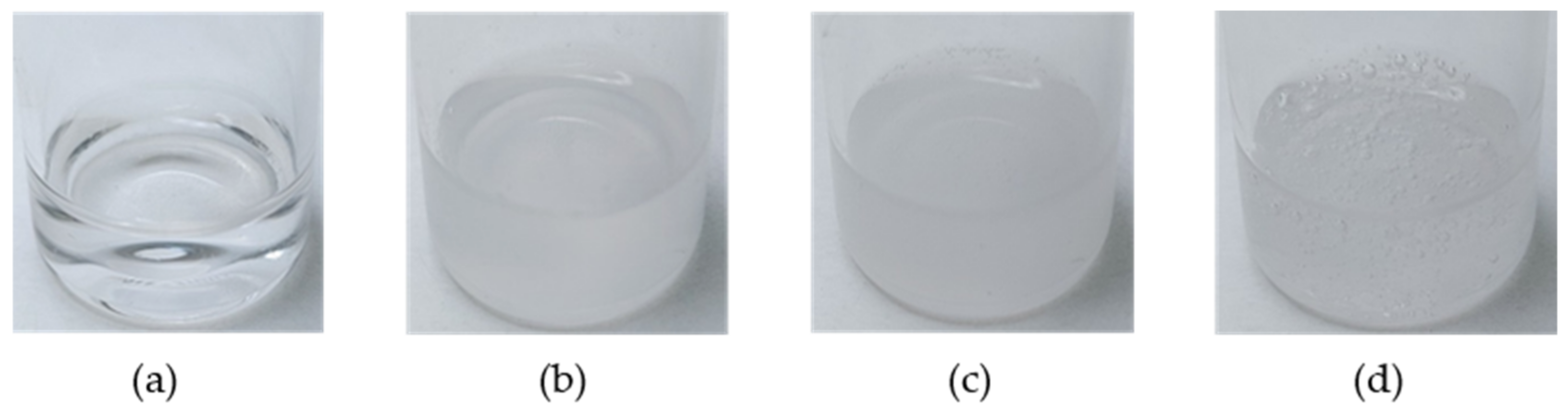
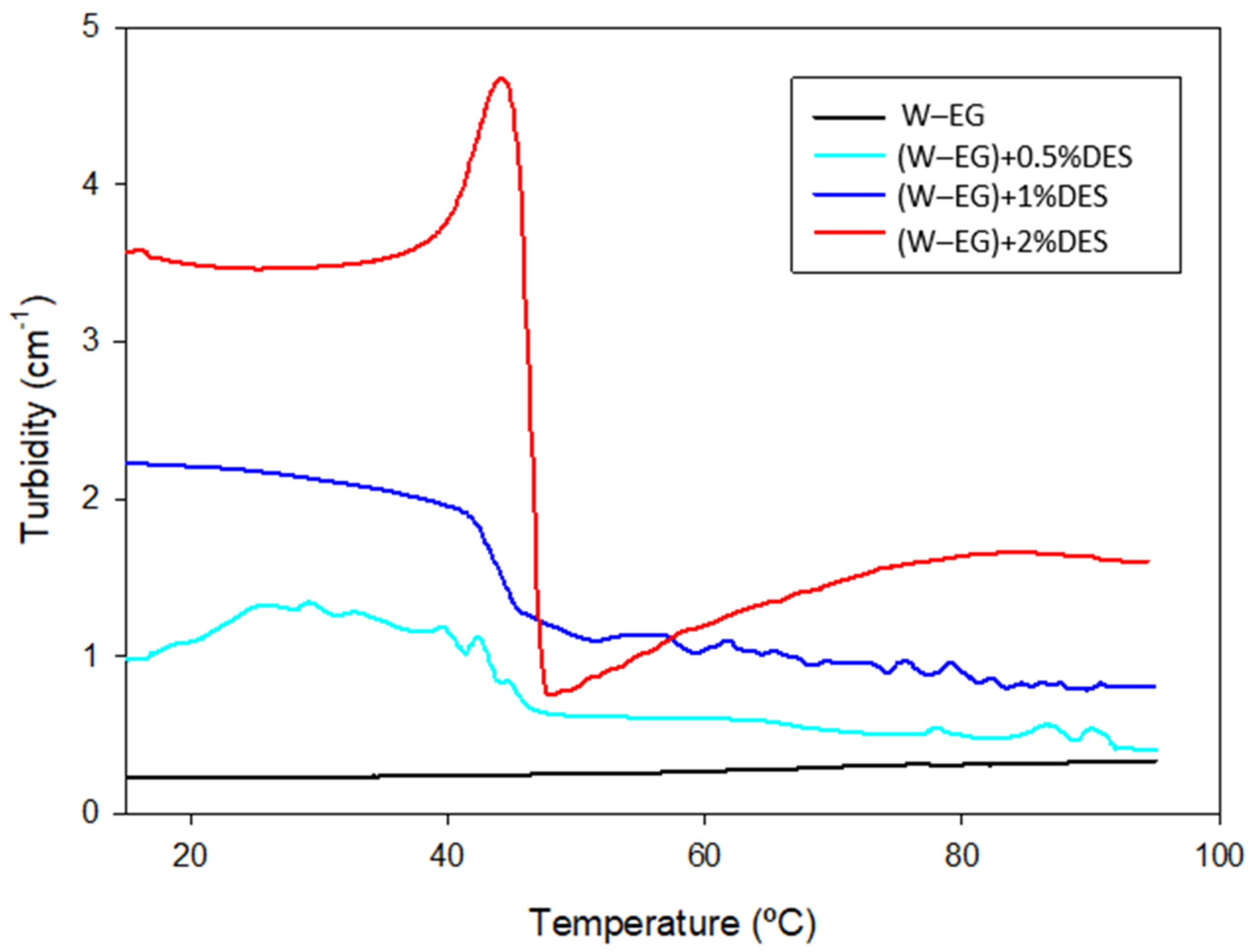



| Lubricant | Turbidity (cm−1) | ||||
|---|---|---|---|---|---|
| 15 °C | 40 °C | 44 °C | 50 °C | 95 °C | |
| W–EG | 0.23 | 0.25 | 0.25 | 0.24 | 0.34 |
| (W–EG) + 0.5%DES | 0.97 | 1.60 | 0.76 | 0.62 | 0.39 |
| (W–EG) + 1%DES | 2.22 | 1.96 | 1.54 | 1.12 | 0.90 |
| (W–EG) + 2%DES | 3.57 | 3.77 | 4.68 | 0.80 | 1.60 |
| Lubricant | Initial | After 5 min |
|---|---|---|
| W–EG |  35.6° (2.1) |  33.8° (1.6) |
| (W–EG) + 0.5%DES |  53.1° (3.9) |  41.9° (2.0) |
| (W–EG) + 1%DES |  51.1° (5.0) |  49.0° (4.7) |
| (W–EG) + 2%DES |  46.4° (4.4) |  41.2° (1.5) |
| Lubricant | COF | Wear Rate (mm3/N·m) |
|---|---|---|
| W–EG | 0.28 (±0.019) | 1.92 × 10−5 (±1.47 × 10−6) |
| (W–EG) + 0.5%DES | 0.08 (±0.004) | 4.01 × 10−6 (±2.22 × 10−7) |
| (W–EG) + 1%DES | 0.07 (±0.007) | 6.40 × 10−6 (±5.80 × 10−7) |
| (W–EG) + 2%DES | 0.09 (±0.009) | 1.06 × 10−5 (±5.32 × 10−7) |
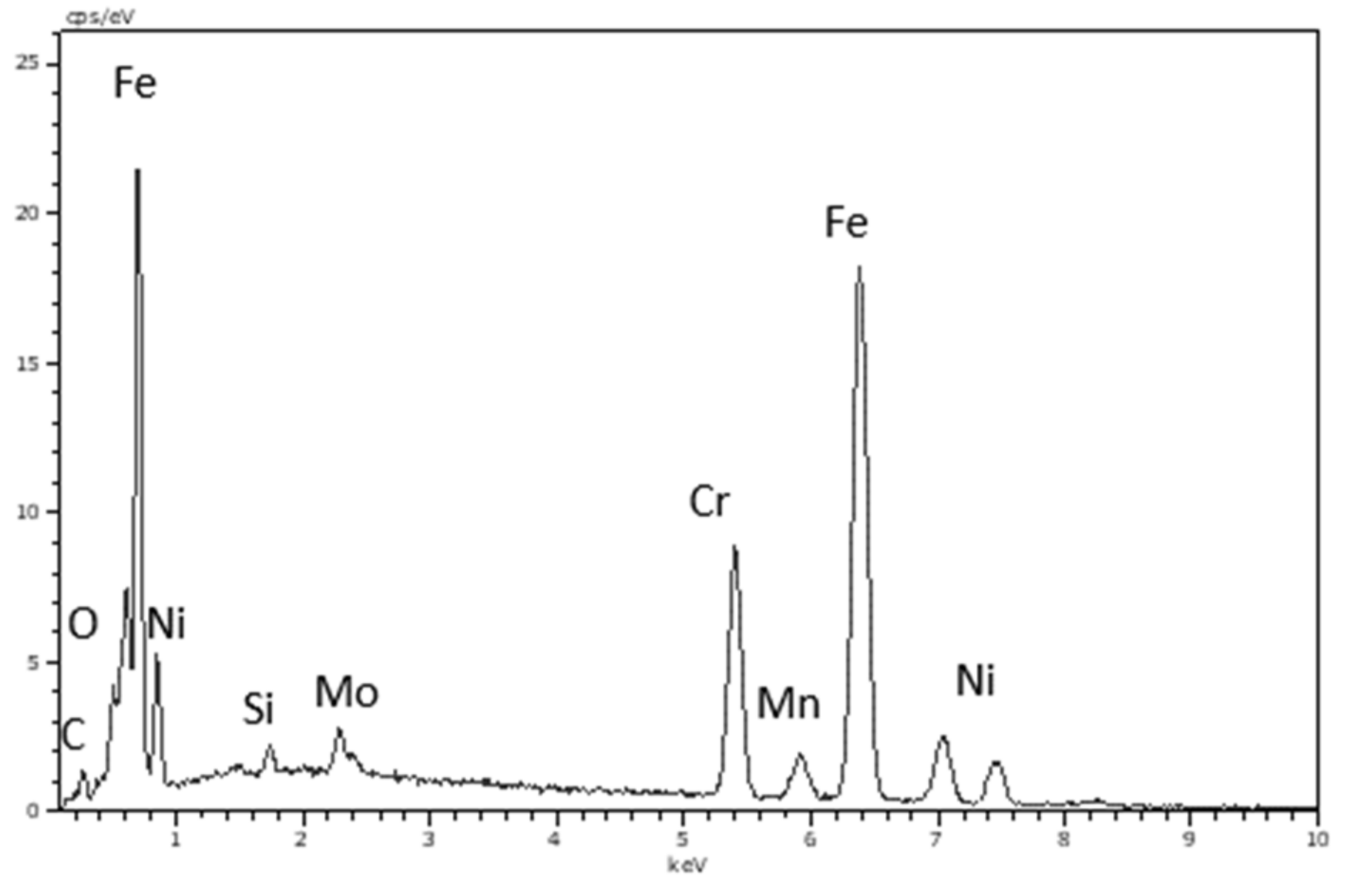
| Lubricant | Element (wt.%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Fe | C | O | Cr | |||||
| Inside | Outside | Inside | Outside | Inside | Outside | Inside | Outside | |
| W–EG | 56.9 | 61.2 | 10.4 | 7.3 | 7.6 | 2.2 | 13.9 | 15.4 |
| (W–EG) + 0.5%DES | 59.7 | 62.0 | 8.7 | 7.8 | 3.3 | 1.4 | 15.1 | 15.6 |
| (W–EG) + 1%DES | 60.9 | 61.6 | 7.5 | 7.7 | 2.8 | 1.7 | 15.4 | 15.5 |
| (W–EG) + 2%DES | 60.4 | 61.1 | 9.1 | 7.9 | 1.8 | 1.6 | 15.1 | 15.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avilés, M.-D.; Sánchez-Rodríguez, C.; Pamies, R.; Bermúdez, M.-D.; Carrión-Vilches, F.-J.; Sanfelix, S.G.; Kjøniksen, A.-L. New Water-Ethylene Glycol Lubricants with Stearate Ionic Liquid Crystal Additive. Lubricants 2022, 10, 241. https://doi.org/10.3390/lubricants10100241
Avilés M-D, Sánchez-Rodríguez C, Pamies R, Bermúdez M-D, Carrión-Vilches F-J, Sanfelix SG, Kjøniksen A-L. New Water-Ethylene Glycol Lubricants with Stearate Ionic Liquid Crystal Additive. Lubricants. 2022; 10(10):241. https://doi.org/10.3390/lubricants10100241
Chicago/Turabian StyleAvilés, María-Dolores, Cristian Sánchez-Rodríguez, Ramón Pamies, María-Dolores Bermúdez, Francisco-José Carrión-Vilches, Susana García Sanfelix, and Anna-Lena Kjøniksen. 2022. "New Water-Ethylene Glycol Lubricants with Stearate Ionic Liquid Crystal Additive" Lubricants 10, no. 10: 241. https://doi.org/10.3390/lubricants10100241
APA StyleAvilés, M.-D., Sánchez-Rodríguez, C., Pamies, R., Bermúdez, M.-D., Carrión-Vilches, F.-J., Sanfelix, S. G., & Kjøniksen, A.-L. (2022). New Water-Ethylene Glycol Lubricants with Stearate Ionic Liquid Crystal Additive. Lubricants, 10(10), 241. https://doi.org/10.3390/lubricants10100241








