Redox–Genomic Crosstalk: Linking Oxidative Stress, Sperm DNA Fragmentation, and Epigenetics in Personalized Management of Male Infertility
Abstract
1. Introduction
2. Novelty and Significance of the Present Review
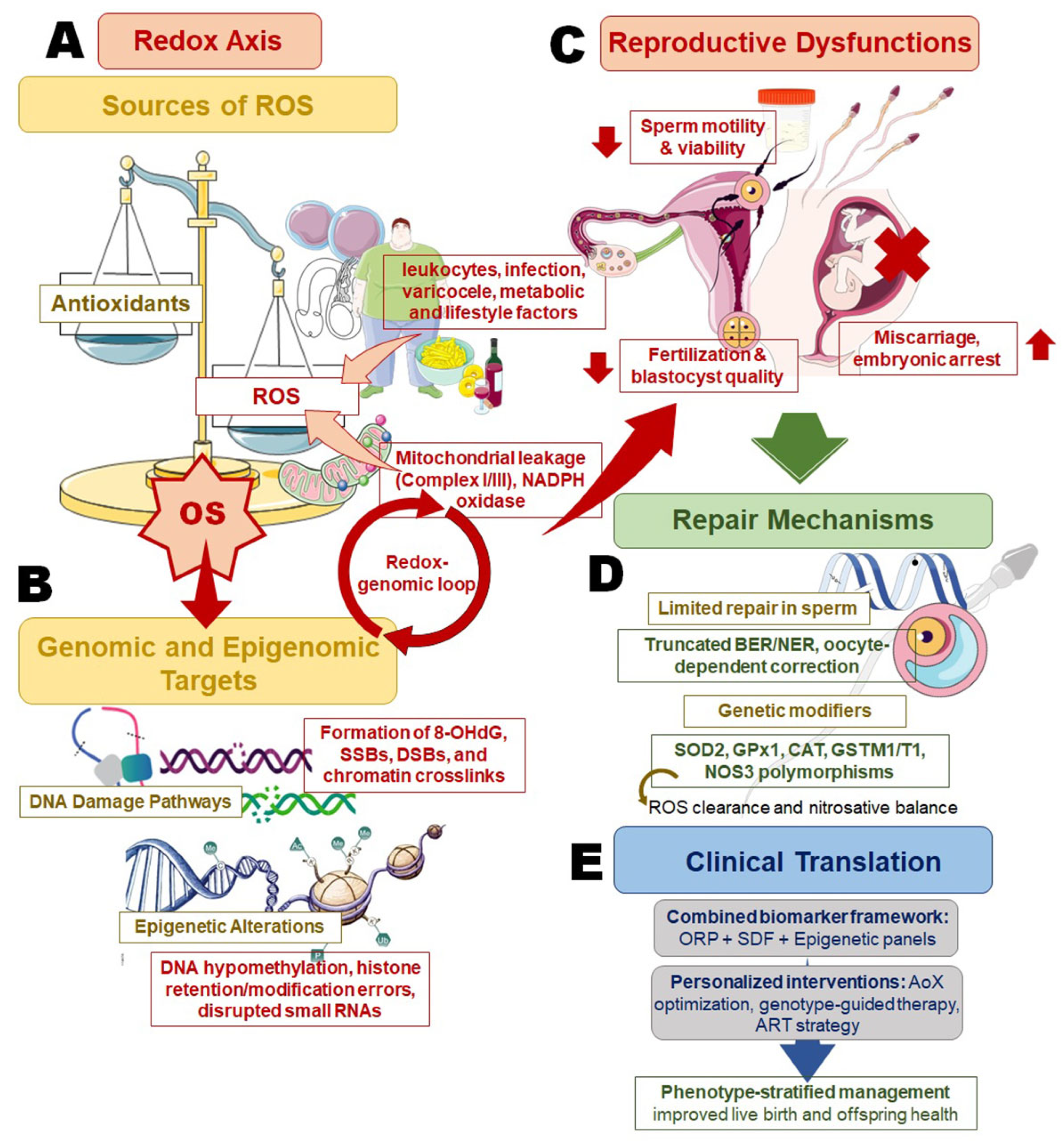
3. Redox Biology Across Spermatogenesis
3.1. Endogenous and Exogenous ROS Sources
3.2. Environmental and Metabolic ROS Amplifiers
3.3. Antioxidant Defense Networks
3.4. Molecular Targets of Oxidative Damage
3.5. Repair Limitations in Sperm
4. Mechanistic Routes to SDF
4.1. Chromatin Remodeling Defects
4.2. Oxidative DNA Lesions and Breaks
4.3. Clinical Consequences of SDF
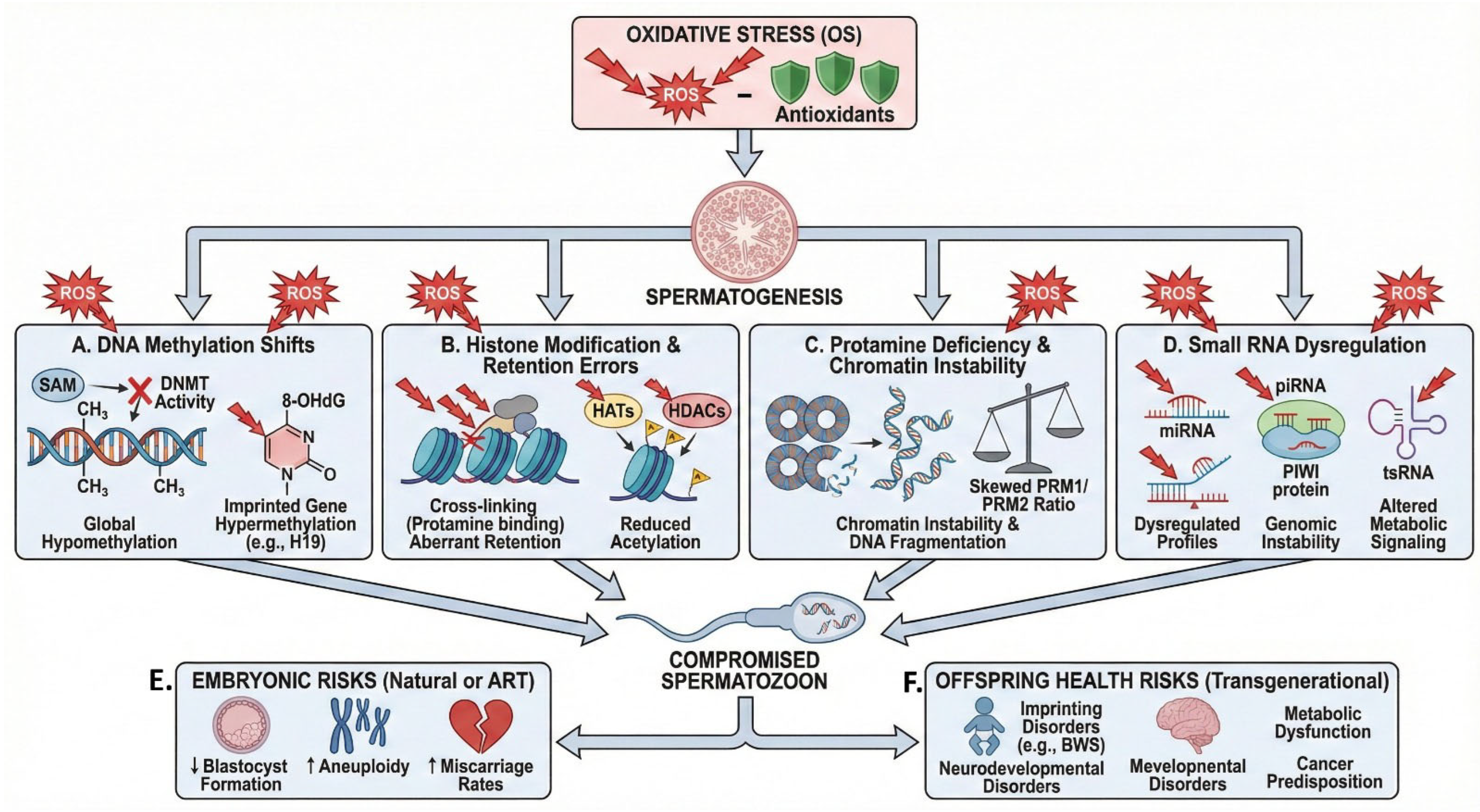
5. Epigenetic Dysregulation Under OS
5.1. Oxidative Shifts in DNA Methylation
5.2. Histone Modification and Retention Errors
5.3. Protamine Deficiency and Chromatin Instability
5.4. Small RNAs as OS Sensors
5.5. Embryonic and Offspring Risks
6. Measurement: From Bench to Clinic
6.1. Redox Biomarkers and Oxidation Stress Testing
6.2. Assays for SDF
6.3. Epigenetic and Chromatin Assays
7. Clinical Endotypes: Who Is the ‘Redox–Genomic’ Patient?
7.1. Redox-Dominant Endotype
7.2. Redox-Driven DNA Fragmentation
7.3. Chromatin/Epigenetic Dysfunction with Redox Overlay
7.4. Epigenetic-Dominant Endotype
7.5. SDF-Isolated Endotype
8. Genetic Modifiers of Redox Damage and Repair
8.1. Antioxidant Enzyme Polymorphisms
8.2. DNA Repair Gene Variants
8.3. Nitric Oxide Synthase Pathway Genes
8.4. Genotype and Ancestry-Specific Cut-Offs
9. Evidence to Outcomes: Natural Conception and ART
9.1. Predictive Value for Natural Fertility
9.2. Impact on ART Outcomes
9.3. Safety and Offspring Health Considerations
10. Future Directions and Trial Blueprints
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 4-HNE | 4-hydroxynonenal |
| 5-hmC | 5-hydroxymethylcytosine |
| 8-OHdG | 8-hydroxy-2′-deoxyguanosine |
| AB | Aniline blue |
| AGEs | Glycation end products |
| AIF | Apoptosis-inducing factor |
| ARG | Arginase |
| ART | Assisted reproductive technologies |
| BER | Base excision repair |
| BRDT | Bromodomain Testis-specific protein |
| CHD5 | Chromodomain Helicase DNA-binding protein 5 |
| CMA3 | Chromomycin A3 |
| DFI | Sperm DNA fragmentation index |
| DNMT | DNA (cytosine-5)-methyltransferase |
| DSBs | Double-strand breaks |
| GATPase | Adenosine Triphosphatase |
| GCH1 | GTP cyclohydrolase 1 |
| GSH | Glutathione |
| GSTM1 | Glutathione S-transferase Mu 1 |
| GSTT1 | Glutathione S-transferase Theta 1 |
| H19/IGF2 | H19/Insulin-like Growth Factor 2 |
| HATs | Histone acetyltransferases |
| HDACs | Histone deacetylases |
| HR | Homologous recombination |
| IL-6 | Interleukin-6 |
| MEST | Mesoderm Specific Transcript |
| Msr | Methionine sulfoxide reductase |
| miRNAs | MicroRNAs |
| MTHFR | Methylenetetrahydrofolate reductase |
| NAP1L4 | Nucleosome Assembly Protein 1-like 4 |
| NADP | Nicotinamide Adenine Dinucleotide Phosphate |
| NER | Nucleotide excision repair |
| NO | Nitric oxide |
| OGG1 | 8-oxoguanine DNA glycosylase 1 |
| ORP | Oxidation-reduction potential |
| PARP1 | Poly (ADP-ribose) polymerase 1 |
| PRDXs | Peroxiredoxins |
| PRM | Protamines |
| PTMs | Post-translational histone modifications |
| Px | Glutathione peroxidase |
| ROS | Reactive oxygen species |
| SAM | S-adenosylmethionine |
| SDF | Sperm DNA fragmentation |
| SNRPN | Small Nuclear Ribonucleoprotein Polypeptide N |
| SOD2 | Superoxide Dismutase 2 |
| SSBs | Single-strand breaks |
| TET enzymes | Ten-Eleven Translocation enzymes |
| TNF-α | Tumor Necrosis Factor-alpha |
| TNP | Transition proteins |
| TUNEL | Terminal deoxynucleotidyl transferase dUTP nick end labeling |
| VNTR | Variable number tandem repeat |
| piRNAs | PIWI-interacting RNAs |
| tsRNAs | Transfer RNA-derived small RNAs |
References
- Huang, B.; Wang, Z.; Kong, Y.; Jin, M.; Ma, L. Global, regional and national burden of male infertility in 204 countries and territories between 1990 and 2019: An analysis of global burden of disease study. BMC Public Health 2023, 23, 2195. [Google Scholar] [CrossRef]
- Esteves, S.C. A clinical appraisal of the genetic basis in unexplained male infertility. J. Hum. Reprod. Sci. 2013, 6, 176–182. [Google Scholar] [CrossRef]
- Gunes, S.; Arslan, M.A.; Hekim, G.N.T.; Asci, R. The role of epigenetics in idiopathic male infertility. J. Assist. Reprod. Genet. 2016, 33, 553–569. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P.; Roychoudhury, S.; Nath, M.; Dutta, S. Oxidative stress and idiopathic male infertility. In Oxidative Stress and Toxicity in Reproductive Biology and Medicine: A Comprehensive Update on Male Infertility—Volume One; Springer: Berlin/Heidelberg, Germany, 2022; pp. 181–204. [Google Scholar]
- Dutta, S.; Henkel, R.; Sengupta, P.; Agarwal, A. Physiological role of ROS in sperm function. In Male Infertility: Contemporary Clinical Approaches, Andrology, Art and Antioxidants; Springer: Berlin/Heidelberg, Germany, 2020; pp. 337–345. [Google Scholar]
- Panner Selvam, M.K.; Sengupta, P.; Agarwal, A. Sperm DNA fragmentation and male infertility. In Genetics of Male Infertility: A Case-Based Guide for Clinicians; Springer: Berlin/Heidelberg, Germany, 2020; pp. 155–172. [Google Scholar]
- Sengupta, P.; Dutta, S.; Liew, F.F.; Dhawan, V.; Das, B.; Mottola, F.; Slama, P.; Rocco, L.; Roychoudhury, S. Environmental and genetic traffic in the journey from sperm to offspring. Biomolecules 2023, 13, 1759. [Google Scholar] [CrossRef] [PubMed]
- Tournaye, H. Male factor infertility and art. Asian J. Androl. 2011, 14, 103. [Google Scholar] [CrossRef]
- Assidi, M. Infertility in men: Advances towards a comprehensive and integrative strategy for precision theranostics. Cells 2022, 11, 1711. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.D.K.; Kuroda, S.; Nguyen, N.T.; Yumura, Y.; Takeshima, T. Redox imbalance in male infertility: From empirical antioxidant therapy to precision medicine and epigenetic insights. Reprod. Med. Biol. 2025, 24, e12693. [Google Scholar] [CrossRef]
- Vassilaki, I.; Potiris, A.; Domali, E.; Karampitsakos, T.; Mavrogianni, D.; Grigoriadis, T.; Zikopoulos, A.; Moustakli, E.; Papadopoulou, A.; Anagnostaki, I.; et al. Micrornas regulating oxidative stress in human fertility: A narrative review of mechanistic insights and clinical potential. Med. Sci. 2025, 13, 254. [Google Scholar] [CrossRef]
- Kurhaluk, N.; Kaminski, P.; Tkaczenko, H. Oxidative stress, antioxidants, gut microbiota and male fertility. Cell Physiol. Biochem. 2025, 59, 82–123. [Google Scholar] [CrossRef]
- Bouhadana, D.; Godin Pagé, M.-H.; Montjean, D.; Bélanger, M.-C.; Benkhalifa, M.; Miron, P.; Petrella, F. The role of antioxidants in male fertility: A comprehensive review of mechanisms and clinical applications. Antioxidants 2025, 14, 1013. [Google Scholar] [CrossRef]
- Moazamian, A.; Saez, F.; Drevet, J.R.; Aitken, R.J.; Gharagozloo, P. Redox-driven epigenetic modifications in sperm: Unraveling paternal influences on embryo development and transgenerational health. Antioxidants 2025, 14, 570. [Google Scholar] [CrossRef] [PubMed]
- Kaltsas, A.; Markou, E.; Kyrgiafini, M.A.; Zikopoulos, A.; Symeonidis, E.N.; Dimitriadis, F.; Zachariou, A.; Sofikitis, N.; Chrisofos, M. Oxidative-stress-mediated epigenetic dysregulation in spermatogenesis: Implications for male infertility and offspring health. Genes 2025, 16, 93. [Google Scholar] [CrossRef]
- Sharma, R.; Agarwal, A. Spermatogenesis: An overview. In Sperm Chromatin: Biological and Clinical Applications in Male Infertility and Assisted Reproduction; Springer: Berlin/Heidelberg, Germany, 2011; pp. 19–44. [Google Scholar]
- Baker, M.A.; Aitken, R.J. The importance of redox regulated pathways in sperm cell biology. Mol. Cell. Endocrinol. 2004, 216, 47–54. [Google Scholar] [CrossRef]
- Sengupta, P.; Dutta, S.; Irez, T. Oxidants and antioxidants in male reproduction: Roles of oxidative and reductive stress. J. Integr. Sci. Technol. 2024, 12, 753. [Google Scholar] [CrossRef]
- Boguenet, M.; Bouet, P.-E.; Spiers, A.; Reynier, P.; May-Panloup, P. Mitochondria: Their role in spermatozoa and in male infertility. Hum. Reprod. Update 2021, 27, 697–719. [Google Scholar] [CrossRef] [PubMed]
- Afzal, S.; Abdul Manap, A.S.; Attiq, A.; Albokhadaim, I.; Kandeel, M.; Alhojaily, S.M. From imbalance to impairment: The central role of reactive oxygen species in oxidative stress-induced disorders and therapeutic exploration. Front. Pharmacol. 2023, 14, 1269581. [Google Scholar] [CrossRef] [PubMed]
- Drevet, J.R.; Hallak, J.; Nasr-Esfahani, M.-H.; Aitken, R.J. Reactive oxygen species and their consequences on the structure and function of mammalian spermatozoa. Antioxid. Redox Signal. 2022, 37, 481–500. [Google Scholar] [CrossRef]
- Piomboni, P.; Focarelli, R.; Stendardi, A.; Ferramosca, A.; Zara, V. The role of mitochondria in energy production for human sperm motility. Int. J. Androl. 2012, 35, 109–124. [Google Scholar] [CrossRef]
- Henkel, R.; Offor, U.; Fisher, D. The role of infections and leukocytes in male infertility. Andrologia 2021, 53, e13743. [Google Scholar] [CrossRef] [PubMed]
- Hanukoglu, I. Antioxidant protective mechanisms against reactive oxygen species (ros) generated by mitochondrial p450 systems in steroidogenic cells. Drug Metab. Rev. 2006, 38, 171–196. [Google Scholar] [CrossRef]
- Leisegang, K.; Dutta, S. Do lifestyle practices impede male fertility? Andrologia 2021, 53, e13595. [Google Scholar] [CrossRef]
- Rodríguez-Martínez, H.; Kvist, U.; Ernerudh, J.; Sanz, L.; Calvete, J.J. Seminal plasma proteins: What role do they play? Am. J. Reprod. Immunol. 2011, 66, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Madhu, N.R.; Sarkar, B.; Slama, P.; Jha, N.K.; Ghorai, S.K.; Jana, S.K.; Govindasamy, K.; Massanyi, P.; Lukac, N.; Kumar, D. Effect of environmental stressors, xenobiotics, and oxidative stress on male reproductive and sexual health. In Oxidative Stress and Toxicity in Reproductive Biology and Medicine: A Comprehensive Update on Male Infertility Volume II; Springer: Berlin/Heidelberg, Germany, 2022; pp. 33–58. [Google Scholar]
- Agarwal, A.; Sengupta, P. Oxidative stress and its association with male infertility. In Male Infertility: Contemporary Clinical Approaches, Andrology, Art and Antioxidants; Springer: Berlin/Heidelberg, Germany, 2020; pp. 57–68. [Google Scholar]
- Durairajanayagam, D.; Sharma, R.K.; du Plessis, S.S.; Agarwal, A. Testicular heat stress and sperm quality. In Male Infertility: A Complete Guide to Lifestyle and Environmental Factors; Springer: Berlin/Heidelberg, Germany, 2014; pp. 105–125. [Google Scholar]
- Sengupta, P. Environmental and occupational exposure of metals and their role in male reproductive functions. Drug Chem. Toxicol. 2013, 36, 353–368. [Google Scholar] [CrossRef] [PubMed]
- Harlev, A.; Agarwal, A.; Gunes, S.O.; Shetty, A.; du Plessis, S.S. Smoking and male infertility: An evidence-based review. World J. Men’s Health 2015, 33, 143–160. [Google Scholar] [CrossRef]
- Akang, E.N.; Oremosu, A.A.; Osinubi, A.; James, A.B.; Biose, I.J.; Dike, S.I.; Idoko, K.M. Alcohol-induced male infertility: Is sperm DNA fragmentation a causative? J. Exp. Clin. Anat. 2017, 16, 53–59. [Google Scholar]
- Bhattacharya, K.; Sengupta, P.; Dutta, S.; Karkada, I.R. Obesity, systemic inflammation and male infertility. Chem. Biol. Lett. 2020, 7, 92–98. [Google Scholar]
- Leisegang, K.; Sengupta, P.; Agarwal, A.; Henkel, R. Obesity and male infertility: Mechanisms and management. Andrologia 2021, 53, e13617. [Google Scholar] [CrossRef]
- Omolaoye, T.S.; Skosana, B.T.; Ferguson, L.M.; Ramsunder, Y.; Ayad, B.M.; Du Plessis, S.S. Implications of exposure to air pollution on male reproduction: The role of oxidative stress. Antioxidants 2024, 13, 64. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Singh, A.K. Impact of environmental factors on human semen quality and male fertility: A narrative review. Environ. Sci. Eur. 2022, 34, 6. [Google Scholar] [CrossRef]
- Deepinder, F.; Makker, K.; Agarwal, A. Cell phones and male infertility: Dissecting the relationship. Reprod. Biomed. Online 2007, 15, 266–270. [Google Scholar] [CrossRef]
- Aronica, L.; Ordovas, J.M.; Volkov, A.; Lamb, J.J.; Stone, P.M.; Minich, D.; Leary, M.; Class, M.; Metti, D.; Larson, I.A.; et al. Genetic biomarkers of metabolic detoxification for personalized lifestyle medicine. Nutrients 2022, 14, 768. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Sengupta, P.; Slama, P.; Roychoudhury, S. Oxidative stress, testicular inflammatory pathways, and male reproduction. Int. J. Mol. Sci. 2021, 22, 10043. [Google Scholar] [CrossRef] [PubMed]
- Asadi, N.; Bahmani, M.; Kheradmand, A.; Rafieian-Kopaei, M. The impact of oxidative stress on testicular function and the role of antioxidants in improving it: A review. J. Clin. Diagn. Res. 2017, 11, IE01. [Google Scholar] [CrossRef]
- Llavanera, M.; Delgado-Bermúdez, A.; Olives, S.; Mateo-Otero, Y.; Recuero, S.; Bonet, S.; Fernández-Fuertes, B.; Yeste, M.; Barranco, I. Glutathione s-transferases play a crucial role in mitochondrial function, plasma membrane stability and oxidative regulation of mammalian sperm. Antioxidants 2020, 9, 100. [Google Scholar] [CrossRef]
- Kowalczyk, A. The role of the natural antioxidant mechanism in sperm cells. Reprod. Sci. 2022, 29, 1387–1394. [Google Scholar] [CrossRef]
- Lacerda-Abreu, M.A.; Meyer-Fernandes, J.R. Extracellular inorganic phosphate-induced release of reactive oxygen species: Roles in physiological processes and disease development. Int. J. Mol. Sci. 2021, 22, 7768. [Google Scholar] [CrossRef]
- Kojo, K.; Oguri, T.; Tanaka, T.; Nakazono, A.; Numahata, D.; Uchida, M.; Yamasaki, K.; Ikeda, A.; Kakinuma, T.; Negoro, H. Seminal plasma metallomics: A new horizon for diagnosing and managing male infertility. Rev. Int. Andrología 2025, 23, 1–11. [Google Scholar]
- Bertelsmann, H.; Sieme, H.; Behne, D.; Kyriakopoulos, A. Is the distribution of selenium and zinc in the sublocations of spermatozoa regulated? Ann. N. Y. Acad. Sci. 2007, 1095, 204–208. [Google Scholar] [CrossRef]
- Yu, B.; Huang, Z. Variations in antioxidant genes and male infertility. BioMed Res. Int. 2015, 2015, 513196. [Google Scholar] [CrossRef]
- Sengupta, P.; Dutta, S.; Alahmar, A.T. Reductive stress and male infertility. In Oxidative Stress and Toxicity in Reproductive Biology and Medicine: A Comprehensive Update on Male Infertility Volume II; Springer: Berlin/Heidelberg, Germany, 2022; pp. 311–321. [Google Scholar]
- Aitken, R.J. Free radicals, lipid peroxidation and sperm function. Reprod. Fertil. Dev. 1995, 7, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.C.; Nogueira-Ferreira, R.; Amado, F.; Alves, M.G.; Ferreira, R.; Oliveira, P.F. Exploring the role of oxidative stress in sperm motility: A proteomic network approach. Antioxid. Redox Signal. 2022, 37, 501–520. [Google Scholar] [CrossRef]
- Almabhouh, F.; Singh, H. Adverse effects of leptin on histone-to-protamine transition during spermatogenesis are prevented by melatonin in sprague-dawley rats. Andrologia 2018, 50, e12814. [Google Scholar] [CrossRef]
- Davies, K.J. Oxidative stress, antioxidant defenses, and damage removal, repair, and replacement systems. IUBMB Life 2000, 50, 279–289. [Google Scholar] [CrossRef]
- Bansal, M.; Kaushal, N. Oxidative Stress Mechanisms and Their Modulation; Springer: Berlin/Heidelberg, Germany, 2014; Volume 9. [Google Scholar]
- Davila, M.P.; Muñoz, P.M.; Bolaños, J.G.; Stout, T.; Gadella, B.; Tapia, J.; Da Silva, C.B.; Ferrusola, C.O.; Peña, F. Mitochondrial atp is required for the maintenance of membrane integrity in stallion spermatozoa, whereas motility requires both glycolysis and oxidative phosphorylation. Reproduction 2016, 152, 683–694. [Google Scholar] [CrossRef]
- Rashki Ghaleno, L.; Alizadeh, A.; Drevet, J.R.; Shahverdi, A.; Valojerdi, M.R. Oxidation of sperm DNA and male infertility. Antioxidants 2021, 10, 97. [Google Scholar] [CrossRef]
- Agarwal, A.; Majzoub, A.; Baskaran, S.; Selvam, M.K.P.; Cho, C.L.; Henkel, R.; Finelli, R.; Leisegang, K.; Sengupta, P.; Barbarosie, C.; et al. Sperm DNA fragmentation: A new guideline for clinicians. World J. Men’s Health 2020, 38, 412. [Google Scholar] [CrossRef]
- Dutta, S.; Henkel, R.; Agarwal, A. Comparative analysis of tests used to assess sperm chromatin integrity and DNA fragmentation. Andrologia 2021, 53, e13718. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wang, H.; Zou, S.; Yu, X.; Li, J. Perspective in the mechanisms for repairing sperm DNA damage. Reprod. Sci. 2025, 32, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Onochie, C.; Evi, K.; O’Flaherty, C. Role of redox-induced protein modifications in spermatozoa in health and disease. Antioxidants 2025, 14, 720. [Google Scholar] [CrossRef] [PubMed]
- Roque, M.; Esteves, S.C. Effect of varicocele repair on sperm DNA fragmentation: A review. Int. Urol. Nephrol. 2018, 50, 583–603. [Google Scholar] [CrossRef]
- Ding, G.-L.; Liu, Y.; Liu, M.-E.; Pan, J.-X.; Guo, M.-X.; Sheng, J.-Z.; Huang, H.-F. The effects of diabetes on male fertility and epigenetic regulation during spermatogenesis. Asian J. Androl. 2015, 17, 948–953. [Google Scholar] [CrossRef] [PubMed]
- Peel, A.; Saini, A.; Deluao, J.C.; McPherson, N.O. Sperm DNA damage: The possible link between obesity and male infertility, an update of the current literature. Andrology 2023, 11, 1635–1652. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Leisegang, K.; Sengupta, P. Oxidative stress in pathologies of male reproductive disorders. In Pathology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 15–27. [Google Scholar]
- Zhang, S.; Tao, W.; Han, J.-D.J. 3d chromatin structure changes during spermatogenesis and oogenesis. Comput. Struct. Biotechnol. J. 2022, 20, 2434–2441. [Google Scholar] [CrossRef]
- Torres-Flores, U.; Hernández-Hernández, A. The interplay between replacement and retention of histones in the sperm genome. Front. Genet. 2020, 11, 780. [Google Scholar] [CrossRef]
- Brykczynska, U.; Hisano, M.; Erkek, S.; Ramos, L.; Oakeley, E.J.; Roloff, T.C.; Beisel, C.; Schübeler, D.; Stadler, M.B.; Peters, A.H. Repressive and active histone methylation mark distinct promoters in human and mouse spermatozoa. Nat. Struct. Mol. Biol. 2010, 17, 679–687. [Google Scholar] [CrossRef]
- Längst, G.; Manelyte, L. Chromatin remodelers: From function to dysfunction. Genes 2015, 6, 299–324. [Google Scholar] [CrossRef]
- Ahmad, S.; Khan, H.; Shahab, U.; Rehman, S.; Rafi, Z.; Khan, M.Y.; Ansari, A.; Siddiqui, Z.; Ashraf, J.M.; Abdullah, S.; et al. Protein oxidation: An overview of metabolism of sulphur containing amino acid, cysteine. Front. Biosci. 2017, 9, 71–87. [Google Scholar] [CrossRef]
- Schneider, S.; Shakeri, F.; Trötschel, C.; Arévalo, L.; Kruse, A.; Buness, A.; Poetsch, A.; Steger, K.; Schorle, H. Protamine-2 deficiency initiates a reactive oxygen species (ros)-mediated destruction cascade during epididymal sperm maturation in mice. Cells 2020, 9, 1789. [Google Scholar] [CrossRef]
- Gavriliouk, D.; Aitken, R.J. Damage to sperm DNA mediated by reactive oxygen species: Its impact on human reproduction and the health trajectory of offspring. In The Male Role in Pregnancy Loss and Embryo Implantation Failure; Springer: Berlin/Heidelberg, Germany, 2015; pp. 23–47. [Google Scholar]
- García-Rodríguez, A.; Gosálvez, J.; Agarwal, A.; Roy, R.; Johnston, S. DNA damage and repair in human reproductive cells. Int. J. Mol. Sci. 2018, 20, 31. [Google Scholar] [CrossRef]
- Gunes, S.; Al-Sadaan, M.; Agarwal, A. Spermatogenesis, DNA damage and DNA repair mechanisms in male infertility. Reprod. Biomed. Online 2015, 31, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Ménézo, Y.; Dale, B.; Cohen, M. DNA damage and repair in human oocytes and embryos: A review. Zygote 2010, 18, 357–365. [Google Scholar] [CrossRef]
- Zhao, R.Z.; Jiang, S.; Zhang, L.; Yu, Z.B. Mitochondrial electron transport chain, ros generation and uncoupling. Int. J. Mol. Med. 2019, 44, 3–15. [Google Scholar] [CrossRef]
- Norberg, E.; Orrenius, S.; Zhivotovsky, B. Mitochondrial regulation of cell death: Processing of apoptosis-inducing factor (aif). Biochem. Biophys. Res. Commun. 2010, 396, 95–100. [Google Scholar] [CrossRef]
- Gill, K.; Kups, M.; Harasny, P.; Machalowski, T.; Grabowska, M.; Lukaszuk, M.; Matuszewski, M.; Duchnik, E.; Fraczek, M.; Kurpisz, M.; et al. The negative impact of varicocele on basic semen parameters, sperm nuclear DNA dispersion and oxidation-reduction potential in semen. Int. J. Environ. Res. Public Health 2021, 18, 5977. [Google Scholar] [CrossRef]
- Jiang, B.; Yang, D.; Peng, H. Environmental toxins and reproductive health: Unraveling the effects on sertoli cells and the blood–testis barrier in animals. Biol. Reprod. 2024, 111, 977–986. [Google Scholar] [CrossRef]
- Zheng, G.; Fu, Y.; He, C. Nucleic acid oxidation in DNA damage repair and epigenetics. Chem. Rev. 2014, 114, 4602–4620. [Google Scholar] [CrossRef] [PubMed]
- Farkouh, A.a.; Salvio, G.; Kuroda, S.; Saleh, R.; Vogiatzi, P.; Agarwal, A. Sperm DNA integrity and male infertility: A narrative review and guide for the reproductive physicians. Transl. Androl. Urol. 2022, 11, 1023. [Google Scholar] [CrossRef] [PubMed]
- Chua, S.C.; Yovich, S.J.; Hinchliffe, P.M.; Yovich, J.L. The sperm DNA fragmentation assay with sdf level less than 15% provides a useful prediction for clinical pregnancy and live birth for women aged under 40 years. J. Pers. Med. 2023, 13, 1079. [Google Scholar] [CrossRef]
- Mangoli, E.; Khalili, M.A. The beneficial role of intra cytoplasmic morphologically selected sperm injection (imsi) in assisted reproduction. J. Reprod. Infertil. 2020, 21, 3. [Google Scholar] [PubMed]
- Dutta, S.; Sengupta, P.; Roychoudhury, S.; Chakravarthi, S.; Wang, C.W.; Slama, P. Antioxidant paradox in male infertility:‘A blind eye’on inflammation. Antioxidants 2022, 11, 167. [Google Scholar] [CrossRef]
- Newman, H.; Catt, S.; Vining, B.; Vollenhoven, B.; Horta, F. DNA repair and response to sperm DNA damage in oocytes and embryos, and the potential consequences in art: A systematic review. Mol. Hum. Reprod. 2022, 28, gaab071. [Google Scholar] [CrossRef]
- Siddeek, B.; Mauduit, C.; Simeoni, U.; Benahmed, M. Sperm epigenome as a marker of environmental exposure and lifestyle, at the origin of diseases inheritance. Mutat. Res./Rev. Mutat. Res. 2018, 778, 38–44. [Google Scholar] [CrossRef]
- Marasco, V.; Boner, W.; Griffiths, K.; Heidinger, B.; Monaghan, P. Intergenerational effects on offspring telomere length: Interactions among maternal age, stress exposure and offspring sex. Proc. R. Soc. B 2019, 286, 20191845. [Google Scholar] [CrossRef]
- Breton, C.V.; Landon, R.; Kahn, L.G.; Enlow, M.B.; Peterson, A.K.; Bastain, T.; Braun, J.; Comstock, S.S.; Duarte, C.S.; Hipwell, A.; et al. Exploring the evidence for epigenetic regulation of environmental influences on child health across generations. Commun. Biol. 2021, 4, 769. [Google Scholar] [CrossRef]
- Esteves, S.C.; Santi, D.; Simoni, M. An update on clinical and surgical interventions to reduce sperm DNA fragmentation in infertile men. Andrology 2020, 8, 53–81. [Google Scholar] [CrossRef]
- Agarwal, A.; Finelli, R.; Selvam, M.K.P.; Leisegang, K.; Majzoub, A.; Tadros, N.; Ko, E.; Parekh, N.; Henkel, R.; Durairajanayagam, D.; et al. A global survey of reproductive specialists to determine the clinical utility of oxidative stress testing and antioxidant use in male infertility. World J. Men’s Health 2021, 39, 470. [Google Scholar] [CrossRef]
- Dutta, S.; Sengupta, P.; Izuka, E.; Menuba, I.; Nwagha, U. Oxidative and nitrosative stress and female reproduction: Roles of oxidants and antioxidants. J. Integr. Sci. Technol. 2024, 12, 754. [Google Scholar] [CrossRef]
- Sengupta, P.; Dutta, S.; Samrot, A.V. Sperm DNA fragmentation testing in infertility. In Genetic Testing in Reproductive Medicine; Springer: Berlin/Heidelberg, Germany, 2024; pp. 47–66. [Google Scholar]
- Tunc, O.; Tremellen, K. Oxidative DNA damage impairs global sperm DNA methylation in infertile men. J. Assist. Reprod. Genet. 2009, 26, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Houshdaran, S.; Cortessis, V.K.; Siegmund, K.; Yang, A.; Laird, P.W.; Sokol, R.Z. Widespread epigenetic abnormalities suggest a broad DNA methylation erasure defect in abnormal human sperm. PLoS ONE 2007, 2, e1289. [Google Scholar] [CrossRef]
- Efimova, O.A.; Pendina, A.A.; Tikhonov, A.V.; Parfenyev, S.E.; Mekina, I.D.; Komarova, E.M.; Mazilina, M.A.; Daev, E.V.; Chiryaeva, O.G.; Galembo, I.A.; et al. Genome-wide 5-hydroxymethylcytosine patterns in human spermatogenesis are associated with semen quality. Oncotarget 2017, 8, 88294–88307. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Sato, A.; Otsu, E.; Hiura, H.; Tomatsu, C.; Utsunomiya, T.; Sasaki, H.; Yaegashi, N.; Arima, T. Aberrant DNA methylation of imprinted loci in sperm from oligospermic patients. Hum. Mol. Genet. 2007, 16, 2542–2551. [Google Scholar] [CrossRef]
- Song, B.; Wang, C.; Chen, Y.; Li, G.; Gao, Y.; Zhu, F.; Wu, H.; Lv, M.; Zhou, P.; Wei, Z.; et al. Sperm DNA integrity status is associated with DNA methylation signatures of imprinted genes and non-imprinted genes. J. Assist. Reprod. Genet. 2021, 38, 2041–2048. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Shen, L.; Dai, Q.; Wu, S.C.; Collins, L.B.; Swenberg, J.A.; He, C.; Zhang, Y. Tet proteins can convert 5-methylcytosine to 5-formylcytosine and 5-carboxylcytosine. Science 2011, 333, 1300–1303. [Google Scholar] [CrossRef]
- Choucair, F.; Saliba, E.; Jaoude, I.A.; Hazzouri, M. Antioxidants modulation of sperm genome and epigenome damage: Fact or fad? Converging evidence from animal and human studies. Middle East Fertil. Soc. J. 2018, 23, 85–90. [Google Scholar] [CrossRef]
- Naderi, N.; Tavalaee, M.; Nasr-Esfahani, M.H. The epigenetic approach of varicocele: A focus on sperm DNA and m6a-rna methylation. Hum. Reprod. Update 2024, 31, 81–101. [Google Scholar] [CrossRef]
- Ucar, V.B.; Nami, B.; Acar, H.; Kılınç, M. Is methylenetetrahydrofolate reductase (mthfr) gene a1298c polymorphism related with varicocele risk? Andrologia 2015, 47, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Hammoud, S.S.; Nix, D.A.; Zhang, H.; Purwar, J.; Carrell, D.T.; Cairns, B.R. Distinctive chromatin in human sperm packages genes for embryo development. Nature 2009, 460, 473–478. [Google Scholar] [CrossRef]
- Mohanty, G.; Swain, N.; Goswami, C.; Kar, S.; Samanta, L. Histone retention, protein carbonylation, and lipid peroxidation in spermatozoa: Possible role in recurrent pregnancy loss. Syst. Biol. Reprod. Med. 2016, 62, 201–212. [Google Scholar] [CrossRef]
- Kouzarides, T. Chromatin modifications and their function. Cell 2007, 128, 693–705. [Google Scholar] [CrossRef]
- Sonnack, V.; Failing, K.; Bergmann, M.; Steger, K. Expression of hyperacetylated histone h4 during normal and impaired human spermatogenesis. Andrologia 2002, 34, 384–390. [Google Scholar] [CrossRef]
- Steilmann, C.; Paradowska, A.; Bartkuhn, M.; Vieweg, M.; Schuppe, H.C.; Bergmann, M.; Kliesch, S.; Weidner, W.; Steger, K. Presence of histone h3 acetylated at lysine 9 in male germ cells and its distribution pattern in the genome of human spermatozoa. Reprod. Fertil. Dev. 2011, 23, 997–1011. [Google Scholar] [CrossRef]
- van de Werken, C.; van der Heijden, G.W.; Eleveld, C.; Teeuwssen, M.; Albert, M.; Baarends, W.M.; Laven, J.S.E.; Peters, A.H.F.M.; Baart, E.B. Paternal heterochromatin formation in human embryos is h3k9/hp1 directed and primed by sperm-derived histone modifications. Nat. Commun. 2014, 5, 5868. [Google Scholar] [CrossRef]
- Oliva, R. Protamines and male infertility. Hum. Reprod. Update 2006, 12, 417–435. [Google Scholar] [CrossRef]
- Francis, S.; Yelumalai, S.; Jones, C.; Coward, K. Aberrant protamine content in sperm and consequential implications for infertility treatment. Hum. Fertil. 2014, 17, 80–89. [Google Scholar] [CrossRef]
- Aoki, V.W.; Liu, L.; Jones, K.P.; Hatasaka, H.H.; Gibson, M.; Peterson, C.M.; Carrell, D.T. Sperm protamine 1/protamine 2 ratios are related to in vitro fertilization pregnancy rates and predictive of fertilization ability. Fertil. Steril. 2006, 86, 1408–1415. [Google Scholar] [CrossRef]
- de Mateo, S.; Gázquez, C.; Guimerà, M.; Balasch, J.; Meistrich, M.L.; Ballescà, J.L.; Oliva, R. Protamine 2 precursors (pre-p2), protamine 1 to protamine 2 ratio (p1/p2), and assisted reproduction outcome. Fertil. Steril. 2009, 91, 715–722. [Google Scholar] [CrossRef]
- Yassin, M.M.; Mwafy, S.N.; Laqqan, M.M. Association of obesity with reproductive hormones alterations, DNA fragmentation and protamine deficiency in human spermatozoa. Middle East Fertil. Soc. J. 2025, 30, 4. [Google Scholar] [CrossRef]
- Sadek, A.; Almohamdy, A.S.; Zaki, A.; Aref, M.; Ibrahim, S.M.; Mostafa, T. Sperm chromatin condensation in infertile men with varicocele before and after surgical repair. Fertil. Steril. 2011, 95, 1705–1708. [Google Scholar] [CrossRef] [PubMed]
- Hekmatdoost, A.; Lakpour, N.; Sadeghi, M.R. Sperm chromatin integrity: Etiologies and mechanisms of abnormality, assays, clinical importance, preventing and repairing damage. Avicenna J. Med. Biotechnol. 2009, 1, 147–160. [Google Scholar] [PubMed]
- Sengul, M.; Hekim, N.; Asci, R.; Gunes, S. The impact of antioxidants on antioxidant capacity, DNA fragmentation, and chromatin quality in subfertile men: A randomized clinical trial study. Rev. Assoc. Medica Bras. 2024, 70, e20240211. [Google Scholar] [CrossRef]
- Hamidian, S.; Talebi, A.R.; Fesahat, F.; Bayat, M.; Mirjalili, A.M.; Ashrafzadeh, H.R.; Rajabi, M.; Montazeri, F.; Babaei, S. The effect of vitamin c on the gene expression profile of sperm protamines in the male partners of couples with recurrent pregnancy loss: A randomized clinical trial. Clin. Exp. Reprod. Med. 2020, 47, 68–76. [Google Scholar] [CrossRef]
- Jodar, M.; Selvaraju, S.; Sendler, E.; Diamond, M.P.; Krawetz, S.A. The presence, role and clinical use of spermatozoal rnas. Hum. Reprod. Update 2013, 19, 604–624. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Xin, Y.; Sun, X.; Zhang, Y.; Chen, Y.; Liu, S.; He, B. Small noncoding rnas contribute to sperm oxidative stress-induced programming of behavioral and metabolic phenotypes in offspring. Oxidative Med. Cell. Longev. 2022, 2022, 6877283. [Google Scholar] [CrossRef]
- Han, X.; Huang, Q. Environmental pollutants exposure and male reproductive toxicity: The role of epigenetic modifications. Toxicology 2021, 456, 152780. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Yu, M.; Guo, T.; Sui, Y.; Tian, Z.; Ni, X.; Chen, X.; Jiang, M.; Jiang, J.; Lu, Y.; et al. Micrornas in spermatogenesis dysfunction and male infertility: Clinical phenotypes, mechanisms and potential diagnostic biomarkers. Front. Endocrinol. 2024, 15, 1293368. [Google Scholar] [CrossRef]
- Aravin, A.A.; Hannon, G.J.; Brennecke, J. The piwi-pirna pathway provides an adaptive defense in the transposon arms race. Science 2007, 318, 761–764. [Google Scholar] [CrossRef] [PubMed]
- Perillo, G.; Shibata, K.; Wu, P.-H. Pirnas in sperm function and embryo viability. Reproduction 2023, 165, R91–R102. [Google Scholar] [CrossRef]
- Stallmeyer, B.; Bühlmann, C.; Stakaitis, R.; Dicke, A.-K.; Ghieh, F.; Meier, L.; Zoch, A.; MacKenzie MacLeod, D.; Steingröver, J.; Okutman, Ö.; et al. Inherited defects of pirna biogenesis cause transposon de-repression, impaired spermatogenesis, and human male infertility. Nat. Commun. 2024, 15, 6637. [Google Scholar] [CrossRef]
- Hwang, Y.E.; Baek, Y.M.; Baek, A.; Kim, D.E. Oxidative stress causes alu rna accumulation via piwil4 sequestration into stress granules. BMB Rep. 2019, 52, 196–201. [Google Scholar] [CrossRef]
- Peng, H.; Shi, J.; Zhang, Y.; Zhang, H.; Liao, S.; Li, W.; Lei, L.; Han, C.; Ning, L.; Cao, Y.; et al. A novel class of trna-derived small rnas extremely enriched in mature mouse sperm. Cell Res. 2012, 22, 1609–1612. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Shi, J.; Tuorto, F.; Li, X.; Liu, Y.; Liebers, R.; Zhang, L.; Qu, Y.; Qian, J.; et al. Dnmt2 mediates intergenerational transmission of paternally acquired metabolic disorders through sperm small non-coding rnas. Nat. Cell Biol. 2018, 20, 535–540. [Google Scholar] [CrossRef]
- Huang, H.-Y.; Hopper, A.K. Multiple layers of stress-induced regulation in trna biology. Life 2016, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Grosso, J.B.; Zoff, L.; Calvo, K.L.; Maraval, M.B.; Perez, M.; Carbonaro, M.; Brignardello, C.; Morente, C.; Spinelli, S.V. Levels of seminal trna-derived fragments from normozoospermic men correlate with the success rate of art. Mol. Hum. Reprod. 2021, 27, gaab017. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.R.; Wang, R.J.; Huang, Z.H.; Wu, H.L.; Huang, X.H.; Bo, H.; Lin, G.; Zhu, W.B.; Huang, C. Sperm trna-derived fragments expression is potentially linked to abstinence-related improvement of sperm quality. Asian J. Androl. 2025, 27, 638–645. [Google Scholar] [CrossRef]
- Li, F.; Duan, X.; Li, M.; Ma, X. Sperm DNA fragmentation index affect pregnancy outcomes and offspring safety in assisted reproductive technology. Sci. Rep. 2024, 14, 356. [Google Scholar] [CrossRef]
- Giacone, F.; Cannarella, R.; Mongioì, L.M.; Alamo, A.; Condorelli, R.A.; Calogero, A.E.; La Vignera, S. Epigenetics of male fertility: Effects on assisted reproductive techniques. World J. Men's Health 2019, 37, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Robinson, L.; Gallos, I.D.; Conner, S.J.; Rajkhowa, M.; Miller, D.; Lewis, S.; Kirkman-Brown, J.; Coomarasamy, A. The effect of sperm DNA fragmentation on miscarriage rates: A systematic review and meta-analysis. Hum. Reprod. 2012, 27, 2908–2917. [Google Scholar] [CrossRef]
- Osman, A.; Alsomait, H.; Seshadri, S.; El-Toukhy, T.; Khalaf, Y. The effect of sperm DNA fragmentation on live birth rate after ivf or icsi: A systematic review and meta-analysis. Reprod. Biomed. Online 2015, 30, 120–127. [Google Scholar] [CrossRef]
- Fu, W.; Cui, Q.; Yang, Z.; Bu, Z.; Shi, H.; Bi, B.; Yang, Q.; Xin, H.; Shi, S.; Hu, L. High sperm DNA fragmentation increased embryo aneuploidy rate in patients undergoing preimplantation genetic testing. Reprod. Biomed. Online 2023, 47, 103366. [Google Scholar] [CrossRef]
- Ounap, K. Silver-russell syndrome and beckwith-wiedemann syndrome: Opposite phenotypes with heterogeneous molecular etiology. Mol. Syndromol. 2016, 7, 110–121. [Google Scholar] [CrossRef]
- Cortessis, V.K.; Azadian, M.; Buxbaum, J.; Sanogo, F.; Song, A.Y.; Sriprasert, I.; Wei, P.C.; Yu, J.; Chung, K.; Siegmund, K.D. Comprehensive meta-analysis reveals association between multiple imprinting disorders and conception by assisted reproductive technology. J. Assist. Reprod. Genet. 2018, 35, 943–952. [Google Scholar] [CrossRef]
- Barberet, J.; Ducreux, B.; Guilleman, M.; Simon, E.; Bruno, C.; Fauque, P. DNA methylation profiles after art during human lifespan: A systematic review and meta-analysis. Hum. Reprod. Update 2022, 28, 629–655. [Google Scholar] [CrossRef]
- Soubry, A.; Murphy, S.K.; Wang, F.; Huang, Z.; Vidal, A.C.; Fuemmeler, B.F.; Kurtzberg, J.; Murtha, A.; Jirtle, R.L.; Schildkraut, J.M.; et al. Newborns of obese parents have altered DNA methylation patterns at imprinted genes. Int. J. Obes. 2015, 39, 650–657. [Google Scholar] [CrossRef]
- Sharma, R.; Agarwal, A.; Rohra, V.K.; Assidi, M.; Abu-Elmagd, M.; Turki, R.F. Effects of increased paternal age on sperm quality, reproductive outcome and associated epigenetic risks to offspring. Reprod. Biol. Endocrinol. 2015, 13, 35. [Google Scholar] [CrossRef]
- Hultman, C.M.; Sandin, S.; Levine, S.Z.; Lichtenstein, P.; Reichenberg, A. Advancing paternal age and risk of autism: New evidence from a population-based study and a meta-analysis of epidemiological studies. Mol. Psychiatry 2011, 16, 1203–1212. [Google Scholar] [CrossRef] [PubMed]
- Gapp, K.; Jawaid, A.; Sarkies, P.; Bohacek, J.; Pelczar, P.; Prados, J.; Farinelli, L.; Miska, E.; Mansuy, I.M. Implication of sperm rnas in transgenerational inheritance of the effects of early trauma in mice. Nat. Neurosci. 2014, 17, 667–669. [Google Scholar] [CrossRef] [PubMed]
- Ji, B.T.; Shu, X.O.; Linet, M.S.; Zheng, W.; Wacholder, S.; Gao, Y.T.; Ying, D.M.; Jin, F. Paternal cigarette smoking and the risk of childhood cancer among offspring of nonsmoking mothers. J. Natl. Cancer Inst. 1997, 89, 238–244. [Google Scholar] [CrossRef]
- Çayan, S.; Farkouh, A.; Agarwal, A.; Atmoko, W.; Wyns, C.; Arafa, M.; Zini, A.; Shah, R.; Alipour, H.; Chung, E.; et al. Global andrology forum clinical guidelines on the relevance of sperm DNA fragmentation in reproductive medicine. World J. Men’s Health 2025, 43, e21. [Google Scholar] [CrossRef]
- Saleh, R.; Sallam, H.; Elsuity, M.A.; Dutta, S.; Sengupta, P.; Nasr, A. Antioxidant therapy for infertile couples: A comprehensive review of the current status and consideration of future prospects. Front. Endocrinol. 2024, 15, 1503905. [Google Scholar] [CrossRef]
- Baskaran, S.; Finelli, R.; Agarwal, A.; Henkel, R. Diagnostic value of routine semen analysis in clinical andrology. Andrologia 2021, 53, e13614. [Google Scholar] [CrossRef] [PubMed]
- Dandekar, S.; Nadkarni, G.; Kulkarni, V.; Punekar, S. Lipid peroxidation and antioxidant enzymes in male infertility. J. Postgrad. Med. 2002, 48, 186–189. [Google Scholar]
- Agarwal, A.; Roychoudhury, S.; Bjugstad, K.B.; Cho, C.-L. Oxidation-reduction potential of semen: What is its role in the treatment of male infertility? Ther. Adv. Urol. 2016, 8, 302–318. [Google Scholar] [CrossRef]
- Panner Selvam, M.K.; Finelli, R.; Agarwal, A.; Henkel, R. Evaluation of seminal oxidation–reduction potential in male infertility. Andrologia 2021, 53, e13610. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Sharma, R.; Roychoudhury, S.; Du Plessis, S.; Sabanegh, E. Mioxsys: A novel method of measuring oxidation reduction potential in semen and seminal plasma. Fertil. Steril. 2016, 106, 566–573.e510. [Google Scholar] [CrossRef]
- Shen, H.-M.; Ong, C.-N. Detection of oxidative DNA damage in human sperm and its association with sperm function and male infertility. Free Radic. Biol. Med. 2000, 28, 529–536. [Google Scholar] [CrossRef]
- Netherton, J.K.; Hetherington, L.; Ogle, R.A.; Gavgani, M.M.; Velkov, T.; Villaverde, A.I.B.; Tanphaichitr, N.; Baker, M.A. Mass spectrometry reveals new insights into the production of superoxide anions and 4-hydroxynonenal adducted proteins in human sperm. Proteomics 2020, 20, 1900205. [Google Scholar] [CrossRef] [PubMed]
- Dalle-Donne, I.; Scaloni, A.; Giustarini, D.; Cavarra, E.; Tell, G.; Lungarella, G.; Colombo, R.; Rossi, R.; Milzani, A. Proteins as biomarkers of oxidative/nitrosative stress in diseases: The contribution of redox proteomics. Mass Spectrom. Rev. 2005, 24, 55–99. [Google Scholar] [CrossRef]
- Gupta, S.; Finelli, R.; Agarwal, A.; Henkel, R. Total antioxidant capacity—Relevance, methods and clinical implications. Andrologia 2021, 53, e13624. [Google Scholar] [CrossRef] [PubMed]
- Organization, W.H. Who laboratory manual for the examination and processing of human semen. In Who Laboratory Manual for the Examination and Processing of Human Semen; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Aitken, R.J.; Gharagozloo, P. The assessment of oxidative stress in human semen: Chaos and confusion in pursuit of diagnostic precision. Reprod. Biomed. Online 2025, 50, 104488. [Google Scholar] [CrossRef]
- Yamasaki, K.; Uchida, M.; Watanabe, N.; Ihana, T.; Ishiguro, Y.; Kuroda, S.; Takeshima, T.; Yumura, Y.; Mieno, M.; Yoshida, K.; et al. Effects of antioxidant co-supplementation therapy on spermatogenesis dysfunction in relation to the basal oxidation–reduction potential levels in spermatozoa: A pilot study. Reprod. Med. Biol. 2022, 21, e12450. [Google Scholar] [CrossRef]
- Fernández, J.L.; Cajigal, D.; López-Fernández, C.; Gosálvez, J. Assessing sperm DNA fragmentation with the sperm chromatin dispersion test. In DNA Damage Detection In Situ, Ex Vivo, and In Vivo: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2010; pp. 291–301. [Google Scholar]
- Agarwal, A.; Farkouh, A.; Parekh, N.; Zini, A.; Arafa, M.; Kandil, H.; Tadros, N.; Busetto, G.M.; Ambar, R.; Parekattil, S.; et al. Sperm DNA fragmentation: A critical assessment of clinical practice guidelines. World J. Men’s health 2021, 40, 30. [Google Scholar] [CrossRef]
- Almekaty, K.; Ghaith, A.; Ragab, M.; Elbardisi, H. Is varicocele repair justified in infertile men with clinical varicocele, normal conventional semen parameters and elevated sperm DNA fragmentation (sdf)? Arab. J. Urol. 2025, 23, 230–236. [Google Scholar] [CrossRef]
- Martinez, M.; Majzoub, A. Best laboratory practices and therapeutic interventions to reduce sperm DNA damage. Andrologia 2021, 53, e13736. [Google Scholar] [CrossRef]
- Szabó, A.; Váncsa, S.; Hegyi, P.; Kói, T.; Ács, J.; Hermánné, R.J.; Ács, N.; Szarvas, T.; Nyirády, P.; Kopa, Z. Assessing the efficacy of varicocelectomy, antioxidants, fsh treatment, and lifestyle modifications on sperm DNA fragmentation: A systematic review and meta-analysis. Sci. Rep. 2025, 15, 10118. [Google Scholar] [CrossRef] [PubMed]
- Gisbert Iranzo, A.; Cano-Extremera, M.; Hervás, I.; Falquet Guillem, M.; Gil Juliá, M.; Navarro-Gomezlechon, A.; Pacheco-Rendón, R.M.; Garrido, N. Sperm selection using microfluidic techniques significantly decreases sperm DNA fragmentation (sdf), enhancing reproductive outcomes: A systematic review and meta-analysis. Biology 2025, 14, 792. [Google Scholar] [CrossRef]
- Dutta, S.; Sengupta, P.; Mottola, F.; Das, S.; Hussain, A.; Ashour, A.; Rocco, L.; Govindasamy, K.; Rosas, I.M.; Roychoudhury, S. Crosstalk between oxidative stress and epigenetics: Unveiling new biomarkers in human infertility. Cells 2024, 13, 1846. [Google Scholar] [CrossRef] [PubMed]
- Åsenius, F.; Danson, A.F.; Marzi, S.J. DNA methylation in human sperm: A systematic review. Hum. Reprod. Update 2020, 26, 841–873. [Google Scholar] [CrossRef] [PubMed]
- Cannarella, R.; Crafa, A.; Barbagallo, F.; Lundy, S.D.; La Vignera, S.; Condorelli, R.A.; Calogero, A.E. H19 sperm methylation in male infertility: A systematic review and meta-analysis. Int. J. Mol. Sci. 2023, 24, 7224. [Google Scholar] [CrossRef]
- Darbandi, M.; Darbandi, S.; Agarwal, A.; Baskaran, S.; Dutta, S.; Sengupta, P.; Khorram Khorshid, H.R.; Esteves, S.; Gilany, K.; Hedayati, M.; et al. Reactive oxygen species-induced alterations in h19-igf2 methylation patterns, seminal plasma metabolites, and semen quality. J. Assist. Reprod. Genet. 2019, 36, 241–253. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Yin, J.; Gao, Y.; Li, Y.; Bai, D.; He, W.; Li, X.; Zhang, P.; Li, R.; et al. Distinct h3k9me3 and DNA methylation modifications during mouse spermatogenesis. J. Biol. Chem. 2019, 294, 18714–18725. [Google Scholar] [CrossRef]
- Bao, J.; Bedford, M.T. Epigenetic regulation of the histone-to-protamine transition during spermiogenesis. Reproduction 2016, 151, R55. [Google Scholar] [CrossRef]
- Capra, E.; Turri, F.; Lazzari, B.; Cremonesi, P.; Gliozzi, T.; Fojadelli, I.; Stella, A.; Pizzi, F. Small rna sequencing of cryopreserved semen from single bull revealed altered mirnas and pirnas expression between high-and low-motile sperm populations. BMC Genom. 2017, 18, 14. [Google Scholar] [CrossRef] [PubMed]
- Dennery, P.A. Effects of oxidative stress on embryonic development. Birth Defects Res. Part C Embryo Today Rev. 2007, 81, 155–162. [Google Scholar] [CrossRef]
- Rodgers, A.B.; Morgan, C.P.; Leu, N.A.; Bale, T.L. Transgenerational epigenetic programming via sperm microrna recapitulates effects of paternal stress. Proc. Natl. Acad. Sci. USA 2015, 112, 13699–13704. [Google Scholar] [CrossRef]
- Jiang, Z.; Wang, Y.; Lin, J.; Xu, J.; Ding, G.; Huang, H. Genetic and epigenetic risks of assisted reproduction. Best Pract. Res. Clin. Obstet. Gynaecol. 2017, 44, 90–104. [Google Scholar] [CrossRef] [PubMed]
- Kaltsas, A.; Giannakodimos, I.; Markou, E.; Stavropoulos, M.; Deligiannis, D.; Kratiras, Z.; Chrisofos, M. The androbactome and the gut microbiota–testis axis: A narrative review of emerging insights into male fertility. Int. J. Mol. Sci. 2025, 26, 6211. [Google Scholar] [CrossRef]
- Dare, B.; Oyeniyi, F.; Olaniyan, O. Role of antioxidant in testicular integrity. Annu. Res. Rev. Biol. 2014, 4, 998. [Google Scholar] [CrossRef]
- Hamada, A.; Esteves, S.C.; Agarwal, A. Genetics and Male Infertility. Infertility-Diagnosis, Management & IVF, 1st ed.; Jaypee Brothers Medical Publishers: New Delhi, India, 2012; pp. 113–160. [Google Scholar]
- Ighodaro, O.; Akinloye, O. First line defence antioxidants-superoxide dismutase (sod), catalase (cat) and glutathione peroxidase (gpx): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Ji, G.; Gu, A.; Wang, Y.; Huang, C.; Hu, F.; Zhou, Y.; Song, L.; Wang, X. Genetic variants in antioxidant genes are associated with sperm DNA damage and risk of male infertility in a chinese population. Free Radic. Biol. Med. 2012, 52, 775–780. [Google Scholar] [CrossRef]
- García Rodríguez, A.; de la Casa, M.; Johnston, S.; Gosálvez, J.; Roy, R. Association of polymorphisms in genes coding for antioxidant enzymes and human male infertility. Ann. Hum. Genet. 2019, 83, 63–72. [Google Scholar] [CrossRef]
- Chabory, E.; Damon, C.; Lenoir, A.; Henry-Berger, J.; Vernet, P.; Cadet, R.; Saez, F.; Drevet, J. Mammalian glutathione peroxidases control acquisition and maintenance of spermatozoa integrity. J. Anim. Sci. 2010, 88, 1321–1331. [Google Scholar] [CrossRef]
- Fallah, F.; Colagar, A.H.; Saleh, H.A.; Ranjbar, M. Variation of the genes encoding antioxidant enzymes sod2 (rs4880), gpx1 (rs1050450), and cat (rs1001179) and susceptibility to male infertility: A genetic association study and in silico analysis. Environ. Sci. Pollut. Res. 2023, 30, 86412–86424. [Google Scholar] [CrossRef]
- Maiorino, M.; Bosello, V.; Ursini, F.; Foresta, C.; Garolla, A.; Scapin, M.; Sztajer, H.; Flohé, L. Genetic variations of gpx-4 and male infertility in humans. Biol. Reprod. 2003, 68, 1134–1141. [Google Scholar] [CrossRef]
- Liu, P.; Zhu, J.; Yuan, G.; Li, D.; Wen, Y.; Huang, S.; Lv, Z.; Guo, Y.; Cheng, J. The effects of selenium on gpx4-mediated lipid peroxidation and apoptosis in germ cells. J. Appl. Toxicol. 2022, 42, 1016–1028. [Google Scholar] [CrossRef] [PubMed]
- Kodydková, J.; Vávrová, L.; Kocík, M.; Zak, A. Human catalase, its polymorphisms, regulation and changes of its activity in different diseases. Folia Biol. 2014, 60, 153. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, G.; Smolenski, S.; Hattis, D.; Guyton, K.Z.; Johns, D.O.; Sonawane, B. Genetic polymorphism in glutathione transferases (gst): Population distribution of gstm1, t1, and p1 conjugating activity. J. Toxicol. Environ. Health Part B 2009, 12, 389–439. [Google Scholar] [CrossRef]
- González-Díaz, C.A.; Suárez-Souto, M.A.; Pérez-Soto, E.; Gómez-López, M.; Munguía-Cervantes, J.E.; Pérez-Vielma, N.M.; Sánchez-Monroy, V. The human 8-oxog DNA glycosylase 1 (ogg1) ser326cys polymorphism in infertile men. Biomedicines 2024, 12, 2286. [Google Scholar] [CrossRef]
- Zheng, L.-r.; Wang, X.-f.; Zhou, D.-x.; Zhang, J.; Huo, Y.-w.; Tian, H. Association between xrcc1 single-nucleotide polymorphisms and infertility with idiopathic azoospermia in northern chinese han males. Reprod. Biomed. Online 2012, 25, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Paladhi, P.; Dutta, S.; Ghosh, P.; Chattopadhyay, R.; Ghosh, S. Men with genetic predisposition face greater fertility challenges when exposed to electromagnetic radiation. Mol. Biol. Rep. 2025, 52, 773. [Google Scholar] [CrossRef]
- Ben Chaaben, A.; Mariaselvam, C.; Salah, S.; Busson, M.; Dulphy, N.; Douik, H.; Ghanem, A.; Boukaouci, W.; Al Daccak, R.; Mamoghli, T.; et al. Polymorphisms in oxidative stress-related genes are associated with nasopharyngeal carcinoma susceptibility. Immunobiology 2015, 220, 20–25. [Google Scholar] [CrossRef]
- Dutta, S.; Sengupta, P. The role of nitric oxide on male and female reproduction. Malays. J. Med. Sci. 2022, 29, 18. [Google Scholar]
- Dutta, S.; Sengupta, P.; Samrot, A.V. Physiological and pathological functions of reactive nitrogen species (rns) and reactive sulphur species (rss) on male reproductive functions. J. Integr. Sci. Technol. 2024, 12, 755. [Google Scholar] [CrossRef]
- Förstermann, U.; Boissel, J.P.; Kleinert, H. Expressional control of the ‘constitutive’isoforms of nitric oxide synthase (nos i and nos iii). FASEB J. 1998, 12, 773–790. [Google Scholar] [CrossRef]
- Buldreghini, E.; Mahfouz, R.Z.; Vignini, A.; Mazzanti, L.; Ricciardo-Lamonica, G.; Lenzi, A.; Agarwal, A.; Balercia, G. Single nucleotide polymorphism (snp) of the endothelial nitric oxide synthase (enos) gene (glu298asp variant) in infertile men with asthenozoospermia. J. Androl. 2010, 31, 482–488. [Google Scholar] [CrossRef]
- Mostafa, T.; Rashed, L.A.; Nabil, N.; Fouad, H.; Sabry, D.; El-Saied, D.M. Endothelial nitric oxide synthase gene polymorphism relationship with semen parameters and oxidative stress in infertile oligoasthenoteratozoospermic men. Urology 2015, 85, 1058–1061. [Google Scholar] [CrossRef]
- Kuchakulla, M.; Masterson, T.; Arora, H.; Kulandavelu, S.; Ramasamy, R. Effect of nitroso-redox imbalance on male reproduction. Transl. Androl. Urol. 2018, 7, 968. [Google Scholar] [CrossRef]
- Nasr, H.B.; Dimassi, S.; M’hadhbi, R.; Debbabi, H.; Kortas, M.; Tabka, Z.; Chahed, K. Functional g894t (rs1799983) polymorphism and intron-4 vntr variant of nitric oxide synthase (nos3) gene are susceptibility biomarkers of obesity among tunisians. Obes. Res. Clin. Pract. 2016, 10, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Vučić, N.L.; Nikolić, Z.Z.; Vukotić, V.D.; Tomović, S.M.; Vuković, I.I.; Kanazir, S.; Savić-Pavićević, D.L.; Brajušković, G.N. Nos 3 gene variants and male infertility: Association of 4a/4b with oligoasthenozoospermia. Andrologia 2018, 50, e12817. [Google Scholar] [CrossRef] [PubMed]
- Qidwai, T.; Jamal, F. Inducible nitric oxide synthase (inos) gene polymorphism and disease prevalence. Scand. J. Immunol. 2010, 72, 375–387. [Google Scholar] [CrossRef]
- Fraczek, M.; Kurpisz, M. Inflammatory mediators exert toxic effects of oxidative stress on human spermatozoa. J. Androl. 2007, 28, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Tatham, A.L.; Crabtree, M.J.; Warrick, N.; Cai, S.; Alp, N.J.; Channon, K.M. Gtp cyclohydrolase i expression, protein, and activity determine intracellular tetrahydrobiopterin levels, independent of gtp cyclohydrolase feedback regulatory protein expression. J. Biol. Chem. 2009, 284, 13660–13668. [Google Scholar] [CrossRef]
- Channon, K.M. Tetrahydrobiopterin and nitric oxide synthase recouplers. In Reactive Oxygen Species: Network Pharmacology and Therapeutic Applications; Springer: Berlin/Heidelberg, Germany, 2020; pp. 339–352. [Google Scholar]
- Gholinezhad, M.; Aliarab, A.; Abbaszadeh-Goudarzi, G.; Yousefnia-Pasha, Y.; Samadaian, N.; Rasolpour-Roshan, K.; Aghagolzadeh-Haji, H.; Mohammadoo-Khorasani, M. Nitric oxide, 8-hydroxydeoxyguanosine, and total antioxidant capacity in human seminal plasma of infertile men and their relationship with sperm parameters. Clin. Exp. Reprod. Med. 2020, 47, 54. [Google Scholar] [CrossRef]
- Gonzalez, M.; Clayton, S.; Wauson, E.; Christian, D.; Tran, Q.-K. Promotion of nitric oxide production: Mechanisms, strategies, and possibilities. Front. Physiol. 2025, 16, 1545044. [Google Scholar] [CrossRef]
- Karahalil, B.; Aygun Kocabas, N. Hogg1 ser326cys genetic polymorphism in a turkish population. Arch. Toxicol. 2005, 79, 377–380. [Google Scholar] [CrossRef]
- Wahid, M. Base excision repair DNA genetic variant ogg1 ser326cys among saudi population: A comparative approach with worldwide ethnic group variations. Minerva Biotechnol. Biomol. Res. 2025, 37, 21. [Google Scholar] [CrossRef]
- Kurashova, N.A.; Dashiev, B.G.; Bairova, T.A.; Labygina, A.V.; Kolesnikova, L.I. Association of polymorphic markers of gstp1 gene with oxidative stress parameters in infertility men. Urologiia 2020, 4, 84–89. [Google Scholar] [CrossRef]
- Panner Selvam, M.K.; Baskaran, S.; O’Connell, S.; Almajed, W.; Hellstrom, W.J.; Sikka, S.C. Association between seminal oxidation-reduction potential and sperm DNA fragmentation—A meta-analysis. Antioxidants 2022, 11, 1563. [Google Scholar] [CrossRef] [PubMed]
- Cissen, M.; Wely, M.v.; Scholten, I.; Mansell, S.; Bruin, J.P.d.; Mol, B.W.; Braat, D.; Repping, S.; Hamer, G. Measuring sperm DNA fragmentation and clinical outcomes of medically assisted reproduction: A systematic review and meta-analysis. PLoS ONE 2016, 11, e0165125. [Google Scholar] [CrossRef] [PubMed]
- Simon, L.; Zini, A.; Dyachenko, A.; Ciampi, A.; Carrell, D.T. A systematic review and meta-analysis to determine the effect of sperm DNA damage on in vitro fertilization and intracytoplasmic sperm injection outcome. Asian J. Androl. 2017, 19, 80–90. [Google Scholar] [CrossRef]
- Deng, C.; Li, T.; Xie, Y.; Guo, Y.; Yang, Q.y.; Liang, X.; Deng, C.h.; Liu, G.h. Sperm DNA fragmentation index influences assisted reproductive technology outcome: A systematic review and meta-analysis combined with a retrospective cohort study. Andrologia 2019, 51, e13263. [Google Scholar] [CrossRef]
- Chen, Y.; Li, W.; Chen, X. The association of sperm DNA fragment and assisted reproductive outcomes: A meta-analysis. Comput. Math. Methods Med. 2022, 2022, 1126616. [Google Scholar] [CrossRef]
- Zhao, G.; Jiang, X.; Zheng, Y.; Bai, H.; Jiang, Z.; Cheng, S.; Li, D. Outcomes comparison of testicular versus ejaculated sperm for intracytoplasmic sperm injection in infertile men with high DNA fragmentation: Updated systematic review and meta-analysis. Transl. Androl. Urol. 2023, 12, 1785. [Google Scholar] [CrossRef]
- Esteves, S.C.; Roque, M.; Bradley, C.K.; Garrido, N. Reproductive outcomes of testicular versus ejaculated sperm for intracytoplasmic sperm injection among men with high levels of DNA fragmentation in semen: Systematic review and meta-analysis. Fertil. Steril. 2017, 108, 456–467.e451. [Google Scholar] [CrossRef] [PubMed]
- Cano-Extremera, M.; Hervas, I.; Gisbert Iranzo, A.; Falquet Guillem, M.; Gil Juliá, M.; Navarro-Gomezlechon, A.; Pacheco-Rendón, R.; Garrido Puchalt, N. Superior live birth rates, reducing sperm DNA fragmentation (sdf), and lowering miscarriage rates by using testicular sperm versus ejaculates in intracytoplasmic sperm injection (icsi) cycles from couples with high sdf: A systematic review and meta-analysis. Biology 2025, 14, 130. [Google Scholar] [CrossRef] [PubMed]
- Awaga, H.A.; Bosdou, J.K.; Goulis, D.G.; Chatzimeletiou, K.; Salem, M.; Roshdy, S.; Grimbizis, G.; Tarlatzis, B.C.; Kolibianakis, E.M. Testicular versus ejaculated spermatozoa for icsi in patients without azoospermia: A systematic review. Reprod. Biomed. Online 2018, 37, 573–580. [Google Scholar] [CrossRef] [PubMed]
| Axis of Interaction | Principal Mechanistic Drivers | Molecular Consequences | Representative Biomarkers/Assays | Clinical Manifestation/Endotype | Therapeutic and Translational Implications |
|---|---|---|---|---|---|
| Redox Imbalance (Oxidative Axis) | Mitochondrial electron leakage (Complex I/III); NADPH oxidase overactivity; leukocyte infiltration; environmental ROS (smoke, metals, heat) | Lipid peroxidation (MDA, 4-HNE); protein carbonylation; oxidative base lesions (8-OHdG) | ORP (MiOXSYS); TAC; SOD, GPx, CAT activity; 8-OHdG ELISA | Redox-dominant or Redox-driven SDF phenotype; often reversible with antioxidant/lifestyle correction | Lifestyle optimization; targeted antioxidants (CoQ10, carnitine, NAC); ORP-guided therapy; monitor to prevent reductive stress |
| DNA Integrity (Genomic Axis) | Oxidative strand scission; protamine deficiency; apoptotic endonuclease activation (EndoG, AIF); defective repair (OGG1, PARP1 loss) | Single-/double-strand breaks; mtDNA mutations; chromatin cross-links | SDF assays: TUNEL, SCSA, Comet, SCD; 8-OHdG quantification | SDF-isolated or mixed oxidative-genomic phenotype; unexplained infertility with normal semen parameters | Varicocelectomy, infection control, antioxidant or mitochondrial stabilizers; SDF-guided ART planning |
| Chromatin Remodeling | Incomplete histone–protamine transition; BRDT/CHD5/TNP/PRM mutations; cysteine oxidation in protamines | Excess histone retention; disulfide bridge loss; open chromatin susceptibility | Protamine 1/2 ratio; Aniline blue staining; ChIP-seq for H3K4me3/H3K9me2 | Chromatin/epigenetic dysfunction with redox overlay | Epigenetic-safe antioxidants (melatonin); sperm chromatin maturity testing before ART |
| Epigenetic Dysregulation | ROS-induced methyl-cytosine oxidation; TET dysfunction; altered histone acetylation; disturbed small RNA cargo | Global hypomethylation; imprinting errors (H19, MEST); dysregulated miRNA/piRNA | Bisulfite-seq; methylation arrays; small RNA-seq | Epigenetic-dominant phenotype; recurrent miscarriage or ART failure despite normal semen | Nutritional-epigenetic modulation (folate, B12, SAMe); avoidance of environmental oxidants; selection of epigenetically stable sperm for ART |
| Genetic Modifiers of Redox Response | SNPs in SOD2 (Val16Ala), GPx1 (Pro198Leu), CAT (–262C>T), GST nulls, NOS3 (Glu298Asp) | Altered enzymatic kinetics; NO–ROS imbalance; increased susceptibility to oxidative injury | Genotyping (PCR/NGS panels); correlation with ORP and SDF | Variable expressivity across ethnic cohorts; defines heritable redox–genomic endotype | Genotype-guided antioxidant or NO-modulating therapy; population-specific reference intervals |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Sengupta, P.; Dutta, S.; Elsuity, M.A.; Saleh, R. Redox–Genomic Crosstalk: Linking Oxidative Stress, Sperm DNA Fragmentation, and Epigenetics in Personalized Management of Male Infertility. J. Pers. Med. 2026, 16, 79. https://doi.org/10.3390/jpm16020079
Sengupta P, Dutta S, Elsuity MA, Saleh R. Redox–Genomic Crosstalk: Linking Oxidative Stress, Sperm DNA Fragmentation, and Epigenetics in Personalized Management of Male Infertility. Journal of Personalized Medicine. 2026; 16(2):79. https://doi.org/10.3390/jpm16020079
Chicago/Turabian StyleSengupta, Pallav, Sulagna Dutta, Mohamed AlaaEldein Elsuity, and Ramadan Saleh. 2026. "Redox–Genomic Crosstalk: Linking Oxidative Stress, Sperm DNA Fragmentation, and Epigenetics in Personalized Management of Male Infertility" Journal of Personalized Medicine 16, no. 2: 79. https://doi.org/10.3390/jpm16020079
APA StyleSengupta, P., Dutta, S., Elsuity, M. A., & Saleh, R. (2026). Redox–Genomic Crosstalk: Linking Oxidative Stress, Sperm DNA Fragmentation, and Epigenetics in Personalized Management of Male Infertility. Journal of Personalized Medicine, 16(2), 79. https://doi.org/10.3390/jpm16020079







