Future of Valvular Heart Disease and Structural Heart Interventions: Why So Much Excitement?
Abstract
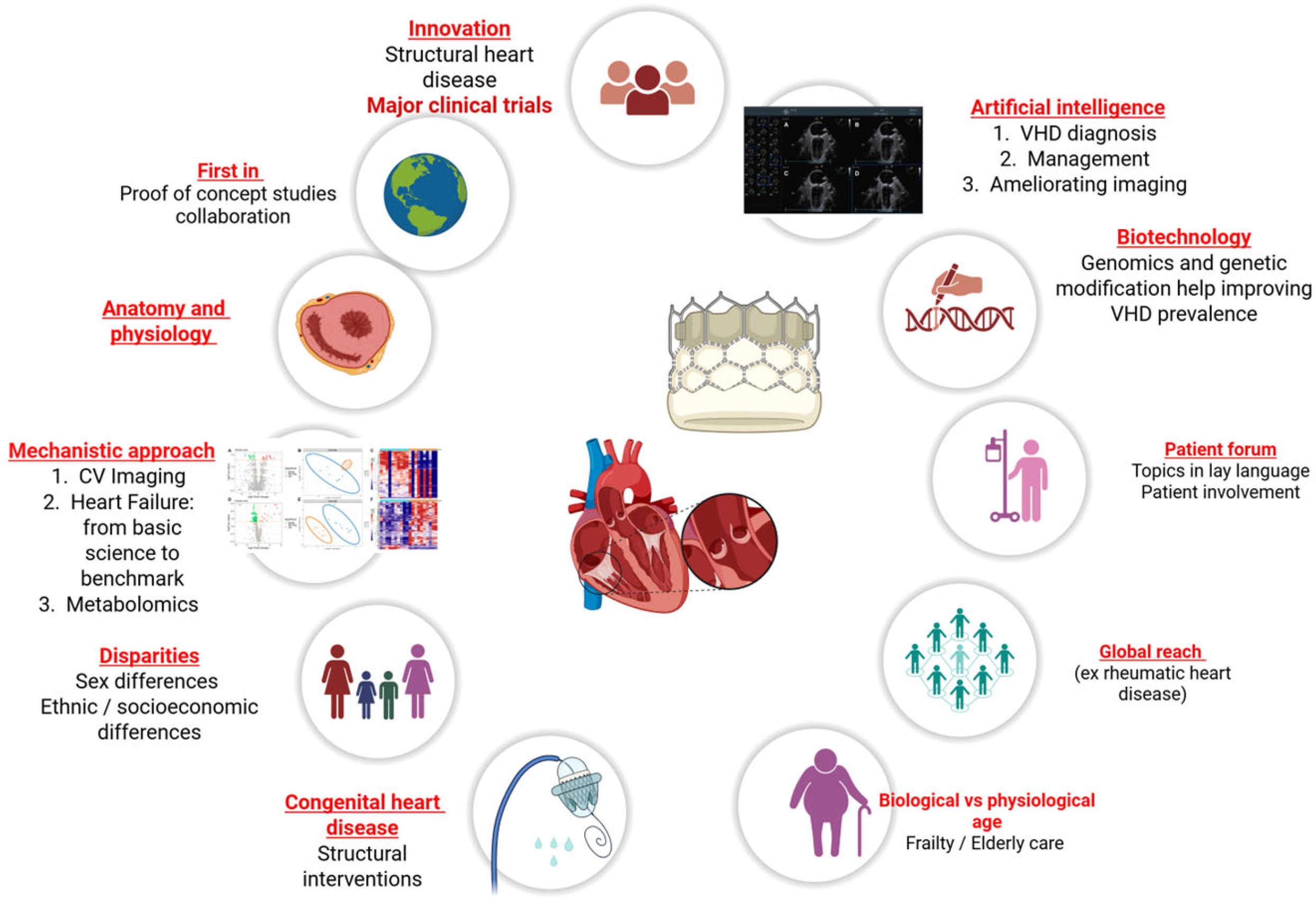
1. Introduction
2. Multimodality Imaging in Structural Heart Disease
3. Clinical Trials and Proof of Concept Studies
4. Multiomics and Metabolomics in SHD
5. Disparities in SHD
6. Frailty in SHD
7. Congenital Heart Disease and VHD Interventions
8. Artificial Intelligence
9. Multidisciplinary Team Decision-Making in VHD Management
10. Going Back the Basics
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| VHD | Valvular heart disease |
| SHD | Structural heart disease |
| CMR | Cardiac magnetic resonance |
| CT | Computed tomography |
| ACHD | Adult congenital heart disease |
| AI | Artificial intelligence |
| ECG | Electrocardiogram |
| 2D | Two-dimensional |
| 3D | Three-dimensional |
| AVA | Aortic valve area |
| LVOT | Left ventricular outflow tract |
| DHM | Digital heart model |
References
- Pibarot, P.; Larose, É.; Dumesnil, J. Imaging of valvular heart disease. Can. J. Cardiol. 2013, 29, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Unger, P.; Pibarot, P.; Tribouilloy, C.; Lancellotti, P.; Maisano, F.; Iung, B.; Piérard, L. European Society of Cardiology Council on Valvular Heart Disease. Multiple and Mixed Valvular Heart Diseases. Circ. Cardiovasc. Imaging 2018, 11, e007862. [Google Scholar] [CrossRef] [PubMed]
- Schlosshan, D.; Aggarwal, G.; Mathur, G.; Allan, R.; Cranney, G. Real-time 3D transesophageal echocardiography for the evaluation of rheumatic mitral stenosis. JACC Cardiovasc. Imaging 2011, 4, 580–588. [Google Scholar] [CrossRef]
- Chu, J.W.; Levine, R.A.; Chua, S.; Poh, K.-K.; Morris, E.; Hua, L.; Ton-Nu, T.-T.; Hung, J. Assessing mitral valve area and orifice geometry in calcific mitral stenosis: A new solution by real-time three-dimensional echocardiography. J. Am. Soc. Echocardiogr. 2008, 21, 1006–1009. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bartel, T.; Bonaros, N.; Edlinger, M.; Velik-Salchner, C.; Feuchtner, G.; Rudnicki, M.; Müller, S. Intracardiac echo and reduced radiocontrast requirements during TAVR. JACC Cardiovasc. Imaging 2014, 7, 319–320. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lancellotti, P.; Pellikka, P.A.; Budts, W.; Chaudhry, F.A.; Donal, E.; Dulgheru, R.; Edvardsen, T.; Garbi, M.; Ha, J.-W.; Kane, G.C.; et al. The clinical use of stress echocardiography in non-ischaemic heart disease: Recommendations from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 1191–1229, Erratum in Eur. Heart J. Cardiovasc. Imaging 2017, 18, 832. https://doi.org/10.1093/ehjci/jex040.. [Google Scholar] [CrossRef] [PubMed]
- Kalam, K.; Otahal, P.; Marwick, T.H. Prognostic implications of global LV dysfunction: A systematic review and meta-analysis of global longitudinal strain and ejection fraction. Heart 2014, 100, 1673–1680. [Google Scholar] [CrossRef]
- Bax, J.J.; Delgado, V. Advanced imaging in valvular heart disease. Nat. Rev. Cardiol. 2017, 14, 209–223. [Google Scholar] [CrossRef] [PubMed]
- Marsan, N.A.; Delgado, V.; Shah, D.J.; Pellikka, P.; Bax, J.J.; Treibel, T.; Cavalcante, J.L. Valvular heart disease: Shifting the focus to the myocardium. Eur. Heart J. 2022, 44, 28–40. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, D.D.; Villablanca, P.A.; So, K.C.-Y.; Cubeddu, R.J.; O’nEill, B.P.; O’nEill, W.W. CT Imaging for Valvular Interventions. Struct. Heart 2025, 9, 100667. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cawley, P.J.; Maki, J.H.; Otto, C.M. Cardiovascular magnetic resonance imaging for valvular heart disease: Technique and validation. Circulation 2009, 119, 468–478. [Google Scholar] [CrossRef] [PubMed]
- Everett, R.J.; Treibel, T.A.; Fukui, M.; Lee, H.; Rigolli, M.; Singh, A.; Bijsterveld, P.; Tastet, L.; Al Musa, T.; Dobson, L.; et al. Extracellular Myocardial Volume in Patients with Aortic Stenosis. J. Am. Coll. Cardiol. 2020, 75, 304–316. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nchimi, A.; Dibato, J.E.; Davin, L.; Schoysman, L.; Oury, C.; Lancellotti, P. Predicting Disease Progression and Mortality in Aortic Stenosis: A Systematic Review of Imaging Biomarkers and Meta-Analysis. Front. Cardiovasc. Med. 2018, 5, 112. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.; Kadem, L.; Larose, E.; Clavel, M.-A.; Pibarot, P. Comparison between cardiovascular magnetic resonance and transthoracic Doppler echocardiography for the estimation of effective orifice area in aortic stenosis. J. Cardiovasc. Magn. Reson. Off. J. Soc. Cardiovasc. Magn. Reson. 2011, 13, 25. [Google Scholar] [CrossRef]
- Troger, F.; Lechner, I.; Reindl, M.; Tiller, C.; Holzknecht, M.; Pamminger, M.; Kremser, C.; Schwaiger, J.; Reinstadler, S.J.; Bauer, A.; et al. A novel approach to determine aortic valve area with phase-contrast cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2022, 24, 7–13. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kietrsunthorn, P.S.; Ghrair, F.; Schelegle, A.R.; Foerst, J.R. Transcatheter Mitral Valve Therapies in Patients with Mitral Annular Calcification. Interv. Cardiol. Clin. 2024, 13, 237–248. [Google Scholar] [CrossRef]
- Marin-Cuartas, M.; Zaid, S.; Kempfert, J.; Borger, M.A.; Akansel, S.; Noack, T.; Holzhey, D.; Kaneko, T.; George, I.; Ailawadi, G.; et al. Mitral valve surgery after failed transcatheter intervention for mitral regurgitation: Surgical techniques, challenges and outcomes. Eur. J. Cardio-Thoracic Surg. 2025, 67, ezaf179. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, S.; Conradi, L.; Cohen, D.J.; Coisne, A.; Scotti, A.; Abraham, W.T.; Ben Ali, W.; Zhou, Z.; Li, Y.; Kar, S.; et al. Transcatheter Mitral Valve Replacement Versus Medical Therapy for Secondary Mitral Regurgitation: A Propensity Score-Matched Comparison. Circ. Cardiovasc. Interv. 2023, 16, e013045. [Google Scholar] [CrossRef] [PubMed]
- Trindade, F.; Almeida-Coelho, J.; Sousa-Mendes, C.; Saraiva, F.; Arbonés, M.L.; Leite-Moreira, A.; Vitorino, R.; Falcão-Pires, I. Myocardial phosphoproteomics unveils a key role of DYRK1A in aortic valve replacement-induced reverse remodelling. Basic Res. Cardiol. 2025. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Gaznabi, S.; Miranda, J.; Lorenzatti, D.; Piña, P.; Balasubramanian, S.S.; Desai, D.; Desai, A.; Ho, E.C.; Scotti, A.; Gongora, C.A.; et al. Multimodality Imaging in Aortic Stenosis: Beyond the Valve—Focusing on the Myocardium. Interv. Cardiol. Clin. 2025, 14, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Jacob, M.; Lopata, A.L.; Dasouki, M.; Rahman, A.M.A. Metabolomics toward personalized medicine. Mass Spectrom. Rev. 2019, 38, 221–238. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G.D.; Asnani, A.; Gerszten, R.E. Application of metabolomics to cardiovascular biomarker and pathway discovery. J. Am. Coll. Cardiol. 2008, 52, 117–123. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Müller, J.; Bertsch, T.; Volke, J.; Schmid, A.; Klingbeil, R.; Metodiev, Y.; Karaca, B.; Kim, S.-H.; Lindner, S.; Schupp, T.; et al. Narrative review of metabolomics in cardiovascular disease. J. Thorac. Dis. 2021, 13, 2532–2550. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Karczewski, K.J.; Snyder, M.P. Integrative omics for health and disease. Nat. Rev. Genet. 2018, 19, 299–310. [Google Scholar] [CrossRef]
- Blaser, M.C.; Kraler, S.; Lüscher, T.F.; Aikawa, E. Multi-Omics Approaches to Define Calcific Aortic Valve Disease Pathogenesis. Circ. Res. 2021, 128, 1371–1397. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- González, A.; Ravassa, S.; Beaumont, J.; López, B.; Díez, J. New targets to treat the structural remodeling of the myocardium. J. Am. Coll. Cardiol. 2011, 58, 1833–1843. [Google Scholar] [CrossRef] [PubMed]
- DesJardin, J.T.; Chikwe, J.; Hahn, R.T.; Hung, J.W.; Delling, F.N. Sex Differences and Similarities in Valvular Heart Disease. Circ. Res. 2022, 130, 455–473. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Munir, M.B.; Khan, S.U.; Subramanian, C.R.; Asad, Z.U.A.; Talluri, S.; Madhanakumar, A.; Lone, A.N.; Khan, M.S.; Michos, E.D.; Alkhouli, M. Representation of women, older patients, ethnic, and racial minorities in trials of atrial fibrillation. Pacing Clin. Electrophysiol. 2021, 44, 423–431. [Google Scholar] [CrossRef] [PubMed]
- WHO Guideline on the Prevention and Diagnosis of Rheumatic Fever and Rheumatic Heart Disease [Internet]; World Health Organization: Geneva, Switzerland, 2024; 4, Recommendations. Available online: https://www.ncbi.nlm.nih.gov/books/NBK609686/?utm_source=chatgpt.com (accessed on 1 September 2025).
- Pavone, N.; Chiariello, G.A.; Bruno, P.; Marzetti, E.; Spalletta, C.; Nesta, M.; Mazza, A.; Cammertoni, F.; Colizzi, C.; Iafrancesco, M.; et al. The “Heart Valve Clinic” Pathway for the Management of Frail Patients with Valvular Heart Disease: From “One for All” to “All for One”. Crit. Pathways Cardiol. 2019, 18, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Valvani, R.; Javed, N.; Vittorio, T.; Mohyeldin, M. Frailty Indices and Their Importance in Elderly Patients: A Perspective Review. J. Community Hosp. Intern. Med. Perspect. 2024, 14, 5–33. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bou-Chaaya, R.G.; Zhu, Z.; Duarte, V.E.; Lin, C.H. Percutaneous Structural Interventions in Adult Congenital Heart Disease. Methodist DeBakey Cardiovasc. J. 2023, 19, 78–90. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kang, S.-L.; Benson, L. Recent advances in cardiac catheterization for congenital heart disease. F1000Research 2018, 7, 370. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Attia, Z.I.; Kapa, S.; Lopez-Jimenez, F.; McKie, P.M.; Ladewig, D.J.; Satam, G.; Pellikka, P.A.; Enriquez-Sarano, M.; Noseworthy, P.A.; Munger, T.M.; et al. Screening for cardiac contractile dysfunction using an artificial intelligence–enabled electrocardiogram. Nat. Med. 2019, 25, 70–74. [Google Scholar] [CrossRef]
- Cohen-Shelly, M.; Attia, Z.I.; Friedman, P.A.; Ito, S.; Essayagh, B.A.; Ko, W.-Y.; Murphree, D.H.; Michelena, H.I.; Enriquez-Sarano, M.; Carter, R.E.; et al. Electrocardiogram screening for aortic valve stenosis using artificial intelligence. Eur. Heart J. 2021, 42, 2885–2896. [Google Scholar] [CrossRef] [PubMed]
- Bamford, P.; Abdelrahman, A.; Malkin, C.J.; Cunnington, M.S.; Blackman, D.J.; Ali, N. Artificial intelligence in heart valve disease: Diagnosis, innovation and treatment. A state-of-the-art review. Br. J. Cardiol. 2024, 31, 031. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mihan, A.; Pandey, A.; Van Spall, H.G. Mitigating the risk of artificial intelligence bias in cardiovascular care. Lancet Digit. Health 2024, 6, e749–e754. [Google Scholar] [CrossRef] [PubMed]
- Praz, F.; Borger, M.A.; Lanz, J.; Marin-Cuartas, M.; Abreu, A.; Adamo, M.; Marsan, N.A.; Barili, F.; Bonaros, N.; Cosyns, B.; et al. 2025 ESC/EACTS Guidelines for the management of valvular heart disease: Developed by the task force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2025, ehaf194. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients with Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, e35–e71, Erratum in Circulation 2021, 143, e228. https://doi.org/10.1161/CIR.0000000000000960; Erratum in Circulation 2021, 143, e784. https://doi.org/10.1161/CIR.0000000000000966.. [Google Scholar] [CrossRef] [PubMed]
- Symersky, P.; Budde, R.P.J.; Westers, P.; de Mol, B.A.J.M.; Prokop, M. Multidetector CT imaging of mechanical prosthetic heart valves: Quantification of artifacts with a pulsatile in-vitro model. Eur. Radiol. 2011, 21, 2103–2110. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Afilalo, J.; Lauck, S.; Kim, D.H.; Lefevre, T.; Piazza, N.; Lachapelle, K.; Martucci, G.; Lamy, A.; Labinaz, M.; Peterson, M.D.; et al. Frailty in older adults undergoing aortic valve replacement: The FRAILTY-AVR Study. J. Am. Coll. Cardiol. 2017, 70, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Hahn, R.T.; Lawlor, M.K.; Davidson, C.J.; Badhwar, V.; Sannino, A.; Spitzer, E.; Lurz, P.; Lindman, B.R.; Topilsky, Y.; Baron, S.J.; et al. Tricuspid Valve Academic Research Consortium Definitions for Tricuspid Regurgitation and Trial Endpoints. Eur. Heart J. 2023, 44, 4508–4532. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- von Stein, P.; Stolz, L.; Haurand, J.M.; Gröger, M.; Rudolph, F.; Mustafa, D.; Jobst, J.; Mues, C.A.; Mahabadi, A.A.; Hoerbrand, I.A.; et al. Transcatheter Edge-to-Edge Repair for Atrial and Ventricular Secondary Mitral Regurgitation: Insights From the REPAIR Study. JACC Cardiovasc. Interv. 2025, 18, 2020–2032. [Google Scholar] [CrossRef] [PubMed]
- Argulian, E.; Grapsa, J. Precision medicine and mitral valve assessment. Eur. Heart J. Imaging Methods Pract. 2025, 3, qyaf073. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Little, S.H. Structural Heart Interventions. Methodist DeBakey Cardiovasc. J. 2017, 13, 96–97. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beneki, E.; Grapsa, J. Future of Valvular Heart Disease and Structural Heart Interventions: Why So Much Excitement? J. Pers. Med. 2025, 15, 443. https://doi.org/10.3390/jpm15090443
Beneki E, Grapsa J. Future of Valvular Heart Disease and Structural Heart Interventions: Why So Much Excitement? Journal of Personalized Medicine. 2025; 15(9):443. https://doi.org/10.3390/jpm15090443
Chicago/Turabian StyleBeneki, Eirini, and Julia Grapsa. 2025. "Future of Valvular Heart Disease and Structural Heart Interventions: Why So Much Excitement?" Journal of Personalized Medicine 15, no. 9: 443. https://doi.org/10.3390/jpm15090443
APA StyleBeneki, E., & Grapsa, J. (2025). Future of Valvular Heart Disease and Structural Heart Interventions: Why So Much Excitement? Journal of Personalized Medicine, 15(9), 443. https://doi.org/10.3390/jpm15090443





