Diabetes Phenotypes in Patients Presenting a Myocardial Infarction: Progress Towards Precision Medicine?
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Cluster Analysis
2.3. Cardiovascular Events
3. Results
3.1. Patients and Clusters
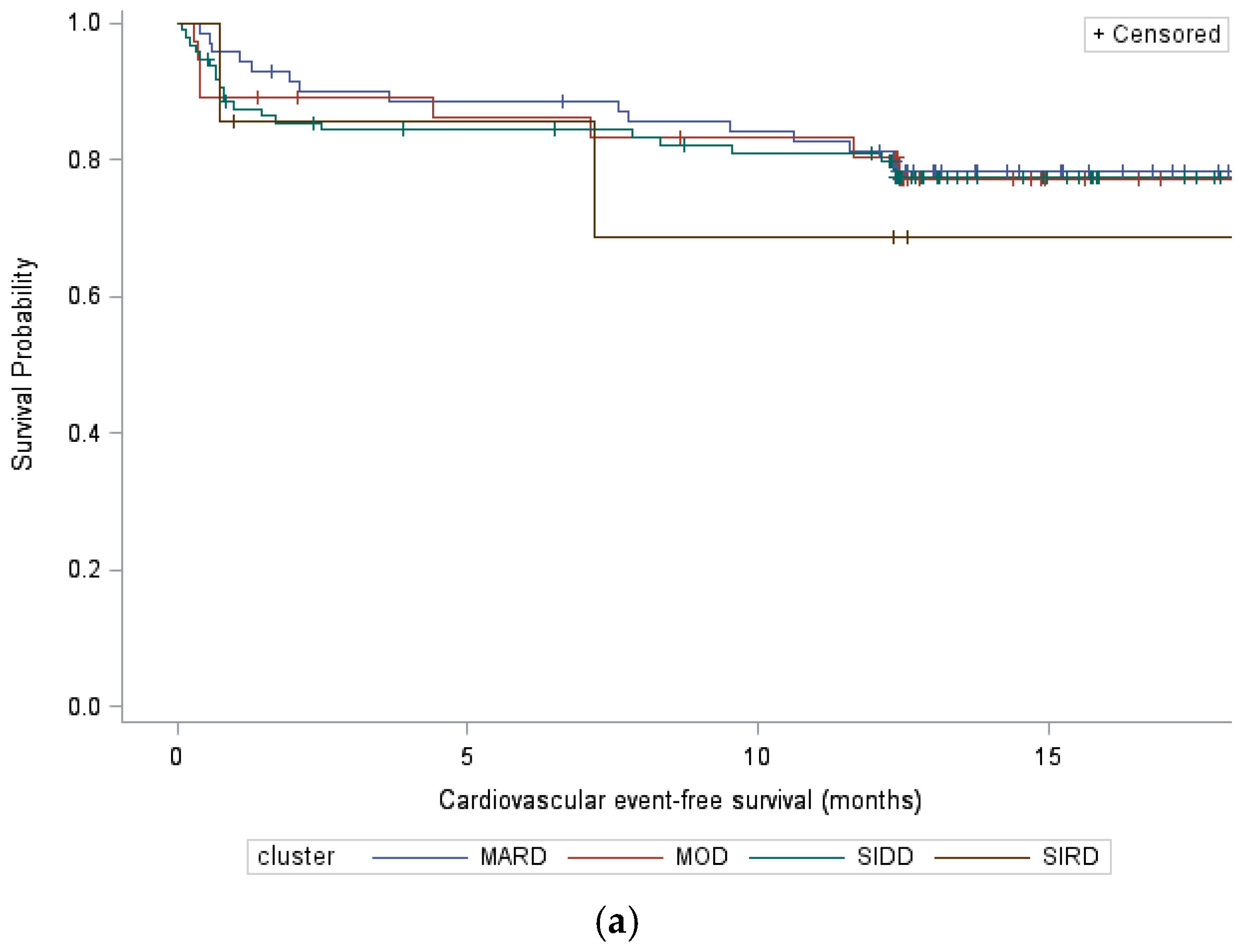
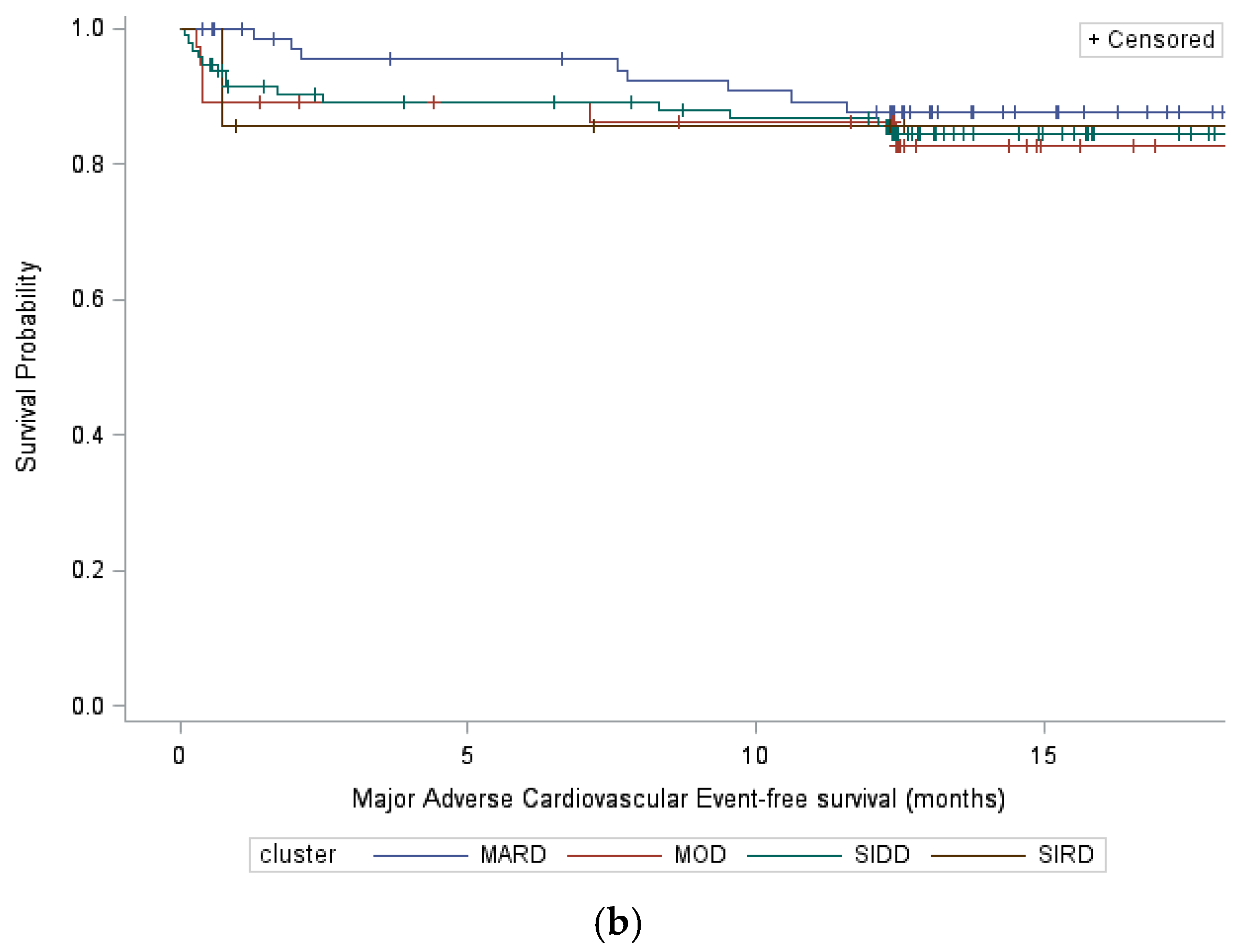
3.2. Cardiovascular Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The Emerging Risk Factors Collaboration. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: A collaborative meta-analysis of 102 prospective studies. Lancet 2010, 375, 2215–2222. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes-2025. Diabetes Care 2025, 48 (Suppl. 1), S27–S49. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Diabetes. Key Facts 2024. Available online: www.who.int/news-room/fact-sheets/detail/diabetes (accessed on 18 August 2025).
- Ahlqvist, E.; Storm, P.; Käräjämäki, A.; Martinell, M.; Dorkhan, M.; Carlsson, A.; Vikman, P.; Prasad, R.B.; Aly, D.M.; Almgren, P.; et al. Novel subgroups of adult-onset diabetes and their association with outcomes: A data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol. 2018, 6, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Kreimer, F.; Schlettert, C.; Abumayyaleh, M.; Akin, I.; Hijazi, M.M.; Hamdani, N.; Gotzmann, M.; Mügge, A.; El-Battrawy, I.; Aweimer, A. The impact of diabetes mellitus on the outcome of troponin-positive patients with non-obstructive coronary arteries. Int. J. Cardiol. Heart Vasc. 2024, 50, 101350. [Google Scholar] [CrossRef] [PubMed]
- Ekemeyong Awong, L.E.; Zielinska, T. Comparative Analysis of the Clustering Quality in Self-Organizing Maps for Human Posture Classification. Sensors 2023, 23, 7925. [Google Scholar] [CrossRef]
- Salimi, A.; Zolghadrasli, A.; Jahangiri, S.; Hatamnejad, M.R.; Bazrafshan, M.; Izadpanah, P.; Dehghani, F.; Askarinejad, A.; Salimi, M.; Bazrafshan Drissi, H. The potential of HEART score to detect the severity of coronary artery disease according to SYNTAX score. Sci. Rep. 2023, 13, 7228. [Google Scholar] [CrossRef]
- Altun, B.; Turkon, H.; Tasolar, H.; Beggı, H.; Altun, M.; Temız, A.; Gazı, E.; Barutcu, A.; Bekler, A.; Colkesen, Y. The relationship between high-sensitive troponin T, neutrophil lymphocyte ratio and SYNTAX Score. Scand. J. Clin. Lab. Invest. 2014, 74, 108–115. [Google Scholar] [CrossRef]
- Herder, C.; Roden, M. A novel diabetes typology: Towards precision diabetology from pathogenesis to treatment. Diabetologia 2022, 65, 1770–1781. [Google Scholar] [CrossRef]
- Li, X.; Chen, H. Characteristics of glucolipid metabolism and complications in novel cluster-based diabetes subgroups: A retrospective study. Lipids Health Dis. 2023, 22, 200. [Google Scholar] [CrossRef]
- Preechasuk, L.; Khaedon, N.; Lapinee, V.; Tangjittipokin, W.; Srivanichakorn, W.; Sriwijitkamol, A.; Plengvidhya, N.; Likitmaskul, S.; Thongtang, N. Cluster analysis of Thai patients with newly diagnosed type 2 diabetes mellitus to predict disease progression and treatment outcomes: A prospective cohort study. BMJ Open Diabetes Res. Care 2022, 10, e003145. [Google Scholar] [CrossRef]
- Humbert, O.; Noirot, E.; Leclerc, T.; Mouhat, B.; Pommier, T.; Cochet, A.; Cottin, Y. Étude et comparaison de la valeur pronostique de différents scores clinique, coronarographique et scintigraphique chez le patient coronarien stable après un syndrome coronarien aigu. Ann. Cardiol. Angiol. 2020, 69, 12–23. [Google Scholar] [CrossRef]
- Lee, C.D.; Folsom, A.R.; Pankow, J.S.; Brancati, F.L.; Atherosclerosis Risk in Communities (ARIC) Study Investigators. Cardiovascular events in diabetic and nondiabetic adults with or without history of myocardial infarction. Circulation 2004, 109, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Gyldenkerne, C.; Olesen, K.K.; Thrane, P.G.; Madsen, M.; Thim, T.; Würtz, M.; O Jensen, L.; Raungaard, B.; Poulsen, P.L.; E Bøtker, H.; et al. Diabetes is not a risk factor for myocardial infarction in patients without coronary artery disease: A study from the Western Denmark Heart Registry. Diab Vasc. Dis. Res. 2020, 17, 1479164120941809. [Google Scholar] [CrossRef] [PubMed]
- Esper, R.B.; Farkouh, M.E.; Ribeiro, E.E.; Hueb, W.; Domanski, M.; Hamza, T.H.; Siami, F.S.; Godoy, L.C.; Mathew, V.; French, J.; et al. SYNTAX Score in Patients With Diabetes Undergoing Coronary Revascularization in the FREEDOM Trial. J. Am. Coll. Cardiol. 2018, 72 Pt A, 2826–2837. [Google Scholar] [CrossRef]
- Tanabe, H.; Masuzaki, H.; Shimabukuro, M. Novel strategies for glycaemic control and preventing diabetic complications applying the clustering-based classification of adult-onset diabetes mellitus: A perspective. Diabetes Res. Clin. Pract. 2021, 180, 109067. [Google Scholar] [CrossRef] [PubMed]
- Action to Control Cardiovascular Risk in Diabetes Study Group; Gerstein, H.C.; Miller, M.E.; Byington, R.P.; Goff, D.C.; Bigger, J.T.; Buse, J.B.; Cushman, W.C.; Genuth, S.; Ismail-Beigi, F.; et al. Effects of intensive glucose lowering in type 2 diabetes. N. Engl. J. Med. 2008, 358, 2545–2559. [Google Scholar]
- Park, S.; Kim, D.-W.; Lee, K.; Park, M.-W.; Chang, K.; Jeong, M.H.; Ahn, Y.K.; Chae, S.C.; Ahn, T.H.; Rha, S.W.; et al. Association between body mass index and three-year outcome of acute myocardial infarction. Sci. Rep. 2024, 14, 365. [Google Scholar] [CrossRef]
- Biswas, S.; Mukherjee, A.; Chakraborty, S.; Chaturvedi, A.; Samanta, B.; Khanra, D.; Ray, S.; Sharma, R.K.; Бисвас, С.; Мукерджи, А.; et al. Impact of plasma glucose and duration of type 2 diabetes mellitus on SYNTAX Score II in patients suffering from non ST-elevation myocardial infarction. Kardiologiia 2022, 62, 40–48. [Google Scholar] [CrossRef]
- Nichols, G.A.; Joshua-Gotlib, S.; Parasuraman, S. Independent Contribution of A1C, Systolic Blood Pressure, and LDL Cholesterol Control to Risk of Cardiovascular Disease Hospitalizations in Type 2 Diabetes: An Observational Cohort Study. J. Gen. Intern. Med. 2013, 28, 691–697. [Google Scholar] [CrossRef]
- Wan, E.Y.F.; Fung, C.S.C.; Yu, E.Y.T.; Chin, W.Y.; Fong, D.Y.T.; Chan, A.K.C.; Lam, C.L.K. Effect of Multifactorial Treatment Targets and Relative Importance of Hemoglobin A1c, Blood Pressure, and Low-Density Lipoprotein-Cholesterol on Cardiovascular Diseases in Chinese Primary Care Patients With Type 2 Diabetes Mellitus: A Population-Based Retrospective Cohort Study. J. Am. Heart Assoc. 2017, 6, e006400. [Google Scholar]
- Kahkoska, A.R.; Geybels, M.S.; Klein, K.R.; Kreiner, F.F.; Marx, N.; Nauck, M.A.; Pratley, R.E.; Wolthers, B.O.; Buse, J.B. Validation of distinct type 2 diabetes clusters and their association with diabetes complications in the DEVOTE, LEADER and SUSTAIN-6 cardiovascular outcomes trials. Diabetes Obes. Metab. 2020, 22, 1537–1547. [Google Scholar] [CrossRef]
- Weight, N.; Moledina, S.; Ullah, M.; Wijeysundera, H.C.; Davies, S.; Chew, N.W.S.; Lawson, C.; Khan, S.U.; Gale, C.P.; Rashid, M.; et al. Impact of Chronic Kidney Disease on the Processes of Care and Long-Term Mortality of Non-ST-Segment-Elevation Myocardial Infarction: A Nationwide Cohort Study and Long-Term Follow-Up. J. Am. Heart Assoc. 2024, 13, e032671. [Google Scholar] [CrossRef]
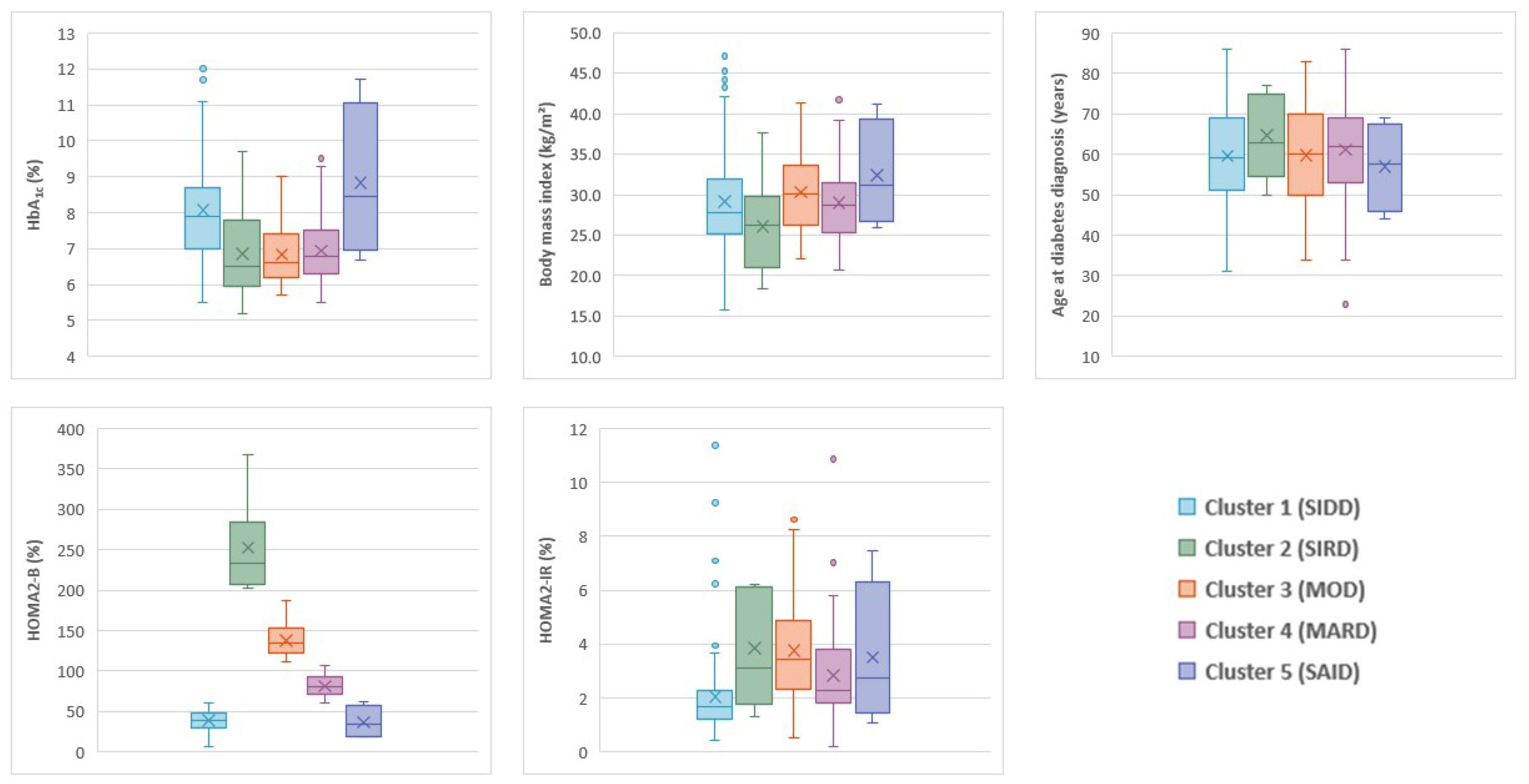
| Cluster | Cluster 1 (SIDD) | Cluster 2 (SIRD) | Cluster 3 (MOD) | Cluster 4 (MARD) | Total | p |
|---|---|---|---|---|---|---|
| n (%) | 115 (47) | 9 (3) | 39 (16) | 83 (34) | 246 (100) | |
| Baseline characteristics | ||||||
| Age (years), median (range) | 71 (45–94) | 79 (72–90) | 70 (48–92) | 71 (41–92) | 71 (41–94) | 0.03 |
| Men, n (%) | 77 (67) | 3 (33) | 32 (82) | 65 (78) | 177 (72) | 0.01 |
| Newly diagnosed diabetes, n (%) | 8 (7) | 0 (0) | 2 (5) | 10 (12.0) | 20 (8) | 0.52 |
| High blood pressure, n (%) | 92 (80) | 8 (89) | 32 (82) | 67 (81) | 199 (81) | 1.00 |
| Smokers, n (%) | 34 (30) | 0 (0) | 18 (46) | 38 (46) | 90 (37) | <10−3 |
| Established atherosclerotic cardiovascular disease, n (%) | 50 (43) | 8 (89) | 21 (54) | 40 (48) | 119 (48) | 0.05 |
| Prior myocardial infarction, n (%) | 20 (17) | 2 (22) | 11 (28) | 18 (22) | 51 (21) | 0.50 |
| Prior stroke, n (%) | 15 (13) | 1 (11) | 1 (3) | 8 (10) | 25 (10) | 0.25 |
| Glomerular function rate (CKD-EPI; mL/min/1.73 m2), median (range) | 84 (6–134) | 25 (4–58) | 54 (6–128) | 74 (13–124) | 73 (4–134) | <10−3 |
| Urinary albumin/creatinine ratio, median (range) | 55 (3–3496) | 219 (4–5520) | 31 (0–2499) | 19 (2–6120) | 38 (0–6120) | 0.04 |
| Heart failure, n (%) | 30 (26) | 2 (22) | 13 (33) | 20 (24) | 65 (26) | 0.73 |
| Left ventricular ejection fraction (%), median (range) | 53 (20–70) | 57 (39–70) | 54 (23–65) | 55 (20–66) | 53 (20–70) | 0.54 |
| Dyslipidemia, n (%) | 85 (74) | 6 (67) | 32 (82) | 57 (69) | 180 (73) | 0.32 |
| Atrial flutter, n (%) | 13 (11) | 2 (22) | 8 (21) | 14 (17) | 37 (15) | 0.33 |
| Low-density lipoprotein cholesterol (g/L), median (range) | 1.1 (0.4–3.6) | 0.9 (0.5–1.6) | 1.0 (0.3–1.9) | 1.0 (0.4–2.0) | 1.0 (0.3–3.6) | 0.51 |
| High-density lipoprotein cholesterol (g/L), median (range) | 0.4 (0.2–0.8) | 0.4 (0.3–0.4) | 0.3 (0.2–0.6) | 0.4 (0.2–0.7) | 0.4 (0.2–0.8) | 0.19 |
| Triglycerides (g/L), median (range) | 1.5 (0.5–9.5) | 2.7 (1.1–3.9) | 1.7 (0.6–5.9) | 1.4 (0.5–16.1) | 1.6 (0.5–16.1) | 0.22 |
| Patients with insulin, n (%) | 37 (32) | 3 (33) | 7 (18) | 15 (18) | 62 (25) | 0.07 |
| Myocardial infarction characteristics | ||||||
| ST-segment elevation myocardial infarction, n (%) | 50 (44) | 0 (0) | 12 (31) | 33 (40) | 95 (39) | 0.04 |
| SYNTAX score, median (range) Mild: SYNTAX ≤ 22, n (%) Medium: 22 < SYNTAX ≤ 32, n (%) Severe: SYNTAX > 32, n (%) | 13 (0–48) 91 (79) 15 (13) 5 (4) | 10 (0–23) 5 (56) 1 (11) 0 (0) | 12 (0–61) 31 (79) 1 (3) 3 (8) | 14 (0–50) 61 (73) 14 (17) 5 (6) | 13 (0–61) 188 (75) 31 (12) 13 (5) | 0.75 |
| Lesions, median (range) | 2 (0–3) | 3 (0–3) | 2 (0–3) | 2 (0–3) | 2 (0–3) | 0.63 |
| Lesions, location | 0.51 | |||||
| Monotruncular, n (%) Bitruncular, n (%) Tritruncular, n (%) | 25 (22) 36 (31) 44 (38) | 1 (11) 1 (11) 4 (44) | 10 (26) 9 (23) 16 (41) | 27 (33) 27 (33) 26 (31) | 63 (26) 73 (30) 90 (37) | |
| Cluster | Cluster 1 (SIDD) | Cluster 2 (SIRD) | Cluster 3 (MOD) | Cluster 4 (MARD) | Total |
|---|---|---|---|---|---|
| n (%) | 115 (47) | 9 (3) | 39 (16) | 83 (34) | 246 (100) |
| All CV events, n (%) | 21 (18) | 2 (22) | 8 (21) | 15 (18) | 46 (19) |
| MACE, n (%) | 14 (12) | 1 (11) | 6 (15) | 7 (8) | 28 (11) |
| Cardiovascular death, n (%) | 9 (8) | 0 (0) | 4 (10) | 5 (6) | 18 (7) |
| Myocardial infarction, n (%) | 2 (2) | 0 (0) | 1 (3) | 2 (2) | 5 (2) |
| Ischemic stroke, n (%) | 3 (3) | 1 (11) | 0 (0) | 0 (0) | 4 (2) |
| Revascularization, n (%) | 0 (0) | 0 (0) | 1 (3) | 0 (0) | 1 (0) |
| Other CV events, n (%) | 7 (6) | 1 (11) | 2 (5) | 8 (10) | 18 (7) |
| Unstable angina, n (%) | 0 (0) | 0 (0) | 0 (0) | 1 (1) | 1 (0) |
| Acute heart failure, n (%) | 2 (2) | 0 (0) | 0 (0) | 3 (4) | 5 (2) |
| Acute pulmonary edema, n (%) | 2 (2) | 0 (0) | 0 (0) | 2 (2) | 4 (2) |
| Cardiogenic shock, n (%) | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 1 (0) |
| Severe hypotension, n (%) | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 1 (0) |
| Pacemaker implantation, n (%) | 1 (1) | 0 (0) | 1 (3) | 0 (0) | 2 (1) |
| Chest pain, n (%) | 0 (0) | 1 (1) | 1 (3) | 0 (0) | 2 (1) |
| Pericarditis, n (%) | 0 (0) | 0 (0) | 0 (0) | 2 (2) | 2 (1) |
| All Cardiovascular Events | Major Adverse Cardiovascular Events | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||
| Variable | HR [CI 95%] | p | HR [CI 95%] | p | HR [CI 95%] | p | HR [CI 95%] | p |
| Cluster | ||||||||
| SIRD vs. SIDD | 1.39 [0.34;5.71] | 0.65 | 0.43 [0.08;2.27] | 0.32 | 1.05 [0.13;8.16] | 0.96 | 0.27 [0.03;2.79] | 0.27 |
| MOD vs. SIDD | 1.04 [0.46;2.36] | 0.92 | 0.62 [0.23;1.66] | 0.34 | 1.17 [0.45;3.06] | 0.75 | 0.66 [0.19;2.24] | 0.50 |
| MARD vs. SIDD | 0.93 [0.48;1.79] | 0.83 | 0.75 [0.37;1.53] | 0.43 | 0.65 [0.27;1.59] | 0.35 | 0.53 [0.21;1.35] | 0.18 |
| Established ASCVD, Yes vs. No | 1.60 [0.89;2.88] | 0.12 | 1.24 [0.62;2.49] | 0.54 | 1.74 [0.81;3.71] | 0.15 | 1.41 [0.65;3.02] | 0.38 |
| Glomerular function rate (CKD-EPI; mL/min/1.73 m2) | 0.99 [0.98;1.00] | 0.003 | 0.98 [0.97;1.00] | 0.01 | 0.98 [0.97;1.00] | 0.01 | 0.98 [0.96;1.00] | 0.03 |
| Low-density lipoprotein cholesterol (g/L) | 0.57 [0.29;1.11] | 0.10 | 0.74 [0.33;1.66] | 0.47 | 0.68 [0.29;1.60] | 0.38 | - | - |
| NSTEMI vs. STEMI | 1.70 [0.90;3.22] | 0.10 | 1.51 [0.76;2.97] | 0.24 | 2.00 [0.85;4.70] | 0.11 | 1.83 [0.76;4.40] | 0.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lacqua, C.; Barbou, A.; Zeller, M.; Aho Glele, L.S.; Adam, H.; Bichat, F.; Petit, J.-M.; Cottin, Y.; Boulin, M. Diabetes Phenotypes in Patients Presenting a Myocardial Infarction: Progress Towards Precision Medicine? J. Pers. Med. 2025, 15, 444. https://doi.org/10.3390/jpm15090444
Lacqua C, Barbou A, Zeller M, Aho Glele LS, Adam H, Bichat F, Petit J-M, Cottin Y, Boulin M. Diabetes Phenotypes in Patients Presenting a Myocardial Infarction: Progress Towards Precision Medicine? Journal of Personalized Medicine. 2025; 15(9):444. https://doi.org/10.3390/jpm15090444
Chicago/Turabian StyleLacqua, Christelle, Arnaud Barbou, Marianne Zeller, Ludwig Serge Aho Glele, Héloïse Adam, Florence Bichat, Jean-Michel Petit, Yves Cottin, and Mathieu Boulin. 2025. "Diabetes Phenotypes in Patients Presenting a Myocardial Infarction: Progress Towards Precision Medicine?" Journal of Personalized Medicine 15, no. 9: 444. https://doi.org/10.3390/jpm15090444
APA StyleLacqua, C., Barbou, A., Zeller, M., Aho Glele, L. S., Adam, H., Bichat, F., Petit, J.-M., Cottin, Y., & Boulin, M. (2025). Diabetes Phenotypes in Patients Presenting a Myocardial Infarction: Progress Towards Precision Medicine? Journal of Personalized Medicine, 15(9), 444. https://doi.org/10.3390/jpm15090444







