Treatment Strategies for Patients with Mitral Regurgitation: A Meta-Analysis of Randomized Controlled Trials
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Eligible and Included Studies
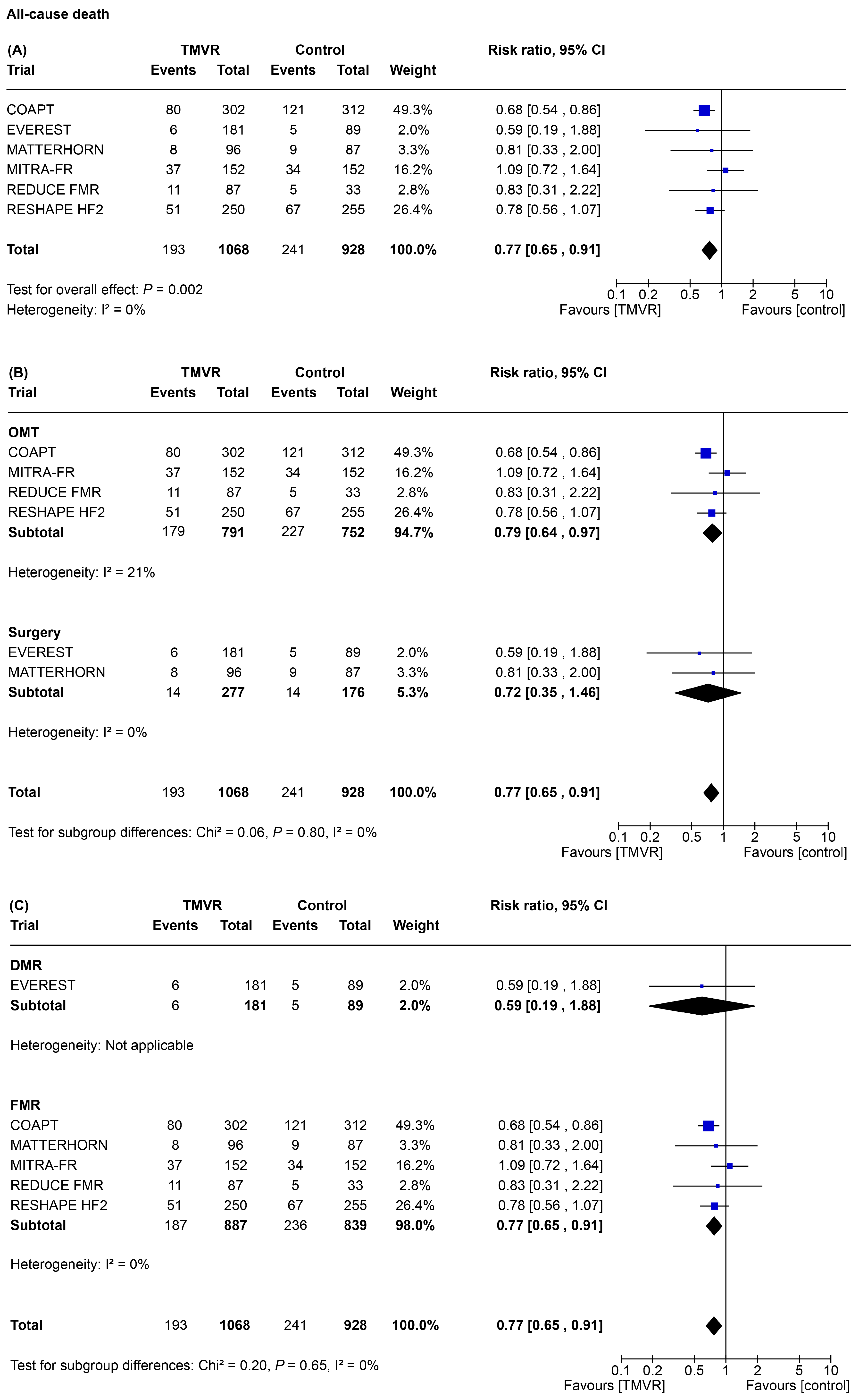
3.2. Primary Outcome
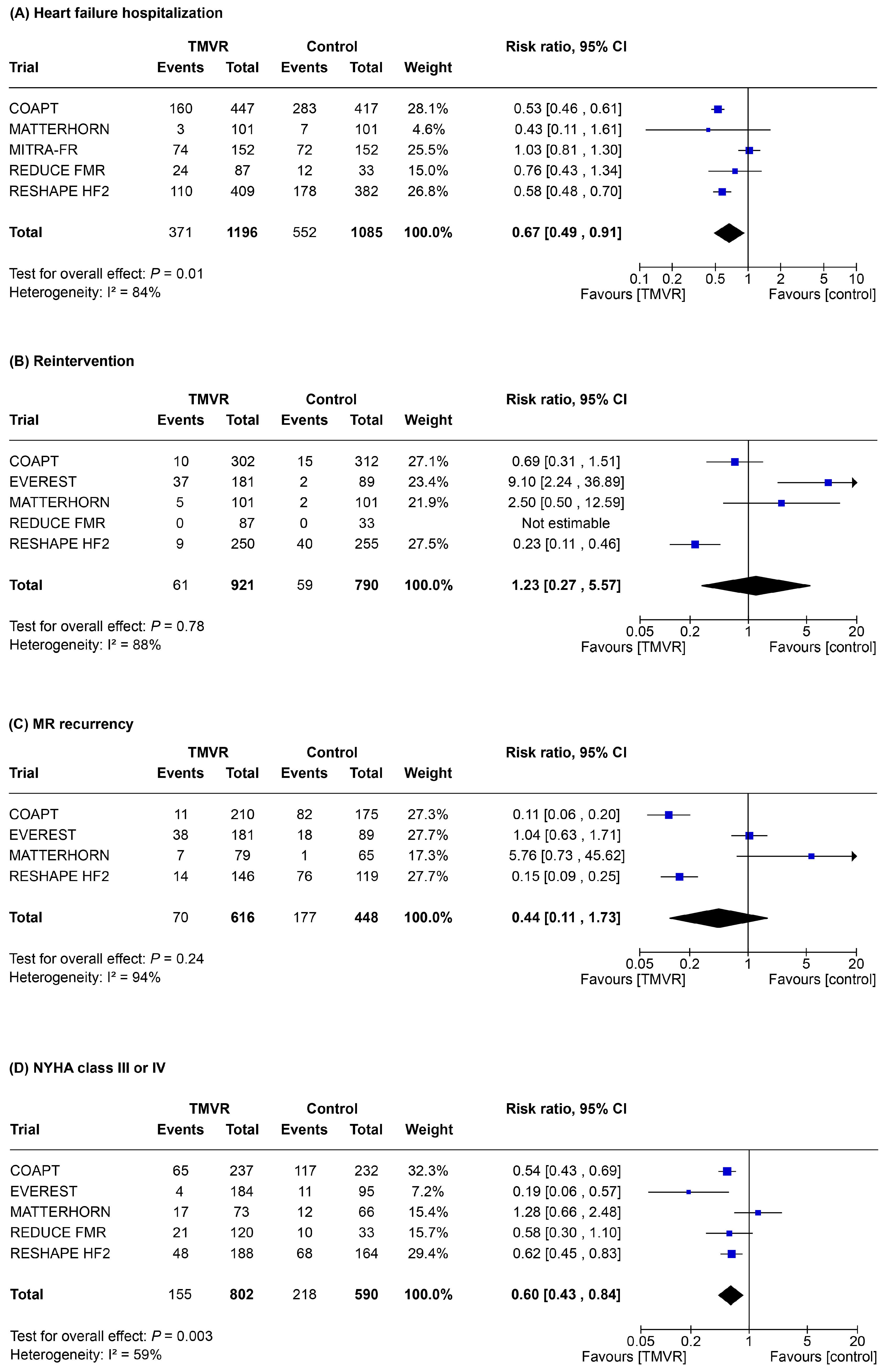
3.3. Secondary Outcomes
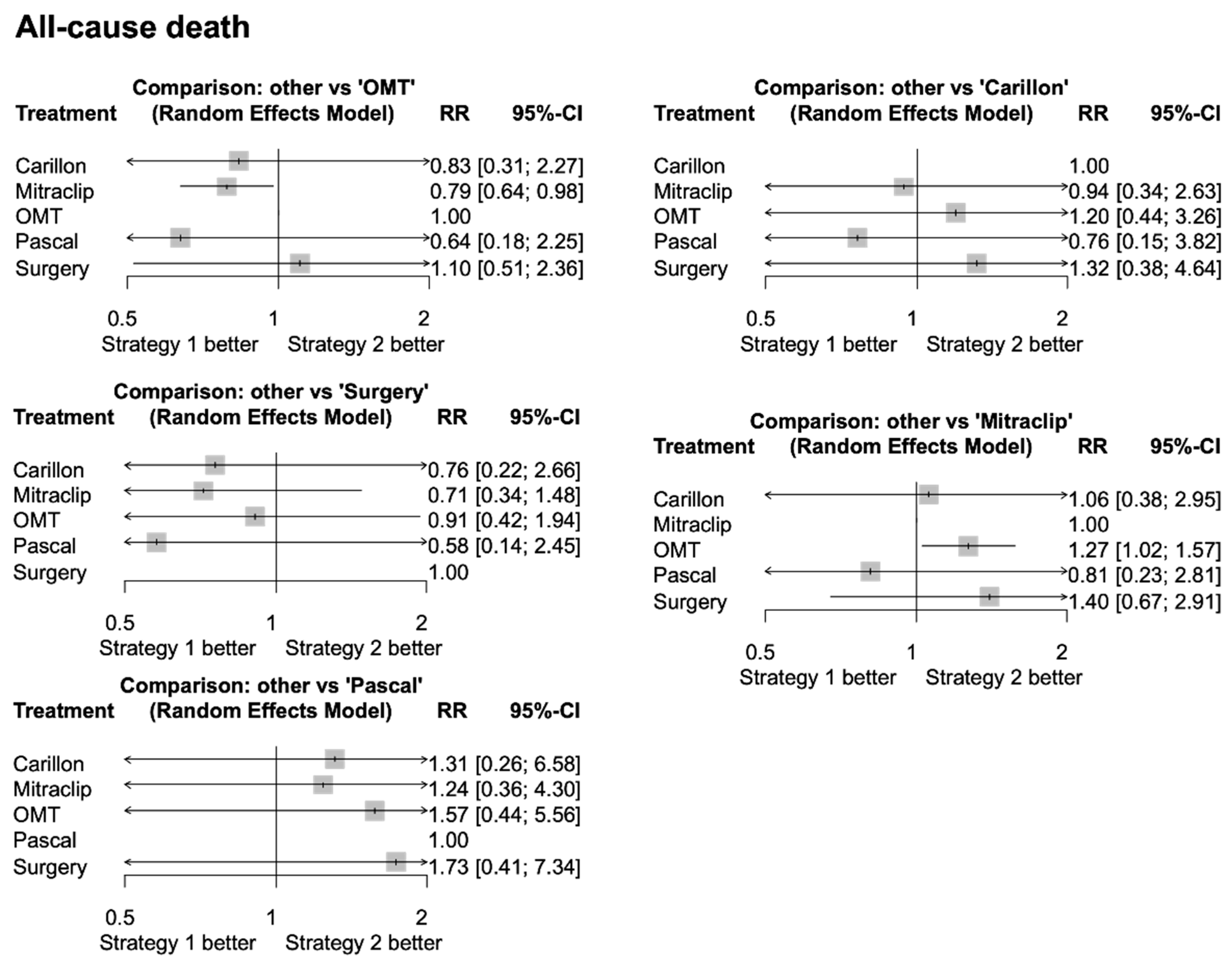
3.4. Network Meta-Analysis
4. Discussion
- (i).
- In symptomatic patients with significant MR, TMVR was associated with a significant reduction of all-cause death, re-hospitalization for heart failure, MR, and improved patient functional class as compared to control therapy;
- (ii).
- These results are mainly driven by trials enrolling patients with FMR. Due to the limited data available, however, they cannot be extended to patients presenting with DMR. For this subgroup of patients, the results of the present meta-analysis should therefore be considered exploratory in nature;
- (iii).
- TEER appears to be the best treatment for mortality reduction, due to the extent of evidence regarding the MitraClip device;
- (iv).
- Further trials are needed to obtain direct stronger evidence in the context of DMR, and to provide direct comparison between different strategies in order to improve the personalized treatment of patients with MR.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DMR | Degenerative mitral regurgitation |
| FMR | Functional mitral regurgitation |
| HF | Heart failure |
| MR | Mitral regurgitation |
| OMT | Optimal medical therapy |
| TMVR | Transcatheter mitral valve repair |
References
- Nkomo, V.T.; Gardin, J.M.; Skelton, T.N.; Gottdiener, J.S.; Scott, C.G.; Enriquez-Sarano, M. Burden of valvular heart diseases: A population-based study. Lancet 2006, 368, 1005–1011. [Google Scholar] [CrossRef]
- Goel, S.S.; Bajaj, N.; Aggarwal, B.; Gupta, S.; Poddar, K.L.; Ige, M.; Bdair, H.; Anabtawi, A.; Rahim, S.; Whitlow, P.L.; et al. Prevalence and outcomes of unoperated patients with severe symptomatic mitral regurgitation and heart failure. J. Am. Coll. Cardiol. 2014, 63, 185–186. [Google Scholar] [CrossRef]
- Dziadzko, V.; Clavel, M.-A.; Dziadzko, M.; Medina-Inojosa, J.R.; Michelena, H.; Maalouf, J.; Nkomo, V.; Thapa, P.; Enriquez-Sarano, M. Outcome and undertreatment of mitral regurgitation: A community cohort study. Lancet 2018, 391, 960–969. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, D.; Moskowitz, A.J.; Gelijns, A.C.; Ailawadi, G.; Parides, M.K.; Perrault, L.P.; Hung, J.W.; Voisine, P.; Dagenais, F.; Gillinov, A.M.; et al. Two-Year Outcomes of Surgical Treatment of Severe Ischemic Mitral Regurgitation. N. Engl. J. Med. 2016, 374, 344–353. [Google Scholar] [CrossRef]
- Michler, R.E.; Smith, P.K.; Parides, M.K.; Ailawadi, G.; Thourani, V.; Moskowitz, A.J.; Acker, M.A.; Hung, J.W.; Chang, H.L.; Perrault, L.P.; et al. Two-Year Outcomes of Surgical Treatment of Moderate Ischemic Mitral Regurgitation. N. Engl. J. Med. 2016, 374, 1932–1941. [Google Scholar] [CrossRef]
- Goliasch, G.; Bartko, P.E.; Pavo, N.; Neuhold, S.; Wurm, R.; Mascherbauer, J.; Lang, I.M.; Strunk, G.; Hülsmann, M. Refining the prognostic impact of functional mitral regurgitation in chronic heart failure. Eur. Heart J. 2018, 39, 39–46. [Google Scholar] [CrossRef]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients with Valvular Heart Disease. Circulation 2021, 143, e72–e227. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef] [PubMed]
- Stone, G.W.; Lindenfeld, J.; Abraham, W.T.; Kar, S.; Lim, D.S.; Mishell, J.M.; Whisenant, B.; Grayburn, P.A.; Rinaldi, M.; Kapadia, S.R.; et al. Transcatheter Mitral-Valve Repair in Patients with Heart Failure. N. Engl. J. Med. 2018, 379, 2307–2318. [Google Scholar] [CrossRef]
- Obadia, J.-F.; Messika-Zeitoun, D.; Leurent, G.; Iung, B.; Bonnet, G.; Piriou, N.; Lefèvre, T.; Piot, C.; Rouleau, F.; Carrié, D.; et al. Percutaneous Repair or Medical Treatment for Secondary Mitral Regurgitation. N. Engl. J. Med. 2018, 379, 2297–2306. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Friede, T.; von Bardeleben, R.-S.; Butler, J.; Khan, M.-S.; Diek, M.; Heinrich, J.; Geyer, M.; Placzek, M.; Ferrari, R.; et al. Transcatheter Valve Repair in Heart Failure with Moderate to Severe Mitral Regurgitation. N. Engl. J. Med. 2024, 391, 1799–1809. [Google Scholar] [CrossRef]
- Feldman, T.; Foster, E.; Glower, D.D.; Kar, S.; Rinaldi, M.J.; Fail, P.S.; Smalling, R.W.; Siegel, R.; Rose, G.A.; Engeron, E.; et al. Percutaneous repair or surgery for mitral regurgitation. N. Engl. J. Med. 2011, 364, 1395–1406. [Google Scholar] [CrossRef]
- Zahr, F.; Smith, R.L.; Gillam, L.D.; Chadderdon, S.; Makkar, R.; von Bardeleben, R.S.; Ruf, T.F.; Kipperman, R.M.; Rassi, A.N.; Szerlip, M.; et al. One-Year Outcomes from the CLASP IID Randomized Trial for Degenerative Mitral Regurgitation. JACC Cardiovasc. Interv. 2023, 16, 2803–2816. [Google Scholar] [CrossRef] [PubMed]
- Makkar, R.R.; Chikwe, J.; Chakravarty, T.; Chen, Q.; O’gAra, P.T.; Gillinov, M.; Mack, M.J.; Vekstein, A.; Patel, D.; Stebbins, A.L.; et al. Transcatheter Mitral Valve Repair for Degenerative Mitral Regurgitation. JAMA 2023, 329, 1778–1788. [Google Scholar] [CrossRef] [PubMed]
- Anker, M.S.; Porthun, J.; Schulze, P.C.; Rassaf, T.; Landmesser, U. Percutaneous Transcatheter Edge-To-Edge Repair for Functional Mitral Regurgitation in Heart Failure. J. Am. Coll. Cardiol. 2024, 84, 2364–2368. [Google Scholar] [CrossRef]
- D’Amario, D.; Laborante, R.; Mennuni, M.; Adamo, M.; Metra, M.; Patti, G. Efficacy and safety of trans-catheter repair devices for mitral regurgitation. Int. J. Cardiol. 2024, 411, 132245. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- McCarthy, P.M.; Whisenant, B.; Asgar, A.W.; Ailawadi, G.; Hermiller, J.; Williams, M.; Morse, A.; Rinaldi, M.; Grayburn, P.; Thomas, J.D.; et al. Percutaneous MitraClip Device or Surgical Mitral Valve Repair in Patients with Primary Mitral Regurgitation Who Are Candidates for Surgery: Design and Rationale of the REPAIR MR Trial. J. Am. Heart Assoc. 2023, 12, e027504. [Google Scholar] [CrossRef]
- Piriou, N.; Al Habash, O.; Donal, E.; Senage, T.; Le Tourneau, T.; Pattier, S.; Guyomarch, B.; Roussel, J.-C.; Trochu, J.N.; Vahanian, A.; et al. The MITRA-HR study: Design and rationale of a randomised study of MitraClip transcatheter mitral valve repair in patients with severe primary mitral regurgitation eligible for high-risk surgery. EuroIntervention 2019, 15, e329–e335. [Google Scholar] [CrossRef]
- Song, C.; Madhavan, M.V.; Lindenfeld, J.; Abraham, W.T.; Kar, S.; Lim, D.S.; Grayburn, P.A.; Kapadia, S.R.; Kotinkaduwa, L.N.; Mack, M.J.; et al. Age-Related Outcomes After Transcatheter Mitral Valve Repair in Patients with Heart Failure: Analysis from COAPT. JACC Cardiovasc. Interv. 2022, 15, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Lindenfeld, J.; Abraham, W.T.; Grayburn, P.A.; Kar, S.; Asch, F.M.; Lim, D.S.; Nie, H.; Singhal, P.; Sundareswaran, K.S.; Weissman, N.J.; et al. Association of Effective Regurgitation Orifice Area to Left Ventricular End-Diastolic Volume Ratio with Transcatheter Mitral Valve Repair Outcomes: A Secondary Analysis of the COAPT Trial. JAMA Cardiol. 2021, 6, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Shahim, B.; Cohen, D.J.; Asch, F.M.; Bax, J.; George, I.; Rück, A.; Ben-Yehuda, O.; Kar, S.; Lim, D.S.; Saxon, J.T.; et al. Repeat Mitral Valve Interventions After Transcatheter Edge-to-Edge Repair: The COAPT Trial. Am. J. Cardiol. 2024, 223, 7–14. [Google Scholar] [CrossRef]
- Witte, K.K.; Lipiecki, J.; Siminiak, T.; Meredith, I.T.; Malkin, C.J.; Goldberg, S.L.; Stark, M.A.; von Bardeleben, R.S.; Cremer, P.C.; Jaber, W.A.; et al. The REDUCE FMR Trial: A Randomized Sham-Controlled Study of Percutaneous Mitral Annuloplasty in Functional Mitral Regurgitation. JACC Heart Fail. 2019, 7, 945–955. [Google Scholar] [CrossRef]
- Baldus, S.; Doenst, T.; Pfister, R.; Gummert, J.; Kessler, M.; Boekstegers, P.; Lubos, E.; Schröder, J.; Thiele, H.; Walther, T.; et al. Transcatheter Repair versus Mitral-Valve Surgery for Secondary Mitral Regurgitation. N. Engl. J. Med. 2024, 391, 1787–1798. [Google Scholar] [CrossRef]
- Grayburn, P.A.; Sannino, A.; Packer, M. Proportionate and Disproportionate Functional Mitral Regurgitation. JACC Cardiovasc. Imaging 2019, 12, 353–362. [Google Scholar] [CrossRef]
- von Bardeleben, R.S.; Mahoney, P.; Morse, M.A.; Price, M.J.; Denti, P.; Maisano, F.; Rogers, J.H.; Rinaldi, M.; De Marco, F.; Rollefson, W.; et al. 1-Year Outcomes with Fourth-Generation Mitral Valve Transcatheter Edge-to-Edge Repair from the EXPAND G4 Study. JACC Cardiovasc. Interv. 2023, 16, 2600–2610. [Google Scholar] [CrossRef]
- Kar, S.; von Bardeleben, R.S.; Rottbauer, W.; Mahoney, P.; Price, M.J.; Grasso, C.; Williams, M.; Lurz, P.; Ahmed, M.; Hausleiter, J.; et al. Contemporary Outcomes Following Transcatheter Edge-to-Edge Repair: 1-Year Results from the EXPAND Study. JACC Cardiovasc. Interv. 2023, 16, 589–602. [Google Scholar] [CrossRef]
- Okuno, T.; Izumo, M.; Shiokawa, N.; Kuwata, S.; Ishibashi, Y.; Sato, Y.; Koga, M.; Okuyama, K.; Suzuki, N.; Kida, K.; et al. Newer versus Early Generation of the MitraClip for Primary Mitral Regurgitation: A Japanese Single-Center Experience. Rev. Cardiovasc. Med. 2023, 24, 138. [Google Scholar] [CrossRef]
- Chakravarty, T.; Makar, M.; Patel, D.; Oakley, L.; Yoon, S.H.; Stegic, J.; Singh, S.; Skaf, S.; Nakamura, M.; Makkar, R.R. Transcatheter Edge-to-Edge Mitral Valve Repair with the MitraClip G4 System. JACC Cardiovasc. Interv. 2020, 13, 2402–2414. [Google Scholar] [CrossRef] [PubMed]
- Saji, M.; Yamamoto, M.; Kubo, S.; Asami, M.; Enta, Y.; Shirai, S.; Izumo, M.; Mizuno, S.; Watanabe, Y.; Amaki, M.; et al. Short-Term Outcomes Following Transcatheter Edge-to-Edge Repair: Insights from the OCEAN-Mitral Registry. JACC Asia 2023, 3, 766–773. [Google Scholar] [CrossRef]
- Geis, N.A.; Schlegel, P.; Heckmann, M.B.; Katus, H.A.; Frey, N.; López, P.C.; Raake, P.W. One-Year Results Following PASCAL-Based or MitraClip-Based Mitral Valve Transcatheter Edge-to-Edge Repair. ESC Heart Fail. 2022, 9, 853–865. [Google Scholar] [CrossRef]
- Haschemi, J.; Haurand, J.M.; Bönner, F.; Kelm, M.; Westenfeld, R.; Horn, P. PASCAL vs MitraClip for Mitral Valve Transcatheter Edge-to-Edge Repair: A Single-Center Real-World Experience. JACC Cardiovasc. Interv. 2022, 15, 1002–1004. [Google Scholar] [CrossRef] [PubMed]
- Mauri, V.; Sugiura, A.; Spieker, M.; Iliadis, C.; Horn, P.; Öztürk, C.; Besler, C.; Riebisch, M.; Al-Hammadi, O.; Ruf, T.; et al. Early Outcomes of 2 Mitral Valve Transcatheter Leaflet Approximation Devices: A Propensity Score-Matched Multicenter Comparison. JACC Cardiovasc. Interv. 2022, 15, 2541–2551. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, M.; Haude, M.; Sievert, H.; Fichtlscherer, S.; Lehmann, R.; Klein, N.; Witte, K.; Degen, H.; Pfeiffer, D.; Goldberg, S.L.; et al. The CINCH-FMR postmarket registry: Real-world long-term outcomes with percutaneous mitral valve repair. Cardiovasc. Revasc. Med. 2024, 60, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Webb, J.G.; Murdoch, D.J.; Boone, R.H.; Moss, R.; Attinger-Toller, A.; Blanke, P.; Cheung, A.; Hensey, M.; Leipsic, J.; Ong, K.; et al. Percutaneous Transcatheter Mitral Valve Replacement: First-in-Human Experience with a New Transseptal System. J. Am. Coll. Cardiol. 2019, 73, 1239–1246. [Google Scholar] [CrossRef]
- Hell, M.M.; Wild, M.G.; Baldus, S.; Rudolph, T.; Treede, H.; Petronio, A.S.; Modine, T.; Andreas, M.; Coisne, A.; Duncan, A.; et al. Transapical Mitral Valve Replacement: 1-Year Results of the Real-World Tendyne European Experience Registry. JACC Cardiovasc. Interv. 2024, 17, 648–661. [Google Scholar] [CrossRef]
- Turner, R.M.; Bird, S.M.; Higgins, J.P.T. The impact of study size on meta-analyses: Examination of underpowered studies in Cochrane reviews. PLoS ONE 2013, 8, e59202. [Google Scholar] [CrossRef]
- Salanti, G.; Del Giovane, C.; Chaimani, A.; Caldwell, D.M.; Higgins, J.P.T. Evaluating the quality of evidence from a network meta-analysis. PLoS ONE 2014, 9, e99682. [Google Scholar] [CrossRef]
- Dias, S.; Welton, N.J.; Caldwell, D.M.; Ades, A.E. Checking consistency in mixed treatment comparison meta-analysis. Stat. Med. 2010, 29, 932–944. [Google Scholar] [CrossRef]
- Rucker, G. Network meta-analysis, electrical networks and graph theory. Res. Synth. Methods 2012, 3, 312–324. [Google Scholar] [CrossRef] [PubMed]
| Trial | Patients, n | Age, Years | Male, % | Diabetes, % | STS Risk Score, % | COPD, % | DMR, % | FMR, % | LVEF, % | EROA, cm2 | NYHA III–IV, % |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CLASP IID | 294 | 81 | 65 | 19 | 3.9 | 18 | 100 | 0 | 59 | 0.50 | 61 |
| COAPT | 614 | 72 | 64 | 37 | 8.15 | 23 | 0 | 100 | 31 | 0.41 | 60 |
| EVEREST | 279 | 67 | 64 | 9 | N/R | 15 | 73 | 27 | 60 | 0.58 | 68 |
| MATTERHORN | 208 | 71 | 60 | 26 | 2 | 17 | 0 | 100 | 43 | 0.22 | 86 |
| MITRA FR | 304 | 70 | 75 | 29 | N/R | N/R | 0 | 100 | 33 | 0.31 | 68 |
| REDUCE FMR | 120 | 70 | 73 | 30 | N/R | N/R | 0 | 100 | 34 | 54 | |
| RESHAPE HF2 | 505 | 70 | 80 | 35 | 5.3 | 14 | 0 | 100 | 32 | 0.23 | 75 |
| Variable | Subgroup | All-Cause Death | Pint | Re-Hospitalization for HF | Pint | Re-Intervention | Pint | MR 3+/4+ | Pint | NYHA III or IV | Pint |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Trial Size, patients | >300 | 0.80 [0.62–1.02] | 0.87 | 0.60 [0.47–0.97] | 0.92 | 0.39 [0.13; 1.16] | 0.003 | 0.13 [0.09–0.2] | 0.002 | 0.58 [0.48–0.70] | 0.97 |
| ≤300 | 0.75 [0.42–1.34] | 0.69 [0.41–1.17] | 5.07 [1.37; 18.79] | 1.83 [0.36–9.19] | 0.57 [0.22–1.47] | ||||||
| Type of MR | FMR | 0.77 [0.65–0.91] | 0.65 | 0.67 [0.47–0.91] | 0.61 [0.19; 1.97] | 0.004 | 0.27 [0.08–0.86] | 0.04 | 0.64 [0.49–0.85] | 0.04 | |
| DMR | 0.59 [0.19–1.88] | Not estimable | 9.10 [2.24; 36.89] | 1.04 [0.63–1.71] | 0.19 [0.06–0.57] | ||||||
| Comparator | OMT | 0.79 [0.64–0.97] | 0.80 | 0.69 [0.50–0.95] | 0.5 | 0.39 [0.13; 1.16] | 0.003 | 0.13 [0.09–0.20] | 0.002 | 0.58 [0.48–0.69] | 0.91 |
| Surgery | 0.72 [0.35–1.46] | 0.43 [0.11–1.61] | 5.07 [1.37; 18.79] | 1.83 [0.36–9.19] | 0.52 [0.08–3.45] | ||||||
| Baseline % of NYHA III/IV | >65% | 0.86 [0.68–1.09] | 0.18 | 0.72 [0.43–1.21] | 0.44 | 1.64 [0.13–20.71] | 0.66 | 0.77 [0.14–4.30] | 0.04 | 0.60 [0.27–1.31] | 0.96 |
| ≤65% | 0.69 [0.55–0.87] | 0.57 [0.43–0.76] | 0.90 [0.41–1.96] | 0.11 [0.06–0.20] | 0.59 [0.45–0.76] | ||||||
| Risk of Bias | High | 0.71 [0.59–0.86] | 0.08 | 0.54 [0.49–0.61] | 0.002 | 1.02 [0.17; 6.12] | 0.47 | 0.26 [0.06–1.08] | 0.02 | 0.53 [0.39–0.74] | 0.27 |
| Low or intermediate | 1.01 [0.71–1.43] | 0.91 [0.67–1.24] | 2.50 [0.50; 12.59] | 1.44 [0.11–1.73] | 0.60 [0.44–0.84] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carassia, C.; Simonetti, F.; Alvarez Covarrubias, H.A.; Wolf, B.; Pellegrini, C.; Rheude, T.; Fuchs, P.; Roski, F.; Kühlein, M.; Blum, E.; et al. Treatment Strategies for Patients with Mitral Regurgitation: A Meta-Analysis of Randomized Controlled Trials. J. Pers. Med. 2025, 15, 383. https://doi.org/10.3390/jpm15080383
Carassia C, Simonetti F, Alvarez Covarrubias HA, Wolf B, Pellegrini C, Rheude T, Fuchs P, Roski F, Kühlein M, Blum E, et al. Treatment Strategies for Patients with Mitral Regurgitation: A Meta-Analysis of Randomized Controlled Trials. Journal of Personalized Medicine. 2025; 15(8):383. https://doi.org/10.3390/jpm15080383
Chicago/Turabian StyleCarassia, Claudia, Fiorenzo Simonetti, Hector A. Alvarez Covarrubias, Bernhard Wolf, Costanza Pellegrini, Tobias Rheude, Patrick Fuchs, Ferdinand Roski, Moritz Kühlein, Edna Blum, and et al. 2025. "Treatment Strategies for Patients with Mitral Regurgitation: A Meta-Analysis of Randomized Controlled Trials" Journal of Personalized Medicine 15, no. 8: 383. https://doi.org/10.3390/jpm15080383
APA StyleCarassia, C., Simonetti, F., Alvarez Covarrubias, H. A., Wolf, B., Pellegrini, C., Rheude, T., Fuchs, P., Roski, F., Kühlein, M., Blum, E., Ndrepepa, G., Trenkwalder, T., Joner, M., Kastrati, A., Cassese, S., & Xhepa, E. (2025). Treatment Strategies for Patients with Mitral Regurgitation: A Meta-Analysis of Randomized Controlled Trials. Journal of Personalized Medicine, 15(8), 383. https://doi.org/10.3390/jpm15080383








