Personalizing Cochlear Implant Care in Single-Sided Deafness: A Distinct Paradigm from Bilateral Hearing Loss
Abstract
1. Introduction
2. Methods
3. Current Clinical Management of SSD and Opportunities for Personalized Medicine
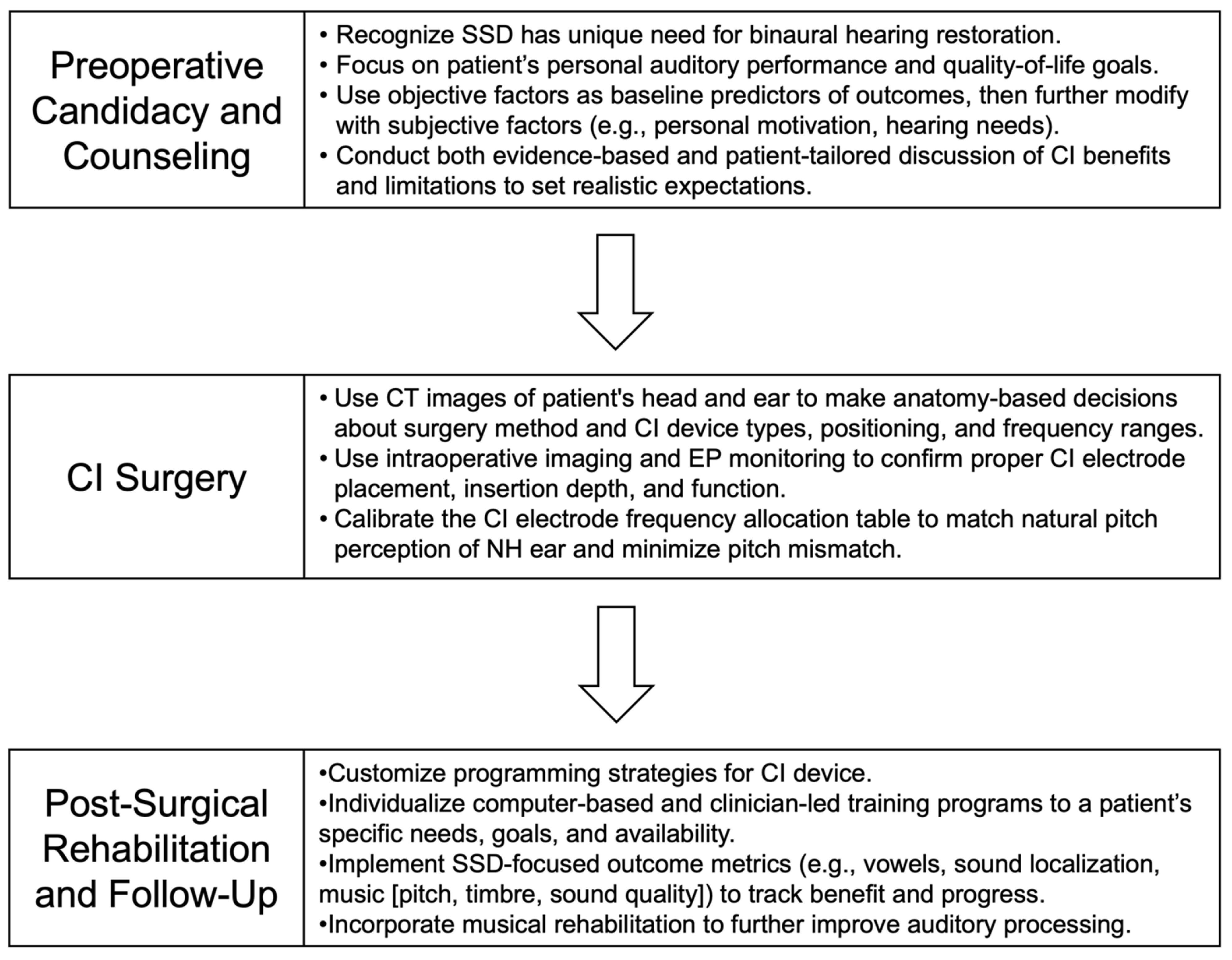
3.1. Treatment Options and Evaluation of CI Candidacy
A Personalized Approach to Determining Candidacy
3.2. Pre-Operative Counseling with Patients
A Personalized Approach to Counseling
3.3. CI Surgery
A Personalized Approach to Surgery
3.4. Post-Surgical Auditory Rehabilitation
A Personalized Approach to Rehabilitation
4. CI Usage Behaviors Among the SSD Population
5. Factors Contributing to Lower CI Usage in SSD
5.1. Reliance on the Contralateral Ear and Ingrained Compensatory Behaviors
5.2. Auditory Processing and Binaural Integration Challenges
5.3. Anatomical, Pathological, and Device-Related Considerations
6. Special Considerations When Treating SSD
6.1. Music Perception as a Tool for SSD
6.1.1. Music as a Uniquely Powerful Auditory Probe
6.1.2. The Aural Mismatch: Deconstructing Music Perception Through a CI
6.1.3. Clinical Applications: A Dual Role in Assessment and Rehabilitation
6.2. The Role of Big Data Within Personalized Medicine for SSD Management
6.2.1. Current Landscape: What Has Been Done
6.2.2. Future Applications and Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CI | cochlear implant. |
| SSD | single-sided deafness. |
| SNHL | sensorineural hearing loss. |
| CPA | cerebellopontine angle. |
| CN | cranial nerve. |
| CROS-HA | contralateral routing of signal hearing aid. |
| BAHA | bone-anchored hearing aid. |
| PTA | pure tone audiometry. |
| MRI | magnetic resonance imaging. |
| CT | computed tomography. |
| ITD | interaural time difference. |
| ILD | interaural level difference. |
| LW | lateral wall. |
| PM | perimodiolar. |
| RWI | round window insertion. |
| DF | default frequency. |
| ABF | anatomy-based fitting. |
| CNC | consonant-nucleus-consonant. |
| CBAT | computer-based auditory training. |
| CIS | continuous interleaved sampling. |
| FSP | fine structure processing. |
| ML | machine learning. |
| AI | artificial intelligence. |
References
- Mudry, A.; Mills, M. The early history of the cochlear implant: A retrospective. JAMA Otolaryngol–Head. Neck Surg. 2013, 139, 446–453. [Google Scholar] [CrossRef]
- Nassiri, A.M.; Sorkin, D.L.; Carlson, M.L. Current estimates of cochlear implant utilization in the United States. Otol. Neurotol. 2022, 43, e558–e562. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.-G. Celebrating the one millionth cochlear implant. JASA Express Lett. 2022, 2, 077201. [Google Scholar] [CrossRef]
- Zeitler, D.M.; Dorman, M.F. Cochlear implantation for single-sided deafness: A new treatment paradigm. J. Neurol. Surg. Part. B Skull Base. 2019, 80, 178–186. [Google Scholar] [CrossRef]
- Kay-Rivest, E.; Irace, A.L.; Golub, J.S.; Svirsky, M.A. Prevalence of single-sided deafness in the United States. Laryngoscope 2022, 132, 1652–1656. [Google Scholar] [CrossRef]
- Dillon, M.T.; Kocharyan, A.; Daher, G.S.; Carlson, M.L.; Shapiro, W.H.; Snapp, H.A.; Firszt, J.B. American Cochlear Implant Alliance Task Force guidelines for clinical assessment and management of adult cochlear implantation for single-sided deafness. Ear Hear. 2022, 43, 1605–1619. [Google Scholar] [CrossRef] [PubMed]
- Purcell, P.L.; Cushing, S.L.; Papsin, B.C.; Gordon, K.A. Unilateral hearing loss and single-sided deafness in children: An update on diagnosis and management. Curr. Otorhinolaryngol. Rep. 2020, 8, 259–266. [Google Scholar] [CrossRef]
- Gordon, K.A.; Cushing, S.L.; Easwar, V.; Polonenko, M.J.; Papsin, B.C. Binaural integration: A challenge to overcome for children with hearing loss. Curr. Opin. Otolaryngol. Head. Neck Surg. 2017, 25, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Friedmann, D.R.; Ahmed, O.H.; McMenomey, S.O.; Shapiro, W.H.; Waltzman, S.B.; Roland, J.T., Jr. Single-sided deafness cochlear implantation: Candidacy, evaluation, and outcomes in children and adults. Otol. Neurotol. 2016, 37, e154–e160. [Google Scholar] [CrossRef]
- Usami, S.-I.; Kitoh, R.; Moteki, H.; Nishio, S.-Y.; Kitano, T.; Kobayashi, M.; Shinagawa, J.; Yokota, Y.; Sugiyama, K.; Watanabe, K. Etiology of single-sided deafness and asymmetrical hearing loss. Acta Oto-Laryngol. 2017, 137, S2–S7. [Google Scholar] [CrossRef]
- Morzaria, S.; Westerberg, B.D.; Kozak, F.K. Systematic review of the etiology of bilateral sensorineural hearing loss in children. Int. J. Pediatr. Otorhinolaryngol. 2004, 68, 1193–1198. [Google Scholar] [CrossRef]
- Cunningham, L.L.; Tucci, D.L. Hearing loss in adults. New Engl. J. Med. 2017, 377, 2465–2473. [Google Scholar] [CrossRef]
- Schilder, A.G.M.; Chonmaitree, T.; Cripps, A.W.; Rosenfeld, R.M.; Casselbrant, M.L.; Haggard, M.P.; Venekamp, R.P. Otitis media. Nat. Rev. Dis. Primers 2016, 2, 16063. [Google Scholar] [CrossRef] [PubMed]
- Carlson, M.L.; Link, M.J. Vestibular schwannomas. New Engl. J. Med. 2021, 384, 1335–1348. [Google Scholar] [CrossRef] [PubMed]
- Sarna, B.; Abouzari, M.; Merna, C.; Jamshidi, S.; Saber, T.; Djalilian, H.R. Perilymphatic fistula: A review of classification, etiology, diagnosis, and treatment. Front. Neurol. 2020, 11, 1046. [Google Scholar] [CrossRef]
- Casselman, J.W.; E Offeciers, F.; Govaerts, P.J.; Kuhweide, R.; Geldof, H.; Somers, T.; D’HOnt, G. Aplasia and hypoplasia of the vestibulocochlear nerve: Diagnosis with MR imaging. Radiology 1997, 202, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Morita, S.; Fujiwara, K.; Fukuda, A.; Fukuda, S.; Nishio, S.-Y.; Kitoh, R.; Hato, N.; Ikezono, T.; Ishikawa, K.; Kaga, K.; et al. The clinical features and prognosis of mumps-associated hearing loss: A retrospective, multi-institutional investigation in Japan. Acta Oto-Laryngol. 2017, 137, S44–S47. [Google Scholar] [CrossRef]
- Xia, W.; Yan, H.; Zhang, Y.; Wang, C.; Gao, W.; Lv, C.; Wang, W.; Liu, Z. Congenital human cytomegalovirus infection inducing sensorineural hearing loss. Front. Microbiol. 2021, 12, 649690. [Google Scholar] [CrossRef]
- De Siati, R.D.; Rosenzweig, F.; Gersdorff, G.; Gregoire, A.; Rombaux, P.; Deggouj, N. Auditory neuropathy spectrum disorders: From diagnosis to treatment: Literature review and case reports. J. Clin. Med. 2020, 9, 1074. [Google Scholar] [CrossRef]
- Shukla, A.; Harper, M.; Pedersen, E.; Goman, A.; Suen, J.J.; Price, C.; Applebaum, J.; Hoyer, M.; Lin, F.R.; Reed, N.S. Hearing loss, loneliness, and social isolation: A systematic review. Otolaryngol–Head. Neck Surg. 2020, 162, 622–633. [Google Scholar] [CrossRef]
- Niazi, Y.; Ejaz, B.; Muazzam, A. Impact of hearing impairment on psychological distress and subjective well-being in older adults. Pak. J. Med. Sci. 2020, 36, 1210. [Google Scholar] [CrossRef]
- Fellinger, J.; Holzinger, D.; Gerich, J.; Goldberg, D. Mental distress and quality of life in the hard of hearing. Acta Psychiatr. Scand. 2007, 115, 243–245. [Google Scholar] [CrossRef] [PubMed]
- Besser, J.; Stropahl, M.; Urry, E.; Launer, S. Comorbidities of hearing loss and the implications of multimorbidity for audiological care. Hear. Res. 2018, 369, 3–14. [Google Scholar] [CrossRef]
- Thomson, R.S.; Auduong, P.; Miller, A.T.; Gurgel, R.K. Hearing loss as a risk factor for dementia: A systematic review. Laryngoscope Investig. Otolaryngol. 2017, 2, 69–79. [Google Scholar] [CrossRef]
- Yeo, B.S.Y.; Song, H.J.J.M.D.; Toh, E.M.S.; Ng, L.S.; Ho, C.S.H.; Ho, R.; Merchant, R.A.; Tan, B.K.J.; Loh, W.S. Association of hearing aids and cochlear implants with cognitive decline and dementia: A systematic review and meta-analysis. JAMA Neurol. 2023, 80, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Dalton, D.S.; Cruickshanks, K.J.; Klein, B.E.K.; Klein, R.; Wiley, T.L.; Nondahl, D.M. The impact of hearing loss on quality of life in older adults. Gerontol. 2003, 43, 661–668. [Google Scholar] [CrossRef]
- Roland, L.; Fischer, C.; Tran, K.; Rachakonda, T.; Kallogjeri, D.; Lieu, J.E.C. Quality of life in children with hearing impairment: Systematic review and meta-analysis. Otolaryngol.–Head. Neck Surg. 2016, 155, 208–219. [Google Scholar] [CrossRef]
- Lieu, J.E. Permanent unilateral hearing loss (UHL) and childhood development. Curr. Otorhinolaryngol. Rep. 2018, 6, 74–81. [Google Scholar] [CrossRef]
- Sangen, A.; Royackers, L.; Desloovere, C.; Wouters, J.; van Wieringen, A. Single-sided deafness affects language and auditory development–a case–control study. Clin. Otolaryngol. 2017, 42, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Kuppler, K.; Lewis, M.; Evans, A.K. A review of unilateral hearing loss and academic performance: Is it time to reassess traditional dogmata? Int. J. Pediatr. Otorhinolaryngol. 2013, 77, 617–622. [Google Scholar] [CrossRef]
- Zhan, K.Y.; Findlen, U.M.; Allen, D.Z.; Shannon, M.K.; Mattingly, J.K.; Adunka, O.F. Therapeutic challenges and clinical characteristics of single-sided deafness in children. Int. J. Pediatr. Otorhinolaryngol. 2020, 135, 110116. [Google Scholar] [CrossRef]
- Tomblin, J.B.; Oleson, J.; Ambrose, S.E.; Walker, E.A.; McCreery, R.W.; Moeller, M.P. Aided hearing moderates the academic outcomes of children with mild to severe hearing loss. Ear Hear. 2020, 41, 775–789. [Google Scholar] [CrossRef]
- Martin, D.; Bat-Chava, Y.; Lalwani, A.; Waltzman, S.B. Peer relationships of deaf children with cochlear implants: Predictors of peer entry and peer interaction success. J. Deaf. Stud. Deaf. Educ. 2011, 16, 108–120. [Google Scholar] [CrossRef]
- Bat-Chava, Y.; Deignan, E. Peer relationships of children with cochlear implants. J. Deaf. Stud. Deaf. Educ. 2001, 6, 186–199. [Google Scholar] [CrossRef]
- Kennedy, V.; Stephens, D.; Fitzmaurice, P. The impact of cochlear implants from the perspective of significant others of adult cochlear implant users. Otol. Neurotol. 2008, 29, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Manrique-Huarte, R.; Calavia, D.; Irujo, A.H.; Girón, L.; Manrique-Rodríguez, M. Treatment for hearing loss among the elderly: Auditory outcomes and impact on quality of life. Audiol. Neurotol. 2016, 21 (Suppl. S1), 29–35. [Google Scholar] [CrossRef]
- Francis, H.W.; Chee, N.; Yeagle, J.; Cheng, A.; Niparko, J.K. Impact of cochlear implants on the functional health status of older adults. Laryngoscope 2002, 112, 1482–1488. [Google Scholar] [CrossRef] [PubMed]
- Rauch, A.-K.; Kagermann, S.; Wesarg, T.; Jakob, T.F.; Aschendorff, A.; Ihorst, G.; Speck, I.; Arndt, S. Data logging evidence of cochlear implant use in single-sided and bilateral deafness. Audiol. Neurotol. 2019, 24, 206–216. [Google Scholar] [CrossRef]
- Távora-Vieira, D.; Acharya, A.; Rajan, G.P. What can we learn from adult cochlear implant recipients with single-sided deafness who became elective non-users? Cochlear Implant. Int. 2020, 21, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Lindquist, N.R.; Holder, J.T.; Patro, A.; Cass, N.D.; Tawfik, K.O.; O’MAlley, M.R.; Bennett, M.L.; Haynes, D.S.; Gifford, R.H.; Perkins, E.L. Cochlear implants for single-sided deafness: Quality of life, daily usage, and duration of deafness. Laryngoscope 2023, 133, 2362–2370. [Google Scholar] [CrossRef] [PubMed]
- Speck, I.; Challier, P.; Wesarg, T.; Jakob, T.F.; Aschendorff, A.; Hassepass, F.; Arndt, S. Is the cochlear implant a successful long-term solution for single-sided deaf and asymmetric hearing-impaired patients? Eur. Arch. Oto-Rhino-Laryngol. 2021, 278, 3257–3265. [Google Scholar] [CrossRef] [PubMed]
- Muigg, F.; Bliem, H.R.; Kühn, H.; Seebacher, J.; Holzner, B.; Weichbold, V.W. Cochlear implantation in adults with single-sided deafness: Generic and disease-specific long-term quality of life. Eur. Arch. Oto-Rhino-Laryngol. 2020, 277, 695–704. [Google Scholar] [CrossRef]
- Macielak, R.J.; Richard, C.; Malhotra, P.S.; Adunka, O.F.; Findlen, U.M. Cochlear Implantation for Single-Sided Deafness in Pediatric Patients: A Critical Assessment of Usage Rate. Otol. Neurotol. 2024, 45, 1122–1127. [Google Scholar] [CrossRef]
- Tan, V.Y.J.; Hollow, R.; Tari, S.; Rousset, A.; Wills, R.; Briggs, R.J.S.; Dowell, R.C. Cochlear implant usage in single sided deafness and factors affecting usage. Cochlear Implant. Int. 2024, 25, 387–393. [Google Scholar] [CrossRef]
- Liu, Y.-W.; Cheng, X.; Chen, B.; Peng, K.; Ishiyama, A.; Fu, Q.-J. Effect of tinnitus and duration of deafness on sound localization and speech recognition in noise in patients with single-sided deafness. Trends Hear. 2018, 22, 2331216518813802. [Google Scholar] [CrossRef]
- Vincent, C.; Arndt, S.; Firszt, J.B.; Fraysse, B.; Kitterick, P.T.; Papsin, B.C.; Snik, A.; Van de Heyning, P.; Deguine, O.; Marx, M. Identification and evaluation of cochlear implant candidates with asymmetrical hearing loss. Audiol. Neurotol. 2015, 20 (Suppl. S1), 87–89. [Google Scholar] [CrossRef]
- Van de Heyning, P.; Távora-Vieira, D.; Mertens, G.; Van Rompaey, V.; Rajan, G.P.; Müller, J.; Hempel, J.M.; Leander, D.; Polterauer, D.; Marx, M.; et al. Towards a unified testing framework for single-sided deafness studies: A consensus paper. Audiol. Neurotol. 2017, 21, 391–398. [Google Scholar] [CrossRef]
- Zwolan, T.A.; Schvartz-Leyzac, K.C.; Pleasant, T. Development of a 60/60 guideline for referring adults for a traditional cochlear implant candidacy evaluation. Otol. Neurotol. 2020, 41, 895–900. [Google Scholar] [CrossRef] [PubMed]
- Sorkin, D.L.; Adunka, O.F.; Westin, N. Health Insurance Coverage of Cochlear Implantation in Single-Sided Deafness and Asymmetric Hearing Loss. Otol. Neurotol. 2023, 44, e628–e634. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.J.; Takkoush, S.; Mendenhall, T.J.; Bramwell, M.L.; Steele, J.L.; Espahbodi, M.; Patel, N.S.; Gurgel, R.K. Hearing Benefits of Cochlear Implantation in Older Adults With Asymmetric Hearing Loss. Otol. Neurotol. 2024, 46, 515–520. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. Cochlear implants for children and adults with severe to profound deafness (NICE Technology Appraisal Guidance TA566). 2019. Available online: https://www.nice.org.uk/guidance/ta566 (accessed on 26 August 2025).
- Lee, C.; Yeung, M.W.; Falk, L.; Ali, A.; Walter, M. Implantable devices for single-sided deafness and conductive or mixed hearing loss: A health technology assessment. Ont. Health Technol. Assess. Ser. 2020, 20, 1–165. [Google Scholar]
- Ji-young, Y. Korean Otological Society calls for expanded insurance coverage for cochlear implants to prevent dementia. 2025. Available online: https://www.koreabiomed.com/news/articleView.html?idxno=27198 (accessed on 26 August 2025).
- German Society of Oto-Rhino-Laryngology, Head and Neck Surgery. S2k Guideline: Cochlear Implantation. 2020. Available online: https://adulthearing.com/wp-content/uploads/2021/02/German_Guidelines_Cochlea-Implantat-Versorgung-zentral-auditorische-Implantate_2020.pdf (accessed on 26 August 2025).
- Seebacher, J.; Muigg, F.; Kühn, H.; Weichbold, V.; Galvan, O.; Zorowka, P.; Schmutzhard, J. Cost-utility analysis of cochlear implantation in adults with single-sided deafness: Austrian and German perspective. Otol. Neurotol. 2021, 42, 799–805. [Google Scholar] [CrossRef]
- Warner-Czyz, A.D.; Roland, J.T.J.; Thomas, D.; Uhler, K.; Zombek, L. American cochlear implant alliance task force guidelines for determining cochlear implant candidacy in children. Ear Hear. 2022, 43, 268–282. [Google Scholar] [CrossRef]
- Vermeire, K.; Van de Heyning, P. Binaural hearing after cochlear implantation in subjects with unilateral sensorineural deafness and tinnitus. Audiol. Neurotol. 2009, 14, 163–171. [Google Scholar] [CrossRef]
- Zeitler, D.M.; Dorman, M.F.; Natale, S.J.; Loiselle, L.; Yost, W.A.; Gifford, R.H. Sound source localization and speech understanding in complex listening environments by single-sided deaf listeners after cochlear implantation. Otol. Neurotol. 2015, 36, 1467–1471. [Google Scholar] [CrossRef] [PubMed]
- Zeitler, D.M.; Dorman, M.F.; Natale, S.J.; Loiselle, L.; Yost, W.A.; Gifford, R.H. Cochlear implantation in adults with asymmetric hearing loss. Ear Hear. 2012, 33, 521–533. [Google Scholar] [CrossRef]
- Dorman, M.F.; Zeitler, D.; Cook, S.J.; Loiselle, L.; Yost, W.A.; Wanna, G.B.; Gifford, R.H. Interaural level difference cues determine sound source localization by single-sided deaf patients fit with a cochlear implant. Audiol. Neurotol. 2015, 20, 183–188. [Google Scholar] [CrossRef]
- Galvin, J.J.; Fu, Q.-J.; Wilkinson, E.P.; Mills, D.; Hagan, S.C.; Lupo, J.E.; Padilla, M.; Shannon, R.V. Benefits of cochlear implantation for single-sided deafness: Data from the House Clinic-University of Southern California-University of California. Los. Angeles Clin. Trial Ear Hear. 2019, 40, 766–781. [Google Scholar]
- Litovsky, R.Y.; Moua, K.; Godar, S.; Kan, A.; Misurelli, S.M.; Lee, D.J. Restoration of spatial hearing in adult cochlear implant users with single-sided deafness. Hear. Res. 2019, 372, 69–79. [Google Scholar] [CrossRef]
- Avan, P.; Giraudet, F.; Büki, B. Importance of binaural hearing. Audiol. Neurotol. 2015, 20 (Suppl. S1), 3–6. [Google Scholar] [CrossRef]
- Wesarg, T.; Kuntz, I.; Jung, L.; Wiebe, K.; Schatzer, R.; Brill, S.; Aschendorff, A.; Arndt, S. Masked speech perception with bone conduction device, contralateral routing of signals hearing aid, and cochlear implant use in adults with single-sided deafness: A prospective hearing device comparison using a unified testing framework. Audiol. Neurotol. 2024, 29, 271–289. [Google Scholar] [CrossRef]
- Gersdorff, G.; Péan, V.; Camby, S.; Barriat, S.; Lefebvre, P.P. Factors predictive of binaural hearing restoration by cochlear implant in single-sided deafness. Audiol. Neurotol. 2024, 29, 228–238. [Google Scholar] [CrossRef]
- Öz, O.; Dinçer D., H.; Batuk, M.Ö.; Sennaroğlu, G.; Govaerts, P.J. Assessment of Binaural Benefits in Hearing and Hearing-Impaired Listeners. J. Speech Lang. Hear. Res. 2023, 66, 3633–3648. [Google Scholar] [CrossRef]
- Brown, K.D.; Dillon, M.T.; Park, L.R. Benefits of cochlear implantation in childhood unilateral hearing loss (CUHL trial). Laryngoscope 2022, 132, S1–S18. [Google Scholar] [CrossRef]
- Schnupp, J.W.H.; Buchholz, S.; Buck, A.N.; Budig, H.; Khurana, L.; Rosskothen-Kuhl, N. Pulse timing dominates binaural hearing with cochlear implants. Proc. Natl. Acad. Sci. USA 2025, 122, e2416697122. [Google Scholar] [CrossRef]
- Cosentino, S.; Carlyon, R.P.; Deeks, J.M.; Parkinson, W.; Bierer, J.A. Rate discrimination, gap detection and ranking of temporal pitch in cochlear implant users. J. Assoc. Res. Otolaryngol. 2016, 17, 371–382. [Google Scholar] [CrossRef]
- Carlyon, R.P.; Deeks, J.M.; Delgutte, B.; Chung, Y.; Vollmer, M.; Ohl, F.W.; Kral, A.; Tillein, J.; Litovsky, R.Y.; Schnupp, J.; et al. Limitations on temporal processing by cochlear implant users: A compilation of viewpoints. Trends Hear. 2025, 29, 23312165251317006. [Google Scholar] [CrossRef]
- Sullivan, C.B.; Al-Qurayshi, Z.; Zhu, V.; Liu, A.; Dunn, C.; Gantz, B.J.; Hansen, M.R. Long-term audiologic outcomes after cochlear implantation for single-sided deafness. Laryngoscope 2020, 130, 1805–1811. [Google Scholar] [CrossRef]
- Harris, M.S.; Capretta, N.R.; Henning, S.C.; Feeney, L.; Pitt, M.A.; Moberly, A.C. Postoperative rehabilitation strategies used by adults with cochlear implants: A pilot study. Laryngoscope Investig. Otolaryngol. 2016, 1, 42–48. [Google Scholar] [CrossRef]
- Boothroyd, A. Adult aural rehabilitation: What is it and does it work? Trends Amplif. 2007, 11, 63–71. [Google Scholar] [CrossRef]
- Morelli, L.; Fancello, V.; Gaino, F.; Cagliero, G.; Caruso, A.; Sanna, M. Cochlear implantation in single-sided deafness: A single-center experience of 138 cases. Eur. Arch. Oto-Rhino-Laryngol. 2023, 280, 4427–4432. [Google Scholar] [CrossRef]
- Shannon, C.M.; Schvartz-Leyzac, K.C.; Dubno, J.R.; McRackan, T.R. Determinants of cochlear implant satisfaction and decisional regret in adult cochlear implant users. Otol. Neurotol. 2023, 44, e722–e729. [Google Scholar] [CrossRef]
- McRackan, T.R.; Reddy, P.; Costello, M.S.; Dubno, J.R. Role of preoperative patient expectations in adult cochlear implant outcomes. Otol. Neurotol. 2021, 42, e130–e136. [Google Scholar] [CrossRef]
- Park, L.R.; Gagnon, E.B.; Dillon, M.T. Factors that influence outcomes and device use for pediatric cochlear implant recipients with unilateral hearing loss. Front. Hum. Neurosci. 2023, 17, 1141065. [Google Scholar] [CrossRef]
- O’COnnell, B.P.; Hunter, J.B.; Wanna, G.B. The importance of electrode location in cochlear implantation. Laryngoscope Investig. Otolaryngol. 2016, 1, 169–174. [Google Scholar] [CrossRef]
- Sennaroglu, L. Cochlear implantation in inner ear malformations–a review article. Cochlear Implant. Int. 2010, 11, 4–41. [Google Scholar] [CrossRef]
- Gibson, P.; Boyd, P. Optimal electrode design: Straight versus perimodiolar, European annals of otorhinolaryngology. Head. Neck Dis. 2016, 133, S63–S65. [Google Scholar]
- Boyd, P.J. Potential benefits from deeply inserted cochlear implant electrodes. Ear Hear. 2011, 32, 411–427. [Google Scholar] [CrossRef]
- Franke-Trieger, A.; Jolly, C.; Darbinjan, A.; Zahnert, T.; Mürbe, D. Insertion depth angles of cochlear implant arrays with varying length: A temporal bone study. Otol. Neurotol. 2014, 35, 58–63. [Google Scholar]
- Canfarotta, M.W.; Dillon, M.T.; Buchman, C.A.; Buss, E.; O’COnnell, B.P.; Rooth, M.A.; King, E.R.; Pillsbury, H.C.; Adunka, O.F.; Brown, K.D. Long-term influence of electrode array length on speech recognition in cochlear implant users. Laryngoscope 2021, 131, 892–897. [Google Scholar]
- Hamzavi, J.; Arnoldner, C. Effect of deep insertion of the cochlear implant electrode array on pitch estimation and speech perception. Acta Oto-Laryngol. 2006, 126, 1182–1187. [Google Scholar] [CrossRef] [PubMed]
- Gani, M.; Valentini, G.; Sigrist, A.; Kós, M.-I.; Boëx, C. Implications of deep electrode insertion on cochlear implant fitting. J. Assoc. Res. Otolaryngol. 2007, 8, 69–83. [Google Scholar] [CrossRef]
- Adunka, O.F.; Pillsbury, H.C.; Adunka, M.C.; Buchman, C.A. Is electric acoustic stimulation better than conventional cochlear implantation for speech perception in quiet? Otol. Neurotol. 2010, 31, 1049–1054. [Google Scholar] [CrossRef] [PubMed]
- Sturm, J.J.; Patel, V.; Dibelius, G.; Kuhlmey, M.; Kim, A.H. Comparative performance of lateral wall and perimodiolar cochlear implant arrays. Otol. Neurotol. 2021, 42, 532–539. [Google Scholar] [CrossRef]
- Briggs, R.J.; McLean, T.; Rousset, A.; Tari, S.; O’LEary, S.J.; Dowell, R.C.; Leigh, J.; Cowan, R. Randomized Controlled Trial Comparing Outcomes for Adult Cochlear Implant Recipients Using a Lateral Wall or Perimodiolar Electrode Array. Otol. Neurotol. 2025, 46, 802–808. [Google Scholar] [CrossRef]
- Dhanasingh, A.; Jolly, C. An overview of cochlear implant electrode array designs. Hear. Res. 2017, 356, 93–103. [Google Scholar] [CrossRef]
- Ramos-Macias, Á.; Briggs, R.; Choi, B.Y.; Friedmann, D.; Ishiyama, A.; Lenarz, T.; Mylanus, E.; O’LEary, S.; Roland, J.T.; Zarowski, A. The importance of the Electrode-Neural Interface in supporting long term outcomes in cochlear implantation, Expert opinion. Audiol. Neurotol. 2025, 23, 1–11. [Google Scholar] [CrossRef]
- Buchman, C.A.; Gifford, R.H.; Haynes, D.S.; Lenarz, T.; O’Donoghue, G.; Adunka, O.; Biever, A.; Briggs, R.J.; Carlson, M.L.; Dai, P. Unilateral Cochlear Implants for Severe, Profound, or Moderate Sloping to Profound Bilateral Sensorineural Hearing Loss: A Systematic Review and Consensus Statements. JAMA Otolaryngol.–Head. Neck Surg. 2020, 146, 942–953. [Google Scholar] [CrossRef]
- Richard, C.; Fayad, J.N.; Doherty, J.; Jr, F.H.L. Round window versus cochleostomy technique in cochlear implantation: Histologic findings. Otol. Neurotol. 2012, 33, 1181–1187. [Google Scholar] [CrossRef]
- Adunka, O.; Gstoettner, W.; Hambek, M.; Unkelbach, M.H.; Radeloff, A.; Kiefer, J. Preservation of basal inner ear structures in cochlear implantation. ORL 2005, 66, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Roland, P.S.; Wright, C.G. Surgical aspects of cochlear implantation: Mechanisms of insertional trauma. Adv. Otorhinolaryngol. 2006, 64, 11. [Google Scholar]
- Wanna, G.B.; Noble, J.H.; Carlson, M.L.; Gifford, R.H.; Dietrich, M.S.; Haynes, D.S.; Dawant, B.M.; Labadie, R.F. Impact of electrode design and surgical approach on scalar location and cochlear implant outcomes. Laryngoscope 2014, 124, S1–S7. [Google Scholar] [CrossRef]
- Jiam, N.T.; Jiradejvong, P.; Pearl, M.S.; Limb, C.J. The effect of round window vs cochleostomy surgical approaches on cochlear implant electrode position: A flat-panel computed tomography study. JAMA Otolaryngol.–Head. Neck Surg. 2016, 142, 873–880. [Google Scholar] [CrossRef]
- Zhou, L.; Friedmann, D.R.; Treaba, C.; Peng, R.; Roland, J.T. Does cochleostomy location influence electrode trajectory and intracochlear trauma? Laryngoscope 2015, 125, 966–971. [Google Scholar] [CrossRef] [PubMed]
- Meshik, X.; Holden, T.A.; Chole, R.A.; Hullar, T.E. Optimal cochlear implant insertion vectors. Otol. Neurotol. 2010, 31, 58–63. [Google Scholar] [CrossRef]
- Jangra, A.; Das, K.N.; Sharma, V.; Timmaraju, S.; Khera, P.; Tiwari, S.; Soni, K.; Choudhury, B.; Ghatak, S.; Dixit, S.G.; et al. Comparison of depth of electrode insertion between cochleostomy and round window approach: A cadaveric study. Eur. Arch. Oto-Rhino-Laryngol. 2024, 281, 3547–3555. [Google Scholar] [CrossRef] [PubMed]
- Hiremath, S.B.; Biswas, A.; Mndebele, G.; Schramm, D.; Ertl-Wagner, B.B.; Blaser, S.I.; Chakraborty, S. Cochlear implantation: Systematic approach to preoperative radiologic evaluation. Radiographics 2023, 43, e220102. [Google Scholar] [CrossRef] [PubMed]
- Naples, J.G.; Ruckenstein, M.J. Cochlear implant. Otolaryngol. Clin. North. Am. 2020, 53, 87–102. [Google Scholar] [CrossRef]
- Appachi, S.; Schwartz, S.; Ishman, S.; Anne, S. Utility of intraoperative imaging in cochlear implantation: A systematic review. Laryngoscope 2018, 128, 1914–1921. [Google Scholar] [CrossRef]
- Greisiger, R.; Bester, C.; Sørensen, T.; Korslund, H.; Bunne, M.; O’LEary, S.; Jablonski, G.E. Intraoperative measured Electrocochleography and fluoroscopy video to detect cochlea trauma. Otol. Neurotol. 2024, 45, 36–45. [Google Scholar] [CrossRef]
- Perazzini, C.; Puechmaille, M.; Saroul, N.; Plainfossé, O.; Montrieul, L.; Bécaud, J.; Gilain, L.; Chabrot, P.; Boyer, L.; Mom, T. Fluoroscopy guided electrode-array insertion for cochlear implantation with straight electrode-arrays: A valuable tool in most cases. Eur. Arch. Oto-Rhino-Laryngol. 2021, 278, 965–975. [Google Scholar] [CrossRef]
- Jia, H.; Torres, R.; Nguyen, Y.; De Seta, D.; Ferrary, E.; Wu, H.; Sterkers, O.; Bernardeschi, D.; Mosnier, I. Intraoperative conebeam CT for assessment of intracochlear positioning of electrode arrays in adult recipients of cochlear implants. Am. J. Neuroradiol. 2018, 39, 768–774. [Google Scholar] [CrossRef]
- Labadie, R.F.; Schefano, A.D.; Holder, J.T.; Dwyer, R.T.; Rivas, A.; O’malley, M.R.; Noble, J.H.; Dawant, B.M. Use of intraoperative CT scanning for quality control assessment of cochlear implant electrode array placement. Acta Oto-Laryngol. 2020, 140, 206–211. [Google Scholar] [CrossRef]
- Tykocinski, M.; Cohen, L.T.; Cowan, R.S. Measurement and analysis of access resistance and polarization impedance in cochlear implant recipients. Otol. Neurotol. 2005, 26, 948–956. [Google Scholar] [CrossRef]
- Weiss, N.M.; Hans, S.; Wozniak, M.; Föger, A.; Dazert, S.; Van Rompaey, V.; Van de Heyning, P.; Schmutzhard, J.; Dierker, A. Performing Repeated Intraoperative Impedance Telemetry Measurements during Cochlear Implantation. J. Vis. Exp. (JoVE) 2023, 4, e65600. [Google Scholar] [CrossRef]
- Bester, C.; Razmovski, T.; Collins, A.; Mejia, O.; Foghsgaard, S.; Mitchell-Innes, A.; Shaul, C.; Campbell, L.; Eastwood, H.; O’lEary, S. Four-point impedance as a biomarker for bleeding during cochlear implantation. Sci. Rep. 2020, 10, 2777. [Google Scholar] [CrossRef]
- Mehta, N.; Chu, P.; Birman, C.S. To Use the Back-up Cochlear Implant or Not? Using Intra-operative Impedance to Guide Your Decisions. Otol. Neurotol. 2022, 43, e408–e413. [Google Scholar] [CrossRef] [PubMed]
- Jiam, N.T.; Podury, A.; Quesnel, A.M.; Handzel, O. Worldwide differences in surgeon intraoperative practices for cochlear implantation. Cochlear Implant. Int. 2024, 25, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Oxenham, A.J. How we hear: The perception and neural coding of sound. Annu. Rev. Psychol. 2018, 69, 27–50. [Google Scholar] [CrossRef] [PubMed]
- Humphries, C.; Liebenthal, E.; Binder, J.R. Tonotopic organization of human auditory cortex. Neuroimage 2010, 50, 1202–1211. [Google Scholar] [CrossRef]
- Canfarotta, M.W.; Dillon, M.T.; Buss, E.; Pillsbury, H.C.; Brown, K.D.; O’cOnnell, B.P. Frequency-to-Place Mismatch: Characterizing Variability and the Influence on Speech Perception Outcomes in Cochlear Implant Recipients. Ear Hear. 2020, 41, 1349–1361. [Google Scholar] [CrossRef]
- Aljazeeri, I.; Hamed, N.; Abdelsamad, Y.; Sharif, T.; Al-Momani, M.; Hagr, A. Anatomy-Based Frequency Allocation in Cochlear Implantation: The Importance of Cochlear Coverage. Laryngoscope 2022, 132, 2224–2231. [Google Scholar] [CrossRef]
- Reiss, L.A.; Gantz, B.J.; Turner, C.W. Cochlear implant speech processor frequency allocations may influence pitch perception. Otol. Neurotol. 2008, 29, 160–167. [Google Scholar] [CrossRef]
- Nie, K.; Barco, A.; Zeng, F.-G. Spectral and temporal cues in cochlear implant speech perception. Ear Hear. 2006, 27, 208–217. [Google Scholar] [CrossRef]
- Podury, A.; Barry, B.; Barrett, K.C.; Jiam, N.T. Perfecting Sensory Restoration and the Unmet Need for Personalized Medicine in Cochlear Implant Users: A Narrative Review. Brain Sci. 2025, 15, 479. [Google Scholar] [CrossRef] [PubMed]
- Tóth, T.F.; Németh, A.; Bakó, P.; Révész, P.; Gerlinger, I.; Szanyi, I. Matching the pitch perception of the cochlear implanted ear with the contralateral ear in patients with single-sided deafness: A novel approach. Eur. Arch. Oto-Rhino-Laryngol. 2023, 280, 4851–4859. [Google Scholar] [CrossRef]
- Bernstein, J.G.; Jensen, K.K.; Stakhovskaya, O.A.; Noble, J.H.; Hoa, M.; Kim, H.J.; Shih, R.; Kolberg, E.; Cleary, M.; Goupell, M.J. Interaural place-of-stimulation mismatch estimates using CT scans and binaural perception, but not pitch, are consistent in cochlear-implant users. J. Neurosci. 2021, 41, 10161–10178. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.P.M.; Bennink, E.; Grolman, W.; van Zanten, G.A. Electro-acoustic pitch matching experiments in patients with single-sided deafness and a cochlear implant: Is there a need for adjustment of the default frequency allocation tables? Hear. Res. 2016, 342, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Brown, K.D.; Overton, A.B.; Thompson, N.J.; Sloop, A.D.; Richter, M.E.; Canfarotta, M.W.; Selleck, A.M.; Dedmon, M.M.; Dillon, M.T. Association of Tonotopic Mismatch With the Speech Recognition of Cochlear Implant Users With Unilateral Hearing Loss. Otol. Neurotol. 2025, 46, e302–e306. [Google Scholar] [CrossRef]
- Alahmadi, A.; Abdelsamad, Y.; Hafez, A.; Hagr, A.; Melo, R.S. X-ray guided anatomy-based fitting: The validity of OTOPLAN. PLoS ONE 2024, 19, e0313567. [Google Scholar] [CrossRef]
- Dessard, L.; Gersdorff, G.; Ivanovik, N.; Zoca-Assadi, M.; Nopp, P.; Camby, S.; Lefebvre, P.P. Cochlear implant: Analysis of the frequency-to-place mismatch with the table-based software OTOPLAN® and its influence on hearing performance. Audiol. Neurotol. 2024, 29, 239–245. [Google Scholar] [CrossRef]
- Wangchuk, P.; Umat, C.; Chong, F.Y.; Zaki, F.M.; Abdullah, A. Speech Perception Outcomes with the Anatomy-Based Fitting Map among Experienced, Adult Cochlear Implant Users: A Longitudinal Study. Audiol. Neurotol. 2025, 30, 222–236. [Google Scholar] [CrossRef] [PubMed]
- Kurz, A.; Müller-Graff, F.-T.; Hagen, R.; Rak, K. One click is not enough: Anatomy-based fitting in experienced cochlear implant users. Otol. Neurotol. 2022, 43, 1176–1180. [Google Scholar] [CrossRef] [PubMed]
- Ross, M. A retrospective look at the future of aural rehabilitation. J. Acad. Rehabil. Audiol. 1997, 30, 11–28. [Google Scholar]
- Zhang, T.; Dorman, M.F.; Fu, Q.-J.; Spahr, A.J. Auditory training in patients with unilateral cochlear implant and contralateral acoustic stimulation. Ear Hear. 2012, 33, e70–e79. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.-J.; Galvin, J.J. Maximizing cochlear implant patients’ performance with advanced speech training procedures. Hear. Res. 2008, 242, 198–208. [Google Scholar] [CrossRef]
- Holder, J.T.; Dwyer, N.C.; Gifford, R.H. Duration of processor use per day is significantly correlated with speech recognition abilities in adults with cochlear implants. Otol. Neurotol. 2020, 41, e227–e231. [Google Scholar] [CrossRef]
- Lindquist, N.R.; Dietrich, M.S.; Patro, A.; Henry, M.R.; DeFreese, A.J.; Freeman, M.H.; Perkins, E.L.; Gifford, R.H.; Haynes, D.S.; Holder, J.T. Early datalogging predicts cochlear implant performance: Building a recommendation for daily device usage. Otol. Neurotol. 2023, 44, e479–e485. [Google Scholar] [CrossRef]
- Holder, J.T.; Gifford, R.H. Effect of increased daily cochlear implant use on auditory perception in adults. J. Speech Lang. Hear. Res. 2021, 64, 4044–4055. [Google Scholar] [CrossRef]
- Schvartz-Leyzac, K.C.; Conrad, C.A.; Zwolan, T.A. Datalogging statistics and speech recognition during the first year of use in adult cochlear implant recipients. Otol. Neurotol. 2019, 40, e686–e693. [Google Scholar] [CrossRef]
- Easwar, V.; Sanfilippo, J.; Papsin, B.; Gordon, K. Impact of consistency in daily device use on speech perception abilities in children with cochlear implants: Datalogging evidence. J. Am. Acad. Audiol. 2018, 29, 835–846. [Google Scholar] [CrossRef]
- Gagnon, E.B.; Eskridge, H.; Brown, K.D.; Park, L.R. The impact of cumulative cochlear implant wear time on spoken language outcomes at age 3 years. J. Speech Lang. Hear. Res. 2021, 64, 1369–1375. [Google Scholar] [CrossRef]
- Park, L.R.; Gagnon, E.B.; Thompson, E.; Brown, K.D. Age at full-time use predicts language outcomes better than age of surgery in children who use cochlear implants. Am. J. Audiol. 2019, 28, 986–992. [Google Scholar] [CrossRef]
- Busch, T.; Vanpoucke, F.; van Wieringen, A. Auditory environment across the life span of cochlear implant users: Insights from data logging. J. Speech Lang. Hear. Res. 2017, 60, 1362–1377. [Google Scholar] [CrossRef]
- Walia, A.; Ortmann, A.J.; Lefler, S.; Holden, T.A.; Puram, S.V.; Herzog, J.A.; Buchman, C.A. Electrocochleography-based tonotopic map: I, Place coding of the human cochlea with hearing loss. Ear Hear. 2025, 46, 253–264. [Google Scholar] [CrossRef]
- Erber, N.P. Evaluating speech perception ability in hearing impaired children. In Childhood Deafness: Causation, Assessment, and Management; Grune & Stratton: New York, NY, USA, 1977; pp. 173–181. [Google Scholar]
- Glade, R.; Taylor, E.; Culbertson, D.S.; Ray, C. Overview of current approaches to aural rehabilitation for adults with cochlear implants. Perspect. ASHA Spec. Interest. Groups 2020, 5, 1175–1187. [Google Scholar] [CrossRef]
- Henshaw, H.; Ferguson, M.A. Efficacy of individual computer-based auditory training for people with hearing loss: A systematic review of the evidence. PLoS ONE 2013, 8, e62836. [Google Scholar] [CrossRef] [PubMed]
- Cambridge, G.; Taylor, T.; Arnott, W.; Wilson, W.J. Auditory training for adults with cochlear implants: A systematic review. Int. J. Audiol. 2022, 61, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.-J.; Galvin, J.; Wang, X.; Nogaki, G. Moderate auditory training can improve speech performance of adult cochlear implant patients. Acoust. Res. Lett. Online 2005, 6, 106–111. [Google Scholar] [CrossRef]
- Schumann, A.; Serman, M.; Gefeller, O.; Hoppe, U. Computer-based auditory phoneme discrimination training improves speech recognition in noise in experienced adult cochlear implant listeners. Int. J. Audiol. 2015, 54, 190–198. [Google Scholar] [CrossRef]
- Stacey, P.C.; Raine, C.H.; O’DOnoghue, G.M.; Tapper, L.; Twomey, T.; Summerfield, A.Q. Effectiveness of computer-based auditory training for adult users of cochlear implants. Int. J. Audiol. 2010, 49, 347–356. [Google Scholar] [CrossRef]
- Miller, S.E.; Zhang, Y.; Nelson, P.B. Efficacy of multiple-talker phonetic identification training in postlingually deafened cochlear implant listeners. J. Speech Lang. Hear. Res. 2016, 59, 90–98. [Google Scholar] [CrossRef]
- Plant, G.; Bernstein, C.M.; Levitt, H. Optimizing performance in adult cochlear implant users through clinician directed auditory training. Semin. Hear. 2015, 36, 296–310. [Google Scholar] [CrossRef]
- Busby, P.A.; Roberts, S.A.; Tong, Y.C.; Clark, G.M. Results of speech perception and speech production training for three prelingually deaf patients using a multiple-electrode cochlear implant. Br. J. Audiol. 1991, 25, 291–302. [Google Scholar] [CrossRef]
- Dawson, P.; Clark, G.M. Changes in synthetic and natural vowel perception after specific training for congenitally deafened patients using a multichannel cochlear implant. Ear Hear. 1997, 18, 488–501. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, C.M.; Brewer, D.M.; Bakke, M.H.; Olson, A.D.; Machmer, E.J.; Spitzer, J.B.; Schauer, P.C.; Sydlowski, S.A.; Levitt, H. Maximizing cochlear implant outcomes with short-term aural rehabilitation. J. Am. Acad. Audiol. 2021, 32, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-H.; Anderson, G.T.; Loizou, P.C. A neural network model for optimizing vowel recognition by cochlear implant listeners. IEEE Trans. Neural Syst. Rehabil. Eng. 2001, 9, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, R.M.; Boermans, P.-P.B.M.; Avenarius, O.F.; Briaire, J.J.; Frijns, J.H.M. Effects of pulse width, pulse rate and paired electrode stimulation on psychophysical measures of dynamic range and speech recognition in cochlear implants. Ear Hear. 2012, 33, 489–496. [Google Scholar] [CrossRef]
- McKay, C.M.; McDermott, H.J. The perceptual effects of current pulse duration in electrical stimulation of the auditory nerve. J. Acoust. Soc. Am. 1999, 106, 998–1009. [Google Scholar] [CrossRef]
- Loizou, P.C.; Poroy, O.; Dorman, M. The effect of parametric variations of cochlear implant processors on speech understanding. J. Acoust. Soc. Am. 2000, 108, 790–802. [Google Scholar] [CrossRef]
- Shader, M.J.; Nguyen, N.; Cleary, M.; Hertzano, R.; Eisenman, D.J.; Anderson, S.; Gordon-Salant, S.; Goupell, M.J. Effect of stimulation rate on speech understanding in older cochlear-implant users. Ear Hear. 2020, 41, 640–651. [Google Scholar] [CrossRef]
- Brochier, T.; McDermott, H.J.; McKay, C.M. The effect of presentation level and stimulation rate on speech perception and modulation detection for cochlear implant users. J. Acoust. Soc. Am. 2017, 141, 4097–4105. [Google Scholar] [CrossRef]
- Carlson, M.L. Cochlear implantation in adults. New Engl. J. Med. 2020, 382, 1531–1542. [Google Scholar] [CrossRef] [PubMed]
- Dornhoffer, J.R.; Khandalavala, K.R.; Saoji, A.A.; Lohse, C.M.; Carlson, M.L. Longitudinal Trends in Cochlear Implant Programming from a Single-Institution Review of Over 400 Adult Implant Recipients: Evidence to Support Selective De-Escalation of Device Programming. Otol. Neurotol. 2025, 46, 796–801. [Google Scholar] [CrossRef]
- Pantaleo, A.; Murri, A.; Cavallaro, G.; Pontillo, V.; Auricchio, D.; Quaranta, N. Single-sided deafness and hearing rehabilitation modalities: Contralateral routing of signal devices, bone conduction devices, and cochlear implants. Brain Sci. 2024, 14, 99. [Google Scholar] [CrossRef]
- Kurz, A.; Zanzinger, M.; Hagen, R.; Rak, K. The impact of cochlear implant microphone settings on the binaural hearing of experienced cochlear implant users with single-sided deafness. Eur. Arch. Oto-Rhino-Laryngol. 2021, 278, 2067–2077. [Google Scholar] [CrossRef]
- Pieper, S.H.; Hamze, N.; Brill, S.; Hochmuth, S.; Exter, M.; Polak, M.; Radeloff, A.; Buschermöhle, M.; Dietz, M. Considerations for fitting cochlear implants bimodally and to the single-sided deaf. Trends Hear. 2022, 26, 23312165221108259. [Google Scholar] [CrossRef]
- Saba, J.N.; Ali, H.; Hansen, J.H. Formant priority channel selection for an “n-of-m” sound processing strategy for cochlear implants. J. Acoust. Soc. Am. 2018, 144, 3371–3380. [Google Scholar] [CrossRef]
- Carlyon, R.P.; Goehring, T. Cochlear implant research and development in the twenty-first century: A critical update. J. Assoc. Res. Otolaryngol. 2021, 22, 481–508. [Google Scholar] [CrossRef] [PubMed]
- Kiefer, J.; Müller, J.; Pfennigdorff, T.; Schön, F.; Helms, J.; von Ilberg, C.; Baumgartner, W.; Gstöttner, W.; Ehrenberger, K.; Arnold, W.; et al. Speech understanding in quiet and in noise with the CIS speech coding strategy (MED-EL Combi-40) compared to the multipeak and spectral peak strategies (nucleus). Orl 1996, 58, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Kan, A.; Yu, G.; Guo, Z.; Zheng, N.; Meng, Q. Pitch perception with the temporal limits encoder for cochlear implants. IEEE Trans. Neural Syst. Rehabil. Eng. 2022, 30, 2528–2539. [Google Scholar] [CrossRef] [PubMed]
- Arnoldner, C.; Riss, D.; Brunner, M.; Durisin, M.; Baumgartner, W.-D.; Hamzavi, J.-S. Speech and music perception with the new fine structure speech coding strategy: Preliminary results. Acta Oto-Laryngol. 2007, 127, 1298–1303. [Google Scholar] [CrossRef]
- Wilson, B.S.; Schatzer, R.; Lopez-Poveda, E.A.; Sun, X.; Lawson, D.T.; Wolford, R.D. Two new directions in speech processor design for cochlear implants. Ear Hear. 2005, 26, 73S–81S. [Google Scholar] [CrossRef] [PubMed]
- Riss, D.; Arnoldner, C.; Baumgartner, W.-D.; Kaider, A.; Hamzavi, J.-S. A new fine structure speech coding strategy: Speech perception at a reduced number of channels. Otol. Neurotol. 2008, 29, 784–788. [Google Scholar] [CrossRef]
- Vermeire, K.; Punte, A.K.; Van de Heyning, P. Better speech recognition in noise with the fine structure processing coding strategy. Orl 2010, 72, 305–311. [Google Scholar] [CrossRef]
- Dorman, M.F.; Gifford, R.H.; Spahr, A.J.; McKarns, S.A. The benefits of combining acoustic and electric stimulation for the recognition of speech, voice and melodies. Audiol. Neurotol. 2008, 13, 105–112. [Google Scholar] [CrossRef]
- Daher, G.S.; Kocharyan, A.; Dillon, M.T.; Carlson, M.L. Cochlear implantation outcomes in adults with single-sided deafness: A systematic review and meta-analysis. Otol. Neurotol. 2023, 44, 297–309. [Google Scholar] [CrossRef]
- Dornhoffer, J.R.; Chidarala, S.; Patel, T.; Khandalavala, K.R.; Nguyen, S.A.; Schvartz-Leyzac, K.C.; Dubno, J.R.; Carlson, M.L.; Moberly, A.C.; McRackan, T.R. Systematic review of auditory training outcomes in adult cochlear implant recipients and meta-analysis of outcomes. J. Clin. Med. 2024, 13, 400. [Google Scholar] [CrossRef]
- Mole, G. Auditory Training for a Cochlear Implant for Single-Sided Deafness: Two Perspectives: A hearing therapist in the United Kingdom and her auditory trainer describe two sides of the CI journey. The ASHA Leader 2016, 21, 1. [Google Scholar] [CrossRef]
- Jiam, N.T.; Deroche, M.L.; Jiradejvong, P.; Limb, C.J. A randomized controlled crossover study of the impact of online music training on pitch and timbre perception in cochlear implant users. J. Assoc. Res. Otolaryngol. 2019, 20, 247–262. [Google Scholar] [CrossRef] [PubMed]
- Boyer, J.; Stohl, J. MELUDIA–Online music training for cochlear implant users. Cochlear Implant. Int. 2022, 23, 257–269. [Google Scholar] [CrossRef]
- Deep, N.L.; Spitzer, E.R.; Shapiro, W.H.; Waltzman, S.B.; Roland, J.T.J.; Friedmann, D.R. Cochlear implantation in adults with single-sided deafness: Outcomes and device use. Otol. Neurotol. 2021, 42, 414–423. [Google Scholar] [CrossRef]
- Tan, V.Y.J.; Zhang, E.Z.Y.; Leem, P.S.; Deepak D, V.; Li, H.; Teng, S.W.; Krishna S, G.; Ong, B.; Tan, B.Y.B. Audiologic and patient perceived benefit in cochlear implantation for single-sided deafness. Proc. Singap. Healthc. 2022, 31, 20101058221083393. [Google Scholar] [CrossRef]
- Ganek, H.V.; Cushing, S.L.; Papsin, B.C.; Gordon, K.A. Cochlear implant use remains consistent over time in children with single-sided deafness. Ear Hear. 2020, 41, 678–685. [Google Scholar] [CrossRef]
- Arras, T.; Boudewyns, A.; Swinnen, F.; Zarowski, A.; Philips, B.; Desloovere, C.; Wouters, J.; van Wieringen, A. Longitudinal auditory data of children with prelingual single-sided deafness managed with early cochlear implantation. Sci. Rep. 2022, 12, 9376. [Google Scholar] [CrossRef]
- Benchetrit, L.; Ronner, E.A.; Anne, S.; Cohen, M.S. Cochlear implantation in children with single-sided deafness: A systematic review and meta-analysis. JAMA Otolaryngol.–Head. Neck Surg. 2021, 147, 58–69. [Google Scholar] [CrossRef]
- Polonenko, M.J.; Papsin, B.C.; Gordon, K.A. Children with single-sided deafness use their cochlear implant. Ear Hear. 2017, 38, 681–689. [Google Scholar] [CrossRef]
- Macías, Á.R.; Borkoski-Barreiro, S.A.; González, J.C.F.; Martínez, I.d.M.; de Miguel, Á.R. Single-sided deafness and cochlear implantation in congenital and acquired hearing loss in children. Clin. Otolaryngol. 2019, 44, 138–143. [Google Scholar] [CrossRef]
- Thomas, J.P.; Neumann, K.; Dazert, S.; Voelter, C. Cochlear implantation in children with congenital single-sided deafness. Otol. Neurotol. 2017, 38, 496–503. [Google Scholar] [CrossRef]
- Greaver, L.; Eskridge, H.; Teagle, H.F. Considerations for pediatric cochlear implant recipients with unilateral or asymmetric hearing loss: Assessment, device fitting, and habilitation. Am. J. Audiol. 2017, 26, 91–98. [Google Scholar] [CrossRef]
- Summerfield, A.Q.; Marshall, D.H. Non-use of cochlear implants by post-lingually deafened adults. Cochlear Implant. Int. 2000, 1, 18–38. [Google Scholar] [CrossRef]
- Warner-Czyz, A.D.; Loy, B.; Pourchot, H.; White, T.; Cokely, E. Effect of hearing loss on peer victimization in school-age children. Except. Child. 2018, 84, 280–297. [Google Scholar] [CrossRef]
- Contrera, K.J.; Choi, J.S.; Blake, C.R.; Betz, J.F.; Niparko, J.K.; Lin, F.R. Rates of long-term cochlear implant use in children. Otol. Neurotol. 2014, 35, 426–430. [Google Scholar] [CrossRef]
- Özdemir, S.; Tuncer, Ü.; Tarkan, Ö.; Kıroğlu, M.; Çetik, F.; Akar, F. Factors contributing to limited or non-use in the cochlear implant systems in children: 11 years experience. Int. J. Pediatr. Otorhinolaryngol. 2013, 77, 407–409. [Google Scholar] [CrossRef]
- Archbold, S.M.; Nikolopoulos, T.P.; Lloyd-Richmond, H. Long-term use of cochlear implant systems in paediatric recipients and factors contributing to non-use. Cochlear Implant. Int. 2009, 10, 25–40. [Google Scholar] [CrossRef]
- Junior, F.C.; Pinna, M.H.; Alves, R.D.; Malerbi, A.F.D.S.; Bento, R.F. Cochlear implantation and single-sided deafness: A systematic review of the literature. Int. Arch. Otorhinolaryngol. 2016, 20, 69–75. [Google Scholar]
- Távora-Vieira, D.; Wedekind, A. Single-sided deafness: Emotional and social handicap, impact on health status and quality of life, functional hearing, and the effects of cochlear implantation. Otol. Neurotol. 2022, 43, 1116–1124. [Google Scholar] [CrossRef] [PubMed]
- Hornsby, B.W.Y.; Naylor, G.; Bess, F.H. A taxonomy of fatigue concepts and their relation to hearing loss. Ear Hear. 2016, 37, 136S–144S. [Google Scholar] [CrossRef]
- Dorman, M.F.; Gifford, R.H. Combining acoustic and electric stimulation in the service of speech recognition. Int. J. Audiol. 2010, 49, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Limb, C.J. Cochlear implant-mediated perception of music. Curr. Opin. Otolaryngol. Head. Neck Surg. 2006, 14, 337–340. [Google Scholar] [CrossRef]
- Mussoi, B.S.; Bentler, R.A. Binaural interference and the effects of age and hearing loss. J. Am. Acad. Audiol. 2017, 28, 005–013. [Google Scholar] [CrossRef]
- Corina, D.P.; Blau, S.; LaMarr, T.; Lawyer, L.A.; Coffey-Corina, S. Auditory and visual electrophysiology of deaf children with cochlear implants: Implications for cross-modal plasticity. Front. Psychol. 2017, 8, 59. [Google Scholar] [CrossRef]
- Glennon, E.; Svirsky, M.A.; Froemke, R.C. Auditory cortical plasticity in cochlear implant users. Curr. Opin. Neurobiol. 2020, 60, 108–114. [Google Scholar] [CrossRef]
- Zhu, C.; Gulati, J.; Swanson, D.; Shah, A.; Chisolm, P.; Hoa, M. A Systematic Review of the Incidence of Cochlear Nerve Deficiency in Pediatric Single-Sided Deafness. Otol. Neurotol. 2024, 46, 668–674. [Google Scholar] [CrossRef]
- Mankekar, G.; Holmes, S. Hearing Rehabilitation in Vestibular Schwannoma. Audiol. Res. 2023, 13, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Zong, F.; Ma, X.; Xu, X.; Chen, W.; Song, Z.; Han, X.; Wang, X.; Zhang, H. Cochlear implantation in patients with ossified cochleas. Am. J. Otolaryngol. 2019, 40, 183–186. [Google Scholar] [CrossRef]
- Holden, L.K.; Finley, C.C.; Firszt, J.B.; Holden, T.A.; Brenner, C.; Potts, L.G.; Gotter, B.D.; Vanderhoof, S.S.; Mispagel, K.; Heydebrand, G.; et al. Factors affecting open-set word recognition in adults with cochlear implants. Ear Hear. 2013, 34, 342–360. [Google Scholar] [CrossRef] [PubMed]
- Nadol, J. B, Jr. Patterns of neural degeneration in the human cochlea and auditory nerve: Implications for cochlear implantation. Otolaryngol.-Head. Neck Surg. 1997, 117, 220–228. [Google Scholar] [CrossRef]
- Jiam, N.T.; Limb, C. Music perception and training for pediatric cochlear implant users. Expert. Rev. Med. Devices 2020, 17, 1193–1206. [Google Scholar] [CrossRef] [PubMed]
- Levitin, D.J. The music instinct. In This is Your Brain on Music: The Science of Human Obsession; Dutton: New York, NY, USA, 2006; pp. 240–261. [Google Scholar]
- Limb, C.J.; Roy, A.T. Technological, biological, and acoustical constraints to music perception in cochlear implant users. Hear. Res. 2014, 308, 13–26. [Google Scholar] [CrossRef]
- McDermott, H.J. Music perception with cochlear implants: A review. Trends Amplif. 2004, 8, 49–82. [Google Scholar] [CrossRef] [PubMed]
- Lassaletta, L.; Castro, A.; Bastarrica, M.; Pérez-Mora, R.; Madero, R.; De Sarriá, J.; Gavilán, J. Does music perception have an impact on quality of life following cochlear implantation? Acta Oto-Laryngol. 2007, 127, 682–686. [Google Scholar] [CrossRef]
- Gfeller, K.; Turner, C.; Oleson, J.; Zhang, X.; Gantz, B.; Froman, R.; Olszewski, C. Accuracy of cochlear implant recipients on pitch perception, melody recognition, and speech reception in noise. Ear Hear. 2007, 28, 412–423. [Google Scholar] [CrossRef]
- Mutlu, B.; Topçu, M.T.; Yüksel, M.; Kalcıoğlu, M.T. Evaluation of the effect of musical perception activities on speech perception in adult Cochlear implant users. Turk. Arch. Otorhinolaryngol. 2023, 60, 188. [Google Scholar] [CrossRef]
- Looi, V.; She, J. Music perception of cochlear implant users: A questionnaire, and its implications for a music training program. Int. J. Audiol. 2010, 49, 116–128. [Google Scholar] [CrossRef]
- Galvin, J.J.I.; Fu, Q.-J.; Nogaki, G. Melodic contour identification by cochlear implant listeners. Ear Hear. 2007, 28, 302–319. [Google Scholar] [CrossRef] [PubMed]
- Ab Shukor, N.F.; Seo, Y.J.; Han, W. Meta-analysis exploring the effects of music training in cochlear implant users by age. J. Audiol. Otol. 2023, 27, 193. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.-J.; GalvinIII, J.J.; Wang, X.; Wu, J.-L. Benefits of music training in Mandarin-speaking pediatric cochlear implant users. J. Speech Lang. Hear. Res. 2015, 58, 163–169. [Google Scholar] [CrossRef]
- Slater, J.; Skoe, E.; Strait, D.L.; O’cOnnell, S.; Thompson, E.; Kraus, N. Music training improves speech-in-noise perception: Longitudinal evidence from a community-based music program. Behav. Brain Res. 2015, 291, 244–252. [Google Scholar] [CrossRef]
- Mellor, J.C.; Stone, M.A.; Keane, J. Application of data mining to “big data” acquired in audiology: Principles and potential. Trends Hear. 2018, 22, 2331216518776817. [Google Scholar] [CrossRef]
- Gaylor, J.M.; Raman, G.; Chung, M.; Lee, J.; Rao, M.; Lau, J.; Poe, D.S. Cochlear implantation in adults: A systematic review and meta-analysis. JAMA Otolaryngol.–Head. Neck Surg. 2013, 139, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Boisvert, I.; Reis, M.; Au, A.; Cowan, R.; Dowell, R.C. Cochlear implantation outcomes in adults: A scoping review. PLoS ONE 2020, 15, e0232421. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.T.; Chong, D.S.; Sun, C.; Mohapatra, N.; Jiam, N.T. Machine-Learning Predictions of Cochlear Implant Functional Outcomes: A Systematic Review. Ear Hear. 2025, 46, 952–962. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.P.S.; Mishra, S.K.; Diaz, P.A.A. A systematic review of machine learning approaches in cochlear implant outcomes. Npj Digit. Med. 2025, 8, 1–12. [Google Scholar] [CrossRef]
- Miller, S.E.; Grisel, J.J.; Schafer, E. The Auditory Implant Initiative. Audiol. Today 2019, 31, 30–38. [Google Scholar]
- American Academy of Otolaryngology-Head and Neck Surgery. Reg-ent℠|Research. 2024. Available online: https://www.entnet.org/quality-practice/reg-ent-clinical-data-registry/reg-ent-research/ (accessed on 20 July 2025).
- Riley, R.D.; Ensor, J.; I E Snell, K.; Archer, L.; Whittle, R.; Dhiman, P.; Alderman, J.; Liu, X.; Kirton, L.; Manson-Whitton, J.; et al. Importance of sample size on the quality and utility of AI-based prediction models for healthcare. Lancet Digit. Health 2025, 7, 100857. [Google Scholar] [CrossRef]
- Zhang, S.; Bamakan, S.M.H.; Qu, Q.; Li, S. Learning for personalized medicine: A comprehensive review from a deep learning perspective. IEEE Rev. Biomed. Eng. 2018, 12, 194–208. [Google Scholar] [CrossRef]
- Crowson, M.G.; Lin, V.; Chen, J.M.; Chan, T.C.Y. Machine learning and cochlear implantation—A structured review of opportunities and challenges. Otol. Neurotol. 2020, 41, e36–e45. [Google Scholar] [CrossRef]
- Ramos-Miguel, A.; Perez-Zaballos, T.; Perez, D.; Falconb, J.C.; Ramosb, A. Use of data mining to predict significant factors and benefits of bilateral cochlear implantation. Eur. Arch. Oto-Rhino-Laryngol. 2015, 272, 3157–3162. [Google Scholar] [CrossRef]
- Tan, L.; Holland, S.K.; Deshpande, A.K.; Chen, Y.; Choo, D.I.; Lu, L.J. A semi-supervised support vector machine model for predicting the language outcomes following cochlear implantation based on pre-implant brain fMRI imaging. Brain Behav. 2015, 5, e00391. [Google Scholar] [CrossRef]
- Shew, M.A.; Pavelchek, C.; Michelson, A.; Ortmann, A.; Lefler, S.; Walia, A.; Durakovic, N.; Phillips, A.; Rejepova, A.; Herzog, J.A.; et al. Machine Learning Feasibility in Cochlear Implant Speech Perception Outcomes—Moving Beyond Single Biomarkers for Cochlear Implant Performance Prediction. Ear Hear. 2025, 46, 1266–1281. [Google Scholar] [CrossRef]
- Shafieibavani, E.; Goudey, B.; Kiral, I.; Zhong, P.; Jimeno-Yepes, A.; Swan, A.; Gambhir, M.; Buechner, A.; Kludt, E.; Eikelboom, R.H.; et al. Predictive models for cochlear implant outcomes: Performance, generalizability, and the impact of cohort size. Trends Hear. 2021, 25, 23312165211066174. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, Y.; Noble, J.H.; Dawant, B.M. Localizing landmark sets in head CTs using random forests and a heuristic search algorithm for registration initialization. J. Med. Imaging 2017, 4, 044007. [Google Scholar] [CrossRef] [PubMed]
- Reda, F.A.; McRackan, T.R.; Labadie, R.F.; Dawant, B.M.; Noble, J.H. Automatic segmentation of intra-cochlear anatomy in post-implantation CT of unilateral cochlear implant recipients. Med. Image Anal. 2014, 18, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Choi, C. Shape optimization of cochlear implant electrode array using genetic algorithms. In Proceedings of the 23rd Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Istanbul, Turkey, 25–28 October 2001. [Google Scholar][Green Version]
- Gao, X.; Grayden, D.B.; McDonnell, M.D. Modeling electrode place discrimination in cochlear implant stimulation. IEEE Trans. Biomed. Eng. 2016, 64, 2219–2229. [Google Scholar] [CrossRef]
- Dhanasingh, A.; Nielsen, S.B.; Beal, F.; Schilp, S.; Hessler, R.; Jolly, C.; Hochmair, I. Cochlear implant electrode design for safe and effective treatment. Front. Neurol. 2024, 15, 1348439. [Google Scholar] [CrossRef]
- Alohali, Y.A.; Fayed, M.S.; Abdelsamad, Y.; Almuhawas, F.; Alahmadi, A.; Mesallam, T.; Hagr, A. Machine learning and cochlear implantation: Predicting the post-operative electrode impedances. Electronics 2023, 12, 2720. [Google Scholar] [CrossRef]
- Essaid, B.; Kheddar, H.; Batel, N.; Chowdhury, M.E.H.; Lakas, A. Artificial intelligence for cochlear implants: Review of strategies, challenges, and perspectives. IEEE Access 2024. [CrossRef]
- Chen, J.; Wang, Y.; Yoho, S.E.; Wang, D.; Healy, E.W. Large-scale training to increase speech intelligibility for hearing-impaired listeners in novel noises. J. Acoust. Soc. Am. 2016, 139, 2604–2612. [Google Scholar] [CrossRef]
- Healy, E.W.; Yoho, S.E.; Chen, J.; Wang, Y.; Wang, D. An algorithm to increase speech intelligibility for hearing-impaired listeners in novel segments of the same noise type. J. Acoust. Soc. Am. 2015, 138, 1660–1669. [Google Scholar] [CrossRef]
- Borjigin, A.; Kokkinakis, K.; Bharadwaj, H.M.; Stohl, J.S. Overcoming Limitations: Deep Neural Network Models Show Promise in Reducing Multi-Talker Noise Interference for Cochlear Implant Listeners. Sci Rep 2024, 14, 13241. [Google Scholar] [CrossRef]
- Demyanchuk, A.; Kludt, E.; Lenarz, T.; Büchner, A. A Machine Learning Model to Predict Postoperative Speech Recognition Outcomes in Cochlear Implant Recipients: Development, Validation, and Comparison with Expert Clinical Judgment. J. Clin. Med. 2025, 14, 3625. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, F.; Cazzador, D.; Gazzola, F.; Cassarà, A.; Ariano, M.; Colombo, A.; Franchella, S.; Trevisi, P.; de Filippis, C.; Marioni, G.; et al. Remote Check as a tele-health instrument for cochlear implant recipients: Analysis of impact and feasibility of application. Am. J. Otolaryngol. 2024, 45, 104294. [Google Scholar] [CrossRef] [PubMed]


| Etiology | Definition |
|---|---|
| Idiopathic SNHL | Cause unknown. May occur suddenly. |
| Chronic Otitis Media [13] | Persistent or recurrent inflammation and/or infection of the middle ear and mastoid cavity. May cause cholesteatoma: the abnormal growth of keratinizing squamous epithelium in the ear, leading to progressive bone erosion. |
| Cerebellopontine angle (CPA) tumor [14] | Tumor located at the CPA, the anatomical region at the junction of the cerebellum, pons, and medulla in the posterior fossa (posterior and inferior portion of the brain). Includes vestibular schwannoma, a benign tumor arising from the Schwann cells of CN VIII, which controls both balance and auditory function. |
| Perilymphatic fistula [15] | An abnormal connection between the fluid-filled inner ear and the air-filled middle ear or mastoid cavity, causing leakage of perilymphatic fluid out of the inner ear. |
| Cochlear nerve deficiency [16] | Having a cochlear nerve (the auditory branch of CN VIII) either smaller in diameter than normal (hypoplasia) or completely absent (aplasia). |
| Viral infections | Mumps virus [17]: causes sudden hearing loss by direct viral invasion and subsequent damage to cochlear structures. Congenital cytomegalovirus [18]: transmitted from mother to fetus through the placenta during pregnancy. Causes hearing loss by directly damaging cells and immune-mediated injury to the cochlea and auditory pathway. |
| Auditory neuropathy spectrum disorder [19] | Hearing loss caused by impaired transmission of sound from the inner ear to the brain, despite intact function of the outer hair cells in the cochlea. Described as “being able to hear but not understand.” |
| Authors | Study Type | Population (n) | Key Findings |
|---|---|---|---|
| Zeitler et al., 2019 [4] | Retrospective cohort study examining pre- and post-op auditory performance and device usage. | Children (9) | (a) Word recognition scores (CNC, MLNT), sentence testing (AzBio, HINT), and bimodal speech reception thresholds all improved after receiving the CI. (b) 8 (88.9%) children were full-time users of their device; the 1 non-user was congenitally deaf but implanted at age 9.5, thus having a long duration of deafness. |
| Rauch et al., 2019 [38] | Retrospective cohort study comparing CI users with SSD (SSD-CI) to bilaterally deaf individuals using unilateral (Uni-CI) or bilateral CI (Bil-CI) | Children and Adults (206): - SSD-CI (27) - Uni-CI (114) - Bil-CI (65) | (a) SSD-CI of all age groups used their device on average for 8.07 h/day, which was less than both Uni-CI (10.82) and Bil-CI (10.60). Differences between groups were only significant in the working age group (ages 18 to 65). (b) CI use time was similar between age groups within SSD-CI but not within Uni-CI or Bil-CI. (c) The auditory environments in which CIs were activated were overall similar between all groups, but adult SSD-CI used their CIs less than the Uni-CI and Bil-CI groups in quiet environments. |
| Tavora-Viera et al., 2020 [39] | Retrospective cohort study investigating CI non-use. | Adults (114) | (a) 5 (4.4%) individuals became elective non-users (defined as completely stopping, or refusing re-implantation if needed), with mean time before discontinuation of 11.5 months (range 1.5 to 60 months). (b) Reasons included: distorted sound quality and poor speech understanding through the CI, perceived irrelevance of the CI, unmet expectations of CI benefit, and unwillingness to participate in rehabilitation sessions leading to lack of improvement. |
| Lindquist et al., 2023 [40] | Retrospective case series examining pre- and post-op auditory performance and CI usage behavior. | Adults (66) | (a) Speech performance scores were significantly higher after CI surgery, peaking at 6 months post-activation. (b) 34 patients (51.5%) had a follow-up appointment with their audiologist after 12 months post-implant. (c) Average daily wear time (mean 8.0 h/day, SD 4.6 h/day) was positively associated with post-operative CNC, AzBio, and CIQoL-10 scores. (d) 9 patients (14%) became non-users or were explanted at last contact. Reasons included: magnet retention issues, lack of perceived benefit in hearing or tinnitus reduction, infection/dehiscence, and magnet pain during MRI scans. |
| Speck et al., 2021 [41] | Prospective cohort study examining pre- and post-op auditory performance and long-term CI usage behavior | Adults (78): - SSD (41) - AHL (37) | (a) CI improved speech recognition in noise, sound localization, and subjective speech intelligibility and spatial hearing in AHL and SSD. (b) Long-term data (≥5 yrs post-implant) from 76 participants demonstrated CI wear time of 6 to 10 h/day (median: 8 h). (c) 4 (9.8%) individuals in the SSD cohort were elective non-users. Reasons included: CI did not improve speech comprehension, fear of contaminating the device at work, lack of practice with the CI, and lack of subjective benefit. |
| Muigg et al., 2020 [42] | Prospective cohort study examining hearing-related QoL (HRQoL) measures pre- and up to 2 years post-implantation. | Adults (20) | (a) Cochlear implantation was associated with increased hearing-specific and generic HRQoL among SSD patients within the first 6 months. (b) 2 (10%) individuals discontinued CI use at 12 and 20 months after activation due to perceived poor benefit from lack of adaptation to the device or unmet expectations. Both had long-term SSD (>10 years). |
| Macielak et al., 2024 [43] | Retrospective cohort study on pre- and post-op audiometric performance and CI usage behavior. | Children (66) | (a) 12 (18%) patients were eventually lost to follow-up. (b) At the last evaluation, only 10 (19%) of the 54 remaining patients were users, 13 (24%) were limited users (>2 but <6 h/day), and 28 (52%) were non-users (≤2 h/day). (c) There was no association between usage and duration of deafness or age of implantation. |
| Tan et al., 2024 [44] | Retrospective cohort study examining CI usage behavior at 12 and 24 months post-op. | Adults (54): - 12-month follow-up (54) - 24-month follow-up (38) | (a) Mean CI usage was 8.2 h/day (SD 4.2) at 12 months, and 7.0 h/day (SD 5.1) at 24 months post-cochlear implantation. (b) At 12 months, 5 (9.3%) out of 54 individuals were non-users (<1 h/day), while 7 out of 38 (18.4%) were non-users at 24 months—a 9.1% increase in non-use. (c) 15 (27.8%) individuals missed their 24-month follow-up appointment, while 1 was explanted due to worsening tinnitus, not finding the CI useful, and magnet retention issues. |
| Smith et al., 2024 [50] | Retrospective cohort study examining pre- and post-op auditory performance and CI usage behavior. | Adults (12): - SSD (2) - AHL (10) | (a) Improvements in PTA, CNC word and phoneme, and AZBio scores were significant after implantation. (b) Mean daily CI use was 9.3 h/day (SD 3.3). |
| Sullivan et al., 2020 [71] | Retrospective cohort study examining pre- and post-op auditory performance and CI usage behavior. | Adults (60) | (a) Improvements in speech understanding, sound localization, and quality of life were found post-implant. (b) 4 subjects became non-users of their CI due to poor device performance after activation. (b) 41.7% (25) of the initial population were lost to follow up by 24 months, 68.3% (41) at 36 months, 76.7% (46) at 48 months, 83.3% (50) at 60 months, and 95% (57) at 72 months post-implant. |
| Deep et al., 2021 [176] | Retrospective case series examining pre- and post-op auditory performance and CI usage behavior. | Adults (53) | (a) Speech perception in both quiet and noise scores and tinnitus suppression improved significantly after CI. (b) Average daily CI usage (n = 47) was 8.3 h/day (SD 3.5; range 1.5–14). 3 subjects were limited users (<4 h/day) due to difficulty adjusting to the signal. (c) 2 individuals became non-users (reasons included other comorbidities and lack of benefit due to cochlear ossification). |
| Tan et al., 2022 [177] | Prospective single-arm study comparing pre- and 1-year post-operative auditory performance, and CI usage behavior 4 years post-op. | Adults (8) | (a) PTA in the deaf ear improved significantly after implantation, with post-operative median reaching near-normal levels of 30 dB. (b) 5 (62%) discontinued CI use within 4 years post-implantation. One patient’s reason was the CI worsened his tinnitus, while another found that his tinnitus improved post-surgery even without the CI on and thus perceived no benefit from the CI. Other reasons included follow-up cost concerns and speech through CI sounding distorted. |
| Ganek et al., 2020 [178] | Retrospective cohort study examining CI datalogging sessions. | Children (23) | (a) Average CI wear time was 6.22 h/day (SD 2.81, range 0.0004 to 14.74). (b) There was no association between wear time and increasing age or hearing experience. |
| Arras et al., 2022 [179] | Prospective cohort study comparing auditory performance and CI usage behavior of early implanted SSD (SSD + CI) with non-implanted SSD (SSD + NoCI) and NH individuals. | Children (47): - SSD + CI (12) - SSD + NoCI (9) - NH (26) | (a) Prelingual (under the age of 2.5) implantation of children with SSD significantly improves speech understanding in noise and sound localization ability. (b) SSD + CI children wore their speech processor on average 8.9 h/day (SD 2.7, range 2.9 to 12.2). (c) At the end of the study, 8 (75%) of the SSD + CI group were considered regular users (≥8 h/day), 3 were limited users (<8 h/day), and 1 became a non-user due to lack of perceived benefit (though objectively, his scores showed otherwise) and insufficient family support. |
| Benchetrit et al., 2021 [180] | Systematic review and meta-analysis of 12 studies examining auditory performance and CI usage rates. | Children (119) | (a) Speech perception in noise and quiet and sound localization improved in the majority if children after receiving the CI. (b) 11 studies (101 children) reported device usage metrics: 75 (74.3%) children used their CI regularly, 21 (20.8%) were limited users, and 5 (4.9%) were nonusers. (c) Nonusers had longer duration of deafness and greater average age of implantation compared to both limited and regular users. |
| Polonenko et al., 2017 [181] | Retrospective cohort study examining CI usage behavior. | Children (7) | (a) Average daily CI use was 7.4 h/day (SD 1.7). (b) Older children had longer average wear times than younger children; a 10% increase in age correlated with a 0.24 h increase in device usage. (c) CIs were used most often in environments that were moderately loud (50–70 dB) or contained speech in noise. |
| Ramos-Macías et al., 2019 [182] | Prospective cohort study examining pre- and post-op auditory performance and CI usage behavior. | Children (23) with different etiologies: - Congenital SSD (4) - Acquired SSD (19) | (a) CI improved sound lateralization in both groups and speech recognition among the acquired SSD group (as individuals in the congenital SSD group were too young to be tested). (b) Increased binaural benefit from CI was associated with post-lingual deafness and shorter duration of deafness. (c) CI wear time ranged from 6–12 to 10–16 h/day for the congenital and acquired SSD groups, respectively. |
| Thomas et al., 2017 [183] | Retrospective cohort study examining binaural hearing measures and subjective benefit. | Children (21) | (a) Speech recognition in noise and sound lateralization ability were significantly better in the CI-aided condition compared to unaided. (b) 3 (60%) of 5 subjects with follow-up ≥3 years became limited users or nonusers of CI, reasons being poor hearing benefit or social stigmatization. |
| Greaver et al., 2017 [184] | Prospective cohort study examining pre- and post-op auditory performance and device usage. | Children (5): - SSD (1) - AHL (4) | (a) Patients were able to achieve speech recognition through their implanted ear even when the better hearing ear was occluded. (b) CI datalogs collected at the most recent follow-up appointment revealed 3 (60%) full-time CI users (≥8 h/day) and 2 (40%) limited CI users (<4 h/day, mostly at school). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, E.Y.; Younan, S.M.; Barrett, K.C.; Jiam, N.T. Personalizing Cochlear Implant Care in Single-Sided Deafness: A Distinct Paradigm from Bilateral Hearing Loss. J. Pers. Med. 2025, 15, 439. https://doi.org/10.3390/jpm15090439
Lin EY, Younan SM, Barrett KC, Jiam NT. Personalizing Cochlear Implant Care in Single-Sided Deafness: A Distinct Paradigm from Bilateral Hearing Loss. Journal of Personalized Medicine. 2025; 15(9):439. https://doi.org/10.3390/jpm15090439
Chicago/Turabian StyleLin, Emmeline Y., Stephanie M. Younan, Karen C. Barrett, and Nicole T. Jiam. 2025. "Personalizing Cochlear Implant Care in Single-Sided Deafness: A Distinct Paradigm from Bilateral Hearing Loss" Journal of Personalized Medicine 15, no. 9: 439. https://doi.org/10.3390/jpm15090439
APA StyleLin, E. Y., Younan, S. M., Barrett, K. C., & Jiam, N. T. (2025). Personalizing Cochlear Implant Care in Single-Sided Deafness: A Distinct Paradigm from Bilateral Hearing Loss. Journal of Personalized Medicine, 15(9), 439. https://doi.org/10.3390/jpm15090439






