The Precision Paradigm in Periodontology: A Multilevel Framework for Tailored Diagnosis, Treatment, and Prevention
Abstract
1. Introduction
1.1. Precision Diagnosis
1.2. Personalized Treatment
1.3. Individualized Prevention
1.4. Integration of Digital Tools and Systems Biology
1.5. Educational and Clinical Workflow Adaptation
- -
- Detect PD earlier and more accurately via sensitive BMs;
- -
- Customize treatment according to patient-specific characteristics and biofilm susceptibility;
- -
- Drive preventive strategies based on individualized risk profiles and behavioral insights;
- -
- Improve clinician–patient engagement using clear risk communication and educational tools;
- -
- Enhance efficiency and sustainability of care by optimizing recall intervals and resource allocation.
2. Materials and Methods
2.1. Protocol and Registration
2.2. Search Processing
2.3. Inclusion Criteria
- Participants: Patients with periodontal diseases.
- Interventions: Precision medicine approaches (personalized diagnostics, treatments, and prevention).
- Comparisons: Standard periodontal care.
- Outcomes: Diagnostic accuracy, treatment effectiveness, and prevention efficacy.
- Study: Clinical studies (RCTs, cohort, case–control, cross-sectional, experimental, and bioinformatic).
2.4. Exclusion Criteria
2.5. Data Processing
2.6. Quality Assessment
- -
- Confounding bias;
- -
- Bias resulting from exposure measurement;
- -
- Bias in a study’s participant selection;
- -
- Bias resulting from post-exposure intervention;
- -
- Bias resulting from missing data;
- -
- Bias resulting from outcome measurement,
- -
- Bias in the presentation of the results.
3. Results
3.1. Study Selection
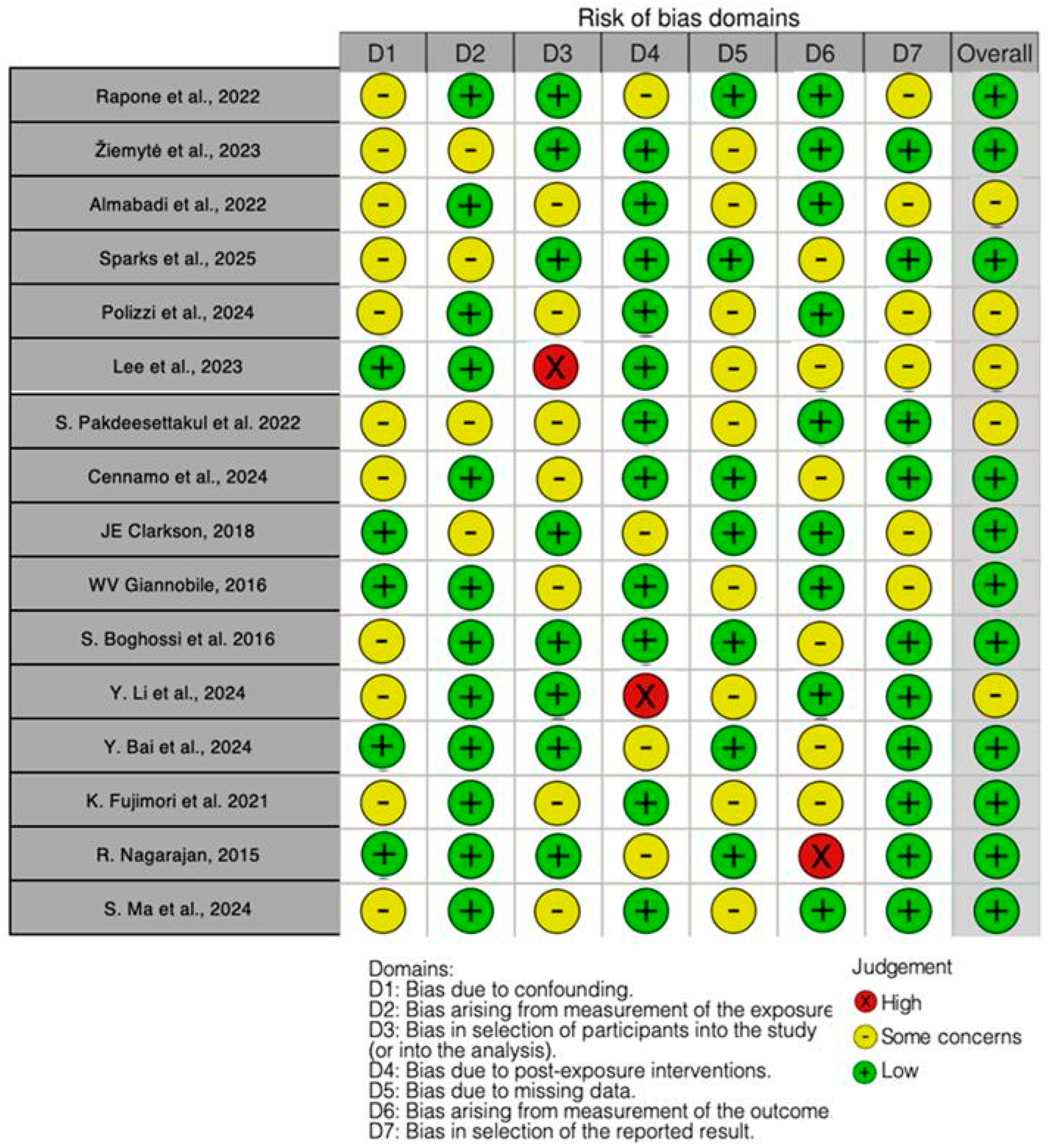
3.2. Quality Assessment and Risk of Bias of Included Articles
4. Discussion
4.1. Advances and Integration of Precision Medicine Approaches in Periodontology
4.2. Integrating Precision Diagnostics into Periodontal Practice: Clinical Tools and Molecular Innovations
4.3. Implementing Precision Prevention in Periodontology: Evidence for Risk-Based and Personalized Care Strategies
4.4. Multi-Omics Discovery of Immune and Genetic Biomarkers in Periodontitis
5. Strengths and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HR | Host response |
| DEGs | Differentially Expressed Genes |
| EDR | Electronic dental records |
| GCF | Gingival crevicular fluid |
| PD | Periodontal disease |
| PM | Precision medicine |
| PP | Precision periodontics |
| PPHCC | Precision Periodontal Health Care Chart |
| PPRA | Periodontal Risk Assessment |
| ROC | Receiver operating characteristic |
| SPR-POF | Surface plasmon resonance–plastic optical fiber |
| WGCNA | Weighted gene co-expression network analysis |
References
- Armitage, G.C. Development of a Classification System for Periodontal Diseases and Conditions. Northwest Dent. 2000, 79, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Belstrøm, D.; Grande, M.A.; Sembler-Møller, M.L.; Kirkby, N.; Cotton, S.L.; Paster, B.J.; Holmstrup, P. Influence of Periodontal Treatment on Subgingival and Salivary Microbiotas. J. Periodontol. 2018, 89, 531–539. [Google Scholar] [CrossRef]
- Buduneli, N.; Kinane, D.F. Host-Derived Diagnostic Markers Related to Soft Tissue Destruction and Bone Degradation in Periodontitis. J. Clin. Periodontol. 2011, 38 (Suppl. S11), 85–105. [Google Scholar] [CrossRef]
- Janakiram, C.; Dye, B.A. A Public Health Approach for Prevention of Periodontal Disease. Periodontology 2000 2020, 84, 202–214. [Google Scholar] [CrossRef]
- Kwon, T.; Lamster, I.B.; Levin, L. Current Concepts in the Management of Periodontitis. Int. Dent. J. 2021, 71, 462–476. [Google Scholar] [CrossRef] [PubMed]
- Morgan, X.C.; Huttenhower, C. Chapter 12: Human Microbiome Analysis. PLoS Comput. Biol. 2012, 8, e1002808. [Google Scholar] [CrossRef]
- Bostanci, N.; Belibasakis, G.N. Precision Periodontal Care: From Omics Discoveries to Chairside Diagnostics. Clin. Oral Investig. 2023, 27, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Ali Alftaikhah, S.A.; Issrani, R.; Alnasser, M.; Almutairi, H.A.; Khattak, O.; Iqbal, A.; Prabhu, N. Salivary Biomarkers in Periodontitis: A Scoping Review. Cureus 2023, 15, e50207. [Google Scholar] [CrossRef]
- Giannobile, W.V. Salivary Diagnostics for Periodontal Diseases. J. Am. Dent. Assoc. 2012, 143, 6S–11S. [Google Scholar] [CrossRef]
- Trindade, D.; Carvalho, R.; Machado, V.; Chambrone, L.; Mendes, J.J.; Botelho, J. Prevalence of Periodontitis in Dentate People between 2011 and 2020: A Systematic Review and Meta-Analysis of Epidemiological Studies. J. Clin. Periodontol. 2023, 50, 604–626. [Google Scholar] [CrossRef]
- Heitz-Mayfield, L.J.A. Conventional Diagnostic Criteria for Periodontal Diseases (Plaque-Induced Gingivitis and Periodontitis). Periodontology 2000 2024, 95, 10–19. [Google Scholar] [CrossRef]
- Chen, C.; Hemme, C.; Beleno, J.; Shi, Z.J.; Ning, D.; Qin, Y.; Tu, Q.; Jorgensen, M.; He, Z.; Wu, L.; et al. Oral Microbiota of Periodontal Health and Disease and Their Changes after Nonsurgical Periodontal Therapy. ISME J. 2018, 12, 1210–1224. [Google Scholar] [CrossRef] [PubMed]
- Gardner, A.; Carpenter, G.; So, P.-W. Salivary Metabolomics: From Diagnostic Biomarker Discovery to Investigating Biological Function. Metabolites 2020, 10, 47. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and Grading of Periodontitis: Framework and Proposal of a New Classification and Case Definition. J. Periodontol. 2018, 89 (Suppl. S1), S159–S172. [Google Scholar] [CrossRef]
- Furlaneto, F.; Ishikawa, K.H.; Messora, M.R.; Mayer, M.P.A. Probiotics During the Therapeutic Management of Periodontitis. Adv. Exp. Med. Biol. 2022, 1373, 353–375. [Google Scholar] [CrossRef]
- Niemczyk, W.; Janik, K.; Żurek, J.; Skaba, D.; Wiench, R. Platelet-Rich Plasma (PRP) and Injectable Platelet-Rich Fibrin (i-PRF) in the Non-Surgical Treatment of Periodontitis—A Systematic Review. Int. J. Mol. Sci. 2024, 25, 6319. [Google Scholar] [CrossRef] [PubMed]
- Glurich, I.; Acharya, A.; Brilliant, M.H.; Shukla, S.K. Progress in Oral Personalized Medicine: Contribution of “Omics”. J. Oral Microbiol. 2015, 7, 28223. [Google Scholar] [CrossRef]
- Lavigne, S.E. The Oral Microbiome and Precision Medicine: A Peek into the Future of Periodontal Diagnostics. Can. J. Dent. Hyg. 2019, 53, 83–85. [Google Scholar]
- Fuentes, L.; Yakob, M.; Wong, D.T.W. Emerging Horizons of Salivary Diagnostics for Periodontal Disease. Br. Dent. J. 2014, 217, 567–573. [Google Scholar] [CrossRef]
- Hirtz, C.; O’Flynn, R.; Voisin, P.M.; Deville de Périère, D.; Lehmann, S.; Guedes, S.; Amado, F.; Ferreira, R.; Trindade, F.; Vitorino, R. The Potential Impact of Salivary Peptides in Periodontitis. Crit. Rev. Clin. Lab. Sci. 2021, 58, 479–492. [Google Scholar] [CrossRef] [PubMed]
- Haas, A.N.; Furlaneto, F.; Gaio, E.J.; Gomes, S.C.; Palioto, D.B.; Castilho, R.M.; Sanz, M.; Messora, M.R. New Tendencies in Non-Surgical Periodontal Therapy. Braz. Oral Res. 2021, 35, e095. [Google Scholar] [CrossRef]
- Lorusso, F.; Tartaglia, G.; Inchingolo, F.; Scarano, A. Early Response and Clinical Efficacy of a Mouthwash Containing Chlorhexidine, Anti Discoloration System, Polyvinylpyrrolidone/Vinyl Acetate and Sodium DNA in Periodontitis Model: A Triple-Blind Randomized Controlled Clinical Trial. Dent. J. 2022, 10, 101. [Google Scholar] [CrossRef] [PubMed]
- Bartold, P.M. Lifestyle and Periodontitis: The Emergence of Personalized Periodontics. Periodontology 2000 2018, 78, 7–11. [Google Scholar] [CrossRef]
- Rakic, M.; Pejcic, N.; Perunovic, N.; Vojvodic, D. A Roadmap towards Precision Periodontics. Medicina 2021, 57, 233. [Google Scholar] [CrossRef] [PubMed]
- Duenas, S.; McGee, Z.; Mhatre, I.; Mayilvahanan, K.; Patel, K.K.; Abdelhalim, H.; Jayprakash, A.; Wasif, U.; Nwankwo, O.; Degroat, W.; et al. Computational Approaches to Investigate the Relationship between Periodontitis and Cardiovascular Diseases for Precision Medicine. Hum. Genom. 2024, 18, 116. [Google Scholar] [CrossRef] [PubMed]
- Ghallab, N.A. Diagnostic Potential and Future Directions of Biomarkers in Gingival Crevicular Fluid and Saliva of Periodontal Diseases: Review of the Current Evidence. Arch. Oral Biol. 2018, 87, 115–124. [Google Scholar] [CrossRef]
- Cobb, C.M.; Sottosanti, J.S. A Re-Evaluation of Scaling and Root Planing. J. Periodontol. 2021, 92, 1370–1378. [Google Scholar] [CrossRef]
- Jepsen, K.; Sculean, A.; Jepsen, S. Complications and Treatment Errors Related to Regenerative Periodontal Surgery. Periodontology 2000 2023, 92, 120–134. [Google Scholar] [CrossRef]
- Theodoro, L.H.; Marcantonio, R.A.C.; Wainwright, M.; Garcia, V.G. LASER in Periodontal Treatment: Is It an Effective Treatment or Science Fiction? Braz. Oral Res. 2021, 35, e099. [Google Scholar] [CrossRef]
- Rapone, B.; Inchingolo, F.; Tartaglia, G.M.; De Francesco, M.; Ferrara, E. Asymmetric Dimethylarginine as a Potential Mediator in the Association between Periodontitis and Cardiovascular Disease: A Systematic Review of Current Evidence. Dent. J. 2024, 12, 297. [Google Scholar] [CrossRef]
- Malcangi, G.; Patano, A.; Guglielmo, M.; Sardano, R.; Palmieri, G.; Di Pede, C.; de Ruvo, E.; Inchingolo, A.D.; Mancini, A.; Inchingolo, F.; et al. Precision Medicine in Oral Health and Diseases: A Systematic Review. J. Pers. Med. 2023, 13, 725. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, A.M.; Malcangi, G.; Piras, F.; Palmieri, G.; Settanni, V.; Riccaldo, L.; Morolla, R.; Buongiorno, S.; de Ruvo, E.; Inchingolo, A.D.; et al. Precision Medicine on the Effects of Microbiota on Head-Neck Diseases and Biomarkers Diagnosis. J. Pers. Med. 2023, 13, 933. [Google Scholar] [CrossRef]
- Lamster, I.B.; Ahlo, J.K. Analysis of Gingival Crevicular Fluid as Applied to the Diagnosis of Oral and Systemic Diseases. Ann. N. Y. Acad. Sci. 2007, 1098, 216–229. [Google Scholar] [CrossRef]
- Pitchika, V.; Büttner, M.; Schwendicke, F. Artificial Intelligence and Personalized Diagnostics in Periodontology: A Narrative Review. Periodontology 2000 2024, 95, 220–231. [Google Scholar] [CrossRef]
- Polverini, P.J. Personalized Medicine and the Future of Dental Practice. Pers. Med. 2018, 15, 449–451. [Google Scholar] [CrossRef]
- Alshihayb, T.S.; Sharma, P.; Dietrich, T.; Heaton, B. Exploring Periodontitis Misclassification Mechanisms under Partial-Mouth Protocols. J. Clin. Periodontol. 2022, 49, 448–457. [Google Scholar] [CrossRef]
- Fischer, R.G.; Gomes Filho, I.S.; Cruz, S.S.d.; Oliveira, V.B.; Lira-Junior, R.; Scannapieco, F.A.; Rego, R.O. What Is the Future of Periodontal Medicine? Braz. Oral Res. 2021, 35, e102. [Google Scholar] [CrossRef] [PubMed]
- Preianò, M.; Savino, R.; Villella, C.; Pelaia, C.; Terracciano, R. Gingival Crevicular Fluid Peptidome Profiling in Healthy and in Periodontal Diseases. Int. J. Mol. Sci. 2020, 21, 5270. [Google Scholar] [CrossRef] [PubMed]
- Sarakbi, R.M.; Varma, S.R.; Muthiah Annamma, L.; Sivaswamy, V. Implications of Artificial Intelligence in Periodontal Treatment Maintenance: A Scoping Review. Front. Oral Health 2025, 6, 1561128. [Google Scholar] [CrossRef]
- Teles, F.R.F.; Chandrasekaran, G.; Martin, L.; Patel, M.; Kallan, M.J.; Furquim, C.; Hamza, T.; Cucchiara, A.J.; Kantarci, A.; Urquhart, O.; et al. Salivary and Serum Inflammatory Biomarkers during Periodontitis Progression and after Treatment. J. Clin. Periodontol. 2024, 51, 1619–1631. [Google Scholar] [CrossRef]
- Teles, F.; Martin, L.; Patel, M.; Hu, W.; Bittinger, K.; Kallan, M.J.; Chandrasekaran, G.; Cucchiara, A.J.; Giannobile, W.V.; Stephens, D.; et al. Gingival Crevicular Fluid Biomarkers During Periodontitis Progression and After Periodontal Treatment. J. Clin. Periodontol. 2025, 52, 40–55. [Google Scholar] [CrossRef] [PubMed]
- Santonocito, S.; Polizzi, A.; Cavalcanti, R.; Ronsivalle, V.; Chaurasia, A.; Spagnuolo, G.; Isola, G. Impact of Laser Therapy on Periodontal and Peri-Implant Diseases. Photobiomodul. Photomed. Laser Surg. 2022, 40, 454–462. [Google Scholar]
- Kinney, J.S.; Morelli, T.; Oh, M.; Braun, T.M.; Ramseier, C.A.; Sugai, J.V.; Giannobile, W.V. Crevicular Fluid Biomarkers and Periodontal Disease Progression. J. Clin. Periodontol. 2014, 41, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Koidou, V.P.; Cavalli, N.; Hagi-Pavli, E.; Nibali, L.; Donos, N. Expression of Inflammatory Biomarkers and Growth Factors in Gingival Crevicular Fluid at Different Healing Intervals Following Non-Surgical Periodontal Treatment: A Systematic Review. J. Periodontal. Res. 2020, 55, 801–809. [Google Scholar] [CrossRef]
- Mendonça, C.D.d.; Mata, A.D.S.P.d.; Azevedo, L.F.R.; Marques, J.F.; Silveira, J.M.L.; Marques, D.N.d.S. Probiotics in the Non-Surgical Treatment of Periodontitis: A Systematic Review and Network Meta-Analysis. BMC Oral Health 2024, 24, 1224. [Google Scholar] [CrossRef]
- Moraes, R.M.; Schlagenhauf, U.; Anbinder, A.L. Outside the Limits of Bacterial Viability: Postbiotics in the Management of Periodontitis. Biochem. Pharmacol. 2022, 201, 115072. [Google Scholar] [CrossRef]
- Lorenzo-Erro, S.M.; Andrade, E.; Massa, F.; Colistro, V.; Asquino, N.; Moliterno, P. Periodontitis Prevalence and Associated Factors: A Comparison of Two Examination Protocols. Acta Odontol. Latinoam. 2022, 35, 178–187. [Google Scholar] [CrossRef]
- Amato, A. Personalized Oral and Dental Care. J. Pers. Med. 2023, 13, 110. [Google Scholar] [CrossRef]
- Kikuchi, T.; Hayashi, J.-I.; Mitani, A. Next-Generation Examination, Diagnosis, and Personalized Medicine in Periodontal Disease. J. Pers. Med. 2022, 12, 1743. [Google Scholar] [CrossRef]
- Gundelly, M.; Pusuluri, S.V.; Koduganti, R.R.; Ambati, M.; Chiluveru, S.; Chandaka, M. Precision Medicine in Periodontics: A Literature Review. Cureus 2024, 16, e68952. [Google Scholar] [CrossRef] [PubMed]
- Collins, F.S.; Varmus, H. A New Initiative on Precision Medicine. N. Engl. J. Med. 2015, 372, 793–795. [Google Scholar] [CrossRef] [PubMed]
- Bartold, P.M.; Ivanovski, S. P4 Medicine as a Model for Precision Periodontal Care. Clin. Oral Investig. 2022, 26, 5517–5533. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Guo, L.; Li, X.; Liu, S.; Du, J.; Xu, J.; Hu, J.; Liu, Y. Challenges and Tissue Engineering Strategies of Periodontal-Guided Tissue Regeneration. Tissue Eng. Part C Methods 2022, 28, 405–419. [Google Scholar] [CrossRef]
- Gomathi, G.D.; Gopalakrishnan, S.; Sudhakar, U. Personalised Medicine—A Recent Revolution in Periodontics. IJPI 2019, 4, 69–72. [Google Scholar] [CrossRef]
- Guzman, Y.A.; Sakellari, D.; Papadimitriou, K.; Floudas, C.A. High-Throughput Proteomic Analysis of Candidate Biomarker Changes in Gingival Crevicular Fluid after Treatment of Chronic Periodontitis. J. Periodontal. Res. 2018, 53, 853–860. [Google Scholar] [CrossRef]
- Rizal, M.I.; Soeroso, Y.; Sulijaya, B.; Assiddiq, B.F.; Bachtiar, E.W.; Bachtiar, B.M. Proteomics Approach for Biomarkers and Diagnosis of Periodontitis: Systematic Review. Heliyon 2020, 6, e04022. [Google Scholar] [CrossRef]
- Sánchez-Medrano, A.G.; Martinez-Martinez, R.E.; Soria-Guerra, R.; Portales-Perez, D.; Bach, H.; Martinez-Gutierrez, F. A Systematic Review of the Protein Composition of Whole Saliva in Subjects with Healthy Periodontium Compared with Chronic Periodontitis. PLoS ONE 2023, 18, e0286079. [Google Scholar] [CrossRef]
- Albeshri, S.; Greenstein, G. Efficacy of Nonsurgical Periodontal Therapy for Treatment of Periodontitis: Practical Application of Current Knowledge. Gen. Dent. 2022, 70, 12–19. [Google Scholar]
- Hashimoto, H.; Hashimoto, S.; Shimazaki, Y. Functional Impairment and Periodontitis in Rheumatoid Arthritis. Int. Dent. J. 2022, 72, 641–647. [Google Scholar] [CrossRef]
- Lundmark, A.; Hu, Y.O.O.; Huss, M.; Johannsen, G.; Andersson, A.F.; Yucel-Lindberg, T. Identification of Salivary Microbiota and Its Association With Host Inflammatory Mediators in Periodontitis. Front. Cell. Infect. Microbiol. 2019, 9, 216. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.-S.; Kook, J.-K.; Park, S.-N.; Lim, Y.K.; Choi, G.H.; Kim, S.; Ji, S. Salivary Microbiota Reflecting Changes in Subgingival Microbiota. Microbiol. Spectr. 2024, 12, e0103024. [Google Scholar] [CrossRef]
- Hartenbach, F.A.R.R.; Velasquez, É.; Nogueira, F.C.S.; Domont, G.B.; Ferreira, E.; Colombo, A.P.V. Proteomic Analysis of Whole Saliva in Chronic Periodontitis. J. Proteom. 2020, 213, 103602. [Google Scholar] [CrossRef] [PubMed]
- Slots, J. Life-Threatening Pathogens in Severe/Progressive Periodontitis: Focal Infection Risk, Future Periodontal Practice, Role of the Periodontology 2000. Periodontology 2000 2020, 84, 215–216. [Google Scholar] [CrossRef]
- Lin, S.-Y.; Sun, J.-S.; Lin, I.-P.; Hung, M.-C.; Chang, J.Z.-C. Efficacy of Adjunctive Local Periodontal Treatment for Type 2 Diabetes Mellitus Patients with Periodontitis: A Systematic Review and Network Meta-Analysis. J. Dent. 2024, 148, 105212. [Google Scholar] [CrossRef]
- Amarasena, N.; Gnanamanickam, E.S.; Miller, J. Effects of Interdental Cleaning Devices in Preventing Dental Caries and Periodontal Diseases: A Scoping Review. Aust. Dent. J. 2019, 64, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Isola, G. Early Biomarkers of Periodontitis: New Challenges for a Personalized Medicine Approach. Int. J. Environ. Res. Public Health 2021, 19, 251. [Google Scholar] [CrossRef] [PubMed]
- Isola, G. Advances in Biomarkers and Diagnostics in Periodontitis and Oral Diseases. Int. J. Environ. Res. Public Health 2021, 18, 1886. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.S.; Ding, X.; Dawson, D.R.; Ebersole, J.L. Salivary Biomarkers for Discriminating Periodontitis in the Presence of Diabetes. J. Clin. Periodontol. 2021, 48, 216–225. [Google Scholar] [CrossRef]
- Jiang, Y.; Feng, J.; Du, J.; Fu, J.; Liu, Y.; Guo, L.; Liu, Y. Clinical and Biochemical Effect of Laser as an Adjunct to Non-Surgical Treatment of Chronic Periodontitis. Oral Dis. 2022, 28, 1042–1057. [Google Scholar] [CrossRef]
- Chew, R.J.J.; Goh, C.E.; Sriram, G.; Preshaw, P.M.; Tan, K.S. Microbial Biomarkers as a Predictor of Periodontal Treatment Response: A Systematic Review. J. Periodontal Res. 2023, 58, 1113–1127. [Google Scholar] [CrossRef]
- Divaris, K.; Moss, K.; Beck, J.D. Biologically Informed Stratification of Periodontal Disease Holds the Key to Achieving Precision Oral Health. J. Periodontol. 2020, 91 (Suppl. S1), S50–S55. [Google Scholar] [CrossRef]
- Beck, J.D.; Philips, K.; Moss, K.; Divaris, K.; Morelli, T.; Offenbacher, S. Advances in Precision Oral Health. Periodontology 2000 2020, 82, 268–285. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, E.T.; de Araújo, J.S.M.; Pires, A.C.; Lira Dos Santos, E.J. Local Delivery Natural Products to Treat Periodontitis: A Systematic Review and Meta-Analysis. Clin. Oral Investig. 2021, 25, 4599–4619. [Google Scholar] [CrossRef]
- Benavides-Reyes, C.; Cabello, I.; Magán-Fernández, A.; Rodríguez-Barranco, M.; Usta, S.N.; Mesa, F. Clinical Effects of Probiotics on the Treatment of Gingivitis and Periodontitis: A Systematic Review and Meta-Analysis. BMC Oral Health 2025, 25, 490. [Google Scholar] [CrossRef]
- Li, J.; Zhao, G.; Zhang, H.M.; Zhu, F.F. Probiotic Adjuvant Treatment in Combination with Scaling and Root Planing in Chronic Periodontitis: A Systematic Review and Meta-Analysis. Benef. Microbes 2023, 14, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Mohammad-Rahimi, H.; Motamedian, S.R.; Pirayesh, Z.; Haiat, A.; Zahedrozegar, S.; Mahmoudinia, E.; Rohban, M.H.; Krois, J.; Lee, J.-H.; Schwendicke, F. Deep Learning in Periodontology and Oral Implantology: A Scoping Review. J. Periodontal Res. 2022, 57, 942–951. [Google Scholar] [CrossRef] [PubMed]
- Lyu, J.; Zhang, Y.; Zhou, R.; Ding, C.; Ye, H.; Fang, Q.; Jiang, C.; Chen, X.; Zhong, L. The Effect of Periodontal Treatments on Endothelial Function in Degrees of Periodontitis Patients: A Systematic Review and Meta-Analysis. PLoS ONE 2024, 19, e0308793. [Google Scholar] [CrossRef] [PubMed]
- Thilagar, S.; Theyagarajan, R.; Mugri, M.H.; Bahammam, H.A.; Bahammam, S.A.; Bahammam, M.A.; Yadalam, P.K.; Raj, A.T.; Bhandi, S.; Patil, S. Periodontal Treatment for Chronic Periodontitis With Rheumatoid Arthritis. Int. Dent. J. 2022, 72, 832–838. [Google Scholar] [CrossRef]
- Morelli, T.; Agler, C.S.; Divaris, K. Genomics of Periodontal Disease and Tooth Morbidity. Periodontology 2000 2020, 82, 143–156. [Google Scholar] [CrossRef]
- Slavkin, H.C. From High Definition Precision Healthcare to Precision Public Oral Health: Opportunities and Challenges. J. Public Health Dent. 2020, 80 (Suppl. S1), S23–S30. [Google Scholar] [CrossRef]
- Bee, S.-L.; Hamid, Z.A.A. Asymmetric Resorbable-Based Dental Barrier Membrane for Periodontal Guided Tissue Regeneration and Guided Bone Regeneration: A Review. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 2157–2182. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Zhao, X.; Zhang, Y.; Liu, Y.; Li, A.; Pei, D. Clinical Effectiveness of Adjunctive Diode Laser on Scaling and Root Planing in the Treatment of Periodontitis: Is There an Optimal Combination of Usage Mode and Application Regimen? A Systematic Review and Meta-Analysis. Lasers Med. Sci. 2022, 37, 759–769. [Google Scholar] [CrossRef]
- Gomes, E.W.B.; Casarin, M.; Martins, T.M.; da Silva, A.F. Local Delivery Therapies as Adjuvants to Non-Surgical Periodontal Treatment of Periodontitis Grade C: A Systematic Review. Clin. Oral Investig. 2020, 24, 4213–4224. [Google Scholar] [CrossRef]
- Sgolastra, F.; Petrucci, A.; Ciarrocchi, I.; Masci, C.; Spadaro, A. Adjunctive Systemic Antimicrobials in the Treatment of Chronic Periodontitis: A Systematic Review and Network Meta-Analysis. J. Periodontal Res 2021, 56, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, S.; Satoh, M.; Takiwaki, M.; Nomura, F. Current Status of Proteomic Technologies for Discovering and Identifying Gingival Crevicular Fluid Biomarkers for Periodontal Disease. Int. J. Mol. Sci. 2018, 20, 86. [Google Scholar] [CrossRef]
- Lin, Z.; Strauss, F.J.; Lang, N.P.; Sculean, A.; Salvi, G.E.; Stähli, A. Efficacy of Laser Monotherapy or Non-Surgical Mechanical Instrumentation in the Management of Untreated Periodontitis Patients. A Systematic Review and Meta-Analysis. Clin. Oral Investig. 2021, 25, 375–391. [Google Scholar] [CrossRef]
- Liu, J.; Huang, Y.; Huang, J.; Yang, W.; Tao, R. Effects of Ozone Therapy as an Adjuvant in the Treatment of Periodontitis: A Systematic Review and Meta-Analysis. BMC Oral Health 2025, 25, 335. [Google Scholar] [CrossRef]
- Wang, P.; Sun, F.; Ling, X. Effectiveness of Photodynamic Therapy as an Adjunctive Treatment for Periodontitis: A Systematic Review and Meta-Analysis. J. Stomatol. Oral Maxillofac. Surg. 2025, 126, 102036. [Google Scholar] [CrossRef]
- Mishra, S.; Misra, S.R.; Panda, S.; Mohanty, N.; Manfredi, B.; Parrini, M.; Giacomello, M.S.; Mortellaro, C.; Greco Lucchina, A.; Annunziata, M.; et al. Role of Probiotics in Adjunct to Non-Surgical Periodontal Therapy in Patients with Chronic Periodontitis: A Systematic Review and Meta-Analysis. J. Biol. Regul. Homeost. Agents 2021, 35, 67–78. [Google Scholar] [CrossRef]
- Jervøe-Storm, P.-M.; Bunke, J.; Worthington, H.V.; Needleman, I.; Cosgarea, R.; MacDonald, L.; Walsh, T.; Lewis, S.R.; Jepsen, S. Adjunctive Antimicrobial Photodynamic Therapy for Treating Periodontal and Peri-Implant Diseases. Cochrane Database Syst. Rev. 2024, 7, CD011778. [Google Scholar] [CrossRef] [PubMed]
- Cyris, M.; Festerling, J.; Kahl, M.; Springer, C.; Dörfer, C.E.; Graetz, C. Guided Biofilm Therapy versus Conventional Protocol-Clinical Outcomes in Non-Surgical Periodontal Therapy. BMC Oral Health 2024, 24, 1105. [Google Scholar] [CrossRef]
- Kluknavská, J.; Rabajdová, M.; Urban, P.; Špaková, I.; Klepcová, Z.; Kalinová, K.; Vašková, J. Expression of Selected Inflammatory Proteins and Metalloproteinases in Periodontitis. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 1825–1831. [Google Scholar] [CrossRef]
- Lopez, M.A.; Passarelli, P.C.; Marra, M.; Lopez, A.; D’Angelo, A.; Moffa, A.; Martinez, S.; Casale, M.; D’Addona, A. Photodynamic Therapy (PDT) in Non-Surgical Treatment of Periodontitis. J. Biol. Regul. Homeost. Agents 2020, 34, 67–78, Technology in Medicine. [Google Scholar] [PubMed]
- Paternò Holtzman, L.; Valente, N.A.; Vittorini Orgeas, G.; Copes, L.; Discepoli, N.; Clementini, M. Change in Clinical Parameters after Subgingival Instrumentation for the Treatment of Periodontitis and Timing of Periodontal Re-Evaluation: A Systematic Review and Meta-Analysis. J. Clin. Periodontol. 2025, 52, 137–158. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, A.D.; Inchingolo, A.M.; Malcangi, G.; Avantario, P.; Azzollini, D.; Buongiorno, S.; Viapiano, F.; Campanelli, M.; Ciocia, A.M.; De Leonardis, N.; et al. Effects of Resveratrol, Curcumin and Quercetin Supplementation on Bone Metabolism-A Systematic Review. Nutrients 2022, 14, 3519. [Google Scholar] [CrossRef]
- Malcangi, G.; Marinelli, G.; Inchingolo, A.D.; Trilli, I.; Ferrante, L.; Casamassima, L.; Nardelli, P.; Inchingolo, F.; Palermo, A.; Inchingolo, A.M.; et al. Salivaomics: New Frontiers in Studying the Relationship Between Periodontal Disease and Alzheimer’s Disease. Metabolites 2025, 15, 389. [Google Scholar] [CrossRef]
- Tsuchida, S.; Satoh, M.; Umemura, H.; Sogawa, K.; Kawashima, Y.; Kado, S.; Sawai, S.; Nishimura, M.; Kodera, Y.; Matsushita, K.; et al. Proteomic Analysis of Gingival Crevicular Fluid for Discovery of Novel Periodontal Disease Markers. Proteomics 2012, 12, 2190–2202. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, A.D.; Cazzolla, A.P.; Di Cosola, M.; Greco Lucchina, A.; Santacroce, L.; Charitos, I.A.; Topi, S.; Malcangi, G.; Hazballa, D.; Scarano, A.; et al. The Integumentary System and Its Microbiota between Health and Disease. J. Biol. Regul. Homeost. Agents 2021, 35, 303–321. [Google Scholar] [CrossRef]
- Sonnenschein, S.; Reccius, I.; Kilian, S.; Kim, T.-S. Ten-Year Changes of Periodontitis Grading Using Direct and Indirect Evidence: A Retrospective Evaluation. Quintessence Int. 2024, 55, 772–779. [Google Scholar] [CrossRef]
- Thomas, J.T.; Joseph, B.; Varghese, S.; Thomas, N.G.; Kamalasanan Vijayakumary, B.; Sorsa, T.; Anil, S.; Waltimo, T. Association between Metabolic Syndrome and Salivary MMP-8, Myeloperoxidase in Periodontitis. Oral Dis. 2025, 31, 225–238. [Google Scholar] [CrossRef]
- Oh, T.-J.; Yu, S.-H. Evidence-Based Clinical Practice Guideline for Treatment of Stage IIII Periodontitis. J. Evid. Based Dent. Pract. 2021, 21, 101638. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, F.; Dipalma, G.; Cirulli, N.; Cantore, S.; Saini, R.S.; Altini, V.; Santacroce, L.; Ballini, A.; Saini, R. Microbiological Results of Improvement in Periodontal Condition by Administration of Oral Probiotics. J. Biol. Regul. Homeost. Agents 2018, 32, 1323–1328. [Google Scholar] [PubMed]
- Inchingolo, F.; Santacroce, L.; Cantore, S.; Ballini, A.; Del Prete, R.; Topi, S.; Saini, R.; Dipalma, G.; Arrigoni, R. Probiotics and EpiCor® in Human Health. J. Biol. Regul. Homeost. Agents 2019, 33, 1973–1979. [Google Scholar] [CrossRef]
- Gürsoy, U.K.; Gürsoy, M.; Könönen, E. Biomarkers and Periodontal Regenerative Approaches. Dent. Clin. N. Am. 2022, 66, 157–167. [Google Scholar] [CrossRef]
- Inchingolo, F.; Inchingolo, A.M.; Latini, G.; Ferrante, L.; Trilli, I.; Del Vecchio, G.; Palmieri, G.; Malcangi, G.; Inchingolo, A.D.; Dipalma, G. Oxidative Stress and Natural Products in Orthodontic Treatment: A Systematic Review. Nutrients 2023, 16, 113. [Google Scholar] [CrossRef]
- Lahoud, P.; Jacobs, R.; Boisse, P.; EzEldeen, M.; Ducret, M.; Richert, R. Precision Medicine Using Patient-Specific Modelling: State of the Art and Perspectives in Dental Practice. Clin. Oral Investig. 2022, 26, 5117–5128. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, A.M.; Malcangi, G.; Ferrante, L.; Del Vecchio, G.; Viapiano, F.; Inchingolo, A.D.; Mancini, A.; Annicchiarico, C.; Inchingolo, F.; Dipalma, G.; et al. Surface Coatings of Dental Implants: A Review. J. Funct. Biomater. 2023, 14, 287. [Google Scholar] [CrossRef]
- Inchingolo, F.; Inchingolo, A.M.; Malcangi, G.; De Leonardis, N.; Sardano, R.; Pezzolla, C.; de Ruvo, E.; Di Venere, D.; Palermo, A.; Inchingolo, A.D.; et al. The Benefits of Probiotics on Oral Health: Systematic Review of the Literature. Pharmaceuticals 2023, 16, 1313. [Google Scholar] [CrossRef]
- Del Corso, M.; Vervelle, A.; Simonpieri, A.; Jimbo, R.; Inchingolo, F.; Sammartino, G.; Dohan Ehrenfest, D.M. Current Knowledge and Perspectives for the Use of Platelet-Rich Plasma (PRP) and Platelet-Rich Fibrin (PRF) in Oral and Maxillofacial Surgery Part 1: Periodontal and Dentoalveolar Surgery. Curr. Pharm. Biotechnol. 2012, 13, 1207–1230. [Google Scholar] [CrossRef]
- Ballini, A.; Dipalma, G.; Isacco, C.G.; Boccellino, M.; Di Domenico, M.; Santacroce, L.; Nguyễn, K.C.D.; Scacco, S.; Calvani, M.; Boddi, A.; et al. Oral Microbiota and Immune System Crosstalk: A Translational Research. Biology 2020, 9, 131. [Google Scholar] [CrossRef]
- Paqué, P.N.; Hjerppe, J.; Zuercher, A.N.; Jung, R.E.; Joda, T. Salivary Biomarkers as Key to Monitor Personalized Oral Healthcare and Precision Dentistry: A Scoping Review. Front. Oral Health 2022, 3, 1003679. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, A.D.; Di Cosola, M.; Inchingolo, A.M.; Greco Lucchina, A.; Malcangi, G.; Pettini, F.; Scarano, A.; Bordea, I.R.; Hazballa, D.; Lorusso, F.; et al. Correlation between Occlusal Trauma and Oral Microbiota: A Microbiological Investigation. J. Biol. Regul. Homeost. Agents 2021, 35, 295–302. [Google Scholar] [CrossRef]
- Minić, I.; Pejčić, A.; Bradić-Vasić, M. Effect of the Local Probiotics in the Therapy of Periodontitis A Randomized Prospective Study. Int. J. Dent. Hyg. 2022, 20, 401–407. [Google Scholar] [CrossRef]
- Inchingolo, F.; Inchingolo, A.M.; Inchingolo, A.D.; Fatone, M.C.; Ferrante, L.; Avantario, P.; Fiore, A.; Palermo, A.; Amenduni, T.; Galante, F.; et al. Bidirectional Association between Periodontitis and Thyroid Disease: A Scoping Review. Int. J. Environ. Res. Public Health 2024, 21, 860. [Google Scholar] [CrossRef] [PubMed]
- Dipalma, G.; Inchingolo, A.D.; Fiore, A.; Balestriere, L.; Nardelli, P.; Casamassima, L.; Di Venere, D.; Palermo, A.; Inchingolo, F.; Inchingolo, A.M. The Differential Impact of Clear Aligners and Fixed Orthodontic Appliances on Periodontal Health: A Systematic Review. Children 2025, 12, 138. [Google Scholar] [CrossRef]
- Steigmann, L.; Kačarević, Ž.P.; Khoury, J.; Nagy, K.; Feres, M. Integration of Precision Medicine into the Dental Care Setting. Front. Dent. Med. 2024, 5, 1398897. [Google Scholar] [CrossRef]
- Matthews, D.C. Prevention and Treatment of Periodontal Diseases in Primary Care. Evid. Based Dent. 2014, 15, 68–69. [Google Scholar] [CrossRef][Green Version]
- Laforgia, A.; Inchingolo, A.D.; Piras, F.; Colonna, V.; Giorgio, R.V.; Carone, C.; Rapone, B.; Malcangi, G.; Inchingolo, A.M.; Inchingolo, F.; et al. Therapeutic Strategies and Genetic Implications for Periodontal Disease Management: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 7217. [Google Scholar] [CrossRef]
- Ballini, A.; Cantore, S.; Farronato, D.; Cirulli, N.; Inchingolo, F.; Papa, F.; Malcangi, G.; Inchingolo, A.D.; Dipalma, G.; Sardaro, N.; et al. Periodontal Disease and Bone Pathogenesis: The Crosstalk between Cytokines and Porphyromonas Gingivalis. J. Biol. Regul. Homeost. Agents 2015, 29, 273–281. [Google Scholar]
- Drisko, C.H. Nonsurgical Periodontal Therapy. Periodontology 2000 2001, 25, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, F.; Inchingolo, A.M.; Avantario, P.; Settanni, V.; Fatone, M.C.; Piras, F.; Di Venere, D.; Inchingolo, A.D.; Palermo, A.; Dipalma, G. The Effects of Periodontal Treatment on Rheumatoid Arthritis and of Anti-Rheumatic Drugs on Periodontitis: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 17228. [Google Scholar] [CrossRef] [PubMed]
- Rapone, B.; Ferrara, E.; Santacroce, L.; Topi, S.; Gnoni, A.; Dipalma, G.; Mancini, A.; Di Domenico, M.; Tartaglia, G.M.; Scarano, A.; et al. The Gaseous Ozone Therapy as a Promising Antiseptic Adjuvant of Periodontal Treatment: A Randomized Controlled Clinical Trial. Int. J. Environ. Res. Public Health 2022, 19, 985. [Google Scholar] [CrossRef]
- Žiemytė, M.; Lopez-Roldan, A.; Carda-Diéguez, M.; Reglero-Santaolaya, M.; Rodriguez, A.; Ferrer, M.D.; Mira, A. Personalized Antibiotic Selection in Periodontal Treatment Improves Clinical and Microbiological Outputs. Front. Cell. Infect. Microbiol. 2023, 13, 1307380. [Google Scholar] [CrossRef]
- Almabadi, E.S.; Bauman, A.; Akhter, R.; Gugusheff, J.; Van Buskirk, J.; Sankey, M.; Palmer, J.E.; Kavanagh, D.J.; Seymour, G.J.; Cullinan, M.P.; et al. The Effect of a Personalized Oral Health Education Program on Periodontal Health in an At-Risk Population: A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2021, 18, 846. [Google Scholar] [CrossRef]
- Sparks, J.A.; Iversen, M.D.; Kroouze, R.M.; Mahmoud, T.G.; Triedman, N.A.; Kalia, S.S.; Atkinson, M.L.; Lu, B.; Deane, K.D.; Costenbader, K.H.; et al. Personalized Risk Estimator for Rheumatoid Arthritis (PRE-RA) Family Study: Rationale and Design for a Randomized Controlled Trial Evaluating Rheumatoid Arthritis Risk Education to First-Degree Relatives. Contemp. Clin. Trials 2014, 39, 145–157. [Google Scholar] [CrossRef]
- Polizzi, A.; Alibrandi, A.; Lo Giudice, A.; Distefano, A.; Orlando, L.; Analazi, A.M.; Pizzo, G.; Volti, G.L.; Isola, G. Impact of Periodontal microRNAs Associated with Alveolar Bone Remodeling during Orthodontic Tooth Movement: A Randomized Clinical Trial. J. Transl. Med. 2024, 22, 1155. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-T.; Meng, H.-W.; Tran, D.; Brandon, R.; Ayilavarapu, S.; Walji, M.F.; Angelov, N. Using Precision Periodontal Health Care Chart (PPHCC) to Improve Periodontal Health. Clin. Oral Investig. 2024, 28, 542. [Google Scholar] [CrossRef]
- Pakdeesettakul, S.; Charatkulangkun, O.; Lertpimonchai, A.; Wang, H.-L.; Sutthiboonyapan, P. Simple Flowcharts for Periodontal Diagnosis Based on the 2018 New Periodontal Classification Increased Accuracy and Clinician Confidence in Making a Periodontal Diagnosis: A Randomized Crossover Trial. Clin. Oral. Investig. 2022, 26, 7021–7031. [Google Scholar] [CrossRef]
- Cennamo, N.; Bencivenga, D.; Annunziata, M.; Arcadio, F.; Stampone, E.; Piccirillo, A.; Della Ragione, F.; Zeni, L.; Guida, L.; Borriello, A. Plasmon Resonance Biosensor for Interleukin-1β Point-of-Care Determination: A Tool for Early Periodontitis Diagnosis. iScience 2024, 27, 108741. [Google Scholar] [CrossRef]
- Clarkson, J.E.; Ramsay, C.R.; Averley, P.; Bonetti, D.; Boyers, D.; Campbell, L.; Chadwick, G.R.; Duncan, A.; Elders, A.; Gouick, J.; et al. IQuaD Dental Trial; Improving the Quality of Dentistry: A Multicentre Randomised Controlled Trial Comparing Oral Hygiene Advice and Periodontal Instrumentation for the Prevention and Management of Periodontal Disease in Dentate Adults Attending Dental Primary Care. BMC Oral Health 2013, 13, 58. [Google Scholar] [CrossRef] [PubMed]
- Giannobile, W.V.; Braun, T.M.; Caplis, A.K.; Doucette-Stamm, L.; Duff, G.W.; Kornman, K.S. Patient Stratification for Preventive Care in Dentistry. J. Dent. Res. 2013, 92, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Silva-Boghossian, C.M.; Colombo, A.P.V.; Tanaka, M.; Rayo, C.; Xiao, Y.; Siqueira, W.L. Quantitative Proteomic Analysis of Gingival Crevicular Fluid in Different Periodontal Conditions. PLoS ONE 2013, 8, e75898. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, B.; Li, D.; Zhang, Y.; Xue, Y.; Hu, K. Machine Learning and Mendelian Randomization Reveal Molecular Mechanisms and Causal Relationships of Immune-Related Biomarkers in Periodontitis. Mediat. Inflamm. 2024, 2024, 9983323. [Google Scholar] [CrossRef]
- Bai, Y.; Xie, P.; Jin, Z.; Qin, S.; Ma, G. Leveraging Genetics to Investigate Causal Effects of Immune Cell Phenotypes in Periodontitis: A Mendelian Randomization Study. Front. Genet. 2024, 15, 1382270. [Google Scholar] [CrossRef]
- Fujimori, K.; Yoneda, T.; Tomofuji, T.; Ekuni, D.; Azuma, T.; Maruyama, T.; Sugiura, Y.; Morita, M. Detection of Salivary miRNAs That Predict Chronic Periodontitis Progression: A Cohort Study. Int. J. Environ. Res. Public Health 2021, 18, 8010. [Google Scholar] [CrossRef]
- Nagarajan, R.; Miller, C.S.; Dawson, D.; Al-Sabbagh, M.; Ebersole, J.L. Patient-Specific Variations in Biomarkers across Gingivitis and Periodontitis. PLoS ONE 2015, 10, e0136792. [Google Scholar] [CrossRef]
- Ma, S.; He, H.; Ren, X. Single-Cell and Transcriptome Analysis of Periodontitis: Molecular Subtypes and Biomarkers Linked to Mitochondrial Dysfunction and Immunity. J. Inflamm. Res. 2024, 17, 11659–11678. [Google Scholar] [CrossRef] [PubMed]

| Author (Year) | Study Design | Number of Patients | Average Age & Gender | Materials and Methods | Outcomes |
|---|---|---|---|---|---|
| Rapone et al. (2022) [122] | Randomized Controlled Clinical Trial | 90 patients (45 in test group, 45 in control group) | Test group (SRP + ozone): 51.62 ± 9.56 years, 87% M, 13% F; Control group (SRP): 49.88 ± 10.54 years, 78% M, 22% F | Test group received SRP plus gaseous ozone therapy (ozonated water rinses and ozone gas applications in three steps), while control group received SRP alone. Clinical parameters (PPD, CAL, BOP) assessed at baseline, 3 months, and 6 months. | The test group showed significant improvements in PPD, CAL, and BOP at both 3 and 6 months compared to SRP alone (p < 0.0001), indicating enhanced periodontal healing and immune modulation with adjunctive ozone therapy. |
| Žiemytė et al. (2023) [123] | Double-blind Randomized Controlled Trial | 64 patients (32 per group) | Adults 40–70 years | Compared antibiotics selected by standard DNA hybridization vs. impedance-based biofilm culture system. | The impedance-based group had greater plaque reduction, reduction in periodontal pathogens, and increase in health-associated bacteria, enabling faster personalized therapy selection. |
| Almabadi et al. (2021) [124] | Randomized Controlled Trial | 233 patients (117 intervention, 116 control) | Adults aged 18–60; majority female | Compared personalized oral health education (tailored messages + motivational interviewing) vs. standard care. | Intervention group showed significant reduction in plaque, gingival inflammation, and bleeding on probing at 12 months vs. control. |
| Sparks et al. (2015) [125] | Randomized controlled trial | Unspecified | Adult first-degree relatives; both genders included | Randomized adults with family history of RA to personalized risk education or standard information; follow-up at 6 and 12 months | Changes in risk perception and preventive behaviors after personalized education |
| Polizzi et al. (2024) [126] | Randomized clinical trial | Unspecified | Unspecified | Patients under orthodontic treatment randomized; gingival crevicular fluid collected; miRNA levels analyzed by qRT-PCR during tooth movement | Identification of miRNAs involved in alveolar bone remodeling; potential biomarkers for personalized orthodontic therapy |
| Lee et al. (2024) [127] | Prospective cohort study | 26 | Unspecified | The study tested the PPHCC, a digital tool for personalized periodontal diagnosis. Patients received treatment and follow-up, while usability was evaluated by both patients and providers. | The PPHCC showed high usability and improved patient engagement and communication, though short-term clinical outcomes were similar to the control group. |
| Pakdeesettakul et al. (2022) [128] | Randomized cross-over controlled trial | 153 | Approximately 60% female; most participants aged 21–25 | The study compared diagnostic flowcharts to 2018 consensus reports using 25 validated cases. Participants with different experience levels evaluated cases in two sessions. The study measured diagnostic accuracy, time, confidence, and user perception. | Flowcharts improved diagnostic accuracy and self-confidence, particularly among less experienced users, without increasing diagnosis time. They were viewed as simple, useful, and preferred for clinical use. |
| Cennamo et al. (2024) [129] | Experimental study; | 2 (1 healthy, 1 with periodontitis) + 1 healthy volunteer for calibration | Males aged 61 and 69 | A SPR-POF biosensor coated with anti-IL-1β antibodies was developed and validated for detecting IL-1β in buffer and saliva. It showed good sensitivity, specificity, and stability, with dose–response curves comparable to ELISA, confirming its reliability for salivary biomarker analysis. | The biosensor accurately detected IL-1β with high sensitivity and specificity, effectively distinguishing health from disease. Its performance, comparable to ELISA, supports its use as a robust point-of-care tool for early, personalized diagnosis of periodontitis. |
| Clarkson et al. (2018) [130] | Pragmatic multi-centre randomized controlled trial with a factorial design | 1860 patients | Unspecified | The study tested personalized oral hygiene advice and different periodontal cleaning intervals in a randomized trial, collecting clinical, patient-reported, and economic data over three years. Results aimed to identify effective, cost-efficient strategies for periodontal disease prevention. | The study found that personalized oral hygiene advice, especially when combined with tailored periodontal care, may enhance clinical outcomes and patient behaviors, offering a potentially cost-effective approach to preventing periodontal disease. |
| WV Giannobile et al. (2026) [131] | Retrospective cohort study | 5117 genotyped participants (from 25,452 eligible) | Mean age 47 years; ~65% female | Participants were classified as low- or high-risk based on smoking, diabetes, and IL-1 genotype. Over 16 years, tooth loss and dental care costs were analyzed in relation to preventive visit frequency using insurance data and stratified statistical models. | Two annual visits significantly reduced tooth loss in high-risk patients, while no benefit was seen in low-risk patients. Personalized prevention based on risk stratification proved more effective than uniform care. |
| Silva-Boghossian et al. (2016) [132] | Cross-sectional proteomic study | 30 | Unspecified | GCF samples from subjects with health, gingivitis, or periodontitis were analyzed via LC-MS/MS to detect differences in protein expression. | Inflammatory proteins were elevated in disease, distinguishing each condition and highlighting GCF proteomics as a promising diagnostic tool for precision periodontology |
| Y. Li et al. (2024) [133] | Bioinformatic study integrating multi-omics, ML, MR & scRNA-seq | Unspecified | Unspecified | Transcriptomic datasets; WGCNA; ML (XGBoost); Mendelian randomization; single-cell RNA-seq | Identified 19 core immune-related genes; CD93, CD69, and CXCL6 confirmed as causal genes in periodontitis. Precision medicine validated for biomarker discovery and stratification. |
| Y. Bai et al. (2024) [134] | Transcriptomic and network-based bioinformatic analysis | Unspecified | Unspecified | GSE10334 dataset; WGCNA; GO/KEGG pathway enrichment; PPI network; external validation datasets | 12 gene modules identified; PLEK, TYROBP, LAPTM5 selected as hub genes. Pathways related to immune response and leukocyte migration highlighted. |
| Fujimori et al. (2021) [135] | Integrative transcriptomic study using two datasets | 44 patients | median age, 67.1 years | GSE10334 and GSE16134 datasets; WGCNA; PPI network; ROC curve analysis; immune cell infiltration profiling | Green-yellow gene module significantly associated with periodontitis; 13 hub genes validated; macrophages and mast cells linked to disease pathogenesis. |
| Nagarajan et al. (2015) [136] | Observational cross-sectional study with statistical clustering | 100 (approx.) | Mixed; age ~varied (NR) | Clinical cohort from University of Kentucky; saliva and serum biomarker analysis; cytokines and chemokines; unsupervised clustering; statistical modeling | Biomarker variation shown across individuals; transitions from health to disease are patient-specific. Supports personalized monitoring using inflammatory profiles. |
| S. Ma et al. (2024) [137] | Single-cell RNA-seq and transcriptomic classification study | 40 patients (scRNA-seq) | Unspecified | Single-cell RNA sequencing; bulk transcriptome; consensus clustering; ML-based biomarker selection; qPCR and IHC validation | Identified 4 molecular subtypes (quiescent, macrophage-dominant, mitochondria-dominant, mixed); validated 5 biomarkers (BNIP3, FAHD1, UNG, etc.); mitochondrial dysfunction linked to immune regulation and periodontitis subtyping. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dipalma, G.; Inchingolo, A.M.; Inchingolo, F.; Palumbo, I.; Riccaldo, L.; Guglielmo, M.; Morolla, R.; Palermo, A.; Marinelli, G.; Inchingolo, A.D. The Precision Paradigm in Periodontology: A Multilevel Framework for Tailored Diagnosis, Treatment, and Prevention. J. Pers. Med. 2025, 15, 440. https://doi.org/10.3390/jpm15090440
Dipalma G, Inchingolo AM, Inchingolo F, Palumbo I, Riccaldo L, Guglielmo M, Morolla R, Palermo A, Marinelli G, Inchingolo AD. The Precision Paradigm in Periodontology: A Multilevel Framework for Tailored Diagnosis, Treatment, and Prevention. Journal of Personalized Medicine. 2025; 15(9):440. https://doi.org/10.3390/jpm15090440
Chicago/Turabian StyleDipalma, Gianna, Angelo Michele Inchingolo, Francesco Inchingolo, Irene Palumbo, Lilla Riccaldo, Mariafrancesca Guglielmo, Roberta Morolla, Andrea Palermo, Grazia Marinelli, and Alessio Danilo Inchingolo. 2025. "The Precision Paradigm in Periodontology: A Multilevel Framework for Tailored Diagnosis, Treatment, and Prevention" Journal of Personalized Medicine 15, no. 9: 440. https://doi.org/10.3390/jpm15090440
APA StyleDipalma, G., Inchingolo, A. M., Inchingolo, F., Palumbo, I., Riccaldo, L., Guglielmo, M., Morolla, R., Palermo, A., Marinelli, G., & Inchingolo, A. D. (2025). The Precision Paradigm in Periodontology: A Multilevel Framework for Tailored Diagnosis, Treatment, and Prevention. Journal of Personalized Medicine, 15(9), 440. https://doi.org/10.3390/jpm15090440












