Frailty as an Independent Predictor of Mortality in Patients with Sepsis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population and Inclusion Criteria
2.3. Sample Size Calculation
2.4. Variables of the Study
2.4.1. Independent Variables
- •
- Sex.
- •
- Age.
- •
- Institutionalization, defined as residence in a long-term care facility before hospital admission.
- •
- Primary infection source: respiratory; urinary; abdominal; unknown or other.
- •
- Type of infection: community acquired or healthcare associated (nosocomial).
- •
- Hospitalization within 30 days prior to the sepsis episode.
- •
- Number of hospital admissions in the 12 months preceding the sepsis episode.
- •
- •
- Individual comorbidities as defined by the CCI.
- •
- Frailty assessed using the Rockwood Clinical Frailty Scale (CFS) [19] only in patients above 65 years (Figure S2). Frailty was evaluated by a trained member of the research team who retrospectively reviewed the medical records of each patient. Whenever the scale score had been documented during admission by the clinical team, that value was used directly. In cases where no explicit score was recorded, the evaluator systematically examined clinical documentation from the six months prior to the sepsis episode—including previous hospital admissions, discharge summaries, progress notes, consultations, nursing comments, and recorded functional scales—to assign the frailty score that best reflected the patient’s baseline condition. Patients with a score ≥ 5 were considered frail. Cases in which the available documentation did not allow for a clear classification were excluded from the frailty analysis.
- •
- Anemia on admission (hemoglobin < 12 g/dL in women and <13 g/dL in men, per World Health Organization criteria [20]).
- •
- SOFA score within the first 24 h.
2.4.2. Outcome Variables
- •
- In-hospital mortality.
- •
- Twelve-month mortality.
- •
- Admission to medical or surgical ICU.
2.5. Statistical Analysis
3. Results
3.1. Age
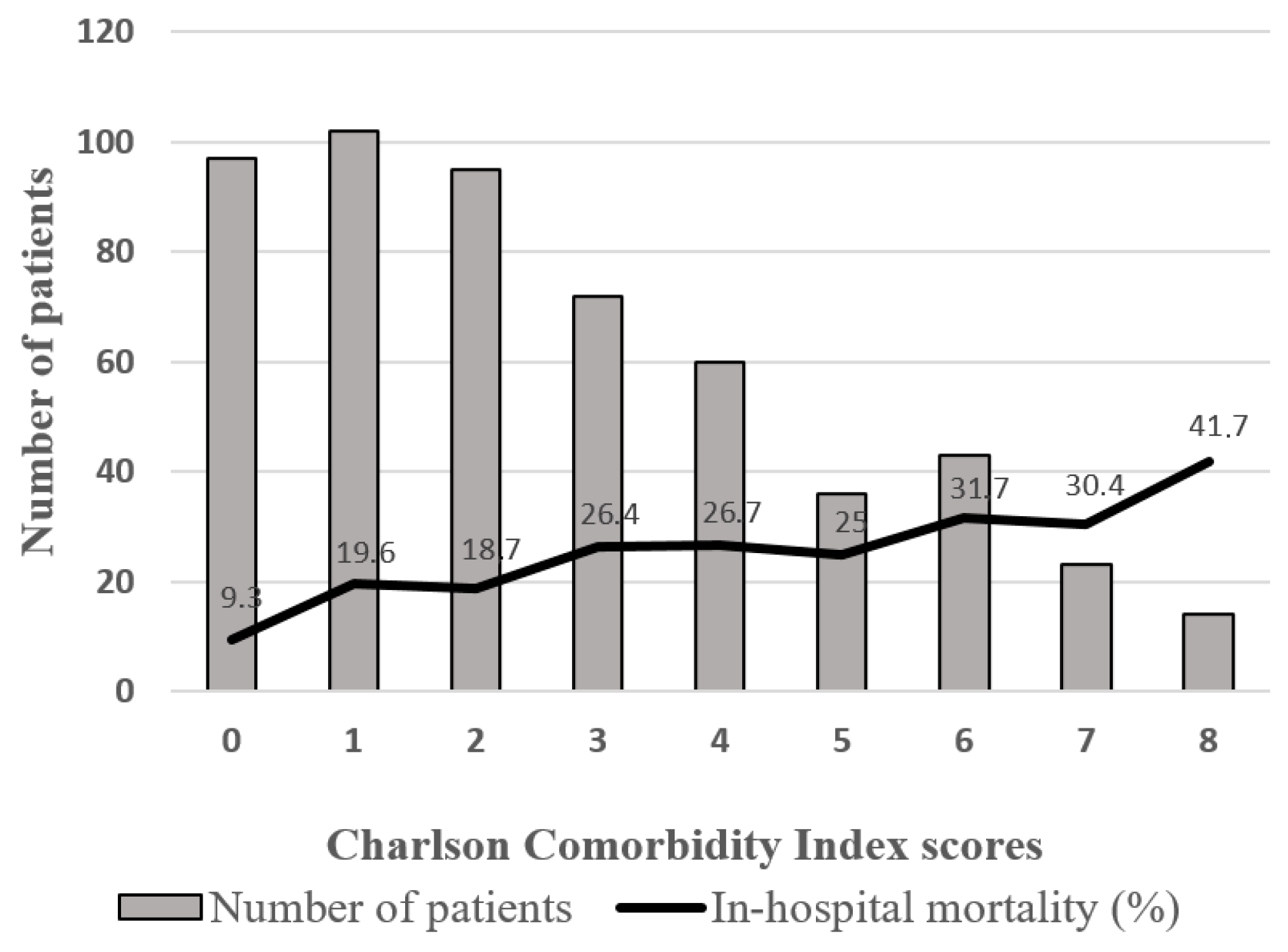
3.2. Charlson Comorbidity Index
3.3. SOFA
3.4. Frailty
3.5. Chronic Diseases
3.6. Anemia
3.7. Infection Source
3.8. ICU Admission
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SOFA | Sequential Organ Failure Assessment |
| ICU | Intensive Care Unit |
| CCI | Charlson Comorbidity Index |
| CFS | Clinical Frailty Score |
| OR | Odds Ratio |
| CI | Confidence Interval |
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef] [PubMed]
- Méndez, R.; Figuerola, A.; Ramasco, F.; Chicot, M.; Pascual, N.F.; García, Í.; von Wernitz, A.; Zurita, N.D.; Semiglia, A.; Pizarro, A.; et al. Decrease in Mortality after the Implementation of a Hospital Model to Improve Performance in Sepsis Care: Princess Sepsis Code. J. Pers. Med. 2024, 14, 149. [Google Scholar] [CrossRef] [PubMed]
- Buchman, T.G.; Simpson, S.Q.; Sciarretta, K.L.; Finne, K.P.B.; Sowers, N.M.; Collier, M.B.; Chavan, S.M.; Oke, I.M.; Pennini, M.E.; Santhosh, A.M.; et al. Sepsis Among Medicare Beneficiaries: 1. The Burdens of Sepsis, 2012–2018. Crit. Care Med. 2020, 48, 276–288. [Google Scholar] [CrossRef]
- Kolodyazhna, A.; Wiersinga, W.J.; van der Poll, T. Aiming for precision: Personalized medicine through sepsis subtyping. Burn. Trauma 2025, 13, tkae073. [Google Scholar] [CrossRef]
- Vincent, J.L.; Moreno, R.; Takala, J.; Willatts, S.; De Mendonça, A.; Bruining, H.; Reinhart, C.K.; Suter, P.M.; Thijs, L.G. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996, 22, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Sarraf, E.; Sadr, A.V.; Abedi, V.; Bonavia, A.S. Enhancing Sepsis prognosis: Integrating social determinants and demographic variables into a comprehensive model for critically ill patients. J. Crit. Care 2024, 83, 154857. [Google Scholar] [CrossRef]
- Alrawashdeh, M.; Klompas, M.; Simpson, S.Q.; Kadri, S.S.; Poland, R.; Guy, J.S.; Perlin, J.B.; Rhee, C. Prevalence and Outcomes of Previously Healthy Adults Among Patients Hospitalized With Community-Onset Sepsis. Chest 2022, 162, 101–110. [Google Scholar] [CrossRef]
- Oud, L.; Garza, J. Previously healthy adults among septic patients: Population-level epidemiology and outcomes. J. Crit. Care 2024, 79, 154427. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Mensa, J.; Barberán, J.; Ferrer, R.; Borges, M.; Rascado, P.; Maseda, E.; Oliver, A.; Marco, F.; Adalia, R.; Aguilar, G.; et al. Recommendations for antibiotic selection for severe nosocomial infections. Rev. Esp. Quimioter. 2021, 34, 511–524. [Google Scholar] [CrossRef]
- Oltean, S.; Ţǎţulescu, D.; Bondor, C.; Slavcovici, A.; Cismaru, C.; Lupşe, M.; Muntean, M.; Jianu, C.; Marcu, C.; Oltean, M. Charlson’s weighted index of comorbidities is useful in assessing the risk of death in septic patients. J. Crit. Care 2012, 27, 370–375. [Google Scholar] [CrossRef]
- Hampshire, P.A.; Guha, A.; Strong, A.; Parsons, D.; Rowan, P. An evaluation of the Charlson co-morbidity score for predicting sepsis after elective major surgery. Indian J. Crit. Care Med. 2011, 15, 30–36. [Google Scholar] [CrossRef]
- Jouffroy, R.; Parfait, P.A.; Gilbert, B.; Tourtier, J.P.; Bloch-Laine, E.; Ecollan, P.; Boularan, J.; Bounes, V.; Vivien, B.; Gueye, P.-N. Relationship between prehospital modified Charlson Comorbidity Index and septic shock 30-day mortality. Am. J. Emerg. Med. 2022, 60, 128–133. [Google Scholar] [CrossRef]
- Walston, J.; Hadley, E.C.; Ferrucci, L.; Guralnik, J.M.; Newman, A.B.; Studenski, S.A.; Ershler, W.B.; Harris, T.; Fried, L.P. Research agenda for frailty in older adults: Toward a better understanding of physiology and etiology: Summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J. Am. Geriatr. Soc. 2006, 54, 991–1001. [Google Scholar] [CrossRef] [PubMed]
- Moïsi, L.; Mino, J.-C.; Guidet, B.; Vallet, H. Frailty assessment in critically ill older adults: A narrative review. Ann. Intensive Care 2024, 14, 93. [Google Scholar] [CrossRef] [PubMed]
- Cecconi, M.; Leaver, S.; Jung, C. Caring for frail patients in the ICU: A multidimensional approach. Intensive Care Med. 2024, 50, 583–586. [Google Scholar] [CrossRef]
- Gordon, J.I.; Brummel, N.E. Implications of frailty before and after intensive care unit admission. Curr. Opin. Crit. Care 2024, 30, 472–478. [Google Scholar] [CrossRef]
- Rockwood, K.; Song, X.; MacKnight, C.; Bergman, H.; Hogan, D.B.; McDowell, I.; Mitnitski, A. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005, 173, 489–495. [Google Scholar] [CrossRef]
- World Health Organization (Ed.) Guideline on Haemoglobin Cutoffs to Define Anaemia in Individuals and Populations; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- Flaatten, H.; De Lange, D.W.; Morandi, A.; Andersen, F.H.; Artigas, A.; Bertolini, G.; Boumendil, A.; Cecconi, M.; Christensen, S.; Faraldi, L.; et al. The impact of frailty on ICU and 30-day mortality and the level of care in very elderly patients (≥80 years). Intensive Care Med. 2017, 43, 1820–1828. [Google Scholar] [CrossRef] [PubMed]
- Guidet, B.; de Lange, D.W.; Boumendil, A.; Leaver, S.; Watson, X.; Boulanger, C.; Szczeklik, W.; Artigas, A.; Morandi, A.; Andersen, F.; et al. The contribution of frailty, cognition, activity of daily life and comorbidities on outcome in acutely admitted patients over 80 years in European ICUs: The VIP2 study. Intensive Care Med. 2020, 46, 57–69. [Google Scholar] [CrossRef]
- Fernando, S.M.; McIsaac, D.I.; Perry, J.J.; Rochwerg, B.; Bagshaw, S.M.; Thavorn, K.; Seely, A.J.E.; Forster, A.J.; Fiest, K.M.; Dave, C.; et al. Frailty and Associated Outcomes and Resource Utilization Among Older ICU Patients With Suspected Infection. Crit. Care Med. 2019, 47, e669–e676. [Google Scholar] [CrossRef] [PubMed]
- McIsaac, D.I.; Taljaard, M.; Bryson, G.L.; Beaulé, P.E.; Gagné, S.; Hamilton, G.; Hladkowicz, E.; Huang, A.; Joanisse, J.A.; Lavallée, L.T.; et al. Frailty as a Predictor of Death or New Disability After Surgery: A Prospective Cohort Study. Ann. Surg. 2020, 271, 283–289. [Google Scholar] [CrossRef]
- Makary, M.A.; Segev, D.L.; Pronovost, P.J.; Syin, D.; Bandeen-Roche, K.; Patel, P.; Takenaga, R.; Devgan, L.; Holzmueller, C.G.; Tian, J.; et al. Frailty as a predictor of surgical outcomes in older patients. J. Am. Coll. Surg. 2010, 210, 901–908. [Google Scholar] [CrossRef]
- Lin, H.-S.; Watts, J.N.; Peel, N.M.; Hubbard, R.E. Frailty and post-operative outcomes in older surgical patients: A systematic review. BMC Geriatr. 2016, 16, 157. [Google Scholar] [CrossRef]
- POSE-Study group∗ Peri-interventional outcome study in the elderly in Europe: A 30-day prospective cohort study. Eur. J. Anaesthesiol. 2022, 39, 198–209. [CrossRef]
- Muscedere, J.; Waters, B.; Varambally, A.; Bagshaw, S.M.; Boyd, J.G.; Maslove, D.; Sibley, S.; Rockwood, K. The impact of frailty on intensive care unit outcomes: A systematic review and meta-analysis. Intensive Care Med. 2017, 43, 1105–1122. [Google Scholar] [CrossRef] [PubMed]
- Stavem, K.; Hoel, H.; Skjaker, S.A.; Haagensen, R. Charlson comorbidity index derived from chart review or administrative data: Agreement and prediction of mortality in intensive care patients. Clin. Epidemiol. 2017, 9, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Nagata, T.; Adachi, Y.; Taniguchi, A.; Kimura, Y.; Iitaka, D.; Iwata, G.; Yamaoka, N. [Impact of the Age-Adjusted Charlson Comorbidity Index on Postoperative Complications after Gastric Cancer Surgery]. Gan To Kagaku Ryoho 2021, 48, 1567–1569. [Google Scholar]
- Cheng, H.; Shao, L.; Wu, H.; Mi, B.; Li, Q.; Zhang, J. Older Adult Sepsis Survivors Discharged to Skilled Nursing Facilities: Age-Adjusted Charlson Comorbidity Index as a Predictor of 6-Month Mortality. Nurs. Crit. Care 2025, 30, e70078. [Google Scholar] [CrossRef]
- Kempker, J.A.; Kramer, M.R.; Waller, L.A.; Martin, G.S. Risk Factors for Septicemia Deaths and Disparities in a Longitudinal US Cohort. Open Forum Infect. Dis. 2018, 5, ofy305. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Su, Y.; Luo, J.; Ding, N. Association between admission hemoglobin level and prognosis in sepsis patients based on a critical care database. Sci. Rep. 2024, 14, 5212. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Ogura, H.; Kushimoto, S.; Shiraishi, A.; Sugiyama, T.; Deshpande, G.A.; Uchida, M.; Nagata, I.; Saitoh, D.; Fujishima, S.; et al. Variations in infection sites and mortality rates among patients in intensive care units with severe sepsis and septic shock in Japan. J. Intensive Care 2019, 7, 28. [Google Scholar] [CrossRef]
- Oliveira, A.M.; Oliveira, A.; Vidal, R.; Gonçalves-Pereira, J. Infectious Foci, Comorbidities and Its Influence on the Outcomes of Septic Critically Ill Patients. Microorganisms 2024, 12, 1705. [Google Scholar] [CrossRef]
- Pieroni, M.; Olier, I.; Ortega-Martorell, S.; Johnston, B.W.; Welters, I.D. In-Hospital Mortality of Sepsis Differs Depending on the Origin of Infection: An Investigation of Predisposing Factors. Front. Med. 2022, 9, 915224. [Google Scholar] [CrossRef]
- Truog, R.D.; Brock, D.W.; Cook, D.J.; Danis, M.; Luce, J.M.; Rubenfeld, G.D.; Levy, M.M. Rationing in the intensive care unit. Crit. Care Med. 2006, 34, 958–963. [Google Scholar] [CrossRef]
- Azoulay, É.; Pochard, F.; Chevret, S.; Vinsonneau, C.; Garrouste, M.; Cohen, Y.; Thuong, M.; Paugam, C.; Apperre, C.; De Cagny, B.; et al. Compliance with triage to intensive care recommendations. Crit. Care Med. 2001, 29, 2132–2136. [Google Scholar] [CrossRef] [PubMed]
- Garrouste-Orgeas, M.; Montuclard, L.; Timsit, J.-F.; Reignier, J.; Desmettre, T.; Karoubi, P.; Moreau, D.; Montesino, L.; Duguet, A.; Boussat, S.; et al. Predictors of intensive care unit refusal in French intensive care units: A multiple-center study. Crit. Care Med. 2005, 33, 750–755. [Google Scholar] [CrossRef]
- Escher, M.; Perneger, T.V.; Chevrolet, J.-C. National questionnaire survey on what influences doctors’ decisions about admission to intensive care. BMJ 2004, 329, 425. [Google Scholar] [CrossRef]
- Strandberg, G.; Walther, S.; Agvald Öhman, C.; Lipcsey, M. Mortality after Severe Sepsis and Septic Shock in Swedish Intensive Care Units 2008-2016-A nationwide observational study. Acta Anaesthesiol. Scand. 2020, 64, 967–975. [Google Scholar] [CrossRef]
- Prescott, H.C.; Osterholzer, J.J.; Langa, K.M.; Angus, D.C.; Iwashyna, T.J. Late mortality after sepsis: Propensity matched cohort study. BMJ 2016, 353, i2375. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, R.S.; Monneret, G.; Payen, D. Immunosuppression in sepsis: A novel understanding of the disorder and a new therapeutic approach. Lancet Infect. Dis. 2013, 13, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Wilhelms, S.B.; Walther, S.M.; Sjöberg, F.; De Geer, L. Causes of late mortality among ICU-treated patients with sepsis. Acta Anaesthesiol. Scand. 2020, 64, 961–966. [Google Scholar] [CrossRef] [PubMed]

| Patient Characteristics | All Patients n = 547 | ICU n = 199 | Non ICU n = 348 |
|---|---|---|---|
| Age, years a | 73.7 ± 17 | 68.9 ± 8.5 | 76 ± 2.1 |
| <65, n (%) | 126 (23) | 62 (31) | 64 (18) |
| ≥65, n (%) | 421 (77) | 137 (69) | 284 (82) |
| Male sex, n (%) | 328 (60) | 130 (65) | 198 (57) |
| Place of residence, n (%) | |||
| Non institutionalized | 496 (91) | 194 (97) | 302 (87) |
| Institutionalized | 46 (8) | 5 (3) | 41 (12) |
| Other | 5 (1) | 0 (0) | 5 (1) |
| Rockwood Frailty Score, n (%) | |||
| <5 | 226 (55) | 104 (78) | 122 (44) |
| ≥5 | 184 (45) | 29 (22) | 155 (56) |
| CCI a | 2.78 ± 1.0 | 2.59 ± 1.4 | 2.88 ± 1.4 |
| <2, n (%) | 199 (36) | 74 (37) | 124 (36) |
| ≥2, n (%) | 348 (64) | 125 (63) | 224 (64) |
| Anemia, n (%) | 271 (50) | 102 (51) | 169 (49) |
| Initial SOFA a | 4.8 ± 2.5 | 5.56 ± 2.8 | 4.35 ± 2.1 |
| <4, n (%) | 190 (35) | 49 (25) | 141 (41) |
| ≥4, n (%) | 357 (65) | 150 (75) | 207 (59) |
| Type of infection, n (%) | |||
| Community-acquired | 447 (82) | 144 (72) | 303 (88) |
| Nosocomial | 96 (18) | 55 (28) | 41 (12) |
| Source of infection, n (%) | |||
| Respiratory | 196 (36) | 51 (26) | 145 (42) |
| Abdominal | 160 (29) | 82 (41) | 78 (22) |
| Urinary | 121 (22) | 38 (19) | 83 (24) |
| Unknown | 32 (6) | 12 (6) | 20 (6) |
| Other | 38 (7) | 16 (8) | 22 (6) |
| Contact with healthcare services | |||
| Admission < 30 days, n (%) | 81 (15) | 25 (13) | 56 (15) |
| Number admissions previous year a | 0.83 ± 0.5 | 0.77 ± 0 | 0.86 ± 0 |
| 0, n (%) | 282 (55) | 107 (58) | 177 (54) |
| 1–3, n (%) | 213 (42) | 72 (39) | 141 (43) |
| ≥4, n (%) | 18 (3) | 6 (3) | 12 (4) |
| In-hospital mortality, n (%) | 116 (21) | 42 (21) | 74 (21) |
| 12-month mortality, n (%) | 184 (34) | 68 (34) | 116 (34) |
| In-Hospital Mortality | 12-Month Mortality | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p Value | OR | 95% CI | p Value | |
| Age ≥ 65 | 2.16 | 1.22–3.83 | 0.007 | 2.41 | 1.50–3.88 | <0.001 |
| CCI ≥ 2 | 2.06 | 1.35–3.13 | 0.001 | 3.05 | 2.10–4.42 | <0.001 |
| SOFA ≥ 4 | 2.47 | 1.62–3.78 | <0.001 | 2.28 | 1.58–3.28 | <0.001 |
| Frailty (CFS ≥ 5) | 2.21 | 1.39–3.50 | 0.001 | 2.19 | 1.46–3.30 | <0.001 |
| Ischemic heart disease | 2.41 | 1.45–4.02 | 0.001 | 2.44 | 1.51–3.96 | <0.001 |
| Dementia | 0.384 | 1.98 | 1.12–3.51 | 0.017 | ||
| Severe liver disease | 2.68 | 1.00–7.21 | 0.042 | 0.261 | ||
| Leukemia | 2.28 | 1.01–5.13 | 0.041 | 2.75 | 1.24–6.12 | 0.010 |
| Disseminated oncologic disease | 2.20 | 1.15–4.20 | 0.015 | 5.12 | 2.65–9.87 | <0.001 |
| Anemia | 1.67 | 1.10–2.54 | 0.016 | 2.14 | 1.48–3.09 | <0.001 |
| Respiratory infection source | 1.90 | 1.22–3.83 | 0.007 | 1.52 | 1.05–2.20 | 0.025 |
| Urinary infection source | 0.30 | 0.16–0.58 | <0.001 | 0.51 | 0.32–0.82 | 0.004 |
| Abdominal infection source | 1.05 | 0.67–1.64 | 0.839 | 1.05 | 0.71–1.55 | 0.825 |
| In-Hospital Mortality | 12-Month Mortality | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p Value | OR | 95% CI | p Value | |
| Age ≥ 65 | 0.18 | 1.98 | 1.09–3.59 | 0.025 | ||
| CCI ≥ 2 | 0.57 | 0.33 | ||||
| SOFA ≥ 4 | 2.13 | 1.34–3.38 | 0.001 | 2.05 | 1.35–3.10 | 0.001 |
| Frailty (CFS ≥ 5) | 2.45 | 1.45–4.15 | 0.001 | 2.02 | 1.24–3.29 | 0.005 |
| Ischemic heart disease | 2.34 | 1.27–4.33 | 0.006 | 2.07 | 1.16–3.70 | 0.014 |
| Dementia | 0.96 | 0.10 | ||||
| Severe liver disease | 3.62 | 1.09–12.10 | 0.036 | 0.25 | ||
| Leukemia | 0.14 | 0.06 | ||||
| Disseminated oncologic disease | 3.14 | 1.43–6.90 | 0.004 | 6.15 | 2.84–13.3 | <0.001 |
| Anemia | 0.10 | 1.85 | 1.21–2.84 | 0.005 | ||
| Respiratory infection source | 0.37 | 0.47 | ||||
| Urinary infection source | 0.37 | 0.15–0.91 | 0.029 | 0.22 | ||
| Abdominal infection source | 0.85 | 0.22 | ||||
| Bivariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p Value | OR | 95% CI | p Value | |
| Age ≥ 65 | 0.5 | 0.33–0.75 | 0.001 | 0.53 | ||
| SOFA ≥ 4 | 2.81 | 1.96–4.02 | <0.001 | 3.69 | 2.40–5.66 | <0.001 |
| Frailty (CFS ≥ 5) | 0.22 | 0.14–0.35 | <0.001 | 0.20 | 0.09–0.41 | <0.001 |
| Institutionalization | 0.18 | 0.07–0.45 | <0.001 | 0.10 | ||
| Dementia | 0.06 | 0.01–0.24 | <0.001 | 0.14 | 0.03–0.65 | 0.012 |
| Leukemia | 2.75 | 1.24–6.12 | 0.010 | 0.49 | ||
| Heart failure | 0.30 | 0.17–0.54 | <0.001 | 0.45 | 0.23–0.89 | 0.022 |
| Solid malignant neoplasm | 2.81 | 1.70–4.63 | <0.001 | 2.72 | 1.45–5.07 | 0.002 |
| Nosocomial infection | 2.82 | 1.80–4.43 | <0.001 | 2.42 | 1.41–4.16 | 0.001 |
| Respiratory infection source | 0.48 | 0.33–0.71 | <0.001 | 0.26 | ||
| Abdominal infection source | 2.43 | 1.66–3.54 | <0.001 | 1.88 | 1.12–3.13 | 0.016 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Interián, A.; Ramasco, F.; Figuerola, A.; Méndez, R. Frailty as an Independent Predictor of Mortality in Patients with Sepsis. J. Pers. Med. 2025, 15, 398. https://doi.org/10.3390/jpm15090398
Interián A, Ramasco F, Figuerola A, Méndez R. Frailty as an Independent Predictor of Mortality in Patients with Sepsis. Journal of Personalized Medicine. 2025; 15(9):398. https://doi.org/10.3390/jpm15090398
Chicago/Turabian StyleInterián, Alejandro, Fernando Ramasco, Angels Figuerola, and Rosa Méndez. 2025. "Frailty as an Independent Predictor of Mortality in Patients with Sepsis" Journal of Personalized Medicine 15, no. 9: 398. https://doi.org/10.3390/jpm15090398
APA StyleInterián, A., Ramasco, F., Figuerola, A., & Méndez, R. (2025). Frailty as an Independent Predictor of Mortality in Patients with Sepsis. Journal of Personalized Medicine, 15(9), 398. https://doi.org/10.3390/jpm15090398






